The Effect of Microstructural Changes Produced by Heat Treatment on the Electromagnetic Interference Shielding Properties of Ti-Based MXenes
Abstract
1. Introduction
2. Materials and Methods
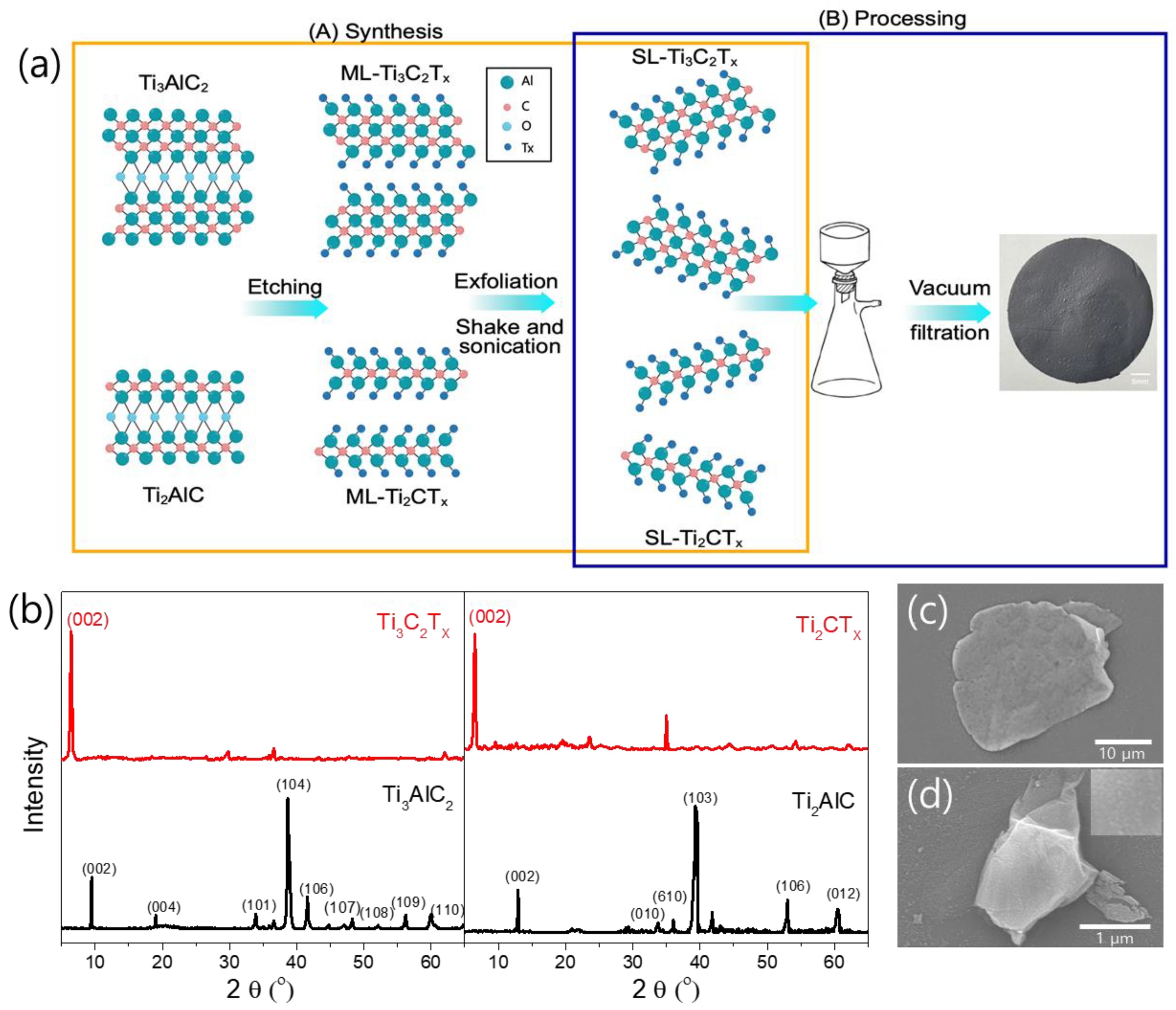
2.1. Synthesis of Ti-Based MXene and Film Preparation
2.2. Characterization of MXene Films
2.3. Measurement and Calculation of EMI Shielding Effectiveness of MXene Films
3. Results and Discussion
3.1. Synthesis of Ti3C2TX and Ti2CTX Nanosheets
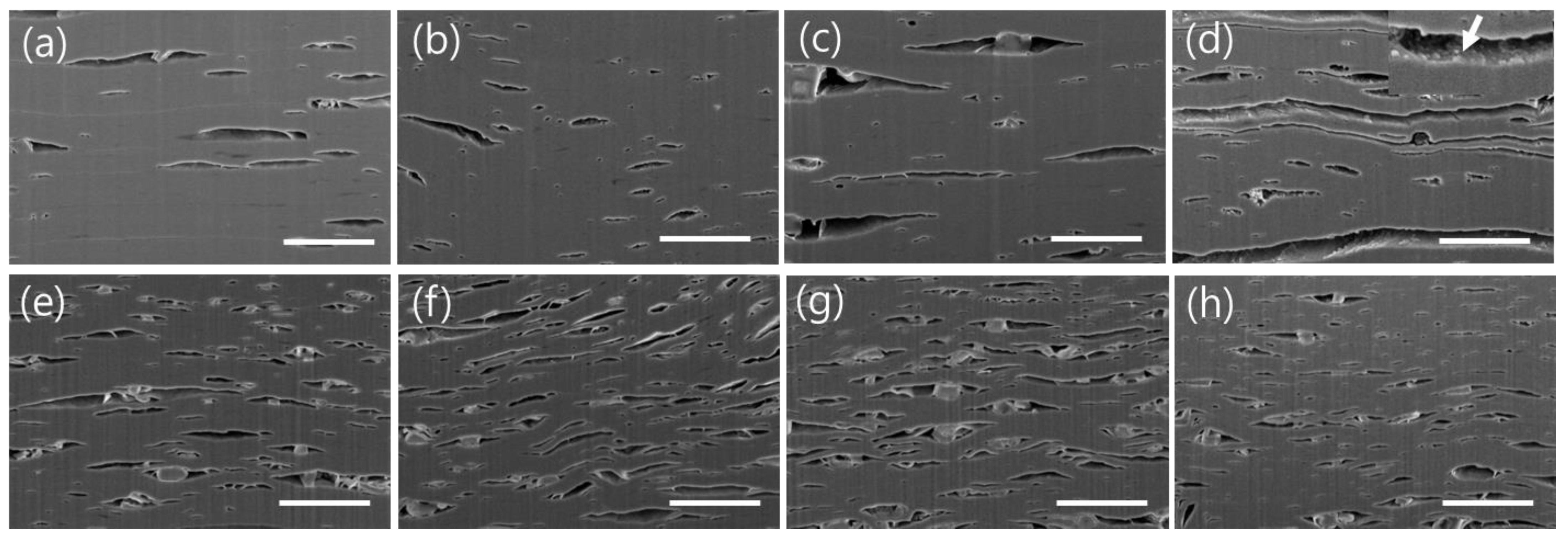
3.2. Structural Changes in Ti3C2TX and Ti2CTX Films Produced by Heat Treatment
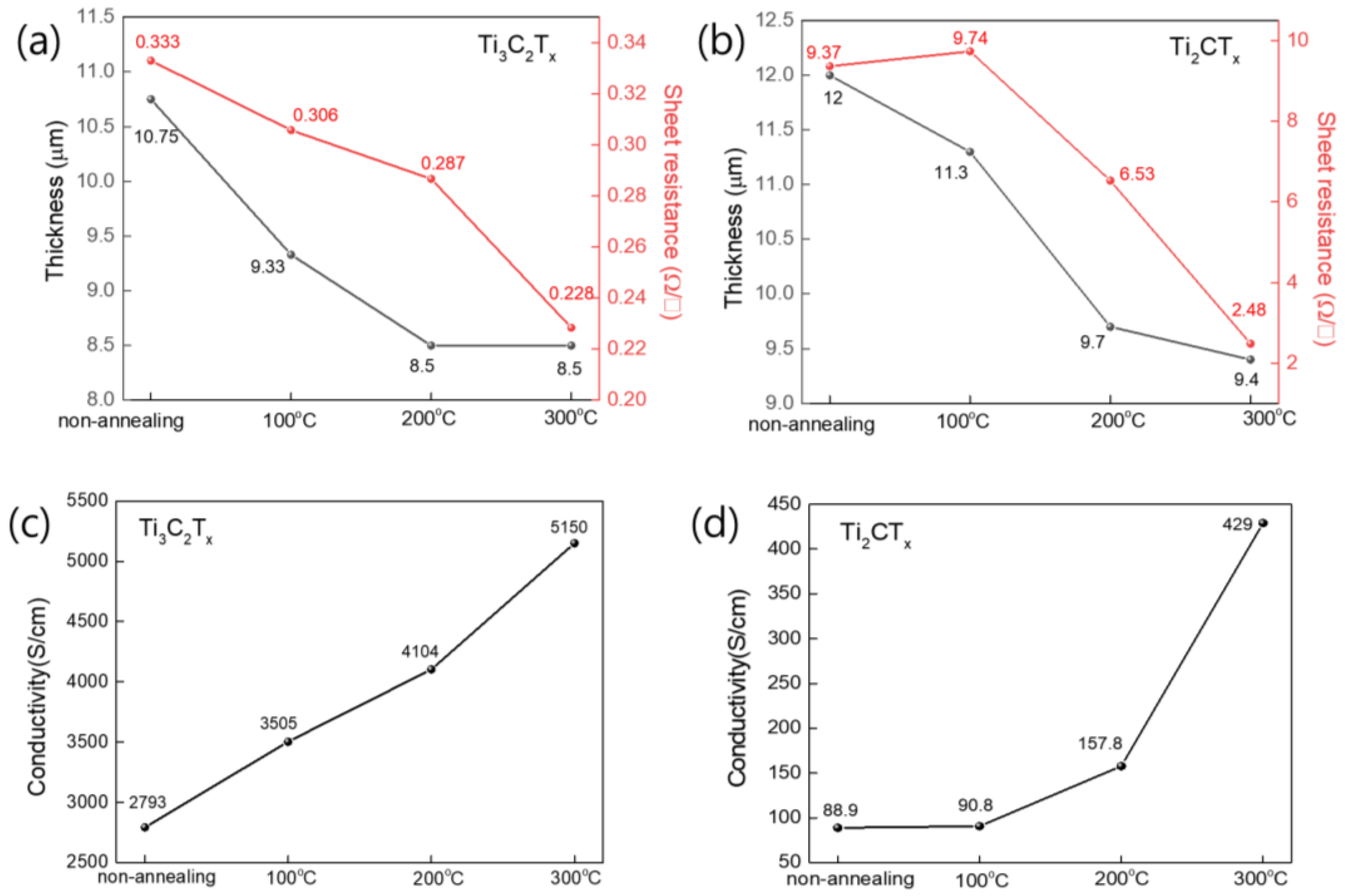
3.3. Electrical Properties of Ti3C2Tx and Ti2CTx After Heat Treatment
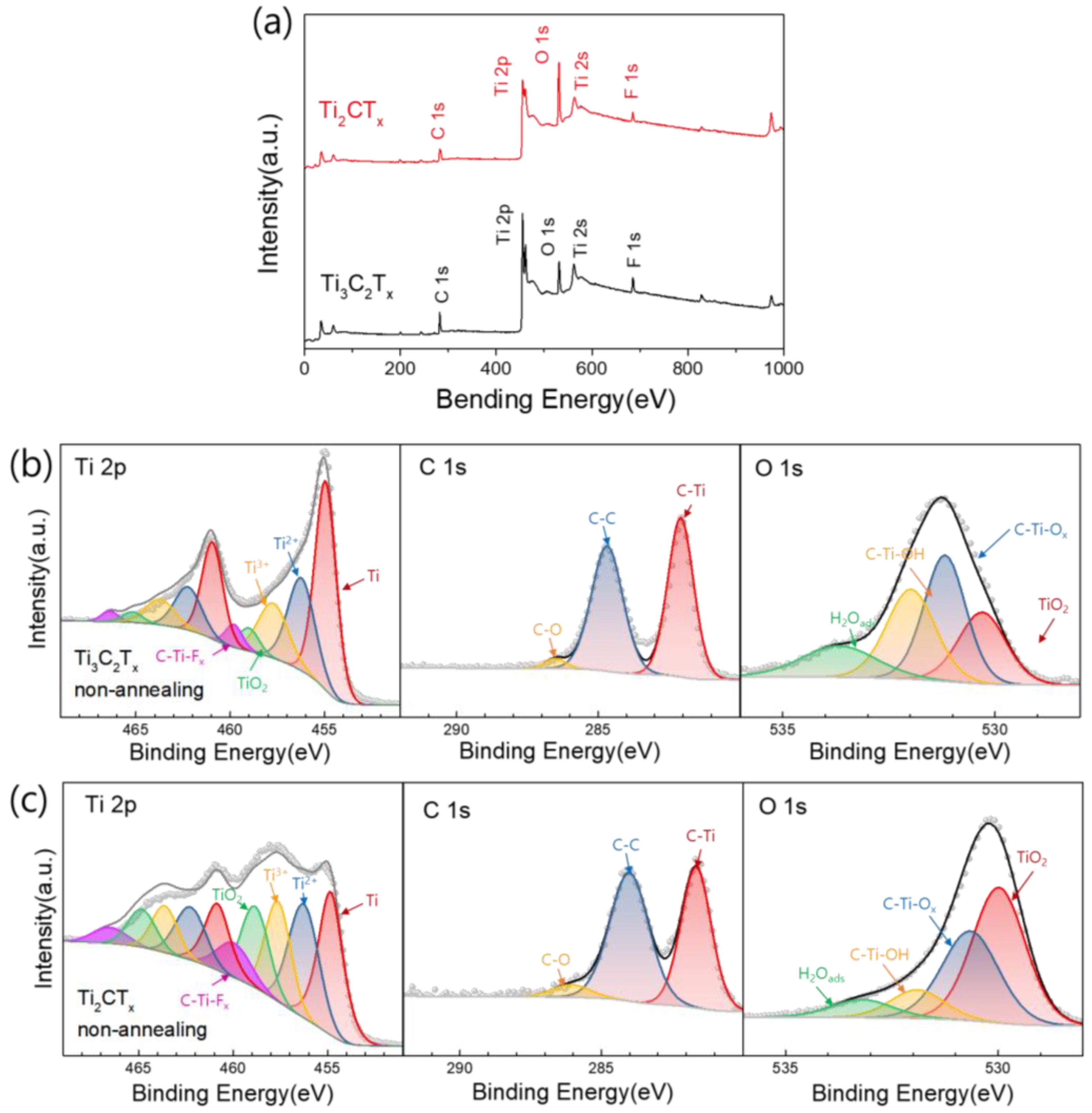
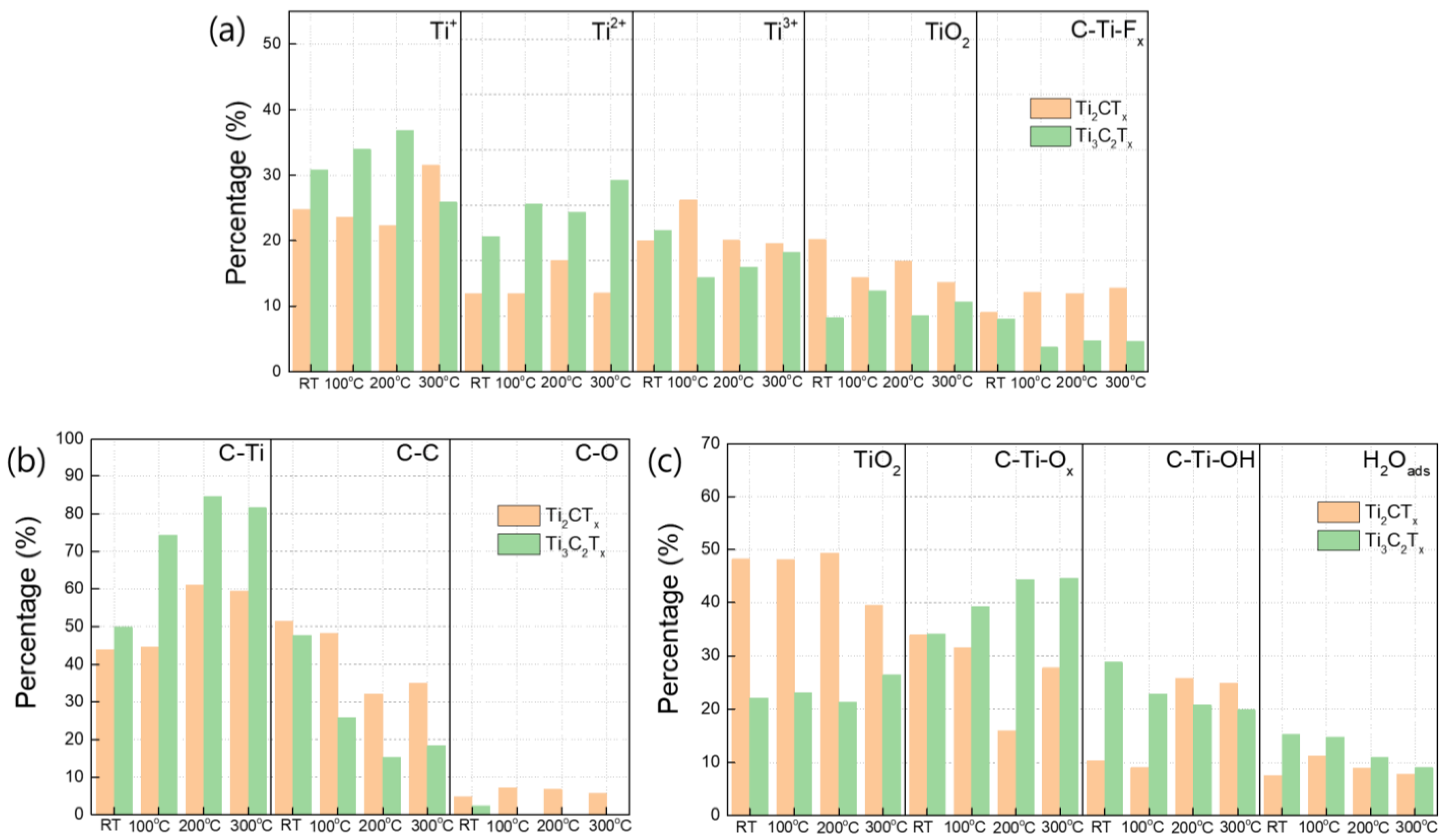
3.4. Compositional Changes in Ti3C2TX and Ti2CTX Films Produced by Heat Treatment
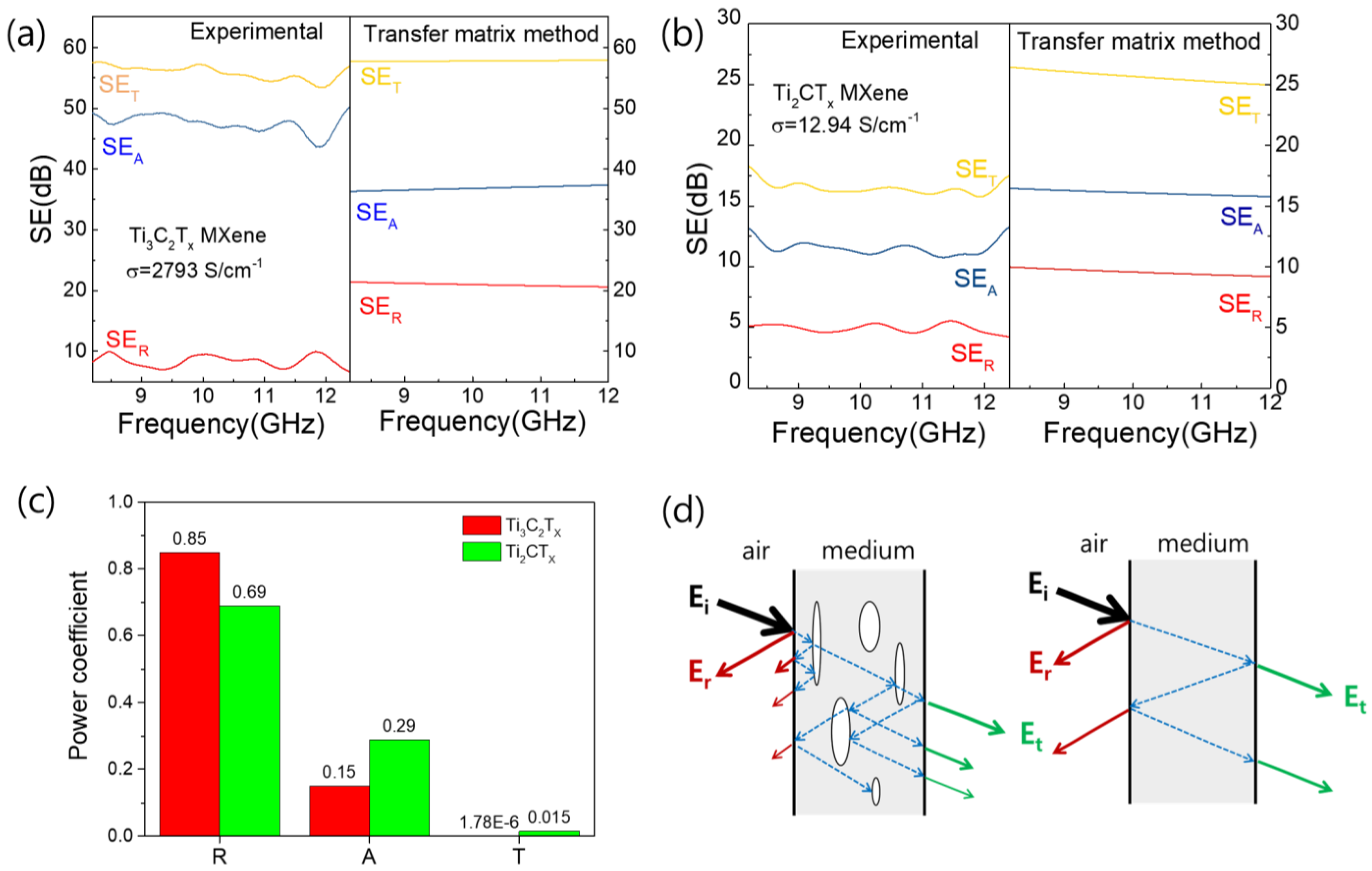
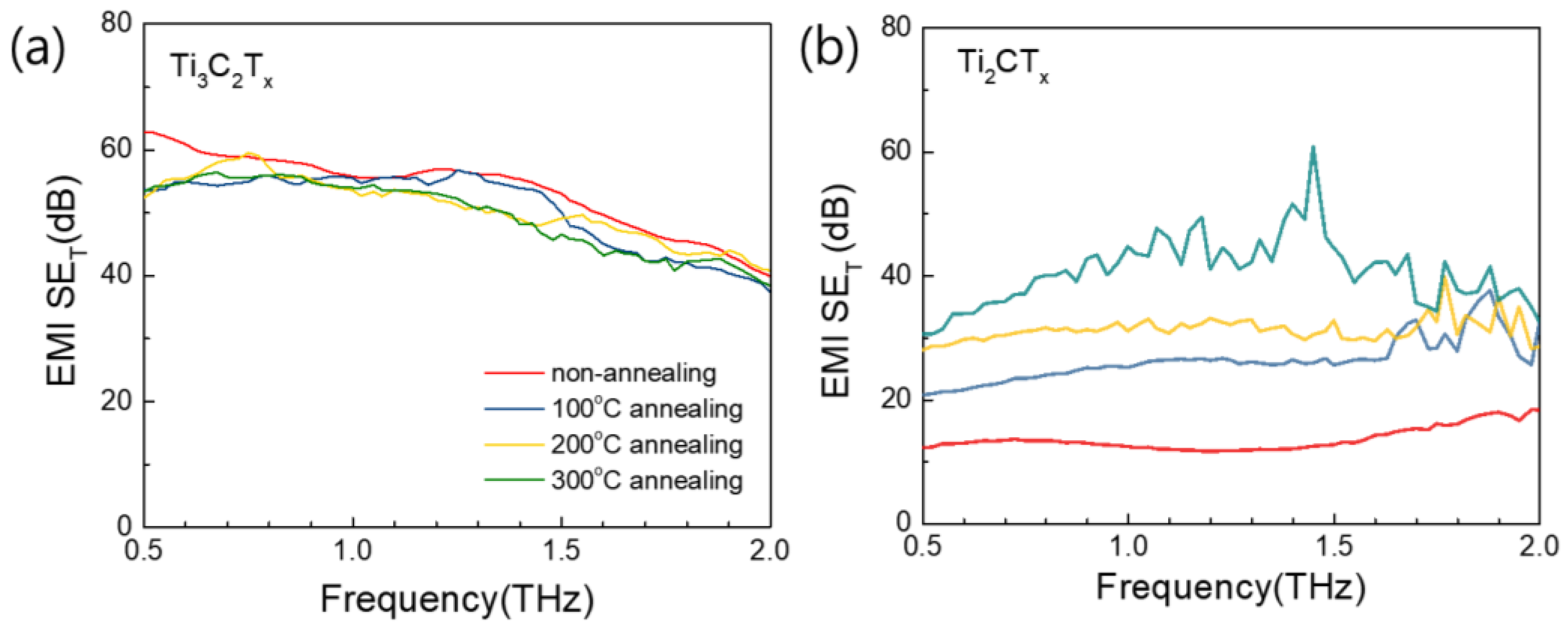
3.5. EMI Shielding Properties of Ti3C2TX and Ti2CTX Films in High-Frequency Range
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Russell, C.L. 5 G wireless telecommunications expansion: Public health and environmental implications. Environ. Res. 2018, 165, 484–495. [Google Scholar] [CrossRef]
- Jiang, Z.-Y.; Huang, W.; Chen, L.-S.; Liu, Y.-H. Ultrathin, lightweight, and freestanding metallic mesh for transparent elec-tromagnetic interference shielding. Opt. Express 2019, 27, 24194–24206. [Google Scholar] [CrossRef]
- Wan, Y.J.; Zhu, P.L.; Yu, S.H.; Sun, R.; Wong, C.P.; Liao, W.H. Anticorrosive, ultralight, and flexible carbon-wrapped metallic nanowire hybrid sponges for highly efficient electromagnetic interference shielding. Small 2018, 14, 1800534. [Google Scholar] [CrossRef]
- Geetha, S.; Satheesh Kumar, K.; Rao, C.R.; Vijayan, M.; Trivedi, D. EMI shielding: Methods and materials—A review. J. Appl. Polym. Sci. 2009, 112, 2073–2086. [Google Scholar] [CrossRef]
- Chung, D. Materials for electromagnetic interference shielding. J. Mater. Eng. Perform. 2000, 9, 350–354. [Google Scholar] [CrossRef]
- Chung, D. Materials for electromagnetic interference shielding. Mater. Chem. Phys. 2020, 255, 123587. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Liao, S.-Y.; Wan, Y.-J.; Zhu, P.-L.; Hu, Y.-G.; Zhao, T.; Sun, R.; Wong, C.-P. Electromagnetic interference shielding materials: Recent progress, structure design, and future perspective. J. Mater. Chem. C 2021, 10, 44–72. [Google Scholar] [CrossRef]
- Li, X.; Yin, X.; Liang, S.; Li, M.; Cheng, L.; Zhang, L. 2D carbide MXene Ti2CTX as a novel high-performance electromagnetic interference shielding material. Carbon 2019, 146, 210–217. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Jiang, Q.; Lei, Y.; Liang, H.; Xi, K.; Xia, C.; Alshareef, H.N. Review of MXene electrochemical microsupercapacitors. Energy Storage Mater. 2020, 27, 78–95. [Google Scholar] [CrossRef]
- Shahzad, F.; Iqbal, A.; Kim, H.; Koo, C.M. 2D Transition Metal Carbides (MXenes): Applications as an Electrically Conducting Material. Adv. Mater. 2020, 32, e2002159. [Google Scholar] [CrossRef] [PubMed]
- Murali, G.; Reddy Modigunta, J.K.; Park, Y.H.; Lee, J.-H.; Rawal, J.; Lee, S.-Y.; In, I.; Park, S.-J. A review on MXene synthesis, stability, and photocatalytic applications. ACS Nano 2022, 16, 13370–13429. [Google Scholar] [CrossRef]
- Li, X.; Huang, Z.; Shuck, C.E.; Liang, G.; Gogotsi, Y.; Zhi, C. MXene chemistry, electrochemistry and energy storage applica-tions. Nat. Rev. Chem. 2022, 6, 389–404. [Google Scholar] [CrossRef]
- Guo, T.; Zhou, D.; Deng, S.; Jafarpour, M.; Avaro, J.; Neels, A.; Heier, J.; Zhang, C. Rational design of Ti3C2Tx MXene inks for conductive, transparent films. ACS Nano 2023, 17, 3737–3749. [Google Scholar] [CrossRef]
- Shahzad, F.; Alhabeb, M.; Hatter, C.B.; Anasori, B.; Hong, S.M.; Koo, C.M.; Gogotsi, Y. Electromagnetic interference shielding with 2D transition metal carbides (MXenes). Science 2016, 353, 1137–1140. [Google Scholar] [CrossRef]
- Verma, R.; Thakur, P.; Chauhan, A.; Jasrotia, R.; Thakur, A. A review on MXene and its’ composites for electromagnetic interference (EMI) shielding applications. Carbon 2023, 208, 170–190. [Google Scholar] [CrossRef]
- Iqbal, A.; Kwon, J.; Kim, M.-K.; Koo, C. MXenes for electromagnetic interference shielding: Experimental and theoretical perspectives. Mater. Today Adv. 2021, 9, 100124. [Google Scholar] [CrossRef]
- Zhao, T.; Xie, P.; Wan, H.; Ding, T.; Liu, M.; Xie, J.; Li, E.; Chen, X.; Wang, T.; Zhang, Q.; et al. Ultrathin MXene assemblies approach the intrinsic absorption limit in the 0.5–10 THz band. Nat. Photonics 2023, 17, 622–628. [Google Scholar] [CrossRef]
- Shi, Y.; Xiang, Z.; Cai, L.; Pan, F.; Dong, Y.; Zhu, X.; Cheng, J.; Jiang, H.; Lu, W. Multi-interface Assembled N-Doped MXene/HCFG/AgNW Films for Wearable Electromagnetic Shielding Devices with Multimodal Energy Conversion and Healthcare Monitoring Performances. ACS Nano 2022, 16, 7816–7833. [Google Scholar] [CrossRef]
- Zhao, Y.; Miao, B.; Nawaz, M.A.; Zhu, Q.; Chen, Q.; Reina, T.R.; Bai, J.; He, D.; Al-Tahan, M.A.; Arsalan, M. Construction of cellulose nanofiber-Ti3C2Tx MXene/silver nanowire nanocomposite papers with gradient structure for efficient electromagnetic interference shielding. Adv. Compos. Hybrid Mater. 2024, 7, 1–16. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, H.-B.; Luo, J.-Q.; Wang, Q.-W.; Xu, B.; Hong, S.; Yu, Z.-Z. Highly electrically conductive three-dimensional Ti3C2Tx MXene/reduced graphene oxide hybrid aerogels with excellent electromagnetic interference shielding performances. ACS Nano 2018, 12, 11193–11202. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Liu, L.; Yang, M.; Zhang, T.; Xu, F.; Lin, Z.; Ding, Y.; Wang, C.; Li, J.; Yin, W.; et al. Lightweight, thermally insulating and stiff carbon honeycomb-induced graphene composite foams with a horizontal laminated structure for electromagnetic interference shielding. Carbon 2017, 123, 223–232. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, H.; Xie, X.; Yang, R.; Liu, Z.; Liu, Y.; Yu, Z. Multifunctional, Superelastic, and Lightweight MXene/Polyimide Aerogels. Small 2018, 14, e1802479. [Google Scholar] [CrossRef] [PubMed]
- Weng, G.M.; Li, J.; Alhabeb, M.; Karpovich, C.; Wang, H.; Lipton, J.; Maleski, K.; Kong, J.; Shaulsky, E.; Elimelech, M. Layer-by-layer assembly of cross-functional semi-transparent MXene-carbon nanotubes composite films for next-generation elec-tromagnetic interference shielding. Adv. Funct. Mater. 2018, 28, 1803360. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, Y.; Qiu, H.; Ma, Z.; Ruan, K.; Gu, J. A mini-review of MXene porous films: Preparation, mechanism and application. J. Mater. Sci. Technol. 2022, 103, 42–49. [Google Scholar] [CrossRef]
- Iqbal, A.; Shahzad, F.; Hantanasirisakul, K.; Kim, M.-K.; Kwon, J.; Hong, J.; Kim, D.; Gogotsi, Y.; Koo, C.M. Anomalous absorption of electromagnetic waves by 2D transition metal carbonitride Ti3CNTx (MXene). Science 2020, 369, 446–450. [Google Scholar] [CrossRef]
- Verger, L.; Natu, V.; Carey, M.; Barsoum, M.W. MXenes: An Introduction of Their Synthesis, Select Properties, and Applications. Trends Chem. 2019, 1, 656–669. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Li, T.; Jabari, E.; McLellan, K.; Naguib, H.E. Review of additive manufacturing with 2D MXene: Techniques, applications, and future perspectives. Prog. Addit. Manuf. 2023, 8, 1587–1617. [Google Scholar] [CrossRef]
- Benchakar, M.; Loupias, L.; Garnero, C.; Bilyk, T.; Morais, C.; Canaff, C.; Guignard, N.; Morisset, S.; Pazniak, H.; Hurand, S.; et al. One MAX phase, different MXenes: A guideline to understand the crucial role of etching conditions on Ti3C2Tx surface chemistry. Appl. Surf. Sci. 2020, 530, 530. [Google Scholar] [CrossRef]
- Yun, T.; Kim, H.; Iqbal, A.; Cho, Y.S.; Lee, G.S.; Kim, M.; Kim, S.J.; Kim, D.; Gogotsi, Y.; Kim, S.O.; et al. Electromagnetic Shielding of Monolayer MXene Assemblies. Adv. Mater. 2020, 32, e1906769. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Z.; Lu, W.; Hou, B. Theoretical Study of Electromagnetic Interference Shielding of 2D MXenes Films. Metals 2018, 8, 652. [Google Scholar] [CrossRef]
- Zou, Q.; Guo, W.; Zhang, L.; Yang, L.; Zhao, Z.; Liu, F.; Ye, X.; Zhang, Y.; Shi, W. MXene-based ultra-thin film for terahertz radiation shielding. Nanotechnology 2020, 31, 505710. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Sambyal, P.; Koo, C.M. 2D MXenes for Electromagnetic Shielding: A Review. Adv. Funct. Mater. 2020, 30, 2000883. [Google Scholar] [CrossRef]
- Han, M.; Shuck, C.E.; Rakhmanov, R.; Parchment, D.; Anasori, B.; Koo, C.M.; Friedman, G.; Gogotsi, Y. Beyond Ti3C2Tx: MXenes for electromagnetic interference shielding. ACS Nano 2020, 14, 5008–5016. [Google Scholar] [CrossRef]
- Zhao, X.; Cao, H.; Coleman, B.J.; Tan, Z.; Echols, I.J.; Pentzer, E.B.; Lutkenhaus, J.L.; Radovic, M.; Green, M.J. The role of antioxidant structure in mitigating oxidation in Ti3C2Tx and Ti2CTx MXenes. Adv. Mater. Interfaces 2022, 9, 2200480. [Google Scholar] [CrossRef]
- Mokrushin, A.S.; Nagornov, I.A.; Gorobtsov, P.Y.; Averin, A.A.; Simonenko, T.L.; Simonenko, N.P.; Simonenko, E.P.; Kuznetsov, N.T. Effect of Ti2CTx MXene oxidation on its gas-sensitive properties. Chemosensors 2022, 11, 13. [Google Scholar] [CrossRef]
- Roy, C.; De, S.K.; Banerjee, P.; Pradhan, S.; Bhattacharyya, S. Investigating suitable medium for the long-duration storage of Ti2CTx MXene. J. Alloy. Compd. 2022, 938, 168471. [Google Scholar] [CrossRef]
- Xu, H.; Yin, X.; Li, X.; Li, M.; Zhang, L.; Cheng, L. Thermal stability and dielectric properties of 2D Ti2C MXenes via annealing under a gas mixture of Ar and H2 atmosphere. Funct. Compos. Struct. 2019, 1, 015002. [Google Scholar] [CrossRef]
- Xue, Q. The influence of particle shape and size on electric conductivity of metal–polymer composites. Eur. Polym. J. 2003, 40, 323–327. [Google Scholar] [CrossRef]
- Kumar, K.; Kim, Y.-S.; Yang, E.-H. The influence of thermal annealing to remove polymeric residue on the electronic doping and morphological characteristics of graphene. Carbon 2013, 65, 35–45. [Google Scholar] [CrossRef]
- Halim, J.; Cook, K.M.; Naguib, M.; Eklund, P.; Gogotsi, Y.; Rosen, J.; Barsoum, M.W. X-ray photoelectron spectroscopy of select multi-layered transition metal carbides (MXenes). Appl. Surf. Sci. 2016, 362, 406–417. [Google Scholar] [CrossRef]
- Näslund, L.; Persson, P.O.Å.; Rosen, J. X-ray Photoelectron Spectroscopy of Ti3AlC2, Ti3C2Tz, and TiC Provides Evidence for the Electrostatic Interaction between Laminated Layers in MAX-Phase Materials. J. Phys. Chem. C 2020, 124, 27732–27742. [Google Scholar] [CrossRef]
- Peng, M.; Qin, F. Clarification of basic concepts for electromagnetic interference shielding effectiveness. J. Appl. Phys. 2021, 130, 225108. [Google Scholar] [CrossRef]
- Zhang, H.; Wan, J.; Wu, R.; Chen, Y.; Yu, H.; Shi, S. MXenes for electromagnetic interference shielding: Insights from structural design. Carbon 2023, 218, 118716. [Google Scholar] [CrossRef]
- Qu, Y.; Li, X.; Wang, X.; Dai, H. Multifunctional AgNWs@MXene/AgNFs electromagnetic shielding composites for flexible and highly integrated advanced electronics. Compos. Sci. Technol. 2022, 230, 109753. [Google Scholar] [CrossRef]
- Huang, M.; Wang, L.; Zhao, B.; Chen, G.; Che, R. Engineering the electronic structure on MXenes via multidimensional component interlayer insertion for enhanced electromagnetic shielding. J. Mater. Sci. Technol. 2022, 138, 149–156. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, H.; Sun, R.; Liu, Y.; Liu, Z.; Zhou, A.; Yu, Z. Hydrophobic, flexible, and lightweight MXene foams for high-performance electromagnetic-interference shielding. Adv. Mater. 2017, 29, 1702367. [Google Scholar] [CrossRef]
- Titova, L.V.; Li, G.; Natu, V.; Huang, S.; Shi, T.; Amer, N.; Hafez, H.A.; Hegmann, F.A.; Turchinovich, D.; Mochalin, V.; et al. 2D MXenes: Terahertz Properties and Applications. In Proceedings of the 2020 45th International Conference on Infrared, Millimeter and Terahertz Waves (IRMMW-THz), Buffalo, NY, USA, 8–13 November 2020; pp. 1–2. [Google Scholar]
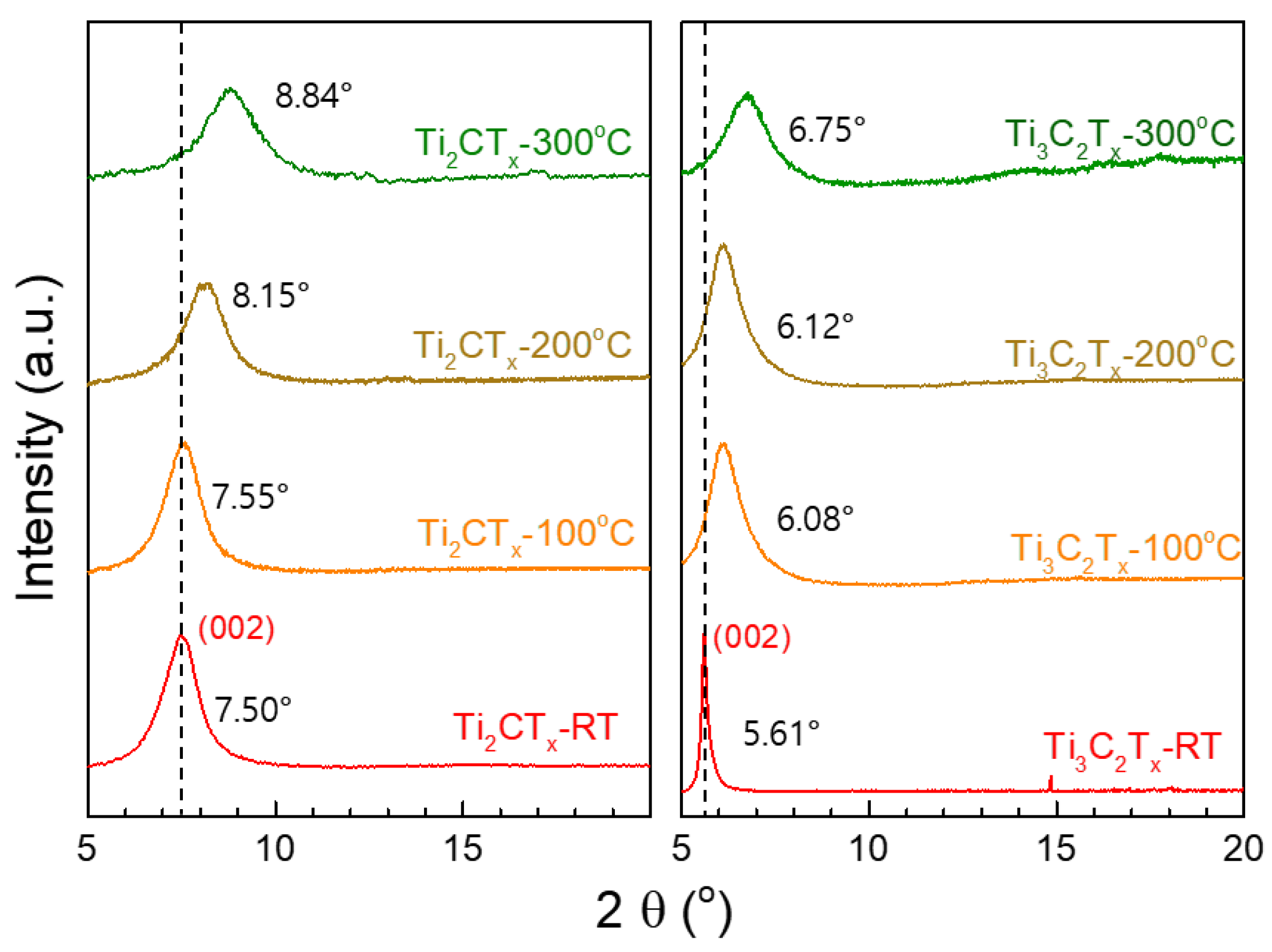

| Heat Treatment Temperature | Ti3C2TX | Ti2CTX |
|---|---|---|
| w/o | 11.78 Å | 15.75 Å |
| 100 °C | 11.71 Å | 14.52 Å |
| 200 °C | 10.85 Å | 14.44 Å |
| 300 °C | 10.00 Å | 13.08 Å |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Lee, J.J.; Kyoung, J.S.; Woo, Y.S. The Effect of Microstructural Changes Produced by Heat Treatment on the Electromagnetic Interference Shielding Properties of Ti-Based MXenes. Nanomaterials 2025, 15, 676. https://doi.org/10.3390/nano15090676
Han X, Lee JJ, Kyoung JS, Woo YS. The Effect of Microstructural Changes Produced by Heat Treatment on the Electromagnetic Interference Shielding Properties of Ti-Based MXenes. Nanomaterials. 2025; 15(9):676. https://doi.org/10.3390/nano15090676
Chicago/Turabian StyleHan, Xue, Jae Jeong Lee, Ji Soo Kyoung, and Yun Sung Woo. 2025. "The Effect of Microstructural Changes Produced by Heat Treatment on the Electromagnetic Interference Shielding Properties of Ti-Based MXenes" Nanomaterials 15, no. 9: 676. https://doi.org/10.3390/nano15090676
APA StyleHan, X., Lee, J. J., Kyoung, J. S., & Woo, Y. S. (2025). The Effect of Microstructural Changes Produced by Heat Treatment on the Electromagnetic Interference Shielding Properties of Ti-Based MXenes. Nanomaterials, 15(9), 676. https://doi.org/10.3390/nano15090676







