Linking Structure to Electrocatalytic Performance: Graphene Nanoplatelets-Derived Novel Mixed Oxide–Carbon Composites as Supports for Pt Electrocatalysts with Enhanced Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Composite Supports and Supported Pt Electrocatalysts
2.3. Physicochemical Characterization of Composite Supports and Supported Pt Electrocatalysts
2.4. Electrochemical Characterization
3. Results
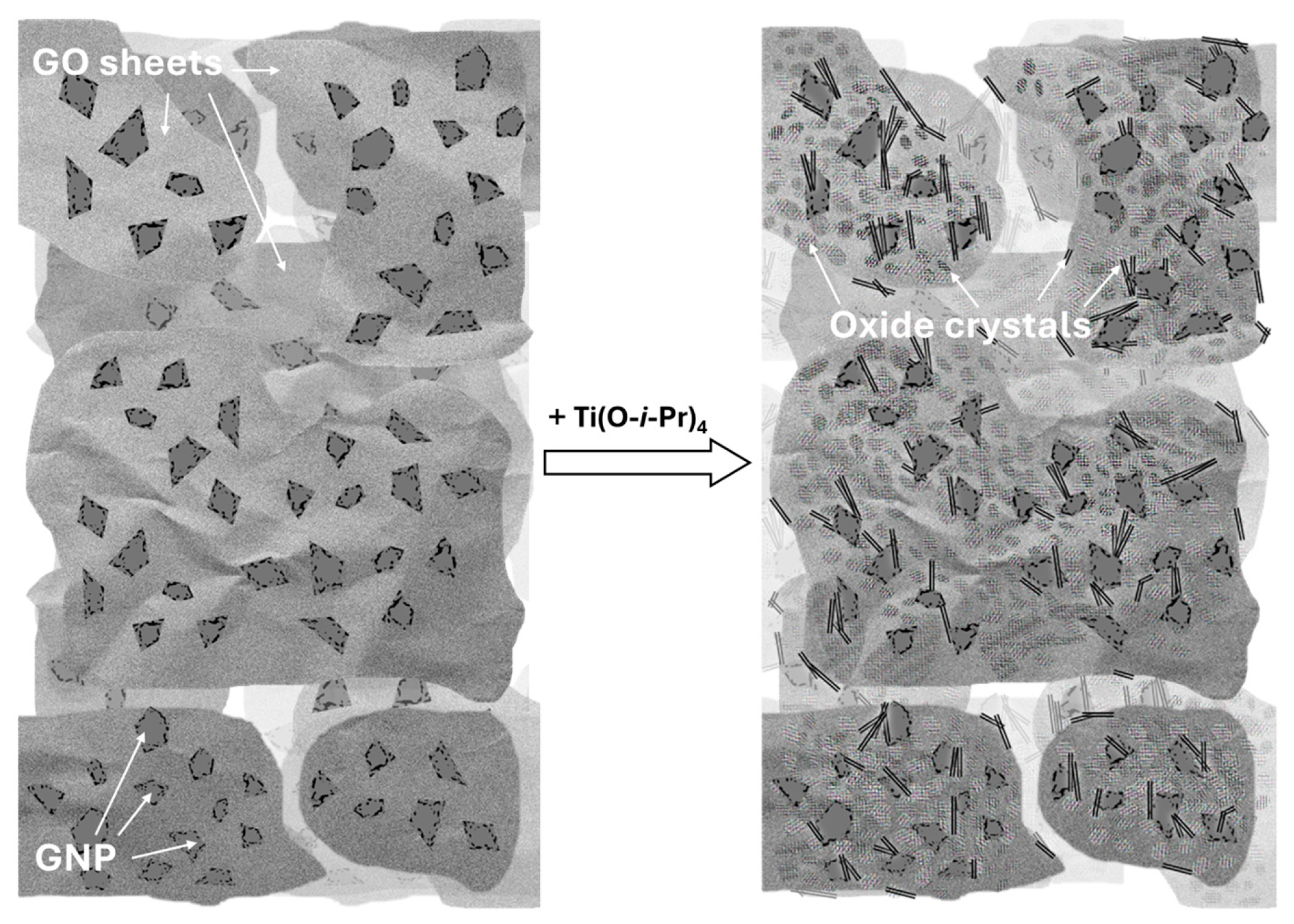
3.1. Preliminary Results Obtained by Use of GNPs from Different Sources
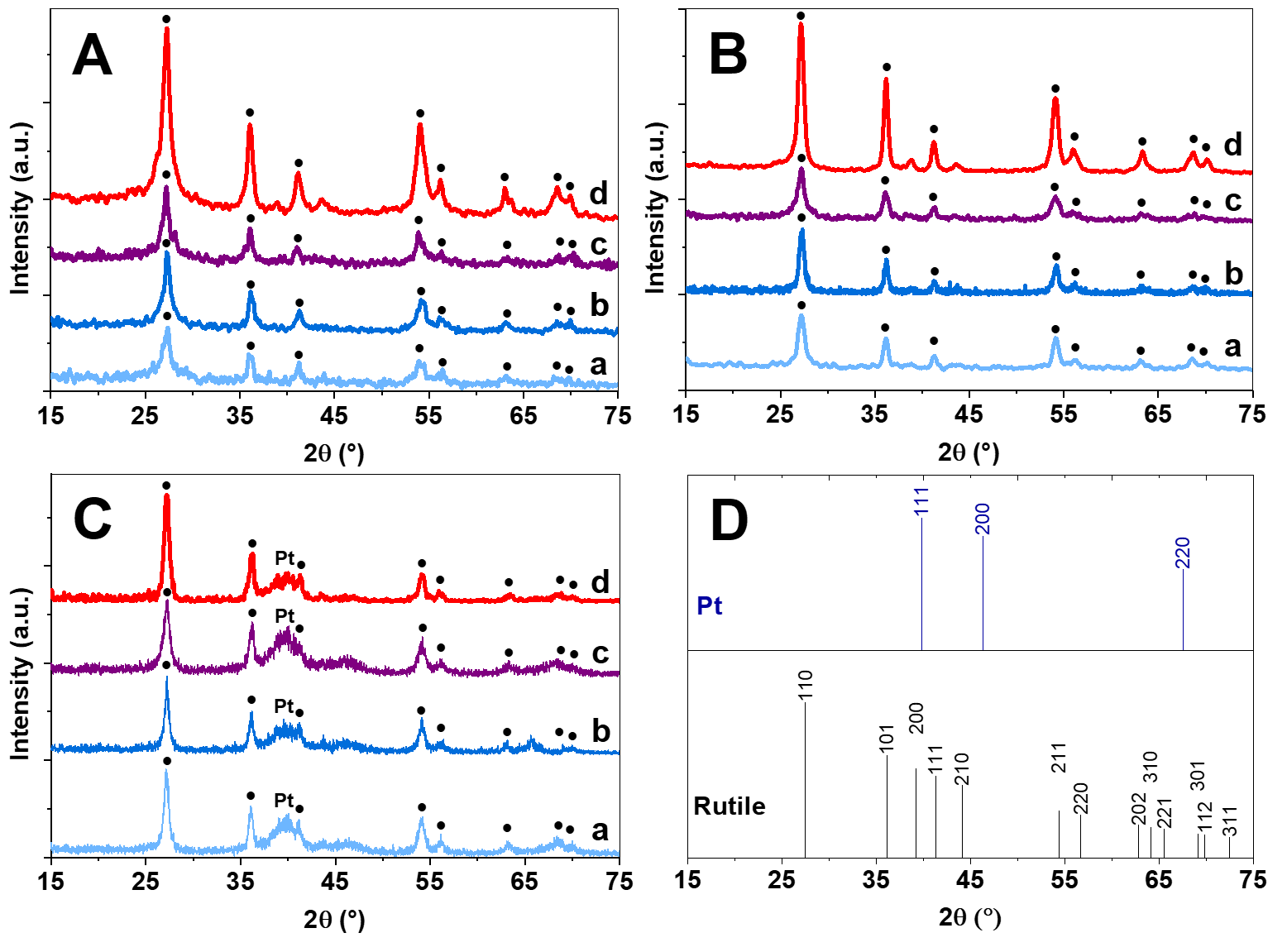
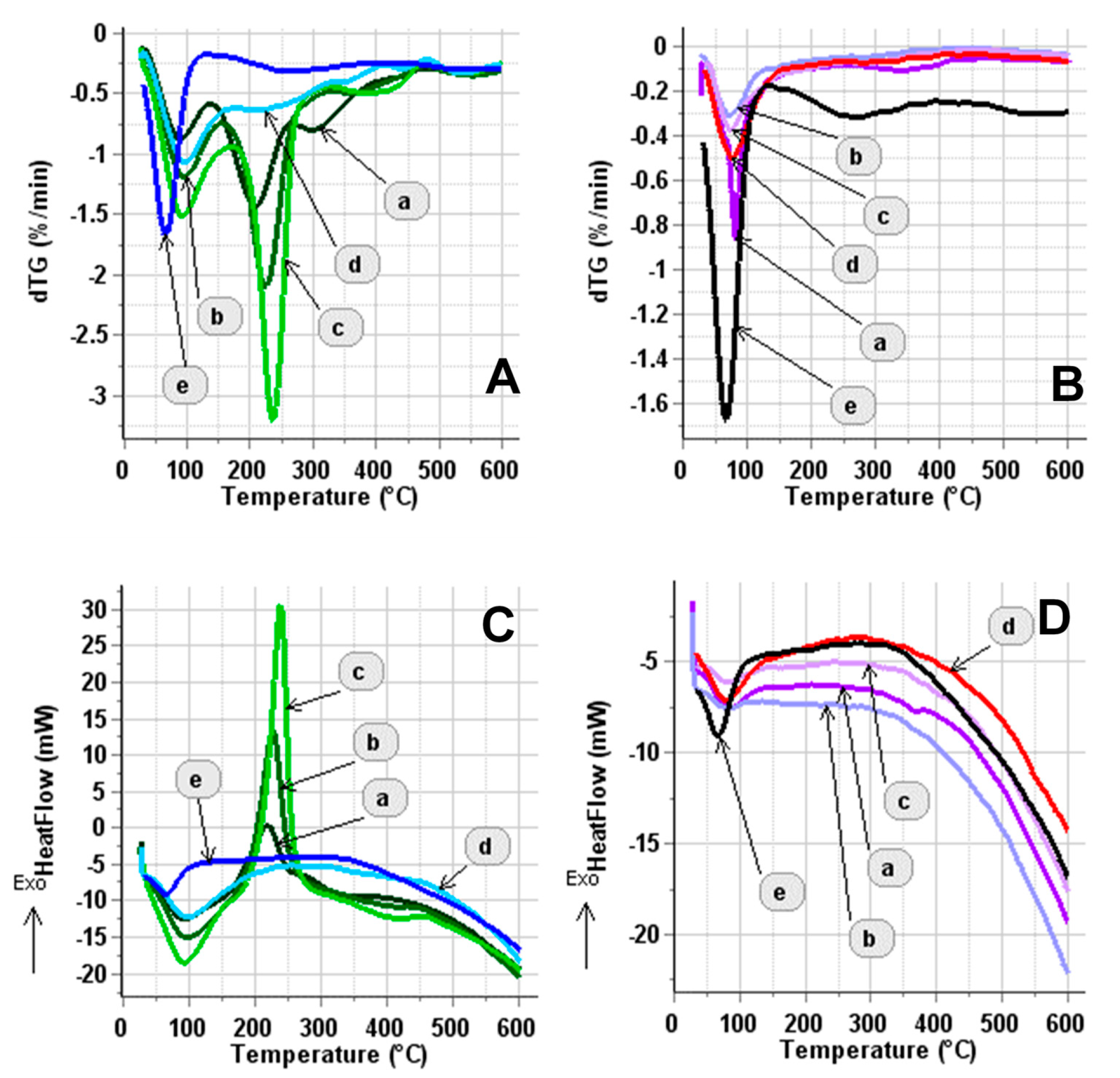
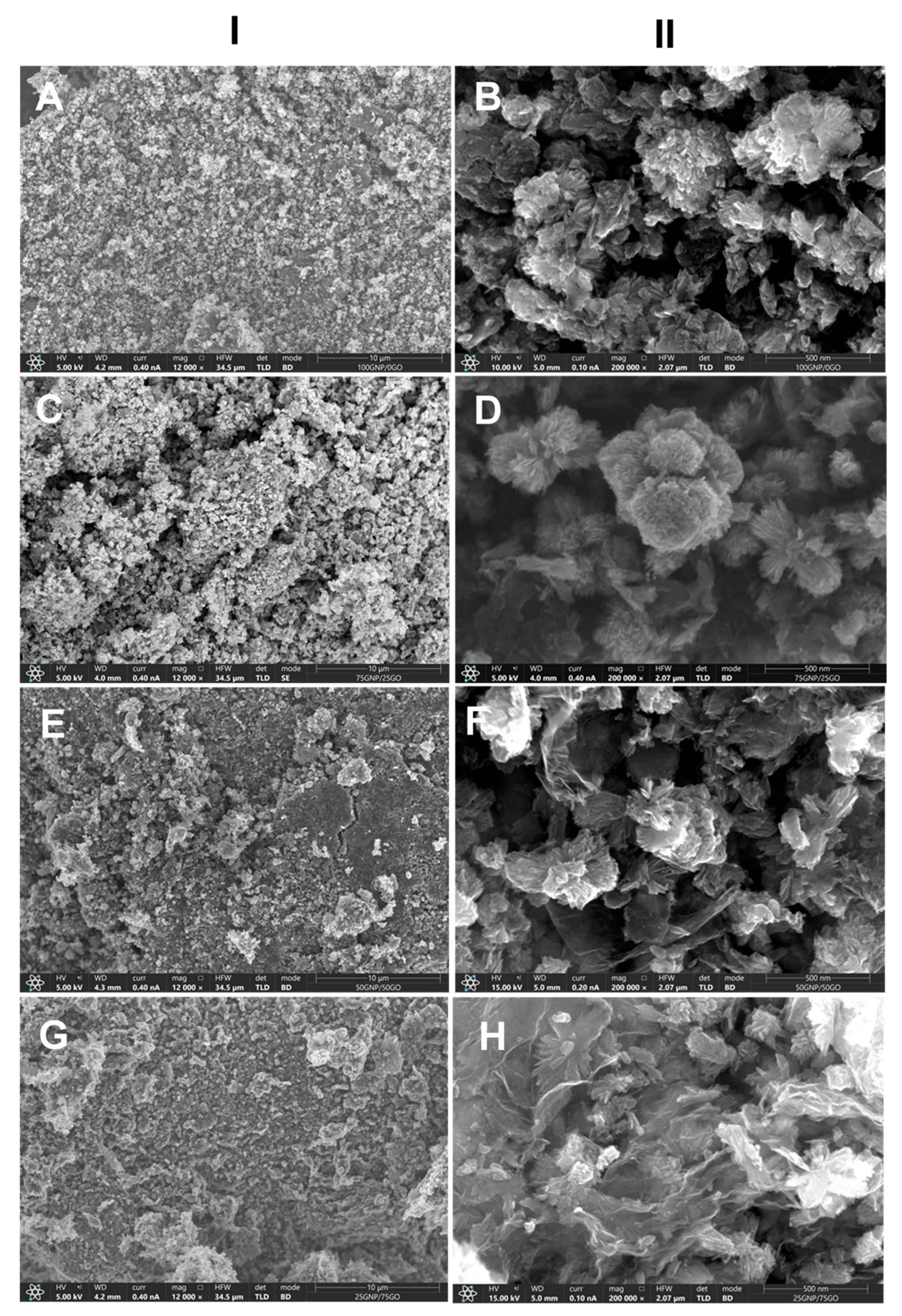
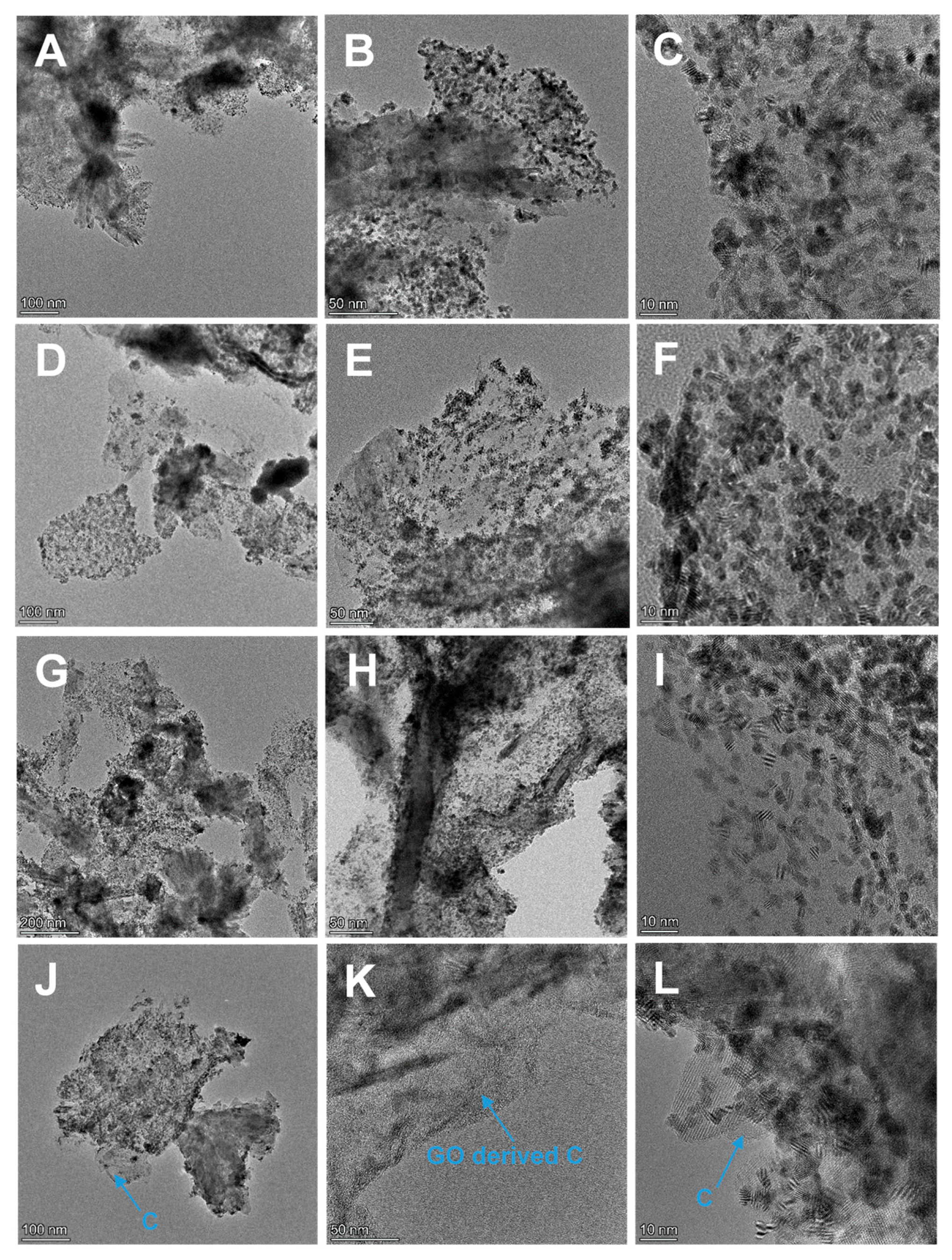
3.2. Physicochemical Characterization of Composite Supports and Supported Pt Electrocatalysts
3.2.1. Characterization of Composite Type of Catalyst Supports
3.2.2. Characterization of Composite-Supported Platinum Catalyst
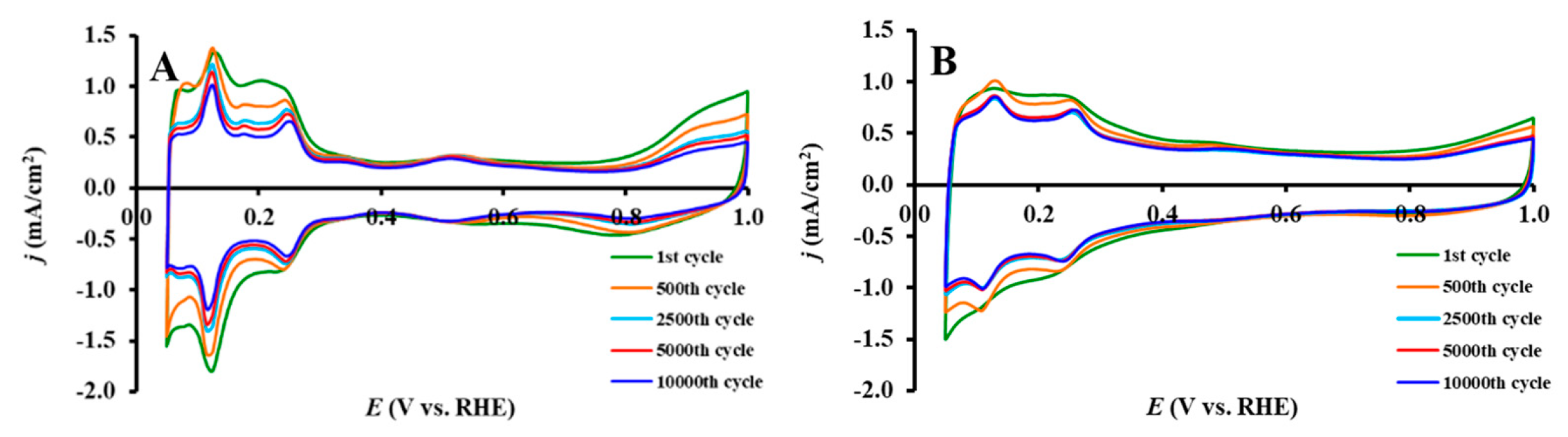
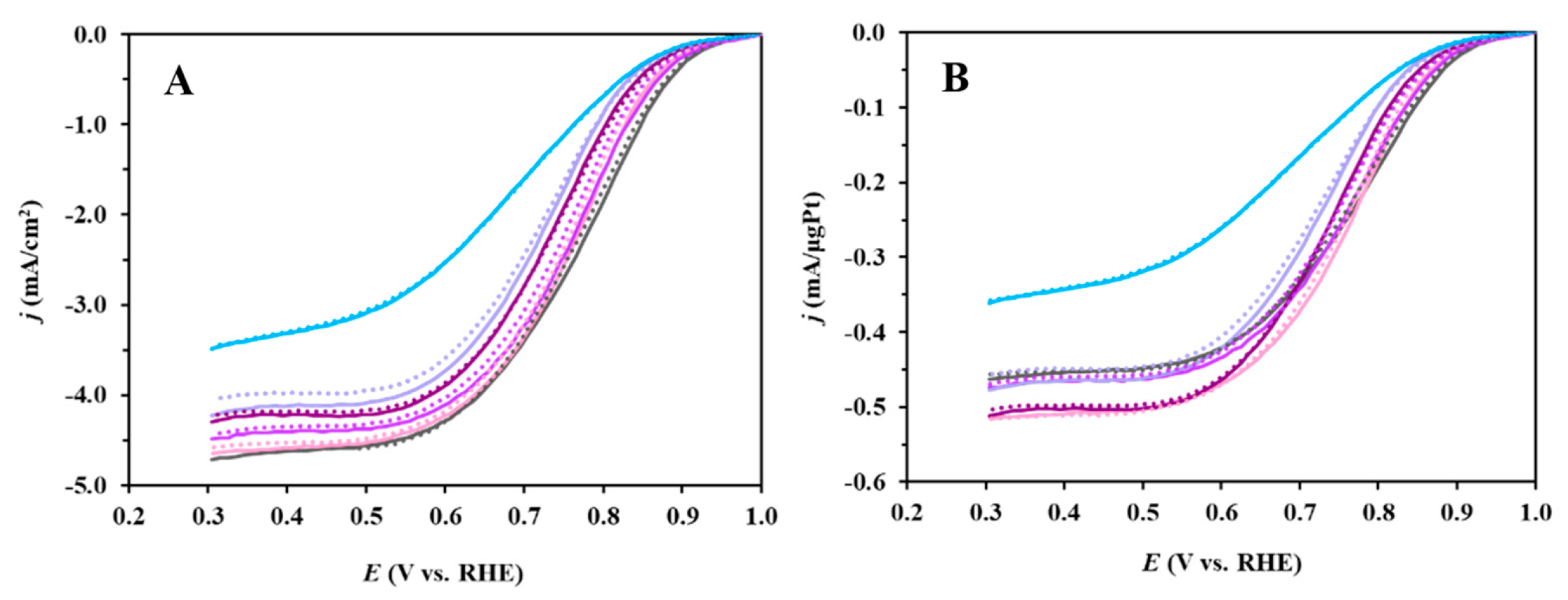
3.3. Electrochemical Behavior of Pt/Ti(1−x)MoxO2-C Composite Type of Catalysts Derived from GNPs-GO Mixtures with Different GNPs/GO Ratios
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agyekum, E.B.; Odoi-Yorke, F.; Abbey, A.A.; Ayetor, G.K. A Review of the Trends, Evolution, and Future Research Prospects of Hydrogen Fuel Cells—A Focus on Vehicles. Int. J. Hydrogen Energy 2024, 72, 918–939. [Google Scholar] [CrossRef]
- Kwon, L.; Kang, J.; Don, K.; Kim, K.; Ahn, C. Advancement and Applications of PEMFC Energy Systems for Large-Class Unmanned Underwater Vehicles: A Review. Int. J. Hydrogen Energy 2024, 79, 277–294. [Google Scholar] [CrossRef]
- Gögebakan, A.B.; Sertsöz, M. The Future of Sustainable Transit: Hydrogen-Powered Trams and PEMFCS. Energy Rep. 2025, 13, 4917–4925. [Google Scholar] [CrossRef]
- Zhang, J. PEM Fuel Cell Electrocatalysts and Catalyst Layers: Fundamentals and Applications; Springer: London, UK, 2008; ISBN 9781848009356. [Google Scholar]
- Okonkwo, P.C.; Ige, O.O.; Barhoumi, E.M.; Uzoma, P.C.; Emori, W.; Benamor, A.; Abdullah, A.M. Platinum Degradation Mechanisms in Proton Exchange Membrane Fuel Cell (PEMFC) System: A Review. Int. J. Hydrogen Energy 2021, 46, 15850–15865. [Google Scholar] [CrossRef]
- Borup, R.L.; Kusoglu, A.; Neyerlin, K.C.; Mukundan, R.; Ahluwalia, R.K.; Cullen, D.A.; More, K.L.; Weber, A.Z.; Myers, D.J. Recent Developments in Catalyst-Related PEM Fuel Cell Durability. Curr. Opin. Electrochem. 2020, 21, 192–200. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, L.; Wang, Y.; Sun, G.; You, J.; Li, T. Multiscale Study of Carbon Corrosion and Pt Degradation in the Cathode Catalyst Layer and Their Effects on PEMFC Performance Decay over 5000 h Operation. Chem. Eng. J. 2025, 522, 167960. [Google Scholar] [CrossRef]
- Niu, F.; Cao, J.; Chen, H.; Shen, S. Recent Advance and Perspectives on CO Tolerant Platinum-Based Alloys in PEMFC Anodes. EnergyChem 2025, 7, 100158. [Google Scholar] [CrossRef]
- Meier, J.C.; Galeano, C.; Katsounaros, I.; Witte, J.; Bongard, H.J.; Topalov, A.A.; Baldizzone, C.; Mezzavilla, S.; Schüth, F.; Mayrhofer, K.J.J. Design Criteria for Stable Pt/C Fuel Cell Catalysts. Beilstein J. Nanotechnol. 2014, 5, 44–67. [Google Scholar] [CrossRef]
- Meier, J.C.; Galeano, C.; Katsounaros, I.; Topalov, A.A.; Kostka, A.; Schu, F.; Mayrhofer, K.J.J.J.; Schüth, F.; Mayrhofer, K.J.J.J. Degradation Mechanisms of Pt/C Fuel Cell Catalysts under Simulated Start-Stop Conditions. ACS Catal. 2012, 2, 832–843. [Google Scholar] [CrossRef]
- Liu, F.; Ji, X.; Gao, Z.; Jiang, J.; Li, Y.; Guo, L. Mechanistic Insights into Anode Gas Exchange Characterization and Carbon Corrosion Mitigation during PEMFC Startup/Shutdown. Int. J. Hydrogen Energy 2025, 172, 151183. [Google Scholar] [CrossRef]
- Valdés-López, V.F.; Mason, T.; Shearing, P.R.; Brett, D.J.L. Carbon Monoxide Poisoning and Mitigation Strategies for Polymer Electrolyte Membrane Fuel Cells—A Review. Prog. Energy Combust. Sci. 2020, 79, 100842. [Google Scholar] [CrossRef]
- Zhao, J.; Tu, Z.; Chan, S.H. Carbon Corrosion Mechanism and Mitigation Strategies in a Proton Exchange Membrane Fuel Cell (PEMFC): A Review. J. Power Sources 2021, 488, 229434. [Google Scholar] [CrossRef]
- Vafeas, N.A.; Slezak, P.; Hitzman, M.W. Analysis of Critical Raw Materials Policy for Electrical and Electronic Equipment: Planning for a Truly Circular Economy. Resour. Policy 2024, 99, 105380. [Google Scholar] [CrossRef]
- Eikeng, E.; Makhsoos, A.; Pollet, B.G. Critical and Strategic Raw Materials for Electrolysers, Fuel Cells, Metal Hydrides and Hydrogen Separation Technologies. Int. J. Hydrogen Energy 2024, 71, 433–464. [Google Scholar] [CrossRef]
- Leader, A.; Gaustad, G.; Babbitt, C. The Effect of Critical Material Prices on the Competitiveness of Clean Energy Technologies. Mater. Renew. Sustain. Energy 2019, 8, 8. [Google Scholar] [CrossRef]
- Borbáth, I.; Tálas, E.; Pászti, Z.; Zelenka, K.; Ayyubov, I.; Salmanzade, K.; Sajó, I.E.; Sáfrán, G.; Tompos, A. Investigation of Ti-Mo Mixed Oxide-Carbon Composite Supported Pt Electrocatalysts: Effect of the Type of Carbonaceous Materials. Appl. Catal. A Gen. 2021, 620, 118155. [Google Scholar] [CrossRef]
- Ayyubov, I.; Tálas, E.; Borbáth, I.; Pászti, Z.; Silva, C.; Szegedi, Á.; Kuncser, A.; Yazici, M.S.; Sajó, I.E.; Szabó, T.; et al. Composites of Titanium–Molybdenum Mixed Oxides and Non-Traditional Carbon Materials: Innovative Supports for Platinum Electrocatalysts for Polymer Electrolyte Membrane Fuel Cells. Nanomaterials 2024, 14, 1053. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Liu, Y.; Wang, X.; Kang, L.; Qiu, T. Graphene-Based Advanced Materials for Energy Storage and Conversion Systems: Progress, Challenges, and Commercial Future. Appl. Energy 2025, 386, 125566. [Google Scholar] [CrossRef]
- Yu, W.; Liu, J.; Lei, H.; Zhou, L.; Cai, W.; Lu, L. Densely Deposited Pt Nanoparticles on Activated Carbon Nanotubes for Improving Oxygen Reduction Reaction Activity and Stability. Next Mater. 2025, 6, 100473. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, G.; Yang, Y.; Song, W.; Yu, H. PtIr/CNT as Anode Catalyst with High Reversal Tolerance in PEMFC. Int. J. Hydrogen Energy 2023, 48, 36500–36511. [Google Scholar] [CrossRef]
- Sahoo, M.; Scott, K.; Ramaprabhu, S. Platinum Decorated on Partially Exfoliated Multiwalled Carbon Nanotubes as High Performance Cathode Catalyst for PEMFC. Int. J. Hydrogen Energy 2015, 40, 9435–9443. [Google Scholar] [CrossRef]
- Lee, I.; Bong, J.; Shokouhimehr, M. Graphene Derivatives Supported Nanocatalysts for Oxygen Reduction Reaction. Chin. J. Catal. 2015, 36, 1799–1810. [Google Scholar] [CrossRef]
- Zahir, M.; Siddique, S.; Khan, A.; Shabhi, S. Recent Developments in Graphene Based Novel Structures for e Ffi Cient and Durable Fuel Cells. Mater. Res. Bull. 2020, 122, 110674. [Google Scholar] [CrossRef]
- Daş, E.; Alkan Gürsel, S.; Bayrakçeken Yurtcan, A. Pt-Alloy Decorated Graphene as an Efficient Electrocatalyst for PEM Fuel Cell Reactions. J. Supercrit. Fluids 2020, 165, 104962. [Google Scholar] [CrossRef]
- Devrim, Y.; Arıca, E.D.; Albostan, A. Graphene Based Catalyst Supports for High Temperature PEM Fuel Cell Application. Int. J. Hydrogen Energy 2018, 43, 11820–11829. [Google Scholar] [CrossRef]
- Şanli, L.I.; Bayram, V.; Yarar, B.; Ghobadi, S.; Gürsel, S.A. Development of Graphene Supported Platinum Nanoparticles for Polymer Electrolyte Membrane Fuel Cells: Effect of Support Type and Impregnation-Reduction Methods. Int. J. Hydrogen Energy 2016, 41, 3414–3427. [Google Scholar] [CrossRef]
- Antolini, E. Graphene as a New Carbon Support for Low-Temperature Fuel Cell Catalysts. Appl. Catal. B Environ. 2012, 123–124, 52–68. [Google Scholar] [CrossRef]
- Shahgaldi, S.; Hamelin, J. Improved Carbon Nanostructures as a Novel Catalyst Support in the Cathode Side of PEMFC: A Critical Review. Carbon N. Y. 2015, 94, 705–728. [Google Scholar] [CrossRef]
- Borbáth, I.; Zelenka, K.; Vass, Á.; Pászti, Z.; Szijjártó, G.P.; Sebestyén, Z.; Sáfrán, G.; Tompos, A. CO Tolerant Pt Electrocatalysts for PEM Fuel Cells with Enhanced Stability against Electrocorrosion. Int. J. Hydrogen Energy 2021, 46, 13534–13547. [Google Scholar] [CrossRef]
- Akalework, N.G.; Pan, C.J.; Su, W.N.; Rick, J.; Tsai, M.C.; Lee, J.F.; Lin, J.M.; Tsai, L.D.; Hwang, B.J. Ultrathin TiO2-Coated MWCNTs with Excellent Conductivity and SMSI Nature as Pt Catalyst Support for Oxygen Reduction Reaction in PEMFCs. J. Mater. Chem. 2012, 22, 20977–20985. [Google Scholar] [CrossRef]
- Nawaz, M.H.; Shahid, M.K.; Gupta, R.K.; Jalil, R.; Chuang, F.C.; Pham, P.V. Flatland of Graphene’s Derivatives: Classification, Synthesis, Mechanisms, Role of Defects, Applications, and Prospectives. Coord. Chem. Rev. 2025, 528, 216421. [Google Scholar] [CrossRef]
- Wick, P.; Louw-Gaume, A.E.; Kucki, M.; Krug, H.F.; Kostarelos, K.; Fadeel, B.; Dawson, K.A.; Salvati, A.; Vázquez, E.; Ballerini, L.; et al. Classification Framework for Graphene-Based Materials. Angew. Chem.-Int. Ed. 2014, 53, 7714–7718. [Google Scholar] [CrossRef]
- Brodie, B.C. On the Atomic Weight of Graphite. Philos. Trans. R. Soc. Lond. 1859, 149, 249–259. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, M.Q.; Liu, S.; Sun, Y.; Xu, Y.J. Waltzing with the Versatile Platform of Graphene to Synthesize Composite Photocatalysts. Chem. Rev. 2015, 115, 10307–10377. [Google Scholar] [CrossRef] [PubMed]
- Lerf, A.; He, H.; Forster, M.; Klinowski, J. Structure of Graphite Oxide Revisited. J. Phys. Chem. B 1998, 102, 4477–4482. [Google Scholar] [CrossRef]
- Dimiev, A.M.; Alemany, L.B.; Tour, J.M. Graphene Oxide. Origin of Acidity, Its Instability in Water, and a New Dynamic Structural Model. ACS Nano 2013, 7, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Ad-Nanotechnologies. Available online: https://ad-nanotech.com/graphene-nanoplatelets/ (accessed on 30 September 2025).
- Techinstro. Available online: https://www.techinstro.com/graphene-nanoplatelets/?srsltid=AfmBOorafupU3fTTo9MLzqssMX5VQwR63cMr10NMJDbbqIP-EHMem23h (accessed on 30 September 2025).
- Nanografi. Available online: https://shop.nanografi.com/graphene/graphene-nanoplatelets/ (accessed on 30 September 2025).
- Shao, Y.; Zhang, S.; Wang, C.; Nie, Z.; Liu, J.; Wang, Y.; Lin, Y. Highly Durable Graphene Nanoplatelets Supported Pt Nanocatalysts for Oxygen Reduction. J. Power Sources 2010, 195, 4600–4605. [Google Scholar] [CrossRef]
- Daş, E.; Alkan Gürsel, S.; Işikel Şanli, L.; Bayrakçeken Yurtcan, A. Comparison of Two Different Catalyst Preparation Methods for Graphene Nanoplatelets Supported Platinum Catalysts. Int. J. Hydrogen Energy 2016, 41, 9755–9761. [Google Scholar] [CrossRef]
- Daş, E.; Alkan Gürsel, S.; Işıkel Şanlı, L.; Bayrakçeken Yurtcan, A. Thermodynamically Controlled Pt Deposition over Graphene Nanoplatelets: Effect of Pt Loading on PEM Fuel Cell Performance. Int. J. Hydrogen Energy 2017, 42, 19246–19256. [Google Scholar] [CrossRef]
- Daş, E.; Gürsel, S.A.; Yurtcan, A.B. Simultaneously Deposited Pt-Alloy Nanoparticles over Graphene Nanoplatelets via Supercritical Carbon Dioxide Deposition for PEM Fuel Cells. J. Alloys Compd. 2021, 874, 159919. [Google Scholar] [CrossRef]
- Arici, E.; Kaplan, B.Y.; Mert, A.M.; Alkan Gursel, S.; Kinayyigit, S. An Effective Electrocatalyst Based on Platinum Nanoparticles Supported with Graphene Nanoplatelets and Carbon Black Hybrid for PEM Fuel Cells. Int. J. Hydrogen Energy 2019, 44, 14175–14183. [Google Scholar] [CrossRef]
- Cataldi, P.; Athanassiou, A.; Bayer, I.S. Graphene Nanoplatelets-Based Advanced Materials and Recent Progress in Sustainable Applications. Appl. Sci. 2018, 8, 1438. [Google Scholar] [CrossRef]
- Kazi, S.N.; Badarudin, A.; Zubir, M.N.M.; Ming, H.N.; Misran, M.; Sadeghinezhad, E.; Mehrali, M.; Syuhada, N.I. Investigation on the Use of Graphene Oxide as Novel Surfactant to Stabilize Weakly Charged Graphene Nanoplatelets. Nanoscale Res. Lett. 2015, 10, 16–18. [Google Scholar] [CrossRef]
- Mani, V.; Chen, S.M.; Lou, B.S. Three Dimensional Graphene Oxide-Carbon Nanotubes and Graphene-Carbon Nanotubes Hybrids. Int. J. Electrochem. Sci. 2013, 8, 11641–11660. [Google Scholar] [CrossRef]
- Qi, Z.; Tan, Y.; Wang, H.; Xu, T.; Wang, L.; Xiao, C. Effects of Noncovalently Functionalized Multiwalled Carbon Nanotube with Hyperbranched Polyesters on Mechanical Properties of Epoxy Composites. Polym. Test. 2017, 64, 38–47. [Google Scholar] [CrossRef]
- He, D.; Zeng, C.; Xu, C.; Cheng, N.; Li, H.; Mu, S.; Pan, M. Polyaniline-Functionalized Carbon Nanotube Supported Platinum Catalysts. Langmuir 2011, 27, 5582–5588. [Google Scholar] [CrossRef]
- Zhang, A.; Tang, M.; Luan, J.; Li, J. Noncovalent Functionalization of Multi-Walled Carbon Nanotubes with Amphiphilic Polymers Containing Pyrene Pendants. Mater. Lett. 2012, 67, 283–285. [Google Scholar] [CrossRef]
- Park, M.; Lee, H.; Jang, J.; Hyuk, J.; Hwan, C.; Yun, S.; Kim, J. Phenyl Glycidyl Ether as an e Ff Ective Noncovalent Functionalization Agent for Multiwalled Carbon Nanotube Reinforced Polyamide 6 Nanocomposite Fi Bers. Compos. Sci. Technol. 2019, 177, 96–102. [Google Scholar] [CrossRef]
- He, J.; Cheng, M.; Jiang, Q.; Angaiah, S.; Shi, M.; Yan, C. Noncovalently Functionalized Organic Graphene Aerogel Composite for High-Performance Proton Storage. Chin. J. Chem. Eng. 2025, 79, 260–268. [Google Scholar] [CrossRef]
- Qiu, L.; Yang, X.; Gou, X.; Yang, W.; Ma, Z.F.; Wallace, G.G.; Li, D. Dispersing Carbon Nanotubes with Graphene Oxide in Water and Synergistic Effects between Graphene Derivatives. Chem.-A Eur. J. 2010, 16, 10653–10658. [Google Scholar] [CrossRef] [PubMed]
- Mypati, S.; Sellathurai, A.; Kontopoulou, M.; Docoslis, A.; Barz, D.P.J. High Concentration Graphene Nanoplatelet Dispersions in Water Stabilized by Graphene Oxide. Carbon N. Y. 2021, 174, 581–593. [Google Scholar] [CrossRef]
- Vass, Á.; Borbáth, I.; Bakos, I.; Pászti, Z.; Sáfrán, G.; Tompos, A. Stability Issues of CO Tolerant Pt-Based Electrocatalysts for Polymer Electrolyte Membrane Fuel Cells: Comparison of Pt/Ti0.8Mo0.2O2–C with PtRu/C. React. Kinet. Mech. Catal. 2019, 126, 679–699. [Google Scholar] [CrossRef]
- Vass, Á.; Borbáth, I.; Pászti, Z.; Bakos, I.; Sajó, I.E.; Németh, P.; Tompos, A. Effect of Mo Incorporation on the Electrocatalytic Performance of Ti–Mo Mixed Oxide–Carbon Composite Supported Pt Electrocatalysts. React. Kinet. Mech. Catal. 2017, 121, 141–160. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Klug, H.P.; Alexander, L.E. X-Ray Diffraction Procedures for Polycrystalline and Amorphous Materials, 2nd ed.; John Wiley & Sons: New York, NY, USA; Sydney, Australia; Toronto, ON, Canada, 1975; ISBN 978-0-471-49369-3. [Google Scholar]
- Vass, Á.; Borbáth, I.; Bakos, I.; Pászti, Z.; Sajó, I.E.; Tompos, A. Novel Pt Electrocatalysts: Multifunctional Composite Supports for Enhanced Corrosion Resistance and Improved CO Tolerance. Top. Catal. 2018, 61, 1300–1312. [Google Scholar] [CrossRef]
- Ayyubov, I.; Vulcu, A.; Berghian-Grosan, C.; Tálas, E.; Borbáth, I.; Sajó, I.E.; Sáfrán, G.; Mihály, J.; Tompos, A. Preparation of Pt Electrocatalyst Supported by Novel, Ti(1-x)MoxO2-C Type of Composites Containing Multi-Layer Graphene. React. Kinet. Mech. Catal. 2021, 135, 49–69. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Williams, R.T. Physisorption Hysteresis Loops and the Characterization of Nanoporous Materials. Adsorpt. Sci. Technol. 2004, 22, 773–782. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J.; Gu, J.; Su, L.; Cheng, L. An Overview of Metal Oxide Materials as Electrocatalysts and Supports for Polymer Electrolyte Fuel Cells. Energy Environ. Sci. 2014, 7, 2535–2558. [Google Scholar] [CrossRef]
- Scholz, W.; Boehm, H.-P. Die Thermische Zersetzung von Graphitoxyd. Naturwissenschaften 1964, 51, 160. [Google Scholar] [CrossRef]
- Gudkov, M.V.; Bazhenov, S.L.; Bekhli, L.S.; Mel’nikov, V.P. Explosive Reduction of Graphite Oxide. Russ. J. Phys. Chem. B 2018, 12, 860–868. [Google Scholar] [CrossRef]
- Yamada, Y.; Yasuda, H.; Murota, K.; Nakamura, M.; Sodesawa, T.; Sato, S. Analysis of Heat-Treated Graphite Oxide by X-Ray Photoelectron Spectroscopy. J. Mater. Sci. 2013, 48, 8171–8198. [Google Scholar] [CrossRef]
- Justin, P.; Ranga Rao, G. Methanol Oxidation on MoO3 Promoted Pt/C Electrocatalyst. Int. J. Hydrogen Energy 2011, 36, 5875–5884. [Google Scholar] [CrossRef]
- Guillén-Villafuerte, O.; García, G.; Rodríguez, J.L.; Pastor, E.; Guil-López, R.; Nieto, E.; Fierro, J.L.G. Preliminary Studies of the Electrochemical Performance of Pt/X@MoO3/C (X = Mo2C, MoO2, Mo0) Catalysts for the Anode of a DMFC: Influence of the Pt Loading and Mo-Phase. Int. J. Hydrogen Energy 2013, 38, 7811–7821. [Google Scholar] [CrossRef]
- Mukerjee, S.; Urian, R.C. Bifunctionality in Pt Alloy Nanocluster Electrocatalysts for Enhanced Methanol Oxidation and CO Tolerance in PEM Fuel Cells: Electrochemical and in Situ Synchrotron Spectroscopy. Electrochim. Acta 2002, 47, 3219–3231. [Google Scholar] [CrossRef]
- Diczházi, D.; Borbáth, I.; Bakos, I.; Szijjártó, G.P.; Tompos, A.; Pászti, Z. Design of Mo-Doped Mixed Oxide–Carbon Composite Supports for Pt-Based Electrocatalysts: The Nature of the Mo-Pt Interaction. Catal. Today 2021, 366, 31–40. [Google Scholar] [CrossRef]
- Woods, R. Electroanalytical Chemistry: A Series of Advances; Bard, A.J., Ed.; M. Dekker: New York, NY, USA; Basel, Switzerland, 1976; Volume 9, pp. 1–162. [Google Scholar]
- Schulenburg, H.; Durst, J.; Müller, E.; Wokaun, A.; Scherer, G.G. Real Surface Area Measurements of Pt3Co/C Catalysts. J. Electroanal. Chem. 2010, 642, 52–60. [Google Scholar] [CrossRef]
- Mayrhofer, K.J.J.; Strmcnik, D.; Blizanac, B.B.; Stamenkovic, V.; Arenz, M.; Markovic, N.M. Measurement of Oxygen Reduction Activities via the Rotating Disc Electrode Method: From Pt Model Surfaces to Carbon-Supported High Surface Area Catalysts. Electrochim. Acta 2008, 53, 3181–3188. [Google Scholar] [CrossRef]
- Silva, C.; Borbáth, I.; Dodony, E.; Olasz, D.; Sáfrán, G.; Szegedi, Á.; Zelenka, K.; Tompos, A.; Pászti, Z. Stability Enhancement of Molybdenum Modified Rutile–Carbon Composite Supported Platinum Electrocatalysts by Reductive Pretreatment: Surface Characteristics and Advanced Electrocatalytic Properties. Mater. Res. Bull. 2025, 182, 113114. [Google Scholar] [CrossRef]
- Micoud, F.; Maillard, F.; Bonnefont, A.; Job, N.; Chatenet, M. The Role of the Support in COAds Monolayer Electrooxidation on Pt Nanoparticles: Pt/WOx vs. Pt/C. Phys. Chem. Chem. Phys. 2010, 12, 1182–1193. [Google Scholar] [CrossRef]
- Gerber, I.C.; Serp, P. A Theory/Experience Description of Support Effects in Carbon-Supported Catalysts. Chem. Rev. 2020, 120, 1250–1349. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.; Pászti, Z.; Salmanzade, K.; Olasz, D.; Dodony, E.; Sáfrán, G.; Szegedi, Á.; Sebestyén, Z.; Tompos, A.; Borbáth, I. Advanced Pt/Ti(1−x)SnxO2–C Composite Supported Electrocatalyst with Functionalized Carbon for Sustainable Energy Conversion Technologies. Nanomaterials 2025, 15, 342. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Subban, C.V.; Wang, H.; Rus, E.; Disalvo, F.J.; Abruña, H.D. Highly Stable and CO-Tolerant Pt/Ti0.7W0.3O2 Electrocatalyst for Proton-Exchange Membrane Fuel Cells. J. Am. Chem. Soc. 2010, 132, 10218–10220. [Google Scholar] [CrossRef]
- Ioroi, T.; Akita, T.; Yamazaki, S.I.; Siroma, Z.; Fujiwara, N.; Yasuda, K. Comparative Study of Carbon-Supported Pt/Mo-Oxide and PtRu for Use as CO-Tolerant Anode Catalysts. Electrochim. Acta 2006, 52, 491–498. [Google Scholar] [CrossRef]
- Subban, C.V.; Zhou, Q.; Hu, A.; Moylan, T.E.; Wagner, F.T.; Disalvo, F.J. Sol-Gel Synthesis, Electrochemical Characterization, and Stability Testing of Ti0.7W0.3O2 Nanoparticles for Catalyst Support Applications in Proton-Exchange Membrane Fuel Cells. J. Am. Chem. Soc. 2010, 132, 17531–17536. [Google Scholar] [CrossRef]
- Gubán, D.; Borbáth, I.; Pászti, Z.; Sajó, I.; Drotár, E.; Hegedűs, M.; Tompos, A. Preparation and Characterization of Novel Ti0.7W0.3O2-C Composite Materials for Pt-Based Anode Electrocatalysts with Enhanced CO Tolerance. Appl. Catal. B Environ. 2015, 174–175, 455–470. [Google Scholar] [CrossRef]
- Knights, S.; Bashyam, R.; He, P.; Lauritzen, M.; Startek, C.; Colbow, V.; Cheng, T.T.H.; Kolodziej, J.; Wessel, S. PEMFC MEA and System Design Considerations. ECS Trans. 2010, 41, 39–53. [Google Scholar] [CrossRef]
- Pan, C.J.; Tsai, M.C.; Su, W.N.; Rick, J.; Akalework, N.G.; Agegnehu, A.K.; Cheng, S.Y.; Hwang, B.J. Tuning/Exploiting Strong Metal-Support Interaction (SMSI) in Heterogeneous Catalysis. J. Taiwan Inst. Chem. Eng. 2017, 74, 154–186. [Google Scholar] [CrossRef]
- Crabb, E.M.; Ravikumar, M.K.; Qian, Y.; Russell, A.E.; Maniguet, S.; Yao, J.; Thompsett, D.; Hurford, M.; Ball, S.C. Controlled Modification of Carbon Supported Platinum Electrocatalysts by Mo. Electrochem. Solid-State Lett. 2002, 5, A5–A9. [Google Scholar] [CrossRef]
- Gubán, D.; Tompos, A.; Bakos, I.; Vass; Pászti, Z.; Szabó, E.G.; Sajó, I.E.; Borbáth, I. Preparation of CO-Tolerant Anode Electrocatalysts for Polymer Electrolyte Membrane Fuel Cells. Int. J. Hydrogen Energy 2017, 42, 13741–13753. [Google Scholar] [CrossRef]
- Yazici, M.S.; Dursun, S.; Borbáth, I.; Tompos, A. Reformate Gas Composition and Pressure Effect on CO Tolerant Pt/Ti0.8Mo0.2O2–C Electrocatalyst for PEM Fuel Cells. Int. J. Hydrogen Energy 2021, 46, 13524–13533. [Google Scholar] [CrossRef]
- Masa, J.; Batchelor-McAuley, C.; Schuhmann, W.; Compton, R.G. Koutecky-Levich Analysis Applied to Nanoparticle Modified Rotating Disk Electrodes: Electrocatalysis or Misinterpretation. Nano Res. 2014, 7, 71–78. [Google Scholar] [CrossRef]
- Batchelor-Mcauley, C.; Compton, R.G. Thin-Film Modified Rotating Disk Electrodes: Models of Electron-Transfer Kinetics for Passive and Electroactive Films. J. Phys. Chem. C 2014, 118, 30034–30038. [Google Scholar] [CrossRef]
- Ayyubov, I.; Tálas, E.; Salmanzade, K.; Kuncser, A.; Pászti, Z.; Neaţu, Ş.; Mirea, A.G.; Florea, M.; Tompos, A.; Borbáth, I. Electrocatalytic Properties of Mixed-Oxide-Containing Composite-Supported Platinum for Polymer Electrolyte Membrane (PEM) Fuel Cells. Materials 2022, 15, 3671. [Google Scholar] [CrossRef] [PubMed]
- Alkan, G.; Košević, M.; Mihailović, M.; Stopic, S.; Friedrich, B.; Stevanović, J.; Panić, V. Characterization of Defined Pt Particles Prepared by Ultrasonic Spray Pyrolysis for One-Step Synthesis of Supported ORR Composite Catalysts. Metals 2022, 12, 290. [Google Scholar] [CrossRef]
- Geppert, T.N.; Bosund, M.; Putkonen, M.; Stühmeier, B.M.; Pasanen, A.T.; Heikkilä, P.; Gasteiger, H.A.; El-Sayed, H.A. HOR Activity of Pt-TiO2-Y at Unconventionally High Potentials Explained: The Influence of SMSI on the Electrochemical Behavior of Pt. J. Electrochem. Soc. 2020, 167, 084517. [Google Scholar] [CrossRef]
- Eckardt, M.; Gebauer, C.; Jusys, Z.; Wassner, M.; Hüsing, N.; Behm, R.J. Oxygen Reduction Reaction Activity and Long-Term Stability of Platinum Nanoparticles Supported on Titania and Titania-Carbon Nanotube Composites. J. Power Sources 2018, 400, 580–591. [Google Scholar] [CrossRef]
- Ayyubov, I.; Borbáth, I.; Pászti, Z.; Sebestyén, Z.; Mihály, J.; Szabó, T.; Illés, E.; Domján, A.; Florea, M.; Radu, D.; et al. Synthesis and Characterization of Graphite Oxide Derived TiO2-Carbon Composites as Potential Electrocatalyst Supports. Top. Catal. 2021, 67, 1348–1367. [Google Scholar] [CrossRef]
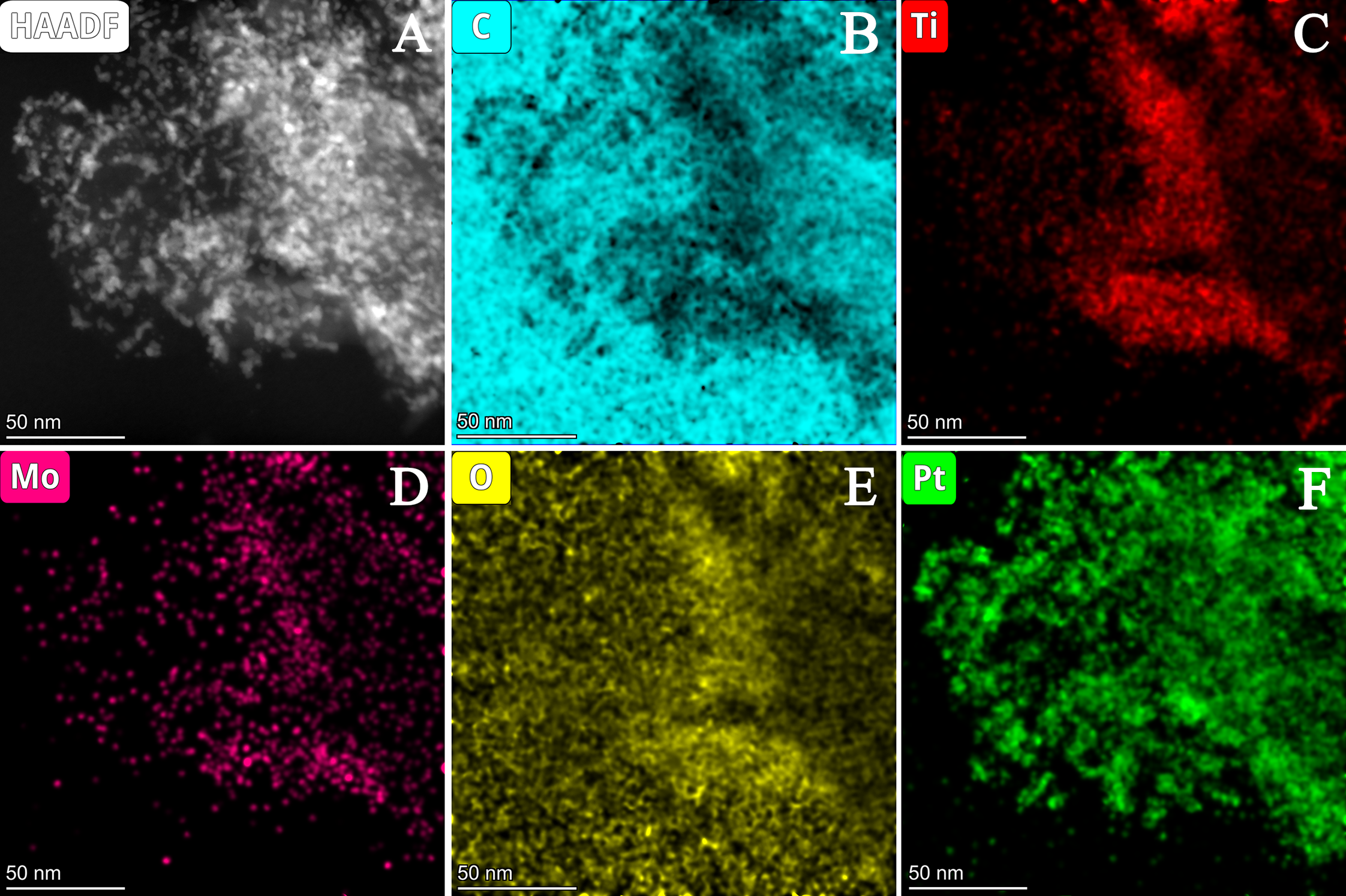
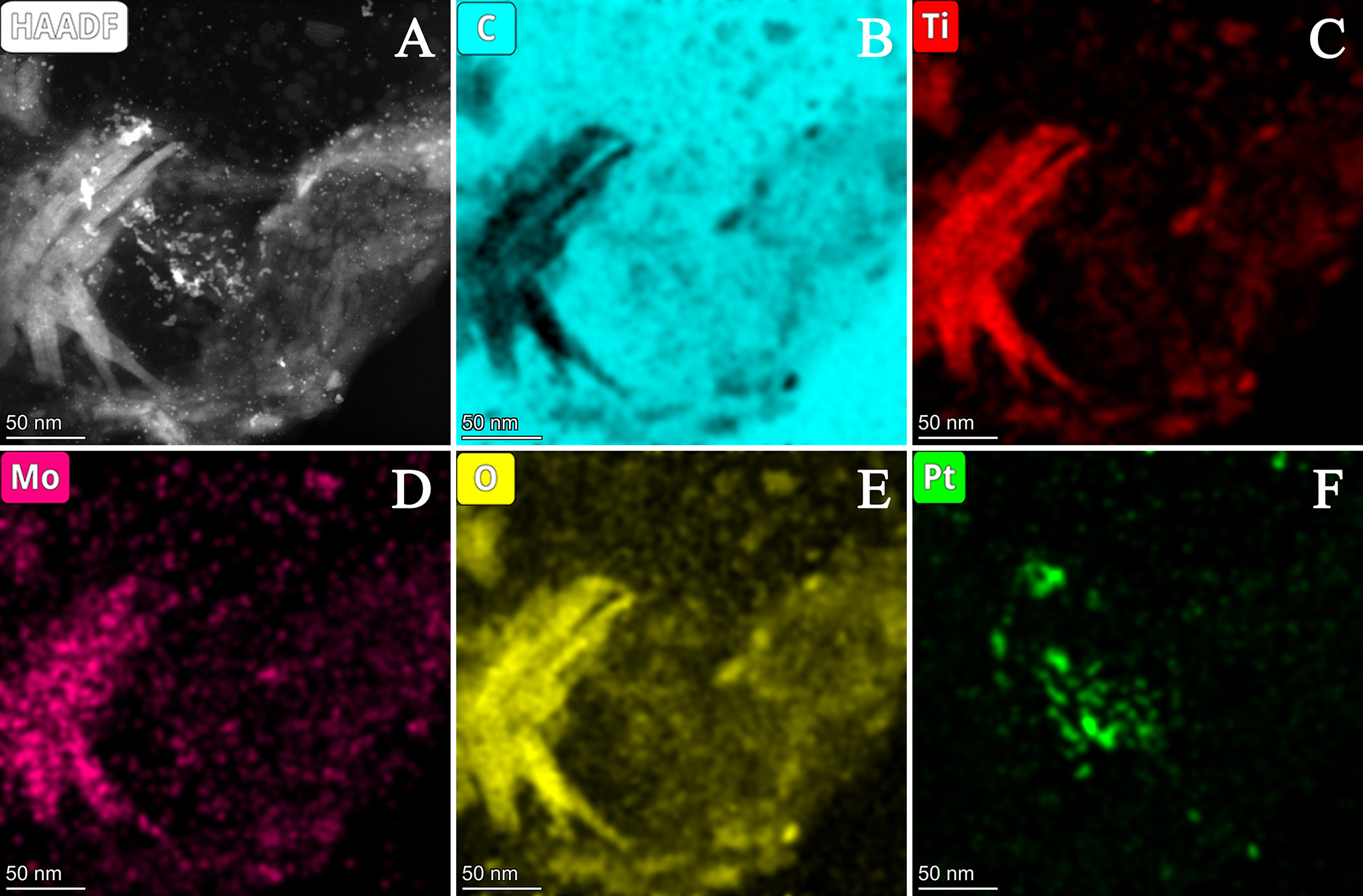
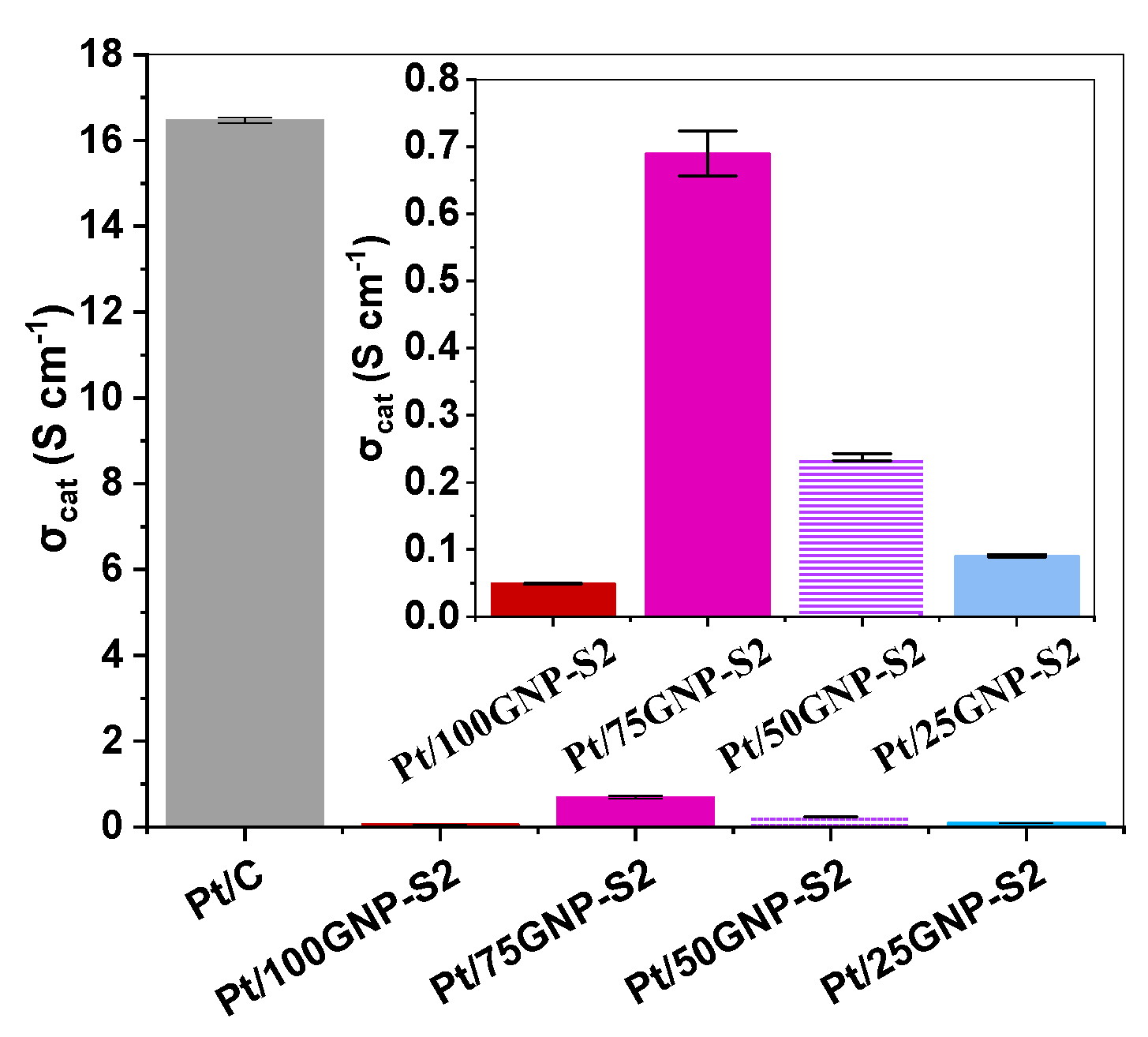
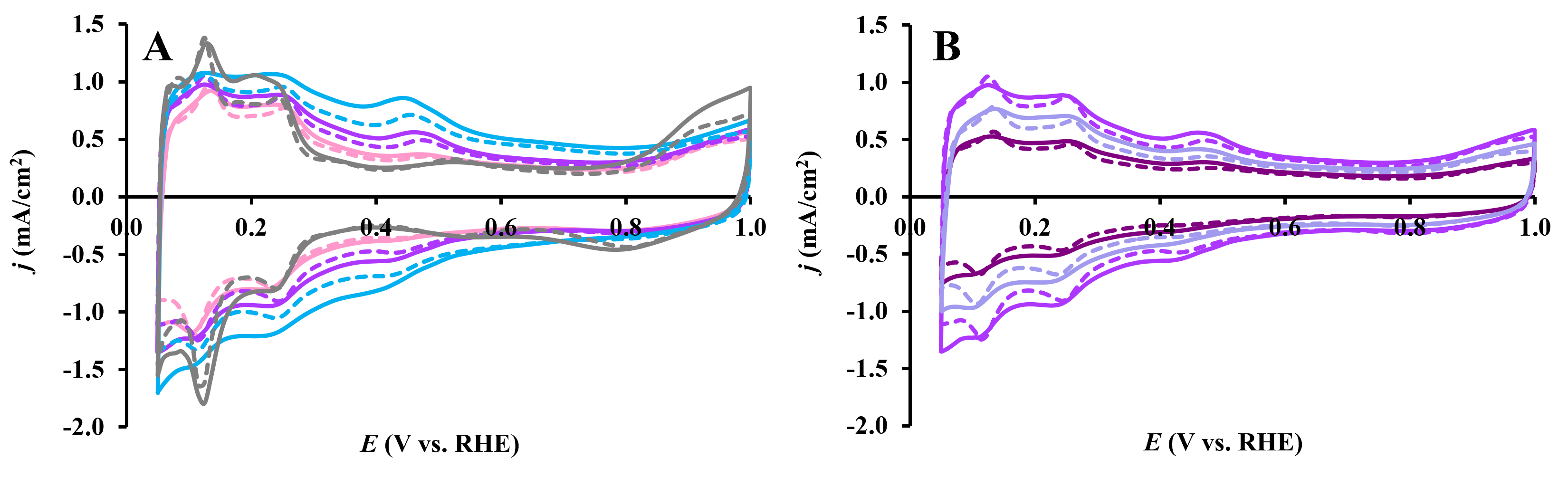

| Composite | Carbon Source | GNPs/GO Ratio 1 | TiO2 Sol | Suspension of Carbonaceous Material 4 | Mo Prec. 2, g | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ti Prec. 2, mL | HNO3 3, mL | H2O, mL | GNP, g | GO Sol, g (% 5) | H2O, mL | |||||
| 100GNP-NG | GNP 6 | 100/0 | 2.100 | 2.35 | 21 | 0.250 | 0 | 10 | 0.299 | [18] |
| 100GNP-S1 | GNP 7 | 100/0 | 2.100 | 2.35 | 21 | 0.250 | 0 | 10 | 0.299 | this work |
| 100GNP-S2 | GNP 8 | 100/0 | 2.100 | 2.35 | 21 | 0.250 | 0 | 10 | 0.299 | this work |
| 75GNP-S2 | GNP 8-GO | 75/25 | 1.079 | 1.52 | 29 | 0.128 | 3.01 (1.4) | 9 | 0.117 9 | this work |
| 50GNP-S2 | GNP 8-GO | 50/50 | 1.079 | 1.52 | 29 | 0.085 | 6.02 (1.4) | 6 | 0.117 9 | this work |
| 25GNP-S2 | GNP 8-GO | 25/75 | 1.079 | 1.52 | 29 | 0.043 | 9.03 (1.4) | 3 | 0.117 9 | this work |
| Composite | Parent GNP | Composites | |||||
|---|---|---|---|---|---|---|---|
| Physisorption 1 | XRD | Physisorption 1 | XRD | ||||
| SSABET, m2/g | Total Pore Volume, cm3/g | D 2, nm | SSABET, m2/g | Total Pore Volume, cm3/g | Lattice Parameters 3, Å | Mo Subst., % | |
| 100GNP-NG | 700 | 1.08 | 9 | 98 4 | 0.14 4 | a~4.63, c~2.94 4 | 18 4 |
| 100GNP-S1 | 347 | 0.62 | 20 | 92 | 0.13 | a~4.63, c~2.94 | 18 |
| 100GNP-S2 | 1068 | 1.64 | 9 | 136 | 0.20 | a~4.63, c~2.94 | 18 |
| Composite | Physisorption 1 | XRD | |
|---|---|---|---|
| SSABET, m2g−1 | Total Pore Volume, cm3g−1 | D 2, nm | |
| 75GNP-S2 | 128 | 0.31 | 11.2 |
| 50GNP-S2 | 105 | 0.33 | 12.5 |
| 25GNP-S2 | 125 | 0.45 | 9.1 |
| Catalyst | XRD | ICP-OES | XPS | |||
|---|---|---|---|---|---|---|
| Pt Average Crystallite Size 1, nm | Pt, wt.% | Ti/Mo, mol/mol | Pt, wt.% | (Ti + Mo + O)/C 2 wt.%/wt.% | Ti/Mo, at%/at% | |
| Pt/100GNP-S2 | 3.7 | 17.7 | 4.8/1 | 44.0 | 66.4/33.6 | 3.8/1 |
| Pt/75GNP-S2 | 3.0 | 16.5 | 5.1/1 | 51.0 | 58.1/41.9 | 3.4/1 |
| Pt/50GNP-S2 | 2.6 | 18.6 | 5.2/1 | 52.5 | 60.7/39.3 | 3.7/1 |
| Pt/25GNP-S2 | 2.5 | 17.4 | 4.8/1 | 44.7 | 58.0/42.0 | 3.7/1 |
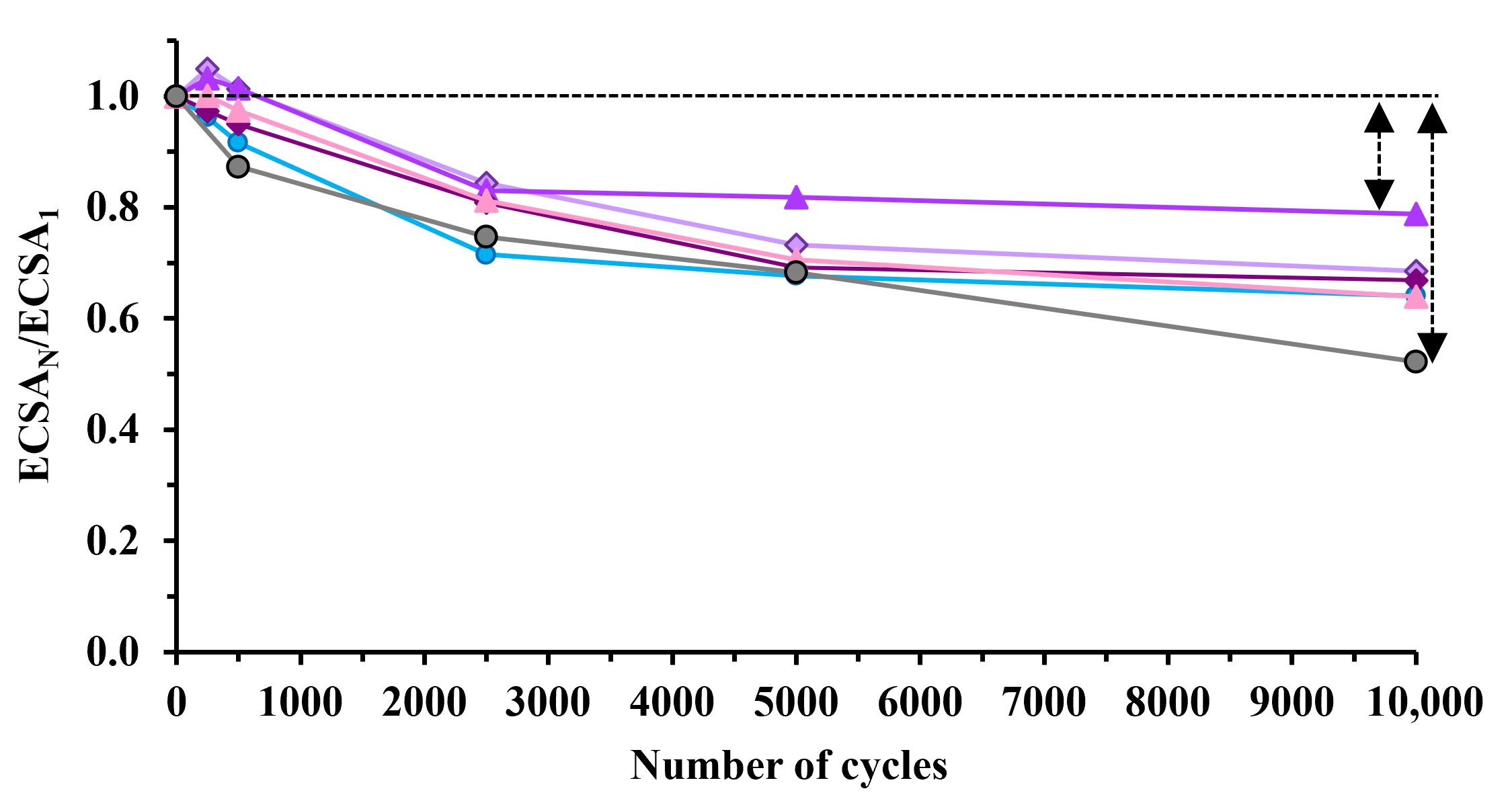
| Sample | ECO,max 1, mV | ECSA1, m2/gPt 2 | ΔECSA500, % 3,4 | ΔECSA10,000, % 3 | MA @ 0.9 V, 5 mA/mgPt |
|---|---|---|---|---|---|
| Pt/100GO | 705 (sh: 745) | 79.7 ± 1.8 | 8.4 | 36.0 | 13.3 |
| Pt/25GNP-S2 | 705 (sh: 745) | 60.8 ± 1.7 | −1.3 | 31.4 | 14.6 |
| Pt/50GNP-S2 | 705 (sh: 745) | 72.4 ± 3.5 | −1.4 | 21.2 | 26.6 |
| Pt/75GNP-S2 | 705 (sh: 745) | 42.6 ± 3.6 | 5.0 | 33.2 | 19.0 |
| Pt/100GNP-S2 | 765 | 63.3 ± 2.3 | 2.5 | 36.1 | 23.8 |
| Pt/C 6 | 805 | 87.2 ± 2.3 | 12.7 | 47.8 | 33.2 |
| Type of Carbon | Preparation Details | Oxide/C Ratio | ΔECSA10,000, % | Ref. |
|---|---|---|---|---|
| GNPs/GO = 50/50 | sol–gel | 75/25 | 21.2 | present work |
| functionalized BP 1 | sol–gel/350 °C reduction 2 | 50/50 | 22.1 | [75] |
| functionalized BP 1 | sol–gel/250 °C reduction 2 | 50/50 | 23.8 | [75] |
| GO-derived carbon | sol–gel + ST 3 | 75/25 | 23.8 | [18] |
| functionalized BP 1 | sol–gel | 25/75 | 24.1 | [17] |
| unmodified BP 1 | sol–gel | 25/75 | 27.6 | [17] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayyubov, I.; Tálas, E.; Borbáth, I.; Pászti, Z.; Trif, L.; Szegedi, Á.; Cannilla, C.; Bonura, G.; Szabó, T.; Dodony, E.; et al. Linking Structure to Electrocatalytic Performance: Graphene Nanoplatelets-Derived Novel Mixed Oxide–Carbon Composites as Supports for Pt Electrocatalysts with Enhanced Stability. Nanomaterials 2025, 15, 1753. https://doi.org/10.3390/nano15231753
Ayyubov I, Tálas E, Borbáth I, Pászti Z, Trif L, Szegedi Á, Cannilla C, Bonura G, Szabó T, Dodony E, et al. Linking Structure to Electrocatalytic Performance: Graphene Nanoplatelets-Derived Novel Mixed Oxide–Carbon Composites as Supports for Pt Electrocatalysts with Enhanced Stability. Nanomaterials. 2025; 15(23):1753. https://doi.org/10.3390/nano15231753
Chicago/Turabian StyleAyyubov, Ilgar, Emília Tálas, Irina Borbáth, Zoltán Pászti, László Trif, Ágnes Szegedi, Catia Cannilla, Giuseppe Bonura, Tamás Szabó, Erzsébet Dodony, and et al. 2025. "Linking Structure to Electrocatalytic Performance: Graphene Nanoplatelets-Derived Novel Mixed Oxide–Carbon Composites as Supports for Pt Electrocatalysts with Enhanced Stability" Nanomaterials 15, no. 23: 1753. https://doi.org/10.3390/nano15231753
APA StyleAyyubov, I., Tálas, E., Borbáth, I., Pászti, Z., Trif, L., Szegedi, Á., Cannilla, C., Bonura, G., Szabó, T., Dodony, E., & Tompos, A. (2025). Linking Structure to Electrocatalytic Performance: Graphene Nanoplatelets-Derived Novel Mixed Oxide–Carbon Composites as Supports for Pt Electrocatalysts with Enhanced Stability. Nanomaterials, 15(23), 1753. https://doi.org/10.3390/nano15231753









