Graphene-Based Organic Semiconductor Film for Highly Selective Photocatalytic CO2 Reduction
Abstract
1. Introduction
2. Experimental Sections
2.1. Materials
2.2. Material Synthesis
2.3. Characterizations
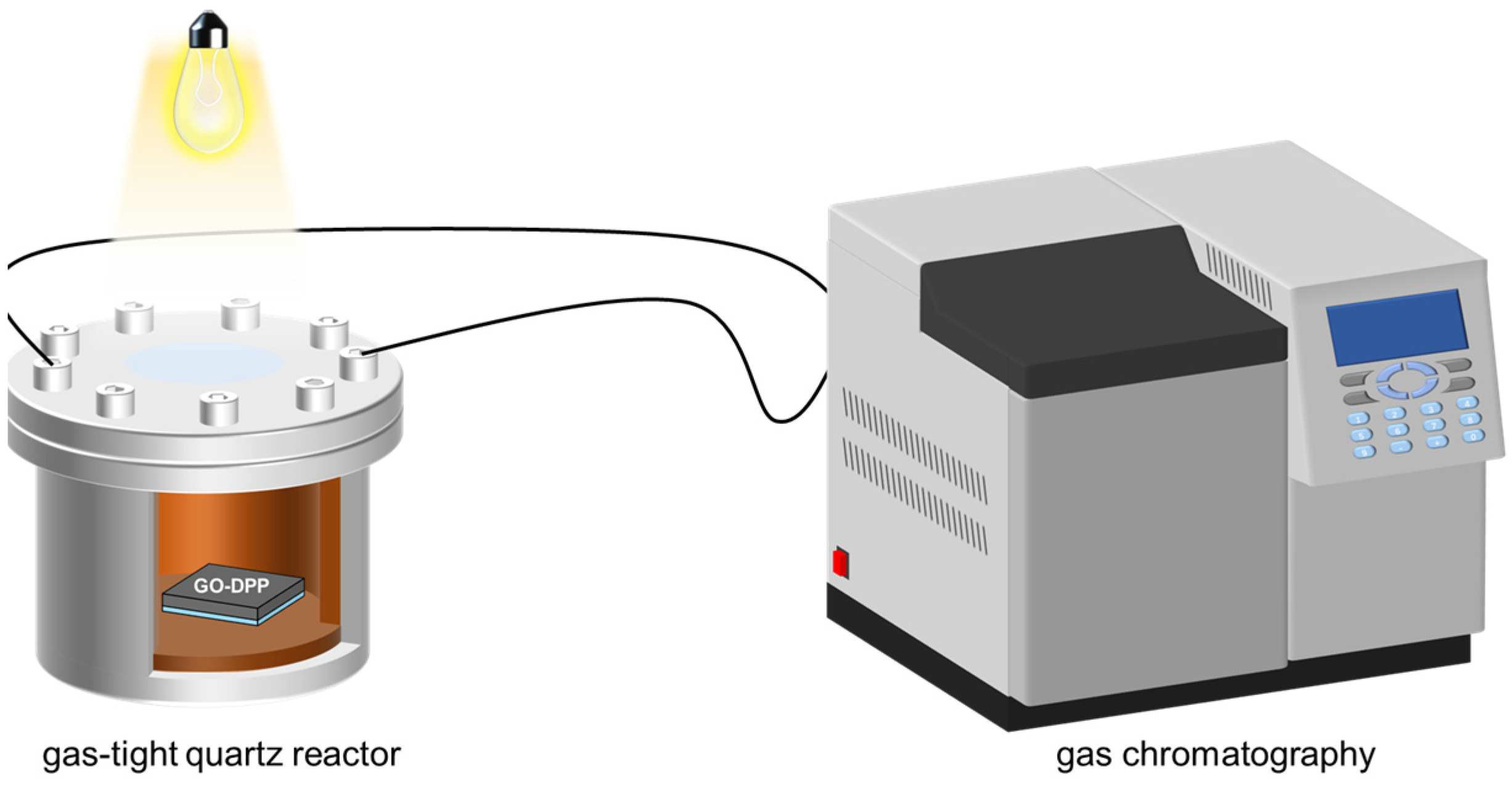
2.4. Photocatalytic Reduction Experiment
3. Results and Discussion
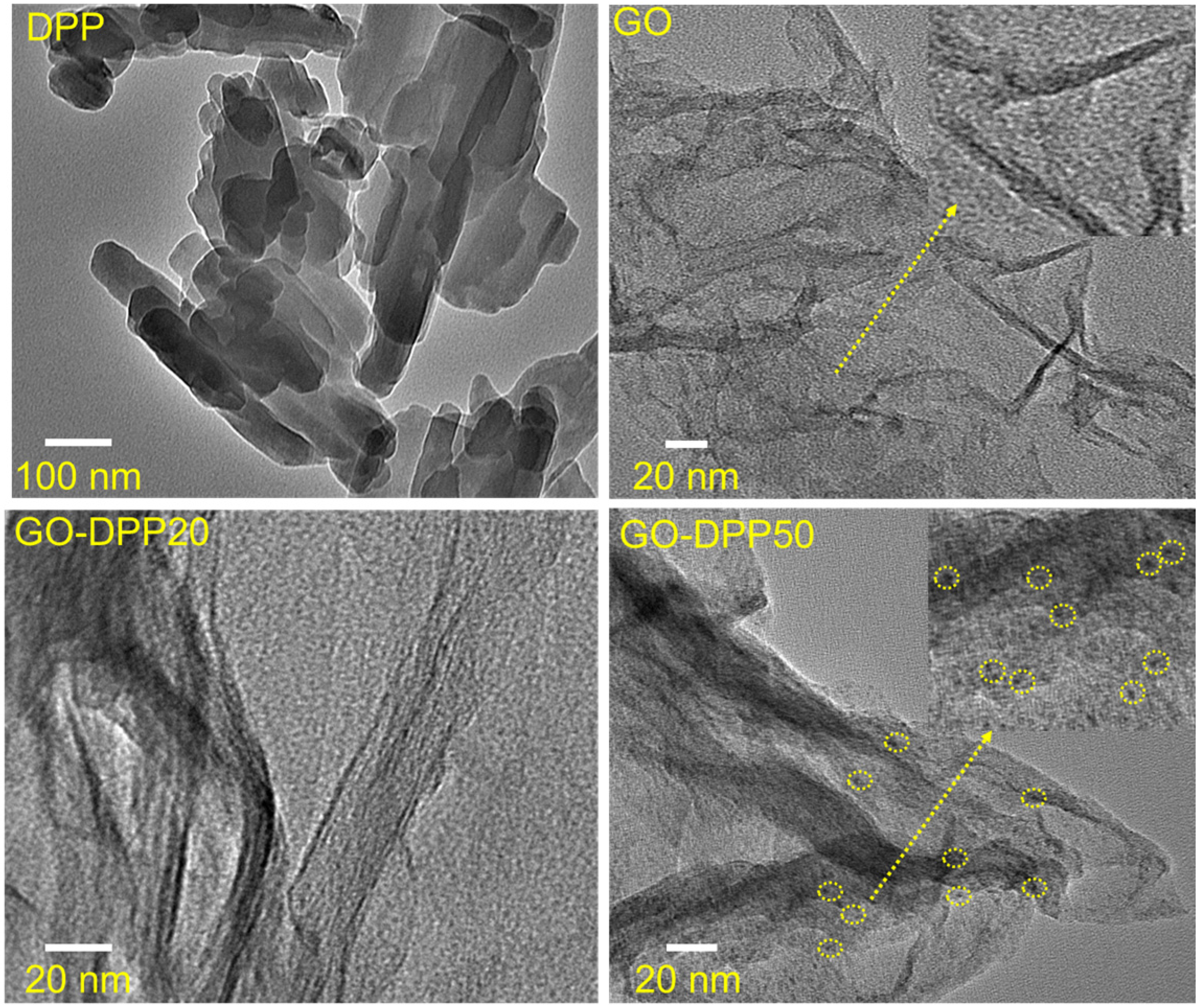
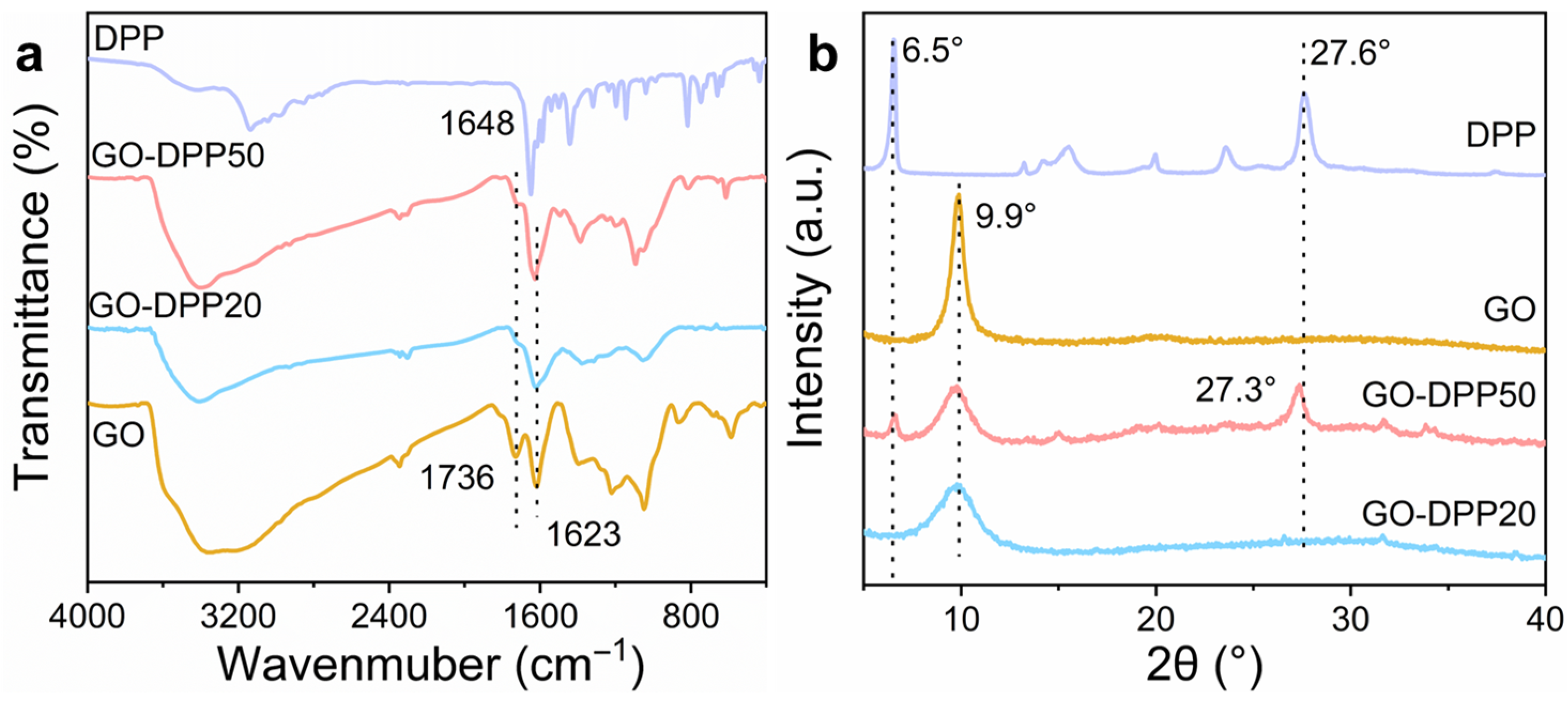
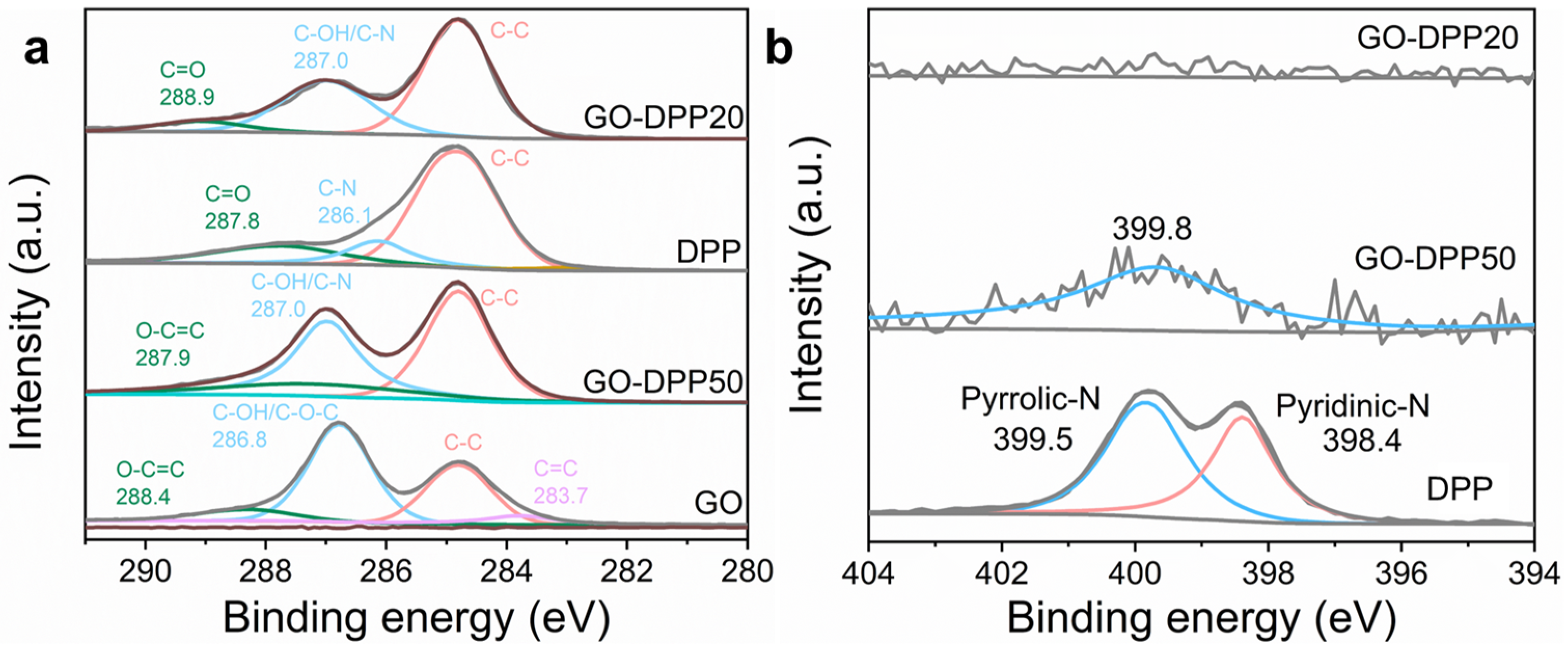
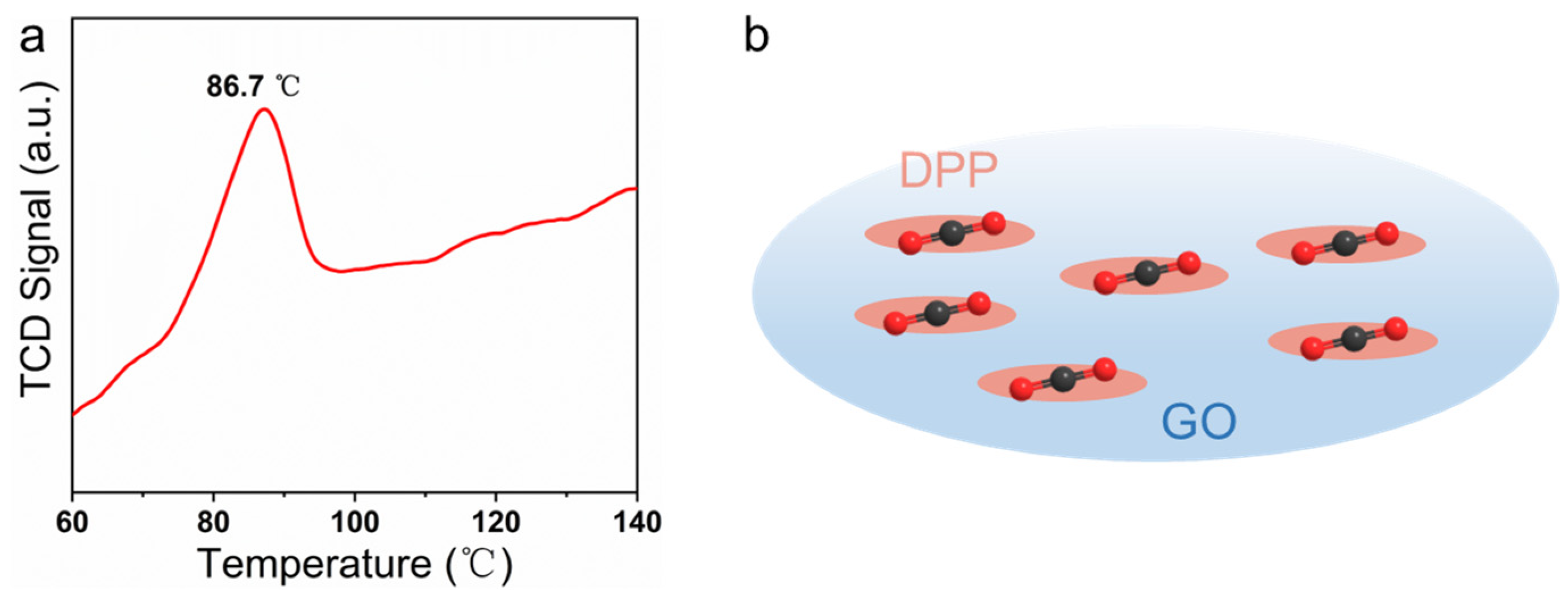
3.1. Morphological and Structural Characterization
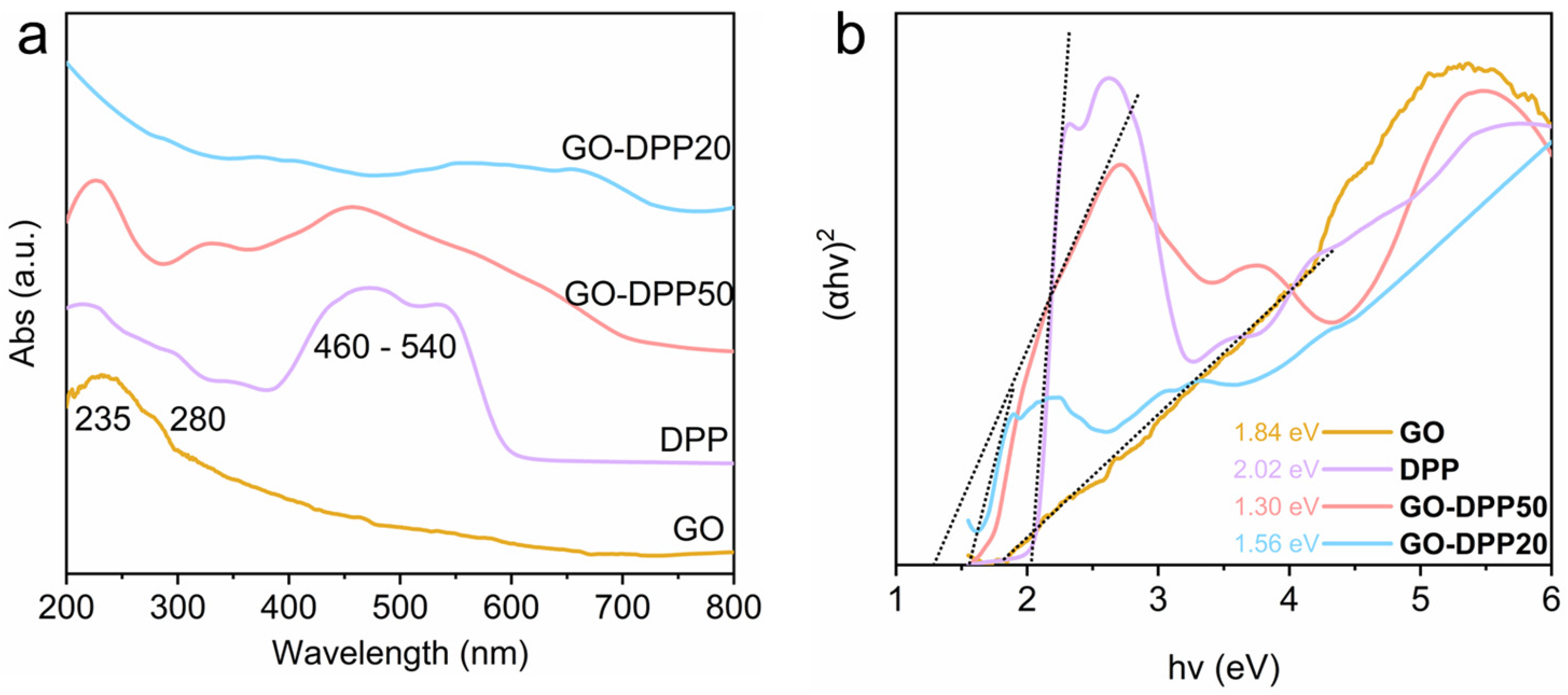
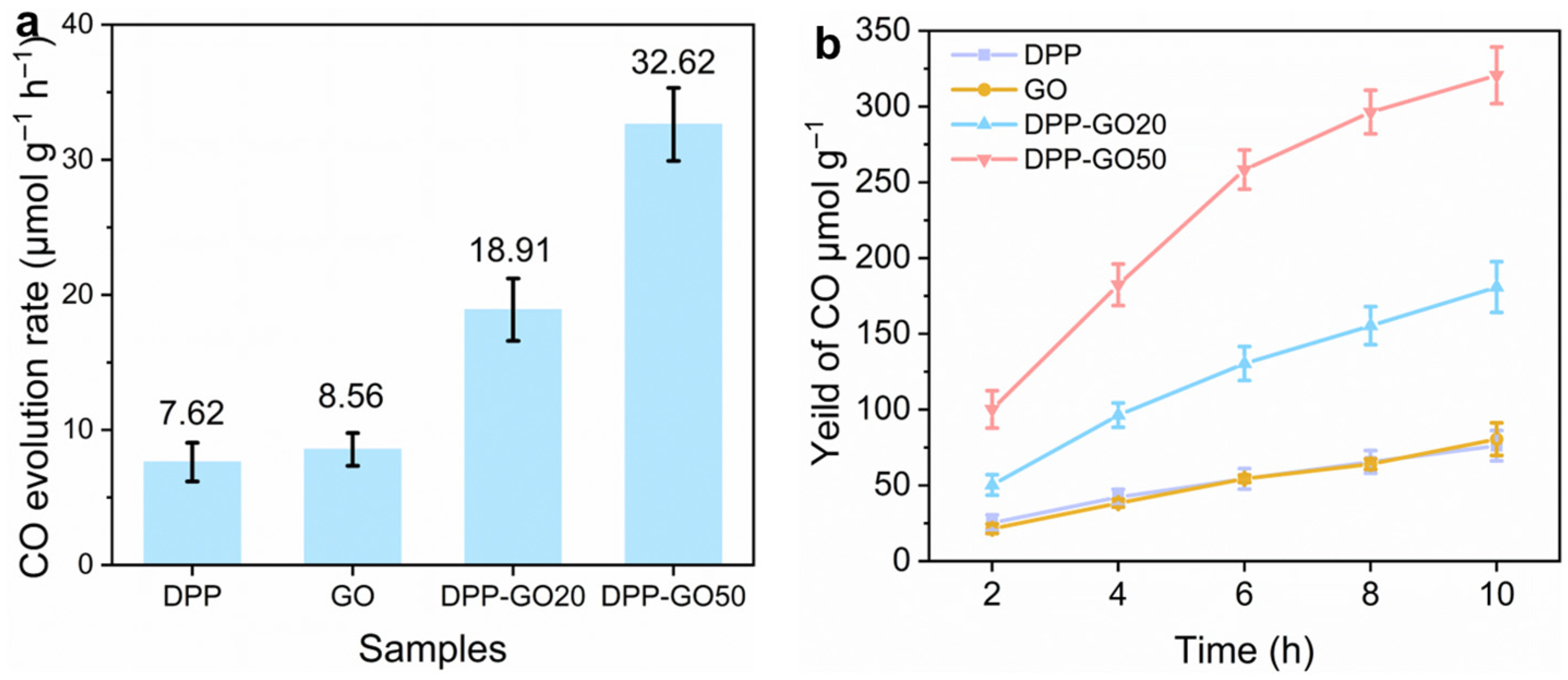
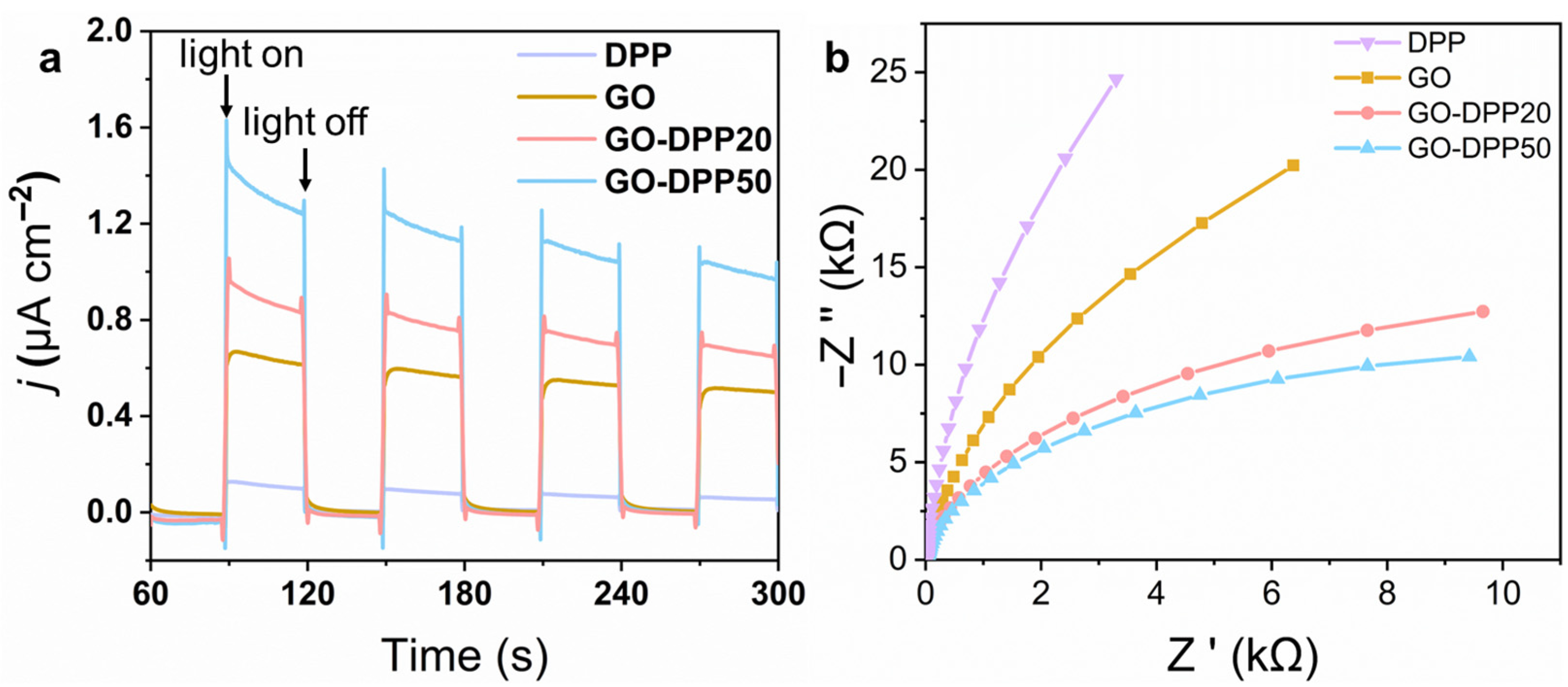
3.2. Optical Properties and Catalytic Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mac Dowell, N.; Fennell, P.S.; Shah, N.; Maitland, G.C. The role of CO2 capture and utilization in mitigating climate change. Nat. Clim. Change 2017, 7, 243–249. [Google Scholar] [CrossRef]
- Wang, T.; Duan, X.; Bai, R.; Li, H.; Qin, C.; Zhang, J.; Duan, Z.; Chen, K.-J.; Pan, F. Ni-Electrocatalytic CO2 Reduction Toward Ethanol. Adv. Mater. 2024, 36, 2410125. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zeng, H.; Feng, C.; Li, L.; Tang, R.; Li, W.; Huang, Y.; Deng, Y. Engineering an annular donor–acceptor reaction chamber with spontaneous feedstock collection for boosting CO2 photoreduction. Energy Environ. Sci. 2024, 17, 5627–5638. [Google Scholar] [CrossRef]
- Nguyen, H.L. Reticular Materials for Artificial Photoreduction of CO2. Adv. Energy Mater. 2020, 10, 2002091. [Google Scholar] [CrossRef]
- Kohse-Höinghaus, K. Combustion, Chemistry, and Carbon Neutrality. Chem. Rev. 2023, 123, 5139–5219. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, X.; Zhang, Z. Application of red mud in carbon capture, utilization and storage (CCUS) technology. Renew. Sustain. Energy Rev. 2024, 202, 114683. [Google Scholar] [CrossRef]
- Kong, T.; Jiang, Y.; Xiong, Y. Photocatalytic CO2 conversion: What can we learn from conventional COx hydrogenation? Chem. Soc. Rev. 2020, 49, 6579–6591. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Y.; Tian, Q.; Li, X.; Pan, A.; Zhao, M.; Zhu, Y.; Wu, T.; Fang, G. Enhancement effect of oxygen vacancy on photocatalytic CO2 reduction. J. Mater. Chem. A 2024, 12, 7207–7214. [Google Scholar] [CrossRef]
- Li, D.; Kassymova, M.; Cai, X.; Zang, S.-Q.; Jiang, H.-L. Photocatalytic CO2 reduction over metal-organic framework-based materials. Coord. Chem. Rev. 2020, 412, 213262. [Google Scholar] [CrossRef]
- Fang, Y.; Gao, Y.; Wen, Y.; He, X.; Meyer, T.J.; Shan, B. Photoelectrocatalytic CO2 Reduction to Methanol by Molecular Self-Assemblies Confined in Covalent Polymer Networks. J. Am. Chem. Soc. 2024, 146, 27475–27485. [Google Scholar] [CrossRef]
- Kaufman, A.J.; Nielander, A.C.; Meyer, G.J.; Maldonado, S.; Ardo, S.; Boettcher, S.W. Absolute band-edge energies are over-emphasized in the design of photoelectrochemical materials. Nat. Catal. 2024, 7, 615–623. [Google Scholar] [CrossRef]
- Li, T.; Huang, H.; Wang, S.; Mi, Y.; Zhang, Y. Recent advances in 2D semiconductor nanomaterials for photocatalytic CO2 reduction. Nano Res. 2023, 16, 8542–8569. [Google Scholar] [CrossRef]
- Li, Y.; Bai, X.; Yuan, D.; Yu, C.; San, X.; Guo, Y.; Zhang, L.; Ye, J. Cu-based high-entropy two-dimensional oxide as stable and active photothermal catalyst. Nat. Commun. 2023, 14, 3171. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yang, C.; Guo, Y.; Feng, J.; Guo, X. Graphene–molecule–graphene single-molecule junctions to detect electronic reactions at the molecular scale. Nat. Protoc. 2023, 18, 1958–1978. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Graphene-based semiconductor photocatalysts. Chem. Soc. Rev. 2012, 41, 782–796. [Google Scholar] [CrossRef] [PubMed]
- Raub, A.A.M.; Hamidah, I.; Nandiyanto, A.B.D.; Ridwan, J.; Mohamed, M.A.; Buyong, M.R.; Yunas, J. ZnO NRs/rGO Photocatalyst in a Polymer-Based Microfluidic Platform. Polymers 2023, 15, 1749. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Dong, H.; Jiang, L.; Hu, W. Organic semiconductor crystals. Chem. Sci. Rev. 2018, 47, 422–500. [Google Scholar] [CrossRef]
- Jin, W.; Yang, C.-Y.; Pau, R.; Wang, Q.; Tekelenburg, E.K.; Wu, H.-Y.; Wu, Z.; Jeong, S.Y.; Pitzalis, F.; Liu, T.; et al. Photocatalytic doping of organic semiconductors. Nature 2024, 630, 96–101. [Google Scholar] [CrossRef]
- Wang, J.-W.; Zhao, F.; Velasco, L.; Sauvan, M.; Moonshiram, D.; Salati, M.; Luo, Z.-M.; He, S.; Jin, T.; Mu, Y.-F.; et al. Molecular catalyst coordinatively bonded to organic semiconductors for selective light-driven CO2 reduction in water. Nat. Commun. 2024, 15, 9779. [Google Scholar] [CrossRef]
- Liu, A.; Zhou, J. Artificial photosynthesis of H2O2 over a self-assembled two-dimensional g-C3N4 film. J. Mater. Chem. A 2025, 13, 8790–8803. [Google Scholar] [CrossRef]
- Zhen, C.; Chen, X.; Chen, R.; Fan, F.; Xu, X.; Kang, Y.; Guo, J.; Wang, L.; Lu, G.Q.; Domen, K.; et al. Liquid metal-embraced photoactive films for artificial photosynthesis. Nat. Commun. 2024, 15, 1672. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Tao, K.; Song, T.; Zhang, Y.; Zhang, T.; Wang, F.; Duan, S.; Chen, Z.; Li, L.; Zhang, X.; et al. Large-Area Conductive MOF Ultrathin Film Controllably Integrating Dinuclear-Metal Sites and Photosensitizers to Boost Photocatalytic CO2 Reduction with H2O as an Electron Donor. J. Am. Chem. Soc. 2024, 146, 6893–6904. [Google Scholar] [CrossRef]
- Yang, L.; Sivasankaran, R.P.; Song, M.K.; Pawar, A.U.; Lee, D.K.; Kang, Y.S. Highly Selective Solar CO2 Conversion into Formic Acid in Nickel-Perylene-C3N4 Semiconductor Photocatalyst. Adv. Energy Mater. 2024, 14, 2402798. [Google Scholar] [CrossRef]
- Yang, L.; Pawar, A.U.; Sivasankaran, R.P.; Lee, D.; Ye, J.; Xiong, Y.; Zou, Z.; Zhou, Y.; Kang, Y.S. Intermediates and their conversion into highly selective multicarbons in photo/electrocatalytic CO2 reduction reactions. J. Mater. Chem. A 2023, 11, 19172–19194. [Google Scholar] [CrossRef]
- Pawar, A.U.; Sivasankaran, R.P.; Yang, L.; Lee, D.K.; Kang, Y.S. A methodical strategy for achieving efficient electro-solar reduction, incorporating appropriate in situ techniques. Chem 2024, 10, 3536–3574. [Google Scholar] [CrossRef]
- Yang, L.; Peng, Y.; Luo, X.; Dan, Y.; Ye, J.; Zhou, Y.; Zou, Z. Beyond C3N4 π-conjugated metal-free polymeric semiconductors for photocatalytic chemical transformations. Chem. Sci. Rev. 2021, 50, 2147–2172. [Google Scholar] [CrossRef]
- Schutting, S.; Borisov, S.M.; Klimant, I. Diketo-Pyrrolo-Pyrrole Dyes as New Colorimetric and Fluorescent pH Indicators for Optical Carbon Dioxide Sensors. Anal. Chem. 2013, 85, 3271–3279. [Google Scholar] [CrossRef]
- Cao, C.; Zhang, Z.; Amirmaleki, M.; Tam, J.; Dou, W.; Filleter, T.; Sun, Y. Local strain mapping of GO nanosheets under in situ TEM tensile testing. Appl. Mater. Today 2019, 14, 102–107. [Google Scholar] [CrossRef]
- Futamura, R.; Iiyama, T.; Ueda, T.; Bonnaud, P.A.; Coudert, F.-X.; Furuse, A.; Tanaka, H.; Pellenq, R.J.M.; Kaneko, K. Staggered structural dynamic-mediated selective adsorption of H2O/D2O on flexible graphene oxide nanosheets. Nat. Commun. 2024, 15, 3585. [Google Scholar] [CrossRef]
- Yaghmaeiyan, N.; Mirzaei, M.; Bamoniri, A. The study of stereoselectivity and mesomeric effect of N-nitrosamines via 1H NMR spectroscopy. Struct. Chem. 2023, 34, 1489–1496. [Google Scholar] [CrossRef]
- Sharma, N.; Kour, M.; Gupta, R.; Bansal, R.K. A new cross-conjugated mesomeric betaine. RSC Adv. 2021, 11, 25296–25304. [Google Scholar] [CrossRef]
- Kumar, P.; Harish; Andersson, G.; Subhedar, K.M.; Dhami, H.S.; Gupta, G.; Mukhopadhyay, A.K.; Joshi, R.P. Utilization of green reductant Thuja Orientalis for reduction of GO to RGO. Ceram. Int. 2021, 47, 14862–14878. [Google Scholar] [CrossRef]
- Chen, J.-X.; Li, J.-W.; Jiang, Z.-J.; Chiu, C.-W. Polymer-assisted dispersion of reduced graphene oxide in electrospun polyvinylidene fluoride nanofibers for enhanced piezoelectric monitoring of human body movement. Chem. Eng. J. 2024, 498, 155244. [Google Scholar] [CrossRef]
- Mahmood, A.; Yuan, Z.; Sui, X.; Riaz, M.A.; Yu, Z.; Liu, C.; Chen, J.; Wang, C.; Zhao, S.; Mahmood, N.; et al. Foldable and scrollable graphene paper with tuned interlayer spacing as high areal capacity anodes for sodium-ion batteries. Energy Storage Mater. 2021, 41, 395–403. [Google Scholar] [CrossRef]
- Jin, T.; Easton, C.D.; Tang, Y.; Yin, H.; Hao, X. Nitrogen-doped graphene oxide monoliths crosslinked by short chain aliphatic amines. J. Hazard. Mater. 2018, 357, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Shang, J.; Zhu, T. Photoactivated Graphene Oxide to Enhance Photocatalytic Reduction of CO2. ACS Appl. Mater. Interfaces 2020, 12, 3580–3591. [Google Scholar] [CrossRef]
- Luo, Y.; Jia, L.; Xu, Y.; Hu, E.; Song, M.; Zhu, X.; Kang, Y.; Huang, Y.; Yang, L. Degradation Benefits Polymerization: Photogenerated Self-Degradable Organocatalyst for Higher-Efficiency ATRP and Pure Polymers. ACS Appl. Polym. Mater. 2024, 6, 5856–5865. [Google Scholar] [CrossRef]
- Yang, L.; Ngo, H.M.; Luo, Y.; Pawar, A.U.; Sivasankaran, R.P.; Ok, K.; Kang, Y.S. Specific Active Sites of Organo-Photocatalysts for Photo-Atom Transfer Radical Polymerization: A Combined Experimental and Theoretical Study of N-Unsubstituted Diketopyrrolopyrrole. J. Phys. Chem. C 2022, 126, 21576–21584. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Arun, M.K.; Thomas, S.; Wu, M.-Y.; Wu, T.; Lin, Y.-W. Hydrothermal and Co-Precipitation Combined with Photo-Reduced Preparation of Ag/AgBr/MgBi2O6 Composites for Visible Light Degradation Toward Organics. Nanomaterials 2024, 14, 1865. [Google Scholar] [CrossRef]
- Ayub, A.; Wani, A.K.; Chopra, C.; Sharma, D.K.; Amin, O.; Wani, A.W.; Singh, A.; Manzoor, S.; Singh, R. Advancing Dye Degradation: Integrating Microbial Metabolism, Photocatalysis, and Nanotechnology for Eco-Friendly Solutions. Bacteria 2025, 4, 15. [Google Scholar] [CrossRef]
- Zhu, Y.; Deng, W.; Chen, L.; Courtois, J.; Tian, Q.; Zhang, X.; Almásy, L.; Yan, M.; Xiong, K. Langmuir-Blodgett-assembled monolayer zinc ferrite nanoparticle film with unique photogenerated charge carrier separation efficiency and charge transfer behavior. Appl. Surf. Sci. 2020, 534, 147646. [Google Scholar] [CrossRef]
- Zhang, W.; Chu, Y.; Wang, C.; Zhao, Y.; Chu, W.; Zhao, J. Enhancing photocatalytic hydrogen production efficiency in all-inorganic lead-free double perovskites via silver doping-induced efficient separation of photogenerated carriers. Sep. Purif. Technol. 2025, 357, 130111. [Google Scholar] [CrossRef]
- Wang, J.-W.; Jiang, L.; Huang, H.-H.; Han, Z.; Ouyang, G. Rapid electron transfer via dynamic coordinative interaction boosts quantum efficiency for photocatalytic CO2 reduction. Nat. Commun. 2021, 12, 4276. [Google Scholar] [CrossRef]
- Cai, J.; Li, H. Electrospun polymer nanofibers coated with TiO2 hollow spheres catalyze for high synergistic photo-conversion of Cr(VI) and As(III) using visible light. Chem. Eng. J. 2020, 398, 125644. [Google Scholar] [CrossRef]
- Feng, F.; Zhou, K.; Zhang, K.; Wang, L.; Wang, R.; Xia, J.; Tang, C. Grain Boundary Guided Folding of Graphene for Twisted Bilayer Graphene. Nanomaterials 2025, 15, 482. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Rooney, C.L.; Shema, H.; Zeng, C.; Panetier, J.A.; Gross, E.; Wang, H.; Baker, L.R. The solvation environment of molecularly dispersed cobalt phthalocyanine determines methanol selectivity during electrocatalytic CO2 reduction. Nat. Catal. 2024, 7, 987–999. [Google Scholar] [CrossRef]
- Jin, Z.; Yang, M.; Dong, Y.; Ma, X.; Wang, Y.; Wu, J.; Fan, J.; Wang, D.; Xi, R.; Zhao, X.; et al. Atomic Dispersed Hetero-Pairs for Enhanced Electrocatalytic CO2 Reduction. Nano-Micro Lett. 2023, 16, 4. [Google Scholar] [CrossRef]
- Gu, J.; Hsu, C.-S.; Bai, L.; Chen, H.M.; Hu, X. Atomically dispersed Fe3+ sites catalyze efficient CO2 electroreduction to CO. Science 2019, 364, 1091–1094. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Tang, H.; Wang, Y.; Zhu, X.; Yang, L. Graphene-Based Organic Semiconductor Film for Highly Selective Photocatalytic CO2 Reduction. Nanomaterials 2025, 15, 677. https://doi.org/10.3390/nano15090677
Xu Y, Tang H, Wang Y, Zhu X, Yang L. Graphene-Based Organic Semiconductor Film for Highly Selective Photocatalytic CO2 Reduction. Nanomaterials. 2025; 15(9):677. https://doi.org/10.3390/nano15090677
Chicago/Turabian StyleXu, Yanghong, Haopeng Tang, Yifei Wang, Xiaofeng Zhu, and Long Yang. 2025. "Graphene-Based Organic Semiconductor Film for Highly Selective Photocatalytic CO2 Reduction" Nanomaterials 15, no. 9: 677. https://doi.org/10.3390/nano15090677
APA StyleXu, Y., Tang, H., Wang, Y., Zhu, X., & Yang, L. (2025). Graphene-Based Organic Semiconductor Film for Highly Selective Photocatalytic CO2 Reduction. Nanomaterials, 15(9), 677. https://doi.org/10.3390/nano15090677







