Unravelling the Thermal Decomposition Parameters for The Synthesis of Anisotropic Iron Oxide Nanoparticles
Abstract
1. Introduction
2. Experimental Details
2.1. Synthesis Methods
2.2. Characterization Methods
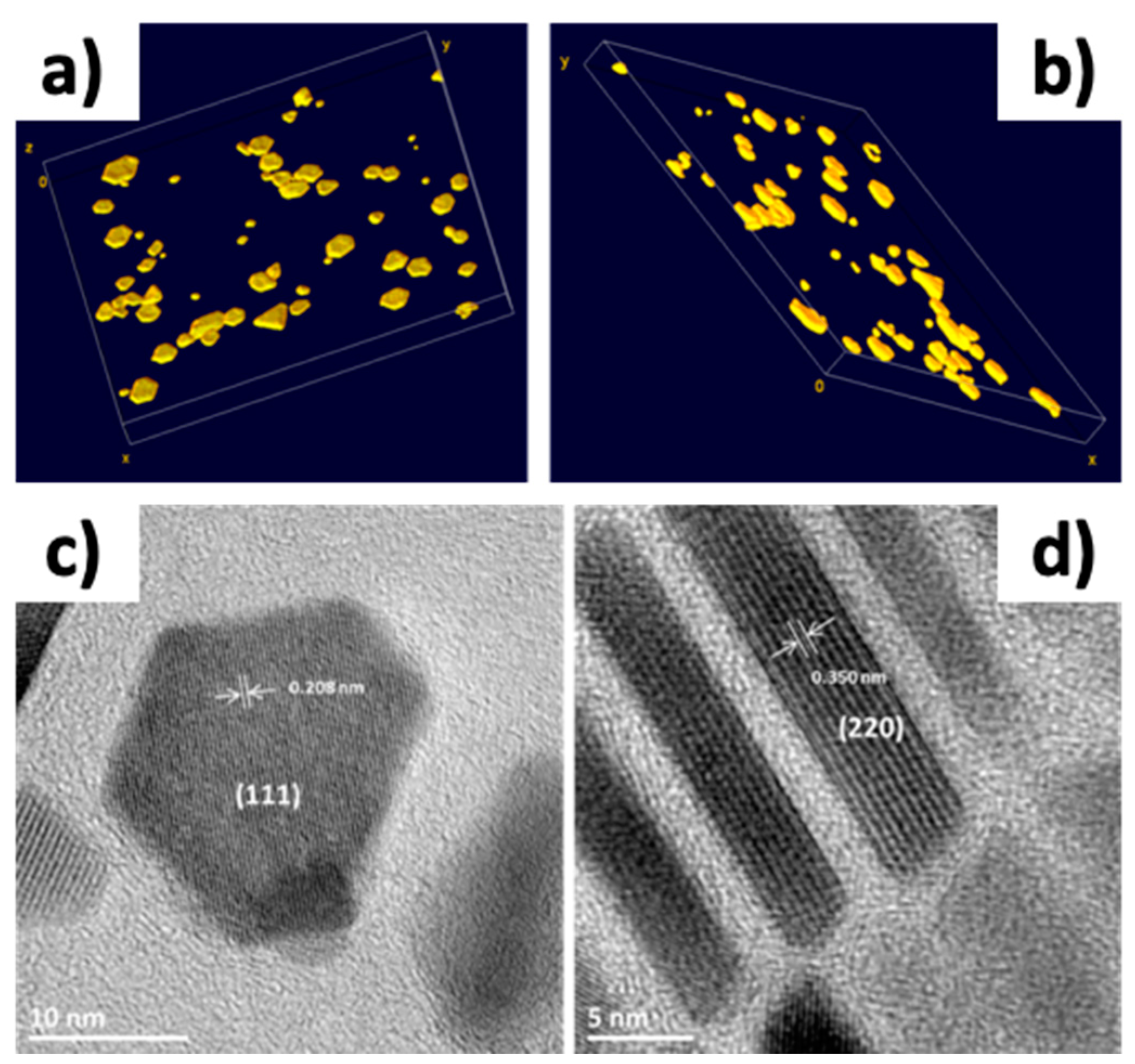
3. Results and Discussion
3.1. Influence of the Structure of Precursors and Sodium Oleate Ligand
3.2. An Optimization of the Cubic Shape NPs Leading to Octopod Shape NPs
3.3. Exploring the Nanoplates Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tartaj, P.; Morales, M.P.; Gonzalez-Carreño, T.; Veintemillas-Verdaguer, S.; Serna, C.J. The Iron Oxides Strike Back: From Biomedical Applications to Energy Storage Devices and Photoelectrochemical Water Splitting. Adv. Mater. 2011, 23, 5243–5249. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Black, C.T.; Sandstrom, R.L.; Rice, P.M.; Murray, C.B.; Sun, S. Magnetotransport of magnetite nanoparticle arrays. Phys. Rev. B 2006, 73, 020402. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Frey, N.A.; Peng, S.; Cheng, K.; Sun, S. Magnetic nanoparticles: synthesis, functionalization, and applications in bioimaging and magnetic energy storage. Chem. Soc. Rev. 2009, 38, 2532–2542. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.-H.; Salabas, E.L.; Schüth, F. Magnetic Nanoparticles: Synthesis, Protection, Functionalization, and Application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.V.M.; Prakash, T.; Kennedy, J.; Chong, S.V.; Rubanov, S. Spin-dependent tunnelling in magnetite nanoparticles. J. Magn. Magn. Mater. 2018, 460, 229–233. [Google Scholar] [CrossRef]
- Prakash, T.; Williams, G.V.M.; Kennedy, J.; Rubanov, S. Formation of magnetic nanoparticles by low energy dual implantation of Ni and Fe into SiO2. J. Alloys Compd. 2016, 667, 255–261. [Google Scholar] [CrossRef]
- Kennedy, J.; Leveneur, J.; Williams, G.V.M.; Mitchell, D.R.G.; Markwitz, A. Fabrication of surface magnetic nanoclusters using low energy ion implantation and electron beam annealing. Nanotechnology 2011, 22, 115602. [Google Scholar] [CrossRef] [PubMed]
- Sayed, F.N.; Polshettiwar, V. Facile and Sustainable Synthesis of Shaped Iron Oxide Nanoparticles: Effect of Iron Precursor Salts on the Shapes of Iron Oxides. Sci. Rep. 2015, 5, 9733. [Google Scholar] [CrossRef] [PubMed]
- Polshettiwar, V.; Baruwati, B.; Varma, R.S. Self-Assembly of Metal Oxides into Three-Dimensional Nanostructures: Synthesis and Application in Catalysis. ACS Nano 2009, 3, 728–736. [Google Scholar] [CrossRef] [PubMed]
- De Montferrand, C.; Hu, L.; Milosevic, I.; Russier, V.; Bonnin, D.; Motte, L.; Brioude, A.; Lalatonne, Y. Iron oxide nanoparticles with sizes, shapes and compositions resulting in different magnetization signatures as potential labels for multiparametric detection. Acta Biomater. 2013, 9, 6150–6157. [Google Scholar] [CrossRef] [PubMed]
- Joshi, H.M.; Lin, Y.P.; Aslam, M.; Prasad, P.V.; Schultz-Sikma, E.A.; Edelman, R.; Meade, T.; Dravid, V.P. Effects of shape and size of cobalt ferrite nanostructures on their MRI contrast and thermal activation. J. Phys. Chem. C 2009, 113, 17761–17767. [Google Scholar] [CrossRef] [PubMed]
- Smolensky, E.D.; Park, H.-Y.E.; Zhou, Y.; Rolla, G.A.; Marjańska, M.; Botta, M.; Pierre, V.C. Scaling laws at the nanosize: the effect of particle size and shape on the magnetism and relaxivity of iron oxide nanoparticle contrast agents. J. Mater. Chem. B 2013, 1, 2818. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Choi, Y.; Lee, Y.; Park, M.; Moon, W.K.; Choi, S.H.; Hyeon, T. Water-Dispersible Ferrimagnetic Iron Oxide Nanocubes with Extremely High r 2 Relaxivity for Highly Sensitive in Vivo MRI of Tumors. Nano Lett. 2012, 12, 3127–3131. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhou, Z.; Bao, J.; Wang, Z.; Hu, J.; Chi, X.; Ni, K.; Wang, R.; Chen, X.; Chen, Z.; et al. Octapod iron oxide nanoparticles as high-performance T2 contrast agents for magnetic resonance imaging. Nat. Commun. 2013, 4, 2266. [Google Scholar] [CrossRef] [PubMed]
- Sathya, A.; Guardia, P.; Brescia, R.; Silvestri, N.; Pugliese, G.; Nitti, S.; Manna, L.; Pellegrino, T. CoxFe3−xO4 Nanocubes for Theranostic Applications: Effect of Cobalt Content and Particle Size. Chem. Mater. 2016, 28, 1769–1780. [Google Scholar] [CrossRef]
- Blanco-Andujar, C.; Walter, A.; Cotin, G.; Bordeianu, C.; Mertz, D.; Felder-Flesch, D.; Begin-Colin, S. Design of iron oxide-based nanoparticles for MRI and magnetic hyperthermia. Nanomedicine 2016, 11, 1889–1910. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Guo, Z.; Gao, F.; Gao, Q.; Wang, D.; Liaw, B.; Cai, Q.; Sun, X.; Wang, X.; Zhao, L. Shape-, size- and structure-controlled synthesis and biocompatibility of iron oxide nanoparticles for magnetic theranostics. Theranostics 2018, 8, 3284–3307. [Google Scholar] [CrossRef] [PubMed]
- Nemati, Z.; Alonso, J.; Rodrigo, I.; Das, R.; Garaio, E.; García, J.Á.; Orue, I.; Phan, M.-H.; Srikanth, H. Improving the Heating Efficiency of Iron Oxide Nanoparticles by Tuning Their Shape and Size. J. Phys. Chem. C 2018, 122, 2367–2381. [Google Scholar] [CrossRef]
- Guardia, P.; Di Corato, R.; Lartigue, L.; Wilhelm, C.; Espinosa, A.; Garcia-Hernandez, M.; Gazeau, F.; Manna, L.; Pellegrino, T. Water-Soluble Iron Oxide Nanocubes with High Values of Specific Absorption Rate for Cancer Cell Hyperthermia Treatment. ACS Nano 2012, 6, 3080–3091. [Google Scholar] [CrossRef] [PubMed]
- Guardia, P.; Riedinger, A.; Nitti, S.; Pugliese, G.; Marras, S.; Genovese, A.; Materia, M.; Lefevre, C.; Manna, L.; Pellegrino, T. One pot synthesis of monodisperse water soluble iron oxide nanocrystals with high values of specific absorption rate. J. Mater. Chem. B 2014, 2, 4426–4434. [Google Scholar] [CrossRef]
- Wetterskog, E.; Tai, C.-W.; Grins, J.; Bergström, L.; Salazar-Alvarez, G. Anomalous Magnetic Properties of Nanoparticles Arising from Defect Structures: Topotaxial Oxidation of Fe1−xO|Fe3−δO4 Core|Shell Nanocubes to Single-Phase Particles. ACS Nano 2013, 7, 7132–7144. [Google Scholar] [CrossRef] [PubMed]
- Wetterskog, E.; Agthe, M.; Mayence, A.; Grins, J.; Wang, D.; Rana, S.; Ahniyaz, A.; Salazar-Alvarez, G.; Bergström, L. Precise control over shape and size of iron oxide nanocrystals suitable for assembly into ordered particle arrays. Sci. Technol. Adv. Mater. 2014, 15, 2802. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhu, X.; Wu, D.; Chen, Q.; Huang, D.; Sun, C.; Xin, J.; Ni, K.; Gao, J. Anisotropic Shaped Iron Oxide Nanostructures: Controlled Synthesis and Proton Relaxation Shortening Effects. Chem. Mater. 2015, 27, 3505–3515. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H. Size-controlled synthesis of magnetite nanoparticles. J. Am. Chem. Soc. 2002, 124, 8204–8205. [Google Scholar] [CrossRef] [PubMed]
- Hyeon, T.; Lee, S.S.; Park, J.; Chung, Y.; Na, H.B. Synthesis of Highly Crystalline and Monodisperse Maghemite Nanocrystallites without a Size-Selection Process. J. Am. Chem. Soc. 2001, 123, 12798–12801. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; An, K.; Hwang, Y.; Park, J.-G.; Noh, H.-J.; Kim, J.-Y.; Park, J.-H.; Hwang, N.-M.; Hyeon, T. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, M.V.; Bodnarchuk, M.I.; Lechner, R.T.; Hesser, G.; Schäffler, F.; Heiss, W. Fatty Acid Salts as Stabilizers in Size- and Shape-Controlled Nanocrystal Synthesis: The Case of Inverse Spinel Iron Oxide. J. Am. Chem. Soc. 2007, 129, 6352–6353. [Google Scholar] [CrossRef] [PubMed]
- Bronstein, L.M.; Huang, X.; Retrum, J.; Schmucker, A.; Pink, M.; Stein, B.D.; Dragnea, B. Influence of Iron Oleate Complex Structure on Iron Oxide Nanoparticle Formation. Chem. Mater. 2007, 19, 3624–3632. [Google Scholar] [CrossRef]
- LaMer, V.K.; Dinegar, R.H. Theory, Production and Mechanism of Formation of Monodispersed Hydrosols. J. Am. Chem. Soc. 1950, 72, 4847–4854. [Google Scholar] [CrossRef]
- Baaziz, W.; Pichon, B.P.; Fleutot, S.; Liu, Y.; Lefevre, C.; Greneche, J.-M.; Toumi, M.; Mhiri, T.; Begin-Colin, S. Magnetic Iron Oxide Nanoparticles: Reproducible Tuning of the Size and Nanosized-Dependent Composition, Defects, and Spin Canting. J. Phys. Chem. C 2014, 118, 3795–3810. [Google Scholar] [CrossRef]
- Kwon, S.G.; Piao, Y.; Park, J.; Angappane, S.; Jo, Y.; Hwang, N.-M.; Park, J.-G.; Hyeon, T. Kinetics of monodisperse iron oxide nanocrystal formation by “heating-up” process. J. Am. Chem. Soc. 2007, 129, 12571–12584. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L. Transmission Electron Microscopy of Shape-Controlled Nanocrystals and Their Assemblies. J. Phys. Chem. B 2000, 104, 1153–1175. [Google Scholar] [CrossRef]
- Yang, C.; Wu, J.; Hou, Y. Fe3O4 nanostructures: synthesis, growth mechanism, properties and applications. Chem. Commun. 2011, 47, 5130. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Xiong, Y.; Lim, B.; Skrabalak, S.E. Shape-controlled synthesis of metal nanocrystals: simple chemistry meets complex physics? Angew. Chem. Int. Ed. Engl. 2009, 48, 60–103. [Google Scholar] [CrossRef] [PubMed]
- Rath, S.S.; Sinha, N.; Sahoo, H.; Das, B.; Mishra, B.K. Molecular modeling studies of oleate adsorption on iron oxides. Appl. Surf. Sci. 2017, 7, 2802. [Google Scholar] [CrossRef]
- Guardia, P.; Labarta, A.; Batlle, X. Tuning the Size, the Shape, and the Magnetic Properties of Iron Oxide Nanoparticles. J. Phys. Chem. C 2011, 115, 390–396. [Google Scholar] [CrossRef]
- Shavel, A.; Liz-Marzán, L.M. Shape control of iron oxide nanoparticles. Phys. Chem. Chem. Phys. 2009, 11, 3762–3766. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, N.; Park, M.; Kim, B.H.; An, K.; Hyeon, T. Synthesis of Uniform Ferrimagnetic Magnetite Nanocubes. J. Am. Chem. Soc. 2009, 131, 454–455. [Google Scholar] [CrossRef] [PubMed]
- Shavel, A.; Rodríguez-González, B.; Spasova, M.; Farle, M.; Liz-Marzán, L.M. Synthesis and Characterization of Iron/Iron Oxide Core/Shell Nanocubes. Adv. Funct. Mater. 2007, 17, 3870–3876. [Google Scholar] [CrossRef]
- Hai, H.T.; Yang, H.T.; Kura, H.; Hasegawa, D.; Ogata, Y.; Takahashi, M.; Ogawa, T. Size control and characterization of wustite (core)/spinel (shell) nanocubes obtained by decomposition of iron oleate complex. J. Colloid Interface Sci. 2010, 346, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Park, J.; An, K.; Yang, N.-K.; Park, J.-G.; Hyeon, T. Synthesis of Hollow Iron Nanoframes. J. Am. Chem. Soc. 2007, 129, 5812–5813. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ogawa, T.; Hasegawa, D.; Takahashi, M. Synthesis and magnetic properties of monodisperse magnetite nanocubes. J. Appl. Phys. 2008, 103, 07D526. [Google Scholar] [CrossRef]
- Cervadoro, A.; Cho, M.; Key, J.; Cooper, C.; Stigliano, C.; Aryal, S.; Brazdeikis, A.; Leary, J.F.; Decuzzi, P. Synthesis of Multifunctional Magnetic NanoFlakes for Magnetic Resonance Imaging, Hyperthermia, and Targeting. ACS Appl. Mater. Interfaces 2014, 6, 12939–12946. [Google Scholar] [CrossRef] [PubMed]
- Cotin, G.; Kiefer, C.; Perton, F.; Boero, M.; Özdamar, B.; Bouzid, A.; Ori, G.; Massobrio, C.; Begin, D.; Pichon, B.; et al. Evaluating the Critical Roles of Precursor Nature and Water Content When Tailoring Magnetic Nanoparticles for Specific Applications. ACS Appl. Nano Mater. 2018, 1, 4306–4316. [Google Scholar] [CrossRef]
- Xu, Z.; Shen, C.; Tian, Y.; Shi, X.; Gao, H.-J. Organic phase synthesis of monodisperse iron oxide nanocrystals using iron chloride as precursor. Nanoscale 2010, 2, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Alivisatos, A.P. Colloidal nanocrystal synthesis and the organic–inorganic interface. Nature 2005, 437, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Pichon, B.P.; Gerber, O.; Lefevre, C.; Florea, I.; Fleutot, S.; Baaziz, W.; Pauly, M.; Ohlmann, M.; Ulhaq, C.; Ersen, O.; et al. Microstructural and Magnetic Investigations of Wüstite-Spinel Core-Shell Cubic-Shaped Nanoparticles. Chem. Mater. 2011, 23, 2886–2900. [Google Scholar] [CrossRef]
- Walter, A.; Billotey, C.; Garofalo, A.; Ulhaq-Bouillet, C.; Lefèvre, C.; Taleb, J.; Laurent, S.; Vander Elst, L.; Muller, R.N.; Lartigue, L.; et al. Mastering the Shape and Composition of Dendronized Iron Oxide Nanoparticles To Tailor Magnetic Resonance Imaging and Hyperthermia. Chem. Mater. 2014, 26, 5252–5264. [Google Scholar] [CrossRef]
- Bronstein, L.M.; Atkinson, J.E.; Malyutin, A.G.; Kidwai, F.; Stein, B.D.; Morgan, D.G.; Perry, J.M.; Karty, J.A. Nanoparticles by Decomposition of Long Chain Iron Carboxylates: From Spheres to Stars and Cubes. Langmuir 2011, 27, 3044–3050. [Google Scholar] [CrossRef] [PubMed]
- Buck, M.R.; Biacchi, A.J.; Schaak, R.E. Insights into the Thermal Decomposition of Co(II) Oleate for the Shape-Controlled Synthesis of Wurtzite-Type CoO Nanocrystals. Chem. Mater. 2014, 26, 1492–1499. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Physica B: Condensed Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Le Bail, A.; Duroy, H.; Fourquet, J.L. Ab Initio Structure Determination of LiSbWO6 by X-ray Powder Diffraction. Mater. Res. Bull. 1988, 23, 447–452. [Google Scholar] [CrossRef]
- Mahieu, N.; Canet, D.; Cases, J.M.; Boubel, J.C. Micellization of sodium oleate in water-d2 as probed by proton longitudinal magnetic relaxation and self-diffusion measurements. J. Phys. Chem. 1991, 95, 1844–1846. [Google Scholar] [CrossRef]
- Tan, T.; Yao, L.; Liu, H.; Li, C.; Wang, C. Precise Control of the Lateral and Vertical Growth of Two-Dimensional Ag Nanoplates. Chem. Eur. J. 2017, 23, 10001–10006. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Tian, D.; Chong, B.; Zhang, X.; Gao, W. Rare-earth doped LaF3 hollow hexagonal nanoplates: hydrothermal synthesis and photoluminescence properties. CrystEngComm 2014, 16, 7106–7114. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhao, Z.; Zhang, H.; Wang, Z.; Chen, X.; Wang, R.; Chen, Z.; Gao, J. Interplay between Longitudinal and Transverse Contrasts in Fe3O4 Nanoplates with (111) Exposed Surfaces. ACS Nano 2014, 8, 7976–7985. [Google Scholar] [CrossRef] [PubMed]
- Palchoudhury, S.; Xu, Y.; Rushdi, A.; Holler, R.A.; Bao, Y. Controlled synthesis of iron oxide nanoplates and nanoflowers. Chem. Commun. 2012, 48, 10499. [Google Scholar] [CrossRef] [PubMed]
- Baaziz, W.; Pichon, B.; Grenèche, J.-M.; Begin-Colin, S. Effect of environment medium and the in situ formation of precursor on the composition-shape of iron oxide nanoparticles synthesized by thermal decomposition method. CrystEngComm 2018. [Google Scholar] [CrossRef]
- McCammon, C.A.; Liu, L. The effects of pressure and temperature on nonstoichiometric wüstite, FexO: The iron-rich phase boundary. Phys. Chem. Miner. 1984, 10, 106–113. [Google Scholar] [CrossRef]
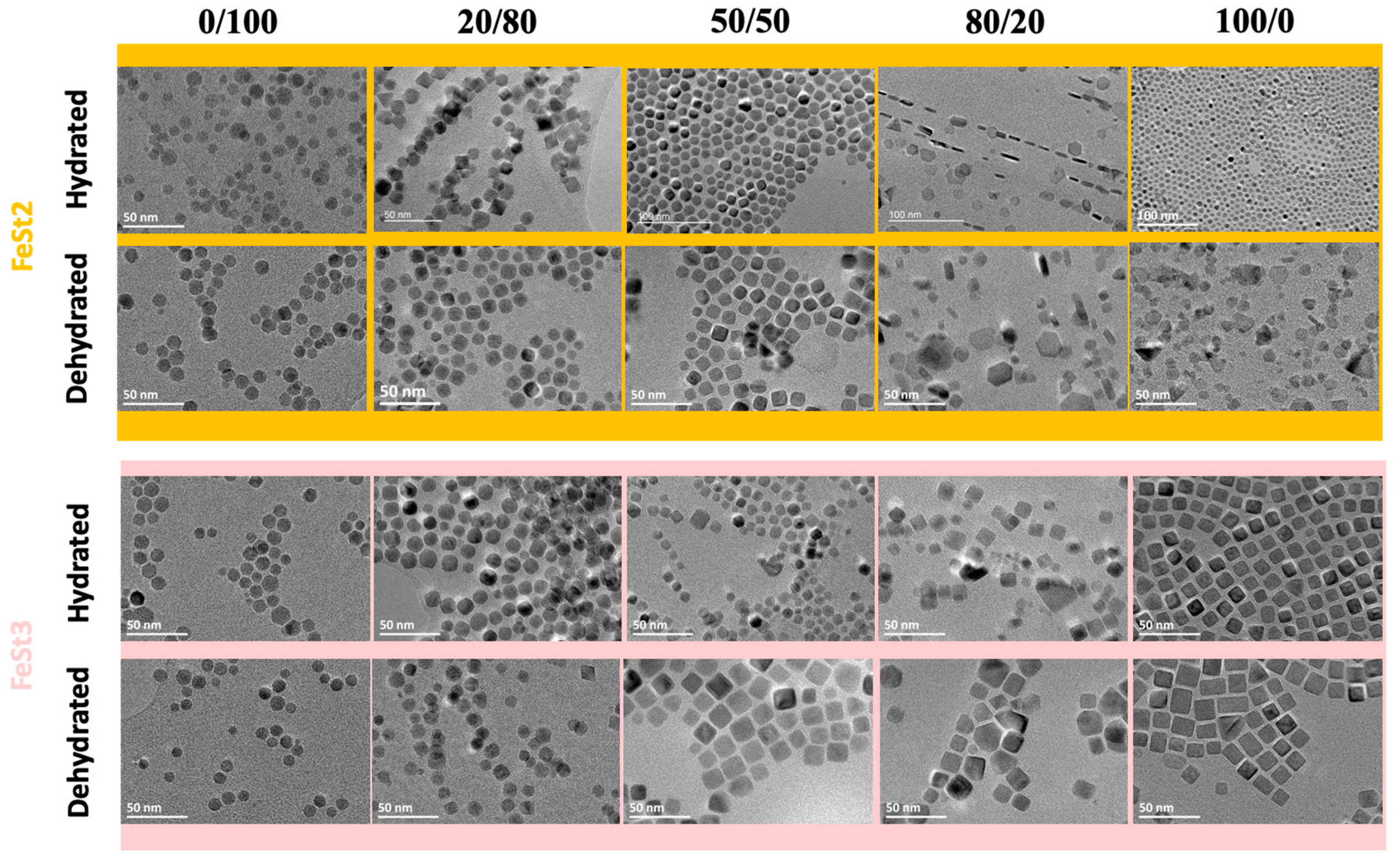
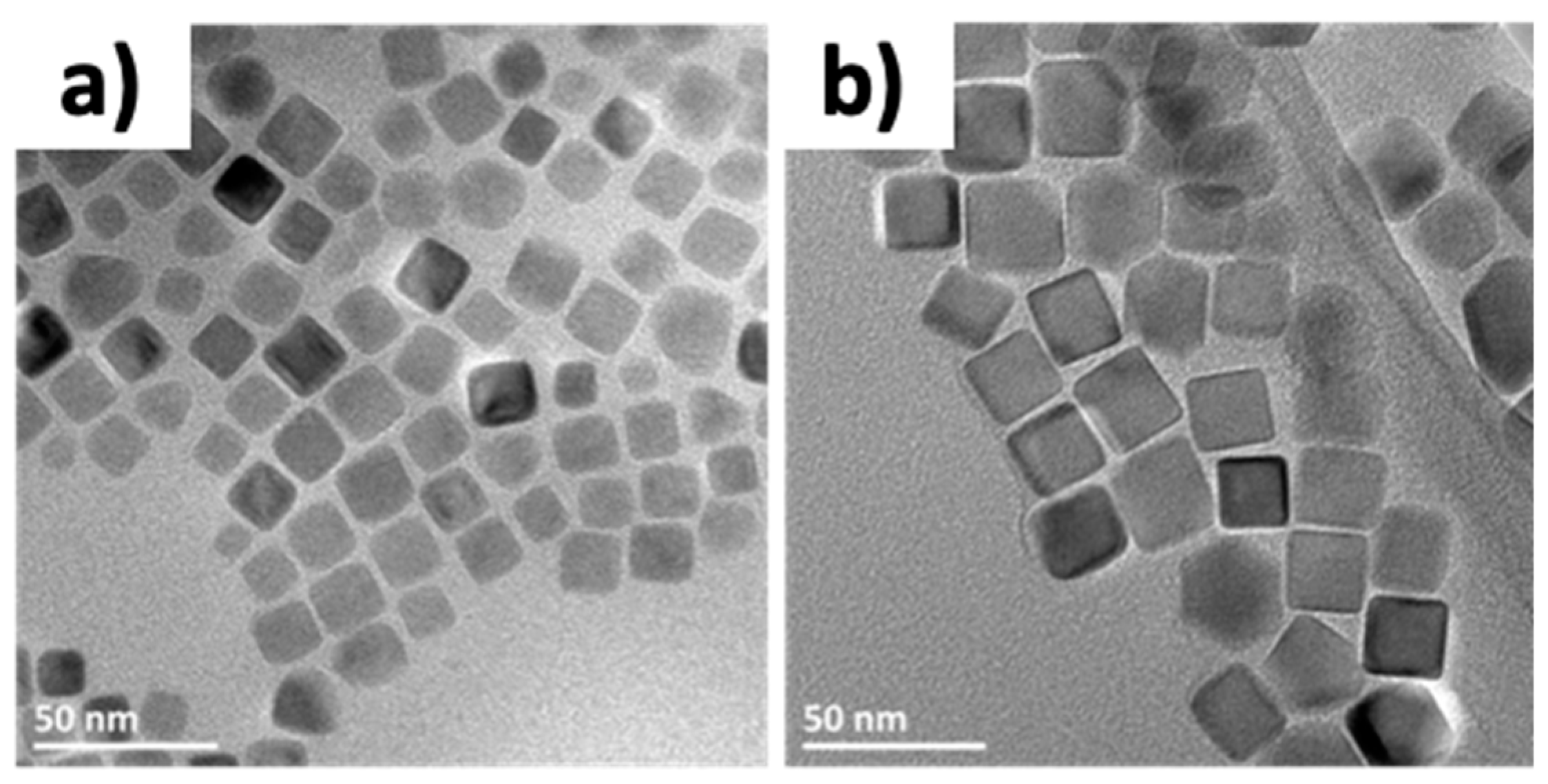
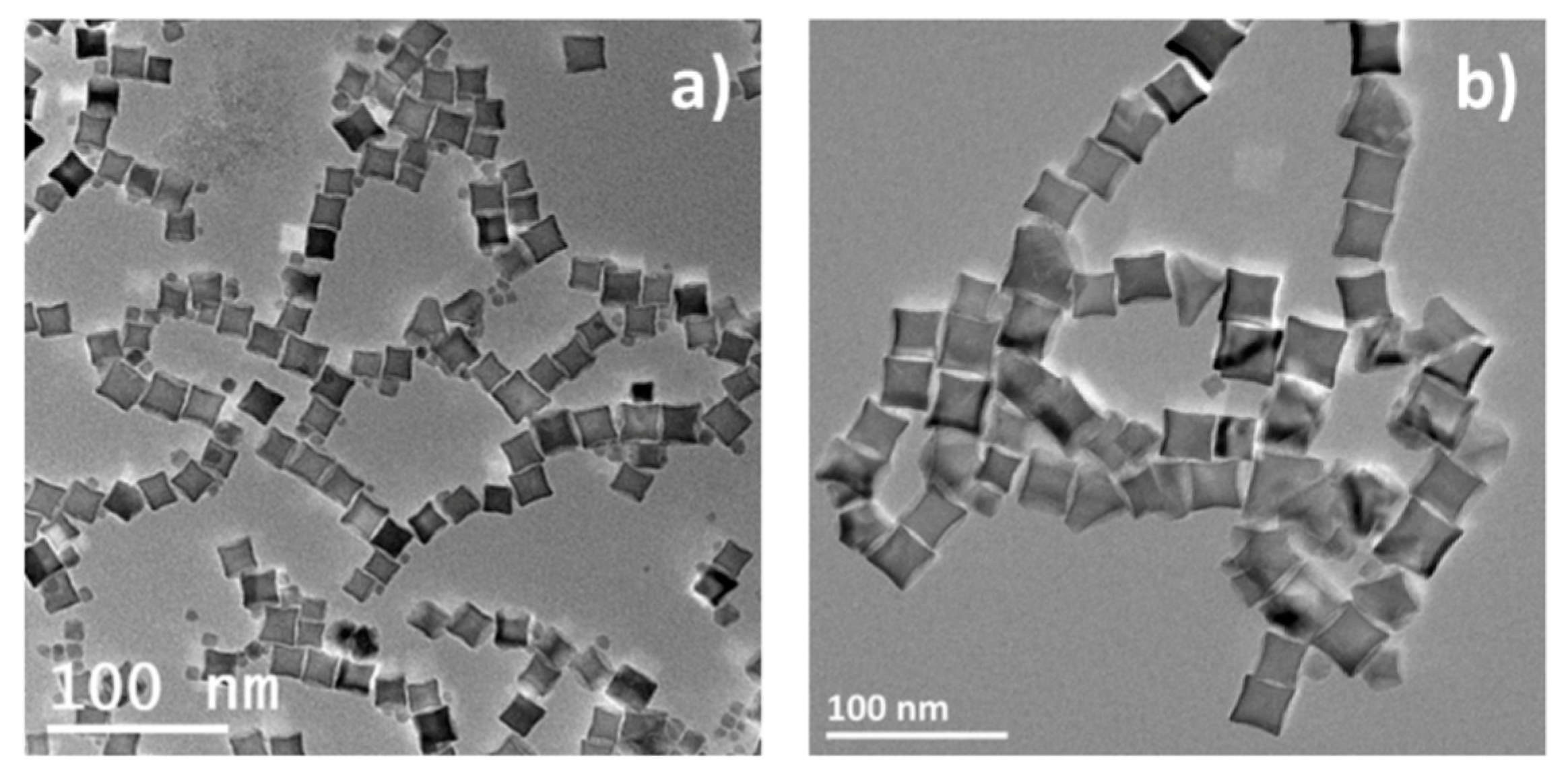
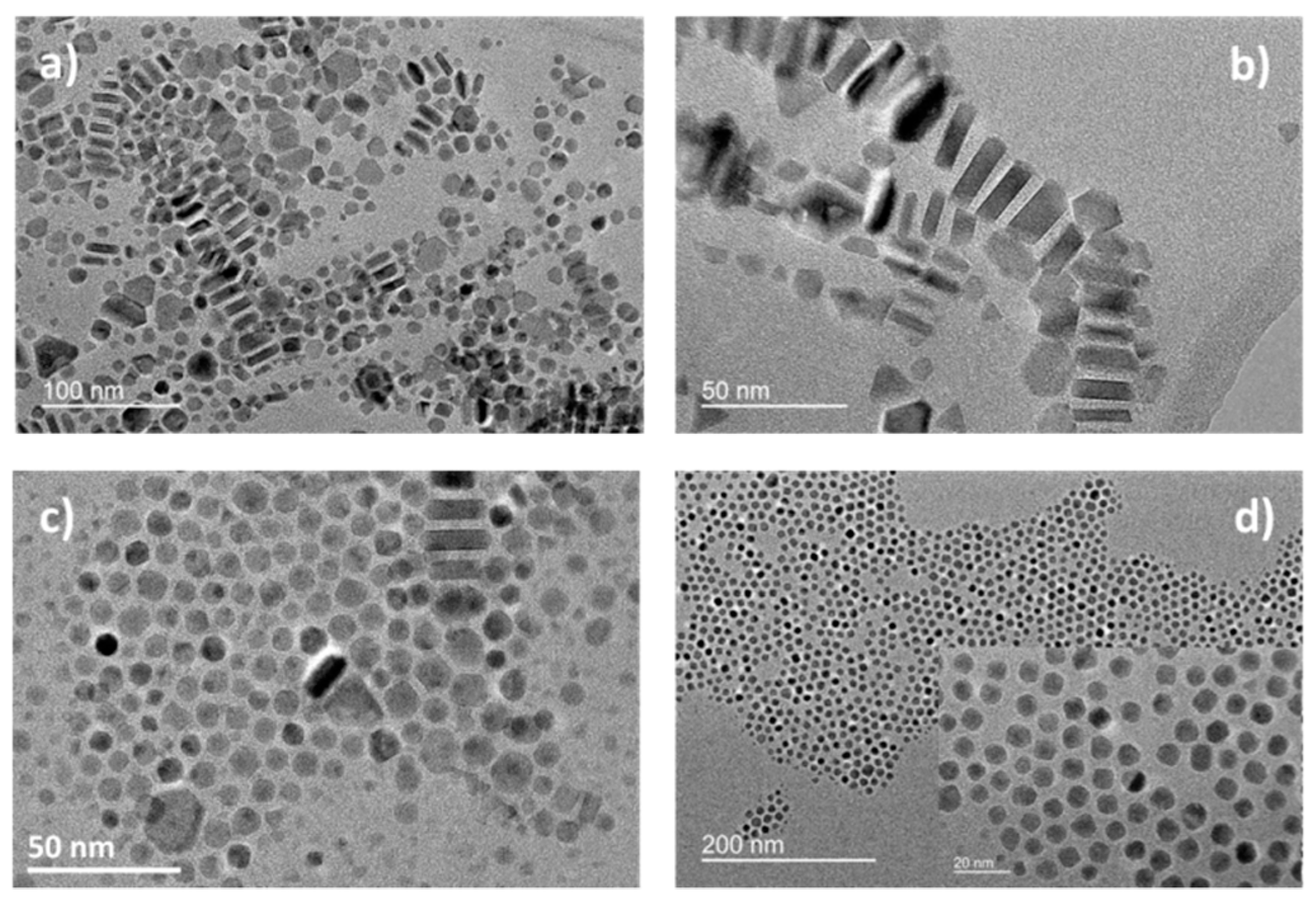
| Reference | Precursor | Solvent | Ligand | Reflux T and Duration | Heating Rate | Observations |
|---|---|---|---|---|---|---|
| [24] | Fe(acac)3 | DBE | OA | 290 °C/30 s | 20 °C/min | Size and shape control with time and quantity of DBE |
| Fe(acac)3 | DBE | OA/4-Bisphenyl carboxilic acid | 290 °C/30 s | 20 °C/min | Smaller cubes | |
| [9] | Fe(Ol)3 | OD or TOA | OA | 340 °C/4 h | 10–15 °C/min | Size controled with T |
| [30] | Fe(acac)3 | DBE | OA/4-Bisphenyl carboxilic acid | 290 °C/30 s | ND | |
| [25,26] | Fe(Ol)3 | Squalane | NaOl/OA | 315 °C/2h | 20 °C/min | Core-shell |
| [29] | Fe(acac)3 | DBE | OA/HDD /OAm | 290 °C/1h | 15 °C/min | If heating rate increases along shorter reflux bigger NPs; on the opposite smaller NPs |
| [7] | Fe(Ol)3 | eicosane | NaOl/OA | 350 °C/30 s | 3.3 °C/min | Core-shell |
| [46] | Fe(Ol)3 in situ | OD | NaOl | 315 °C/ 2 h | ND | Shape control through amount of NaOl |
| [47] | Fe(Ol)? | OD | OA | 320 °C/30 s | 5.5 °C/min | |
| [12] | Fe(Ol)3 | OD | NaOl/OA or DBAOL | 315 °C/30’ | 3.3 °/min | |
| [27] | Fe(Ol)3 | OD | NaOl /OA | 315 °C/30 s | 4 °C/min | Ratio Fe(Ol)/NaOl control the size |
| [28] | Fe(St)2 | OA | NaOl /OA | 380 °C/2 h | 5 °C/min | |
| [23] | Fe(acac)3 | Squalane | Decanoic acid/DBE | 310 °C/1 h | 7 °C /min | Size controled with ratio squalane/DBE |
| Precursor | 0/100 | 20/80 | 50/50 | 80/20 | 100/0 | |
|---|---|---|---|---|---|---|
| FeSt2 | Size (nm) | 9.4 ± 1.9 | 11.7 ± 2.5 | 13.5 ± 2.5 | 17.5 ± 4.4 (L) 6.3 ± 1.3 (t) | 7.2 ± 2.4 |
| Shape | Spheres | Quasi octahedron | Deformed Cubes | Plates | Faceted | |
| FeSt2,d | Size (nm) | 10.0 ± 1.5 | 10.9 ± 1.4 | 10 ± 0.6 | 13.5 ± 5.2 (L) 66.5 ± 1.7 (t) | 9 ± 2.7 |
| Shape | Spheres | Quasi spherical | Cubes | Plates | Various faceted plates | |
| FeSt3 | Size (nm) | 10.8 ± 1.7 | 12.4 ± 1.7 | 8.3 ± 2.2 | 11.3 ± 4.3 | 11.7 ± 1.5 |
| Shape | Spheres | Quasi spherical | Faceted | Faceted | Cubes | |
| FeSt3,d | Size (nm) | 9.2 ± 1.5 | 9.8 ± 1.4 | 15.3 ± 1.8 | 20 ± 1.9/14 ± 1.4 | 13.9 ± 2.2 |
| Shape | Spheres | Quasi spherical (M)/Octahedrons© | Cubes | Cubes (M)/Plates (m) | Elongated cubes | |
| Sample | TEM (nm) | Lattice Parameter (Å) | Crystallite Size According to Crystallographic Direction ± 1 (nm) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 220 | 311 | 222 | 400 | 331 | 422 | 511 | 333 | 440 | 531 | 442 | |||
| NO18 | 17.2 ± 2.2 | 8.364 | 15 | 13 | 11 | 11 | 11 | 12 | 13 | 11 | 15 | 12 | 11 |
| NO28 | 27.8 ± 4.2 | 8.370 | 19 | 20 | 13 | 25 | 18 | 17 | 23 | 12 | 19 | 21 | 16 |
| NPl17 | 16.7 ± 5.2 | 8.384 | 11 | 9 | 17 | 8 | 11 | 10 | 8 | 17 | 11 | 9 | 12 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cotin, G.; Kiefer, C.; Perton, F.; Ihiawakrim, D.; Blanco-Andujar, C.; Moldovan, S.; Lefevre, C.; Ersen, O.; Pichon, B.; Mertz, D.; et al. Unravelling the Thermal Decomposition Parameters for The Synthesis of Anisotropic Iron Oxide Nanoparticles. Nanomaterials 2018, 8, 881. https://doi.org/10.3390/nano8110881
Cotin G, Kiefer C, Perton F, Ihiawakrim D, Blanco-Andujar C, Moldovan S, Lefevre C, Ersen O, Pichon B, Mertz D, et al. Unravelling the Thermal Decomposition Parameters for The Synthesis of Anisotropic Iron Oxide Nanoparticles. Nanomaterials. 2018; 8(11):881. https://doi.org/10.3390/nano8110881
Chicago/Turabian StyleCotin, Geoffrey, Céline Kiefer, Francis Perton, Dris Ihiawakrim, Cristina Blanco-Andujar, Simona Moldovan, Christophe Lefevre, Ovidiu Ersen, Benoit Pichon, Damien Mertz, and et al. 2018. "Unravelling the Thermal Decomposition Parameters for The Synthesis of Anisotropic Iron Oxide Nanoparticles" Nanomaterials 8, no. 11: 881. https://doi.org/10.3390/nano8110881
APA StyleCotin, G., Kiefer, C., Perton, F., Ihiawakrim, D., Blanco-Andujar, C., Moldovan, S., Lefevre, C., Ersen, O., Pichon, B., Mertz, D., & Bégin-Colin, S. (2018). Unravelling the Thermal Decomposition Parameters for The Synthesis of Anisotropic Iron Oxide Nanoparticles. Nanomaterials, 8(11), 881. https://doi.org/10.3390/nano8110881






