Advancements in Ti3C2 MXene-Integrated Various Metal Hydrides for Hydrogen Energy Storage: A Review
Abstract
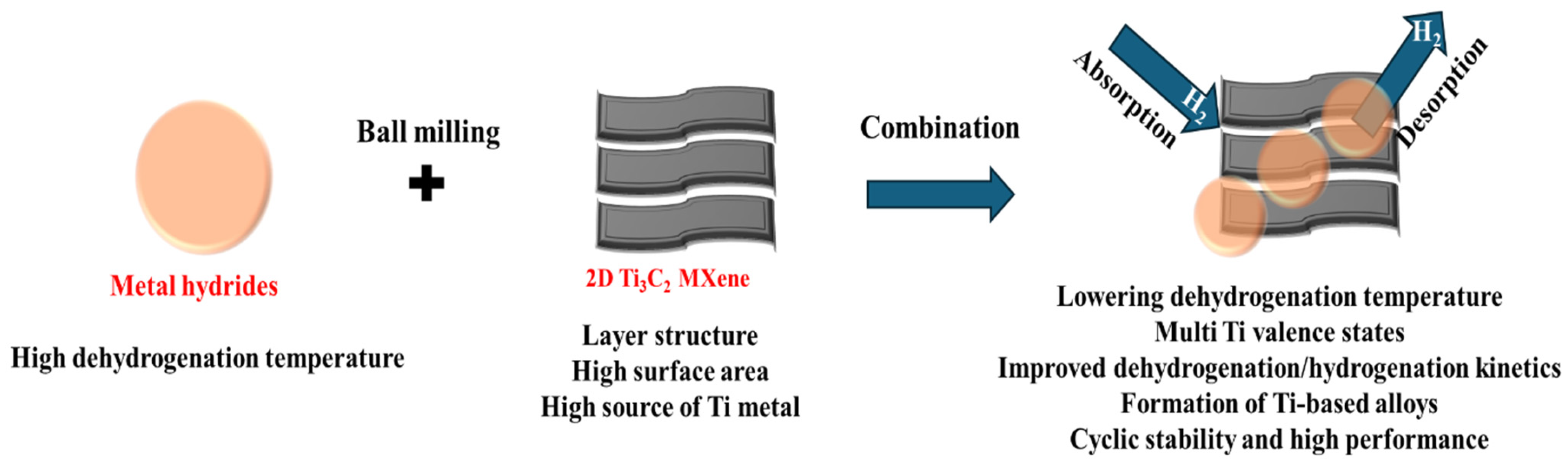
1. Introduction
2. Importance of Ti3C2 MXene for H2 Storage
3. Role of Ti3C2 MXene/Metal Hydride Interface for H2 Storage
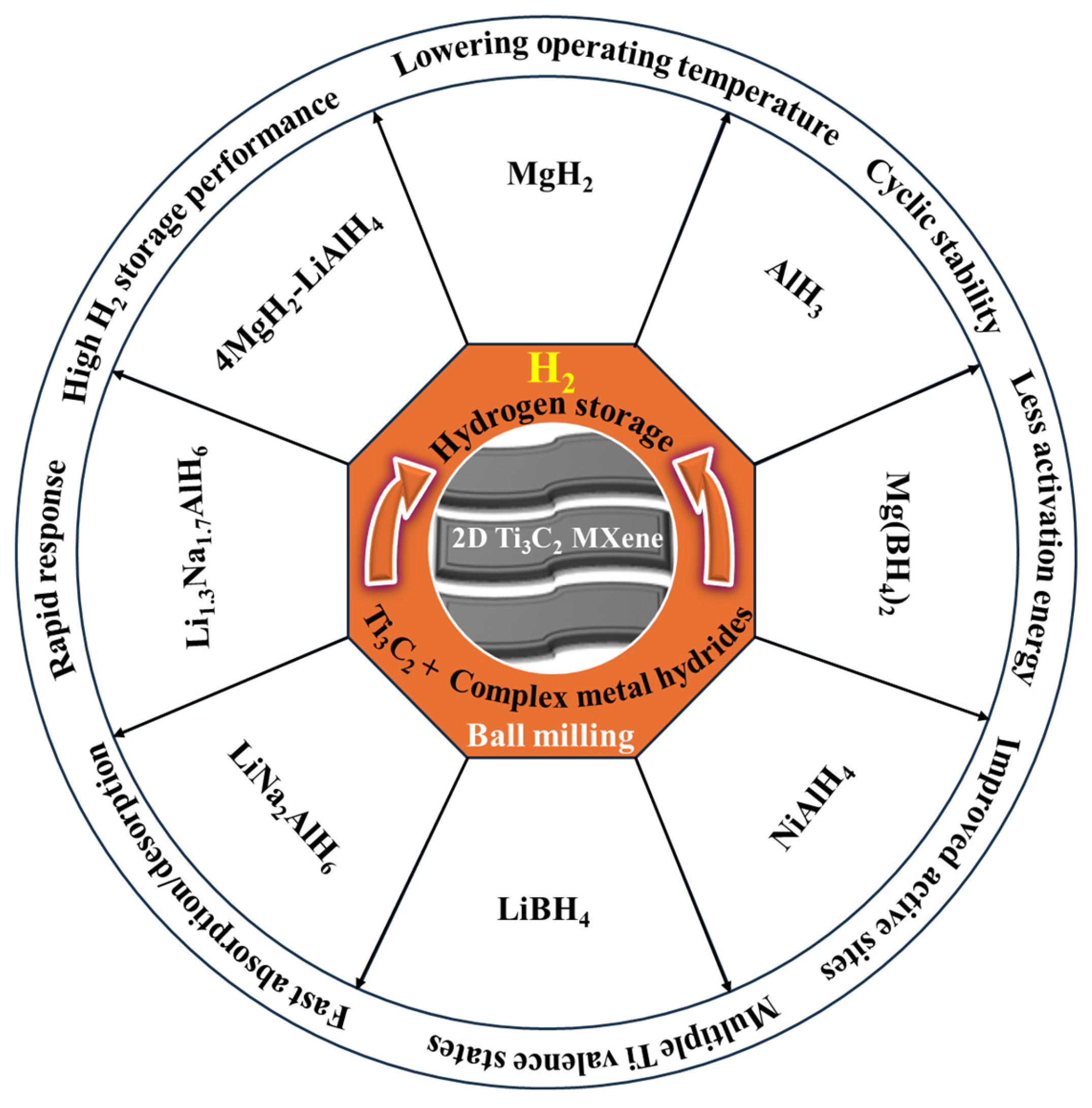
4. Ti3C2 MXene-Based H2 Storage Materials
4.1. Binary Metal Hydrides
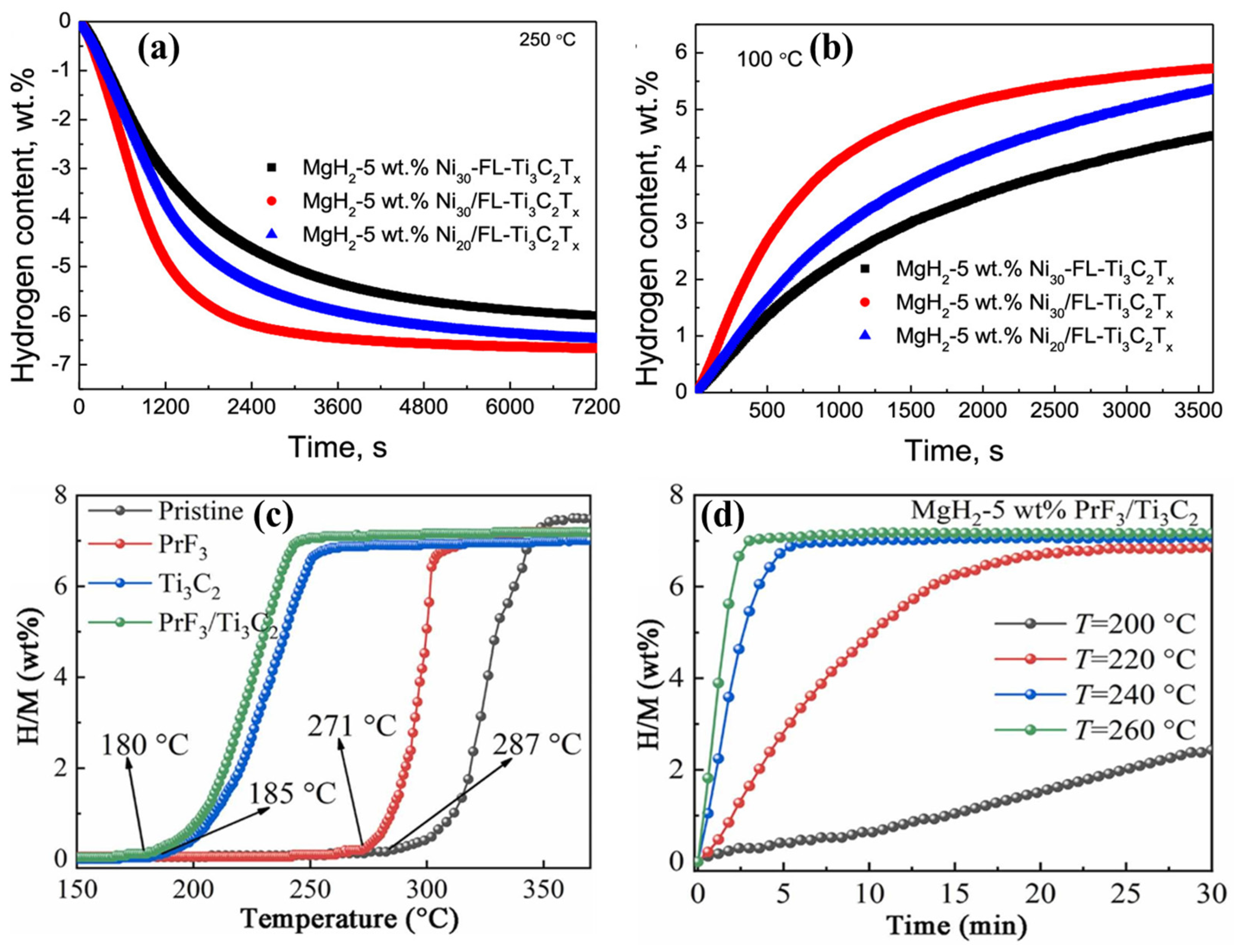
4.1.1. MgH2
4.1.2. AlH3
4.2. Ternary Metal Hydrides
4.2.1. Mg(BH4)2
4.2.2. NiAlH4

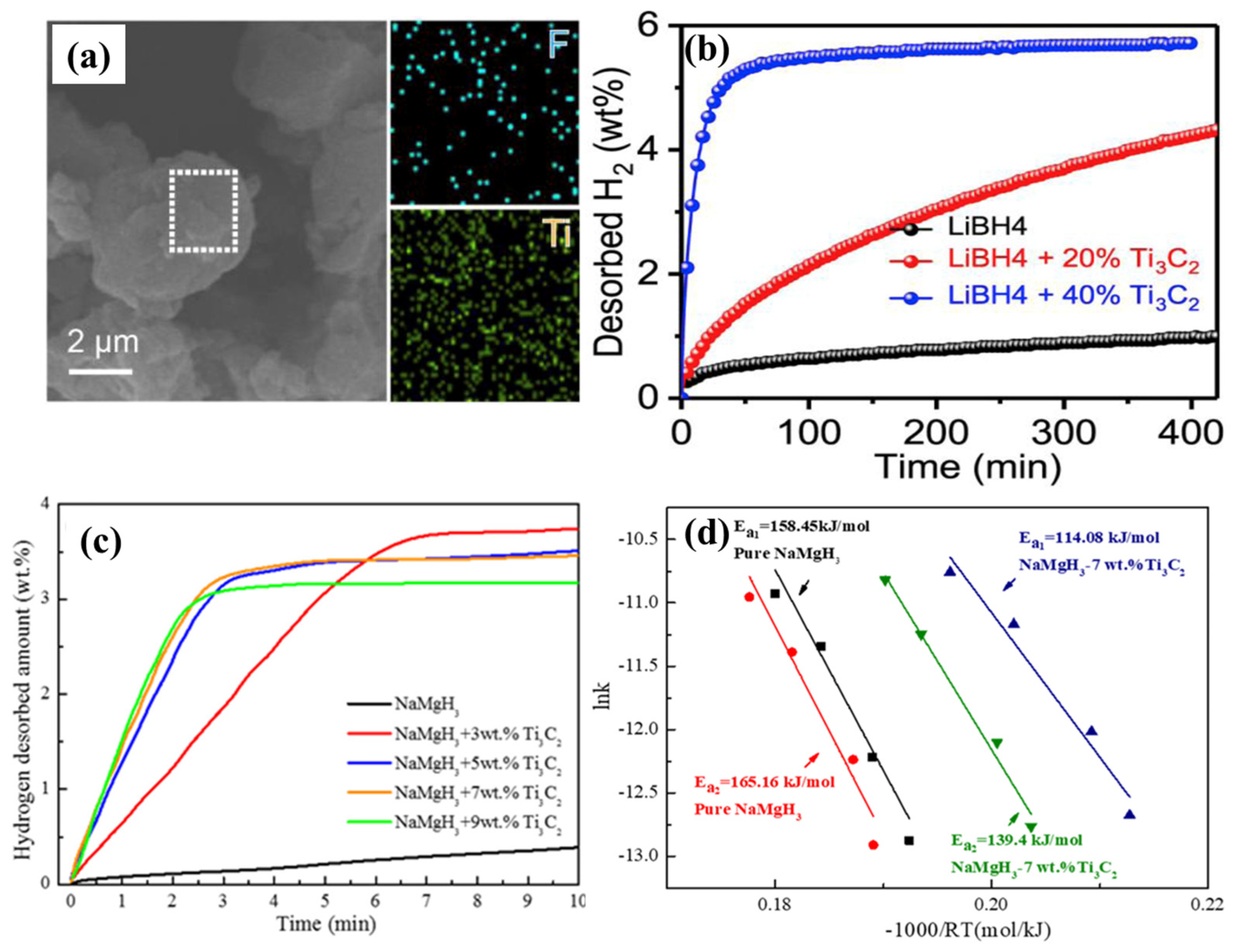
4.2.3. LiBH4
4.2.4. NaMgH3
5. Complex Metal Hydrides
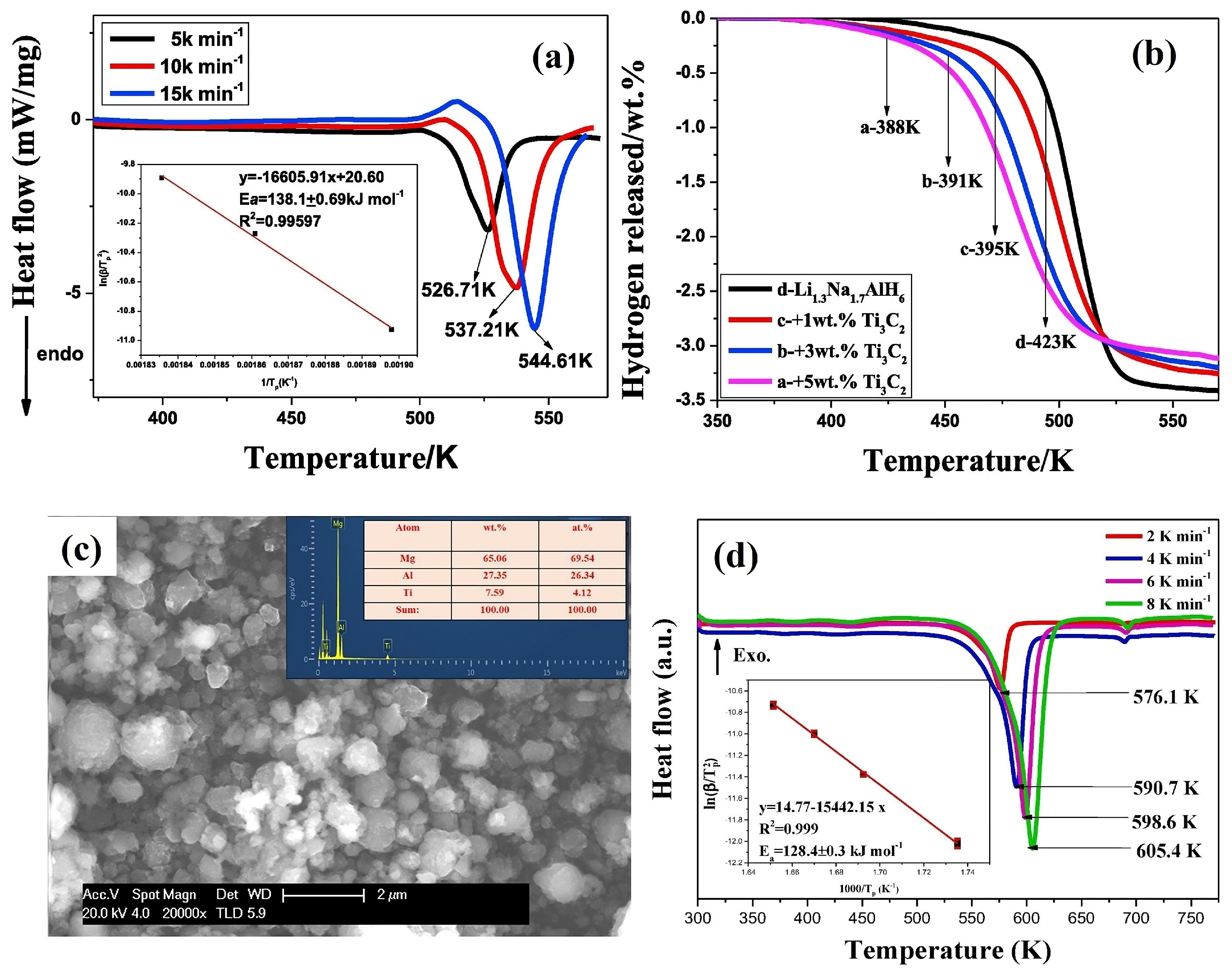
5.1. LiNa2AlH6 and Li1.3Na1.7AlH6
5.2. MgH2-LiAlH4
6. Challenges and Future Perspectives
- (i)
- The role of physisorption or chemisorption of Ti3C2 MXenes is still challenging. An in-depth discussion on the chemisorption versus physisorption of Ti3C2 MXenes is needed to improve the H2 storage capacity of metal hydrides.
- (ii)
- Surface termination groups provide the room for active sites for hydrogen molecules. The role of surface termination groups in the H2 absorption/desorption process of Ti3C2 MXenes remains insufficiently explored. The role of -OH and -O terminated Ti3C2 MXene should be studied to strengthen the H2 storage performance of metal hydride.
- (iii)
- The precise involvement of Ti3C2 MXene content in the H2 storage process at the interface of metal hydrides is not well understood. Specifically, the roles of edge sites, interlayer spacing, and surface terminations should be addressed.
- (iv)
- A detailed comparison between monolayer, few-layer, and multilayer Ti3C2 MXenes is necessary to enhance the H2 storage process through the variation in the surface area, binding strength, storage and release ability. The direction of morphological tuning has the ability to strengthen the H2 absorption and release kinetics during the H2 storage process.
- (v)
- Expansion of interlayer spacing and surface area of Ti3C2 MXene by the doping of single metal atoms have a high chance of accommodating the hydrogen molecules between the layers. These features provide a chance to enhance the H2 absorption/desorption processes at the interface of metal hydrides.
- (vi)
- Metal oxides such as SnO2, WO3, MnO2, MoO3, and ZrO2 gained their importance in H2 storage [76]. Thus, the integration of metal oxides with Ti3C2 MXene has potential room for further improvement in H2 storage of various metal hydrides.
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Balat, M. Potential importance of hydrogen as a future solution to environmental and transportation problems. Int. J. Hydrogen Energy 2008, 33, 4013–4029. [Google Scholar] [CrossRef]
- Hassan, I.A.; Ramadan, H.S.; Saleh, M.A.; Hissel, D. Hydrogen storage technologies for stationary and mobile applications: Review, analysis and perspectives. Renew. Sustain. Energy Rev. 2021, 149, 111311. [Google Scholar] [CrossRef]
- Son, W.; Lee, S.; Woo, J. Community acceptance of hydrogen power plant projects: The case of South Korea. Renew. Sustain. Energy Rev. 2023, 187, 113778. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, S.; Hashmi, S.A.R.; Kim, K.-H. MXenes: Emerging 2D materials for hydrogen storage. Nano Energy 2021, 85, 105989. [Google Scholar] [CrossRef]
- Huang, Y.; Cheng, Y.; Zhang, J. A Review of High Density Solid Hydrogen Storage Materials by Pyrolysis for Promising Mobile Applications. Ind. Eng. Chem. Res. 2021, 60, 2737–2771. [Google Scholar] [CrossRef]
- Züttel, A. Materials for hydrogen storage. Mater. Today 2003, 6, 24–33. [Google Scholar] [CrossRef]
- Liu, S.; Shui, J. Mechanism and properties of emerging nanostructured hydrogen storage materials. Batter. Energy 2022, 1, 20220033. [Google Scholar] [CrossRef]
- Sreedhar, A.; Noh, J.-S. Recent advances in partially and completely derived 2D Ti3C2 MXene based TiO2 nanocomposites towards photocatalytic applications: A review. Sol. Energy 2021, 222, 48–73. [Google Scholar] [CrossRef]
- Sreedhar, A.; Ta, Q.T.H.; Noh, J.-S. Rational engineering of morphology modulated Ti-ZnO thin films coupled monolayer Ti3C2 MXene for efficient visible light PEC water splitting activity. J. Electroanal. Chem. 2022, 921, 116703. [Google Scholar] [CrossRef]
- Züttel, A.; Remhof, A.; Borgschulte, A.; Friedrichs, O. Hydrogen: The future energy carrier. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 3329–3342. [Google Scholar] [CrossRef]
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen Storage for Mobility: A Review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef] [PubMed]
- Faye, O.; Szpunar, J.; Eduok, U. A critical review on the current technologies for the generation, storage, and transportation of hydrogen. Int. J. Hydrogen Energy 2022, 47, 13771–13802. [Google Scholar] [CrossRef]
- Lai, Q.; Sun, Y.; Wang, T.; Modi, P.; Cazorla, C.; Demirci, U.B.; Ares Fernandez, J.R.; Leardini, F.; Aguey-Zinsou, K. How to Design Hydrogen Storage Materials? Fundamentals, Synthesis, and Storage Tanks. Adv. Sustain. Syst. 2019, 3, 1900043. [Google Scholar] [CrossRef]
- Jena, P. Materials for Hydrogen Storage: Past, Present, and Future. J. Phys. Chem. Lett. 2011, 2, 206–211. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, W.; Chen, X.; Jin, J.; Yan, X.; Huang, J. Single-Atom Ni Supported on TiO2 for Catalyzing Hydrogen Storage in MgH2. J. Am. Chem. Soc. 2024, 146, 10432–10442. [Google Scholar] [CrossRef]
- Ding, Z.; Lu, Y.; Li, L.; Shaw, L. High reversible capacity hydrogen storage through Nano-LiBH4 + Nano-MgH2 system. Energy Storage Mater. 2019, 20, 24–35. [Google Scholar] [CrossRef]
- Kwak, Y.-J.; Song, M.-Y.; Lee, K.-T. Improvement in the Hydrogen Storage Properties of MgH2 by Adding NaAlH4. Metals 2024, 14, 227. [Google Scholar] [CrossRef]
- Mao, J.F.; Yu, X.B.; Guo, Z.P.; Liu, H.K.; Wu, Z.; Ni, J. Enhanced hydrogen storage performances of NaBH4–MgH2 system. J. Alloys Compd. 2009, 479, 619–623. [Google Scholar] [CrossRef]
- Lang, C.; Yao, X. Enhancing hydrogen storage performance of magnesium-based materials: A review on nanostructuring and catalytic modification. J. Magnes. Alloy. 2025, 13, 510–538. [Google Scholar] [CrossRef]
- Zhou, D.; Zheng, C.; Zhang, Y.; Sun, H.; Sheng, P.; Zhang, X.; Li, J.; Guo, S.; Zhao, D. An overview of RE-Mg-based alloys for hydrogen storage: Structure, properties, progresses and perspectives. J. Magnes. Alloy. 2025, 13, 41–70. [Google Scholar] [CrossRef]
- Li, Q.; Lu, Y.; Luo, Q.; Yang, X.; Yang, Y.; Tan, J.; Dong, Z.; Dang, J.; Li, J.; Chen, Y.; et al. Thermodynamics and kinetics of hydriding and dehydriding reactions in Mg-based hydrogen storage materials. J. Magnes. Alloy. 2021, 9, 1922–1941. [Google Scholar] [CrossRef]
- Liu, H.; Lu, L.; Luo, H.; Deng, J.; Li, G.; Ning, H.; Fan, Y.; Huang, C.; Lan, Z.; Zhou, W.; et al. Hybrid of bulk NbC and layered Nb4C3 MXene for tailoring the hydrogen storage kinetics and reversibility of Li–Mg–B–H composite: An experimental and theoretical study. J. Mater. Sci. Technol. 2024, 194, 225–235. [Google Scholar] [CrossRef]
- Lu, L.-W.; Luo, H.; Li, G.-X.; Li, Y.; Wang, X.-H.; Huang, C.-K.; Lan, Z.-Q.; Zhou, W.-Z.; Guo, J.; Ismail, M.; et al. Layered niobium carbide enabling excellent kinetics and cycling stability of Li-Mg-B-H hydrogen storage material. Rare Met. 2024, 43, 1153–1166. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, Y.; Zu, A.; Liu, Y.; Zhang, Z.; Guo, J.; Lian, C.; Zou, M.; Wang, S. One-step synthesis of fluorine-functionalized intercalated graphene with adjustable layer spacing for both enhanced physical and chemical hydrogen storage. Mater. Today Catal. 2024, 7, 100074. [Google Scholar] [CrossRef]
- Klechikov, A.G.; Mercier, G.; Merino, P.; Blanco, S.; Merino, C.; Talyzin, A.V. Hydrogen storage in bulk graphene-related materials. Microporous Mesoporous Mater. 2015, 210, 46–51. [Google Scholar] [CrossRef]
- Sreedhar, A.; Reddy, I.N.; Noh, J.-S. Photocatalytic and electrocatalytic reduction of CO2 and N2 by Ti3C2 MXene supported composites for a cleaner environment: A review. J. Clean. Prod. 2021, 328, 129647. [Google Scholar] [CrossRef]
- Hu, Q.; Sun, D.; Wu, Q.; Wang, H.; Wang, L.; Liu, B.; Zhou, A.; He, J. MXene: A New Family of Promising Hydrogen Storage Medium. J. Phys. Chem. A 2013, 117, 14253–14260. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; Liu, X.; Shang, J.; Xu, L.; Yu, R.; Shui, J. Hydrogen storage in incompletely etched multilayer Ti2CTx at room temperature. Nat. Nanotechnol. 2021, 16, 331–336. [Google Scholar] [CrossRef]
- Amani, A.M.; Tayebi, L.; Vafa, E.; Abbasi, M.; Vaez, A.; Kamyab, H.; Chelliapan, S.; Azizli, M.J.; Bazargan-Lari, R. On the horizon of greener pathways to travel into a greener future portal: Green MXenes, environment-friendly synthesis, and their innovative applications. J. Clean. Prod. 2024, 436, 140606. [Google Scholar] [CrossRef]
- Lu, C.; Liu, H.; Xu, L.; Luo, H.; He, S.; Duan, X.; Huang, X.; Wang, X.; Lan, Z.; Guo, J. Two-dimensional vanadium carbide for simultaneously tailoring the hydrogen sorption thermodynamics and kinetics of magnesium hydride. J. Magnes. Alloy. 2022, 10, 1051–1065. [Google Scholar] [CrossRef]
- Deng, J.; Li, Y.; Ning, H.; Qing, P.; Huang, X.; Luo, H.; Zhang, L.; Li, G.; Huang, C.; Lan, Z.; et al. MXenes as catalysts for lightweight hydrogen storage materials: A review. Mater. Today Catal. 2024, 7, 100073. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, B.; Ren, L.; Li, Y.; Lin, X.; Zhang, Q.; Hu, Z.; Zou, J. Nanostructured MXene-based materials for boosting hydrogen sorption properties of Mg/MgH2. Mater. Rep. Energy 2024, 4, 100255. [Google Scholar] [CrossRef]
- Sahoo, R.K.; Sahu, S. Transition from chemisorption to physisorption of H2 on Ti functionalized [2,2,2]paracyclophane: A computational search for hydrogen storage. J. Energy Storage 2023, 63, 106951. [Google Scholar] [CrossRef]
- Tian, W.; Ren, G.; Wu, Y.; Lu, S.; Huan, Y.; Peng, T.; Liu, P.; Sun, J.; Su, H.; Cui, H. Machine-learning-assisted hydrogen adsorption descriptor design for bilayer MXenes. J. Clean. Prod. 2024, 450, 141953. [Google Scholar] [CrossRef]
- Thomas, T.; Bontha, S.; Bishnoi, A.; Sharma, P. MXene as a hydrogen storage material? A review from fundamentals to practical applications. J. Energy Storage 2024, 88, 111493. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, M. Recent progress of MXene as an energy storage material. Nanoscale Horiz. 2024, 9, 215–232. [Google Scholar] [CrossRef]
- Liu, Y.; Du, H.; Zhang, X.; Yang, Y.; Gao, M.; Pan, H. Superior catalytic activity derived from a two-dimensional Ti3C2 precursor towards the hydrogen storage reaction of magnesium hydride. Chem. Commun. 2016, 52, 705–708. [Google Scholar] [CrossRef]
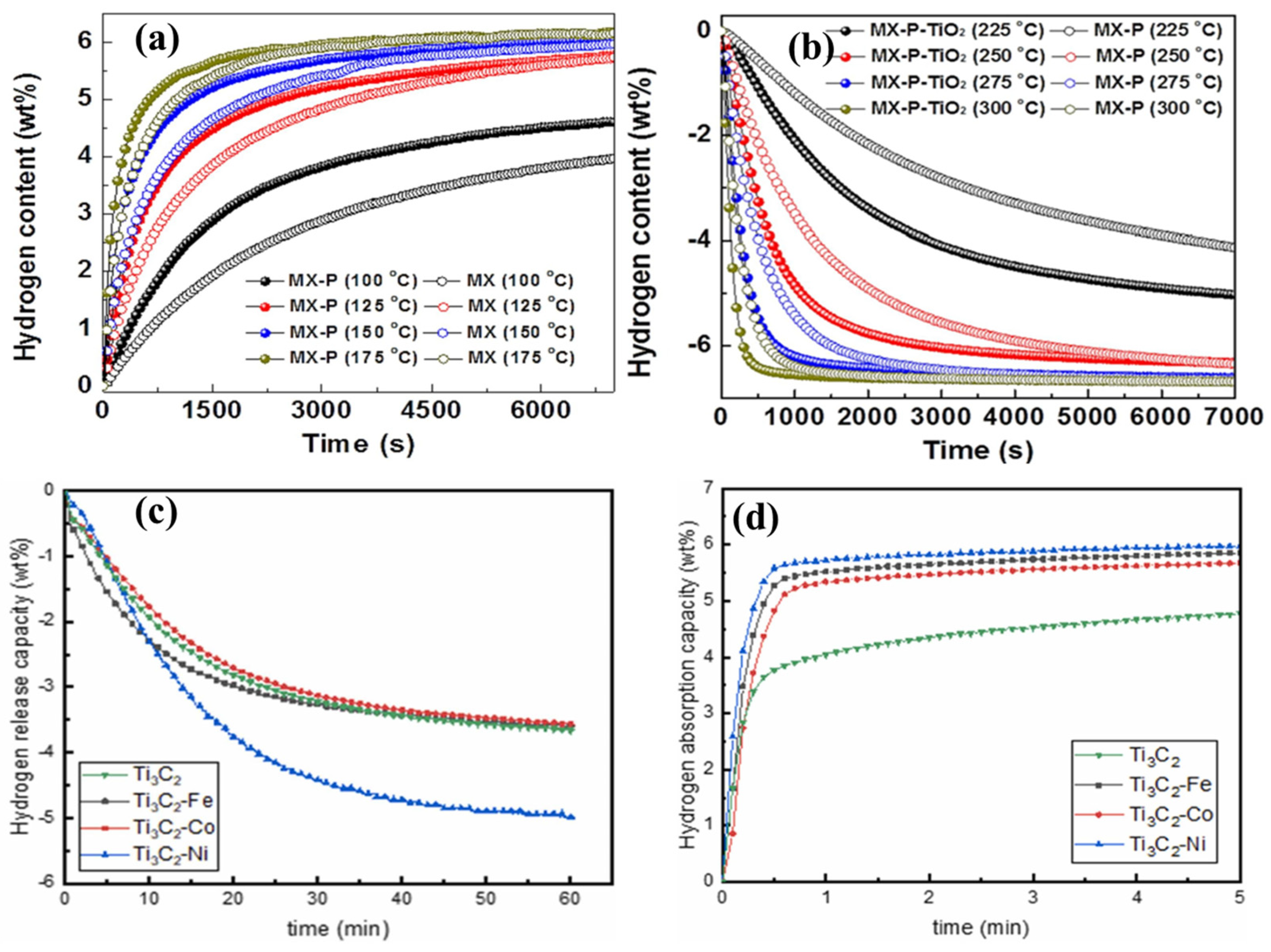
- Gao, H.; Liu, Y.; Zhu, Y.; Zhang, J.; Li, L. Catalytic effect of sandwich-like Ti3C2/TiO2 (A)-C on hydrogen storage performance of MgH2. Nanotechnology 2020, 31, 115404. [Google Scholar] [CrossRef]
- Gao, H.; Shao, Y.; Shi, R.; Liu, Y.; Zhu, J.; Liu, J.; Zhu, Y.; Zhang, J.; Li, L.; Hu, X. Effect of Few-Layer Ti3C2Tx Supported Nano-Ni via Self-Assembly Reduction on Hydrogen Storage Performance of MgH2. ACS Appl. Mater. Interfaces 2020, 12, 47684–47694. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, G.; Zhang, D.; Fan, Y.; Liu, B. Striking enhanced effect of PrF3 particles on Ti3C2 MXene for hydrogen storage properties of MgH2. J. Alloys Compd. 2022, 914, 165291. [Google Scholar] [CrossRef]
- Zhu, W.; Panda, S.; Lu, C.; Ma, Z.; Khan, D.; Dong, J.; Sun, F.; Xu, H.; Zhang, Q.; Zou, J. Using a Self-Assembled Two-Dimensional MXene-Based Catalyst (2D-Ni@Ti3C2) to Enhance Hydrogen Storage Properties of MgH2. ACS Appl. Mater. Interfaces 2020, 12, 50333–50343. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, H.; Zhu, Y.; Li, S.; Zhang, J.; Li, L. Excellent catalytic activity of a two-dimensional Nb4C3Tx (MXene) on hydrogen storage of MgH2. Appl. Surf. Sci. 2019, 493, 431–440. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.; Chen, J.; Guo, X.; Zhu, Y.; Li, L. Enhancing hydrogen storage performances of MgH 2 by Ni nano-particles over mesoporous carbon CMK-3. Nanotechnology 2018, 29, 265705. [Google Scholar] [CrossRef]
- Kong, Q.; Zhang, H.; Yuan, Z.; Liu, J.; Li, L.; Fan, Y.; Fan, G.; Liu, B. Hamamelis -like K2Ti6O13 Synthesized by Alkali Treatment of Ti3C2 MXene: Catalysis for Hydrogen Storage in MgH2. ACS Sustain. Chem. Eng. 2020, 8, 4755–4763. [Google Scholar] [CrossRef]
- Liu, H.; Lu, C.; Wang, X.; Xu, L.; Huang, X.; Wang, X.; Ning, H.; Lan, Z.; Guo, J. Combinations of V2C and Ti3C2 MXenes for Boosting the Hydrogen Storage Performances of MgH2. ACS Appl. Mater. Interfaces 2021, 13, 13235–13247. [Google Scholar] [CrossRef]
- Wu, Z.; Fang, J.; Liu, N.; Wu, J.; Kong, L. The Improvement in Hydrogen Storage Performance of MgH2 Enabled by Multilayer Ti3C2. Micromachines 2021, 12, 1190. [Google Scholar] [CrossRef]
- Gao, H.; Shi, R.; Zhu, J.; Liu, Y.; Shao, Y.; Zhu, Y.; Zhang, J.; Li, L.; Hu, X. Interface effect in sandwich like Ni/Ti3C2 catalysts on hydrogen storage performance of MgH2. Appl. Surf. Sci. 2021, 564, 150302. [Google Scholar] [CrossRef]
- Gu, C.; Gao, H.G.; Tan, P.; Liu, Y.N.; Liu, X.Q.; Hu, X.H.; Zhu, Y.F.; Sun, L.B. Cheese-like Ti3C2 for enhanced hydrogen storage. Chem. Eng. J. 2023, 473, 145462. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Wu, Y.; Chen, W.; Yuan, H.; Hao, L.; Wang, S.; Wang, S. Synergetic effect of Ti3C2-X (X = Fe, Co, Ni) on enhanced hydrogen storage performance of MgH2-TiCrV composite. J. Alloys Compd. 2024, 976, 173274. [Google Scholar] [CrossRef]
- Shi, W.; Hong, F.; Li, R.; Zhao, R.; Ding, S.; Liu, Z.; Qing, P.; Fan, Y.; Liu, H.; Guo, J.; et al. Improved hydrogen storage properties of MgH2 by Mxene (Ti3C2) supported MnO2. J. Energy Storage 2023, 72, 108738. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, M.; Mu, D.; Gao, Y.; He, L.; Ma, L. Synergistic Combination of Ni Nanoparticles and Ti3 C2 MXene Nanosheets with MgH2 Particles for Hydrogen Storage. ACS Appl. Nano Mater. 2023, 6, 21521–21531. [Google Scholar] [CrossRef]
- Wang, L.; Rawal, A.; Quadir, M.Z.; Aguey-Zinsou, K.-F. Formation of aluminium hydride (AlH3) via the decomposition of organoaluminium and hydrogen storage properties. Int. J. Hydrogen Energy 2018, 43, 16749–16757. [Google Scholar] [CrossRef]
- Wang, L.; Rawal, A.; Aguey-Zinsou, K.-F. Hydrogen storage properties of nanoconfined aluminium hydride (AlH3). Chem. Eng. Sci. 2019, 194, 64–70. [Google Scholar] [CrossRef]
- Liu, H.; He, S.; Li, G.; Wang, Y.; Xu, L.; Sheng, P.; Wang, X.; Jiang, T.; Huang, C.; Lan, Z.; et al. Directed Stabilization by Air-Milling and Catalyzed Decomposition by Layered Titanium Carbide Toward Low-Temperature and High-Capacity Hydrogen Storage of Aluminum Hydride. ACS Appl. Mater. Interfaces 2022, 14, 42102–42112. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, D.; Zheng, J.; Xia, A.; Zhang, Q.; Wang, L.; Zhang, L. Heterostructured VF4@Ti3C2 catalyst improving reversible hydrogen storage properties of Mg(BH4)2. Chem. Eng. J. 2023, 460, 141690. [Google Scholar] [CrossRef]
- Chen, J.; Li, C.; Chen, W.; Zhang, X.; Yu, X.; Xia, G. Tailoring reversible hydrogen storage performance of NaAlH4 through NiTiO3 nanorods. J. Alloys Compd. 2024, 971, 172689. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, Z.; Zhang, X.; Jian, N.; Yang, Y.; Gao, M.; Pan, H. Development of Catalyst-Enhanced Sodium Alanate as an Advanced Hydrogen-Storage Material for Mobile Applications. Energy Technol. 2018, 6, 487–500. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Wang, K.; Gao, M.; Pan, H. Remarkably improved hydrogen storage properties of nanocrystalline TiO2-modified NaAlH4 and evolution of Ti-containing species during dehydrogenation/hydrogenation. Nano Res. 2015, 8, 533–545. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Wang, K.; Yang, Y.; Gao, M.; Pan, H. Achieving ambient temperature hydrogen storage in ultrafine nanocrystalline TiO2 @C-doped NaAlH 4. J. Mater. Chem. A 2016, 4, 1087–1095. [Google Scholar] [CrossRef]
- Wu, R.; Du, H.; Wang, Z.; Gao, M.; Pan, H.; Liu, Y. Remarkably improved hydrogen storage properties of NaAlH4 doped with 2D titanium carbide. J. Power Sources 2016, 327, 519–525. [Google Scholar] [CrossRef]
- Zou, G.; Liu, B.; Guo, J.; Zhang, Q.; Fernandez, C.; Peng, Q. Synthesis of Nanoflower-Shaped MXene Derivative with Unexpected Catalytic Activity for Dehydrogenation of Sodium Alanates. ACS Appl. Mater. Interfaces 2017, 9, 7611–7618. [Google Scholar] [CrossRef]
- Fan, Y.; Yuan, Z.; Zou, G.; Zhang, Q.; Liu, B.; Peng, Q. Two-dimensional MXene/A-TiO2 composite with unprecedented catalytic activation for sodium alanate. Catal. Today 2018, 318, 167–174. [Google Scholar] [CrossRef]
- Jiang, R.; Xiao, X.; Zheng, J.; Chen, M.; Chen, L. Remarkable hydrogen absorption/desorption behaviors and mechanism of sodium alanates in-situ doped with Ti-based 2D MXene. Mater. Chem. Phys. 2020, 242, 122529. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhang, D.; Fan, G.; Chen, Y.; Fan, Y.; Liu, B. Synergistic Effect of CeF3 Nanoparticles Supported on Ti3C2 MXene for Catalyzing Hydrogen Storage of NaAlH4. ACS Appl. Energy Mater. 2021, 4, 2820–2827. [Google Scholar] [CrossRef]
- Yuan, Z.; Fan, Y.; Chen, Y.; Liu, X.; Liu, B.; Han, S. Two-dimensional C@TiO2/Ti3C2 composite with superior catalytic performance for NaAlH4. Int. J. Hydrogen Energy 2020, 45, 21666–21675. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhang, D.; Fan, G.; Chen, Y.; Fan, Y.; Liu, B. N-doped carbon coated Ti3C2 MXene as a high-efficiency catalyst for improving hydrogen storage kinetics and stability of NaAlH4. Renew. Energy 2022, 188, 778–787. [Google Scholar] [CrossRef]
- Li, Z.; Xian, K.; Chen, H.; Gao, M.; Qu, S.; Wu, M.; Yang, Y.; Sun, W.; Gao, C.; Liu, Y.; et al. Enhanced reversible hydrogen storage properties of wrinkled graphene microflowers confined LiBH4 system with high volumetric hydrogen storage capacity. Mater. Reports Energy 2024, 4, 100249. [Google Scholar] [CrossRef]
- Li, Z.; Gao, M.; Wang, S.; Zhang, X.; Gao, P.; Yang, Y.; Sun, W.; Liu, Y.; Pan, H. In-situ introduction of highly active TiO for enhancing hydrogen storage performance of LiBH4. Chem. Eng. J. 2022, 433, 134485. [Google Scholar] [CrossRef]
- Wang, S.; Wu, M.H.; Zhu, Y.Y.; Li, Z.L.; Yang, Y.X.; Li, Y.Z.; Liu, H.F.; Gao, M.X. Reactive destabilization and bidirectional catalyzation for reversible hydrogen storage of LiBH4 by novel waxberry-like nano-additive assembled from ultrafine Fe3O4 particles. J. Mater. Sci. Technol. 2024, 173, 63–71. [Google Scholar] [CrossRef]
- Zang, L.; Sun, W.; Liu, S.; Huang, Y.; Yuan, H.; Tao, Z.; Wang, Y. Enhanced Hydrogen Storage Properties and Reversibility of LiBH4 Confined in Two-Dimensional Ti3C2. ACS Appl. Mater. Interfaces 2018, 10, 19598–19604. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, D.; Liu, X.; Fan, G.; Liu, B. Improving the hydrogen storage performance of lithium borohydride by Ti3C2 MXene. Int. J. Hydrogen Energy 2019, 44, 29297–29303. [Google Scholar] [CrossRef]
- Hang, Z.; Hu, Z.; Xiao, X.; Jiang, R.; Zhang, M. Enhancing Hydrogen Storage Kinetics and Cycling Properties of NaMgH3 by 2D Transition Metal Carbide MXene Ti3C2. Processes 2021, 9, 1690. [Google Scholar] [CrossRef]
- Cheng, H.; Li, K.; Fan, X.; Lou, H.; Liu, Y.; Qi, Q.; Zhang, J.; Liu, J.; Yan, K.; Zhang, Y. The enhanced de/re-hydrogenation performances of LiNa 2 AlH 6 combined with two-dimension lamellar Ti3C2. Int. J. Hydrogen Energy 2017, 42, 25285–25293. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, Y.; Zhu, Y.; Guo, X.; Chen, J.; Li, L. Hydrogen storage performances and reaction mechanism of non-stoichiometric compound Li1.3Na1.7AlH6 doped with Ti3C2. Chem. Phys. 2018, 513, 135–140. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.; Cheng, H.; Zhu, Y.; Li, L.; Lin, H. Effects of two-dimension MXene Ti3C2 on hydrogen storage performances of MgH2-LiAlH4 composite. Chem. Phys. 2019, 522, 178–187. [Google Scholar] [CrossRef]
- Goncalves, R.B.; Snurr, R.Q.; Hupp, J.T. Computational Investigation of Metal Oxides as Candidate Hydrogen Storage Materials. J. Phys. Chem. C 2022, 126, 18661–18669. [Google Scholar] [CrossRef]
| Additive (Selective Content) | Isothermal Dehydrogenation | Isothermal Hydrogenation | Activation Energy (kJ mol−1) Absorption/Desorption | Key Parameters For H2 Storage | Ref. |
|---|---|---|---|---|---|
| Temperature—H2 Proportion—Time | Temperature—H2 Proportion—Time | ||||
| MgH2 | |||||
| Ti3C2 (5 wt%) | 300 °C—6.2 wt%—60 s | 150 °C—6.1 wt%—30 s | ---/98.9 (MgH2-155) | Metallic Ti formation | [37] |
| Ti3C2/TiO2-C (5 wt%) | 250 °C—5.0 wt%—1700 s | 125 °C—4.0 wt%—800 s | 42.32 (MgH2-71)/77.69 | Anatase TiO2, Ti0, Ti2+ Ti3+, and Ti4+ | [38] |
| Ni/Ti3C2Tx (5 wt%) | 250 °C—5.83 wt%—1800 s | 100 °C—5.0 wt%—1700 s | 41.36 (MgH2-71)/96.36 | Ti0, Ti2+ Ti3+, and Ti4+ | [39] |
| Ni@Ti3C2 | 250 °C—5.2 wt%—15 min | 125 °C—5.4 wt%—25 s | 73 ± 3.5 (MgH2-141 ± 5.3)/56 ± 4 | Metallic Ti along with Ni | [41] |
| Ti3C2 MXene derived K2Ti6O13 (5 wt%) | 280 °C—6.7 wt%—3 min | 200 °C—6.5 wt%—30 s | ---/105.67 (MgH2-175.34) | Ti and Ti2+ | [44] |
| 2V2C/Ti3C2 (10 wt%) | 225 °C—5.1 wt%—60 min | 40 °C—5.1 wt%—20 s | ---/79.4 (MgH2-127.7) | Uniform distribution of Mg, Ti, and V | [45] |
| Ti3C2 (6 wt%) | 240 °C—6.45 wt%—10 min | 150 °C—6.47 wt%—480 s | ---/99 (MgH2-153) | Metallic Ti formation | [46] |
| Ni/Ti3C2 (5 wt%) | 100 °C—4.59 wt%—1200 s | 250 °C—5.87 wt%—2400 s | 42.38 (MgH2-71)/91.64 | Ti valence states (Ti0, Ti2+ Ti3+, and Ti4+) | [47] |
| PrF3/Ti3C2 (5 wt%) | 260 °C—7.0 wt%—3 min | 150 °C—6.16 wt%—10 min | ---/78.11(MgH2-117.98) | Ti valence states (Ti0, Ti2+, Ti3+, and Ti4+) | [40] |
| Ti3C2 (5 wt%) | --- | 275 °C—6.6 wt%--- | 36.22/79.46 | Internal metallic Ti active edge sites | [48] |
| Ti3C2@MnO2 | 275 °C—6.4 wt%—484 s | 30 °C—4.4 wt%—150 s | ---/61.8 ± 2.2 (MgH2-142.4 ± 0.9) | Multiple interfaces, Ti3C2/MgH2, TiO2/MgH2, MnO2/MgH2, MnO/MgH2, and Mn/MgH2 | [50] |
| Ni/Ti3C2 (Ti3C2 @5 wt%) | 300 °C—3.96 wt%--- | 300 °C—4.46 wt%--- | ---/75.0(Mg-135.4) | Synergy between Ni and Ti3C2 | [51] |
| Ti3C2-Ni | 523 K—4.982 wt%—60 min | 453 K—5.72 wt%—1 min | 80.54/--- | Mg-TiCrV/Ti3C2-Ni interface | [49] |
| AlH3 | |||||
| Ti3C2 (4 wt%) | 100 °C—6.9 wt%—20 min | --- | ---/40 | High surface area and active sites of Ti3C2 | [54] |
| Additive (Content) | Isothermal Dehydrogenation | Isothermal Hydrogenation | Activation Energy (kJ mol−1) Absorption/Desorption | Key Parameters for H2 Storage | Ref. |
|---|---|---|---|---|---|
| Temperature—H2 Proportion—Time | Temperature—H2 Proportion—Time | ||||
| Mg(BH4)2 | |||||
| VF4@Ti3C2 (20 wt%) | 275 °C—8.2 wt%—300 min | --- | ---/172.9 (Mg(BH4)2-374.1) | Formation of metallic Ti and VH2.01 | [55] |
| NiAlH4 | |||||
| Ti3C2 (7 wt%) | 140 °C—4.7 wt%—100 min | 120 °C—4.6 wt%—60 min | ---/87.3 ± 6.7 (First step) | Ti metal and Ti3+ | [60] |
| Ti3C2 (OH0.8F1.2)2 | 100 °C—3.11 wt%—90 min | --- | --- | Ti3C2 MXene-derived alanates and rutile TiO2 | [61] |
| MXene/A-TiO2 (15 wt%) | 140 °C—3.0 wt%—7 min | --- | ---/78.32 (First step) | Homogeneous distribution of Ti and C | [62] |
| Ti3C2 (8 wt%) | 110 °C—4.1 wt%—119 min | 110 °C—4.2 wt%—4.5 min | ---/92.5 (First step) | Ti and TiFx particles | [63] |
| CeF3/Ti3C2 | 100 °C—3.0 wt%—80 min | --- | ---/81.39 (First step) | Ti-F-Ce bonding | [64] |
| C@TiO2/Ti3C2 (10 wt%) | 140 °C—4.0 wt%—13 min | --- | ---/72.41 (First step) | Ti0 and Ti3+ states | [65] |
| Ti3C2/N doped carbon (10 wt%) | 140 °C—4.61 wt%—60 min | --- | ---/76.66 (First step) | Interaction between pyridinic-N and Ti0 | [66] |
| LiBH4 | |||||
| Ti3C2 | 300 °C—8.2 wt%—8 h | --- | ---/94.44 (50% of LiBH4) | Ti-containing defect sites | [70] |
| Ti3C2 (40 wt%) | 300 °C—3.0 wt%—6 h | --- | ---/70.3 (LiBH4-187 ± 24) | Ti metal and high surface area | [71] |
| NaMgH3 | |||||
| Ti3C2 (7 wt%) | 350 °C—3.4 wt%—5 min | 300 °C—3.5 wt%—6 s | ---/114.08 (NaMgH3-158.45) (First step) | Lamellar-structure Ti3C2 | [72] |
| Complex Metal Hydrides | Key Parameters for H2 Storage | Ref. | |||||
|---|---|---|---|---|---|---|---|
| Additive | Dehydrogenation Temperature | Hydrogenation Activation Energy (kJ mol−1) | H2 Release/ Absorption (wt%) | ||||
| Ti3C2 | LiNa2AlH6 + 5 wt% Ti3C2 (385 K) | LiNa2AlH6 (453 K) | LiNa2AlH6 + 5 wt% Ti3C2 (58.28) | LiNa2AlH6 (63.19) | --- | Ti0 species | [73] |
| Ti3C2 | Li1.3Na1.7AlH6 + 5 wt% Ti3C2 (388 K) | Li1.3Na1.7AlH6 (423 K) | Li1.3Na1.7AlH6 + 5 wt% Ti3C2 (56.3) | Li1.3Na1.7AlH6 (59.8) | --- | Ti2+ and Ti0 | [74] |
| Ti3C2 | 4MgH2-LiAlH4-Ti3C2 (400 K) | 4MgH2-LiAlH4 (610 K) | 4MgH2-LiAlH4-Ti3C2 (65.7) | 4MgH2-LiAlH4 (99.2) | 6.6/3.5 | metallic Ti, TiH1.942 (Ti2+) | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sreedhar, A.; Noh, J.-S. Advancements in Ti3C2 MXene-Integrated Various Metal Hydrides for Hydrogen Energy Storage: A Review. Nanomaterials 2025, 15, 673. https://doi.org/10.3390/nano15090673
Sreedhar A, Noh J-S. Advancements in Ti3C2 MXene-Integrated Various Metal Hydrides for Hydrogen Energy Storage: A Review. Nanomaterials. 2025; 15(9):673. https://doi.org/10.3390/nano15090673
Chicago/Turabian StyleSreedhar, Adem, and Jin-Seo Noh. 2025. "Advancements in Ti3C2 MXene-Integrated Various Metal Hydrides for Hydrogen Energy Storage: A Review" Nanomaterials 15, no. 9: 673. https://doi.org/10.3390/nano15090673
APA StyleSreedhar, A., & Noh, J.-S. (2025). Advancements in Ti3C2 MXene-Integrated Various Metal Hydrides for Hydrogen Energy Storage: A Review. Nanomaterials, 15(9), 673. https://doi.org/10.3390/nano15090673







