This study examines whether specific material choices in ternary systems can be translated into measurable performance gains. D–D–A and D–A–A blends were prepared under identical processing and evaluated under AM 1.5G and TL84 indoor illumination. Changes in spectra, energetics, and morphology/transport were tracked to establish causal links between energetic alignment, molecular compatibility, percolation continuity, and the device metrics including VOC, JSC, FF, and EQE. We evaluate whether gains from spectral broadening and cascade alignment appear only with balanced, continuous transport; otherwise, CT states, trap-assisted recombination, and disrupted percolation nullify them. The following sections establish the conditions under which a third component delivers overall performance gains rather than merely altering the active layer.
3.1. Ternary D–D–A System
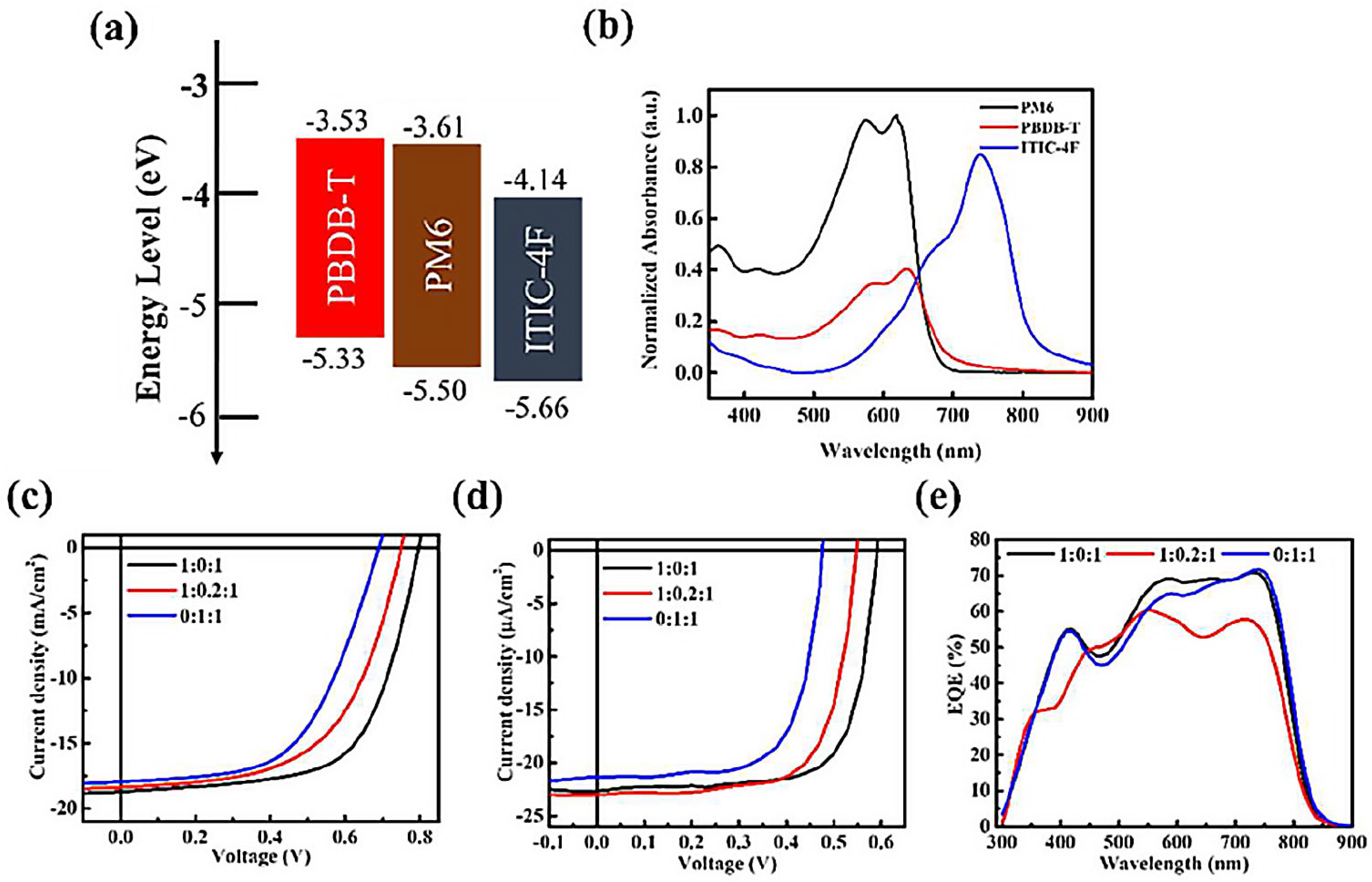
The ternary PM6:PBDB-T:ITIC-4F system was selected to investigate the donor–donor–acceptor (D–D–A) configuration under both solar and indoor illumination, with PM6:ITIC-4F serving as the binary system due to its well-established performance, and PBDB-T incorporated as a secondary donor. The energy levels of PBDB-T are reasonably aligned with those of PM6 and ITIC-4F (
Figure 1a), suggesting the possibility of cascade-assisted charge transfer. In addition, PBDB-T provides an absorption shoulder in the 550–650 nm region (
Figure 1b), which could extend the spectral response of the device. However, since this region overlaps strongly with that of PM6, the intention of introducing PBDB-T was not to increase photocurrent directly but to test whether donor–donor mixing could mitigate recombination losses and stabilize performance, particularly under low-intensity indoor conditions. The current–voltage (J–V) results under both standard solar illumination and indoor light are shown in
Figure 1c,d and summarized in
Table 1. It is evident that the addition of PBDB-T does not enhance the photocurrent, consistent with expectations. On the contrary, with 20% PBDB-T, V
OC decreases under both light sources, from 0.79 V to 0.75 V under AM 1.5G illumination, and from 0.60 V to 0.55 V under TL84 indoor light. The corresponding EQE spectra (
Figure 1e) reveal that the extra absorption from PBDB-T is not effectively converted into photocurrent; instead, a consistent reduction in EQE is observed across the visible region. These findings highlight a fundamental limitation of D–D–A blending. Although the optical absorption appears broadened, there is no genuine spectral complementarity between PBDB-T and PM6. Excitons generated in PBDB-T are not efficiently dissociated at the donor–acceptor interface. Instead, PM6 and PBDB-T compete for exciton transfer to ITIC-4F, resulting in exciton crowding, extended exciton lifetimes within donor domains, and a higher likelihood of bound-pair recombination and trap-assisted recombination. Moreover, because PM6 and PBDB-T possess similar HOMO levels, the driving force for charge separation is reduced, destabilizing energy-level alignment. This creates suboptimal interfacial states that promote non-radiative recombination pathways. The photovoltaic parameters in
Table 1 corroborate these mechanisms, showing concurrent losses in V
OC, J
SC, and FF. Under indoor illumination, where photon flux is inherently low and the spectrum is narrowly distributed, these deficiencies become even more pronounced. Redundant absorption provides no net photocurrent gain, while even minor recombination channels substantially reduce both V
OC and J
SC, reflecting the fragile balance of carrier generation, dissociation, and extraction at low light intensity.
We hypothesize that incorporating PBDB-T into PM6:ITIC-4F increases the likelihood of charge recombination during transport, thereby causing reductions in both V
OC and J
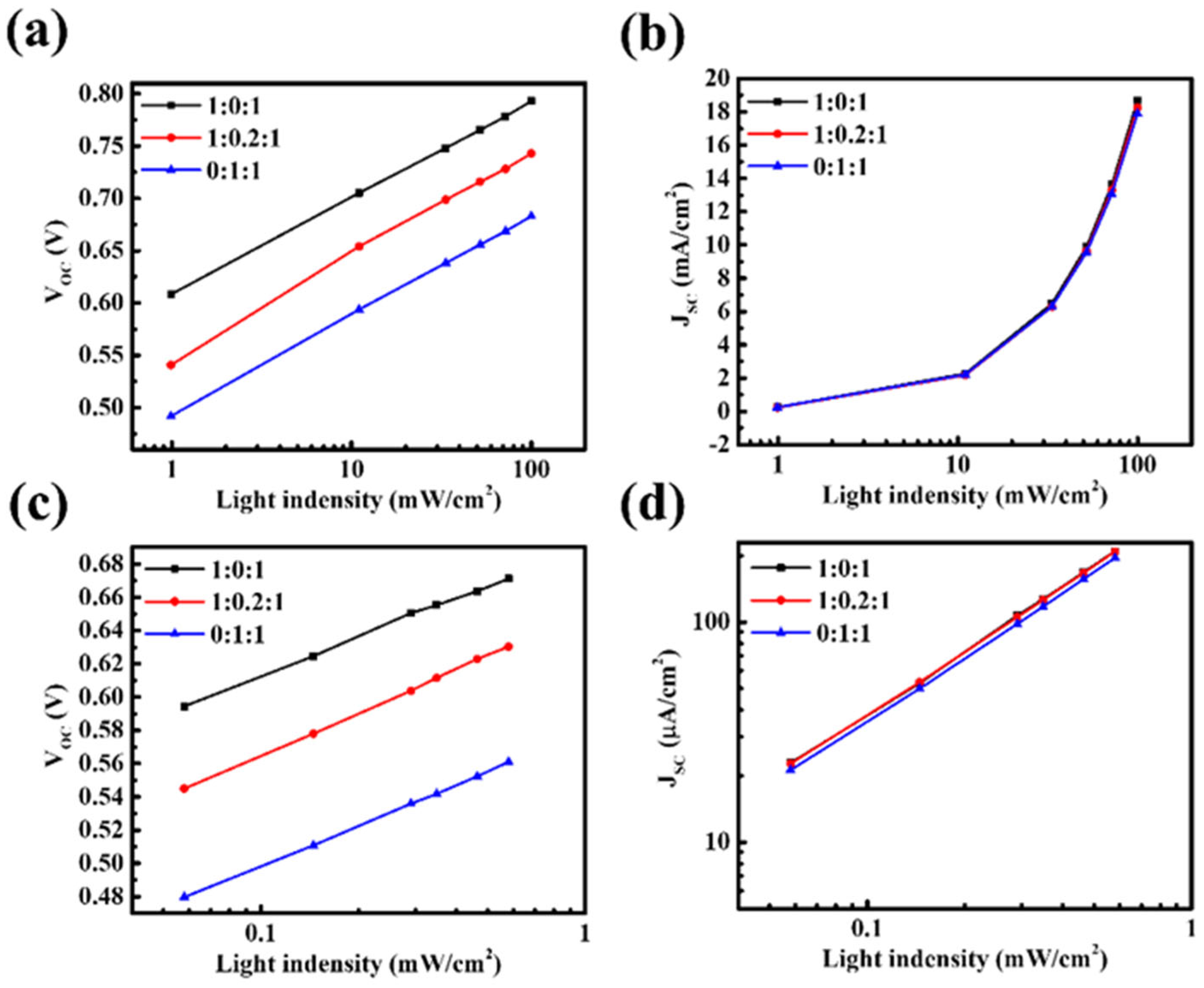
SC. To verify this, light-intensity-dependent measurements were performed to analyze charge recombination behavior. Under both solar and indoor illumination, V
OC and J
SC were measured as a function of light intensity (P) to differentiate between recombination mechanisms. The relationship between V
OC and light intensity reflects trap-assisted (monomolecular) recombination. When the ideality factor n approaches 1, the probability of non-radiative recombination through trap states is low; in contrast, n values significantly greater than 1 indicate pronounced trap-assisted recombination, where charges are more likely to recombine at trap states in the bulk or at interfaces. On the other hand, the dependence of J
SC on light intensity is associated with bimolecular recombination. An exponent α close to 1 indicates that photogenerated carriers can be efficiently transported to the electrodes without recombination, whereas α values significantly below 1 suggest that increased carrier accumulation in the active layer enhances bimolecular recombination. The results show that, under both AM 1.5G solar illumination and TL84 indoor light, the n and α values of the ternary system deviate markedly from unity (
Figure 2 and
Table S1). This indicates the simultaneous presence of severe trap-assisted and bimolecular recombination. The deterioration can be attributed to several mechanistic factors: (i) the energy levels of PBDB-T and PM6 are too close, leading to insufficient driving force for charge separation, unfavorable interfacial states, and enhanced trap-related recombination. (ii) Exciton transfer competition between the two donors extends exciton lifetimes and causes charge accumulation, thereby increasing the probability of bimolecular recombination. (iii) The incorporation of PBDB-T may disrupt the energy level alignment between the active layer and transport layers, further limiting efficient charge extraction. In summary, these results demonstrate that in the ternary D–D–A system, charges cannot be effectively extracted from the active layer but instead suffer from severe recombination losses in both the bulk and interfacial regions, leading to the simultaneous reduction in V
OC and J
SC.
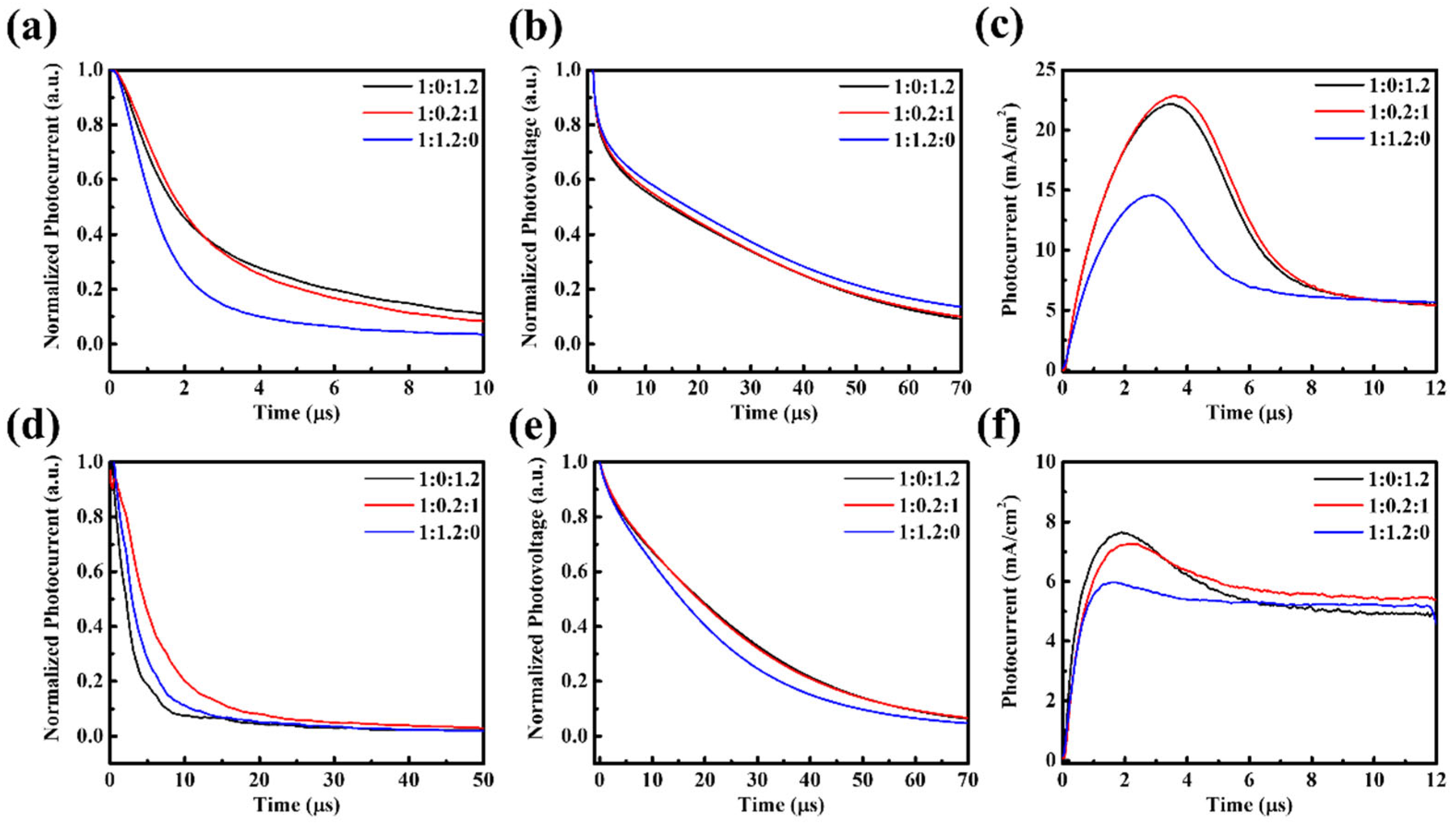
AFM analysis (
Figure 3) indicates that the surface morphologies of PM6:PBDB-T:IT-4F active layers remain relatively smooth, with RMS roughness values of 2.33, 2.30, and 4.27 nm for different ternary ratios, comparable to the binary control. This excludes large-scale surface roughness or phase segregation as the dominant cause of performance loss. Instead, the deterioration originates from molecular-level interactions. Specifically, partial miscibility between PM6 and PBDB-T may disrupt the optimized packing of PM6, while the introduction of PBDB-T creates unfavorable energetic offsets that promote recombination-prone CT states. TPC, TPV, and Photo-CELIV results (
Figure 4) together with the quantitative transport parameters (
Table S2) provide direct evidence. Under solar illumination, ternary blends exhibit prolonged charge extraction times (e.g., 1.90 μs for 1:0.2:1 vs. 0.66 μs for the binary) and markedly reduced mobilities (1.25 vs. 3.50 × 10
−5 cm
2/Vs), while under indoor light the mobility reduction is nearly an order of magnitude. Such slower extraction indicates that carriers are forced into less favorable percolation pathways, where they are more likely to encounter traps or undergo bimolecular recombination before being collected. Moreover, the imbalance between the two donor networks produces asymmetric transport: PBDB-T contributes to charge generation but fails to form continuous conductive channels, thereby disturbing percolation and amplifying the disparity between electron and hole mobilities. This asymmetry manifests as elongated carrier decay times (up to 24.21 μs for 1:0.2:1 under TL84) and directly translates into reduced FF and suppressed J
SC. Hence, the efficiency loss in ternary blends is not caused by surface roughness but by disrupted molecular packing, donor–donor miscibility limits, and unfavorable energetic landscapes that collectively hinder balanced charge transport and extraction.
A similar trend was also identified in another D–D–A configuration, namely PCDTBT:PM6:PC
71BM. Here, the binary PCDTBT:PC
71BM system absorbs primarily in the 350–650 nm range, while the incorporation of PM6 extends absorption into the 650–800 nm region (
Figure S1b). However, the broadened spectral coverage did not lead to improved photocurrent. As shown in the current–voltage characteristics (
Figure S1c,d;
Table S3), the addition of PM6 initially reduced both V
OC and J
SC under both solar and indoor illumination. For instance, under 1-Sun, moving from the baseline binary 1:0:2 device (V
OC = 0.90 V, J
SC = 10.41 mA cm
−2, FF = 61.84%, PCE = 5.77%) to a ternary blend with 0.7:0.3:2 led to pronounced losses (V
OC = 0.88 V, J
SC = 9.01 mA cm
−2, FF = 55.96%, PCE = 4.43%). These losses can be attributed to the increasingly aligned HOMO and LUMO levels of PCDTBT and PM6 at intermediate mixing ratios (
Figure S1a), which reduce the energetic offset, diminish the driving force for exciton dissociation, and thereby enhance non-radiative recombination. Interestingly, as the PM6 fraction continued to increase, device performance recovered. At the 0:1:2 endpoint, the restored energetic offset between PM6 and PC
71BM facilitated more effective charge transfer and reduced recombination, yielding significantly improved characteristics (J
SC = 11.52 mA cm
−2, FF = 70.41%, PCE = 7.32%). A similar trend was observed under TL84 indoor illumination: relative to the binary 1:0:2 device (V
OC = 0.67 V, PCE = 14.28%), the ternary 0.7:0.3:2 blend suffered clear deterioration (V
OC = 0.57 V, PCE = 9.13%), while the 0:1:2 device again outperformed both baselines, reaching V
OC = 0.70 V and PCE = 16.27% (
Table S3). Collectively, these results demonstrate that simply broadening the donor manifold does not guarantee higher photocurrent. At intermediate donor–donor mixing ratios, spectral redundancy, reduced energetic offsets, and disturbed transport pathways amplify trap-assisted and bimolecular recombination, leading to suppressed V
OC, J
SC, and FF. Only when one donor dominates does the system regain efficient charge separation and balanced transport, explaining why the single-donor endpoints outperform the mixed-donor ternary devices.
By comparing the two representative D–D–A systems, PM6:PBDB-T:ITIC-4F and PCDTBT:PM6:PC71BM, a consistent picture emerges highlighting the intrinsic limitations in donor–donor–acceptor ternary configurations. First, spectral overlap between the donors leads to redundant absorption that is not effectively converted into photocurrent. Second, the reduced energetic offset and the presence of multiple exciton transfer pathways decrease exciton dissociation efficiency and increase non-radiative recombination. Third, the charge-transport network becomes disrupted, resulting in reduced carrier mobilities and imbalanced electron–hole transport, which ultimately deteriorate FF and JSC. These effects are particularly pronounced under indoor illumination, where the combination of low photon flux and longer carrier lifetimes magnifies the impact of recombination losses. Taken together, these findings demonstrate that the drawbacks observed are not restricted to a specific material set but represent fundamental challenges of the D–D–A architecture itself. Importantly, they also underscore that successful ternary design requires more than spectral complementarity; careful consideration of energy-level alignment, donor–donor miscibility, and percolation continuity is essential if D–D–A blends are to overcome their inherent limitations.
3.2. Ternary D–A–A System
The ternary PM6:BT-CIC:ITIC-4F system was designed to examine the donor–acceptor–acceptor (D–A–A) configuration under both solar and indoor illumination, with PM6:ITIC-4F serving as the binary reference, and BT-CIC incorporated as a secondary acceptor. The energy levels of BT-CIC are relatively well aligned with those of PM6 and ITIC-4F (
Figure 5a), suggesting a potential cascade pathway for electron transfer from PM6 to BT-CIC and subsequently to ITIC-4F. In addition, BT-CIC exhibits a distinct absorption feature in the near-infrared region (700–800 nm,
Figure 5b), which may be expected to extend the spectral response and enhance photocurrent generation. However, as confirmed by the J–V and EQE measurements shown in
Figure 5c–e and summarized in
Table 2, these anticipated benefits were not observed. The incorporation of BT-CIC does not produce a net photocurrent gain; instead, the ternary devices exhibit performance comparable to or slightly lower than the binary PM6:ITIC-4F reference under AM 1.5G illumination. The EQE spectra reveal that the additional absorption arising from BT-CIC does not contribute effectively to charge generation but instead results in a suppressed spectral response across the visible region. This indicates that excitons generated in BT-CIC are not efficiently dissociated at the donor–acceptor interface due to insufficient energetic offsets. Rather than forming a cascade-assisted charge transfer pathway, BT-CIC introduces redundant excitation channels that promote exciton accumulation and the formation of shallow CT states with limited driving force for separation. These non-ideal CT states act as recombination centers, facilitating non-radiative decay and reducing carrier extraction efficiency. Under TL84 indoor illumination, these effects become even more pronounced, as the reduced photon flux and narrower spectral distribution intensify the imbalance between exciton generation and dissociation. Although V
OC remains nearly unchanged, both J
SC and FF decrease substantially, leading to a notable drop in overall PCE (from 15.45% to 13.48%).
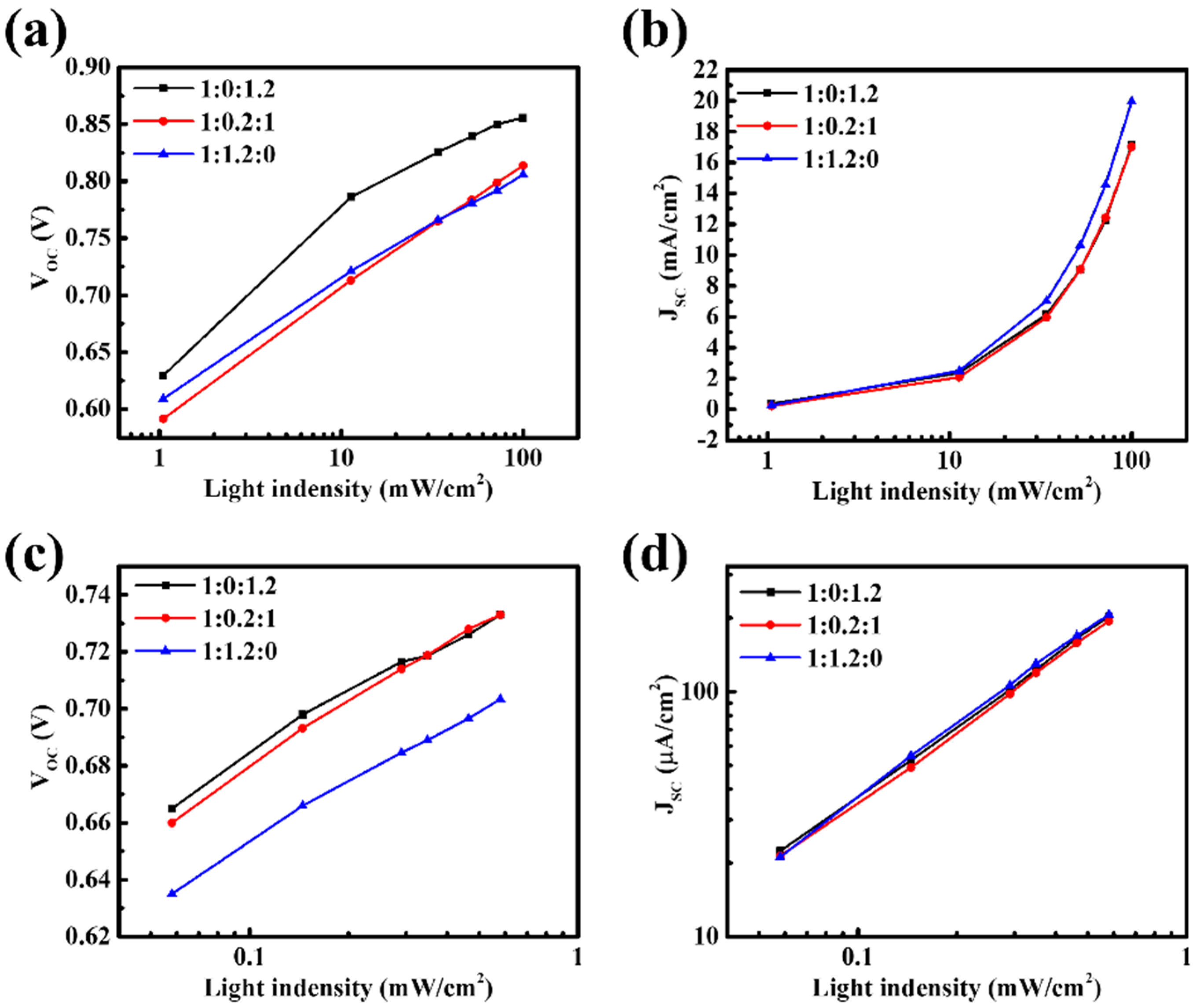
Light-intensity-dependent measurements were further conducted to elucidate the recombination behavior of the ternary PM6:BT-CIC:ITIC-4F system (
Figure 6,
Table S4). Under AM 1.5G illumination, the ternary devices exhibit α values approaching unity, indicating that bimolecular recombination is partially suppressed. This improvement can be ascribed to the formation of additional percolation pathways within the BT-CIC domains, which facilitate carrier transport. However, the consistently high ideality factor n reveals that trap-assisted recombination remains significant. This suggests that while BT-CIC provides auxiliary electron-transport channels, it simultaneously introduces interfacial trap states or perturbs the donor–acceptor energetic alignment, thereby promoting trap-mediated recombination. Under TL84 indoor illumination, the imbalance between these two recombination processes becomes more pronounced. While α remains comparable to that of the binary reference, the n values increase markedly, confirming that carrier trapping dominates over bimolecular recombination under low-light conditions. Such behavior implies the formation of trap-rich interfacial regions or shallow CT states that extend carrier lifetimes but hinder efficient charge extraction. As a result, photogenerated carriers accumulate within localized energy minima and undergo non-radiative decay before reaching the electrodes. Collectively, these results demonstrate that in the D–A–A configuration, the introduction of BT-CIC fails to mitigate recombination losses; instead, it alters the interfacial energetics in a manner that exacerbates trap-assisted processes, thereby accounting for the observed degradation in device performance, particularly under indoor illumination.
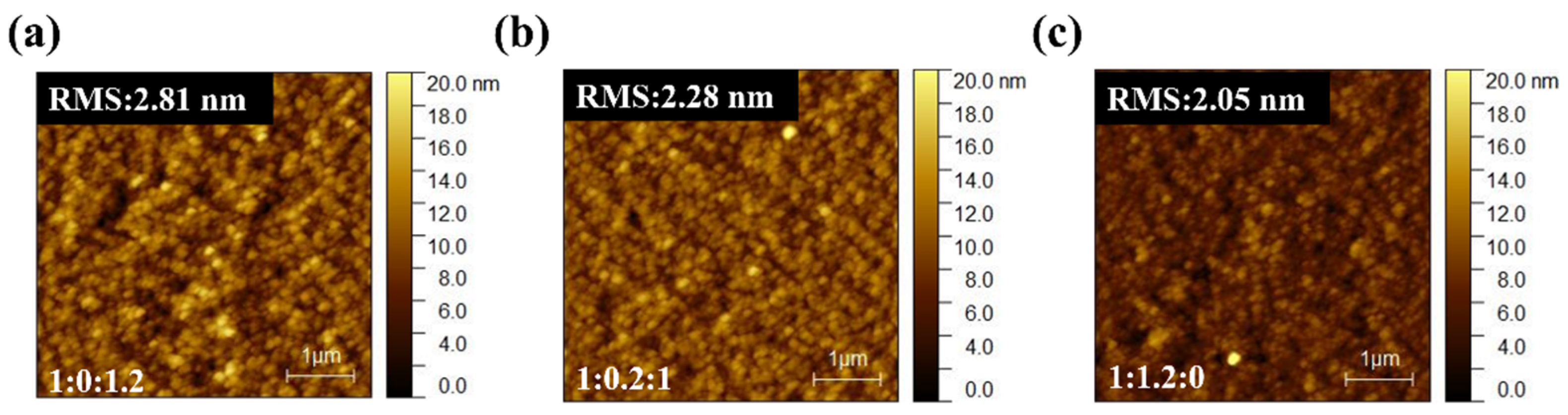
AFM analysis (
Figure 7) reveals that the surface morphologies of PM6:BT-CIC:ITIC-4F active layers remain smooth and homogeneous, with RMS roughness values ranging from 2.0 to 2.8 nm—comparable to the binary control. This excludes macroscopic phase segregation or surface roughness as the origin of the performance deterioration. Instead, the degradation arises from molecular-scale miscibility and packing disruptions within the acceptor framework. Partial miscibility between BT-CIC and ITIC-4F perturbs the optimized molecular ordering of the host acceptor, producing heterogeneous energetic landscapes and locally disordered percolation pathways that hinder efficient carrier transport. TPC and TPV analyses provide further corroboration (
Figure 8,
Table S5). Under AM 1.5G illumination, charge extraction and decay dynamics of the ternary devices are similar to those of the binary reference, consistent with their similar efficiencies. Under TL84 indoor light, however, pronounced transport asymmetry emerges. The charge extraction time of the ternary device (5.66 μs) is nearly twice that of the binary (3.01 μs), accompanied by a substantial drop in carrier mobility (2.29 vs. 5.62 × 10
−5 cm
2/V s). These results indicate that BT-CIC interrupts continuous electron percolation channels, forcing charge carriers to traverse less favorable transport routes with higher trap densities. As a result, space-charge accumulation intensifies, leading to increased carrier-lifetime asymmetry, reduced fill factor, and enhanced trap-assisted recombination. Therefore, despite its morphological uniformity, the BT-CIC-based ternary blend exhibits intrinsic microstructural and energetic disorder that collectively impede balanced charge transport and extraction, ultimately limiting device performance under low-light operation.
A similar inefficiency was observed in the PM6:Y6:PC
71BM system. While PM6:Y6 blends already harvest photons up to the near-infrared, PC
71BM was added to extend visible-light absorption and stabilize V
OC (
Figure S2). However, PCE consistently decreased upon PC
71BM incorporation—from 12.52% to 10.73% under AM 1.5G, and from 17.79% to 15.04% under TL84 indoor light (
Table S6). The primary degradation pathway was the reduction in FF, likely arising from PC
71BM aggregation and poor miscibility with the Y6-rich host. Such clustering disrupts percolation, creating isolated domains and charge-blocking regions. As a result, photocurrent generation becomes spatially inhomogeneous, and recombination is amplified. In the PTB7-Th:3TT-FIC:PC
71BM system, PC
71BM was added to supplement visible absorption (400–550 nm), complementing the broad but near-infrared-shifted absorption of PTB7-Th:3TT-FIC blends (
Figure S3). Under solar illumination, modest improvements were observed when a small fraction of PC
71BM was incorporated, raising PCE to ~10% at the 1:0.7:0.5 ratio (
Figure S4;
Table S7). However, when PC
71BM fully replaced 3TT-FIC, V
OC increased (0.78 V) but JSC sharply declined, limiting PCE to only 6.88%. More critically, under TL84 indoor light, ternary devices underperformed compared with binary PTB7-Th:PC
71BM, with efficiencies peaking at 13.84% but still lower than the expected contribution from spectral broadening. Mechanistically, this discrepancy stems from the mismatch between indoor light spectra and the absorption of 3TT-FIC, which lies predominantly in the near-infrared and thus contributes little indoors. Additionally, excess PC
71BM induces crystalline rearrangements, lowering FF and introducing transport asymmetry.
Taken together, the results from PM6:BT-CIC:ITIC-4F, PM6:Y6:PC71BM, and PTB7-Th:3TT-FIC:PC71BM highlight the intrinsic limitations of D–A–A ternary strategies. While the introduction of a second acceptor can nominally broaden optical absorption and, in principle, provide cascade-assisted electron transfer, these anticipated benefits are consistently undermined by redundant excitations, incomplete cascade formation, and destabilized energetic offsets. The competing charge-transfer pathways extend exciton lifetimes and enhance geminate or trap-assisted recombination, while poor miscibility and domain mismatch disrupt percolation networks and introduce transport asymmetry between electrons and holes. These molecular- and mesoscopic-scale instabilities manifest as reduced mobilities, imbalanced carrier extraction, and suppressed VOC, JSC, and FF. The detrimental effects are particularly severe under indoor illumination, where the combination of low photon flux and long carrier lifetimes magnifies recombination penalties. Collectively, these findings indicate that simply broadening absorption through an additional acceptor is insufficient to achieve performance gains. Instead, successful D–A–A design requires precise control over energy-level alignment, donor–acceptor miscibility, and percolation continuity to prevent recombination-prone CT states and enable efficient charge transport.
By comparatively analyzing five representative ternary systems, two based on donor–donor–acceptor (D–D–A) configurations and three based on donor–acceptor–acceptor (D–A–A) configurations, we can clearly distinguish the mechanistic origins of their limitations. In D–D–A systems, the addition of a second donor introduces spectral redundancy and narrows the driving force for charge separation, while also disturbing percolation pathways and creating transport asymmetry. In D–A–A systems, the inclusion of a secondary acceptor extends absorption into the near-infrared and in principle offers cascade-assisted electron transfer, but in practice it generates non-ideal CT states, energetic disorder, and disrupted electron percolation due to poor miscibility with the primary acceptor. Despite these mechanistic differences, both architectures converge to similar device-level outcomes: enhanced recombination, transport imbalance, and reduced photovoltaic parameters under both solar and indoor illumination. The comparative summary is presented in
Table 3.