Chiral Materials: Multidisciplinary Progress and Emerging Frontier Application Prospects
Abstract
1. Introduction
2. The Application of Chiral Materials in the Field of Optics
2.1. Chiral Materials Emit Circularly Polarized Light
2.1.1. The Definition, Classification, and Generation Mechanism of Circularly Polarized Light
2.1.2. The Principle of Circularly Polarized Light Emitted by Chiral Materials
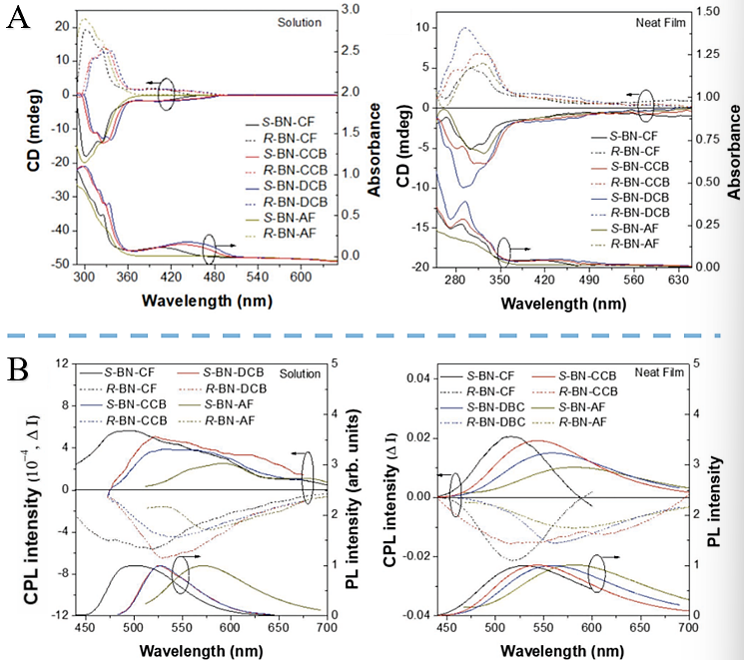
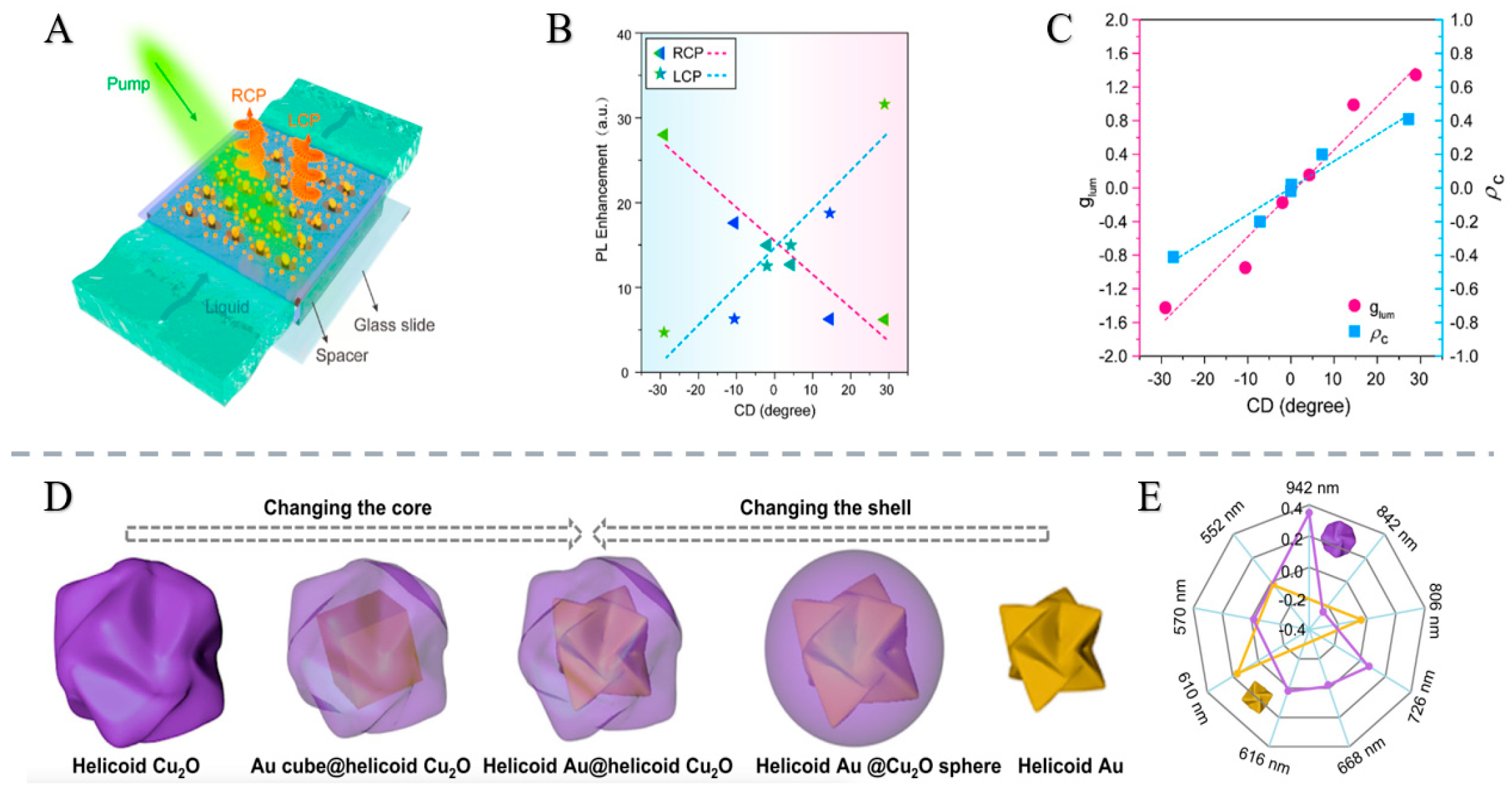
2.1.3. Research Status and Application Examples
2.2. Detection of Circularly Polarized Light by Chiral Materials
2.2.1. The Principle of Detecting Circularly Polarized Light by Chiral Materials
2.2.2. Detection Methods and Techniques of Chiral Materials
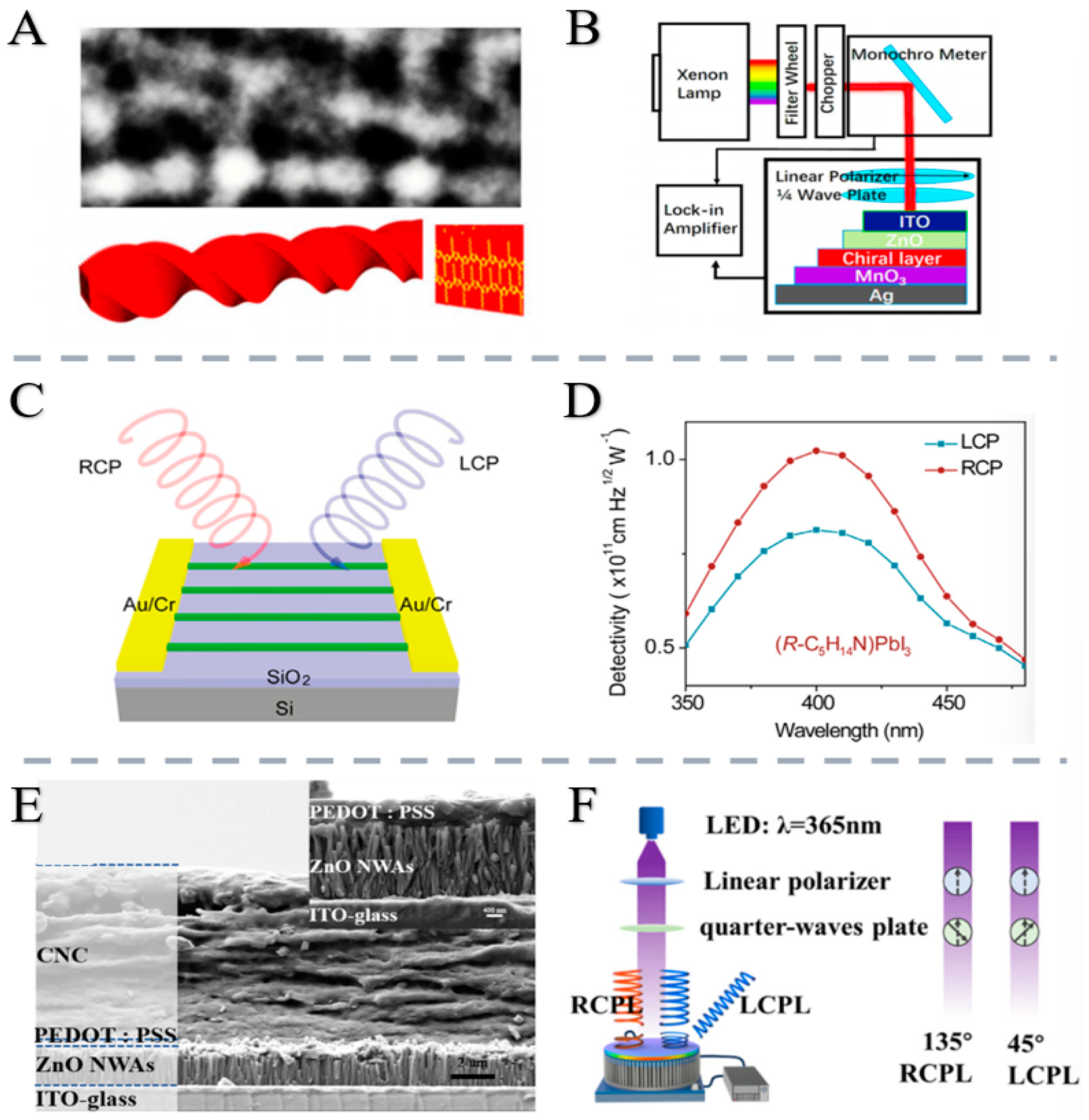
2.2.3. Research Status and Application Examples
2.3. Selective Response of Chiral Materials to Circularly Polarized Light
2.3.1. Selective Response of Circularly Polarized Light
2.3.2. Research Status and Application Examples
2.4. Chiral Materials Have a Giant Photovoltaic Effect
2.4.1. Definition of Giant Photovoltaic Effect
2.4.2. Giant Photovoltaic Effect in Chiral Materials
2.4.3. The Physical Mechanism of the Giant Photovoltaic Effect in Chiral Materials
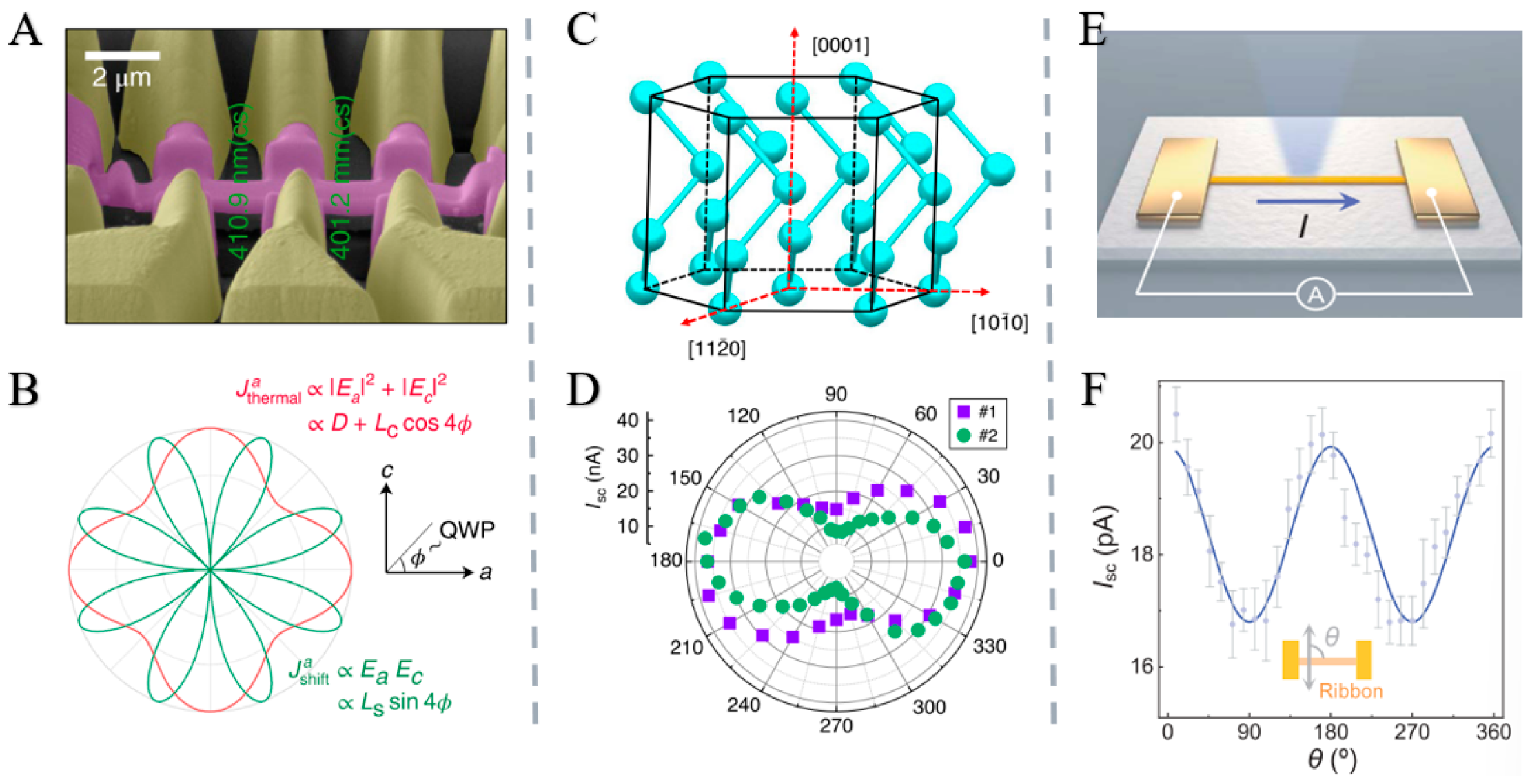
2.4.4. Research Status and Application Examples
2.5. Chiral Materials Achieve an Adjustable Chiral Optical Response
2.6. The Applications of Chiral Liquid Crystal Materials
3. The Application of Chiral Materials in the Field of Quantum Science
3.1. Chiral-Induced Spin Selectivity (CISS)
3.1.1. The Origin and Research Development of the CISS Phenomenon
3.1.2. The Theoretical Basis of CISS
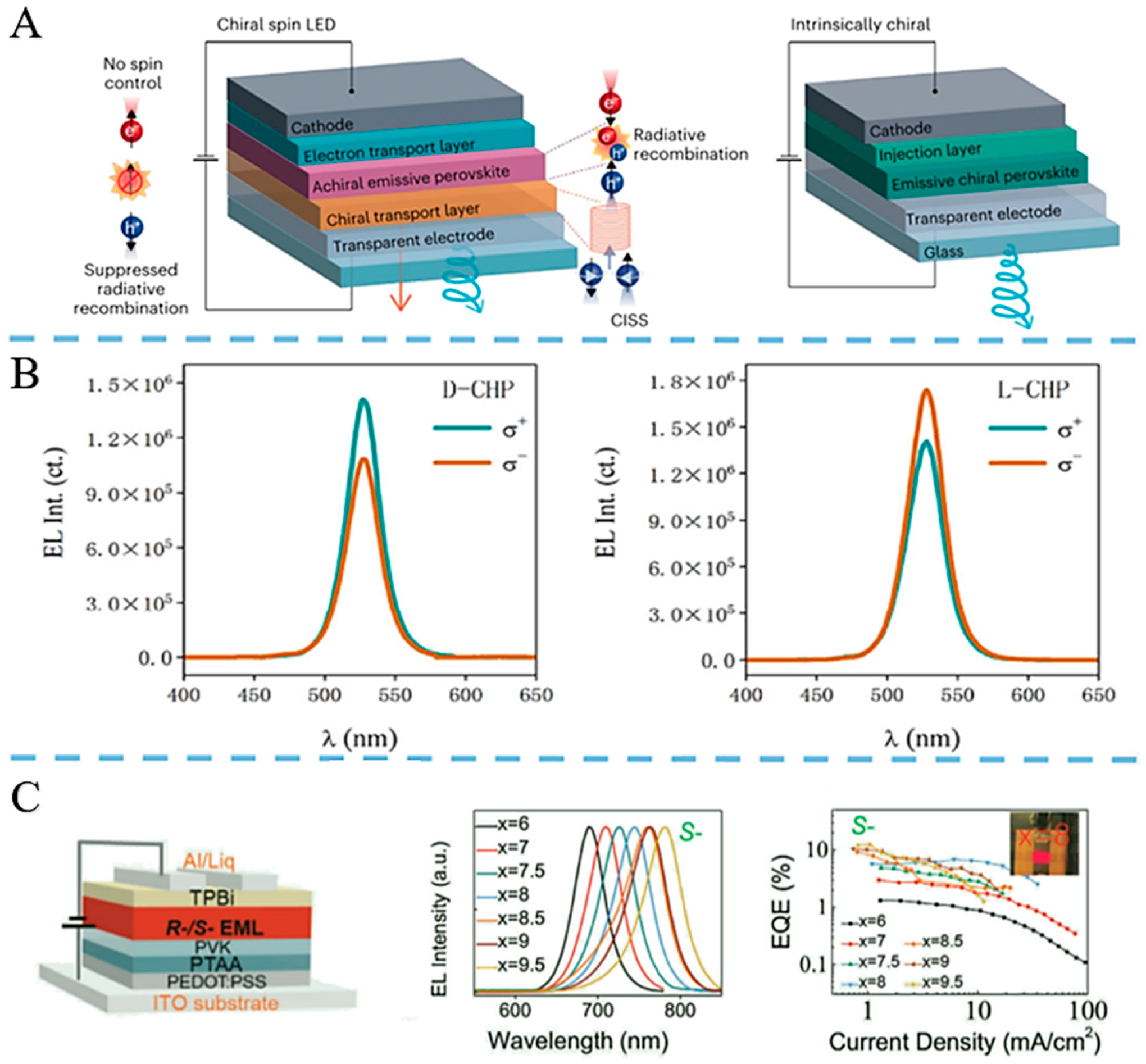
3.1.3. Research Status and Application Examples
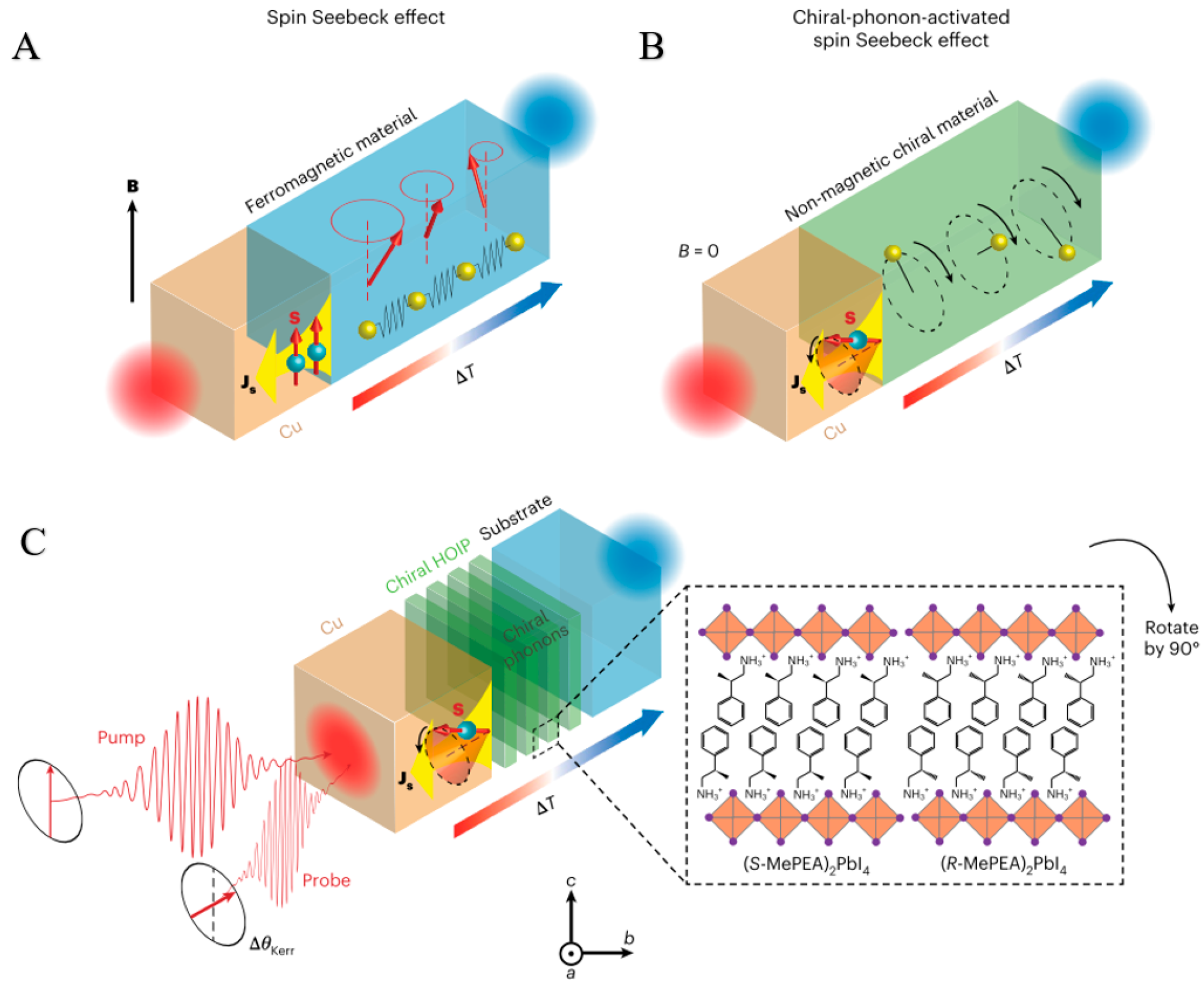
3.2. Chiral Materials Have Chiral-Phonon-Activated Spin Seebeck Effect (CPASS)
3.2.1. The Origin of the CPASS Phenomenon
3.2.2. The Theoretical Basis of CPASS
3.2.3. CPASS-Related Specific Applications
3.2.4. Research Status and Application Examples
3.3. Chiral Materials Can Be Used to Make Topological Insulators
3.4. Chiral Superconductivity
3.4.1. Chiral Superconductors
3.4.2. Chiral Topological Superconductors
4. The Application of Chiral Materials in the Field of Electricity
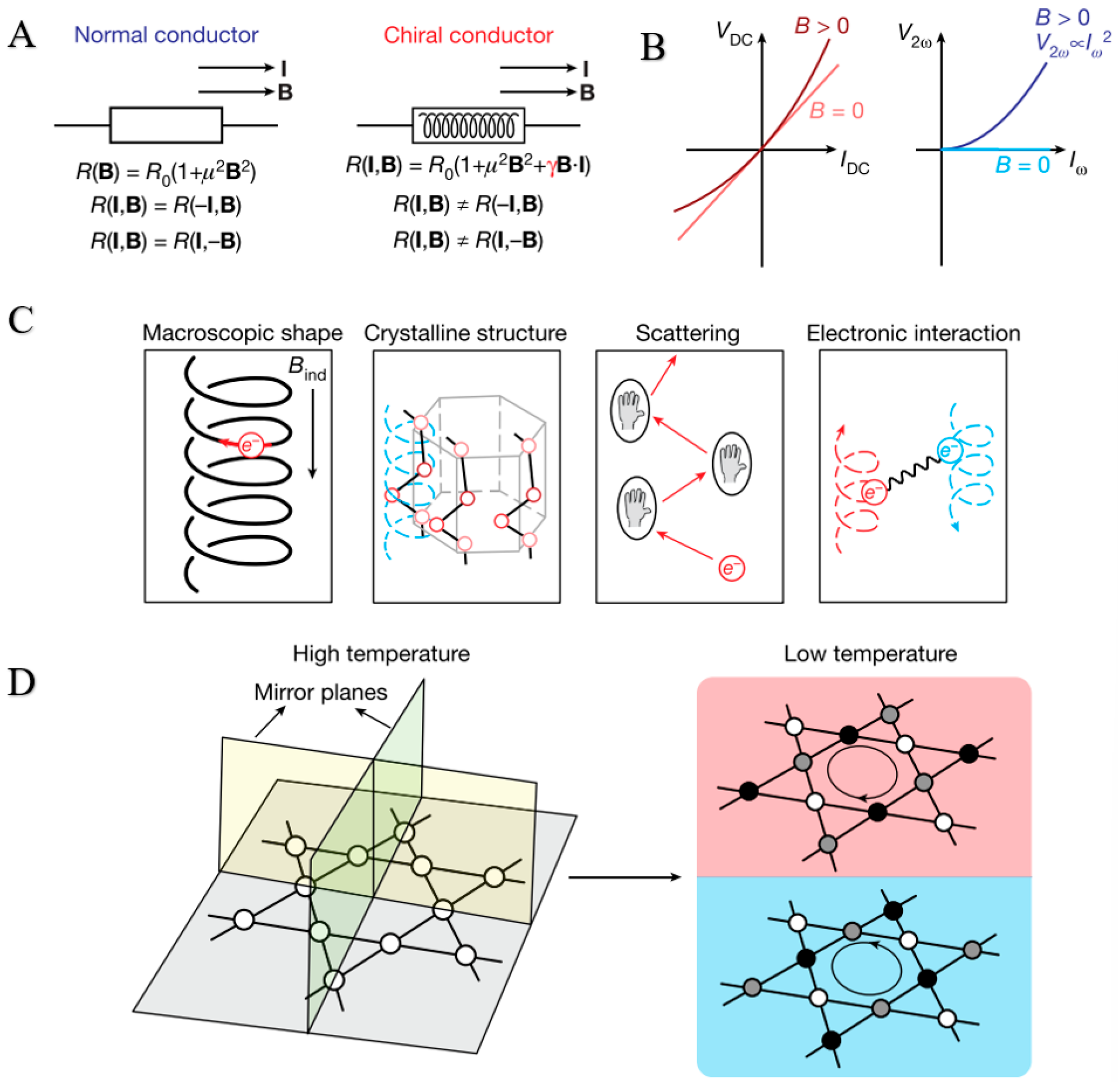
4.1. Chiral Materials Have Electromagnetic Chiral Anisotropy
4.1.1. Electromagnetic Chiral Anisotropy
4.1.2. Application Example
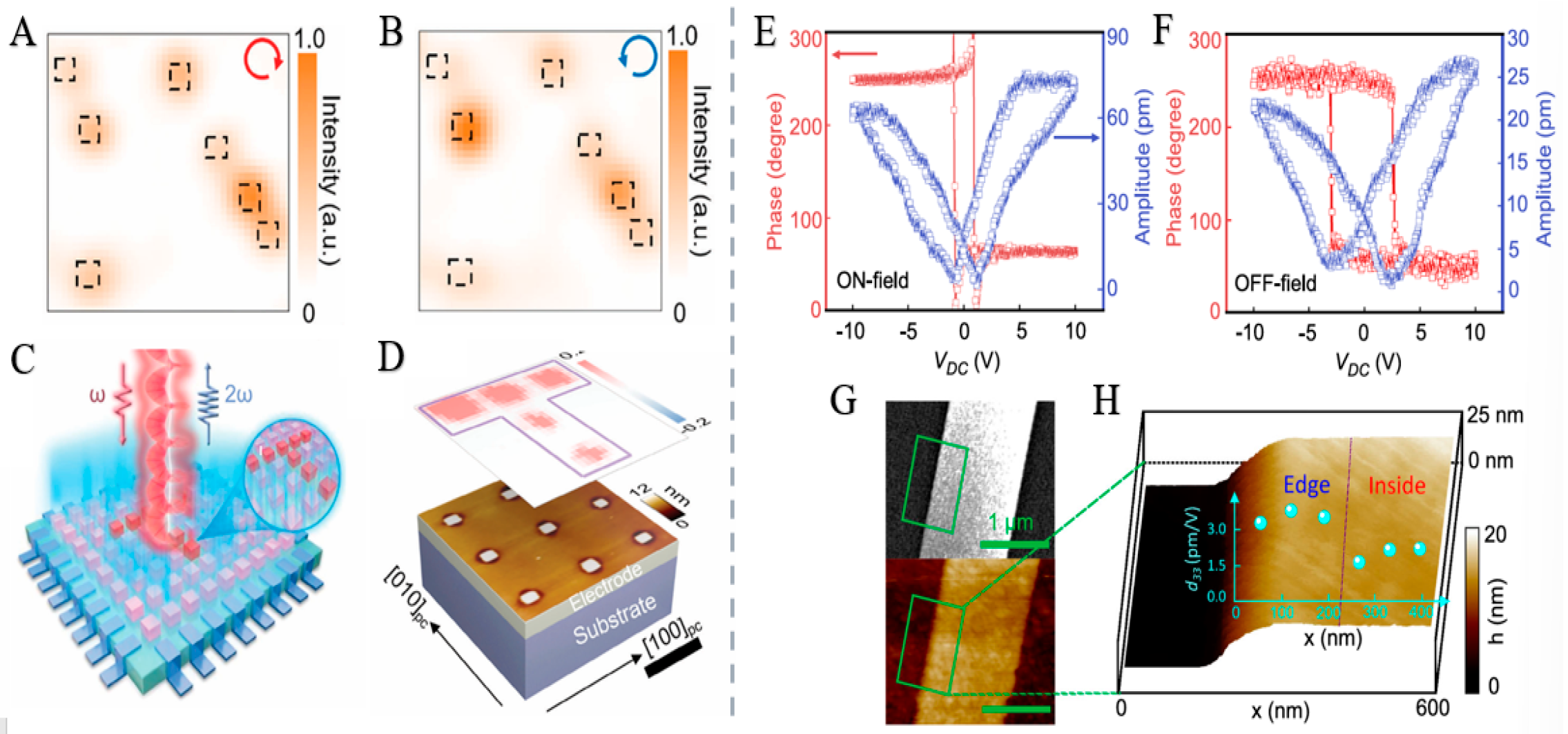
4.2. Chiral Materials Have a Ferroelectric Effect
4.2.1. Ferroelectric Effect
4.2.2. Ferroelectric Effect in Chiral Materials
4.2.3. Research Status and Application Examples
4.3. Chiral Materials Are Used for Chiral Recognition, Analysis, and Detection
4.3.1. Chiral Recognition
4.3.2. Research Status and Application
4.4. Chiral Materials for Electrocatalytic Reactions
5. Application of Chiral Materials in the Field of Biology
5.1. Chiral Materials Are Used for Enantiomeric Separation
5.2. Chiral Materials for Asymmetric Catalysis
5.3. Chiral Materials for Biomarkers
5.4. Chiral Materials Can Be Used for Disease Treatment
6. Conclusions and Foresight
6.1. Research Progress and Main Achievements of Chiral Materials
6.2. Future Research Directions and Application Prospects of Chiral Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CD | Circular dichroism |
| VCD | Vibrational circular dichroism |
| ROA | Raman optical activity |
| CPL | Circularly polarized light |
| LCPL | Left-handed circularly polarized light |
| RCPL | Right-handed circularly polarized light |
| CISS | Chiral-induced spin selectivity |
| SSE | The spin Seebeck effect |
| CPASS | Chiral-phonon-activated spin Seebeck effect |
| BPVE | Bulk photovoltaic effect |
References
- Collins, J.T.; Kuppe, C.; Hooper, D.C.; Sibilia, C.; Centini, M.; Valev, V.K. Chirality and Chiroptical Effects in Metal Nanostructures: Fundamentals and Current Trends. Adv. Opt. Mater. 2017, 5, 1700182. [Google Scholar] [CrossRef]
- Niu, X.; Zhao, R.; Yan, S.; Pang, Z.; Li, H.; Yang, X.; Wang, K. Chiral Materials: Progress, Applications, and Prospects. Small 2023, 19, e2303059. [Google Scholar] [CrossRef]
- Zhou, M.; Guo, S.; Li, J.; Luo, X.; Liu, Z.; Zhang, T.; Cao, X.; Long, M.; Lu, B.; Pan, A.; et al. Surface-Preferred Crystal Plane for a Stable and Reversible Zinc Anode. Adv. Mater. 2021, 33, e2100187. [Google Scholar] [CrossRef]
- Fent, K.; Weston, A.A.; Caminada, D. Erratum to “Ecotoxicology of human pharmaceuticals”. Aquat. Toxicol. 2006, 78, 207. [Google Scholar] [CrossRef]
- Carlsson, C.; Johansson, A.K.; Alvan, G.; Bergman, K.; Kuhler, T. Are pharmaceuticals potent environmental pollutants? Part II: Environmental risk assessments of selected pharmaceutical excipients. Sci. Total Environ. 2006, 364, 88–95. [Google Scholar] [CrossRef]
- Chen, T.; Wang, D.; Wan, L.-J. Two-dimensional chiral molecular assembly on solid surfaces: Formation and regulation. Natl. Sci. Rev. 2015, 2, 205–216. [Google Scholar] [CrossRef]
- Banville, D.L.; Marzilli, L.G.; Strickland, J.A.; Wilson, W.D. Comparison of the effects of cationic porphyrins on DNA properties: Influence of GC content of native and synthetic polymers. Biopolymers 1986, 25, 1837–1858. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, B.; Gill, P. Circular dichroism techniques: Biomolecular and nanostructural analyses—A review. Chem. Biol. Drug Des. 2009, 74, 101–120. [Google Scholar] [CrossRef] [PubMed]
- Arad, E.; Bhunia, S.K.; Jopp, J.; Kolusheva, S.; Rapaport, H.; Jelinek, R. Lysine—Derived Carbon Dots for Chiral Inhibition of Prion Peptide Fibril Assembly. Adv. Ther. 2018, 1, 1800006. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, G.; Xia, H.; Zou, G.; Zhang, Q.; Gao, J. Enantioselective synthesis of helical polydiacetylene by application of linearly polarized light and magnetic field. Nat. Commun. 2014, 5, 5050. [Google Scholar] [CrossRef]
- Teichert, J.F.; Feringa, B.L. Phosphoramidites: Privileged ligands in asymmetric catalysis. Angew. Chem. Int. Ed. Engl. 2010, 49, 2486–2528. [Google Scholar] [CrossRef]
- Zhang, J.H.; Xie, S.-M.; Chen, L.; Wang, B.-J.; He, P.-G.; Yuan, L.-M. Homochiral Porous Organic Cage with High Selectivity for the Separation of Racemates in Gas Chromatography. Anal. Chem. 2015, 87, 7817–7824. [Google Scholar] [CrossRef]
- Mulder, D.J.; Schenning, A.P.H.J.; Bastiaansen, C.W.M. Chiral-nematic liquid crystals as one dimensional photonic materials in optical sensors. J. Mater. Chem. C 2014, 2, 6695–6705. [Google Scholar] [CrossRef]
- Trojanowicz, M.; Kaniewska, M. Electrochemical Chiral Sensors and Biosensors. Electroanalysis 2009, 21, 229–238. [Google Scholar] [CrossRef]
- Farshchi, R.; Ramsteiner, M.; Herfort, J.; Tahraoui, A.; Grahn, H.T. Optical communication of spin information between light emitting diodes. Appl. Phys. Lett. 2011, 98, 162508. [Google Scholar] [CrossRef]
- He, Y.-M.; Cheng, Y.-Z.; Duan, Y.; Zhang, Y.-D.; Fan, Q.-H.; You, S.-L.; Luo, S.; Zhu, S.-F.; Fu, X.-F.; Zhou, Q.-L. Recent Progress of Asymmetric Catalysis from a Chinese Perspective. CCS Chem. 2023, 5, 2685–2716. [Google Scholar] [CrossRef]
- Zhou, Y.; Yue, X.; Jiang, F.; Sun, J.; Guo, W. Catalytic asymmetric synthesis of alpha-tertiary aminoketones from sulfoxonium ylides bearing two aryl groups. Chem. Commun. 2023, 59, 1193–1196. [Google Scholar] [CrossRef]
- Wagenknecht, C.; Li, C.-M.; Reingruber, A.; Bao, X.-H.; Goebel, A.; Chen, Y.-A.; Zhang, Q.; Chen, K.; Pan, J.-W. Experimental demonstration of a heralded entanglement source. Nat. Photonics 2010, 4, 549–552. [Google Scholar] [CrossRef]
- Xue, G.; Zhou, Z.; Guo, Q.; Zuo, Y.; Wei, W.; Yang, J.; Yin, P.; Zhang, S.; Zhong, D.; You, Y.; et al. WS2 ribbon arrays with defined chirality and coherent polarity. Science 2024, 384, 1100–1104. [Google Scholar] [CrossRef]
- Qian, Q.; Ren, H.; Zhou, J.; Wan, Z.; Zhou, J.; Yan, X.; Cai, J.; Wang, P.; Li, B.; Sofer, Z.; et al. Chiral molecular intercalation superlattices. Nature 2022, 606, 902–908. [Google Scholar] [CrossRef]
- Kim, K.; Vetter, E.; Yan, L.; Yang, C.; Wang, Z.; Sun, R.; Yang, Y.; Comstock, A.H.; Li, X.; Zhou, J.; et al. Chiral-phonon-activated spin Seebeck effect. Nat. Mater. 2023, 22, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Legg, H.F.; Rößler, M.; Münning, F.; Fan, D.; Breunig, O.; Bliesener, A.; Lippertz, G.; Uday, A.; Taskin, A.A.; Loss, D.; et al. Giant magnetochiral anisotropy from quantum-confined surface states of topological insulator nanowires. Nat. Nanotechnol. 2022, 17, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Qiu, G.; Ren, H.; Qian, Q.; Li, Y.; Xu, D.; Zhou, J.; Zhou, J.; Zhou, B.; Wang, L.; et al. Unconventional superconductivity in chiral molecule-TaS2 hybrid superlattices. Nature 2024, 632, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, J.; Qi, Y.; Gong, S.; Xu, H.; Liu, Z.; Zhang, R.; Sadi, M.A.; Sychev, D.; Zhao, R.; et al. Room-temperature ferroelectric, piezoelectric and resistive switching behaviors of single-element Te nanowires. Nat. Commun. 2024, 15, 7648. [Google Scholar] [CrossRef]
- Liang, Y.; Banjac, K.; Martin, K.; Zigon, N.; Lee, S.; Vanthuyne, N.; Garcés-Pineda, F.A.; Galán-Mascarós, J.R.; Hu, X.; Avarvari, N.; et al. Enhancement of electrocatalytic oxygen evolution by chiral molecular functionalization of hybrid 2D electrodes. Nat. Commun. 2022, 13, 3356. [Google Scholar] [CrossRef]
- Lv, X.; Tian, Y.; Wu, F.; Luan, X.; Li, F.; Shen, Z.; Xu, G.; Liu, K.; Niu, W. Chiral plasmonic-dielectric coupling enables strong near-infrared chiroptical responses from helicoidal core-shell nanoparticles. Nat. Commun. 2024, 15, 9234. [Google Scholar] [CrossRef]
- Niu, X.; Liu, Y.; Zhao, R.; Yuan, M.; Zhao, H.; Li, H.; Wang, K. Enhancing Electrochemical Signal for Efficient Chiral Recognition by Encapsulating C60 Fullerene into Chiral Lanthanum-Based MOFs. ACS Appl. Mater. Interfaces 2024, 16, 17361–17370. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Z.; Armstrong, D.W.; Wolosker, H.; Zheng, Y. Detection and analysis of chiral molecules as disease biomarkers. Nat. Rev. Chem. 2023, 7, 355–373. [Google Scholar] [CrossRef]
- Antil, N.; Akhtar, N.; Newar, R.; Begum, W.; Kumar, A.; Chauhan, M.; Manna, K. Chiral Iron(II)-Catalysts within Valinol-Grafted Metal–Organic Frameworks for Enantioselective Reduction of Ketones. ACS Catal. 2021, 11, 10450–10459. [Google Scholar] [CrossRef]
- Wang, X.; Xiang, S.; Qi, C.; Chen, M.; Su, X.; Yang, J.-C.; Tian, J.; Feng, H.-T.; Tang, B.Z. Visualization of Enantiorecognition and Resolution by Chiral AIEgens. ACS Nano 2022, 16, 8223–8232. [Google Scholar] [CrossRef]
- Rikken, G.; Avarvari, N. Dielectric magnetochiral anisotropy. Nat. Commun. 2022, 13, 3564. [Google Scholar] [CrossRef]
- Trippe, S. Polarization and Polarimetry: A Review. J. Korean Astron. Soc. 2014, 47, 15–39. [Google Scholar] [CrossRef]
- Zhang, G.; Lyu, X.; Qin, Y.; Li, Y.; Fan, Z.; Meng, X.; Cheng, Y.; Cao, Z.; Xu, Y.; Sun, D.; et al. High discrimination ratio, broadband circularly polarized light photodetector using dielectric achiral nanostructures. Light. Sci. Appl. 2024, 13, 275. [Google Scholar] [CrossRef]
- Yu, N.; Aieta, F.; Genevet, P.; Kats, M.A.; Gaburro, Z.; Capasso, F. A broadband, background-free quarter-wave plate based on plasmonic metasurfaces. Nano Lett. 2012, 12, 6328–6333. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tang, Y.; Fan, Q.; Li, Z.; Zhang, X.; Wang, J.; Guo, J.; Li, Q. When quantum dots meet blue phase liquid crystal elastomers: Visualized full-color and mechanically-switchable circularly polarized luminescence. Light Sci. Appl. 2024, 13, 140. [Google Scholar] [CrossRef]
- Pan, R.; Tao, S.; Kan, L.; Hu, J.; Li, J.; Li, Y.; Zhang, X.; Wang, K. Tunable and Large Magneto—Photoluminescence for Single—Crystalline Chiral Perovskites. Adv. Opt. Mater. 2022, 10, 2200064. [Google Scholar] [CrossRef]
- Di Nuzzo, D.; Cui, L.; Greenfield, J.L.; Zhao, B.; Friend, R.H.; Meskers, S.C.J. Circularly Polarized Photoluminescence from Chiral Perovskite Thin Films at Room Temperature. ACS Nano 2020, 14, 7610–7616. [Google Scholar] [CrossRef]
- Ma, J.; Fang, C.; Chen, C.; Jin, L.; Wang, J.; Wang, S.; Tang, J.; Li, D. Chiral 2D Perovskites with a High Degree of Circularly Polarized Photoluminescence. ACS Nano 2019, 13, 3659–3665. [Google Scholar] [CrossRef] [PubMed]
- Martin, S. Liquid Crystal Materials And Liquid Crystal Displays. Annu. Rev. Mater. Sci. 1997, 27, 305–379. [Google Scholar]
- Song, F.; Xu, Z.; Zhang, Q.; Zhao, Z.; Zhang, H.; Zhao, W.; Qiu, Z.; Qi, C.; Zhang, H.; Sung, H.H.Y.; et al. Highly Efficient Circularly Polarized Electroluminescence from Aggregation—Induced Emission Luminogens with Amplified Chirality and Delayed Fluorescence. Adv. Funct. Mater. 2018, 28, 1800051. [Google Scholar] [CrossRef]
- Furlan, F.; Moreno-Naranjo, J.M.; Gasparini, N.; Feldmann, S.; Wade, J.; Fuchter, M.J. Chiral materials and mechanisms for circularly polarized light-emitting diodes. Nat. Photonics 2024, 18, 658–668. [Google Scholar] [CrossRef]
- Tang, J.; Tao, S.; Li, Y.; Zhang, X.; Kan, L.; Zhang, G.; Jiang, L.; Zhou, J.; Qin, Y.; Sun, X.; et al. Chiral Ionic Liquids Enable High—Performance Room Temperature Single Junction Spin—Light Emitting Diodes. Laser Photonics Rev. 2024, 19, 2401008. [Google Scholar] [CrossRef]
- Yao, J.; Wang, Z.; Huang, Y.; Xue, J.; Zhang, D.; Chen, J.; Chen, X.; Dong, S.-C.; Lu, H. Efficient Green Spin Light-Emitting Diodes Enabled by Ultrafast Energy- and Spin-Funneling in Chiral Perovskites. J. Am. Chem. Soc. 2024, 146, 14157–14165. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Huang, Y.; Sun, H.; Wang, Z.; Xue, J.; Huang, Z.; Dong, S.; Chen, X.; Lu, H. Efficient Spin—Light—Emitting Diodes With Tunable Red to Near—Infrared Emission at Room Temperature. Adv. Mater. 2025, 37, 2413669. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; da Costa, R.C.; Fuchter, M.J.; Campbell, A.J. Circularly polarized light detection by a chiral organic semiconductor transistor. Nat. Photonics 2013, 7, 634–638. [Google Scholar] [CrossRef]
- Berova, N.; Di Bari, L.; Pescitelli, G. Application of electronic circular dichroism in configurational and conformational analysis of organic compounds. Chem. Soc. Rev. 2007, 36, 914–931. [Google Scholar] [CrossRef]
- Zheng, H.; Ju, B.; Wang, X.; Wang, W.; Li, M.; Tang, Z.; Zhang, S.X.; Xu, Y. Circularly Polarized Luminescent Carbon Dot Nanomaterials of Helical Superstructures for Circularly Polarized Light Detection. Adv. Opt. Mater. 2018, 6, 1801246. [Google Scholar] [CrossRef]
- Wei, J.; Chen, Y.; Li, Y.; Li, W.; Xie, J.; Lee, C.; Novoselov, K.S.; Qiu, C.-W. Geometric filterless photodetectors for mid-infrared spin light. Nat. Photonics 2022, 17, 171–178. [Google Scholar] [CrossRef]
- Liu, S.; Yu, F.; Liu, X.; Zhang, H.; Ma, M.; Zhang, S.; Guo, H.; Hu, H.; Yuan, C.; Zheng, Z.; et al. High-performance integrated circularly polarized light detection using soft-helix-decorated perovskite diodes. Newton 2025, 1, 100003. [Google Scholar] [CrossRef]
- Chen, C.; Gao, L.; Gao, W.; Ge, C.; Du, X.; Li, Z.; Yang, Y.; Niu, G.; Tang, J. Circularly polarized light detection using chiral hybrid perovskite. Nat. Commun. 2019, 10, 1927. [Google Scholar] [CrossRef]
- Valev, V.K.; Baumberg, J.J.; Sibilia, C.; Verbiest, T. Chirality and chiroptical effects in plasmonic nanostructures: Fundamentals, recent progress, and outlook. Adv. Mater. 2013, 25, 2517–2534. [Google Scholar] [CrossRef]
- Xu, C.; Ren, Z.; Zhou, H.; Zhou, J.; Ho, C.P.; Wang, N.; Lee, C. Expanding chiral metamaterials for retrieving fingerprints via vibrational circular dichroism. Light Sci. Appl. 2023, 12, 154. [Google Scholar] [CrossRef] [PubMed]
- Liebel, M.; Toninelli, C.; van Hulst, N.F. Room-temperature ultrafast nonlinear spectroscopy of a single molecule. Nat. Photonics 2017, 12, 45–49. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, M.; Hao, X.; Qin, W. Helical-chiroptical nanowires generated orbital angular momentum for the detection of circularly polarized light. Appl. Phys. Lett. 2020, 116, 053301. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Feng, J.; Zhao, J.; Guo, Y.; Yuan, M.; Chen, G.; Gao, H.; Jiang, L.; Wu, Y. Chiral 1D perovskite microwire arrays for circularly polarized light detection. Giant 2022, 9, 100086. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Qiu, L. Circularly Polarized Photodetectors Based on Chiral Materials: A Review. Front. Chem. 2021, 9, 711488. [Google Scholar] [CrossRef]
- Dou, L.; Yang, Y.; You, J.; Hong, Z.; Chang, W.-H.; Li, G.; Yang, Y. Solution-processed hybrid perovskite photodetectors with high detectivity. Nat. Commun. 2014, 5, 5404. [Google Scholar] [CrossRef]
- Lu, J.; Carvalho, A.; Liu, H.; Lim, S.X.; Neto, A.H.C.; Sow, C.H. Hybrid Bilayer WSe2 -CH3 NH3 PbI3 Organolead Halide Perovskite as a High-Performance Photodetector. Angew. Chem. Int. Ed. Engl. 2016, 55, 11945–11949. [Google Scholar] [CrossRef]
- Yang, J.; Huang, Y.; He, S.; Dang, C.; Li, M.; Zhao, L.; Gao, L. High-performance and environmentally friendly circularly polarized light direct detection based on ZnO nanowires and chiral cellulose nanocrystals. J. Mater. Chem. C 2024, 12, 2505–2514. [Google Scholar] [CrossRef]
- Sanchez-Carnerero, E.M.; Agarrabeitia, A.R.; Moreno, F.; Maroto, B.L.; Muller, G.; Ortiz, M.J.; de la Moya, S. Circularly Polarized Luminescence from Simple Organic Molecules. Chemistry 2015, 21, 13488–13500. [Google Scholar] [CrossRef]
- Zinna, F.; Di Bari, L. Lanthanide circularly polarized luminescence: Bases and applications. Chirality 2015, 27, 1–13. [Google Scholar] [CrossRef]
- Wong, H.-Y.; Lo, W.-S.; Yim, K.-H.; Law, G.-L. Chirality and Chiroptics of Lanthanide Molecular and Supramolecular Assemblies. Chem. 2019, 5, 3058–3095. [Google Scholar] [CrossRef]
- Ikai, T.; Shimizu, S.; Awata, S.; Shinohara, K.-i. Chiral Amplification in π-Conjugated Helical Polymers with Circularly Polarized Luminescence. Macromolecules 2018, 51, 2328–2334. [Google Scholar] [CrossRef]
- Wu, Y.; You, L.H.; Yu, Z.-Q.; Wang, J.-H.; Meng, Z.; Liu, Y.; Li, X.-S.; Fu, K.; Ren, X.-K.; Tang, B.Z. Rational Design of Circularly Polarized Luminescent Aggregation-Induced Emission Luminogens (AIEgens): Promoting the Dissymmetry Factor and Emission Efficiency Synchronously. ACS Mater. Lett. 2020, 2, 505–510. [Google Scholar] [CrossRef]
- Kumar, J.; Nakashima, T.; Kawai, T. Circularly Polarized Luminescence in Chiral Molecules and Supramolecular Assemblies. J. Phys. Chem. Lett. 2015, 6, 3445–3452. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Zhao, Z.; Liu, Z.; Lam, J.W.Y.; Tang, B.Z. Circularly polarized luminescence from AIEgens. J. Mater. Chem. C 2020, 8, 3284–3301. [Google Scholar] [CrossRef]
- Ai, Y.; Fei, Y.; Shu, Z.; Zhu, Y.; Liu, J.; Li, Y. Visible-light-controlled ternary chiroptical switches with high-performance circularly polarized luminescence for advanced optical information storage and anti-counterfeiting materials. Chem. Eng. J. 2022, 450, 138390. [Google Scholar] [CrossRef]
- Hou, A.; Chen, H.; Zheng, C.; Xie, K.; Gao, A. Assembly of a Fluorescent Chiral Photonic Crystal Membrane and Its Sensitive Responses to Multiple Signals Induced by Small Molecules. ACS Nano 2020, 14, 7380–7388. [Google Scholar] [CrossRef]
- Lv, J.; Ding, D.; Yang, X.; Hou, K.; Miao, X.; Wang, D.; Kou, B.; Huang, L.; Tang, Z. Biomimetic Chiral Photonic Crystals. Angew. Chem. Int. Ed. Engl. 2019, 58, 7783–7787. [Google Scholar] [CrossRef]
- Han, D.; Yang, X.; Han, J.; Zhou, J.; Jiao, T.; Duan, P. Sequentially amplified circularly polarized ultraviolet luminescence for enantioselective photopolymerization. Nat. Commun. 2020, 11, 5659. [Google Scholar] [CrossRef]
- Zhang, D.W.; Li, M.; Chen, C.F. Recent advances in circularly polarized electroluminescence based on organic light-emitting diodes. Chem. Soc. Rev. 2020, 49, 1331–1343. [Google Scholar] [CrossRef]
- Kunnen, B.; Macdonald, C.; Doronin, A.; Jacques, S.; Eccles, M.; Meglinski, I. Application of circularly polarized light for non-invasive diagnosis of cancerous tissues and turbid tissue-like scattering media. J. Biophotonics 2015, 8, 317–323. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, L.E.; Pal, R. Circularly polarized lanthanide luminescence for advanced security inks. Nat. Rev. Chem. 2021, 5, 109–124. [Google Scholar] [CrossRef]
- Yang, X.; Lv, J.; Zhang, J.; Shen, T.; Xing, T.; Qi, F.; Ma, S.; Gao, X.; Zhang, W.; Tang, Z. Tunable Circularly Polarized Luminescence from Inorganic Chiral Photonic Crystals Doped with Quantum Dots. Angew. Chem. Int. Ed. Engl. 2022, 61, e202201674. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, X.; Khan, T.; Ji, C.; Asghar, M.A.; Zhao, S.; Li, L.; Hong, M.; Luo, J. A Photoferroelectric Perovskite-Type Organometallic Halide with Exceptional Anisotropy of Bulk Photovoltaic Effects. Angew. Chem. Int. Ed. Engl. 2016, 55, 6545–6550. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, Q.-Z.; Yeats, A.L.; Pillsbury, T.; Flanagan, T.C.; Richardella, A.; Zhang, H.; Awschalom, D.D.; Liu, C.-X.; Samarth, N. Helicity dependent photocurrent in electrically gated (Bi1−xSbx)2Te3 thin films. Nat. Commun. 2017, 8, 1037. [Google Scholar] [CrossRef]
- Tan, L.Z.; Zheng, F.; Young, S.M.; Wang, F.; Liu, S.; Rappe, A.M. Shift current bulk photovoltaic effect in polar materials—Hybrid and oxide perovskites and beyond. npj Comput. Mater. 2016, 2, 16026. [Google Scholar] [CrossRef]
- Glazov, M.M.; Ganichev, S.D. High frequency electric field induced nonlinear effects in graphene. Phys. Rep. 2014, 535, 101–138. [Google Scholar] [CrossRef]
- Osterhoudt, G.B.; Diebel, L.K.; Gray, M.J.; Yang, X.; Stanco, J.; Huang, X.; Shen, B.; Ni, N.; Moll, P.J.W.; Ran, Y.; et al. Colossal mid-infrared bulk photovoltaic effect in a type-I Weyl semimetal. Nat. Mater. 2019, 18, 471–475. [Google Scholar] [CrossRef]
- Ma, Q.; Xu, S.-Y.; Chan, C.-K.; Zhang, C.-L.; Chang, G.; Lin, Y.; Xie, W.; Palacios, T.; Lin, H.; Jia, S.; et al. Direct optical detection of Weyl fermion chirality in a topological semimetal. Nat. Phys. 2017, 13, 842–847. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, C.; Peng, M.; Yu, Y.; Zhong, F.; Wang, P.; He, T.; Wang, Y.; Zhang, Z.; Xie, R.; et al. Giant infrared bulk photovoltaic effect in tellurene for broad-spectrum neuromodulation. Light Sci. Appl. 2024, 13, 277. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, H.; Li, D. Recent Progress of Chiral Perovskites: Materials, Synthesis, and Properties. Adv. Mater. 2021, 33, e2008785. [Google Scholar] [CrossRef] [PubMed]
- Cen, M.; Liu, J.; Wang, J.; Li, Y.; Cai, W.; Cheng, M.; Kong, D.; Tang, X.; Cao, T.; Lu, Y.-Q.; et al. Chirally Selective and Switchable Luminescence from Achiral Quantum Emitters on Suspended Twisted Stacking Metasurfaces. ACS Nano 2024, 18, 20556–20566. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zeng, H.; Cheng, M.; Wang, Z.; Wang, J.; Cen, M.; Luo, D.; Priimagi, A.; Liu, Y.J. Photoelastic plasmonic metasurfaces with ultra-large near infrared spectral tuning. Mater. Horiz. 2022, 9, 942–951. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, Y.; Ji, C.; Chen, S.; Li, X.; Zhang, X.; Yao, Y.; Li, J. Fano-Enhanced Circular Dichroism in Deformable Stereo Metasurfaces. Adv. Mater. 2020, 32, e1907077. [Google Scholar] [CrossRef]
- Jun, Y.C.; Huang, K.C.; Brongersma, M.L. Plasmonic beaming and active control over fluorescent emission. Nat. Commun. 2011, 2, 283. [Google Scholar] [CrossRef]
- Lv, X.; Wu, F.; Tian, Y.; Zuo, P.; Li, F.; Xu, G.; Niu, W. Engineering the Intrinsic Chirality of Plasmonic Au@Pd Metamaterials for Highly Sensitive Chiroplasmonic Hydrogen Sensing. Adv. Mater. 2023, 35, e2305429. [Google Scholar] [CrossRef]
- Wu, F.; Tian, Y.; Luan, X.; Lv, X.; Li, F.; Xu, G.; Niu, W. Synthesis of Chiral Au Nanocrystals with Precise Homochiral Facets for Enantioselective Surface Chemistry. Nano Lett. 2022, 22, 2915–2922. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Wang, Y.; Cao, G.; Liang, Q.; Zhang, X.; Sun, H.; Zhang, Y.; Wang, Z.; Liu, X.; et al. Dynamic optical chirality based on liquid crystal-embedded nano-cilia photonic structures. Nat. Commun. 2025, 16, 6569. [Google Scholar] [CrossRef]
- Sun, R.; Liu, S.; Zhang, Y.; Xu, P.; Zhu, L.; Zhu, D.; Chen, W.; Wang, Y.; Ding, S.; Ge, S.; et al. Touch-Driven Bi-Chiral Superstructures for Nested Encryption of Multiplexed Optical Information. Adv. Mater. 2025, 15, e13318. [Google Scholar] [CrossRef]
- Baibich, M.N.; Broto, J.M.; Fert, A.; Van Dau, F.N.; Petroff, F.; Etienne, P.; Creuzet, G.; Friederich, A.; Chazelas, J. Giant magnetoresistance of (001)Fe/(001)Cr magnetic superlattices. Phys. Rev. Lett. 1988, 61, 2472–2475. [Google Scholar] [CrossRef]
- Binasch, G.; Grunberg, P.; Saurenbach, F.; Zinn, W. Enhanced magnetoresistance in layered magnetic structures with antiferromagnetic interlayer exchange. Phys. Rev. B Condens. Matter 1989, 39, 4828–4830. [Google Scholar] [CrossRef]
- Naaman, R.; Waldeck, D.H. Spintronics and chirality: Spin selectivity in electron transport through chiral molecules. Annu. Rev. Phys. Chem. 2015, 66, 263–281. [Google Scholar] [CrossRef]
- Medina, E.; Gonzalez-Arraga, L.A.; Finkelstein-Shapiro, D.; Berche, B.; Mujica, V. Continuum model for chiral induced spin selectivity in helical molecules. J. Chem. Phys. 2015, 142, 194308. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-H.; Naaman, R.; Paltiel, Y.; Parkin, S.S.P. Chiral spintronics. Nat. Rev. Phys. 2021, 3, 328–343. [Google Scholar] [CrossRef]
- Dalum, S.; Hedegard, P. Theory of Chiral Induced Spin Selectivity. Nano Lett. 2019, 19, 5253–5259. [Google Scholar] [CrossRef] [PubMed]
- Koretsune, T.; Arita, R.; Aoki, H. Magneto-orbital effect without spin-orbit interactions in a noncentrosymmetric zeolite-templated carbon structure. Phys. Rev. B 2012, 86, 125207. [Google Scholar] [CrossRef]
- Yeganeh, S.; Ratner, M.A.; Medina, E.; Mujica, V. Chiral electron transport: Scattering through helical potentials. J. Chem. Phys. 2009, 131, 014707. [Google Scholar] [CrossRef]
- Medina, E.; López, F.; Ratner, M.A.; Mujica, V. Chiral molecular films as electron polarizers and polarization modulators. Europhys. Lett. 2012, 99, 17006. [Google Scholar] [CrossRef]
- Gutierrez, R.; Díaz, E.; Naaman, R.; Cuniberti, G. Spin-selective transport through helical molecular systems. Phys. Rev. B 2012, 85, 081404. [Google Scholar] [CrossRef]
- Guo, A.-M.; Sun, Q.F. Sequence-dependent spin-selective tunneling along double-stranded, DNA. Phys. Rev. B 2012, 86, 115441. [Google Scholar] [CrossRef]
- Guo, A.M.; Sun, Q.F. Spin-dependent electron transport in protein-like single-helical molecules. Proc. Natl. Acad. Sci. USA 2014, 111, 11658–11662. [Google Scholar] [CrossRef]
- Rai, D.; Galperin, M. Electrically Driven Spin Currents in DNA. J. Phys. Chem. C 2013, 117, 13730–13737. [Google Scholar] [CrossRef]
- Ben Dor, O.; Yochelis, S.; Mathew, S.P.; Naaman, R.; Paltiel, Y. A chiral-based magnetic memory device without a permanent magnet. Nat. Commun. 2013, 4, 2256. [Google Scholar] [CrossRef]
- Gohler, B.; Hamelbeck, V.; Markus, T.Z.; Kettner, M.; Hanne, G.F.; Vager, Z.; Naaman, R.; Zacharias, H. Spin selectivity in electron transmission through self-assembled monolayers of double-stranded DNA. Science 2011, 331, 894–897. [Google Scholar] [CrossRef]
- Xie, Z.; Markus, T.Z.; Cohen, S.R.; Vager, Z.; Gutierrez, R.; Naaman, R. Spin specific electron conduction through DNA oligomers. Nano Lett. 2011, 11, 4652–4655. [Google Scholar] [CrossRef]
- Bloom, B.P.; Paltiel, Y.; Naaman, R.; Waldeck, D.H. Chiral Induced Spin Selectivity. Chem. Rev. 2024, 124, 1950–1991. [Google Scholar] [CrossRef]
- Jaworski, C.M.; Yang, J.; Mack, S.; Awschalom, D.D.; Heremans, J.P.; Myers, R.C. Observation of the spin-Seebeck effect in a ferromagnetic semiconductor. Nat. Mater. 2010, 9, 898–903. [Google Scholar] [CrossRef]
- Uchida, K.; Adachi, H.; An, T.; Ota, T.; Toda, M.; Hillebrands, B.; Maekawa, S.; Saitoh, E. Long-range spin Seebeck effect and acoustic spin pumping. Nat. Mater. 2011, 10, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.M.; Pearson, J.E.; Bhattacharya, A. Paramagnetic spin seebeck effect. Phys. Rev. Lett. 2015, 114, 186602. [Google Scholar] [CrossRef]
- Bauer, G.E.; Saitoh, E.; van Wees, B.J. Spin caloritronics. Nat. Mater. 2012, 11, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Bader, S.D. Opportunities at the Frontiers of Spintronics. Phys. Rev. Appl. 2015, 4, 047001. [Google Scholar] [CrossRef]
- Xiao, J.; Bauer, G.E.W.; Uchida, K.-C.; Saitoh, E.; Maekawa, S. Theory of magnon-driven spin Seebeck effect. Phys. Rev. B 2010, 81, 099904. [Google Scholar] [CrossRef]
- Rikken, G.L.; Wyder, P. Magnetoelectric anisotropy in diffusive transport. Phys. Rev. Lett. 2005, 94, 016601. [Google Scholar] [CrossRef]
- Tokura, Y.; Nagaosa, N. Nonreciprocal responses from non-centrosymmetric quantum materials. Nat. Commun. 2018, 9, 3740. [Google Scholar] [CrossRef] [PubMed]
- Breunig, O.; Ando, Y. Opportunities in topological insulator devices. Nat. Rev. Phys. 2021, 4, 184–193. [Google Scholar] [CrossRef]
- Guo, C.; Putzke, C.; Konyzheva, S.; Huang, X.; Gutierrez-Amigo, M.; Errea, I.; Chen, D.; Vergniory, M.G.; Felser, C.; Fischer, M.H.; et al. Switchable chiral transport in charge-ordered kagome metal CsV3Sb5. Nature 2022, 611, 461–466. [Google Scholar] [CrossRef]
- Qi, X.-L.; Zhang, S.-C. Topological insulators and superconductors. Rev. Mod. Phys. 2011, 83, 1057–1110. [Google Scholar] [CrossRef]
- Tanaka, Y.; Yokoyama, T.; Balatsky, A.V.; Nagaosa, N. Theory of topological spin current in noncentrosymmetric superconductors. Phys. Rev. B 2009, 79, 060505. [Google Scholar] [CrossRef]
- Yip, S. Noncentrosymmetric Superconductors. Annu. Rev. Condens. Matter Phys. 2014, 5, 15–33. [Google Scholar] [CrossRef]
- Bian, Z.; Kato, K.; Ogoshi, T.; Cui, Z.; Sa, B.; Tsutsui, Y.; Seki, S.; Suda, M. Hybrid Chiral MoS2 Layers for Spin-Polarized Charge Transport and Spin-Dependent Electrocatalytic Applications. Adv. Sci. 2022, 9, e2201063. [Google Scholar] [CrossRef]
- Kallin, C.; Berlinsky, J. Chiral superconductors. Rep. Prog. Phys. 2016, 79, 054502. [Google Scholar] [CrossRef] [PubMed]
- Schnyder, A.P.; Ryu, S.; Furusaki, A.; Ludwig, A.W.W. Classification of topological insulators and superconductors in three spatial dimensions. Phys. Rev. B 2008, 78, 195125. [Google Scholar] [CrossRef]
- Ramires, A. Symmetry aspects of chiral superconductors. Contemp. Phys. 2022, 63, 71–86. [Google Scholar] [CrossRef]
- Ning, Z.; Ma, D.S.; Zeng, J.; Xu, D.H.; Wang, R. Flexible Control of Chiral Superconductivity in Optically Driven Nodal Point Superconductors with Antiferromagnetism. Phys. Rev. Lett. 2024, 133, 246606. [Google Scholar] [CrossRef] [PubMed]
- Bazarnik, M.; Conte, R.L.; Mascot, E.; von Bergmann, K.; Morr, D.K.; Wiesendanger, R. Antiferromagnetism-driven two-dimensional topological nodal-point superconductivity. Nat. Commun. 2023, 14, 614. [Google Scholar] [CrossRef]
- Li, J.; Neupert, T.; Wang, Z.; MacDonald, A.H.; Yazdani, A.; Bernevig, B.A. Two-dimensional chiral topological superconductivity in Shiba lattices. Nat. Commun. 2016, 7, 12297. [Google Scholar] [CrossRef]
- Kezilebieke, S.; Huda, N.; Vaňo, V.; Aapro, M.; Ganguli, S.C.; Silveira, O.J.; Głodzik, S.; Foster, A.S.; Ojanen, T.; Liljeroth, P. Topological superconductivity in a van der Waals heterostructure. Nature 2020, 588, 424–428. [Google Scholar] [CrossRef]
- Mascot, E.; Bedow, J.; Graham, M.; Rachel, S.; Morr, D.K. Topological superconductivity in skyrmion lattices. npj Quantum Mater. 2021, 6, 6. [Google Scholar] [CrossRef]
- Wong, K.H.; Hirsbrunner, M.R.; Gliozzi, J.; Malik, A.; Bradlyn, B.; Hughes, T.L.; Morr, D.K. Higher order topological superconductivity in magnet-superconductor hybrid systems. npj Quantum Mater. 2023, 8, 31. [Google Scholar] [CrossRef]
- Kieu, T.; Mascot, E.; Bedow, J.; Wiesendanger, R.; Morr, D.K. Topological nodal point superconductivity in checkerboard magnet-superconductor hybrid systems. Phys. Rev. B 2023, 108, L060509. [Google Scholar] [CrossRef]
- Kang, M.; Zhang, Z.; Wu, T.; Zhang, X.; Xu, Q.; Krasnok, A.; Han, J.; Alù, A. Coherent full polarization control based on bound states in the continuum. Nat. Commun. 2022, 13, 4536. [Google Scholar] [CrossRef]
- Xiang, Y.; Li, Q.; Li, Y.; Xie, W.; Yang, H.; Wang, Z.; Yao, Y.; Wen, H.-H. Twofold symmetry of c-axis resistivity in topological kagome superconductor CsV(3)Sb(5) with in-plane rotating magnetic field. Nat. Commun. 2021, 12, 6727. [Google Scholar] [CrossRef]
- Ortiz, B.R.; Gomes, L.C.; Morey, J.R.; Winiarski, M.; Bordelon, M.; Mangum, J.S.; Oswald, I.W.H.; Rodriguez-Rivera, J.A.; Neilson, J.R.; Wilson, S.D.; et al. New kagome prototype materials: Discovery of KV3Sb5, RbV3Sb5, and CsV3Sb5. Phys. Rev. Mater. 2019, 3, 094407. [Google Scholar] [CrossRef]
- Ortiz, B.R.; Teicher, S.M.L.; Hu, Y.; Zuo, J.L.; Sarte, P.M.; Schueller, E.C.; Abeykoon, A.M.M.; Krogstad, M.J.; Rosenkranz, S.; Osborn, R.; et al. CsV_3Sb_5: A Z_2 Topological Kagome Metal with a Superconducting Ground State. Phys. Rev. Lett. 2020, 125, 247002. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, B.R.; Sarte, P.M.; Kenney, E.M.; Graf, M.J.; Teicher, S.M.L.; Seshadri, R.; Wilson, S.D. Superconductivity in the Z2 kagome metal KV3Sb5. Phys. Rev. Mater. 2021, 5, 034801. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Y.; Li, Z.; Chen, W.; Xu, Q.; He, D.; Xi, D.; Zhang, Q.; Yuan, T.; Qu, Y.; et al. Solid-Diffusion Synthesis of Single-Atom Catalysts Directly from Bulk Metal for Efficient CO2 Reduction. Joule 2019, 3, 584–594. [Google Scholar] [CrossRef]
- Wu, Y.-R.; Dong, R.-Y.; Xu, J.; Zhang, H.-F. A novel CPA-based layered photonic structure for multipurpose sensing applications. Opt. Laser Technol. 2023, 163, 109422. [Google Scholar] [CrossRef]
- Sanchez-Santolino, G.; Rouco, V.; Puebla, S.; Aramberri, H.; Zamora, V.; Cabero, M.; Cuellar, F.A.; Munuera, C.; Mompean, F.; Garcia-Hernandez, M.; et al. A 2D ferroelectric vortex pattern in twisted BaTiO(3) freestanding layers. Nature 2024, 626, 529–534. [Google Scholar] [CrossRef]
- Chen, S.; Yuan, S.; Hou, Z.; Tang, Y.; Zhang, J.; Wang, T.; Li, K.; Zhao, W.; Liu, X.; Chen, L.; et al. Recent Progress on Topological Structures in Ferroic Thin Films and Heterostructures. Adv. Mater. 2021, 33, e2000857. [Google Scholar] [CrossRef]
- Liu, Y.; Haibibu, A.; Xu, W.; Han, Z.; Wang, Q. Observation of a Negative Thermal Hysteresis in Relaxor Ferroelectric Polymers. Adv. Funct. Mater. 2020, 30, 2000648. [Google Scholar] [CrossRef]
- Zhang, Q.; Prokhorenko, S.; Nahas, Y.; Xie, L.; Bellaiche, L.; Gruverman, A.; Valanoor, N. Deterministic Switching of Ferroelectric Bubble Nanodomains. Adv. Funct. Mater. 2019, 29, 1808573. [Google Scholar] [CrossRef]
- Qi, L.; Ruan, S.; Zeng, Y.J. Review on Recent Developments in 2D Ferroelectrics: Theories and Applications. Adv. Mater. 2021, 33, e2005098. [Google Scholar] [CrossRef] [PubMed]
- Si, M.; Saha, A.K.; Gao, S.; Qiu, G.; Qin, J.; Duan, Y.; Jian, J.; Niu, C.; Wang, H.; Wu, W.; et al. A ferroelectric semiconductor field-effect transistor. Nat. Electron. 2019, 2, 580–586. [Google Scholar] [CrossRef]
- Qian, Z.; Zhou, J.; Wang, H.; Liu, S. Shift current response in elemental two-dimensional ferroelectrics. npj Comput. Mater. 2023, 9, 67. [Google Scholar] [CrossRef]
- Gou, J.; Bai, H.; Zhang, X.; Huang, Y.L.; Duan, S.; Ariando, A.; Yang, S.A.; Chen, L.; Lu, Y.; Wee, A.T.S. Two-dimensional ferroelectricity in a single-element bismuth monolayer. Nature 2023, 617, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Li, W.; Zhang, Q.; Tang, S.; Wang, Y.; Xu, Z.; Liu, Y.; Chen, H.; Gu, J.; Wang, J.; et al. Electric Field-Manipulated Optical Chirality in Ferroelectric Vortex Domains. Adv. Mater. 2024, 36, e2408400. [Google Scholar] [CrossRef]
- Yue, L.; Zhang, X.; Wang, Y.; Li, W.; Tang, Y.; Bai, Y. Cellulose nanocomposite modified conductive self-healing hydrogel with enhanced mechanical property. Eur. Polym. J. 2021, 146, 110258. [Google Scholar] [CrossRef]
- Chen, L.; Wang, L.; Wan, Y.; Zhang, Y.; Qi, Z.; Wu, X.; Xu, H. Acetylene and Diacetylene Functionalized Covalent Triazine Frameworks as Metal-Free Photocatalysts for Hydrogen Peroxide Production: A New Two-Electron Water Oxidation Pathway. Adv. Mater. 2020, 32, e1904433. [Google Scholar] [CrossRef]
- Zor, E.; Bingol, H.; Ersoz, M. Chiral sensors. TrAC Trends Anal. Chem. 2019, 121, 115662. [Google Scholar] [CrossRef]
- Scriba, G.K.E. Chiral Recognition Mechanisms in Analytical Separation Sciences. Chromatographia 2012, 75, 815–838. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y. Chiral Gating for Size- and Shape-Selective Asymmetric Catalysis. J. Am. Chem. Soc. 2019, 141, 13749–13752. [Google Scholar] [CrossRef]
- Peng, Y.; Huang, H.; Zhang, Y.; Kang, C.; Chen, S.; Song, L.; Liu, D.; Zhong, C. A versatile MOF-based trap for heavy metal ion capture and dispersion. Nat. Commun. 2018, 9, 187. [Google Scholar] [CrossRef]
- Gong, W.; Chen, Z.; Dong, J.; Liu, Y.; Cui, Y. Chiral Metal-Organic Frameworks. Chem. Rev. 2022, 122, 9078–9144. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Li, L.; Xiao, Y.; Wang, Y. Recent advances in cyclodextrins-based chiral-recognizing platforms. TrAC Trends Anal. Chem. 2019, 121, 115691. [Google Scholar] [CrossRef]
- Zhu, G.; Kingsford, O.J.; Yi, Y.; Wong, K.-Y. Review—Recent Advances in Electrochemical Chiral Recognition. J. Electrochem. Soc. 2019, 166, H205–H217. [Google Scholar] [CrossRef]
- Chen, L.; Liu, S.; Chang, F.; Xie, X.; Zhu, Z. A Gold Nanoparticles–Enhanced Carbon Nanotubes Electrochemical Chiral Sensor. Electroanalysis 2017, 29, 955–959. [Google Scholar] [CrossRef]
- Brandt, J.R.; Salerno, F.; Fuchter, M.J. The added value of small-molecule chirality in technological applications. Nat. Rev. Chem. 2017, 1, 0045. [Google Scholar] [CrossRef]
- Kiran, V.; Mathew, S.P.; Cohen, S.R.; Delgado, I.H.; Lacour, J.; Naaman, R. Helicenes—A New Class of Organic Spin Filter. Adv. Mater. 2016, 28, 1957–1962. [Google Scholar] [CrossRef]
- Kettner, M.; Maslyuk, V.V.; Nürenberg, D.; Seibel, J.; Gutierrez, R.; Cuniberti, G.; Ernst, K.-H.; Zacharias, H. Chirality-Dependent Electron Spin Filtering by Molecular Monolayers of Helicenes. J. Phys. Chem. Lett. 2018, 9, 2025–2030. [Google Scholar] [CrossRef]
- Naaman, R.; Paltiel, Y.; Waldeck, D.H. Chiral molecules and the electron spin. Nat. Rev. Chem. 2019, 3, 250–260. [Google Scholar] [CrossRef]
- Zhu, F.; Li, C.-X.; Wu, Z.-L.; Cai, T.; Wen, W.; Guo, Q.-X. Chiral aldehyde-nickel dual catalysis enables asymmetric alpha-propargylation of amino acids and stereodivergent synthesis of NP25302. Nat. Commun. 2022, 13, 7290. [Google Scholar] [CrossRef]
- Narmadha, M.; Fang, W.; Zhu, Y.; Jin, J. A Chiral COFs Membrane for Enantioselective Amino Acid Separation. Angew. Chem. Int. Ed. 2025, 64, e202417088. [Google Scholar] [CrossRef]
- Lopez-Serna, R.; Kasprzyk-Hordern, B.; Petrovic, M.; Barcelo, D. Multi-residue enantiomeric analysis of pharmaceuticals and their active metabolites in the Guadalquivir River basin (South Spain) by chiral liquid chromatography coupled with tandem mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 5859–5873. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.-N.; Zhang, Y.; Song, S.-Y.; Zhang, H.-J. Design strategies and applications of charged metal organic frameworks. Coord. Chem. Rev. 2019, 398, 113007. [Google Scholar] [CrossRef]
- Li, J.R.; Kuppler, R.J.; Zhou, H.C. Selective gas adsorption and separation in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Zhang, M.; Duan, A.; Zhang, J.; Yang, R.; Yuan, L. Homochiral metal-organic framework used as a stationary phase for high-performance liquid chromatography. J. Sep. Sci. 2015, 38, 556–561. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, H.; Chan, J.Y.; Ou, R.; Zhu, H.; Forsyth, M.; Marijanovic, E.M.; Doherty, C.M.; Marriott, P.J.; Holl, M.M.B.; et al. Homochiral MOF–Polymer Mixed Matrix Membranes for Efficient Separation of Chiral Molecules. Angew. Chem. 2019, 131, 17084–17091. [Google Scholar] [CrossRef]
- Xie, S.; Hu, C.; Li, L.; Zhang, J.; Fu, N.; Wang, B.; Yuan, L. Homochiral metal-organic framework for HPLC separation of enantiomers. Microchem. J. 2018, 139, 487–491. [Google Scholar] [CrossRef]
- Huang, K.; Dong, X.; Ren, R.; Jin, W. Fabrication of homochiral metal—Organic framework membrane for enantioseparation of racemic diols. AIChE J. 2013, 59, 4364–4372. [Google Scholar] [CrossRef]
- Maurin, G.; Serre, C.; Cooper, A.; Ferey, G. The new age of MOFs and of their porous-related solids. Chem. Soc. Rev. 2017, 46, 3104–3107. [Google Scholar] [CrossRef]
- Navarro-Sanchez, J.; Argente-García, A.I.; Moliner-Martínez, Y.; Roca-Sanjuán, D.; Antypov, D.; Campíns-Falcó, P.; Rosseinsky, M.J.; Martí-Gastaldo, C. Peptide Metal-Organic Frameworks for Enantioselective Separation of Chiral Drugs. J. Am. Chem. Soc. 2017, 139, 4294–4297. [Google Scholar] [CrossRef]
- Tang, B.; Zhang, J.H.; Zi, M.; Chen, X.X.; Yuan, L.M. Solid-phase extraction with metal-organic frameworks for the analysis of chiral compounds. Chirality 2016, 28, 778–783. [Google Scholar] [CrossRef]
- Casini, A.; Woods, B.; Wenzel, M. The Promise of Self-Assembled 3D Supramolecular Coordination Complexes for Biomedical Applications. Inorg. Chem. 2017, 56, 14715–14729. [Google Scholar] [CrossRef]
- Assavapanumat, S.; Ketkaew, M.; Kuhn, A.; Wattanakit, C. Synthesis, Characterization, and Electrochemical Applications of Chiral Imprinted Mesoporous Ni Surfaces. J. Am. Chem. Soc. 2019, 141, 18870–18876. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, Y.; Zhang, Y.; Liu, Y. Photo-Controllable Catalysis and Chiral Monosaccharide Recognition Induced by Cyclodextrin Derivatives. Angew. Chem. Int. Ed. Engl. 2021, 60, 7654–7658. [Google Scholar] [CrossRef]
- Zhang, J.; Han, X.; Wu, X.; Liu, Y.; Cui, Y. Chiral DHIP- and Pyrrolidine-Based Covalent Organic Frameworks for Asymmetric Catalysis. ACS Sustain. Chem. Eng. 2019, 7, 5065–5071. [Google Scholar] [CrossRef]
- Von Zelewsky, A. Stereochemistry of coordination compounds. From alfred werner to the 21st century. Chimia 2014, 68, 297–298. [Google Scholar] [CrossRef] [PubMed]
- Lacour, J.; Jodry, J.J.; Ginglinger, C.; Torche-Haldimann, S. Diastereoselective Ion Pairing of TRISPHAT Anions and Tris(4,4′-dimethyl-2,2′-bipyridine)iron(II). Angew. Chem. Int. Ed. 1998, 37, 2379–2380. [Google Scholar] [CrossRef]
- Brunner, H. Optically Active Organometallic Compounds of Transition Elements with Chiral Metal Atoms. Angew. Chem. Int. Ed. 1999, 38, 1194–1208. [Google Scholar] [CrossRef]
- Monchaud, D.; Jodry, J.J.; Pomeranc, D.; Heitz, V.; Chambron, J.-C.; Sauvage, J.-P.; Lacour, J. Ion-pair-mediated asymmetric synthesis of a configurationally stable mononuclear tris(diimine)-iron(II) complex. Angew. Chem. Int. Ed. Engl. 2002, 41, 2317–2319. [Google Scholar] [CrossRef]
- Steinlandt, P.S.; Zhang, L.; Meggers, E. Metal Stereogenicity in Asymmetric Transition Metal Catalysis. Chem. Rev. 2023, 123, 4764–4794. [Google Scholar] [CrossRef]
- Zu, B.; Guo, Y.; He, C. Catalytic Enantioselective Construction of Chiroptical Boron-Stereogenic Compounds. J. Am. Chem. Soc. 2021, 143, 16302–16310. [Google Scholar] [CrossRef]
- Swords, W.B.; Lee, H.; Park, Y.; Llamas, F.; Skubi, K.L.; Park, J.; Guzei, I.A.; Baik, M.-H.; Yoon, T.P. Highly Enantioselective 6pi Photoelectrocyclizations Engineered by Hydrogen Bonding. J. Am. Chem. Soc. 2023, 145, 27045–27053. [Google Scholar] [CrossRef]
- Chu, Y.P.; Yue, X.L.; Liu, D.H.; Wang, C.; Ma, J. Asymmetric synthesis of stereogenic-at-iridium(III) complexes through Pd-catalyzed kinetic resolution. Nat. Commun. 2025, 16, 1177. [Google Scholar] [CrossRef]
- Kehm, R.; Baldensperger, T.; Raupbach, J.; Hohn, A. Protein oxidation—Formation mechanisms, detection and relevance as biomarkers in human diseases. Redox Biol. 2021, 42, 101901. [Google Scholar] [CrossRef]
- Wolfender, J.L.; Marti, G.; Thomas, A.; Bertrand, S. Current approaches and challenges for the metabolite profiling of complex natural extracts. J. Chromatogr. A 2015, 1382, 136–164. [Google Scholar] [CrossRef] [PubMed]
- Lecoeur-Lorin, M.; Delepee, R.; Ribet, J.P.; Morin, P. Chiral analysis of milnacipran by a nonchiral HPLC—Circular dichroism: Improvement of the linearity of dichroic response by temperature control. J. Sep. Sci. 2008, 31, 3009–3014. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Xu, L.; Qu, A.; Hao, C.; Sun, M.; Xu, C.; Hu, S.; Kuang, H. Chiral Inorganic Nanomaterial-Based Diagnosis and Treatments for Neurodegenerative Diseases. Adv. Mater. 2025, 37, e2418723. [Google Scholar] [CrossRef] [PubMed]
- Kepiro, I.E.; Marzuoli, I.; Hammond, K.; Ba, X.; Lewis, H.; Shaw, M.; Gunnoo, S.B.; De Santis, E.; Łapińska, U.; Pagliara, S.; et al. Engineering Chirally Blind Protein Pseudocapsids into Antibacterial Persisters. ACS Nano 2020, 14, 1609–1622. [Google Scholar] [CrossRef]
- Gao, R.; Xu, L.; Sun, M.; Xu, M.; Hao, C.; Guo, X.; Colombari, F.M.; Zheng, X.; Král, P.; de Moura, A.F.; et al. Site-selective proteolytic cleavage of plant viruses by photoactive chiral nanoparticles. Nat. Catal. 2022, 5, 694–707. [Google Scholar] [CrossRef]
- Fan, X.; Ren, C.; Ning, K.; Shoala, M.A.; Ke, Q.; Zhou, Y.; Wu, Y.; Qiu, R.; Liang, J.; Xiao, S. Enantioselective Antiviral Activities of Chiral Zinc Oxide Nanoparticles. ACS Appl. Mater. Interfaces 2023, 15, 58251–58259. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Zangabad, P.S.; Ghasemi, A.; Amiri, M.; Bahrami, M.; Malekzad, H.; Asl, H.G.; Mahdieh, Z.; Bozorgomid, M.; Ghasemi, A.; et al. Temperature-Responsive Smart Nanocarriers for Delivery Of Therapeutic Agents: Applications and Recent Advances. ACS Appl. Mater. Interfaces 2016, 8, 21107–21133. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Ren, J.; Sun, Y.; Lu, L.; Gao, J.; Chen, L.; Yan, S.; Li, Z. Harnessing chirality: A new dawn in inorganic nanomaterial synthesis and biomedical applications. Chin. Chem. Lett. 2024, 2024, 110791. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, J.; Hao, C.; Hu, S.; Chen, C.; Cao, Y.; Xu, Z.; Guo, J.; Xu, L.; Sun, M.; et al. The Development of Chiral Nanoparticles to Target NK Cells and CD8+ T Cells for Cancer Immunotherapy. Adv. Mater. 2022, 34, e2109354. [Google Scholar] [CrossRef]
- Ding, J.; Wang, T.; Lin, Z.; Li, Z.; Yang, J.; Li, F.; Rong, Y.; Chen, X.; He, C. Chiral polypeptide hydrogels regulating local immune microenvironment and anti-tumor immune response. Nat. Commun. 2025, 16, 1222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, P.; Shi, W.; Qu, A.; Sun, M.; Kuang, H. Renal Clearable Chiral Manganese Oxide Supraparticles for In Vivo Detection of Metalloproteinase-9 in Early Cancer Diagnosis. Adv. Mater. 2025, 37, e2415656. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, F.; Liu, H.; Jin, Z.; Huang, T.; Tang, C.; Tan, C.L.; Shi, Y.; Yan, S. Chiral Materials: Multidisciplinary Progress and Emerging Frontier Application Prospects. Nanomaterials 2025, 15, 1701. https://doi.org/10.3390/nano15221701
Xu F, Liu H, Jin Z, Huang T, Tang C, Tan CL, Shi Y, Yan S. Chiral Materials: Multidisciplinary Progress and Emerging Frontier Application Prospects. Nanomaterials. 2025; 15(22):1701. https://doi.org/10.3390/nano15221701
Chicago/Turabian StyleXu, Feifan, Hao Liu, Zhihan Jin, Tianci Huang, Chuanqi Tang, Chee Leong Tan, Yi Shi, and Shancheng Yan. 2025. "Chiral Materials: Multidisciplinary Progress and Emerging Frontier Application Prospects" Nanomaterials 15, no. 22: 1701. https://doi.org/10.3390/nano15221701
APA StyleXu, F., Liu, H., Jin, Z., Huang, T., Tang, C., Tan, C. L., Shi, Y., & Yan, S. (2025). Chiral Materials: Multidisciplinary Progress and Emerging Frontier Application Prospects. Nanomaterials, 15(22), 1701. https://doi.org/10.3390/nano15221701






