Abstract
Although fast-paced ongoing industrial growth, on the one hand, enhances the lifestyle of the population, on the other hand, it affects human health and the environment as a result of the discharge of pollutants. To address this, designing a novel and effective photocatalyst is necessary to mitigate increasing environmental pollutants. In the present work, we aim to synthesize a single-phase high-entropy zirconate pyrochlore oxide (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 using a modified Pechini method. The physicochemical properties of the prepared nanoparticles were investigated using X-ray diffraction, UV-visible spectroscopy, field emission scanning electron microscopy, and X-ray photoelectron spectroscopy. The photocatalytic properties were examined using cationic dye (methylene blue), anionic dye (Congo red), and Cr(VI). Photocatalytic degradation experiments demonstrate exceptional efficiency in the removal of persistent organic pollutants. The photocatalytic results indicate that the prepared high-entropy (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 zirconate pyrochlore oxide could effectively degrade dyes and reduce Cr(VI). Radical trapping experiments indicate that the degradation of dyes was driven by the hydroxyl radicals, superoxide radicals, and holes. Furthermore, the position of the valence band and conduction band promoted efficient photocatalytic reaction kinetics. The prepared photocatalyst remains structurally stable and can be reused three times without losing activity.
1. Introduction
Drinking water is of utmost importance to human survival. However, with only 3% of fresh water available to humans, fast-paced rapid industrialization further reduces the availability of fresh drinking water. This is the result of effluent being discharged straight into the water streams without being appropriately treated. To ensure the safety of human drinking water, hazardous wastewater must be treated before it is released into the environment [1]. Several dyes and heavy metals, such as hexavalent Cr(VI), are released directly into water bodies such as rivers, particularly by the dyeing, tanning, and ore processing industries [2,3,4,5]. Severe health implications have been reported when water is contaminated with dyes and heavy metals. For example, drinking water contaminated with Cr(VI) levels more than the permissible limit is likely to increase the risk of bladder, liver, kidney, and skin cancer in people [6,7]. When contamination infiltrates aquatic systems, it affects aquatic creatures, and it may indirectly affect humans when consumed [8]. Similarly, when the concentration of Congo red and methylene blue is sufficiently high, the probability of light penetration is attenuated, leading to increased biochemical oxygen demand (BOD) [9]. Apart from this, the uncontrolled release of toxic dyes may lead to severe issues like allergies, cancer, gene mutation, and lung and kidney infection [10,11].
To remediate this, it is essential to find innovative and sustainable wastewater treatment methods. Several wastewater treatment strategies, including coagulation, membrane filtration, adsorption, ion exchange, and photocatalysis, were employed to remediate contamination from dyes and heavy elements [11,12]. Among them, photocatalysis has emerged as a promising green technology for environmental remediation. Functional semiconductor materials can convert light energy into chemical energy, which facilitates the degradation of pollutants through the advanced oxidation process (AOP) [13,14]. In the AOP, reactive oxygen species (ROS), such as hydroxyl and superoxide radicals, are primarily involved in the degradation of toxic components [14,15]. Numerous photocatalytic materials are available, including metals, oxides, carbon-based materials, etc., for degrading a number of industrial dyes and heavy metals [16,17,18]. Of these, we are most interested in pyrochlore oxides, which have the general formula A2B2O7. In these, A-site metal cations form an eightfold coordination with oxygen, while the B-site metal cations form a sixfold coordination with oxygen anions [19]. The compositional versatility of pyrochlore-type oxides enables the easy tuning of electron/hole mobility in the lattice. As a result, pyrochlore oxides offer structural flexibility, higher oxygen mobility, and adjustable properties. These features make them a suitable candidate for catalysis, photocatalysis, solid oxide fuel cells, thermal barrier coating, and dielectric materials [20,21,22].
In general, the performance of photocatalysts depends on various factors such as particle size, bandgap, position of valence and conduction bands, recombination rate, etc. For example, TiO2 possesses a wide bandgap (3.2 eV), which can be excited only with UV light irradiation. In addition, during photoexcitation, the faster recombination of charge carriers explicitly slows down photocatalytic performance [23]. Therefore, modification strategies such as doping and forming heterojunctions have been employed to tune the bandgap and position of the valence and conduction bands and to delay the recombination rates. Consequently, the modified photocatalyst exhibits enhanced reaction kinetics, and a visible light source may be employed to destroy harmful contaminants. Likewise, we are particularly interested in designing a photocatalyst without doping and heterojunction formulation strategies, as a more favorable alternative to traditional reported photocatalysts, in order to simplify the synthesis process.
In recent times, the concept of high entropy has established new methods for designing a number of novel and interesting compositions for diverse functional applications. In general, high-entropy materials (HEMs) are constructed from five or more principal elements with near equimolar ratios and favor solid solution formation. High configurational entropy favors solid solution formation and hinders/lowers the possibility of the formation of secondary phases. Several classes of HEMs have been explored, including alloys, oxides, sulfides, carbides, borides, etc. [24,25,26]. In particular, high-entropy oxides designed for a pyrochlore structure offer a versatile platform for designing advanced functional materials due to their robust crystal structure and their ability to incorporate multiple cations at the A and B sites, leading to a vast compositional space and potentially novel synergistic effects.
For example, (La0.3Gd0.3Ca0.4)2(Ti0.2Zr0.2Hf0.2Nb0.2Ta0.2)2O7 pyrochlore oxide possesses enhanced fracture toughness and low thermal conductivity (1.45 W m−1 K−1 at 900 °C), and therefore could be suitable as a prospective thermal barrier coating material [27]. In another study, 20 new compositions of high-entropy pyrochlore were designed, while eight compositions formed a single-phase structure, and their physical properties were explored [8]. The thermal diffusivity properties of (La0.2Y0.2Gd0.2Nd0.2Sm0.2)Zr2O7 were investigated and found to be approximately 0.5 mm2/s, which could make it a potential candidate for a thermal barrier coating material [28]. Very limited literature exists on high-entropy pyrochlore oxide used for photocatalytic applications. Du et al. investigated the photocatalytic applications of (La0.2Nd0.2Sm0.2Gd0.2Y0.2)2Zr2O7 in terms of the degradation of Rhodamine B dye. Photocatalytic activity was enhanced with a decrease in particle size [29]. Recognizing that the exploration in this area has been very limited, we would like to take advantage of high-entropy pyrochlore oxide for photocatalytic applications.
Therefore, this study focuses on the synthesis and characterization of a novel high-entropy (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 zirconate pyrochlore and investigates its potential as a multifunctional photocatalyst for environmental remediation applications, including the degradation of organic pollutants and the reduction of quantities of heavy metal ions.
2. Materials and Methods
2.1. Materials and Reagents
Cerium(III) nitrate hexahydrate (Ce(NO3)3·6H2O, 99.99%, Sigma Aldrich, Moscow, Russia), praseodymium(III) nitrate hexahydrate (Pr(NO3)3·6H2O, 99.9%, Sigma Aldrich), zinc nitrate hexahydrate (Zn(NO3)2·6H2O, 98%, Sigma Aldrich), neodymium(III) nitrate hexahydrate (Nd(NO3)3·6H2O, 99.9%, Sigma Aldrich), terbium(III) nitrate hexahydrate (Tb(NO3)3·6H2O, 99.99% Sigma Aldrich), Zirconium(IV) oxynitrate hydrate (ZrO(NO3)2·H2O, technical grade, Sigma Aldrich), Ethylene glycol (EG), and citric acid (CA). All the materials were used as received, without any further purification. Deionized water (DI) was used for the synthesis.
2.2. Synthesis of (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 Pyrochlore Oxide Nanoparticles
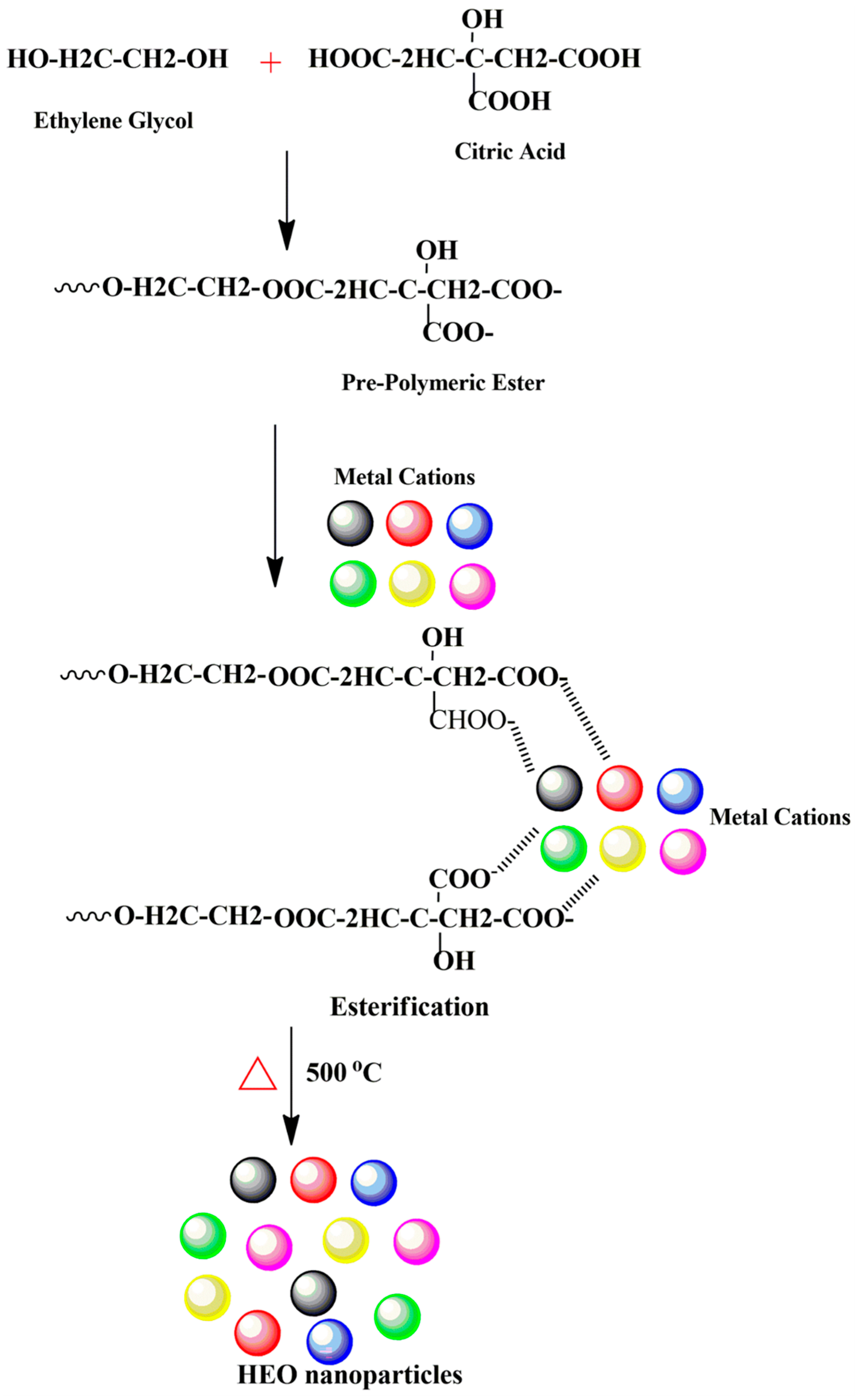
The modified Pechini method was followed to synthesize (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 nanoparticles [30]. Initially, 0.001 moles of individual metal salts were weighed and mixed together in a 250 mL glass beaker. Five mL of distilled water was added to the salt mixture and stirred continuously, followed by the addition of citric acid (0.576 g) and ethylene glycol (3.35 mL). The temperature of the hotplate was fixed at 110 °C to initiate the esterification reaction, and the reaction continued until a viscous gel formed. The resultant gel was dried at 300 °C in an oven for 2 h, followed by calcination at 500 °C for 4 h, and cooled naturally to room temperature to remove organics. The calcined powders were used for further characterization and photocatalytic investigations. The process of nanoparticle formation is described in Scheme 1. Overall, two chemical reactions occur: the chelation reaction between metal cations and citric acid and polyesterification with ethylene glycol. The advantage of using this synthesis technique stems from the ability to form a solid solution containing more principal elements while maintaining good elemental stoichiometry.
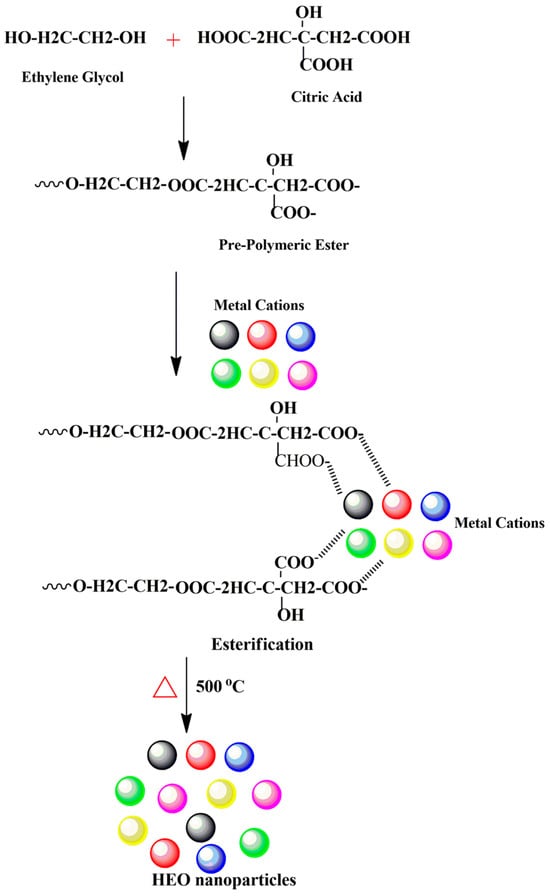
Scheme 1.
Schematic displaying the synthesis of high-entropy oxide nanoparticles prepared using the modified Pechini method.
2.3. Characterization
XRD measurements were performed on the powder samples to investigate the nature of the phase formed using a powder XRD diffractometer. The powdered samples were scanned from 20° to 80° with a scan speed of 5° per minute. Williamson–Hall (W-H) analysis was performed to assess the contribution of crystallite size and the lattice strain in XRD peak broadening. FESEM images were captured using a JEOL (JEOL JSM-7001F, JEOL, Peabody, MA, USA) microscope operated at 20 kV. To investigate the electronic structure, ultraviolet photoelectron spectroscopy (UPS) was performed using a He discharge UV lamp (He I source at 21.2 eV, AXIS SUPRA, Kratos Analytical, a Shimadzu Group company, Manchetser, UK). High-resolution Raman spectral analysis was performed to investigate the structural and chemical properties of the material using a 532 nm laser connected to a WITec Alpha 300R confocal Raman microscope (WITec Alpha 300R, WITec, Ulm, Germany). X-ray photoelectron spectroscopy (XPS, K-alpha, Kratos Analytical, a Shimadzu Group company, Manchetser, UK) was performed using a monochromated Al Kα X-ray source (ℏν = 1486.6 eV), a 180° double-focusing hemispherical analyzer with a 128-channel detector, and a dual-beam flood gun.
2.4. Photocatalytic Investigation
For the photocatalytic investigations, we utilized a batch reaction containing dyes/Cr(VI) and a photocatalyst. Briefly, 50 mL of dye/metal ion (Congo red (50 ppm), Methylene blue (50 ppm), and Cr(VI) (50 ppm)) were added separately to a 100 mL glass beaker. Next, 25 mg of photocatalyst was added to the above solution. Then, 0.5 mL of H2O2 was added to the individual dye solutions, and 1 mL of formic acid was added to the Cr(VI) solution. The mixture was stirred in the dark for 30 min to achieve an adsorption–desorption equilibrium. Next, the solution was irradiated under a UV light source (λ = 365 nm, 100 W, Guangzhou Jiguang Lighting Co., Ltd., China) to initiate the photocatalytic reaction. Meanwhile, to investigate the photocatalytic activity of the photocatalyst, stock solutions were drawn at regular intervals (20 min) from the solution, centrifuged (5000 rpm for 10 min), and the supernatant was monitored using a UV-visible spectrophotometer (Shimadzu UV-2700, Shimadzu, Japan). The decrease in the absorbance monitored at 353 nm for Cr(VI), 499 nm for CR, and 664 nm for MB was used to estimate the degradation/reduction behavior.
3. Results
3.1. Physicochemical Properties
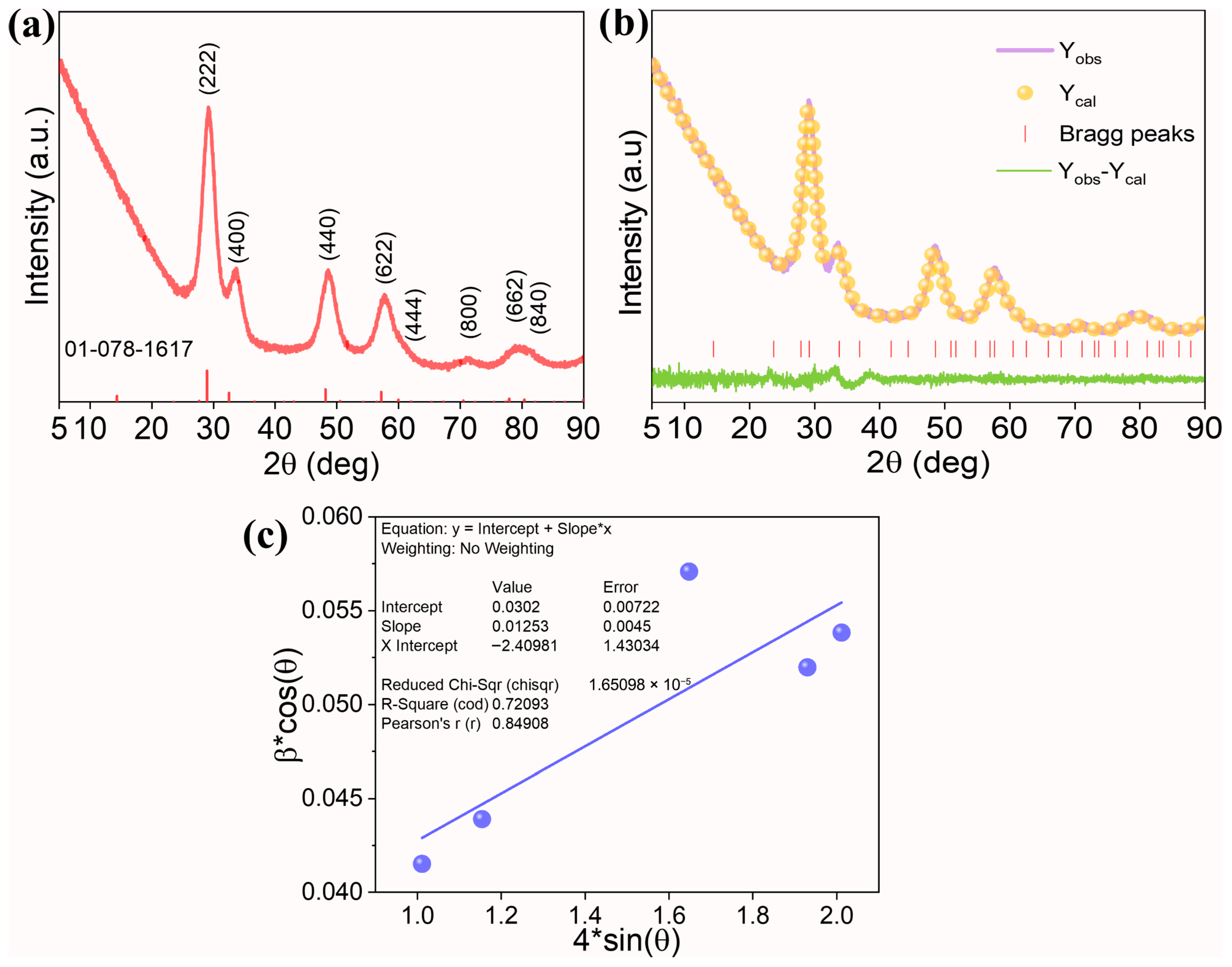
The XRD diffractogram was acquired to investigate the crystal purity and structure (Figure 1a). The strong diffraction peaks observed around 29.2°, 33.6°, 48.7°, 57.6°, 60.5°, 71.2°, 78.8°, and 81.6° were indexed to a cubic pyrochlore structure (ICDD Card No: 01-078-1617, Nd2Zr2O7) containing planes (222), (400), (440), (622), (444), (800), (662), and (840), respectively. In addition, no other diffraction peaks were observed, indicating good solubility of principal elements into the pyrochlore lattice, facilitating the formation of a single-phase solid solution. In general, to form a stable pyrochlore oxide, the ratio of A-site cation to B-site cation (rA/rB) must be in the range of 1.46 to 1.78 [20]. Except for Zn2+, all A-site cations have an oxidation state of +3 with a coordination number of 8, whereas B-site cations have a +4 oxidation state and six-fold coordination. In the current system, based on the ionic radii of, r(Ce3+) = 1.143 Å, r(Pr3+) = 1.126 Å, r(Zn2+) = 0.900 Å, r(Nd3+) = 1.109 Å, r(Tb3+) = 1.040 Å, and r(Zr4+) = 0.720 Å, a value of 1.477 was obtained, indicating a higher possibility of pyrochlore formation.
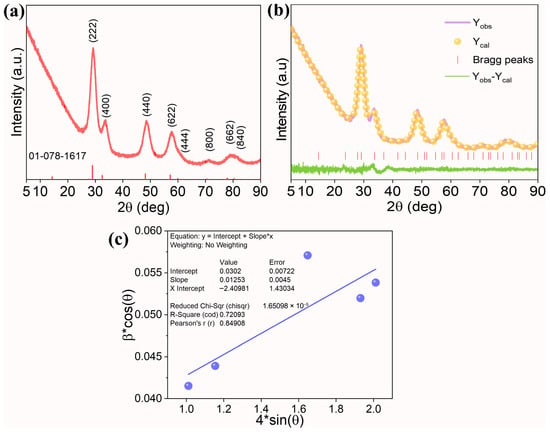
Figure 1.
(a) XRD pattern of (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 nanoparticles, (b) Rietveld refinement plot of the experimental XRD data, and (c) W-H plot to estimate the crystallite size and microstrain in the system. In the plot, “*” represent a multiple sign.
Rietveld refinement was performed on the raw XRD data using a pyrochlore structure (space group: Fd-3m) and the atomic positions of the A-site cations (0.5, 0.5, 0.5), B-site cations (0,0,0), O1 (0.35, 0.125, 0.125), and O2 (0.375, 0.375, 0.375). The output of the refinement results is displayed in Figure 1b and Table 1. The experimental data fits well with the calculated structure, underscoring the formation of single-phase high-entropy pyrochlore oxide. The lattice parameter of 10.594 Å is calculated from the Rietveld refinement result. While using the W-H plot (Figure 1c), the crystallite size and microstrain of 4.5 nm and 1.25% are obtained. Higher values of microstrain result from the contribution of different metal cations possessing different ionic radii trying to accommodate into the pyrochlore lattice. Moreover, the ionic radii of Tb and Zn are smaller than Nd, resulting in a reduced lattice parameter compared to the standard Nd2Zr2O7 oxide (10.676 Ả).

Table 1.
Rietveld refinement results of high-entropy (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 zirconate nanoparticles.
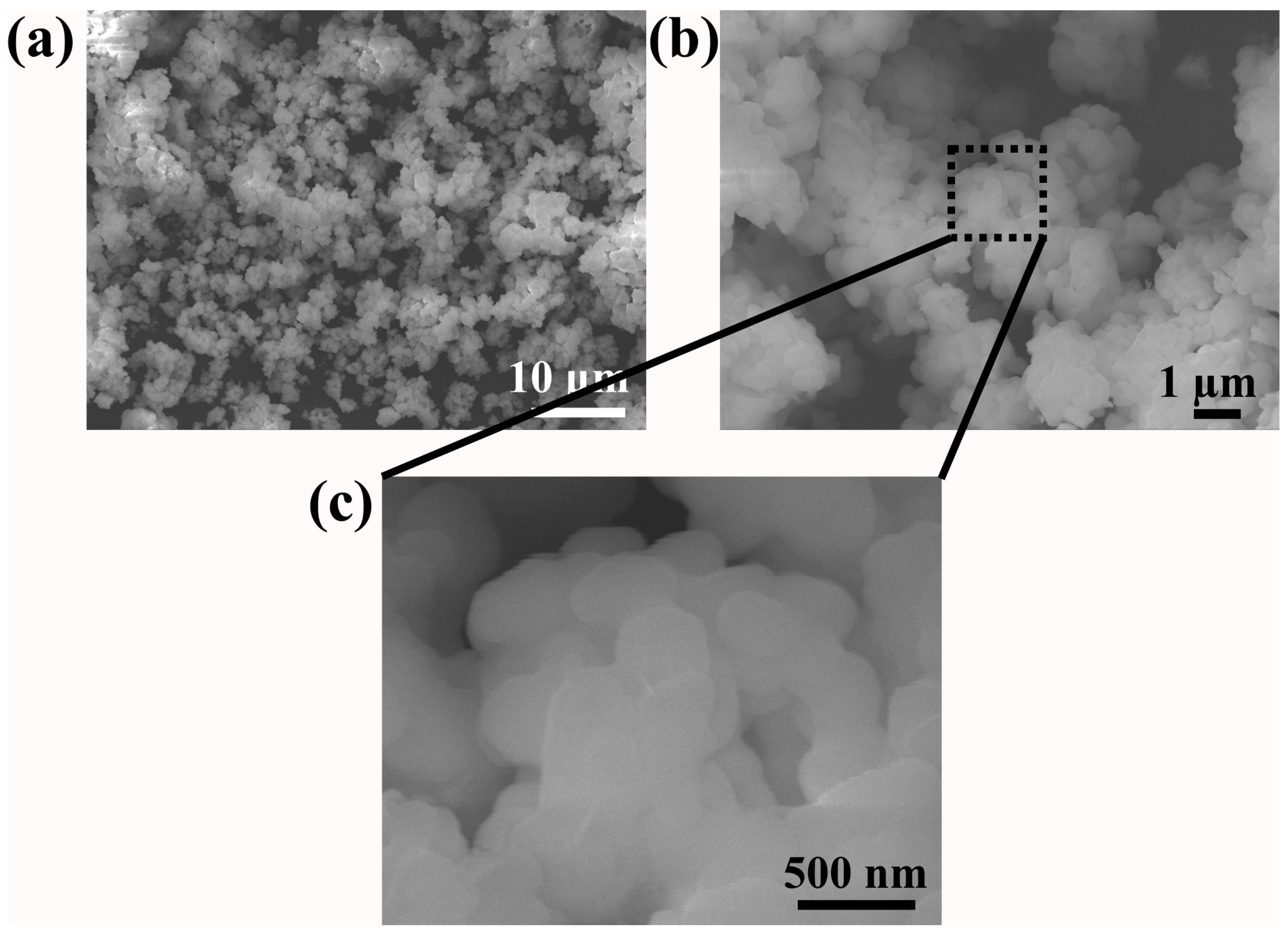
The surface morphology of the prepared high-entropy zirconate was examined using FESEM (Figure 2). Highly agglomerated nanoparticles with an irregular and asymmetric shape are observed in the calcined samples. In general, nanoparticles possess high surface area and surface energy. During the calcination process, the available thermal energy drove the particle size to increase through a diffusion process by consuming the neighboring particles, thus lowering the surface energy [31]. This leads to particle agglomeration, increasing its size, which is observed in the present study.
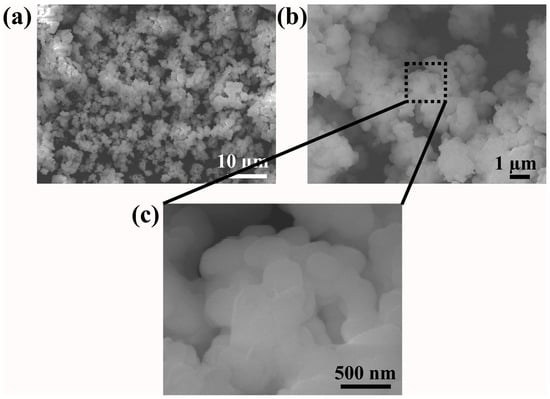
Figure 2.
FESEM image of prepared (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 zirconate nanoparticles: (a) low magnification, (b) high magnification, and (c) enlarged image.
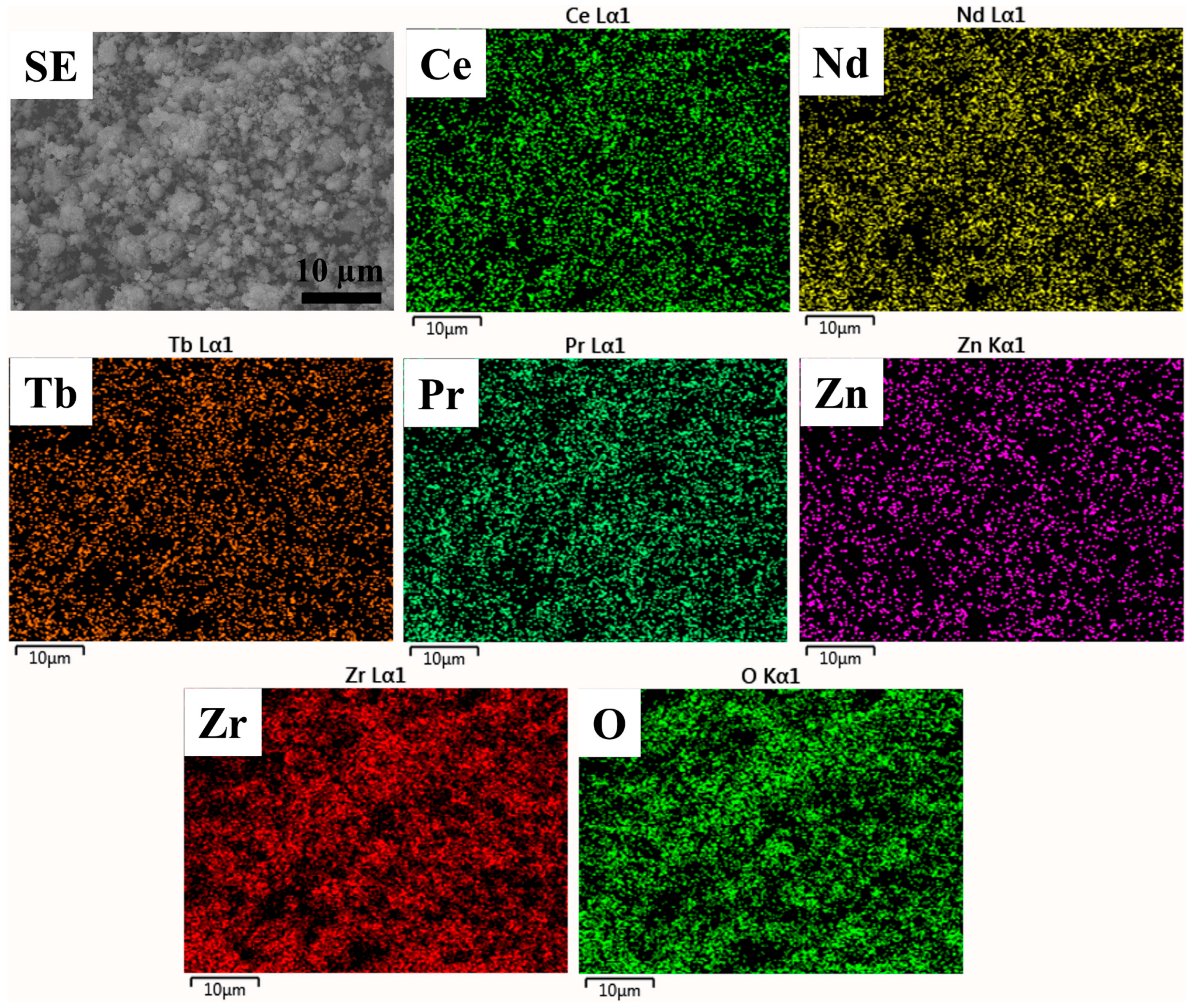
Since high-entropy oxide contains multiple principal elements, it is necessary to investigate the nature of elemental distribution. Here, EDS mapping was carried out to investigate the nature of the elemental distribution of the prepared high-entropy (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 zirconate nanoparticles (Figure 3). The results indicate that the prepared nanoparticles contain an even distribution of principal elements, underscoring the formation of a solid solution. Moreover, the elemental quantification estimated from the EDS spectra (Table 2) indicates near stoichiometry of the elements designed for the pyrochlore oxide system. Overall, the Pechini method would be a viable synthesis technique to produce single-phase high-entropy oxide systems.
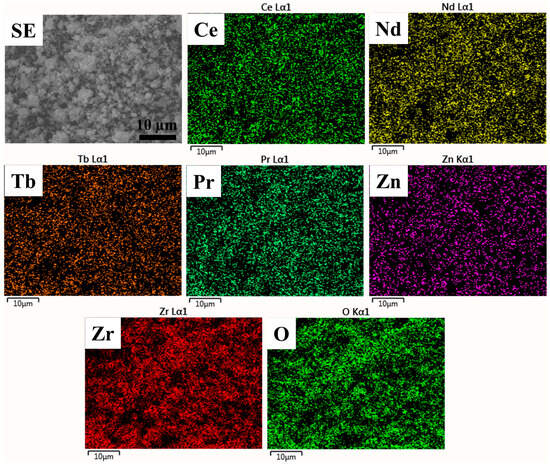
Figure 3.
EDS elemental mapping of prepared (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 zirconate nanoparticles. The scale bar represents 10 µm.

Table 2.
EDS quantification of metal cations and anions present in (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 nanoparticles.
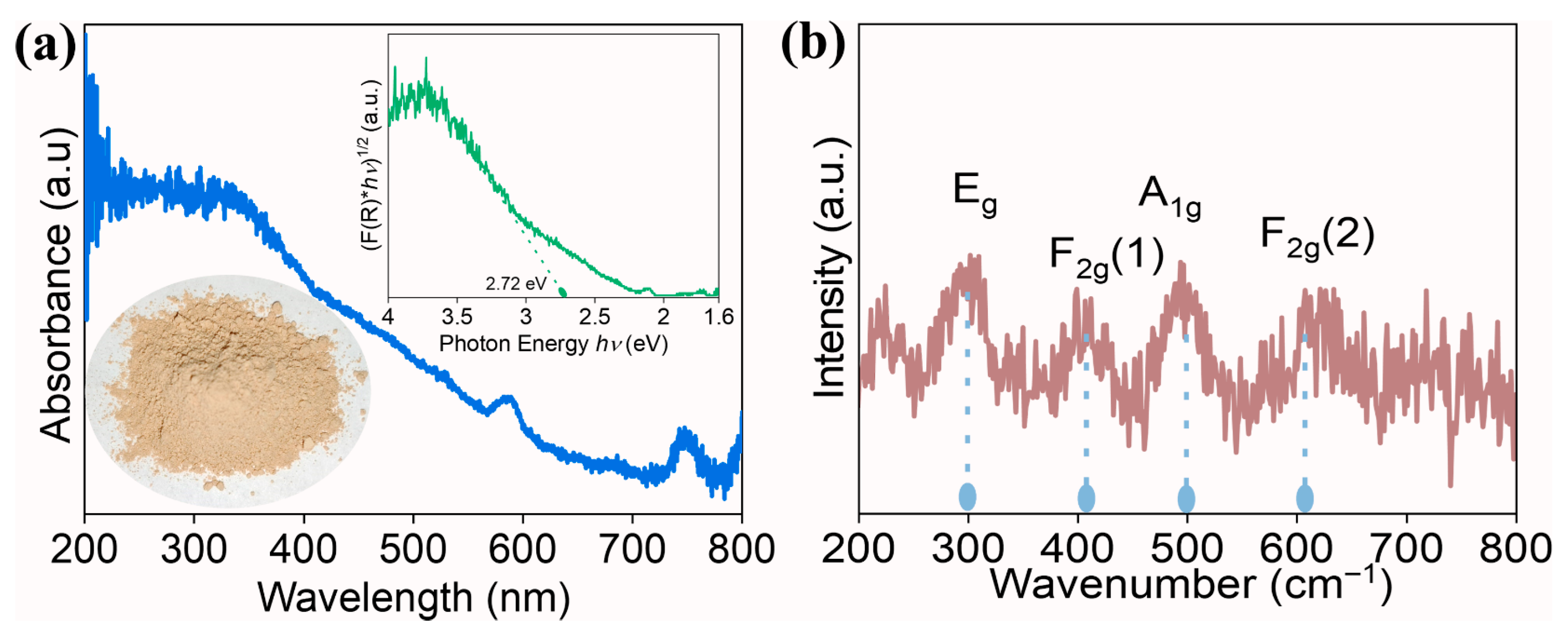
Optical properties of the prepared oxide were investigated using UV-visible spectroscopy, and the plot is displayed in Figure 4. The spectrum shows a well-defined absorption band in the UV region from 400 nm, which arises due to the promotion of electrons from O2− to M3+/4+ during photon interaction. To estimate the bandgap, Kubelka-Munk (K-M) analysis (inset of Figure 4) was performed, and the bandgap value of 2.72 eV was obtained. Compared to pristine oxides, the obtained bandgap is lower, and this can be attributed to either quantum confinement or oxygen defects. In general, high-entropy oxides contain a number of principal elements and demonstrate synergistic interactions. Moreover, in the current investigation, elements such as Ce and Pr usually coexist in +3 and +4 oxidation states. The mixed valence nature of elements facilitates the creation of oxygen vacancies through charge compensation [32]. These bandgap values will assist in estimating the conduction band energy and valence band energy later on.
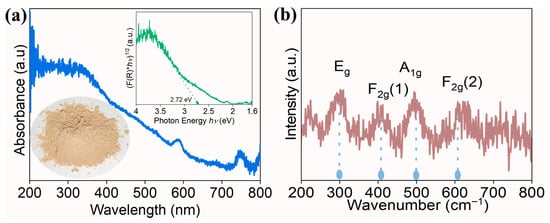
Figure 4.
(a) UV-visible absorption spectra of high-entropy (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 zirconate nanoparticles, (inset) K-M plot to estimate the bandgap and visual appearance of the prepared nanoparticles, and (b) Raman spectrum recorded on high-entropy (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 zirconate nanoparticles.
Raman spectroscopy was conducted to identify the phase purity of the prepared high-entropy (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 zirconate nanoparticles. This stage provides information regarding lattice disorder, especially with regard to oxygen anions, which are highly sensitive to the local environment. In general, according to the group theory, pyrochlore oxides possess six active Raman vibrational modes, including (A-g + Eg + 4 F2g). However, only four modes Eg, F2g(1), A1g, and F2g(2) exist for typical pyrochlore systems [33,34]. In our system, we observe four Raman vibrations centered around 296 cm−1, 405 cm−1, 495 cm−1, and 605 cm−1, corresponding to Eg, F2g(1), A1g, and F2g(2), respectively. No additional Raman modes are present, demonstrating the phase purity of the prepared high-entropy (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 zirconate nanoparticles. We observed a shift in Raman vibrations compared to the reported pyrochlore oxides [34]. This is attributed to the variation in chemical bonds existing between different metal cations and the oxygen anions.
3.2. Photocatalytic Reaction
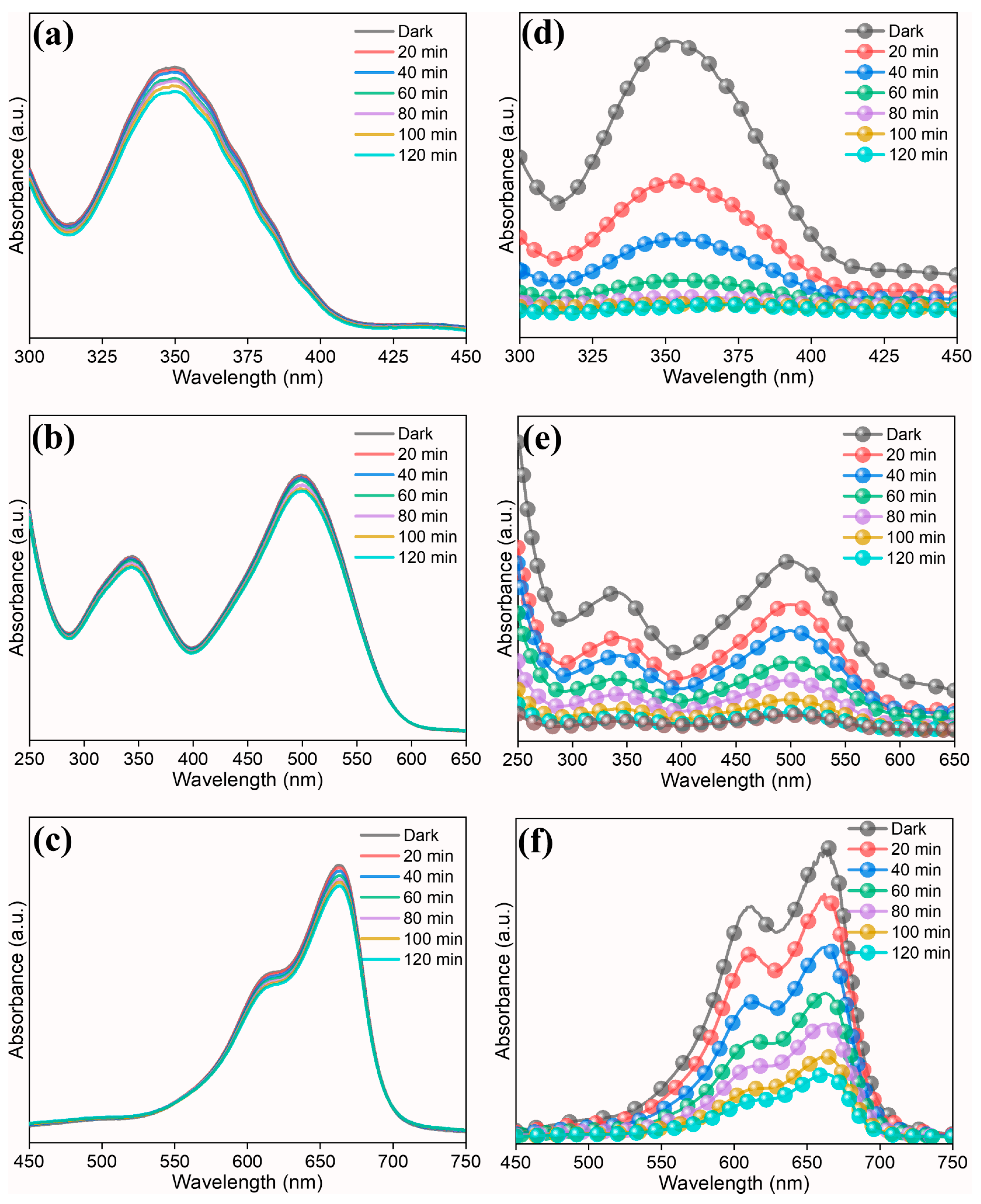
To investigate the photocatalytic properties of prepared high-entropy (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 zirconate nanoparticles, two model pollutants (anionic dye–Congo red and cationic dye—methylene blue) and a heavy metal (Cr(VI)) were chosen. Since the bandgap of the prepared nanoparticle possesses good absorbance behavior in the UV region, we have used a UV light source for the photocatalytic studies. Figure 5a–c display a control experiment in the absence of a photocatalyst. The impact of photolysis on the degradation of dyes and Cr(VI) is low; however, when a photocatalyst is added (Figure 5d–f), one can observe the degradation of dyes and reduction in Cr(VI). This proves that the prepared high-entropy oxide acts as a photocatalyst, improving the overall reaction kinetics.
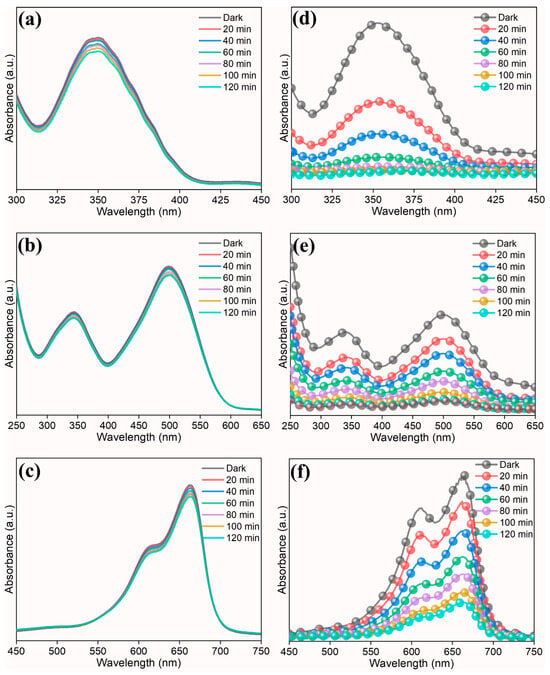
Figure 5.
UV-visible spectra showing photocatalytic reaction. (a,d) Cr(VI), (b,e) Congo red, (c,f) methylene blue. (left—control experiment without photocatalyst and right—with photocatalyst).
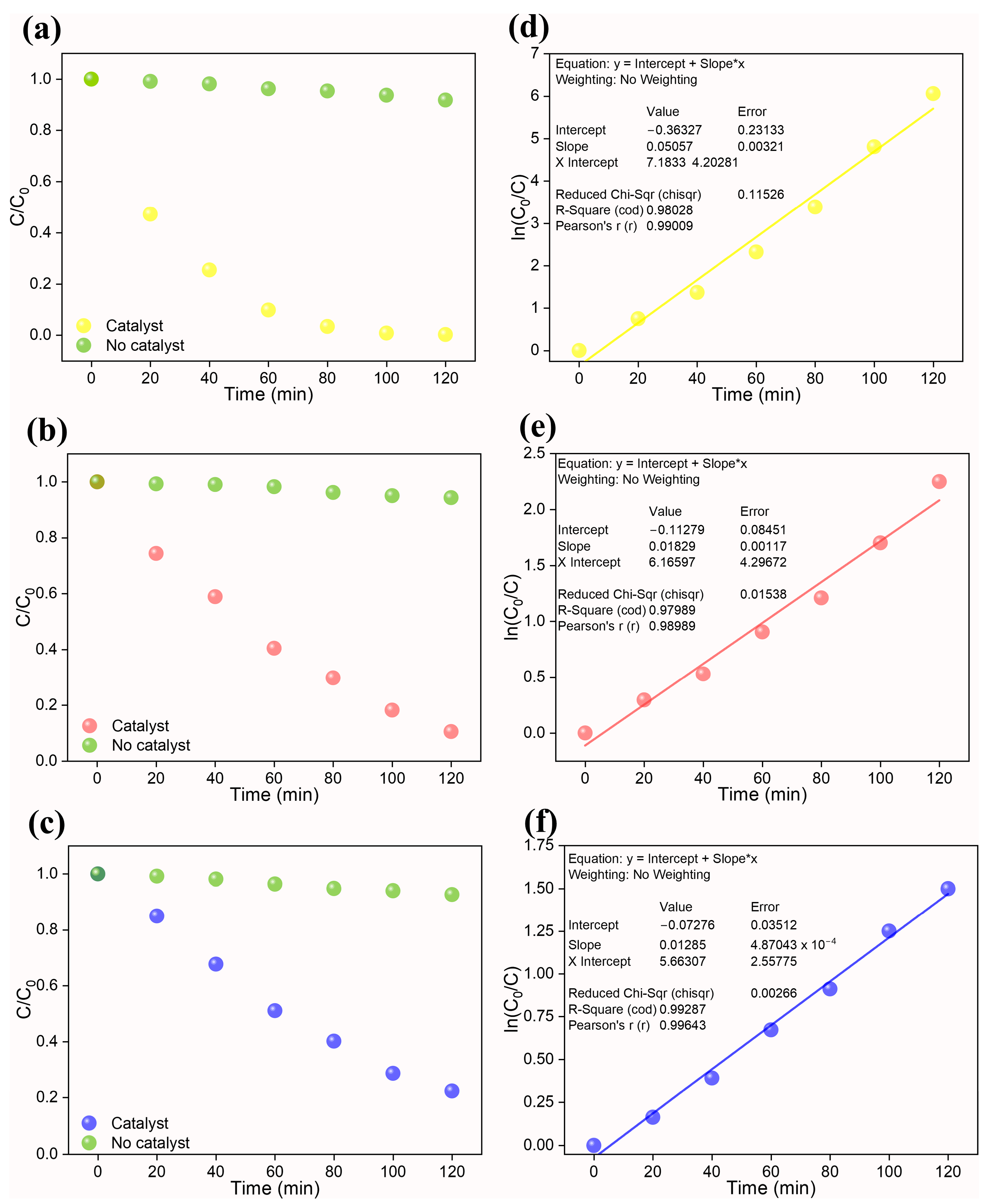
In the current photocatalytic investigation, the reaction kinetics were investigated using a pseudo-first-order reaction given by k*t = ln(C0/C). The plot C/C0 plot during the photocatalytic reaction is displayed in Figure 6a–c. Here, C corresponds to the concentration of dye/Cr(VI) at time t, while C0 corresponds to the concentration of dye/Cr(VI) at time t = 0. The rate constant (k) was estimated from the slope of the pseudo-first-order reaction kinetic fit (ln(C0/C)), and the results are tabulated in Table 3.
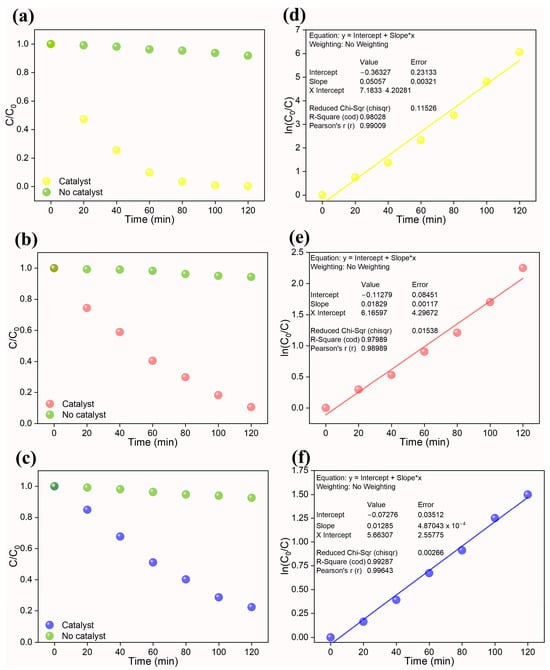
Figure 6.
Left—C/C0 plots of (a) Cr(VI), (b) Congo red, and (c) methylene blue and Right—their corresponding pseudo-first-order kinetic plot ((d) Cr(VI), (e) Congo red, and (f) methylene blue). In the plot, “*” represent a multiple sign.

Table 3.
Pseudo-first-order reaction kinetics of various dyes and Cr(VI).
The k values of 0.0506 min−1, 0.0183 min−1, and 0.0128 min−1 were obtained, respectively, for, Cr(VI), CR, and MB dye. Based on the rate constant values, the prepared high-entropy pyrochlore oxide showcased better photocatalytic activity toward the degradation of dyes and reduction in Cr(VI) ions. A comparison table (Table 4) is presented to evaluate the performance of the synthesized photocatalyst with respect to reported photocatalysts. The performance of recently reported photocatalysts was lower than that of our high-entropy (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 oxide photocatalyst. In the majority of the described studies, heterojunction photocatalysts were constructed. This emphasizes the necessity of high-entropy materials for prospective photocatalytic applications. Further research directions for the research community would be modifying the high-entropy oxides by combining them with other metal oxides to form heterojunctions to tailor the functional properties.

Table 4.
Comparison of photocatalytic degradation of dyes and reduction in Cr(VI) with reported photocatalysts.
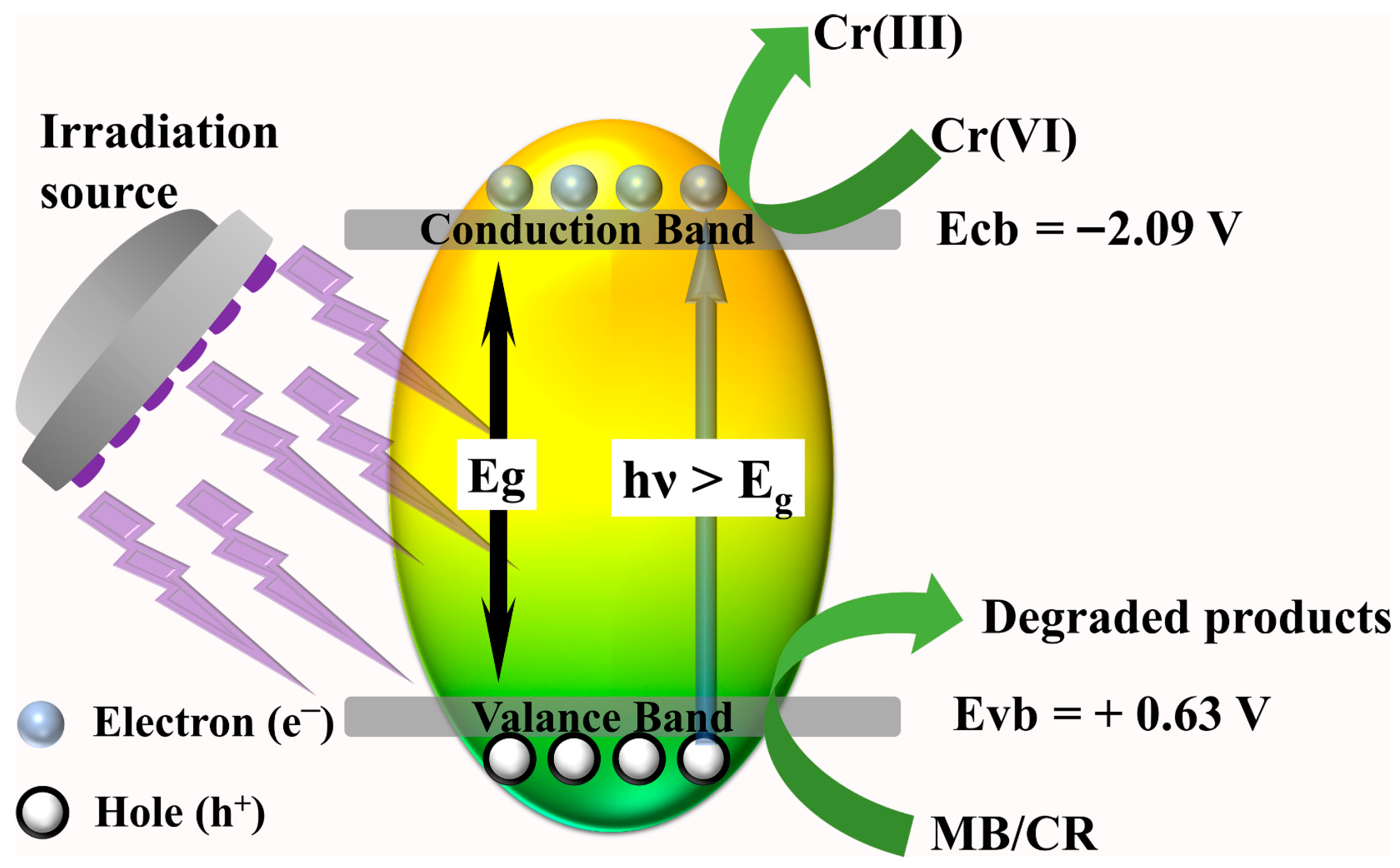
3.3. Proposed Photocatalyst Mechanism
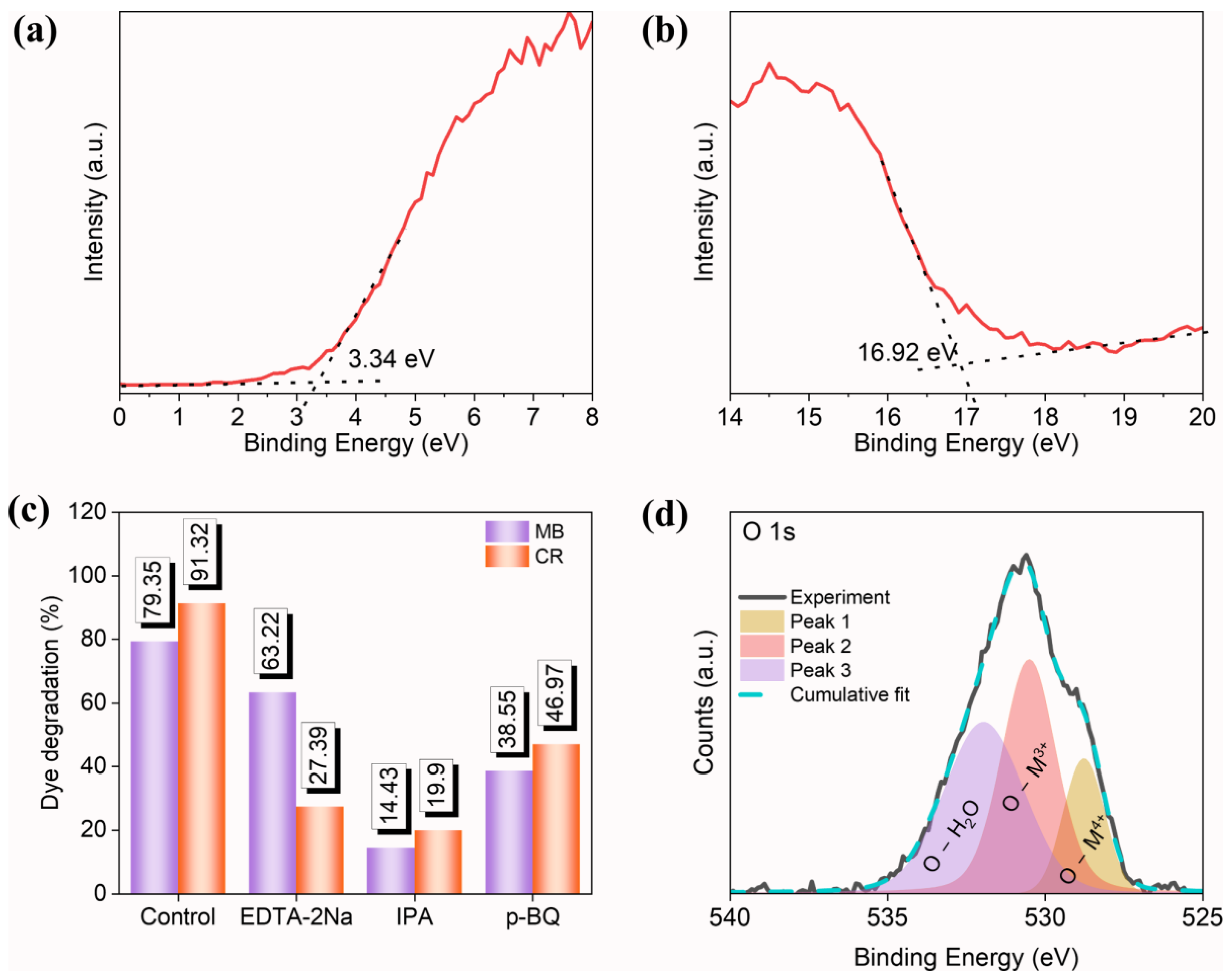
To investigate the photocatalytic reaction mechanism, it is necessary to estimate the conduction band energy and valence band energy of the prepared high-entropy (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 zirconate pyrochlore oxide nanoparticles. The reaction kinetics of typical semiconductor photocatalysts depend on the position of the valence band (Evb) and the conduction band (Ecb) [46]. The optimal positions of the Ecb and the Evb decides the selectivity of free radical generations, which are mostly involved in the degradation of dyes, especially in the generation of reactive oxygen species. Each reactive oxygen species has a distinct redox potential, and the positions of the Ecb and the Evb are crucial and affect the photocatalytic activity. Ultraviolet photoelectron spectroscopy (UPS) analysis was carried out to identify the Evb and Ecb positions (Figure 7a,b). With the help of the bandgap (estimated from the UV-visible plot) and the UPS plot, the work function, Ecb, and Evb with reference to vacuum energy were found to be 4.3 eV, 2.35 eV, and 5.07 eV, respectively. For photocatalytic investigations, the reference must be with respect to NHE, and the conversion of (ENHE = Evacuum − ENHE, absolute), where ENHE is the potential with respect to NHE, Evacuum is the potential with respect to vacuum, and ENHE, absolute is the absolute potential of NHE with respect to vacuum (4.44 eV) [47]. Therefore, the band positions of the Ecb and the Evb are −2.09 V and 0.63 V vs. NHE.
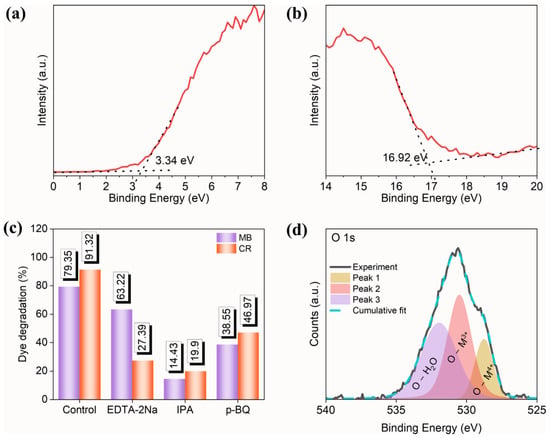
Figure 7.
UPS spectra of (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 zirconate nanoparticles at (a) low binding energy region and (b) high binding energy region, (c) radical trap experiment using various quenchers, and (d) XPS core spectra of O1s.
Radical trapping experiments were carried out to identify the selectivity of free radical species involved in the degradation of MB and CR dye. Before initiating the photocatalytic reactions, 1 mM each of isopropyl alcohol (IPA), p-benzoquinone (p-BQ), and ethylenediaminetetraacetic acid disodium salt (EDTA 2Na) were added to the reaction mixture separately. During the photocatalytic reactions, IPA, p-BQ, and EDTA 2Na act as scavengers towards hydroxyl radicals, superoxide radicals, and holes, respectively. The radical trapping results (Figure 7c) indicate that degradation is facilitated by hydroxyl radicals, superoxide radicals, and holes.
When the photocatalyst is irradiated with a light source whose energy (hʋ) is larger than the bandgap values (Eg), electron–hole pairs are produced (Equation (1)). Next, the photogenerated holes (h+) stay in the valence band while the electron (e−) is placed in the conduction band of the photocatalyst. The lifetime of an electron–hole pair is too small, and most of the generated electron–hole pairs are recombined, hindering the reaction kinetics. To prevent the unrewarding recombination process, either an electron scavenger or a hole scavenger must be added to facilitate faster reaction kinetics [48,49]. Here, H2O2 serves as a source of hydroxyl radicals for the degradation of CR and MB dyes, which are produced by interaction with electrons accessible in the conduction band (Equation (2)). Similarly, holes interact with adsorbed water to form hydroxyl radicals (Equation (3)). Superoxide radicals are generated when adsorbed water is reduced by the available electrons in the conduction band (Equation (4)). Likewise, the contribution of oxygen vacancies cannot be neglected, as they play an important role in photocatalytic reactions. The oxygen vacancies were investigated for the synthesized high-entropy (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 pyrochlore zirconate oxide using XPS analysis (Figure 7d). The high-resolution oxygen spectrum contains three peaks at 532.94 eV, 531.50 eV, and 529.76 eV, labeled to surface hydroxyl groups (adsorbed water), oxygen vacancies, and lattice oxygen, respectively. Accordingly, 39.84% of oxygen vacancies are available in the prepared system. Metal oxides containing aliovalent cations inherently possess anionic vacancies compensating for charge neutrality. These vacancies have a favorable influence on the bandgap, and they may act as charge-trapping centers to capture charge carriers [50,51].
Therefore, by effectively engineering oxygen vacancies of metal oxides, one could tailor the luring ability of holes at the valence band of the semiconductor. As a result, the lifetime charge carrier recombination is delayed, facilitating favorable reaction kinetics. Cumulatively, the produced free radicals successfully target the dye molecules and decompose them (Equation (5)). In the case of photocatalytic reduction in Cr(VI), available holes are scavenged by the added formic acid, forming CO2 and H+ (Equation (6)). This process creates a surplus of available electrons, and the process of Cr(VI) reduction to less toxic Cr(III) occurs as shown in Equation (7). Overall, the favorable band positions of the valence band and conduction band of the prepared high-entropy (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 zirconate pyrochlore oxide photocatalyst could degrade dyes and reduce toxic Cr(VI), demonstrating its multifunctional applications, and the proposed scheme is displayed in Figure 8.
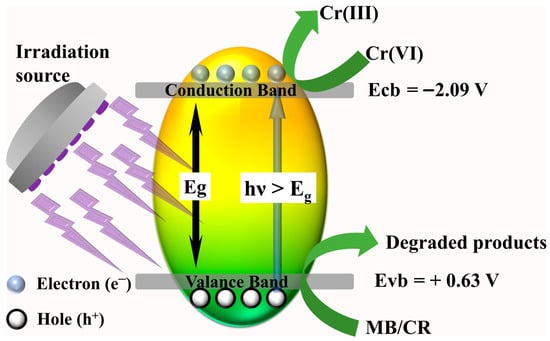
Figure 8.
Proposed mechanism for the degradation of dyes and reduction in Cr(VI) by a high-entropy (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 pyrochlore zirconate oxide photocatalyst.
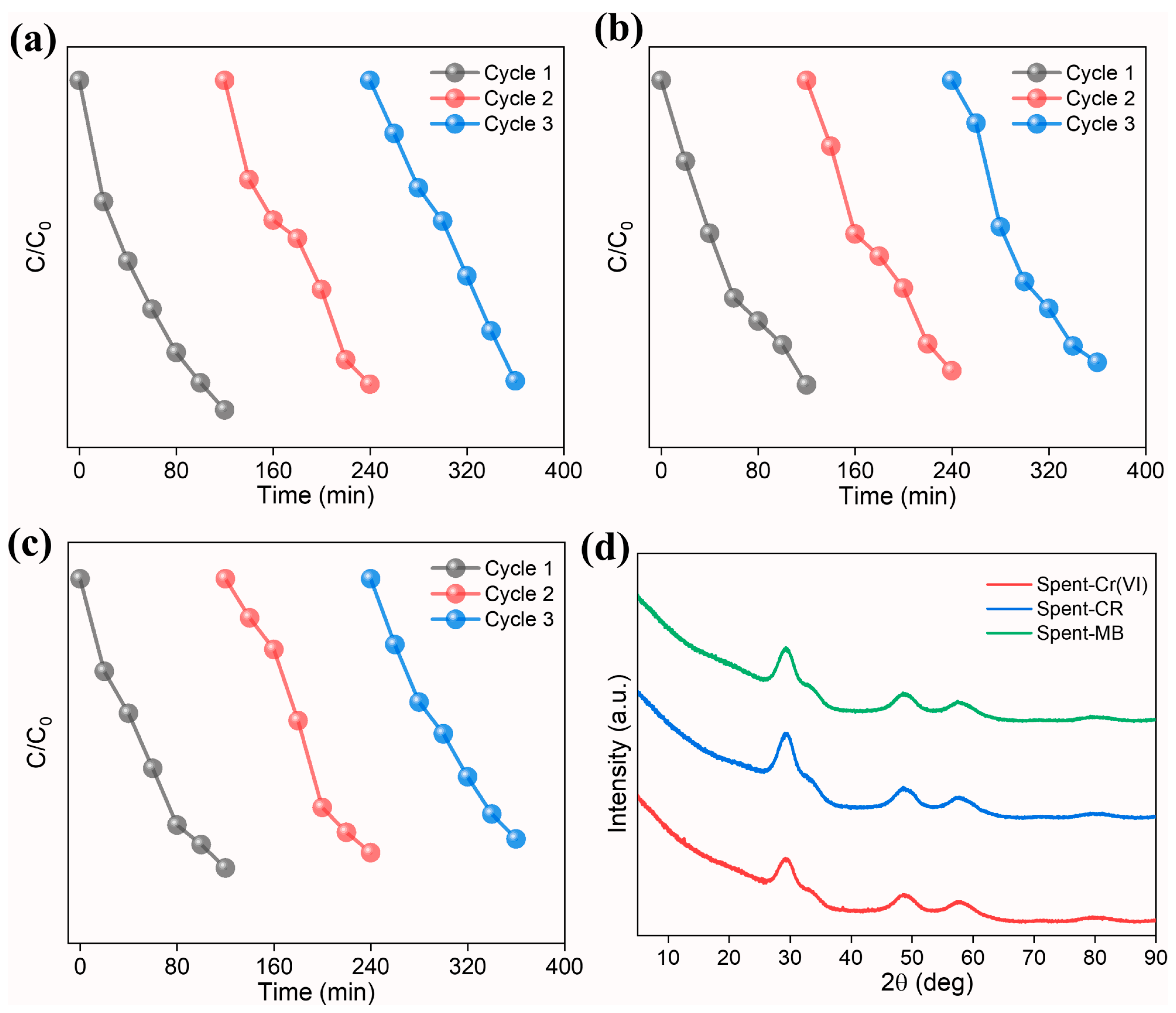
3.4. Photocatalyst Recyclability and Stability
The recyclability and stability of the prepared photocatalyst were investigated for three repetitive cycles. At the end of the first cycle, the photocatalyst was recovered from the reaction solution by centrifugation (5000 rpm for 10 min). The resultant powder was washed several times with DI and dried at 80 °C overnight in an oven and used as such for the subsequent cycles. The degradation efficiency was calculated using (Equation (8)):
The results indicate that the photocatalyst achieved good degradation performance (Figure 9a–c). We observe a marginal decline in the degradation (Table 5) in all three cases, which can be ascribed to the leaching of the photocatalyst during the recovery process. The phase stability of the spent photocatalyst (Figure 9d), characterized using XRD studies, reveals good phase stability without any phase separation. The studies highlight the use of high-entropy (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 pyrochlore zirconate oxide as a photocatalyst towards effective degradation of dyes and reduction in Cr(VI).
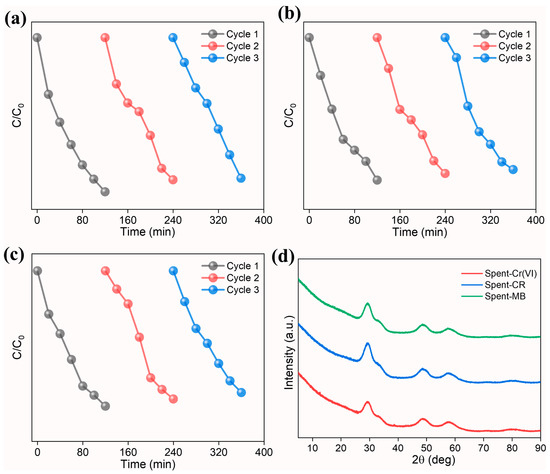
Figure 9.
Normalized concentration (C/C0) as a function of irradiation time for three consecutive cycles using high-entropy (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 pyrochlore zirconate oxide photocatalyst (a) Cr(VI), (b) Congo red, and (c) MB. (d) XRD pattern of spent photocatalyst.

Table 5.
Recyclability studies using the prepared (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 oxide towards the degradation of MB, CR, and reduction in Cr(VI).
4. Conclusions
In the present work, we prepared a single-phase high-entropy zirconate pyrochlore oxide with composition (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 using a simple Pechini method. The prepared high-entropy oxide was characterized to investigate the crystal phase, morphology, elemental composition and distribution, and optical properties. The results indicate that the prepared high-entropy (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 zirconate pyrochlore oxide was phase pure, while the bandgap of 2.72 eV was obtained. The prepared high-entropy (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 zirconate pyrochlore oxide was used as a photocatalyst to degrade various pollutants such as Cr(VI), Congo red, and methylene blue dye. The photocatalysts performed better in degrading Congo red and methylene blue and reducing Cr(VI) separately. A radical trapping experiment underscores the involvement of hydroxyl radicals, superoxide radicals, and holes in the degradation of dyes and the reduction in Cr(VI). Notably, the photocatalyst could be used effectively three times, and the leaching of the photocatalyst during the recovery step dampened the subsequent degradation activity. However, the recycled photocatalyst was structurally stable without any phase separation, retaining its single phase. The study will serve as a guide for constructing prospective photocatalysts based on the principle of high entropy, and the desired characteristics may be tuned to meet specific application requirements. Beyond that, the prepared high-entropy (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 zirconate pyrochlore oxide could be a potential candidate for other functional applications, such as thermal barrier coatings, and requires possible exploration.
Author Contributions
Conceptualization, M.A. and E.A.T.; methodology, M.A.; validation, M.A.; investigation, M.A., S.S., V.R.N., N.K.B., S.G.S., and K.S.L.; writing—original draft preparation, M.A., S.S.; writing—review and editing, M.A. and V.R.N.; visualization, M.A. and V.R.N.; supervision, M.A., E.A.T.; project administration, E.A.T.; funding acquisition, E.A.T. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the Russian Science Foundation and the Government of the Chelyabinsk Region, Grant No. 24-13-20009, https://rscf.ru/en/project/24-13-20009/ (accessed on 3 October 2025).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
Mariappan Anandkumar acknowledges the Indian Institute of Technology Hyderabad, India, for providing analytical instrumental facilities.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AOP | Advanced oxidation process |
| BOD | Biochemical oxygen demand |
| CA | Citric acid |
| CR | Congo red |
| DI | Deionized water |
| EDS | Energy-Dispersive X-ray Spectroscopy |
| EDTA-2Na | Ethylenediaminetetraacetic acid disodium salt |
| EG | Ethylene glycol |
| FESEM | Field emission scanning electron microscopy |
| HEMs | High-entropy materials |
| ICDD | International Centre for Diffraction Data |
| IPA | Isopropyl alcohol |
| K-M | Kubelka-Munk |
| MB | Methylene blue |
| NHE | Normal Hydrogen Electrode |
| p-BQ | p-benzoquinone |
| ROS | Reactive oxygen species |
| S.D. | Standard Deviation |
| UPS | Ultraviolet photoelectron spectroscopy |
| W-H | Williamson-Hall |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
References
- Shan, Z.; Yang, Y.; Shi, H.; Zhu, J.; Tan, X.; Luan, Y.; Jiang, Z.; Wang, P.; Qin, J. Hollow Dodecahedra Graphene Oxide- Cuprous Oxide Nanocomposites With Effective Photocatalytic and Bactericidal Activity. Front. Chem. 2021, 9, 755836. [Google Scholar] [CrossRef] [PubMed]
- Alias, N.; Hussain, Z.; Tan, W.K.; Kawamura, G.; Matsuda, A.; Lockman, Z. Anodic growth of nanotubular TiO2/Nb2O5 on Ti-Nb alloys for photocatalytic Cr(VI) removal. Adv. Powder Technol. 2025, 36, 105052. [Google Scholar] [CrossRef]
- Anandkumar, M.; Lathe, A.; Palve, A.M.; Deshpande, A.S. Single-phase Gd0.2La0.2Ce0.2Hf0.2Zr0.2O2 and Gd0.2La0.2Y0.2Hf0.2Zr0.2O2 nanoparticles as efficient photocatalysts for the reduction of Cr(VI) and degradation of methylene blue dye. J. Alloys Compd. 2021, 850, 156716. [Google Scholar] [CrossRef]
- Wang, X.; Wang, F.; Xu, B.; Yang, B. Bi2S3/Bi heterojunction for efficient removal of Cr(VI) via photocatalytic reduction and adsorption. Appl. Surf. Sci. 2025, 693, 162748. [Google Scholar] [CrossRef]
- Rana, A.; Sonu, S.; Soni, V.; Chawla, A.; Sudhaik, A.; Raizada, P.; Ahamad, T.; Thakur, P.; Thakur, S.; Singh, P. Novel S-scheme derived Mo–Bi2WO6/WO3/Biochar composite for photocatalytic removal of Methylene Blue dye. J. Phys. Chem. Solids 2024, 196, 112385. [Google Scholar] [CrossRef]
- Duan, Y.; Xue, J.; Ma, Y.; Zhao, L.; Zeng, B.; Feng, S.; Ma, J. Fabrication of ZnO quantum dots and In2O3 nanofiber composite photocatalysts for selective Cr(VI) detection and reduction. Environ. Res. 2025, 285, 122706. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, H.; Qin, C.; Li, X.; Xu, D.; Zhao, Y. Groundwater Cr(VI) contamination and remediation: A review from 1999 to 2022. Chemosphere 2024, 360, 142395. [Google Scholar] [CrossRef]
- He, H.; Peng, W.; Jiang, J.; Xia, X.; Luo, J.; Luo, S.; Wang, X.; Yang, K.; Yu, C. Surface modifying ZnWO4 with Zn QDs and oxygen vacancies for enhanced photocatalytic Cr(VI) reduction. Colloids Surfaces A Physicochem. Eng. Asp. 2025, 723, 137420. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Bamigboye, M.O.; Ogunbiyi, O.D.; Akano, M.T. Toxicity and decontamination strategies of Congo red dye. Groundw. Sustain. Dev. 2022, 19, 100844. [Google Scholar] [CrossRef]
- He, J.; Gao, R.; Wang, Z.; Li, W.; Cai, X.; Li, G.; Yang, B. Preparation of GO/BiOI composites and their degradation performance in Congo red solution. Chem. Phys. Lett. 2024, 860, 141771. [Google Scholar] [CrossRef]
- Mondal, A.; Islam, S.; Zaman, S.M.; Sultana, M.; Abedin, M.; Chakraborty, A.K.; Rahman, M.; Rahaman, H.; Sumi, M.S.A.; Nur, A.S. Fabrication of Ca-doped TiO2 for enhanced methylene blue degradation under UV-Vis irradiation. Next Mater. 2024, 7, 100392. [Google Scholar] [CrossRef]
- Gupta, G.K.; Mondal, M.K. 20-Fundamentals and mechanistic pathways of dye degradation using photocatalysts. In Photocatalytic Degradation of Dyes; Shah, M., Dave, S., Das, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 527–545. [Google Scholar]
- Wang, Y.; Wang, J.; Long, Z.; Sun, Z.; Lv, L.; Liang, J.; Zhang, G.; Wang, P.; Gao, W. MnCe-based catalysts for removal of organic pollutants in urban wastewater by advanced oxidation processes—A critical review. J. Environ. Manag. 2024, 370, 122773. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.R.; Kayani, K.F. A comparative review of Fenton-like processes and advanced oxidation processes for Methylene Blue degradation. Inorg. Chem. Commun. 2024, 170, 113467. [Google Scholar] [CrossRef]
- Li, Y.; Bu, J.; Sun, Y.; Huang, Z.; Zhu, X.; Li, S.; Chen, P.; Tang, Y.; He, G.; Zhong, S. Efficient degradation of norfloxacin by synergistic activation of PMS with a three-dimensional electrocatalytic system based on Cu-MOF. Sep. Purif. Technol. 2024, 356, 129945. [Google Scholar] [CrossRef]
- Zhang, C.; Ahmad, I.; Ahmed, S.B.; Ali, M.D.; Karim, M.R.; Bayahia, H.; Khasawneh, M.A. A review of rare earth oxides-based photocatalysts: Design strategies and mechanisms. J. Water Process Eng. 2024, 63, 105548. [Google Scholar] [CrossRef]
- Xu, X.; Sui, Y.; Chen, W.; Li, X.; Huang, W.; Chai, L.; Li, Y.; Zhong, H. Photocatalytic Cr(VI) reduction by metal-free photocatalysts under visible-light irradiation. J. Environ. Chem. Eng. 2024, 12, 114306. [Google Scholar] [CrossRef]
- Saputra, A.M.A.; Piliang, A.F.R.; Dellyansyah; Marpongahtun; Andriayani; Goei, R.; H.T.S., R.R.; Gea, S. Synthesis, properties, and utilization of carbon quantum dots as photocatalysts on degradation of organic dyes: A mini review. Catal. Commun. 2024, 187, 106914. [Google Scholar] [CrossRef]
- Anandkumar, M.; Kesavan, K.P.; Sudarsan, S.; Zhivulin, D.E.; Shaburova, N.A.; Moghaddam, A.O.; Litvinyuk, K.S.; Trofimov, E.A. Phase Evolution of High-Entropy Stannate Pyrochlore Oxide Synthesized via Glycine-Assisted Sol–Gel Synthesis as a Thermal Barrier Coating Material. Nanomaterials 2025, 15, 939. [Google Scholar] [CrossRef]
- Xu, J.; Xi, R.; Xu, X.; Zhang, Y.; Feng, X.; Fang, X.; Wang, X. A2B2O7 pyrochlore compounds: A category of potential materials for clean energy and environment protection catalysis. J. Rare Earths 2020, 38, 840–849. [Google Scholar] [CrossRef]
- Choudhary, L.; Besra, S.; Anwar, S.; Anwar, S. La2Ce2O7 based materials for next generation proton conducting solid oxide cells: Progress, opportunity and future prospects. Int. J. Hydrogen Energy 2023, 48, 28460–28501. [Google Scholar] [CrossRef]
- Sharifnattaj, A.; Ghaziasgar, S.; Bahadori, Y.; Saidi, M. Recent progress in catalysts development of dry reforming of methane process: Review of active phases and supports of catalyst. Mol. Catal. 2025, 582, 115060. [Google Scholar] [CrossRef]
- El Mchaouri, M.; Mallah, S.; Abouhajjoub, D.; Boumya, W.; Elmoubarki, R.; Essadki, A.; Barka, N.; Elhalil, A. Engineering TiO2 photocatalysts for enhanced visible-light activity in wastewater treatment applications. Tetrahedron Green Chem 2025, 6, 100084. [Google Scholar] [CrossRef]
- Torralba, J.M.; Meza, A.; Kumaran, S.V.; Mostafaei, A.; Mohammadzadeh, A. From high-entropy alloys to alloys with high entropy: A new paradigm in materials science and engineering for advancing sustainable metallurgy. Curr. Opin. Solid State Mater. Sci. 2025, 36, 101221. [Google Scholar] [CrossRef]
- Bolar, S.; Ito, Y.; Fujita, T. Future prospects of high-entropy alloys as next-generation industrial electrode materials. Chem. Sci. 2024, 15, 8664–8722. [Google Scholar] [CrossRef]
- Anandkumar, M.; Trofimov, E. Synthesis, Properties, and Applications of High-Entropy Oxide Ceramics: Current Progress and Future Perspectives. J. Alloys Compd. 2023, 960, 170690. [Google Scholar] [CrossRef]
- Zhao, Z.; Ruan, Z.; Li, R.; Yan, S.; Sun, X.; Liu, C.; Zhang, D.; Xu, B.; Ren, Z.; Wang, M.; et al. High entropy pyrochlore (La0.3Gd0.3Ca0.4)2(Ti0.2Zr0.2Hf0.2Nb0.2Ta0.2)2O7 ceramic with amorphous-like thermal conductivity for environmental/thermal barrier coating applications. J. Mater. Sci. Technol. 2024, 205, 315–326. [Google Scholar] [CrossRef]
- Matović, B.; Zagorac, D.; Cvijović-Alagić, I.; Zagorac, J.; Butulija, S.; Erčić, J.; Hanzel, O.; Sedlák, R.; Lisnichuk, M.; Tatarko, P. Fabrication and characterization of high entropy pyrochlore ceramics. Boletín de la Sociedad Española de Cerámica y Vidrio 2023, 62, 66–76. [Google Scholar] [CrossRef]
- Du, M.; Liu, S.; Ge, Y.; Li, Z.; Wei, T.; Yang, X.; Dong, J. Preparation and effect of grain size on the thermal stability, phase transition, mechanical property, and photocatalytic property of pyrochlore (La0.2Nd0.2Sm0.2Gd0.2Y0.2)2Zr2O7 high-entropy oxide. Ceram. Int. 2022, 48, 20667–20674. [Google Scholar] [CrossRef]
- Mnasri, W.; Bérardan, D.; Tusseau-Nenez, S.; Gacoin, T.; Maurin, I.; Dragoe, N. Synthesis of (MgCoNiCuZn)O entropy-stabilized oxides using solution-based routes: Influence of composition on phase stability and functional properties. J. Mater. Chem. C 2021, 9, 15121–15131. [Google Scholar] [CrossRef]
- Alshora, D.H.; Ibrahim, M.A.; Alanazi, F.K. Chapter 6—Nanotechnology from particle size reduction to enhancing aqueous solubility. In Surface Chemistry of Nanobiomaterials; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 163–191. [Google Scholar]
- Anandkumar, M.; Kesavan, K.P.; Sudarsan, S.; Zaitseva, O.V.; Moghaddam, A.O.; Iarushina, D.V.; Trofimov, E.A. Band-Gap Engineering of High-Entropy Fluorite Metal Oxide Nanoparticles Facilitated by Pr3+ Incorporation by Gel Combustion Synthesis. Gels 2025, 11, 117. [Google Scholar] [CrossRef]
- Teng, Z.; Tan, Y.; Zhang, H. High-Entropy Pyrochlore A2B2O7 with Both Heavy and Light Rare-Earth Elements at the A Site. Materials 2022, 15, 129. [Google Scholar] [CrossRef]
- Teng, Z.; Zhu, L.; Tan, Y.; Zeng, S.; Xia, Y.; Wang, Y.; Zhang, H. Synthesis and structures of high-entropy pyrochlore oxides. J. Eur. Ceram. Soc. 2020, 40, 1639–1643. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, X.; Liu, X.; Zhang, W.; Tian, Y.; Li, X.; Li, B.; Su, S. Construction of direct Z-scheme Sn3O4/WO3 heterostructure photocatalyst for enhanced Congo red degradation and Cr(VI) reduction performance. J. Photochem. Photobiol. A Chem. 2024, 460, 116122. [Google Scholar] [CrossRef]
- Shi, D.; Guo, J.; Ma, Y.; Zhang, B.; Zhang, Y. One-pot solvothermal synthesis of magnetic high-performance Zn2+ doped CoFe2O4 visible light photocatalyst. J. Alloys Compd. 2025, 1028, 180699. [Google Scholar] [CrossRef]
- Saeed, M.; Asghar, H.; Khan, I.; Akram, N.; Usman, M. Usman, Synthesis of TiO2-g-C3N4 for efficient photocatalytic degradation of Congo Red dye. Catal. Today 2024, 447, 115154. [Google Scholar] [CrossRef]
- Khan, A.; Chen, H.-Y.; Rusly, C. Visible-light-driven removal of mixed dye pollutants by a novel ZnO/CNT/GO ternary nanocomposite: Synergistic degradation of Congo red and methylene blue. Environ. Res. 2025, 283, 122156. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, Y.; Xiao, T.; Zhu, X.; Jiao, Y.; Yu, Q.; Qi, Z. Construction of flower-like BiOI/Bi2O2CO3 p-n heterojunction for photocatalytic degradation of Congo Red dye. J. Mol. Struct. 2025, 1336, 142076. [Google Scholar] [CrossRef]
- Wahba, M.A.; Abdelkader, M.M.G.; El-Mossalamy, E. Tailoring optical, dielectric, and magnetic properties of highly efficient visible-light LaFeO3 photocatalysts via Ca/Ba mono and Co-doping. Mater. Chem. Phys. 2025, 341, 130916. [Google Scholar] [CrossRef]
- Kumar, K.; Rath, C. Mn doping induced band gap narrowing and enhanced photocatalytic degradation in GdCoO3 perovskite. Vacuum 2025, 242, 114749. [Google Scholar] [CrossRef]
- Chandel, A.; Choudhary, R.; Sharma, P.; Kaur, G.; Pandey, O. Atmosphere-dependent synthesis of BiVO4 and its photocatalytic performance on methylene blue dye degradation. Chem. Phys. 2025, 598, 112798. [Google Scholar] [CrossRef]
- Rao, M.S.; Chaudhary, Y.; Jaihindh, D.P.; Lin, Y.-F.; Rakesh, B.; Sankaran, K.J. ZnO-graphene nanohybrids for photocatalytic degradation of methylene blue dye. Diam. Relat. Mater. 2025, 159, 112791. [Google Scholar] [CrossRef]
- Sharma, Y.; Anand, V.; Dhiman, V.; Heera, P. Biogenic CuO-ZnO nanocomposites synthesized from Jatropha curcas for methylene blue dye degradation. JCIS Open 2025, 19, 100146. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, Y.; Yan, J.; Duan, Z.; Han, H.; Jiang, D. Enhanced BiVO4 photocatalyst anchored on construction waste bricks powder for efficient formaldehyde and methylene blue degradation. Constr. Build. Mater. 2025, 469, 140536. [Google Scholar] [CrossRef]
- Anandkumar, M.; Kannan, P.; Sudarsan, S.; Trofimov, E. High-entropy oxide (CeGdHfPrZr)O2 nanoparticles as reusable photocatalyst for wastewater remediation. Surf. Interfaces 2024, 51, 104815. [Google Scholar] [CrossRef]
- Trasatti, S. The absolute electrode potential: An explanatory note (Recommendations 1986). Pure Appl. Chem. 1986, 58, 955–966. [Google Scholar] [CrossRef]
- Subramanian, Y.; Dhanasekaran, A.; Omeiza, L.A.; Zaini, J.H.; Irvine, J.T.; Azad, A.K. Influence of various sacrificial reagents on congo red degradation and H2O2 production based on heteroanionic titanium oxycarbide photocatalyst and its mechanism. Ionics 2023, 29, 2435–2447. [Google Scholar] [CrossRef]
- Xie, X.; Liu, M.; Wang, C.; Chen, L.; Xu, J.; Cheng, Y.; Dong, H.; Lu, F.; Wang, W.-H.; Liu, H.; et al. Efficient photo-degradation of dyes using CuWO4 nanoparticles with electron sacrificial agents: A combination of experimental and theoretical exploration. RSC Adv. 2015, 6, 953–959. [Google Scholar] [CrossRef]
- Teja, Y.N.; Prakash, R.M.; Murali, A.; Sakar, M. Chapter 5—Defective photocatalysts. In Photocatalytic Systems by Design; Sakar, M., Balakrishna, R.G., Do, T.-O., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 131–163. [Google Scholar]
- Hu, Y.; Anandkumar, M.; Joardar, J.; Wang, X.; Deshpande, A.S.; Reddy, K.M. Effective band gap engineering in multi-principal oxides (CeGdLa-Zr/Hf)Ox by temperature-induced oxygen vacancies. Sci. Rep. 2023, 13, 2362. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).