Abstract
All-solid-state batteries have garnered significant attention due to their potential to exceed the energy density of conventional lithium-ion batteries, particularly when alloying-based materials or lithium metal anodes are used. However, achieving compatibility with lithium metal remains a persistent bottleneck. In this study, we shed light on the potential of SnHPO3 tin phosphite and Ni3.4Sn4 intermetallic as novel conversion/alloying anode materials for all-solid-state lithium batteries using Li6PS5Cl as the solid electrolyte. The two Sn-based active materials were nanostructured by ball-milling to demonstrate considerable promise for application in all-solid-state half-cells. Galvanostatic cycling at room temperature revealed electrochemical behavior based on conversion/alloying reactions akin to those observed in conventional lithium-ion batteries. Promisingly, both materials exhibited satisfying electrochemical stability, with coulombic efficiencies exceeding 97%. These findings indicate that Li6PS5Cl solid electrolyte is compatible with Sn-based alloying anodes.
1. Introduction
The development of high-energy density lithium-ion batteries (LiBs) presents a significant technological challenge, particularly in the context of the widespread electrification of vehicles and nomadic applications. While commercial liquid organic electrolytes exhibit high ionic conductivity, ~10 mS cm−1 at room temperature (RT), they present substantial safety risks that compromise the reliability of LiBs [1,2]. These risks stem primarily from the inherent flammability and volatility of these electrolytes, which can result in severe hazards such as short-circuiting, leakage, and even explosions. Additionally, the limited thermal stability of these electrolytes exacerbates the risk of thermal runaway under conditions of elevated temperatures or mechanical stress. The all-solid-state lithium-ion battery (ASSLiB) represents a promising technology to address these safety concerns, offering the potential for the use of high-capacity electrode materials along with the elimination of flammable liquid electrolytes [3]. Among the various solid electrolytes being researched, sulfide-based solid electrolytes have demonstrated outstanding ionic conductivities with wide electrochemical window stability [4]. A special interest is addressed to Li6PS5Cl, a chlorine argyrodite-type electrolyte, owing to its high ionic conductivity, ~1 mS cm−1 at RT. Furthermore, its application in all-solid-state lithium-ion batteries (ASSLiBs) with a range of active materials has yielded highly promising results [1,5].
One of the most crucial components of the ASSLiBs, the anode, has recently shown remarkable progress, paving the way for new possibilities in battery research. Hitherto, the development of high-capacity anode materials for ASSLiBs has largely been focused on lithium metal [6,7,8,9]. This focus is driven by lithium’s high theoretical capacity (3862 mAh g−1) in tandem with its lowest working potential (−3.04 V vs. SHE), which maximizes the energy density of ASSLiB [7]. However, the lithium metal anode faces significant drawbacks, including lithium deposition at the electrode/electrolyte interface, which triggers the propagation of lithium dendrites into the solid electrolyte, leading to short circuits and cell failure issues [7,8]. Moreover, the high chemical reactivity of lithium metal with the solid electrolyte usually leads to the formation of various by-products at the electrode/electrolyte interface. For example, Liang et al. reported that coupling Li3PS4 solid electrolyte and lithium metal leads to in situ decomposition of the Li3PS4, generating Li2S and Li3P, which subsequently increases interfacial resistance [10]. This undesirable reaction reduces the coulombic efficiency, shortens cycle life, and causes capacity decay, ultimately reducing the overall energy density of the ASSLiB.
These issues can be mitigated by utilizing alternative anode materials that offer not only reduced reactivity toward the solid electrolyte but also higher energy density of the battery. Given that most studies in ASSLiBs have focused on Si-based anodes [8,11,12,13,14,15,16,17,18,19], we aimed to broaden the scope by exploring the potential of Sn-based materials, an underrepresented yet promising alternative. While silicon offers high capacity, its severe volume expansion and interfacial instability pose significant challenges in ASSLiBs. In contrast, Sn-based materials provide a more balanced trade-off between capacity, mechanical stability, and compatibility with solid electrolytes. In particular, Sn anode has gained significant attention owing to its high specific capacity, reaching a theoretical value of 990 mAh g−1 for the formation of Li4.4Sn alloy at an average potential of 0.4 V vs. Li+/Li [20]. Within this framework, prior studies have emphasized the properties of Sn powder as an anode material for ASSLiBs [8,15,21]. For example, Miyazaki et al. synthesized the Sn-based composite anode by milling Sn commercial powder together with 80Li2S·20P2S5 glass solid electrolyte [15]. The composite anode delivers a reversible capacity of 600 mAh g−1 over 80 cycles at 0.03 C. Nonetheless, as it is widely recognized, the Sn anodes undergo considerable volume changes during lithiation (up to 250% for Sn to Li4.4Sn) [20], a common issue in all alloying reactions. This leads to particle agglomeration along with mechanical instability on cycling.
A prevalent strategy to circumvent this issue involves embedding capacitive Sn elements into a non-active matrix capable of accommodating volumetric changes. This matrix can be either metallic, such as intermetallic compounds, or polyanionic materials, such as phosphite compounds. For the former, in a conventional liquid LiB, the nanostructured Ni3.4Sn4 compound is reported to provide a reversible capacity of 250 mAh g−1 over 100 cycles at 0.2 C through a two-step lithium insertion process enabled by a conversion-alloying mechanism [22]. As for the nanostructured SnHPO3 tin phosphite compound, it has demonstrated a reversible capacity of 677 mAh g−1 over 70 cycles at 0.2 C, attributed mainly to the LixSn alloys [23].
To the best of our knowledge, no previous studies have yet examined the electrochemical performance of Ni3.4Sn4 intermetallic as well as SnHPO3 phosphite compounds in ASSLiBs. Thereby, in this work, we investigate the electrochemical performance of SnHPO3 and Ni3.4Sn4 as novel conversion-alloying anode materials for ASSLiBs with a one-to-one comparison with their electrochemical behavior in conventional LiB with liquid electrolyte. Leveraging the excellent mechanical ductility of argyrodite electrolytes, Li6PS5Cl chlorine argyrodite was selected as the solid electrolyte, for which its crystallographic, morphological, and electrochemical properties are investigated.
2. Materials and Methods
2.1. Synthesis of Nanostructured Tin-Based Anodes
Tin phosphite compound SnHPO3 was synthesized by hydrothermal route: a mixture of H3PO3 (Sigma Aldrich, Saint Louis, MO, USA, 99%), SnCl2·2H2O (Sigma-Aldrich, Saint Louis, MO, USA, 98%), and oxalic acid C2H2O4 (SDS company, Minchinbury, Australia, 99.5%) was prepared with a molar ratio of 10:1:2. Oxalic acid addition prevents oxidation of phosphite to phosphate. The mixture was placed into a 50 mL Teflon-lined autoclave, then sealed and heated at 180 °C for 72 h, followed by natural cooling to RT. The resulting powder was washed, dried, and subsequently milled in a planetary mill under air atmosphere for 1 h. The milling was conducted in an 80 mL jar using 7 mm diameter balls made of hardened steel and stainless steel, respectively. The ball-to-powder weight ratio was 5:1, and the mill was operated at a rotational speed of 600 rpm. It is worth noting that a planetary mill (Pulverisette 7, Fritsch, FRITSCH GmbH, Idar-Oberstein, Germany) was utilized for all milling processes of this work.
Since the chemical homogeneity domain of Ni3+xSn4 lies within the range 0.08 ≤ x ≤ 0.6 [24], the Ni-rich compound Ni3.4Sn4 (x = 0.4) was targeted for synthesis. First, pristine Ni3.4Sn4 alloy was synthesized through induction melting of high-purity nickel (99.99%) and tin (99.9%) metals in a stoichiometric molar ratio. The melting process was conducted in a water-cooled copper crucible under a high-purity argon atmosphere. To ensure homogeneity, the ingot was turned over and re-melted three times. Subsequently, the ingot underwent annealing at 700 °C for 7 days under argon atmosphere within a silica tube, followed by quenching in RT water. Annealing conditions were selected based on the characteristics of the Ni-Sn phase diagram [25]. The annealed alloy was manually pulverized to a particle size of approximately 125 μm using an agate mortar. Afterward, nanostructuration of Ni3.4Sn4 was achieved by mechanical milling under argon atmosphere for 20 h, following the same protocol as for SnHPO3. To avoid excessive thermal heating within the jar, a 10 min break was performed after each hour of milling.
2.2. Preparation of Li6PS5Cl Solid Electrolyte
Chlorine argyrodite Li6PS5Cl was synthesized from Li2S (Sigma Aldrich, Saint Louis, MO, USA, 99.98%), P2S5 (Sigma Aldrich, Saint Louis, MO, USA, 98%), and LiCl (Sigma Aldrich, Saint Louis, MO, USA, 99.99%) reagents in stoichiometric ratio to obtain the nominal composition. The mixture was milled for 10 h with 10 min break per milling hour, under argon atmosphere, using a 45 mL jar with 10 mm balls. Both the jar and balls were made of tungsten carbide (WC). The ball-to-powder weight ratio was 39:1, and the milling speed was set to 600 rpm.
2.3. Materials Characterization
2.3.1. Structural, Morphological, and Microstructural Characterization
For all the studied materials, X-ray diffraction (XRD) patterns were obtained in Bragg–Brentano geometry on a D8 Advance DaVinci Bruker diffractometer (Karlsruhe, Germany) equipped with LynxEye detector and Cu-Kα radiation (λ = 0.15418 nm). The Li6PS5Cl containing samples were prepared in an argon-filled glove box using an airtight XRD holder to prevent reaction with moisture and oxygen. The XRD patterns were analyzed by the Rietveld method using FULLPROF suite software [26]. The crystallite size was evaluated from the Lorentzian contribution to the “Thompson-Cox-Hastings pseudo-Voigt” profile shape function after considering the instrumental resolution [27].
Chemical composition of nanostructured Ni3.4Sn4 material was analyzed using inductively coupled plasma-optical emission spectrometry (ICP-OES) using Agilent 5800 device (Agilent Technologies, Santa Clara, CA, USA). Morphology of materials was examined by Scanning Electron Microscopy (EDX-SEM) using a SEM-FEG MERLIN from Zeiss (Jena, Germany). Powder was spread on a double-sided carbon tape affixed to a SEM stub. Preparation was conducted in ambient air, except for the Li6PS5Cl argyrodite-containing powders, which were prepared in an argon-filled glove box and promptly transferred to the SEM device.
Particle size distribution (PSD) of both SnHPO3 and Ni3.4Sn4 nanostructured samples was determined using laser diffraction (LD) with the Mastersizer 3000 particle size analyzer (Malvern Panalytical, Malvern, UK). Powder was gradually added to distilled water under ultrasonic agitation at 1500 rpm until the recommended obscuration level was achieved (<25%).
2.3.2. Electrochemical Measurements
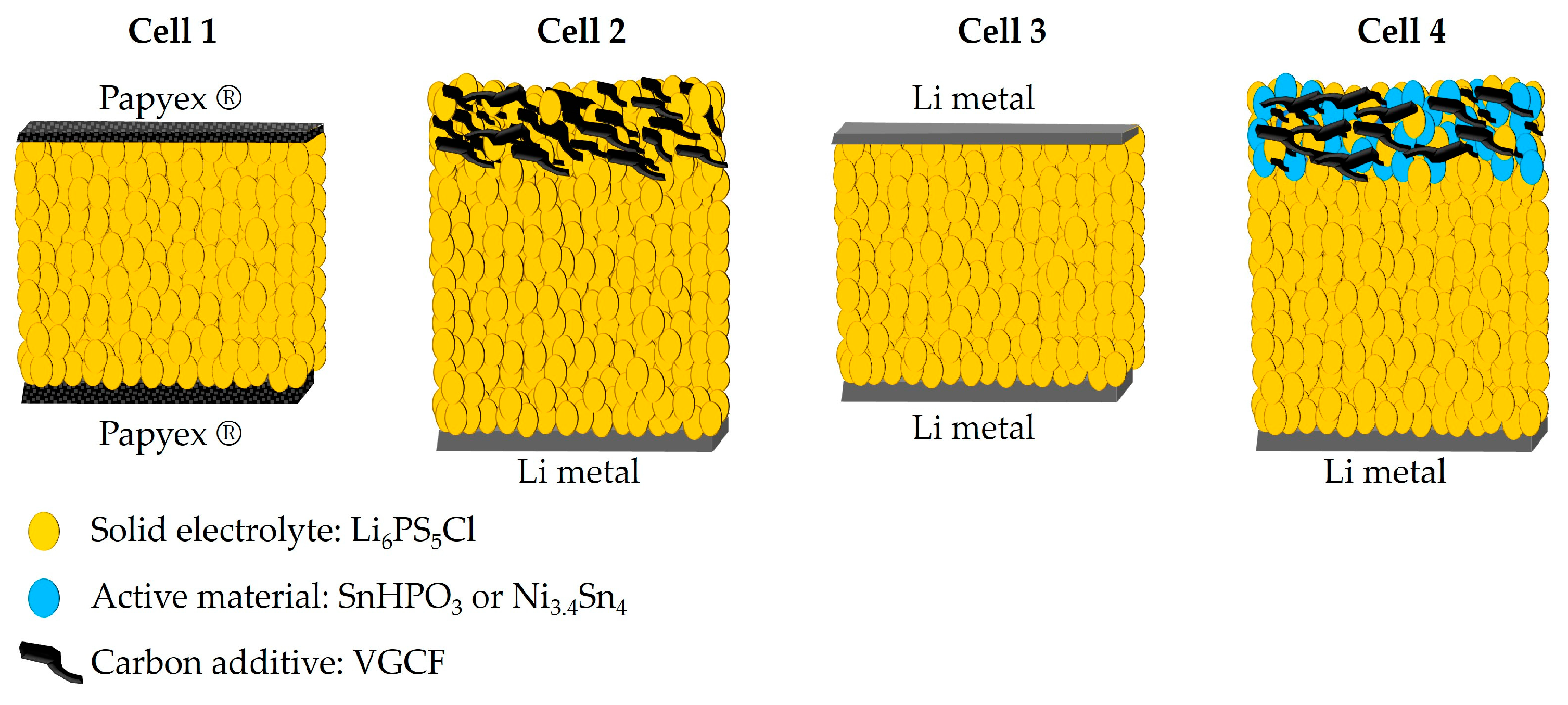
All electrochemical preparation processes were conducted in an argon-filled glove box (O2 < 4 ppm, H2O < 1 ppm), and all electrochemical performances were evaluated on a CR2032 coin cell using a VMP3 potentiostat (Biologic, Seyssinet-Pariset, France). Ionic conductivity of the Li6PS5Cl solid electrolyte was measured using electrochemical impedance spectroscopy technique (EIS). The solid electrolyte powder was cold pressed at 200 MPa for 2 min in a tungsten carbide die (Ø = 6 mm). The argyrodite pellet, with a thickness of 0.88 (3) mm, was then transferred to a symmetrical coin cell between two Papyex® flexible graphite to ensure good electrical contact (cell 1 of Figure 1). EIS data were collected using CESH-e sample holder from Biologic (Seyssinet-Pariset, France) over a frequency range from 400 kHz to 100 Hz and with a voltage amplitude of 30 mV. Data fitting was performed using a suitable equivalent circuit model implemented in the EC-Lab V11.43 software. The activation energy (Ea) was calculated from the Arrhenius plot of ionic conductivity recorded over the temperature range of 25 to 80 °C, using the enhanced intermediate temperature system (ITS-e) from Biologic (Seyssinet-Pariset, France).
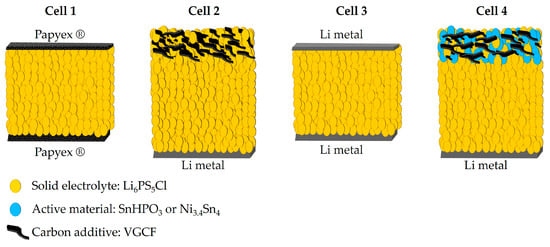
Figure 1.
Schematic illustration of the cell configurations for the electrochemical measurements.
The electrochemical stability window of Li6PS5Cl was assessed using cyclic voltammetry (CV) with the configuration depicted in Cell 2 of Figure 1. A mixture of 75 wt.% Li6PS5Cl and 25 wt.% vapor-grown carbon fibers (VGCF, VGCFTM-H, Showa Denko K.K., Tokyo, Japan) was spread on top of 38 (4) mg Li6PS5Cl pellet and then cold-pressed together under a pressure of 200 MPa. Li metal (0.25 mm thickness, 99.8%, ChemPure Chemicals, Westland, MI, USA) was attached to the other side of the Li6PS5Cl pellet. The carbon additive was added to ensure effective electronic percolation within the electrode and to mitigate the poor electronic conducting nature of the solid electrolyte. CV measurements were conducted at a scan rate of 100 µV s⁻1 over a voltage range from 0 to 5 V vs. Li+/Li.
Lithium symmetric cells were assembled using the configuration shown in Cell 3 of Figure 1 in order to evaluate the interface stability between lithium metal and Li6PS5Cl argyrodite through lithium-ion migration. The galvanostatic mode was employed at different current densities, increasing from 20 to 160 µA cm−2, with a time step of 30 min. In addition, a constant current density of 35 µA cm−2 with a time step of 4 h was conducted to evaluate the long-term interface stability.
All solid-state half-cells were assembled according to the configuration in Cell 4 of Figure 1, with lithium metal serving as both the counter and reference electrode. The composite electrode mixture (mass loading of 7 mg cm−2) was homogeneously distributed over the Li6PS5Cl electrolyte pellet and co-pressed together at 200 MPa for 2 min. To ensure ion percolation within both composites, the electrolyte volume fraction was fixed at 0.5. The composites were prepared by mixing active materials, Li6PS5Cl and VGCF, in an agate mortar with a weight ratio of 40:50:10 and 78:20:2 for SnHPO3 and Ni3.4Sn4, respectively. Carbon fibers (VGCF) have been incorporated into each electrode formulation to enhance electronic conductivity and improve lithium-ion transport within the working electrode. Half-cells were cycled in galvanostatic mode at 5 mA g−1 constant current within the potential range of [0.1–2] and [0.2–2] V vs. Li+/Li for SnHPO3 and Ni3.4Sn4 composites, respectively. The lower voltage cutoff was set above 0 V vs. Li+/Li to prevent argyrodite degradation. For comparative purposes, galvanostatic cycling was conducted for conventional lithium-ion half-cells, Li|LP30|SnHPO3 and Li|LP30|Ni3.4Sn4, at 108 mA g−1 using 1M LiPF6 dissolved in Ethylene Carbonate (EC)/Dimethyl Carbonate (DMC) (1:1 v/v, LP30, Sigma Aldrich, Saint Louis, MO, USA) liquid electrolyte, as described in our previous study [23].
3. Results
3.1. Structure and Morphology of Sn-Based Active Materials
3.1.1. Nanostructured SnHPO3 Tin Phosphite
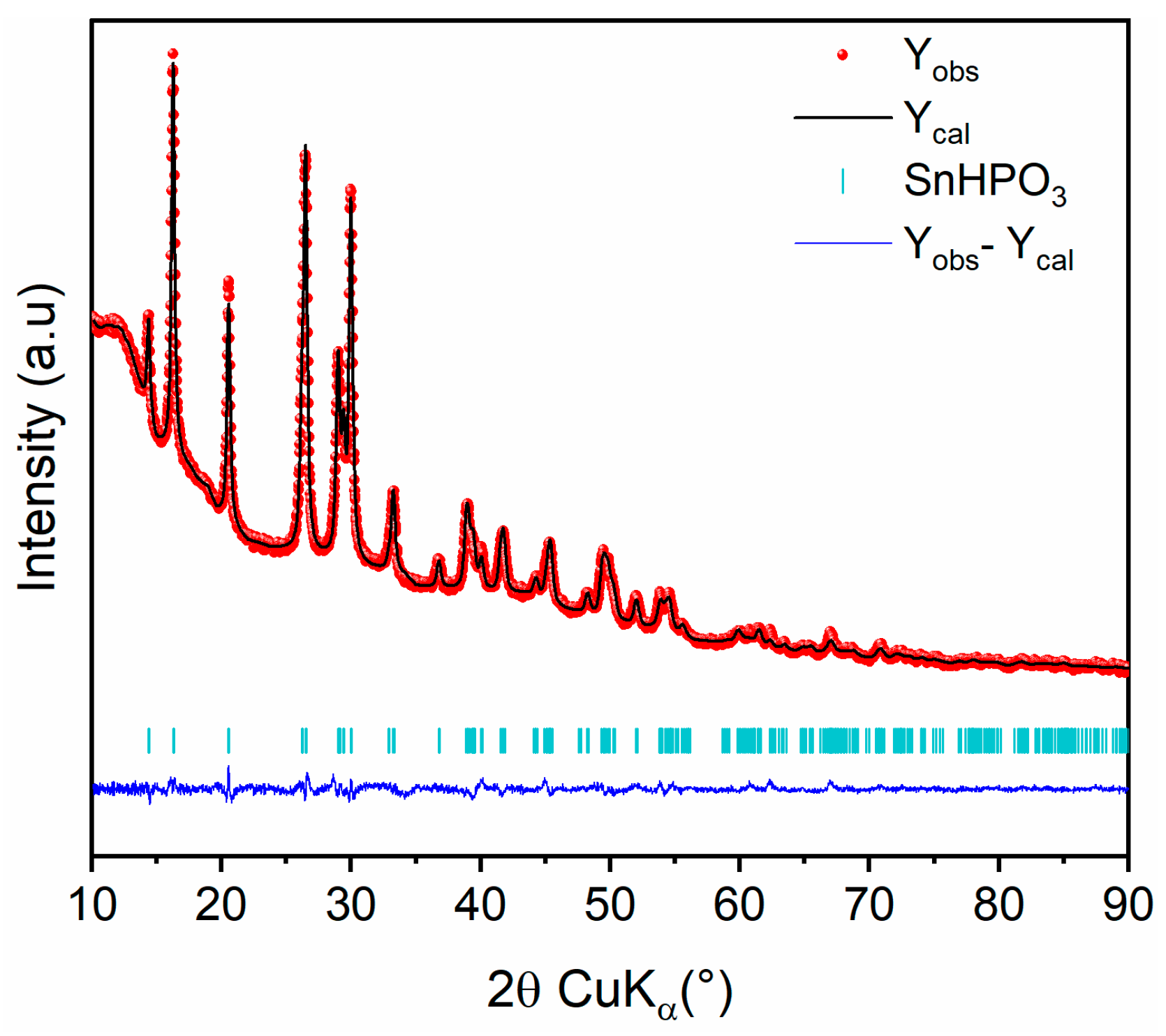
The crystal structure of the nanostructured SnHPO3 was analyzed using XRD analysis. The Rietveld refinement of the XRD pattern is shown in Figure 2. All diffraction peaks can be indexed in a monoclinic symmetry within the C1c1 space group in agreement with the crystal structure reported by McDonald el al. [28]. The refined structural parameters are reported in Table 1. The crystallite size was evaluated from peak broadening to 24 (2) nm.
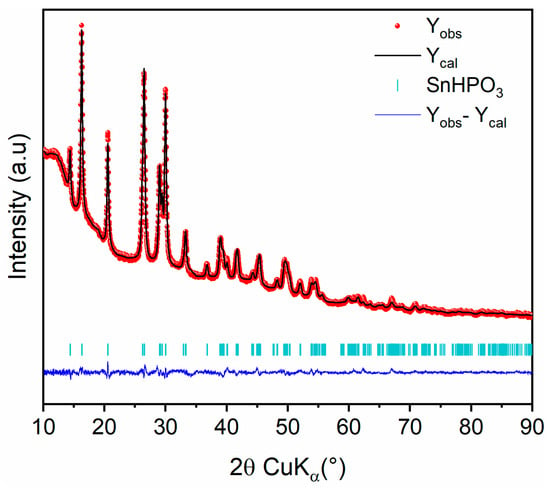
Figure 2.
Rietveld refinement of nanostructured SnHPO3 XRD pattern: Observed (red dots), calculated (black line), and difference (blue line) curves are shown. The vertical marks show the Bragg positions for SnHPO3 phase.

Table 1.
Refined structural parameters by Rietveld method of nanostructured SnHPO3. Rietveld agreement factors (RBragg, Rp, Rwp, and χ2) are provided. Standard deviations referred to the last digit are given in parentheses.
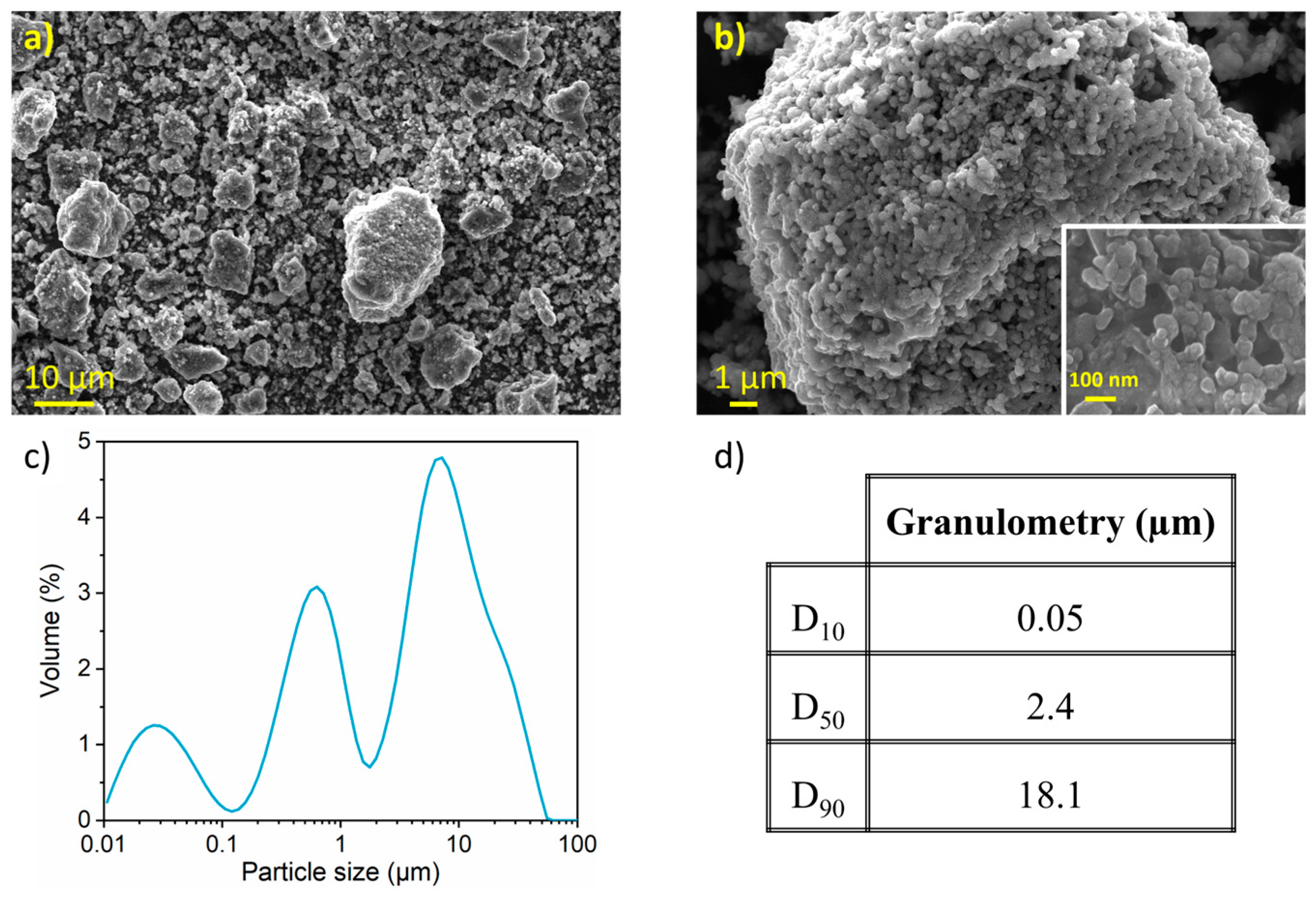
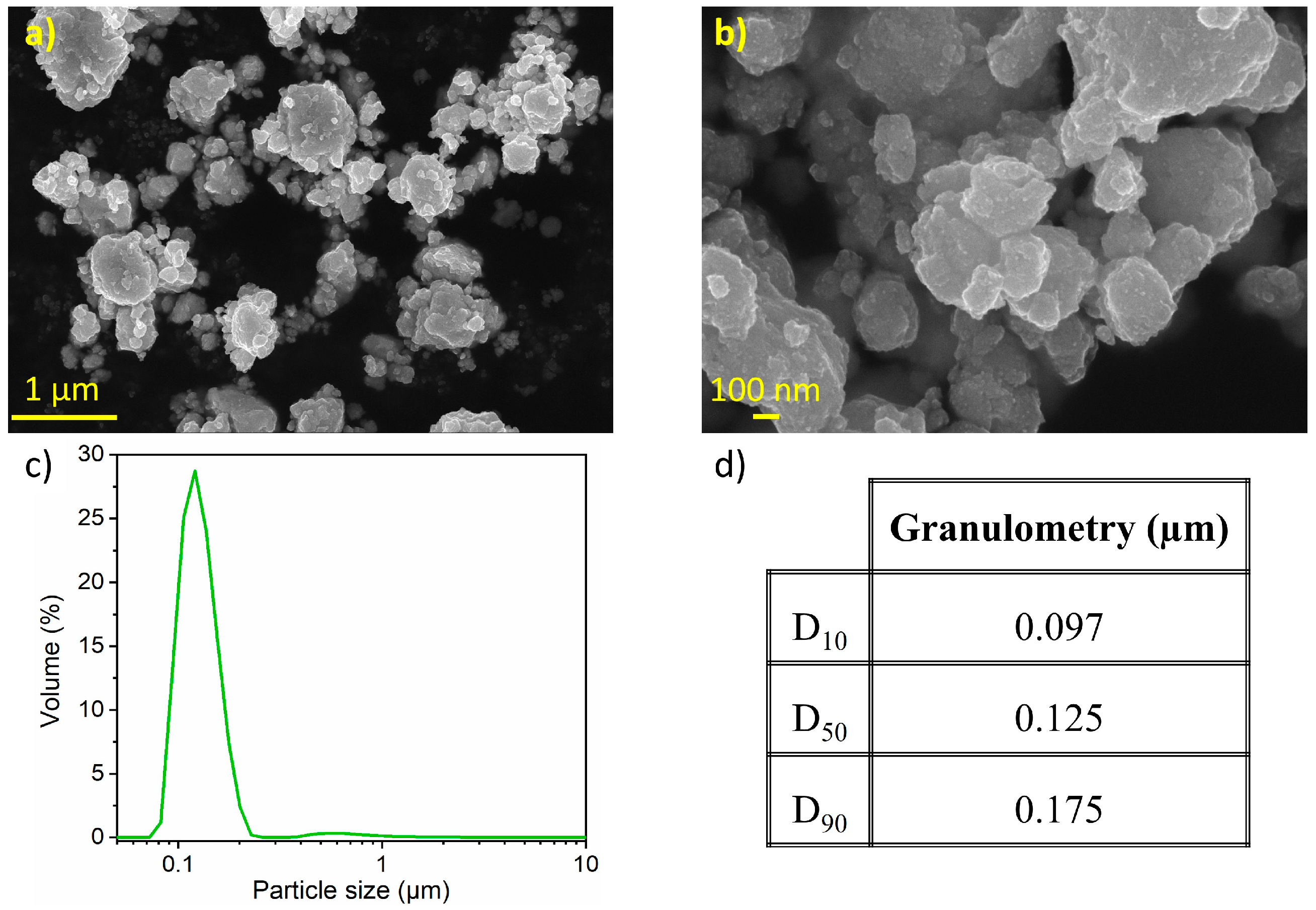
Figure 3 presents SEM micrographs of the nanostructured SnHPO3 powder. Dense and spherical secondary particles are visible, with sizes ranging from 1 to 20 μm (Figure 3a). At higher magnification (Figure 3b), granular primary particles are observed with particle size around 100 nm (inset of Figure 3b). Figure 3c shows the particle size distribution as determined by LD with its corresponding cumulative volume distribution in Figure 3d. The material exhibits a multimodal curve with a wide particle size distribution (10 nm to 60 μm) with a median particle size of 2.4 µm and a considerable extent of agglomeration.
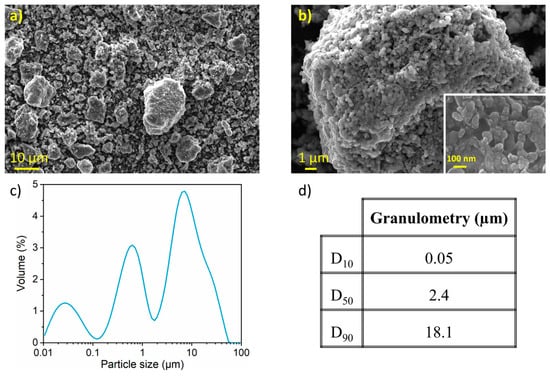
Figure 3.
(a,b) Secondary electron image obtained with in-lens detector at three magnifications (c) Particle size distribution curve from LD measurements (d) Particle size percentiles of the nanostructured SnHPO3.
3.1.2. Nanostructured Ni3.4Sn4 Intermetallic
The crystal structure and chemical composition of the non-milled Ni3+xSn4 intermetallic were analyzed using XRD and ICP analyses. Rietveld refinement of the XRD data is presented in Figure S1, and the crystallographic data are summarized in Table S1. All diffraction peaks can be indexed with monoclinic symmetry in the C2/m space group, as reported by Furuseth et al. for Ni3+xSn4 phases [24]. The over-stoichiometry is accommodated through the partial occupation of the 2a sites, with Rietveld refinement yielding a stoichiometry of Ni3.47(2)Sn4. This result is consistent with the ICP analysis (Ni3.46(4)Sn4) displayed in Table 2. Accordingly, the composition for further study has been set to the nominal Ni3.4Sn4.

Table 2.
Chemical composition determined by ICP analysis of non-milled and 20 h milled Ni3.4Sn4.
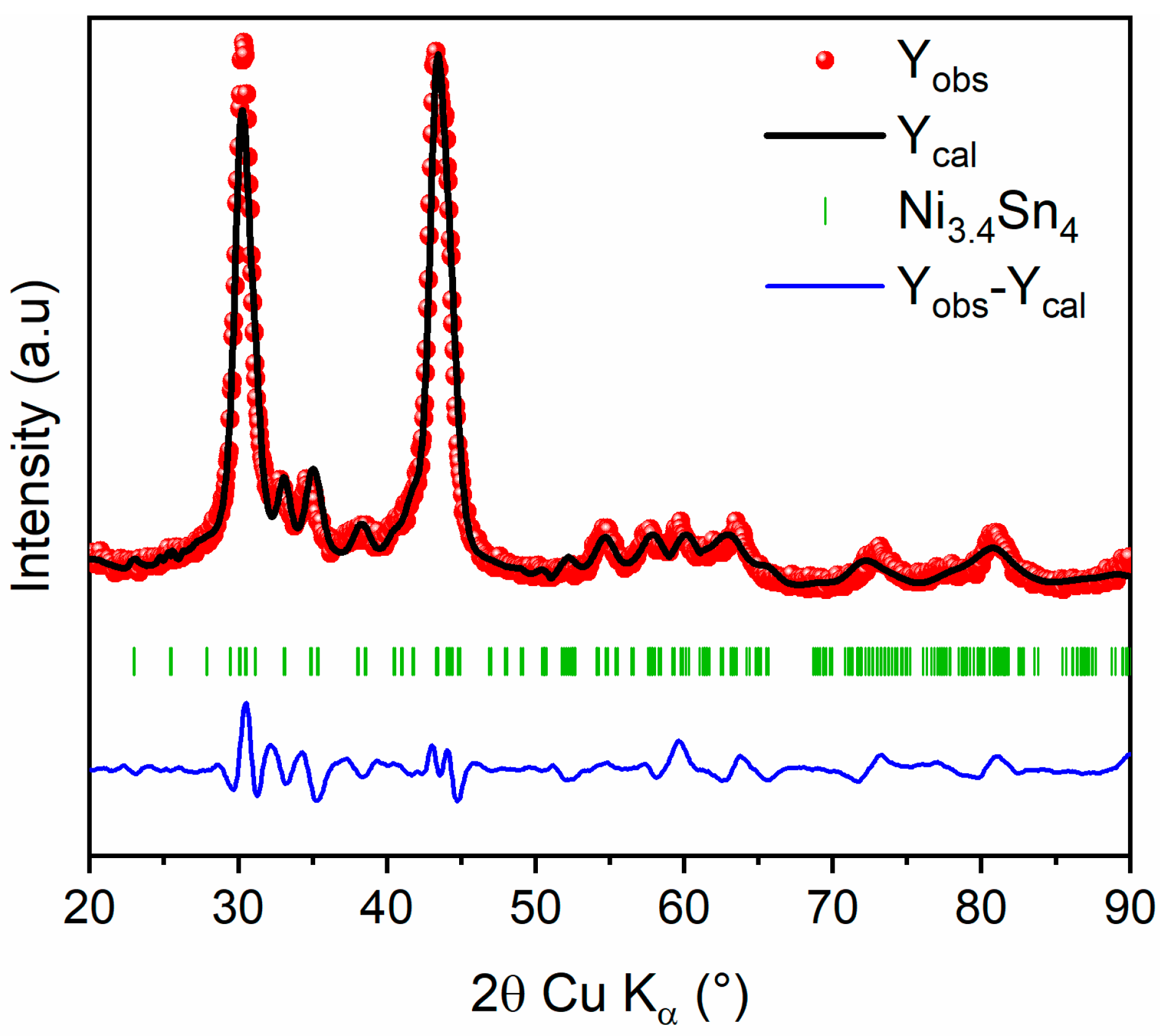
Afterward, the evolution of XRD patterns of Ni3.4Sn4 with milling time is depicted in Figure S2 and Table S2. The Ni3.4Sn4 phase is preserved during milling with gradual broadening of all diffraction peaks. The refined cell parameters and crystallite sizes are gathered in Table S1. Based on previous findings, the 20 h milled Ni3.4Sn4 sample was selected for further investigation owing to its superior electrochemical performance [22]. The refined XRD pattern for 20 h milled Ni3.4Sn4 and the corresponding refined structural parameters are presented in Figure 4 and Table 3, respectively. The unit cell parameters align well with those previously reported [22]. The slight expansion may be attributed to the plastic deformation induced by ball milling, which also leads to a significant reduction in crystallite size, down to ~7 (1) nm, as inferred from the large peak broadening.
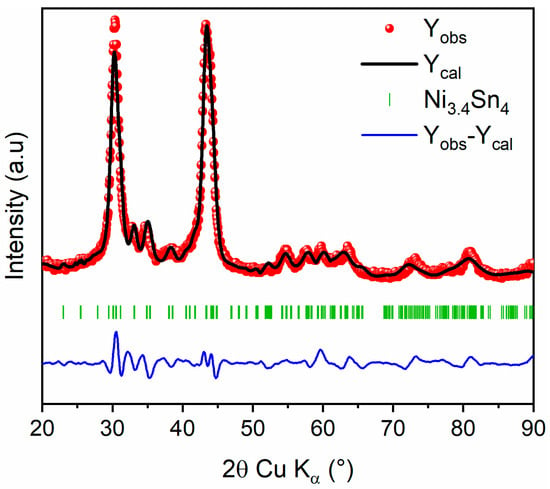
Figure 4.
Rietveld refinement of XRD pattern of 20 h milled Ni3.4Sn4: Observed (red dots), calculated (black line), and difference (blue line) curves are shown. The vertical marks show the Bragg positions for Ni3.4Sn4 phase.

Table 3.
Refined structural parameters of 20 h milled Ni3.4Sn4. Rietveld agreement factors (RBragg, Rp, Rwp, and χ2) are provided. Standard deviations referred to the last digit are given in parentheses.
The average chemical composition of 20 h milled Ni3.4Sn4, as determined by ICP analysis, is gathered in Table 3. A minor Fe contamination from the milling tools, ≤0.09 at.%, was detected. The measured atomic ratio between Ni and Sn concurs fairly well with the targeted values for Ni3.4Sn4.
Figure 5 presents the SEM morphology of 20 h milled Ni3.4Sn4. The sample consists of secondary particles with an average size of ~1 μm (Figure 5a). At higher magnification, the size of primary particles can be estimated to range from 100 to 200 nm (Figure 5b), matching the particle size distribution in Figure 5c. The D10, D50, and D90 values indicate a main particle size of 0.15 µm (Figure 5d). It is worth noting that the LD analysis only captures the primary particle sizes, with no indication of larger agglomerates likely due to their disruption by ultrasonic agitation or their weight preventing suspension during analysis.
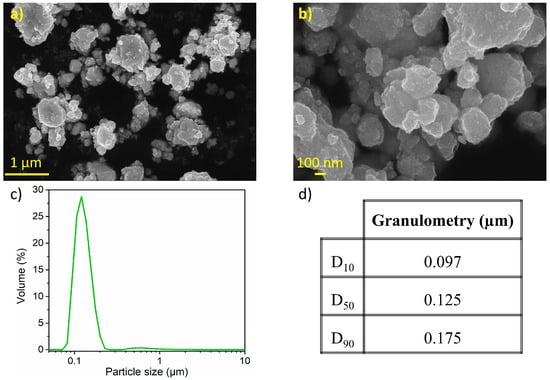
Figure 5.
(a,b) Secondary electron image with in-lens detector with two magnifications; (c) particle size distribution curve from LD measurements; and (d) particle size percentiles of the 20 h milled Ni3.4Sn4.
3.2. Li6PS5Cl Solid Electrolyte
3.2.1. Structural and Morphological Characterization
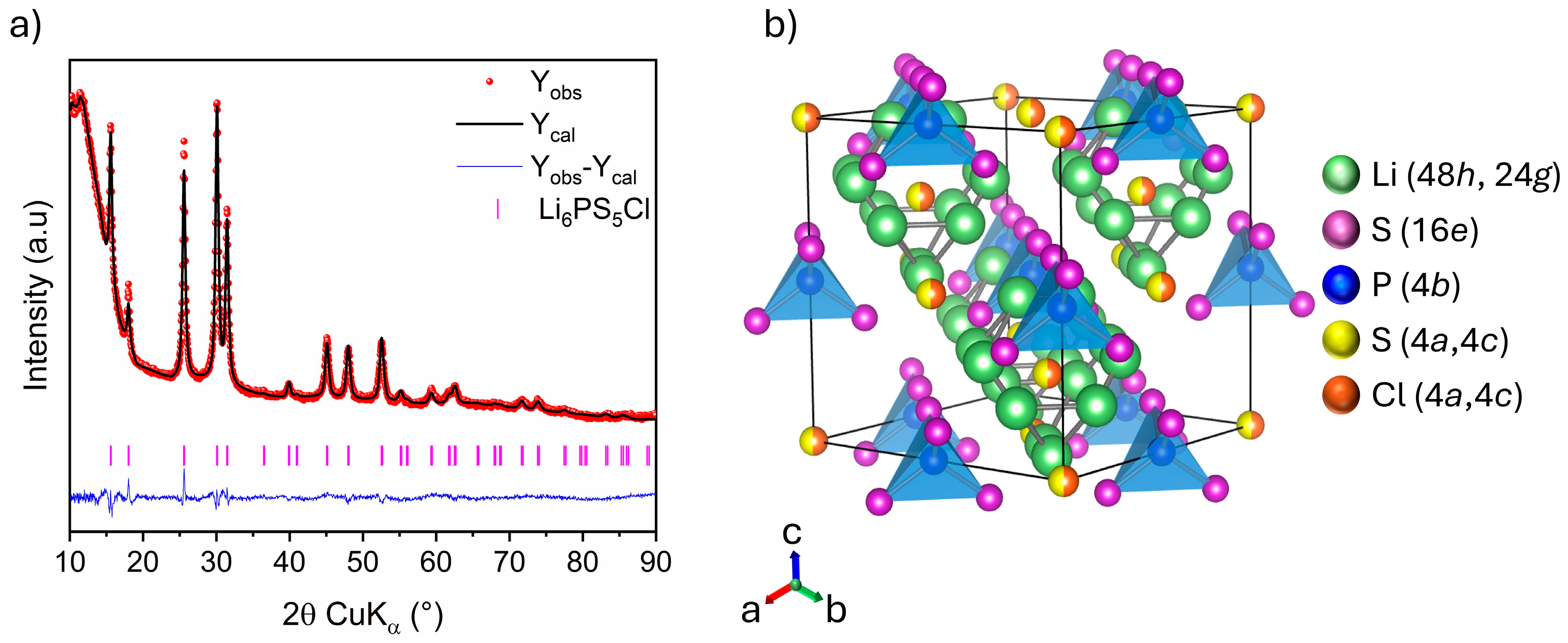
Rietveld analysis of the XRD pattern of Li6PS5Cl chlorine argyrodite is displayed in Figure 6a, with the corresponding crystallographic data gathered in Table 4. The halo patterns at low angles result from the dome-like X-ray transparent cap used to prevent the sample from air exposure. Rietveld refinement indicates that Li6PS5Cl crystallizes in a cubic system with the space group. The lattice parameter and average crystallite size are determined to be 9.8495 (5) Å and 15 (1) nm, respectively, consistent with previously reported values [29,30]. The crystal structure of Li6PS5Cl, illustrated in Figure 6b, reveals a cubic-close-packed lattice. The backbone consists of PS4− polyhedra centered at 4b sites and formed by S2− at 16e sites. The remaining S2− and Cl− occupy the 4a and 4c sites, of which approximately 50% of the surrounding 48h sites are occupied by lithium-ions. The lithium distribution across the 48h and 24g sites creates a cage-like structure centered at 4c sites. In this context, molecular dynamic simulations revealed that the occupancy ratio of halide anions on 4a and 4c sites (disorder) significantly influences the frequency of lithium-ion mobility in the argyrodite lattice [31,32,33,34]. This, in turn, affects the argyrodite’s overall ionic conductivity. Unfortunately, S2−/Cl− occupancies were not refined for disorder in our case since Cl− and S2− are indistinguishable via XRD due to their similar scattering factors [33].
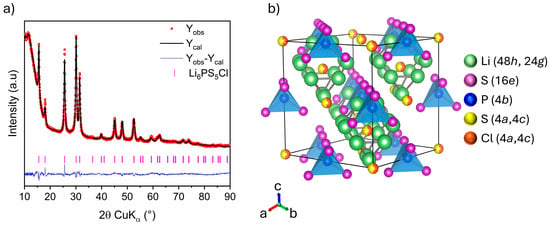
Figure 6.
(a) Rietveld refinement of XRD pattern: Observed (red dots), calculated (black line), and difference (blue line) curves are shown. The vertical marks show the Bragg positions for Li6PS5Cl phase, (b) Crystal structure schema of Li6PS5Cl.

Table 4.
Refined crystallographic data of Li6PS5Cl. Rietveld agreement factors (RBragg, Rp, Rwp, and χ2) are provided. Standard deviations referred to the last digit are given in parentheses.
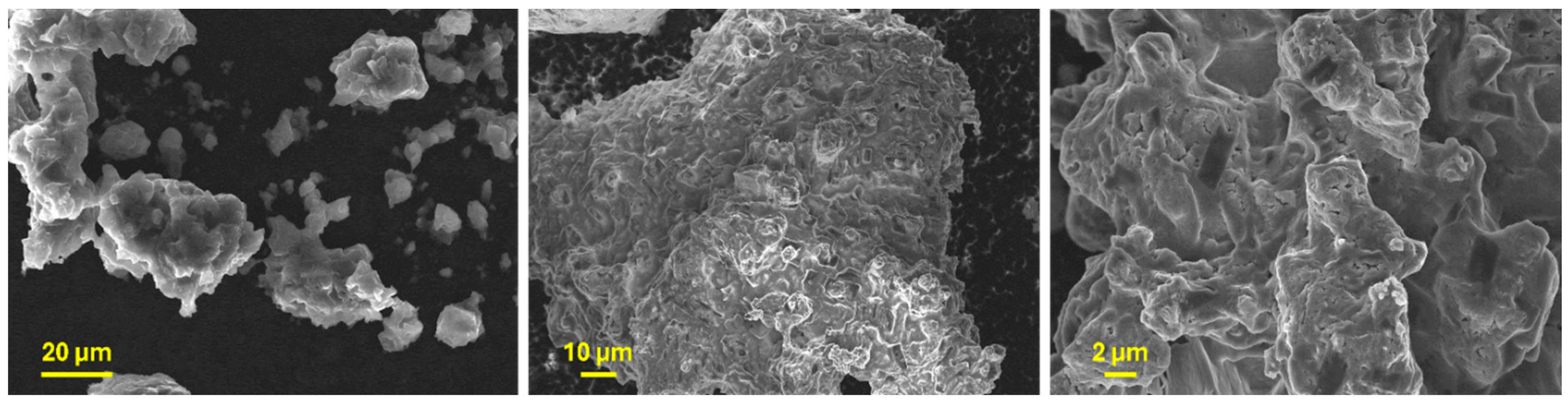
The SEM micrographs in Figure 7 show that Li6PS5Cl, obtained through mechanical milling, is composed of coal-grain-like particles displaying irregular shapes and sizes, with a propensity to agglomerate. This latter underscores the ductile nature of the argyrodite.

Figure 7.
SEM micrographs at different magnifications showing the morphology of Li6PS5Cl powder.
3.2.2. Electrochemical Characterization
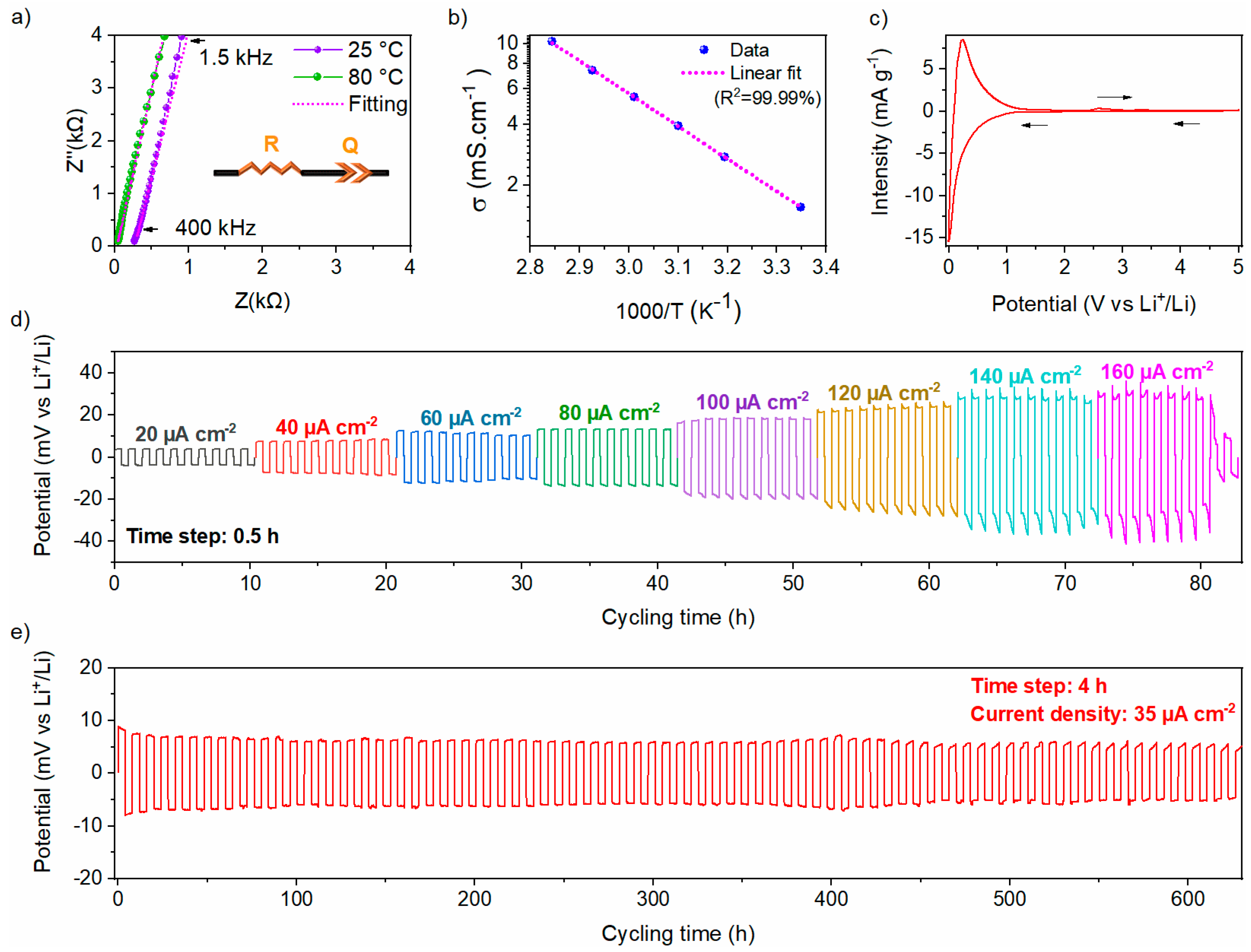
Ionic conductivity is one of the uppermost properties of a solid electrolyte. Nyquist plots of EIS for the Li6PS5Cl electrolyte at 25 and 80 °C are shown in Figure 8a. The impedance data are mostly composed of a steep linear spike attributed to a blocking behavior between the Li6PS5Cl conductor and the carbon electrodes. The impedance data were fit with an R-Q series equivalent circuit model, illustrated in the inset of Figure 8a, where R stands for the ionic resistance of the solid electrolyte, and Q represents the constant phase element (CPE) used to model the non-ideal capacitor. The ionic conductivity of the Li6PS5Cl solid electrolyte increases from 1.5 mS cm−1 at 25 °C to 10.2 mS cm−1 at 80 °C, consistent with reported values for the chlorine argyrodite [29,30]. The shift of the Nyquist plots toward higher frequencies with increasing temperature, as more clearly displayed in Figure S3a, is attributed to Arrhenius-type thermally activated ionic transport phenomena. For clarity, the high-frequency regions at 25 °C and 80 °C are enlarged in Figure S3b. The thermal dependence of the ionic conductivity, as shown in Figure 8b, gives an activation energy of 0.32 (1) eV, aligning with previously reported values [30,34,35]. The electronic conductivity at RT of the Li6PS5Cl solid electrolyte is found to be 3.2 (5) × 10−5 mS cm−1, calculated using the DC chronoamperometry method (Figure S3c).
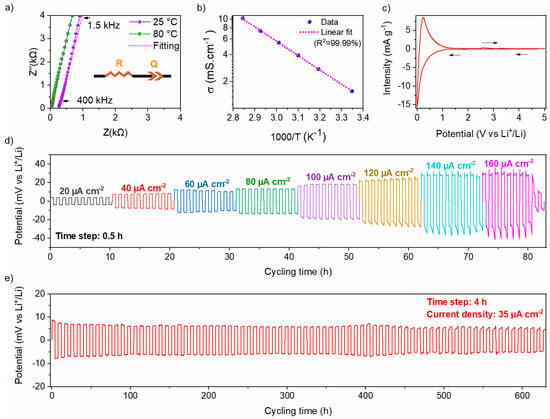
Figure 8.
(a) Nyquist plots at 25 °C and 80 °C with the corresponding equivalent circuit, (b) Arrhenius plot from EIS measurements, (c) Cyclic voltammetry curve with a scan rate of 10 µV s−1 of Li6PS5Cl. Voltage responses of the Li|Li6PS5Cl|Li symmetric cell under repeated polarization with (d) rate capability from 20 to 160 μA cm−2 with 0.5 h per step, and (e) under a constant current density of 35 μA cm−2 with 4h per step.
To study the electrochemical window stability of Li6PS5Cl chlorine argyrodite, a CV measurement was performed as displayed in Figure 8c. A reversible redox activity toward lithium was observed within the electrochemical window between 0 and 5 V vs. Li+/Li. A pronounced reduction peak is observed within the 0–0.5 V vs. Li+/Li potential range, indicating significant degradation of the chlorine argyrodite, which appears to be partially reversible, as evidenced by the prominent oxidation peak observed at 0.23 V vs. Li+/Li. Afterward, a small oxidation peak appears at 2.6 V vs. Li+/Li, with a corresponding reduction peak at 1.5 V vs. Li+/Li, which is due to a limited redox decomposition of the chlorine argyrodite electrolyte [36]. Beyond these observations, no other redox peaks were detected, indicating that the electrolyte can be effectively coupled with active materials operating at various potentials.
The electrochemical stability of Li6PS5Cl toward lithium metal was evaluated using a Li|Li6PS5Cl|Li symmetric cell at RT and measured in terms of critical current density (CCD) and rate capability of lithium-ions plating/stripping performance. As displayed in Figure 8d, the symmetric cell was evaluated by step-ascending current densities with a step gap of 20 µA cm−2. The polarization voltage increases with the applied current density, according to Ohm’s law. From 20 to 140 µA cm−2, the potential increases during the plating/stripping step. The total resistance was determined to be 661 ± 55 Ω, which exceeds the intrinsic resistance of the bulk Li6PS5Cl estimated at 255 ± 5 Ω. This discrepancy can be attributed to the formation of a resistive interfacial layer upon contact with lithium metal, arising from the electrochemical instability of argyrodite at extremely low potentials. At a current density of 160 µA cm−2, an internal short circuit of the cell was recorded, indicated by a sharp decrease of the overpotential. Thus, the CCD is determined to be 140 µA cm−2 at RT.
Afterward, the long-term stability of lithium plating and stripping was evaluated by cycling a Li|Li6PS5Cl|Li symmetric cell at a constant current density of 35 µA cm−2 at RT. This current density was selected based on the conditions under which the solid-state half-cell was cycled in this study (~5 mA g−1). The voltage profile and magnified voltage profiles are shown in Figure 8e and Figure S4, respectively. The symmetric cell demonstrated a flat axisymmetric polarization curve, suggesting highly stable and reversible lithium plating/stripping behavior at the Li/Li6PS5Cl interface over 600 hours. It is worth mentioning that Li6PS5Cl exhibits a relatively stable polarization voltage of 8.2 (±0.5) mV over 600 h of cycling, indicating good stability at the interface between Li6PS5Cl and lithium under the tested conditions.
3.3. Electrochemical Characterization of Sn-Based All-Solid-State Half-Cell
Assembling an ASSLiB using solid electrolyte presents significant challenges, notably regarding chemical compatibility within the composite electrode. Thus, the compatibility of the intimate mixture of each anode active material and Li6PS5Cl, aged for two weeks at RT, was investigated by XRD analysis. Rietveld refinement of the XRD patterns for both composites is shown in Figure S5 with the corresponding refined structural parameters gathered in Table S3. The calculated patterns correspond to the sum of the individual components’ patterns, with no crystalline impurity phases observed. This suggests that no massive reaction occurs between the active material and solid electrolyte after mixing and aging at RT. These preliminary tests indicate that Li6PS5Cl is likely chemically compatible as a solid electrolyte with SnHPO3 and Ni3.4Sn4 electrode active materials.
Solid-state half-cells were cycled in galvanostatic mode at a constant current of 5 mA g−1 within the potential ranges of [0.1–2] and [0.2–2] V vs. Li+/Li for SnHPO3 and Ni3.4Sn4 respectively. It is crucial to point out that the lower voltage cutoff was set above 0 V vs. Li+/Li to mitigate the degradation of the argyrodite electrolyte. Consequently, an incomplete lithiation of SnHPO3 and Ni3.4Sn4 occurs, resulting in a predicted initial capacity reduction of approximately 10% and 40% of the solid-state half-cells compared with their LP30 liquid counterparts.
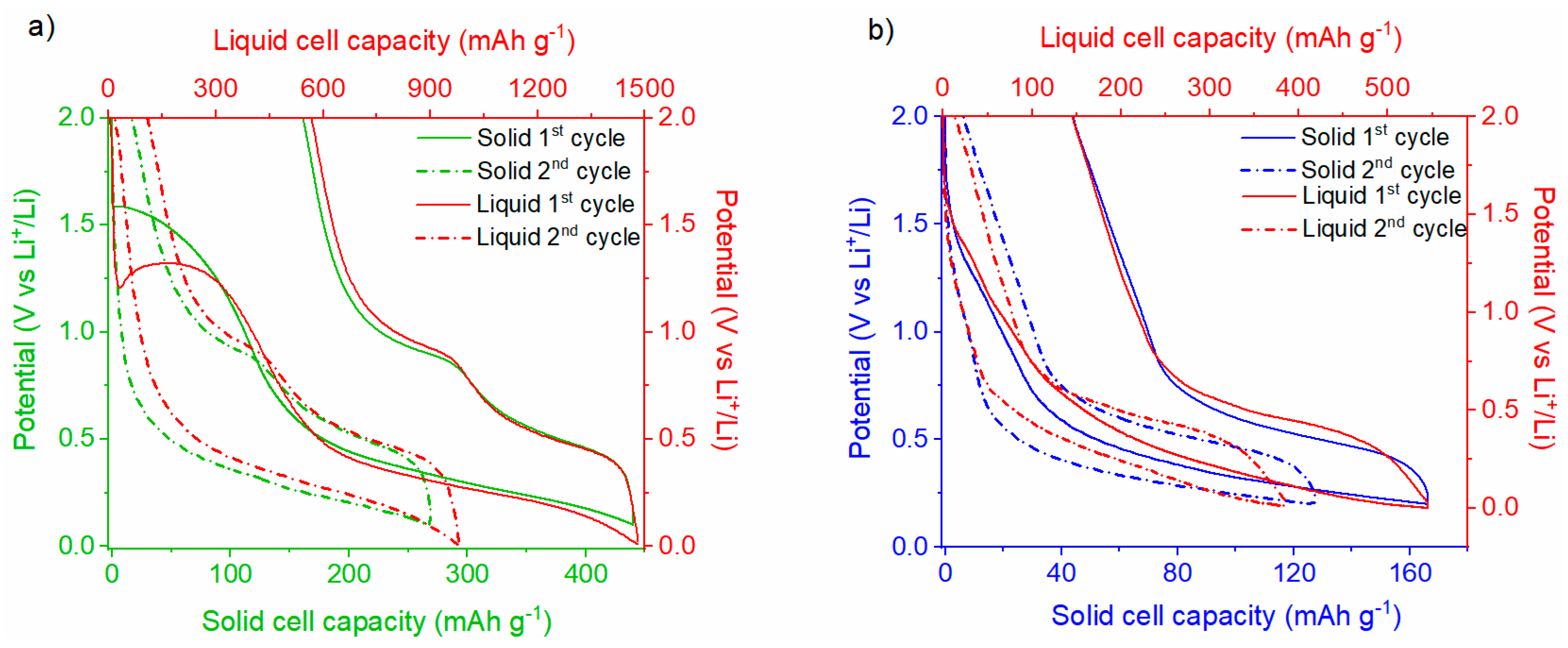
The voltage profiles for the 1st and 2nd cycles of the Li|Li6PS5Cl|SnHPO3 solid half-cell, compared with those of the Li|LP30| SnHPO3 liquid half-cell, are presented in Figure 9a. The potential profiles for the solid half-cell exhibit an analogous trend to that observed in the liquid half-cell. Albeit the variations in the extent of the lithiation reaction between both cells, they exhibit a similar reaction mechanism as better displayed in Figure S6a. Considering the solid-state half-cell, an irreversible pseudo-plateau emerges at ~1.5 V vs. Li+/Li during the first lithiation, which is notably higher than that observed in the liquid half-cell (~1.3 V vs. Li+/Li). This plateau, involving the exchange of 0.7 Li+ per SnHPO3 (100 mAh g−1), is attributed to the irreversible reduction of a fraction of Sn(II) in SnHPO3 to metallic tin Sn(0), as reported in previous studies [23]. Notably, this plateau is not related to the solid electrolyte interphase (SEI) formation since the cyclic voltammogram of chloride argyrodite demonstrates its electrochemical stability at this potential. Following the first lithiation, an extended plateau down to 0.1 V vs. Li+/Li corresponds to the alloying process of the metallic tin, resulting in the formation of LixSn alloys. A total of 3.2 Li+ per SnHPO3 is exchanged during this process, with the initial lithiation capacity reaching 440 mAh g−1, much lower than the 1480 mAh g−1 achieved in the liquid half-cell. During the subsequent delithiation, two distinct plateaus at 0.48 and 0.90 V vs. Li+/Li are observed, attributed to the dealloying of LixSn alloys [23]. The initial reversible capacity for the solid half-cell is 280 mAh g−1, corresponding to 2.1 Li+ per SnHPO3. The irreversible capacity fraction, approximately 40%, is comparable to that of the liquid cell, as it is mainly ascribed to the irreversible reduction in Sn(II) to Sn(0) and SEI formation at low voltages.
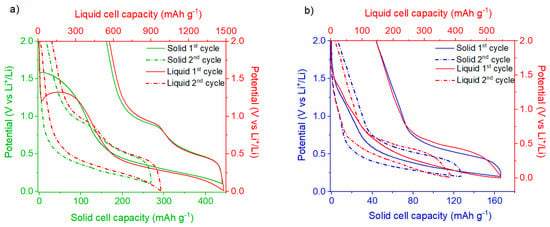
Figure 9.
First and second galvanostatic discharge/charge profiles of (a) Li|Li6PS5Cl|SnHPO3 and (b) Li|Li6PS5Cl|Ni3.4Sn4 half-cells at 5 mA g−1 in comparison with their corresponding liquid half-cells at 108 mA g−1.
The voltage profiles for the 1st and 2nd cycles of the Li|Li6PS5Cl|Ni3.4Sn4 solid-state half-cell compared with the Li|LP30|Ni3.4Sn4 liquid half-cell are shown in Figure 9b. The initial galvanostatic cycles for both the liquid and solid half-cells display no significant differences, indicating similar electrochemical behavior despite the lithiation extent, as displayed in Figure S6b. Starting the lithiation of the solid half-cell, the voltage quickly drops to 1.2 V vs. Li+/Li, followed by an initial potential step that is associated with the formation of the SEI interphase, as reported elsewhere [22]. Subsequently, a broad plateau at [0.2–0.6] V vs. Li+/Li potential range is observed, corresponding to the reversible formation of LixSn alloys. Upon the subsequent delithiation, a prominent plateau at 0.5 V vs. Li+/Li is discernible and attributed to the deformation of LixSn alloys. From the 2nd cycle onward, the potential profiles show no further signs of SEI formation and, similarly to the liquid half-cell, remain smooth. The 1st cycle shows a reversible capacity of 120 mAh g−1, which is lower than the 400 mAh g−1 observed in the liquid half-cell.
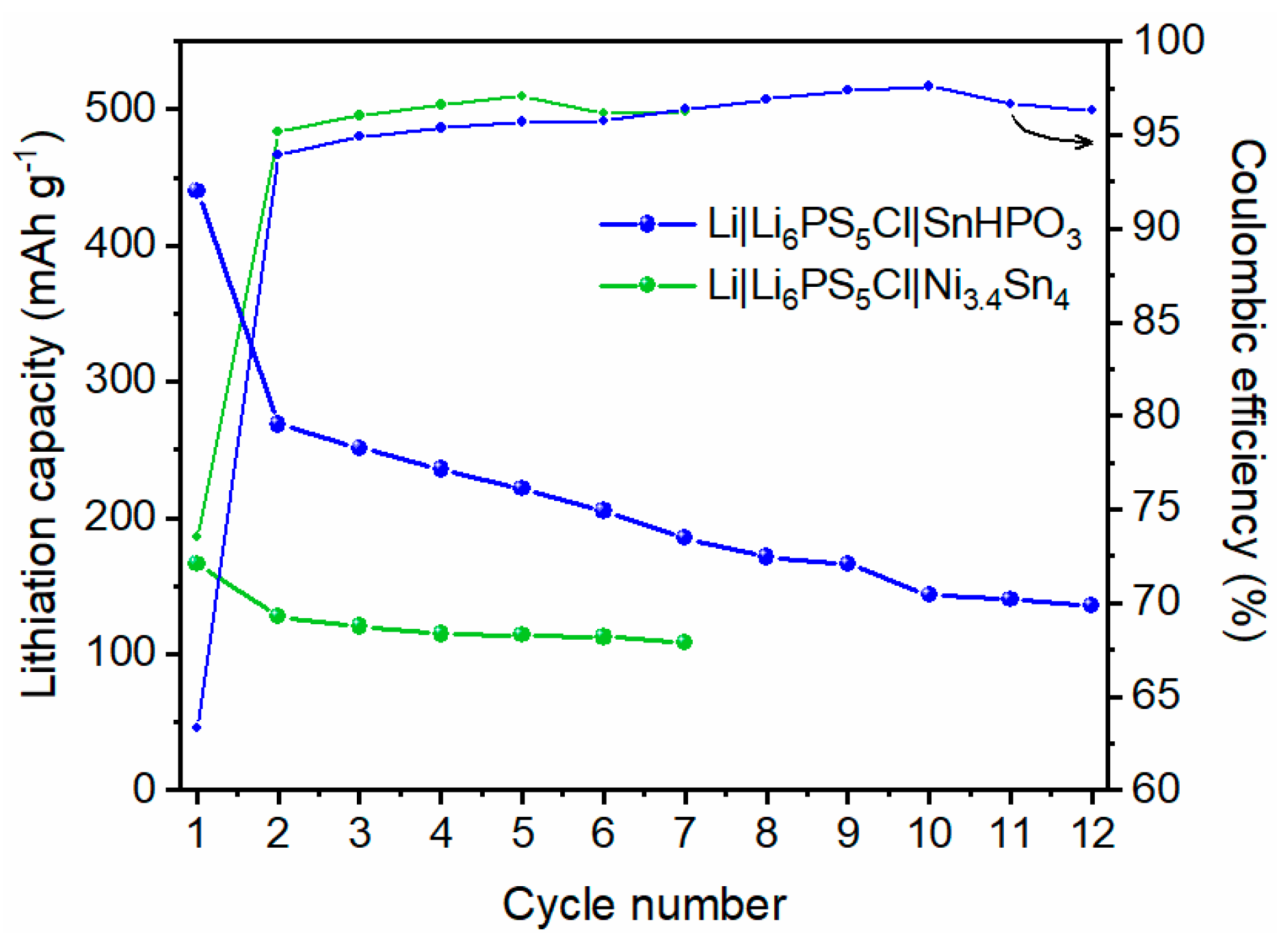
The evolution of lithiation capacity and coulombic efficiency over multiple cycles for the two solid-state half cells is depicted in Figure 10. The evolution of galvanostatic profiles is shown in Figure S7. For SnHPO3 material, a significant capacity decay is observed during the 1st cycle, followed by a gradual decline over the subsequent cycles. In contrast, the Ni3.4Sn4 material begins with a lower lithiation capacity of 170 mAh g−1; nonetheless, it maintains a more stable capacity on cycling, showing better capacity retention. The coulombic efficiencies improve significantly by the 2nd cycle, stabilizing above 95% for SnHPO3 and 97% for Ni3.4Sn4.
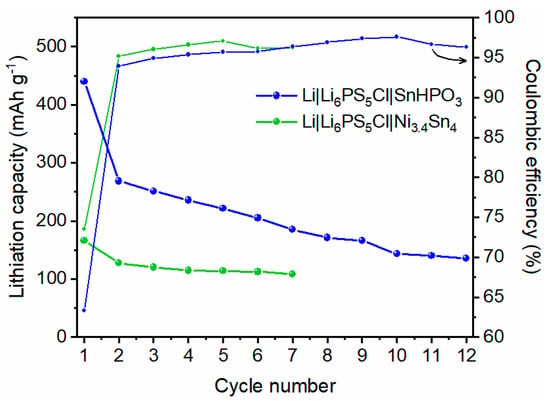
Figure 10.
Evolution of the specific lithiation capacity and coulombic efficiency of Li|Li6PS5Cl|SnHPO3 and Li|Li6PS5Cl|Ni3.4Sn4 solid-state half-cells during galvanostatic cycling at 5 mA g−1.
4. Discussion
In this study, the conversion alloying-based materials SnHPO3 and Ni3.4Sn4 were tested for the first time as anode materials for ASSLiBs using argyrodite Li6PS5Cl as a solid electrolyte. Ball milling yielded nanostructured SnHPO3 (24(1) nm)and Ni3.4Sn4 (7(1) nm) after 1 h and 20 h of milling, respectively. The obtained nanometric primary particles are crucial for ASSLiBs, as they shorten lithium-ion diffusion paths within the active material, minimizing internal resistance.
The morphological analysis of the chlorine argyrodite revealed a ductile nature, facilitating its mechanical interlocking with the active material. Additionally, the solid electrolyte exhibits high ionic conductivity (up to 1.5 mS cm–1) with negligible electronic conductivity. Interestingly, although neutron diffraction data to quantitatively assess the structural disorder are unavailable, this exceptional ionic conductivity is ascribed to the presence of antisite disorder between the 4a and 4c crystallographic sites, which facilitates enhanced lithium-ion mobility by generating additional diffusion pathways. Moreover, these properties were achieved through a simple 10 h ball milling process, offering performance comparable to methods involving extended milling (>24 h) and/or high-temperature sintering (>550 °C). This synthesis approach is, therefore, both efficient and cost-effective, requiring less energy and equipment compared with the reported methods [1,30,37,38]. Furthermore, the symmetric cell exhibited a CCD of 140 μA cm−2, representing a limiting factor for the application of high C-rates in subsequent solid-state half-cells. In this regard, it is important to emphasize that the symmetric cell was cycled without external pressure, as was the case for all manipulations in this study. This condition may lead to local current inhomogeneities, promote dendritic lithium growth, and ultimately accelerate short-circuiting, thereby contributing to the relatively moderate CCD observed [1,2,5,31,35].
The electrochemical performances of SnHPO3 and Ni3.4Sn4 in solid-state half-cells were compared with those in liquid half-cells. The potential profiles for the solid-state half-cells exhibit a trend similar to that of the liquid half-cells, suggesting that both systems undergo the same electrochemical mechanisms. This is an encouraging result, as it indicates that switching from liquid to solid electrolytes does not alter the fundamental electrochemical behavior of the active materials. Moreover, more broadly, the electrochemical profiles are in excellent agreement with those reported for other Sn-based negative electrodes in ASSLiBs [8,15,21]. However, the first lithiation capacities were 440 mAh g−1 for SnHPO3 and 170 mAh g−1 for Ni3.4Sn4, both significantly lower than the capacities achieved in liquid counterparts. It is important to remember that the nanostructured SnHPO3 demonstrated an initial capacity of 1480 mAh g−1 at 108 mA g−1 in a liquid half-cell, attributed not only to the conversion-alloying mechanism reported in Equations (1) and (2), but also to a surface-related supercapacitive contribution and a possible Sn overoxidation upon delithiation [23]. The conversion process is, however, limited in the solid half-cell (0.7 Li+ per SnHPO3) as compared with the liquid half-cell, which involves the exchange of 2.7 Li+ per SnHPO3. In the liquid half-cell, a complete conversion of Sn(II) to Sn(0) occurs, accompanied by the formation of the SEI layer. This process results in the insertion of ~2.7 Li+ during the conversion plateau at potentials exceeding 1.1 V vs. Li+/Li. In contrast, the conversion process is significantly limited in the solid-state half-cell, with only 0.7 Li+ exchanged per SnHPO3 unit and no SEI formation arising from argyrodite degradation. This observation suggests that only a fraction of SnHPO3 (~35%) undergoes conversion to metallic tin, as described by Equation (1). Consequently, the total reversible capacity in the solid-state half-cell is reduced by a factor of three, decreasing from 1480 to 440 mAh g−1. The limited ductility of chlorine argyrodite Li6PS5Cl is seemingly insufficient to accommodate the volume changes associated with the conversion process, thereby resulting in partial conversion.
As for the 20 h-milled Ni3.4Sn4, it delivered an initial lithiation capacity of 546 mAh g−1 at 0.2 C in a liquid half-cell compared with 166 mAh g−1 in the solid-state counterpart. This capacity results from a two-step lithiation process enabled by the conversion-alloying mechanism gathered in Equation (3) [22].
A possible reason for the reduced initial lithiation capacities obtained in the solid-state half-cells may be the low cutoff potential set well above 0 V vs. Li+/Li, a methodical choice made to mitigate the degradation of the Li6PS5Cl argyrodite electrolyte at low potential. This precaution inevitably limits the lithiation extent of SnHPO3 and Ni3.4Sn4, resulting in the anticipated capacity reductions of approximately 40% and 10%, respectively, based on liquid half-cell galvanostatic cycling. However, the solid-state half-cells reveal a more pronounced reduction of approximately 70% for both materials. This finding suggests that the lower cutoff potential cannot be considered the sole factor limiting capacity in the solid-state half-cells. Specifically, the performance gap points to the influence of structural challenges within the composite electrode architecture on the accessible charge storage.
According to this approach, the low initial capacities observed in solid-state half-cells may result from the formation of isolated or disconnected regions of active material within the composite electrode. These disjointed regions hinder the efficient transport of lithium-ions and electrons throughout the composite electrode. Within an ideal composite electrode architecture, interconnected pathways are induced by lithium-ions and electron-conducting phases, leading to efficient charge transfer. Disconnected regions, on the other hand, lead to incomplete conductive pathways, where lithium-ions may be trapped, and electrons may not reach all active sites, resulting in poor utilization of the active material. As part of this investigation, the utilization of both active materials was assessed through SEM/EDX morphological characterization of each composite anode prior to cycling. The SEM images and phase mappings are presented in (Figures S8 and S9). For both electrodes, SEM analysis reveals composite particles consisting of distinct phase domains: the light-toned regions are attributed to the anode active material (SnHPO3 or Ni3.4Sn4) embedded within a dark-grey matrix corresponding to the argyrodite and VGCF matrix. The ductile nature of the argyrodite is evident; however, the anode active material appears as agglomerates. This agglomeration hinders electrochemical percolation within the composite anode, prolonging diffusion paths to the bulk of large particle agglomerates and resulting in portions of the active material being isolated from the argyrodite. Consequently, these isolated regions do not contribute to the overall capacity, leading to reduced electrochemical performance.
The initial coulombic efficiency (irreversible capacity) observed in the solid half-cells is of the same order of magnitude as that of the liquid half-cells. One potential contributor is the degradation of the active materials, which may arise from the substantial volume expansion of Sn during lithiation [20]. This expansion can lead to pulverization, cracking, and stress generation within the active material, which compromises the structural integrity of the electrode, resulting in irreversible disconnection between the electrode and the solid electrolyte. Another plausible factor is the formation of an SEI interphase at the interface between the Li6PS5Cl solid electrolyte and the electrode at low potential [5,39]. The SEI layer is thus composed of the decomposition products of the solid electrolyte consuming lithium and contributing as well to the irreversible capacity.
Upon cycling, both solid-state half-cells exhibit lower reversible capacity compared with their corresponding liquid half-cells. Nonetheless, despite the reduced performance, the reversible capacity of the Li|Li6PS5Cl|Ni3.4Sn4 solid half-cell stabilizes at 110 mAh g−1 with a coulombic efficiency exceeding 97%. In contrast, a more pronounced degradation is observed for the Li|Li6PS5Cl| SnHPO3 solid half-cell, pointing to specific challenges that may arise from the intrinsic properties of SnHPO3. In fact, the more substantial volume changes experienced by SnHPO3 during the lithiation/delithiation process are according to its higher expected capacity. This structural instability can lead to increased mechanical stress, which may loosen the contact between the active material and the solid electrolyte. Moreover, the reduced performance may come from a substantial continuous degradation of Li6PS5Cl triggered by the presence of a higher amount of carbon (10 wt.%) in the composite electrode. This thoughtful choice made for enhancing SnHPO3′s electronic conductivity [23] may have accelerated the decomposition of Li6PS5Cl, as the extent of solid electrolyte degradation correlates with carbon content [40,41]. Furthermore, it is important to note the studied solid-state cells were cycled without external pressure. In the absence of sufficient pressure, the repeated expansion and contraction of the active materials can loosen the contact between the active material and the solid electrolyte, impeding lithium-ion transport and leading to electrode fragmentation. These findings could be further clarified through in-depth morphological characterization of the interface between the argyrodite and the working electrode. Unfortunately, this was not possible in the current study, as the bilayer pellet architecture prevents post-mortem analysis without compromising the integrity of the system. Future studies focusing on the evolution of solid-solid interfaces represent a promising direction for further investigation.
5. Conclusions
This study demonstrates the first investigation of conversion/alloying materials SnHPO3 and Ni3.4Sn4 as potential anode materials for ASSLiBs employing Li6PS5Cl as the solid electrolyte. The ball milling produced nanostructured materials (24(1) nm for SnHPO3 and 7(1) nm for Ni3.4Sn4), enhancing lithium-ion diffusion and reducing mechanical stress in the composite electrode. Despite the similarity in electrochemical reaction mechanisms to those observed in liquid half-cells, the extent of lithiation in solid-state half-cells remains limited, yielding initial capacities of 440 mAh g−1 (SnHPO3) and 170 mAh g−1 (Ni3.4Sn4). The reaction mechanism is the same as the liquid cells can be operated upon without any external pressure; the extent of the lithiation reaction is lower for solid cells with initial capacities of 440 mAh g−1 (SnHPO3) and 170 mAh g−1 (Ni3.4Sn4). This capacity reduction goes beyond the limited low-potential cutoff, underlining additional limitations such as the agglomeration of the active material within the electrode, leading to isolated regions that impede lithium-ion transport. Mechanical degradation, particularly with SnHPO3, further exacerbates these drawbacks. With its higher lithiation capacity, SnHPO3 undergoes higher volume changes during cycling, generating substantial stress.
These results, obtained without the application of external pressure, highlight the need to optimize pressure conditions to prevent electrode fragmentation and ensure optimal interfacial contact between the active material and the solid electrolyte. Future studies should focus on refining electrode architecture to enhance the electrochemical performance and long-term stability of Sn-based anodes in ASSLiBs. Additionally, the lower cutoff potential should be carefully adjusted to allow sufficient lithiation while maintaining the electrochemical stability of the argyrodite at low potential. Further exploration of alternative composite electrode preparation methods, such as high-energy ball milling with controlled parameters or chemical routes, could refine the particle size distribution between the active material and the argyrodite solid electrolyte, thereby improving electrochemical kinetics. These research directions provide a framework for future investigations, fostering the advancement of Sn-based anodes for next-generation ASSLiBs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano15070512/s1, Figure S1: Rietveld refinement of XRD pattern of non-milled Ni3+xSn4. Table S1: Refined crystallographic data of non-milled Ni3+xSn4. Figure S2: Evolution of (a) XRD patterns and (b) crystallite size of Ni3.4Sn4 with milling time. Table S2: Evolution of refined cell parameters and crystallite sizes of Ni3.4Sn4 with milling time. Figure S3: (a) Nyquist plots at different temperatures with the corresponding equivalent circuit, (b) Enlarged Nyquist plots at high frequencies at 25 °C and 80 °C, and (c) current evolution under 0.6 V vs. Li+/Li polarization of the Li6PS5Cl chlorine argyrodite. Figure S4: Magnification of the voltage responses of the Li| Li6PS5Cl |Li symmetric cell under a constant current density of 35 μA cm−2 with 4 h per step. Figure S5: Rietveld refinement of XRD patterns of 1:1 in weight of (a) SnHPO3: Li6PS5Cl and (b) Ni3.4Sn4: Li6PS5Cl composites. Table S3: Refined structural parameters by Rietveld method of composites (1:1 in weight SnHPO3: Li6PS5Cl and Ni3.4Sn4:Li6PS5Cl). Figure S6: Comparison of the 4th galvanostatic profiles in solid and liquid half-cells for (a) SnHPO3 and (b) Ni3.4Sn4 active materials. Figure S7: Galvanostatic discharge/charge profiles of (a) Li|Li6PS5Cl|SnHPO3 and (b) Li|Li6PS5Cl|Ni3.4Sn4 solid-state half-cells. Figure S8: (a) Back-scattered electron micrograph and elemental mapping for SnHPO3: Li6PS5Cl:VGCF/40:50:10 wt.% with (b) Sn (L-edge), (c) S (K-edge), and (d) C (K-edge). Figure S9: (a) Back-scattered electron micrograph and elemental mapping for Ni3.4Sn4: Li6PS5Cl:VGCF/78:20:2 wt.% with (b) Sn (L-edge), (c) S (K-edge), and (d) C (K-edge). (See Refs. [24,30,35,42]).
Author Contributions
Conceptualization, W.T., F.C., M.M., F.C., Z.E. and J.Z.; methodology, W.T. and J.Z.; validation, W.T., M.M. and J.Z.; formal analysis, W.T., M.M., M.O. and F.C.E.M.; investigation, W.T., M.M., J.Z. and M.O.; resources, F.C., Z.E. and J.Z.; data curation, W.T.; writing—original draft preparation, W.T.; writing—review and editing, W.T., F.C., M.M., Z.E., M.O., F.C.E.M. and J.Z.; visualization, W.T.; supervision, F.C., M.M., Z.E., F.C.E.M. and J.Z.; project administration, F.C., Z.E. and J.Z.; funding acquisition, F.C., Z.E. and J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the partnership Hubert Curien PHC Toubkal, via Campus France, under project TBK 21-126.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
WT is grateful to Tahar Azib for LiBs electrochemistry guidelines, Valérie Lalanne for the technical support, and Remy Pires Brazuna for SEM/EDX analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yu, C.; Zhao, F.; Luo, J.; Zhang, L.; Sun, X. Recent Development of Lithium Argyrodite Solid-State Electrolytes for Solid-State Batteries: Synthesis, Structure, Stability and Dynamics. Nano Energy 2021, 83, 105858. [Google Scholar] [CrossRef]
- Wang, D.; Shi, H.; Cui, W.; Li, H.; Niu, J.; Wang, S.; Xu, Z. Li-Argyrodite Solid-State Electrolytes with Lithium Compatibility and Air Stability for All-Solid-State Batteries. J. Mater. Chem. A 2024, 12, 10863–10874. [Google Scholar] [CrossRef]
- Zoubir, O.; Lallaoui, A.; Edfouf, Z. Electrolyte Additives for Bulk-Type All-Oxide Solid-State Lithium-Ion Batteries. J. Energy Storage 2024, 104, 114592. [Google Scholar] [CrossRef]
- Wu, J.; Shen, L.; Zhang, Z.; Liu, G.; Wang, Z.; Zhou, D.; Wan, H.; Xu, X.; Yao, X. All-Solid-State Lithium Batteries with Sulfide Electrolytes and Oxide Cathodes. Electrochem. Energy Rev. 2021, 4, 101–135. [Google Scholar] [CrossRef]
- Park, J.-S.; Jo, C.-H.; Myung, S.-T. Comprehensive Understanding on Lithium Argyrodite Electrolytes for Stable and Safe All-Solid-State Lithium Batteries. Energy Storage Mater. 2023, 61, 102869. [Google Scholar] [CrossRef]
- Divakaran, A.M.; Minakshi, M.; Bahri, P.A.; Paul, S.; Kumari, P.; Divakaran, A.M.; Manjunatha, K.N. Rational Design on Materials for Developing next Generation Lithium-Ion Secondary Battery. Prog. Solid State Chem. 2021, 62, 100298. [Google Scholar] [CrossRef]
- Divakaran, A.M.; Hamilton, D.; Manjunatha, K.N.; Minakshi, M. Design, Development and Thermal Analysis of Reusable Li-Ion Battery Module for Future Mobile and Stationary Applications. Energies 2020, 13, 1477. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, Q.; Martínez-Alanis, P.R.; Chen, G.; Li, J.; Zeng, G.; Biendicho, J.J.; Ci, L.; Cabot, A. Towards Flame Retardant High-Performance Solid-State Lithium Metal Batteries: Poly(Ionic Liquid)-Based Lithiophilic Ion-Conductive Interfaces and Humidity Tolerant Binders. Nano Energy 2025, 133, 110424. [Google Scholar] [CrossRef]
- Sung, J.; Kim, S.Y.; Harutyunyan, A.; Amirmaleki, M.; Lee, Y.; Son, Y.; Li, J. Ultra-Thin Lithium Silicide Interlayer for Solid-State Lithium-Metal Batteries. Adv. Mater. 2023, 35, 2210835. [Google Scholar] [CrossRef]
- Liang, J.; Li, X.; Zhao, Y.; Goncharova, L.V.; Wang, G.; Adair, K.R.; Wang, C.; Li, R.; Zhu, Y.; Qian, Y.; et al. In Situ Li3PS4 Solid-State Electrolyte Protection Layers for Superior Long-Life and High-Rate Lithium-Metal Anodes. Adv. Mater. 2018, 30, 1804684. [Google Scholar] [CrossRef]
- Palaniselvam, T.; Freytag, A.I.; Moon, H.; Janßen, K.A.; Passerini, S.; Adelhelm, P. Tin–Graphite Composite as a High-Capacity Anode for All-Solid-State Li-Ion Batteries. J. Phys. Chem. C 2022, 126, 13043–13052. [Google Scholar] [CrossRef]
- Kreissl, J.J.A.; Dang, H.A.; Mogwitz, B.; Rohnke, M.; Schröder, D.; Janek, J. Implementation of Different Conversion/Alloy Active Materials as Anodes for Lithium-Based Solid-State Batteries. ACS Appl. Mater. Interfaces 2024, 16, 26195–26208. [Google Scholar] [CrossRef] [PubMed]
- Ueda, A.; Nagao, M.; Inoue, A.; Hayashi, A.; Seino, Y.; Ota, T.; Tatsumisago, M. Electrochemical Performance of All-Solid-State Lithium Batteries with Sn4P3 Negative Electrode. J. Power Sources 2013, 244, 597–600. [Google Scholar] [CrossRef]
- Sun, Q.; Zeng, G.; Xu, X.; Li, J.; Biendicho, J.J.; Wang, S.; Tian, Y.; Ci, L.; Cabot, A. Are Sulfide-Based Solid-State Electrolytes the Best Pair for Si Anodes in Li-Ion Batteries? Adv. Energy Mater. 2024, 14, 2402048. [Google Scholar] [CrossRef]
- Miyazaki, R.; Hihara, T. Charge-Discharge Performances of Sn Powder as a High-Capacity Anode for All-Solid-State Lithium Batteries. J. Power Sources 2019, 427, 15–20. [Google Scholar] [CrossRef]
- Huang, Y.; Shao, B.; Wang, Y.; Han, F. Solid-State Silicon Anode with Extremely High Initial Coulombic Efficiency. Energy Environ. Sci. 2023, 16, 1569–1580. [Google Scholar] [CrossRef]
- Li, J.; Su, H.; Liu, Y.; Zhong, Y.; Wang, X.; Tu, J. Li Alloys in All Solid-State Lithium Batteries: A Review of Fundamentals and Applications. Electrochem. Energy Rev. 2024, 7, 18. [Google Scholar] [CrossRef]
- Bezza, I.; Trouillet, V.; Fiedler, A.; Bruns, M.; Indris, S.; Ehrenberg, H.; Saadoune, I. Understanding the Lithiation/Delithiation Process in SnP2O7 Anode Material for Lithium-Ion Batteries. Electrochim. Acta 2017, 252, 446–452. [Google Scholar] [CrossRef]
- Jeong, W.J.; Wang, C.; Yoon, S.G.; Liu, Y.; Chen, T.; McDowell, M.T. Electrochemical Behavior of Elemental Alloy Anodes in Solid-State Batteries. ACS Energy Lett. 2024, 9, 2554–2563. [Google Scholar] [CrossRef]
- Ehinon, K.K.D.; Naille, S.; Dedryvère, R.; Lippens, P.-E.; Jumas, J.-C.; Gonbeau, D. Ni3Sn4 Electrodes for Li-Ion Batteries: Li−Sn Alloying Process and Electrode/Electrolyte Interface Phenomena. Chem. Mater. 2008, 20, 5388–5398. [Google Scholar] [CrossRef]
- Wu, X.; Billaud, J.; Jerjen, I.; Marone, F.; Ishihara, Y.; Adachi, M.; Adachi, Y.; Villevieille, C.; Kato, Y. Operando Visualization of Morphological Dynamics in All-Solid-State Batteries. Adv. Energy Mater. 2019, 9, 1901547. [Google Scholar] [CrossRef]
- Edfouf, Z.; Fariaut-Georges, C.; Cuevas, F.; Latroche, M.; Hézèque, T.; Caillon, G.; Jordy, C.; Sougrati, M.T.; Jumas, J.C. Nanostructured Ni3.5Sn4 Intermetallic Compound: An Efficient Buffering Material for Si-Containing Composite Anodes in Lithium Ion Batteries. Electrochim. Acta 2013, 89, 365–371. [Google Scholar] [CrossRef]
- Tout, W.; Mateos, M.; Zhang, J.; Oubla, M.; Emery, N.; Leroy, E.; Dubot, P.; El Moursli, F.C.; Edfouf, Z.; Cuevas, F. Unraveling the energy storage mechanism in nanostructured SnHPO3 anode through advanced operando and ex-situ characterizations. J. Energy Storage 2025, 112, 115502. [Google Scholar] [CrossRef]
- Furuseth, S.; Fjellvag, H. Structural Properties of Ni3+xSn4. Acta Chem. Scand. A 1986, 40, 695–700. [Google Scholar]
- Schmetterer, C.; Flandorfer, H.; Richter, K.W.; Saeed, U.; Kauffman, M.; Roussel, P.; Ipser, H. A New Investigation of the System Ni–Sn. Intermetallics 2007, 15, 869–884. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent Advances in Magnetic Structure Determination by Neutron Powder Diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Ares, J.R.; Cuevas, F.; Percheron-Guégan, A. Mechanical Milling and Subsequent Annealing Effects on the Microstructural and Hydrogenation Properties of Multisubstituted LaNi5 Alloy. Acta Mater. 2005, 53, 2157–2167. [Google Scholar] [CrossRef]
- McDonald, R.C.; Eriks, K. Crystallographic Studies of Tin(II) Compounds. 2. Structures of Tin(II) Hydrogen Phosphate and Tin(II) Phosphite, SnHPO4 and SnHPO3. Inorg. Chem. 1980, 19, 1237–1241. [Google Scholar] [CrossRef]
- Rao, R.P.; Adams, S. Studies of Lithium Argyrodite Solid Electrolytes for All-Solid-State Batteries. Phys. Status Solidi (A) 2011, 208, 1804–1807. [Google Scholar] [CrossRef]
- Boulineau, S.; Courty, M.; Tarascon, J.-M.; Viallet, V. Mechanochemical Synthesis of Li-Argyrodite Li6PS5X (X = Cl, Br, I) as Sulfur-Based Solid Electrolytes for All Solid-State Batteries Application. Solid State Ion. 2012, 221, 1–5. [Google Scholar] [CrossRef]
- Liu, Y.; Peng, H.; Su, H.; Zhong, Y.; Wang, X.; Xia, X.; Gu, C.; Tu, J. Ultrafast Synthesis of I-Rich Lithium Argyrodite Glass–Ceramic Electrolyte with High Ionic Conductivity. Adv. Mater. 2022, 34, 2107346. [Google Scholar] [CrossRef] [PubMed]
- De Klerk, N.J.; Rosłoń, I.; Wagemaker, M. Diffusion mechanism of Li argyrodite solid electrolytes for Li-ion batteries and prediction of optimized halogen doping: The effect of Li vacancies, halogens, and halogen disorder. Chem. Mater. 2016, 28, 7955–7963. [Google Scholar] [CrossRef]
- Kraft, M.A.; Culver, S.P.; Calderon, M.; Böcher, F.; Krauskopf, T.; Senyshyn, A.; Dietrich, C.; Zevalkink, A.; Janek, J.; Zeier, W.G. Influence of Lattice Polarizability on the Ionic Conductivity in the Lithium Superionic Argyrodites Li6PS5X (X = Cl, Br, I). J. Am. Chem. Soc. 2017, 139, 10909–10918. [Google Scholar] [CrossRef] [PubMed]
- Hanghofer, I.; Gadermaier, B.; Wilkening, H.M.R. Fast Rotational Dynamics in Argyrodite-Type Li6PS5X (X: Cl, Br, I) as Seen by 31P Nuclear Magnetic Relaxation-On Cation–Anion Coupled Transport in Thiophosphates. Chem. Mater. 2019, 31, 4591–4597. [Google Scholar] [CrossRef]
- Peng, L.; Yu, C.; Zhang, Z.; Ren, H.; Zhang, J.; He, Z.; Yu, M.; Zhang, L.; Cheng, S.; Xie, J. Chlorine-Rich Lithium Argyrodite Enabling Solid-State Batteries with Capabilities of High Voltage, High Rate, Low-Temperature and Ultralong Cyclability. Chem. Eng. J. 2022, 430, 132896. [Google Scholar] [CrossRef]
- Tan, D.H.S.; Wu, E.A.; Nguyen, H.; Chen, Z.; Marple, M.A.T.; Doux, J.-M.; Wang, X.; Yang, H.; Banerjee, A.; Meng, Y.S. Elucidating Reversible Electrochemical Redox of Li6PS5Cl Solid Electrolyte. ACS Energy Lett. 2019, 4, 2418–2427. [Google Scholar] [CrossRef]
- Haruna, A.B.; Mofokeng, T.P.; Ogada, J.J.; Zoubir, O.; Lallaoui, A.; El Moursli, F.C.; Ozoemena, K.I. Recent advances in the cathode materials and solid-state electrolytes for lithium sulfur batteries. Electrochem. Commun. 2022, 136, 107248. [Google Scholar] [CrossRef]
- Lee, H.; Oh, P.; Kim, J.; Cha, H.; Chae, S.; Lee, S.; Cho, J. Advances and Prospects of Sulfide All-Solid-State Lithium Batteries via One-to-One Comparison with Conventional Liquid Lithium-Ion Batteries. Adv. Mater. 2019, 31, 1900376. [Google Scholar] [CrossRef]
- Liang, Z.; Xiang, Y.; Wang, K.; Zhu, J.; Jin, Y.; Wang, H.; Zheng, B.; Chen, Z.; Tao, M.; Liu, X.; et al. Understanding the Failure Process of Sulfide-Based All-Solid-State Lithium Batteries via Operando Nuclear Magnetic Resonance Spectroscopy. Nat. Commun. 2023, 14, 259. [Google Scholar] [CrossRef]
- Zhang, W.; Leichtweiß, T.; Culver, S.P.; Koerver, R.; Das, D.; Weber, D.A.; Janek, J. The Detrimental Effects of Carbon Additives in Li10GeP2S12-Based Solid-State Batteries. ACS Appl. Mater. Interfaces 2017, 9, 35888–35896. [Google Scholar] [CrossRef]
- Tan, D.H.S.; Chen, Y.-T.; Yang, H.; Bao, W.; Sreenarayanan, B.; Doux, J.-M.; Li, W.; Lu, B.; Ham, S.-Y.; Sayahpour, B.; et al. Carbon-Free High-Loading Silicon Anodes Enabled by Sulfide Solid Electrolytes. Science 2021, 373, 1494–1499. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).