ZrBr4-Mediated Phase Engineering in CsPbBr3 for Enhanced Operational Stability of White-Light-Emitting Diodes
Abstract
1. Introduction
2. Results and Discussion
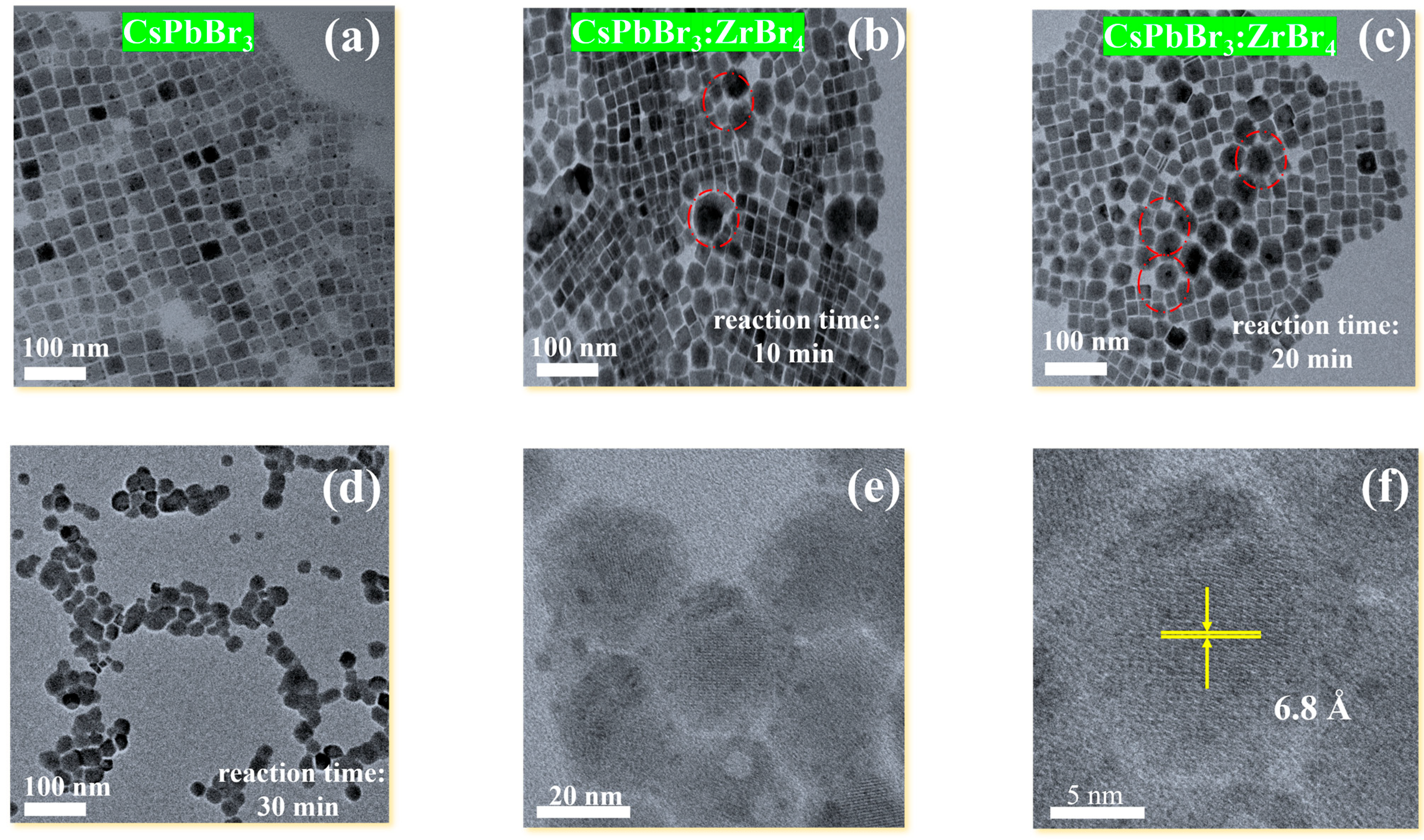
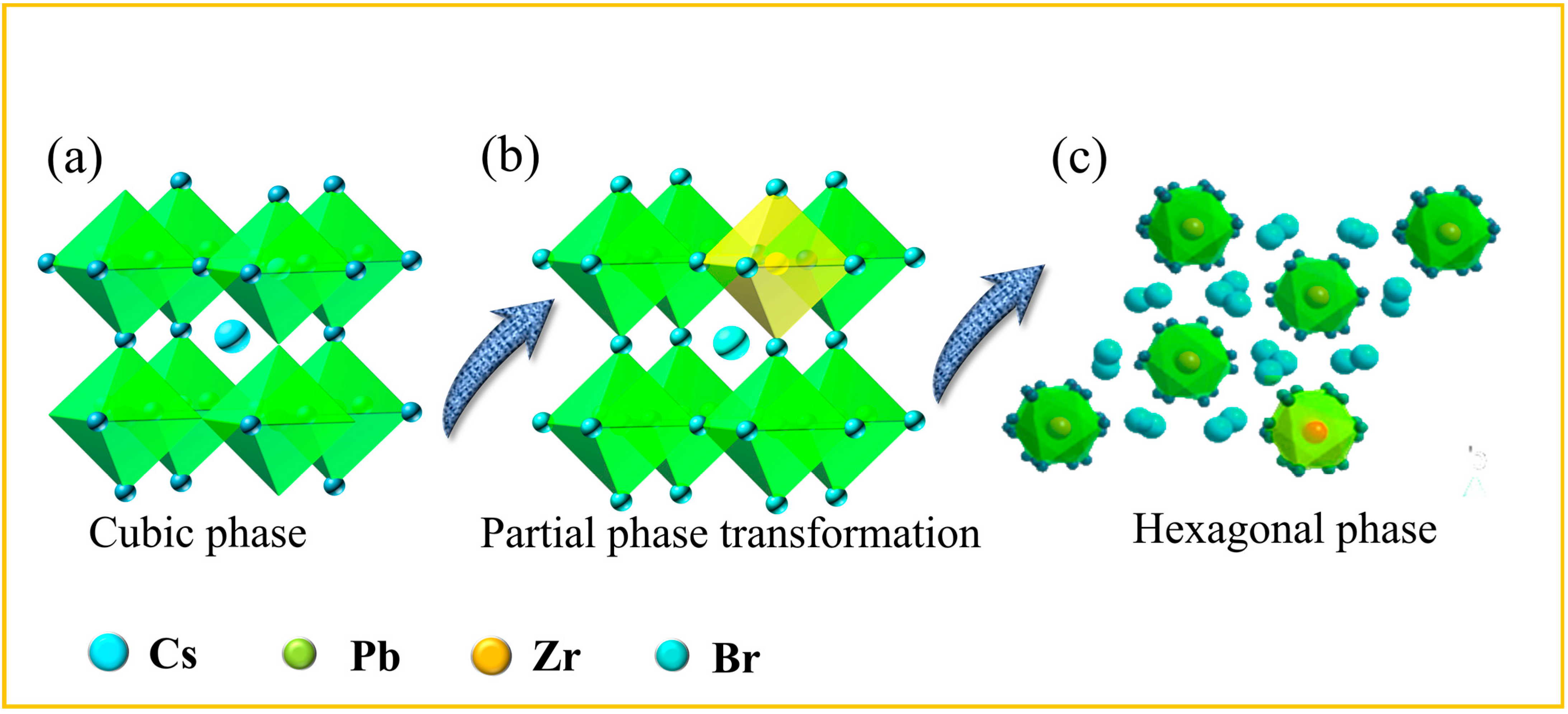
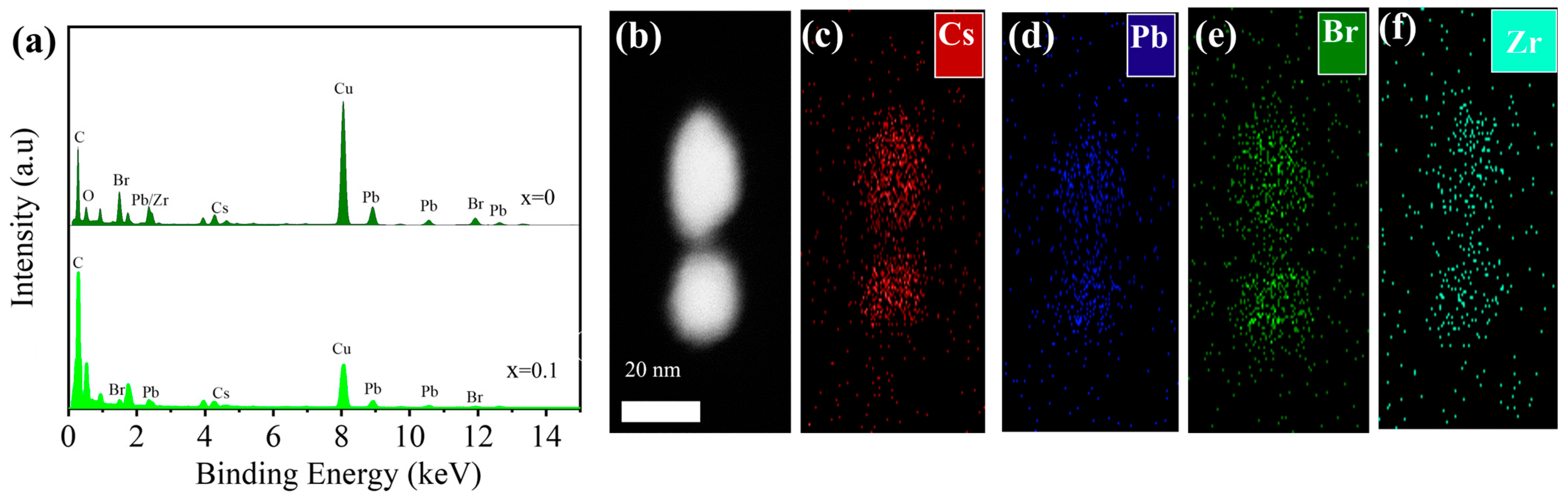
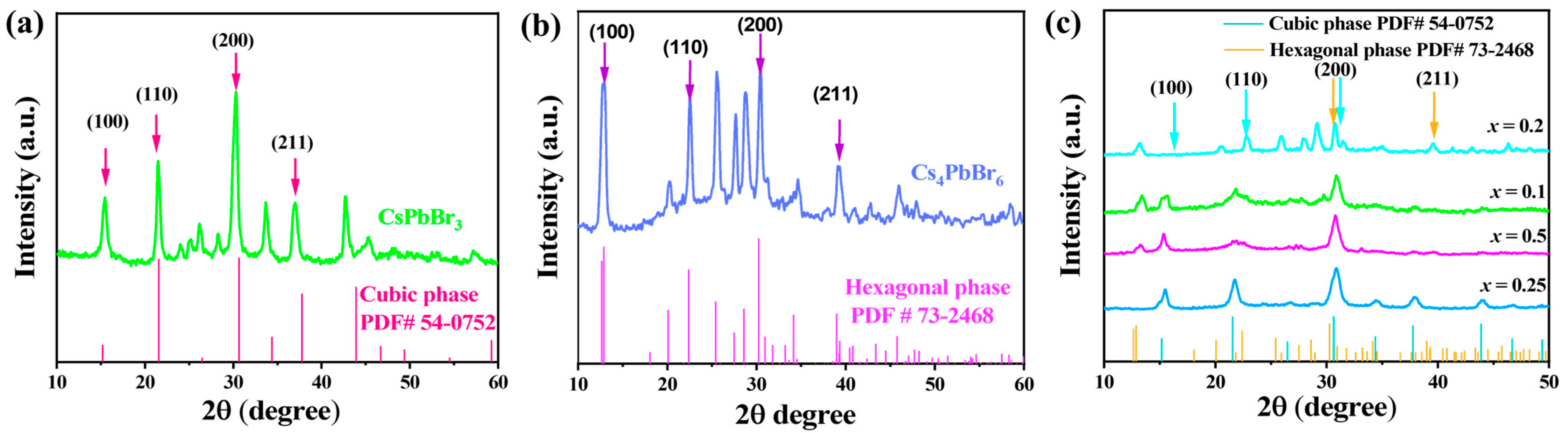
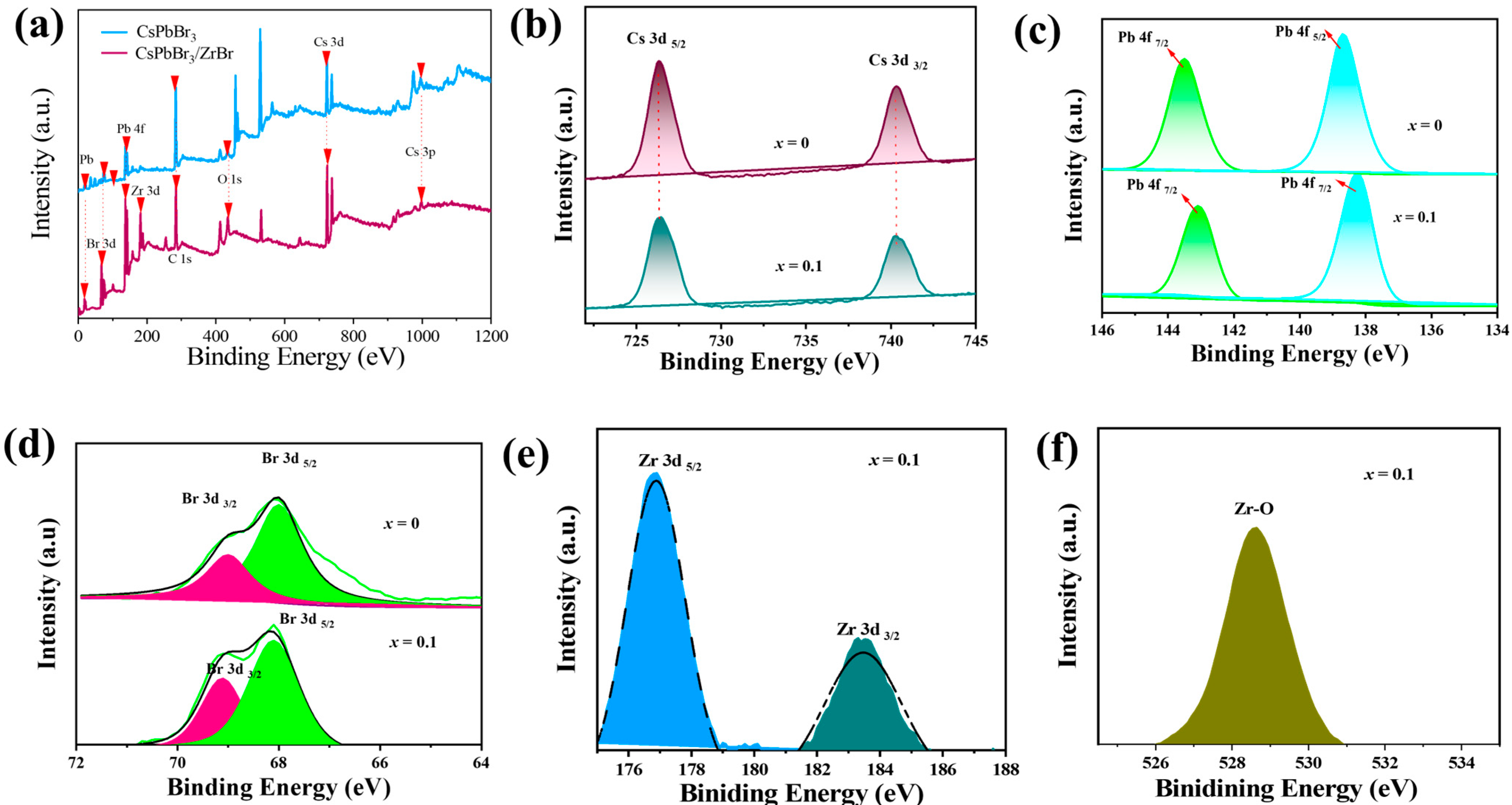
2.1. Morphological Analysis
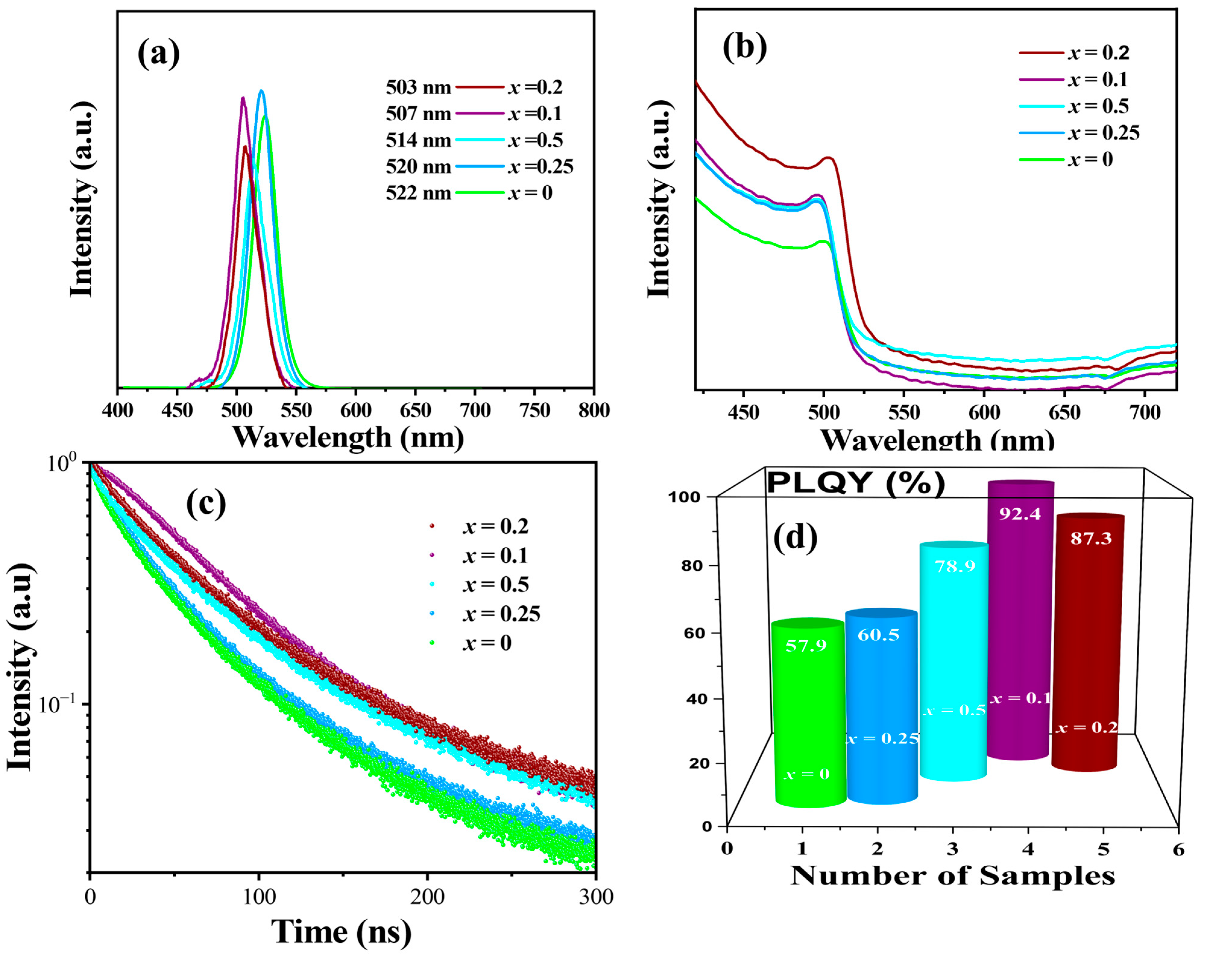
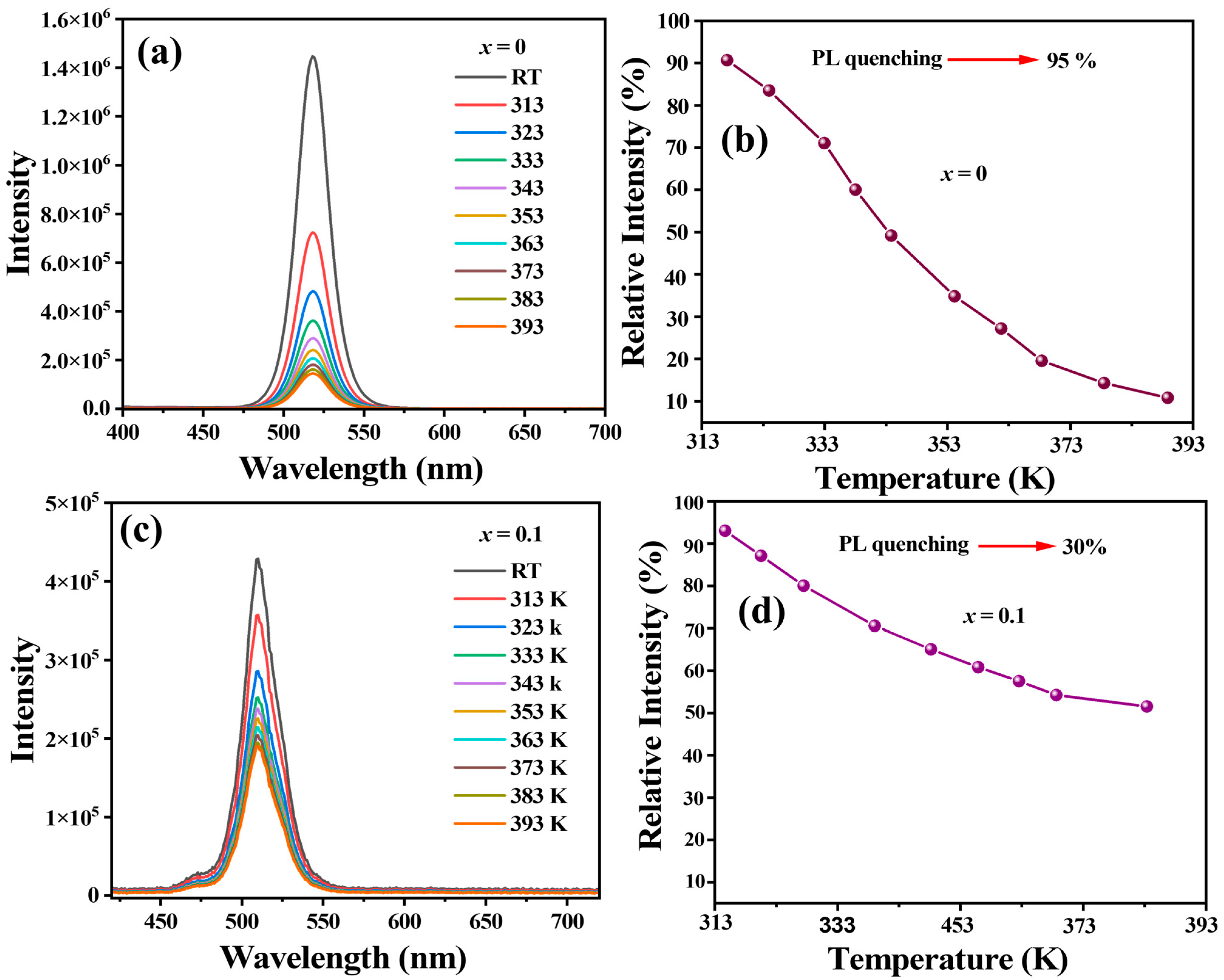
2.2. Optical Properties
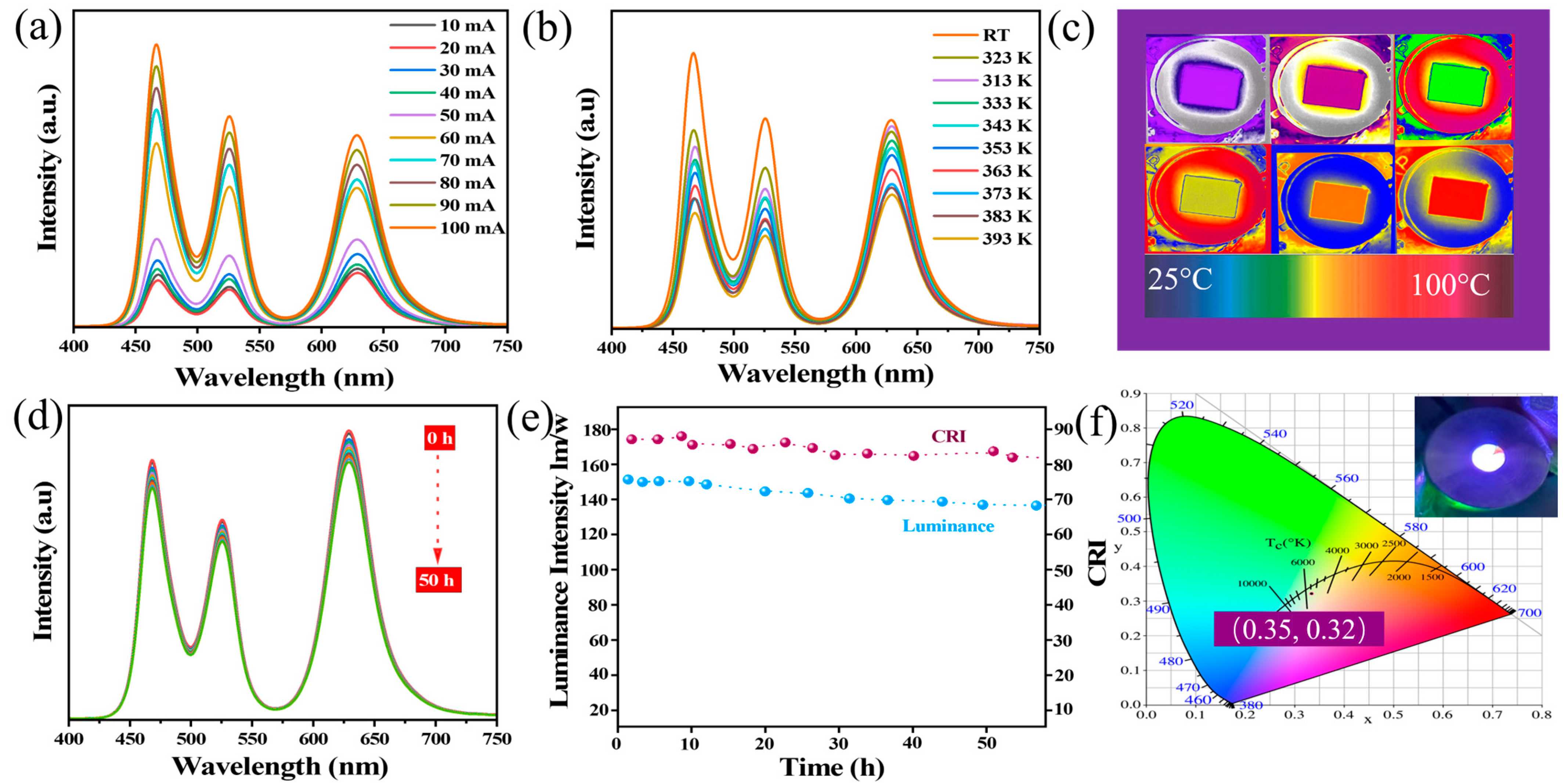
2.3. Device Performance
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shi, D.; Adinolfi, V.; Comin, R.; Yuan, M.; Alarousu, E.; Buin, A.; Chen, Y.; Hoogland, S.; Rothenberger, A.; Katsiev, K.; et al. Low Trap-State Density and Long Carrier Diffusion in Organolead Trihalide Perovskite Single Crystals. Science 2015, 347, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Xue, Q.; Yao, Q.; Li, N.; Brabec, C.J.; Yip, H.-L. Inorganic Halide Perovskite Solar Cells: Progress and Challenges. Adv. Energy Mater. 2020, 10, 2000183. [Google Scholar] [CrossRef]
- Chen, D.; Fang, G.; Chen, X. Silica-Coated Mn-Doped CsPb(Cl/Br)3 Inorganic Perovskite Quantum Dots: Exciton-to-Mn Energy Transfer and Blue-Excitable Solid-State Lighting. ACS Appl. Mater. Interfaces 2017, 9, 40477–40487. [Google Scholar] [CrossRef]
- Li, F.; Xia, Z.; Pan, C.; Gong, Y.; Gu, L.; Liu, Q.; Zhang, J.Z. High Br– Content CsPb(ClyBr1–y)3 Perovskite Nanocrystals with Strong Mn2+ Emission Through Diverse Cation/Anion Exchange Engineering. ACS Appl. Mater. Interfaces 2018, 10, 11739–11746. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.K.; Kim, I.J.; Park, Y.; Kim, M.K.; Lee, J.S. Inorganic Perovskite Quantum Dot-Mediated Photonic Multimodal Synapse. ACS Appl. Mater. Interfaces 2023, 15, 18055–18064. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-Z.; Guo, L.-C.; Ding, G.-L.; Zhou, K.; Xiong, Z.-Y.; Han, S.-T.; Zhou, Y. Inorganic Perovskite Quantum Dot-Based Strain Sensors for Data Storage and In-Sensor Computing. ACS Appl. Mater. Interfaces 2021, 13, 30861–30873. [Google Scholar] [CrossRef] [PubMed]
- Moyen, E.; Jun, H.; Kim, H.-M.; Jang, J. Surface Engineering of Room Temperature-Grown Inorganic Perovskite Quantum Dots for Highly Efficient Inverted Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2018, 10, 42647–42656. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Sun, C.; Bai, X.; Zhang, X.; Wang, Y.; Wang, Y.; Song, H.; Yu, W.W. Efficient and Stable CsPb(Br/I)3@Anthracene Composites for White Light-Emitting Devices. ACS Appl. Mater. Interfaces 2018, 10, 16768–16775. [Google Scholar] [CrossRef]
- Zhang, X.; Qian, Y.; Ling, X.; Wang, Y.; Zhang, Y.; Shi, J.; Shi, Y.; Yuan, J.; Ma, W. α-CsPbBr3 Perovskite Quantum Dots for Application in Semitransparent Photovoltaics. ACS Appl. Mater. Interfaces 2020, 12, 27307–27315. [Google Scholar] [CrossRef]
- Gong, R.; Wang, F.; Cheng, J.; Wang, Z.; Lu, Y.; Wang, J.; Wang, H. Weak-solvent-modulated optical encryption based on perovskite nanocrystals/polymer composites. Chem. Eng. J. 2022, 446, 137212. [Google Scholar] [CrossRef]
- Yoon, S.J.; Draguta, S.; Manser, J.S.; Sharia, O.; Schneider, W.F.; Kuno, M.; Kamat, P.V. Tracking Iodide and Bromide Ion Segregation in Mixed Halide Lead Perovskites During Photoirradiation. ACS Energy Lett. 2016, 1, 290–296. [Google Scholar] [CrossRef]
- Wu, H.; Yao, L.; Cao, W.; Yang, Y.; Cui, Y.; Yang, D.; Qian, G. Stable and Wide-Wavelength Tunable Luminescence of CsPbX3 Nanocrystals Encapsulated in Metal–Organic Frameworks. J. Mater. Chem. C 2022, 10, 5550–5558. [Google Scholar] [CrossRef]
- Chen, T.; Xu, Y.; Xie, Z.; Jiang, W.; Wang, L.; Jiang, W. Ionic Liquid Assisted Preparation and Modulation of the Photoluminescence Kinetics for Highly Efficient CsPbX3 Nanocrystals with Improved Stability. Nanoscale 2020, 12, 9569–9580. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, J.; Bakr, O.M.; Sun, H.-T. Metal-Doped Lead Halide Perovskites: Synthesis, Properties, and Optoelectronic Applications. Chem. Mater. 2018, 30, 6589–6613. [Google Scholar] [CrossRef]
- Padhiar, M.A.; Wang, M.; Ji, Y.; Yang, Z.; Zhou, Y.; Qiu, H.; Wang, H.; Shah, A.A.; Bhatti, A.S. Stable CsPbX3 (Br/Cl) Perovskite Nanocrystal Layer Passivated with Al-Doped CdSe for Blue Light-Emitting Diodes. ACS Appl. Nano Mater. 2022, 5, 908–916. [Google Scholar] [CrossRef]
- Otero-Martínez, C.; Fiuza-Maneiro, N.; Polavarapu, L. Enhancing the Intrinsic and Extrinsic Stability of Halide Perovskite Nanocrystals for Efficient and Durable Optoelectronics. ACS Appl. Mater. Interfaces 2022, 14, 34291–34302. [Google Scholar] [CrossRef] [PubMed]
- Lou, S.; Xuan, T.; Wang, J. (INVITED) Stability: A Desiderated Problem for the Lead Halide Perovskites. Opt. Mater. X 2019, 1, 100023. [Google Scholar] [CrossRef]
- Hu, H.; Wu, L.; Tan, Y.; Zhong, Q.; Chen, M.; Qiu, Y.; Yang, D.; Sun, B.; Zhang, Q.; Yin, Y. Interfacial Synthesis of Highly Stable CsPbX(3)/Oxide Janus Nanoparticles. J. Am. Chem. Soc. 2018, 140, 406–412. [Google Scholar] [CrossRef]
- Chen, W.; Hao, J.; Hu, W.; Zang, Z.; Tang, X.; Fang, L.; Niu, T.; Zhou, M. Enhanced Stability and Tunable Photoluminescence in Perovskite CsPbX3/ZnS Quantum Dot Heterostructure. Small 2017, 13, 1604085. [Google Scholar] [CrossRef]
- Huang, S.; Li, Z.; Kong, L.; Zhu, N.; Shan, A.; Li, L. Enhancing the Stability of CH3NH3PbBr3 Quantum Dots by Embedding in Silica Spheres Derived from Tetramethyl Orthosilicate in “Waterless” Toluene. J. Am. Chem. Soc. 2016, 138, 5749–5752. [Google Scholar] [CrossRef]
- Jiang, M.-C.; Lin, C.-Q.; Yang, Z.; Pan, C.-Y. Silica-Coated CsPbBr3 Nanocrystals with High Stability for Bright White-Emitting Displays. J. Solid-State Chem. 2023, 318, 123724. [Google Scholar] [CrossRef]
- Kumar, A.; Tripathi, S.K.; Shkir, M.; Alqahtani, A.; AlFaify, S. Prospective and Challenges for Lead-Free Pure Inorganic Perovskite Semiconductor Materials in Photovoltaic Application: A Comprehensive Review. Appl. Surf. Sci. Adv. 2023, 18, 100495. [Google Scholar] [CrossRef]
- Gahlot, K.; di Mario, L.; Bosma, R.; Loi, M.A.; Protesescu, L. Air-Stable Thin Films of Tin Halide Perovskite Nanocrystals by Polymers and Al2O3 Encapsulation. Chem. Mater. 2024, 36, 11227–11235. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Cao, M.; Hu, H.; Yang, D.; Chen, M.; Li, P.; Wu, L.; Zhang, Q. One-Pot Synthesis of Highly Stable CsPbBr3@SiO2 Core–Shell Nanoparticles. ACS Nano 2018, 12, 8579–8587. [Google Scholar] [CrossRef]
- Padhiar, M.A.; Ji, Y.; Wang, M.; Pan, S.; Khan, S.A.; Khan, N.Z.; Zhao, L.; Qin, F.; Zhao, Z.; Zhang, S. Sr2+ Doped CsPbBrI2 Perovskite Nanocrystals Coated with ZrO2 for Applications as White LEDs. Nanotechnology 2023, 34, 275201. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; De, A.; Samanta, A. Ambient Condition Mg2+ Doping Producing Highly Luminescent Green- and Violet-Emitting Perovskite Nanocrystals with Reduced Toxicity and Enhanced Stability. J. Phys. Chem. Lett. 2020, 11, 1178–1188. [Google Scholar] [CrossRef]
- Chen, C.; Xuan, T.; Bai, W.; Zhou, T.; Huang, F.; Xie, A.; Wang, L.; Xie, R.-J. Highly Stable CsPbI3:Sr2+ Nanocrystals with Near-Unity Quantum Yield Enabling Perovskite Light-Emitting Diodes with An External Quantum Efficiency of 17.1%. Nano Energy 2021, 85, 106033. [Google Scholar] [CrossRef]
- Yao, J.-S.; Ge, J.; Han, B.-N.; Wang, K.-H.; Yao, H.-B.; Yu, H.-L.; Li, J.-H.; Zhu, B.-S.; Song, J.-Z.; Chen, C.; et al. Ce3+-Doping to Modulate Photoluminescence Kinetics for Efficient CsPbBr3 Nanocrystals Based Light-Emitting Diodes. J. Am. Chem. Soc. 2018, 140, 3626–3634. [Google Scholar] [CrossRef]
- Xu, L.; Yuan, S.; Zeng, H.; Song, J. A Comprehensive Review of Doping in Perovskite Nanocrystals/Quantum Dots: Evolution Of Structure, Electronics, Optics, And Light-Emitting Diodes. Mater. Today Nano 2019, 6, 100036. [Google Scholar] [CrossRef]
- Ahmad, I.; Abohashrh, M.; Aftab, A.; Aziz, H.; Fatima, I.; Shahzadi, N.; Ahmad, S.; Muhmood, T. Manganese and Copper Doped Perovskites Nanocrystals and Their Optoelectronic Applications. Appl. Mater. Today 2023, 32, 101827. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, F.; Huang, Q.; Wang, K.; Li, C.; Wang, R.; Liu, C.; Xu, W.; Liu, R.; Zhu, H.; et al. Shape-Controlled Synthesis of CsPbBr3 Nanorods with Bright Pure Blue Emission and High Stability. J. Mater. Chem. C 2024, 12, 4234–4242. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Z. In-situ fabrication of Cu doped dual-phase CsPbBr3–Cs4PbBr6 Inorganic Perovskite Nanocomposites for Efficient and Selective Photocatalytic CO2 reduction. Chem. Eng. J. 2022, 434, 134811. [Google Scholar] [CrossRef]
- Padhiar, M.A.; Zhang, S.; Qin, F.; Wang, M.; Ji, Y.; Khan, N.Z.; Muhammad, N.; Khan, S.A.; Ahmed, J.; Pan, S. Lead-Free Cs₂NaInCl₆:Bi3⁺/Mn2⁺ Double Perovskite Nanocrystals to Nanosheets with Improved Photoluminescence Quantum Yield for Anti-Counterfeit Marks And Led Applications. Ceram. Int. 2024, 50, 19552–19560. [Google Scholar] [CrossRef]
- Ornelas-Cruz, I.; Santos, R.M.D.; González, J.E.; Lima, M.P.; Da Silva, J.L.F. Cubic-to-Hexagonal Structural Phase Transition in Metal Halide Compounds: A DFT study. J. Mater. Chem. A 2024, 12, 12564–12580. [Google Scholar] [CrossRef]
- Zhang, B.; Liang, Q.; Yong, X.; Wu, H.; Chu, Z.; Ma, Y.; Brovelli, S.; Manna, L.; Lu, S. Facet-Defect Tolerant Bi-Doped Cs2AgxNa1–xInCl6 Nanoplatelets with a Near-Unity Photoluminescence Quantum Yield. Nano Lett. 2023, 23, 9050–9055. [Google Scholar] [CrossRef]
- Chan, W.K.; Zhou, D.; Yu, Z.; Tan, T.T.Y. Mechanistic studies of CsPbBr3 superstructure formation. J. Mater. Chem. C 2021, 9, 14699–14708. [Google Scholar] [CrossRef]
- Ghorai, A.; Singh, S.; Roy, B.; Bose, S.; Mahato, S.; Mukhin, N.; Jha, P.; Ray, S.K. Suppression of Light-Induced Phase Segregations in Mixed Halide Perovskites Through Ligand Passivation. J. Phys. Chem. Lett. 2025, 16, 1760–1768. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Y.; Cai, Y.; Zhang, Y.; Zhou, P.; Liu, B.; Li, Y. Phase Stability and Electronic Structure of CsPbBr3 Perovskites Under Rare-Earth Doping and Hydrostatic Pressure. J. Mater. Sci. 2024, 59, 4586–4595. [Google Scholar] [CrossRef]
- Zeng, Y.-T.; Li, Z.-R.; Chang, S.-P.; Ansay, A.; Wang, Z.-H.; Huang, C.-Y. Bright CsPbBr3 Perovskite Nanocrystals with Improved Stability by In-Situ Zn-Doping. Nanomaterials 2022, 12, 759. [Google Scholar] [CrossRef]
- Keeble, D.J.; Wiktor, J.; Pathak, S.K.; Phillips, L.J.; Dickmann, M.; Durose, K.; Snaith, H.J.; Egger, W. Identification of Lead Vacancy Defects in Lead Halide Perovskites. Nat. Commun. 2021, 12, 5566. [Google Scholar] [CrossRef]
- Varnakavi, N.; Gupta, K.; Lee, K.; Yang, J.; Cha, P.-R.; Lee, N. Compositional Engineering of ZnBr2-Doped CsPbBr3 Perovskite Nanocrystals: Insights into Structure Transformation, Optical Performance, and Charge-Carrier Dynamics. J. Mater. Chem. C 2023, 11, 14248–14259. [Google Scholar] [CrossRef]
- Mączka, M.; Sieradzki, A.; Bondzior, B.; Dereń, P.; Hanuza, J.; Hermanowicz, K. Effect of Aliovalent Doping on the Properties of Perovskite-Like Multiferroic Formates. J. Mater. Chem. C 2015, 3, 9337–9345. [Google Scholar] [CrossRef]
- Luo, L.; Hu, W.; Liang, X.; Ding, Y.; Yuan, H.; Song, Y.; Deng, S.; Kang, K. Study of Phase Transition, Structural Stability and Mechanical Properties of CsPbBr3 Under High Pressure by First Principles. Mater. Today Commun. 2024, 41, 110586. [Google Scholar] [CrossRef]
- Xie, S.; Yang, D.; Li, Z.; Ma, X.; Wang, H.; Liu, S.; Liu, Y.; Yue, S. The Evolution of Electrical, Optical, and Mechanical Properties of CsPbBr3 Perovskites During Continuous Phase Transitions. Chem. Eng. J. 2025, 505, 159524. [Google Scholar] [CrossRef]
- Lawal, S.O.; Takahashi, Y.; Nagasawa, H.; Tsuru, T.; Kanezashi, M. Microporous Structure Control of SiO2-ZrO2 Composite Membranes via Yttrium Doping and an Evaluation of Thermal Stability. J. Sol-Gel Sci. Technol. 2022, 104, 566–579. [Google Scholar] [CrossRef]
- Chen, R.; Xu, Y.; Wang, S.; Xia, C.; Liu, Y.; Yu, B.; Xuan, T.; Li, H. Zinc Ions Doped Cesium Lead Bromide Perovskite Nanocrystals with Enhanced Efficiency and Stability for White Light-Emitting Diodes. J. Alloys Compd. 2021, 866, 158969. [Google Scholar] [CrossRef]
- Guo, X.; Lv, Y.; Wang, L.; Liu, H.; Wang, W. CsPbBr3 Perovskite Quantum Dots by Mesoporous Silica Encapsulated for Enhancing Water and Thermal Stability via High Temperature Solid State Method. Opt. Mater. 2024, 157, 116097. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, L.; Zhou, T.; Zheng, P.; Li, Y.; Xie, R.-J. Improved Stability of CsPbBr3 Perovskite Quantum Dots Achieved by Suppressing Inter ligand Proton Transfer and Applying a Polystyrene Coating. Nanoscale 2018, 10, 21441–21450. [Google Scholar] [CrossRef]
- Schryver, S.; Lamichhane, A. Temperature-driven structural phase transitions in CsPbBr3. Solid State Commun. 2023, 371, 115237. [Google Scholar] [CrossRef]
- Li, Y.; Dong, L.; Patterson, R.; Teh, Z.L.; Hu, Y.; Huang, S.; Chen, C. Stabilizing CsPbBr3 perovskite quantum dots on zirconium phosphate nanosheets through an ion exchange/surface adsorption strategy. Chem. Eng. J. 2020, 381, 122735. [Google Scholar] [CrossRef]
- Yan, D.; Zhao, S.; Zhang, Y.; Wang, H.; Zang, Z. Highly efficient emission and high-CRI warm white light-emitting diodes from ligand-modified CsPbBr3 quantum dots. Opto-Electron. Adv. 2022, 5, 200075. [Google Scholar] [CrossRef]
- Rao, L.; Sun, B.; Liu, Y.; Zhong, G.; Wen, M.; Zhang, J.; Fu, T.; Wang, S.; Wang, F.; Niu, X. Highly Stable and Photoluminescent CsPbBr3/Cs4PbBr6 Composites for White-Light-Emitting Diodes and Visible Light Communication. Nanomaterials 2023, 13, 355. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lv, Y.; Guo, Z.; Dong, L.; Zheng, J.; Chai, C.; Chen, N.; Lu, Y.; Chen, C. One-Step Preparation of Long-Term Stable and Flexible CsPbBr3 Perovskite Quantum Dots/Ethylene Vinyl Acetate Copolymer Composite Films for White Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2018, 10, 15888–15894. [Google Scholar] [CrossRef]
- Guan, H.; Zhao, S.; Wang, H.; Yan, D.; Wang, M.; Zang, Z. Room temperature synthesis of stable single silica-coated CsPbBr3 quantum dots combining tunable red emission of Ag–In–Zn–S for High-CRI white light-emitting diodes. Nano Energy 2020, 67, 104279. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padhiar, M.A.; Ji, Y.; Wang, J.; Khan, N.Z.; Xiong, M.; Wang, S. ZrBr4-Mediated Phase Engineering in CsPbBr3 for Enhanced Operational Stability of White-Light-Emitting Diodes. Nanomaterials 2025, 15, 674. https://doi.org/10.3390/nano15090674
Padhiar MA, Ji Y, Wang J, Khan NZ, Xiong M, Wang S. ZrBr4-Mediated Phase Engineering in CsPbBr3 for Enhanced Operational Stability of White-Light-Emitting Diodes. Nanomaterials. 2025; 15(9):674. https://doi.org/10.3390/nano15090674
Chicago/Turabian StylePadhiar, Muhammad Amin, Yongqiang Ji, Jing Wang, Noor Zamin Khan, Mengji Xiong, and Shuxin Wang. 2025. "ZrBr4-Mediated Phase Engineering in CsPbBr3 for Enhanced Operational Stability of White-Light-Emitting Diodes" Nanomaterials 15, no. 9: 674. https://doi.org/10.3390/nano15090674
APA StylePadhiar, M. A., Ji, Y., Wang, J., Khan, N. Z., Xiong, M., & Wang, S. (2025). ZrBr4-Mediated Phase Engineering in CsPbBr3 for Enhanced Operational Stability of White-Light-Emitting Diodes. Nanomaterials, 15(9), 674. https://doi.org/10.3390/nano15090674








