High-Entropy (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 Zirconate Pyrochlore: A Promising Photocatalyst for Diverse Environmental Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 Pyrochlore Oxide Nanoparticles
2.3. Characterization
2.4. Photocatalytic Investigation
3. Results
3.1. Physicochemical Properties
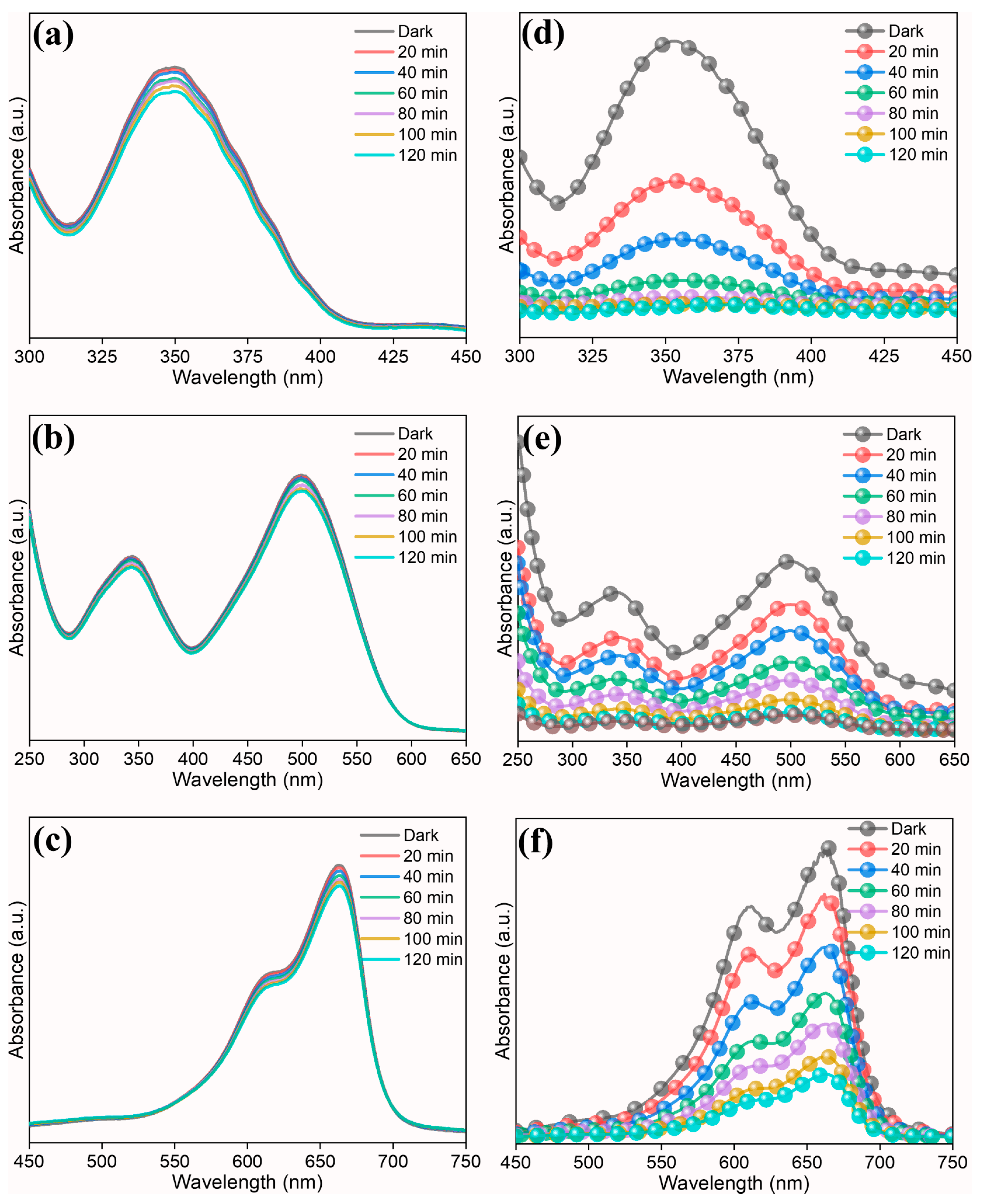
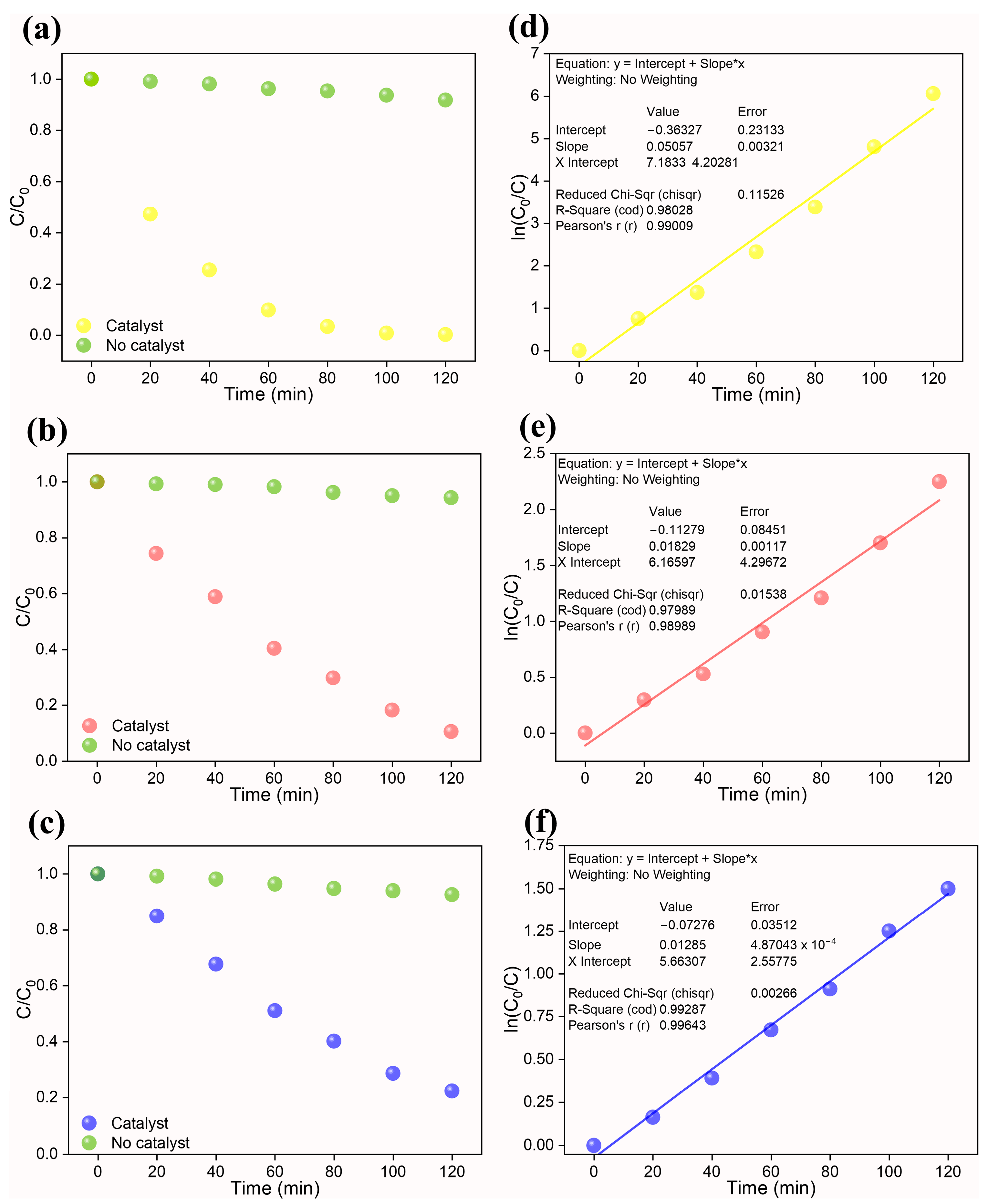
3.2. Photocatalytic Reaction
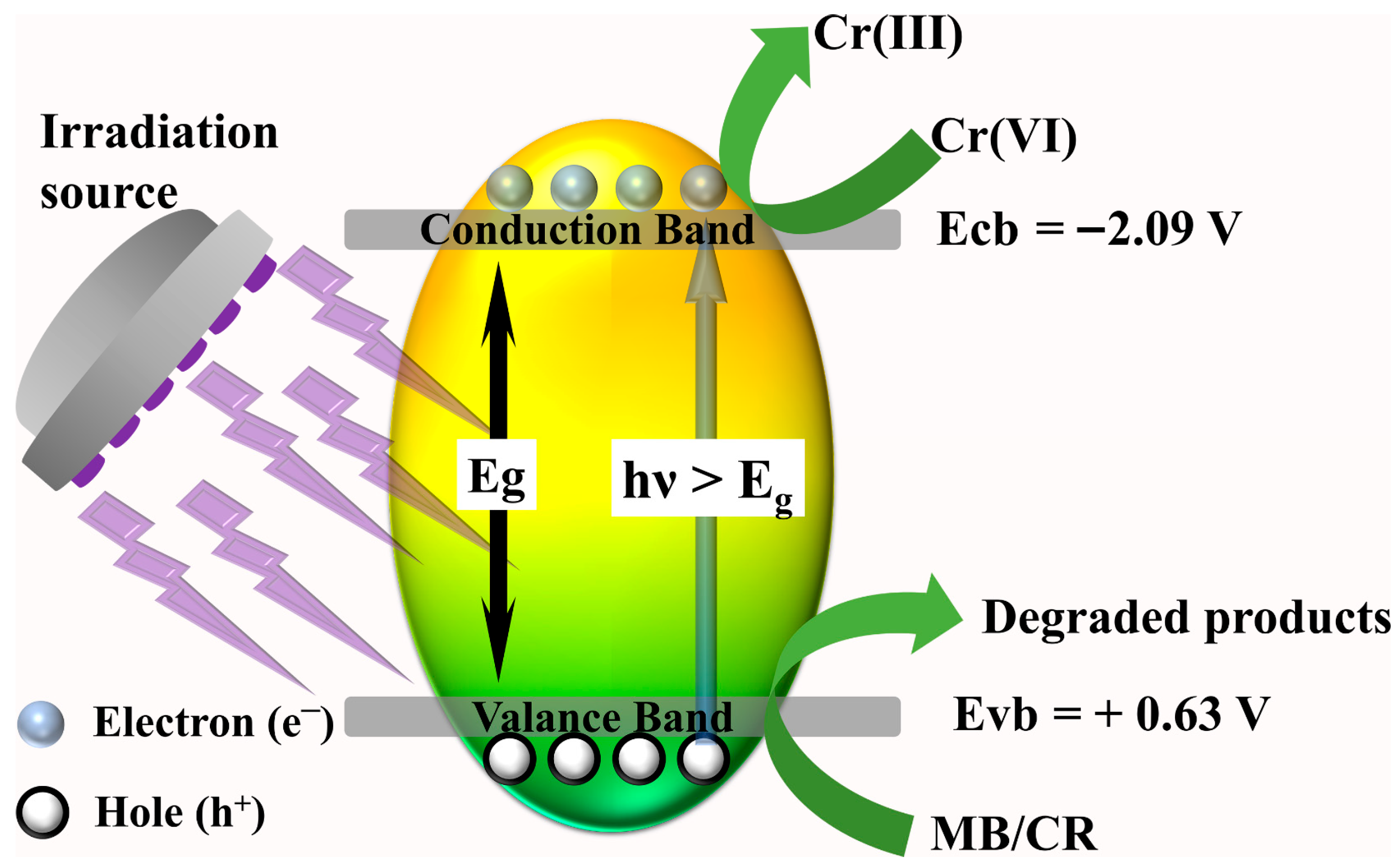
3.3. Proposed Photocatalyst Mechanism
3.4. Photocatalyst Recyclability and Stability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AOP | Advanced oxidation process |
| BOD | Biochemical oxygen demand |
| CA | Citric acid |
| CR | Congo red |
| DI | Deionized water |
| EDS | Energy-Dispersive X-ray Spectroscopy |
| EDTA-2Na | Ethylenediaminetetraacetic acid disodium salt |
| EG | Ethylene glycol |
| FESEM | Field emission scanning electron microscopy |
| HEMs | High-entropy materials |
| ICDD | International Centre for Diffraction Data |
| IPA | Isopropyl alcohol |
| K-M | Kubelka-Munk |
| MB | Methylene blue |
| NHE | Normal Hydrogen Electrode |
| p-BQ | p-benzoquinone |
| ROS | Reactive oxygen species |
| S.D. | Standard Deviation |
| UPS | Ultraviolet photoelectron spectroscopy |
| W-H | Williamson-Hall |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
References
- Shan, Z.; Yang, Y.; Shi, H.; Zhu, J.; Tan, X.; Luan, Y.; Jiang, Z.; Wang, P.; Qin, J. Hollow Dodecahedra Graphene Oxide- Cuprous Oxide Nanocomposites With Effective Photocatalytic and Bactericidal Activity. Front. Chem. 2021, 9, 755836. [Google Scholar] [CrossRef] [PubMed]
- Alias, N.; Hussain, Z.; Tan, W.K.; Kawamura, G.; Matsuda, A.; Lockman, Z. Anodic growth of nanotubular TiO2/Nb2O5 on Ti-Nb alloys for photocatalytic Cr(VI) removal. Adv. Powder Technol. 2025, 36, 105052. [Google Scholar] [CrossRef]
- Anandkumar, M.; Lathe, A.; Palve, A.M.; Deshpande, A.S. Single-phase Gd0.2La0.2Ce0.2Hf0.2Zr0.2O2 and Gd0.2La0.2Y0.2Hf0.2Zr0.2O2 nanoparticles as efficient photocatalysts for the reduction of Cr(VI) and degradation of methylene blue dye. J. Alloys Compd. 2021, 850, 156716. [Google Scholar] [CrossRef]
- Wang, X.; Wang, F.; Xu, B.; Yang, B. Bi2S3/Bi heterojunction for efficient removal of Cr(VI) via photocatalytic reduction and adsorption. Appl. Surf. Sci. 2025, 693, 162748. [Google Scholar] [CrossRef]
- Rana, A.; Sonu, S.; Soni, V.; Chawla, A.; Sudhaik, A.; Raizada, P.; Ahamad, T.; Thakur, P.; Thakur, S.; Singh, P. Novel S-scheme derived Mo–Bi2WO6/WO3/Biochar composite for photocatalytic removal of Methylene Blue dye. J. Phys. Chem. Solids 2024, 196, 112385. [Google Scholar] [CrossRef]
- Duan, Y.; Xue, J.; Ma, Y.; Zhao, L.; Zeng, B.; Feng, S.; Ma, J. Fabrication of ZnO quantum dots and In2O3 nanofiber composite photocatalysts for selective Cr(VI) detection and reduction. Environ. Res. 2025, 285, 122706. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, H.; Qin, C.; Li, X.; Xu, D.; Zhao, Y. Groundwater Cr(VI) contamination and remediation: A review from 1999 to 2022. Chemosphere 2024, 360, 142395. [Google Scholar] [CrossRef]
- He, H.; Peng, W.; Jiang, J.; Xia, X.; Luo, J.; Luo, S.; Wang, X.; Yang, K.; Yu, C. Surface modifying ZnWO4 with Zn QDs and oxygen vacancies for enhanced photocatalytic Cr(VI) reduction. Colloids Surfaces A Physicochem. Eng. Asp. 2025, 723, 137420. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Bamigboye, M.O.; Ogunbiyi, O.D.; Akano, M.T. Toxicity and decontamination strategies of Congo red dye. Groundw. Sustain. Dev. 2022, 19, 100844. [Google Scholar] [CrossRef]
- He, J.; Gao, R.; Wang, Z.; Li, W.; Cai, X.; Li, G.; Yang, B. Preparation of GO/BiOI composites and their degradation performance in Congo red solution. Chem. Phys. Lett. 2024, 860, 141771. [Google Scholar] [CrossRef]
- Mondal, A.; Islam, S.; Zaman, S.M.; Sultana, M.; Abedin, M.; Chakraborty, A.K.; Rahman, M.; Rahaman, H.; Sumi, M.S.A.; Nur, A.S. Fabrication of Ca-doped TiO2 for enhanced methylene blue degradation under UV-Vis irradiation. Next Mater. 2024, 7, 100392. [Google Scholar] [CrossRef]
- Gupta, G.K.; Mondal, M.K. 20-Fundamentals and mechanistic pathways of dye degradation using photocatalysts. In Photocatalytic Degradation of Dyes; Shah, M., Dave, S., Das, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 527–545. [Google Scholar]
- Wang, Y.; Wang, J.; Long, Z.; Sun, Z.; Lv, L.; Liang, J.; Zhang, G.; Wang, P.; Gao, W. MnCe-based catalysts for removal of organic pollutants in urban wastewater by advanced oxidation processes—A critical review. J. Environ. Manag. 2024, 370, 122773. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.R.; Kayani, K.F. A comparative review of Fenton-like processes and advanced oxidation processes for Methylene Blue degradation. Inorg. Chem. Commun. 2024, 170, 113467. [Google Scholar] [CrossRef]
- Li, Y.; Bu, J.; Sun, Y.; Huang, Z.; Zhu, X.; Li, S.; Chen, P.; Tang, Y.; He, G.; Zhong, S. Efficient degradation of norfloxacin by synergistic activation of PMS with a three-dimensional electrocatalytic system based on Cu-MOF. Sep. Purif. Technol. 2024, 356, 129945. [Google Scholar] [CrossRef]
- Zhang, C.; Ahmad, I.; Ahmed, S.B.; Ali, M.D.; Karim, M.R.; Bayahia, H.; Khasawneh, M.A. A review of rare earth oxides-based photocatalysts: Design strategies and mechanisms. J. Water Process Eng. 2024, 63, 105548. [Google Scholar] [CrossRef]
- Xu, X.; Sui, Y.; Chen, W.; Li, X.; Huang, W.; Chai, L.; Li, Y.; Zhong, H. Photocatalytic Cr(VI) reduction by metal-free photocatalysts under visible-light irradiation. J. Environ. Chem. Eng. 2024, 12, 114306. [Google Scholar] [CrossRef]
- Saputra, A.M.A.; Piliang, A.F.R.; Dellyansyah; Marpongahtun; Andriayani; Goei, R.; H.T.S., R.R.; Gea, S. Synthesis, properties, and utilization of carbon quantum dots as photocatalysts on degradation of organic dyes: A mini review. Catal. Commun. 2024, 187, 106914. [Google Scholar] [CrossRef]
- Anandkumar, M.; Kesavan, K.P.; Sudarsan, S.; Zhivulin, D.E.; Shaburova, N.A.; Moghaddam, A.O.; Litvinyuk, K.S.; Trofimov, E.A. Phase Evolution of High-Entropy Stannate Pyrochlore Oxide Synthesized via Glycine-Assisted Sol–Gel Synthesis as a Thermal Barrier Coating Material. Nanomaterials 2025, 15, 939. [Google Scholar] [CrossRef]
- Xu, J.; Xi, R.; Xu, X.; Zhang, Y.; Feng, X.; Fang, X.; Wang, X. A2B2O7 pyrochlore compounds: A category of potential materials for clean energy and environment protection catalysis. J. Rare Earths 2020, 38, 840–849. [Google Scholar] [CrossRef]
- Choudhary, L.; Besra, S.; Anwar, S.; Anwar, S. La2Ce2O7 based materials for next generation proton conducting solid oxide cells: Progress, opportunity and future prospects. Int. J. Hydrogen Energy 2023, 48, 28460–28501. [Google Scholar] [CrossRef]
- Sharifnattaj, A.; Ghaziasgar, S.; Bahadori, Y.; Saidi, M. Recent progress in catalysts development of dry reforming of methane process: Review of active phases and supports of catalyst. Mol. Catal. 2025, 582, 115060. [Google Scholar] [CrossRef]
- El Mchaouri, M.; Mallah, S.; Abouhajjoub, D.; Boumya, W.; Elmoubarki, R.; Essadki, A.; Barka, N.; Elhalil, A. Engineering TiO2 photocatalysts for enhanced visible-light activity in wastewater treatment applications. Tetrahedron Green Chem 2025, 6, 100084. [Google Scholar] [CrossRef]
- Torralba, J.M.; Meza, A.; Kumaran, S.V.; Mostafaei, A.; Mohammadzadeh, A. From high-entropy alloys to alloys with high entropy: A new paradigm in materials science and engineering for advancing sustainable metallurgy. Curr. Opin. Solid State Mater. Sci. 2025, 36, 101221. [Google Scholar] [CrossRef]
- Bolar, S.; Ito, Y.; Fujita, T. Future prospects of high-entropy alloys as next-generation industrial electrode materials. Chem. Sci. 2024, 15, 8664–8722. [Google Scholar] [CrossRef]
- Anandkumar, M.; Trofimov, E. Synthesis, Properties, and Applications of High-Entropy Oxide Ceramics: Current Progress and Future Perspectives. J. Alloys Compd. 2023, 960, 170690. [Google Scholar] [CrossRef]
- Zhao, Z.; Ruan, Z.; Li, R.; Yan, S.; Sun, X.; Liu, C.; Zhang, D.; Xu, B.; Ren, Z.; Wang, M.; et al. High entropy pyrochlore (La0.3Gd0.3Ca0.4)2(Ti0.2Zr0.2Hf0.2Nb0.2Ta0.2)2O7 ceramic with amorphous-like thermal conductivity for environmental/thermal barrier coating applications. J. Mater. Sci. Technol. 2024, 205, 315–326. [Google Scholar] [CrossRef]
- Matović, B.; Zagorac, D.; Cvijović-Alagić, I.; Zagorac, J.; Butulija, S.; Erčić, J.; Hanzel, O.; Sedlák, R.; Lisnichuk, M.; Tatarko, P. Fabrication and characterization of high entropy pyrochlore ceramics. Boletín de la Sociedad Española de Cerámica y Vidrio 2023, 62, 66–76. [Google Scholar] [CrossRef]
- Du, M.; Liu, S.; Ge, Y.; Li, Z.; Wei, T.; Yang, X.; Dong, J. Preparation and effect of grain size on the thermal stability, phase transition, mechanical property, and photocatalytic property of pyrochlore (La0.2Nd0.2Sm0.2Gd0.2Y0.2)2Zr2O7 high-entropy oxide. Ceram. Int. 2022, 48, 20667–20674. [Google Scholar] [CrossRef]
- Mnasri, W.; Bérardan, D.; Tusseau-Nenez, S.; Gacoin, T.; Maurin, I.; Dragoe, N. Synthesis of (MgCoNiCuZn)O entropy-stabilized oxides using solution-based routes: Influence of composition on phase stability and functional properties. J. Mater. Chem. C 2021, 9, 15121–15131. [Google Scholar] [CrossRef]
- Alshora, D.H.; Ibrahim, M.A.; Alanazi, F.K. Chapter 6—Nanotechnology from particle size reduction to enhancing aqueous solubility. In Surface Chemistry of Nanobiomaterials; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 163–191. [Google Scholar]
- Anandkumar, M.; Kesavan, K.P.; Sudarsan, S.; Zaitseva, O.V.; Moghaddam, A.O.; Iarushina, D.V.; Trofimov, E.A. Band-Gap Engineering of High-Entropy Fluorite Metal Oxide Nanoparticles Facilitated by Pr3+ Incorporation by Gel Combustion Synthesis. Gels 2025, 11, 117. [Google Scholar] [CrossRef]
- Teng, Z.; Tan, Y.; Zhang, H. High-Entropy Pyrochlore A2B2O7 with Both Heavy and Light Rare-Earth Elements at the A Site. Materials 2022, 15, 129. [Google Scholar] [CrossRef]
- Teng, Z.; Zhu, L.; Tan, Y.; Zeng, S.; Xia, Y.; Wang, Y.; Zhang, H. Synthesis and structures of high-entropy pyrochlore oxides. J. Eur. Ceram. Soc. 2020, 40, 1639–1643. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, X.; Liu, X.; Zhang, W.; Tian, Y.; Li, X.; Li, B.; Su, S. Construction of direct Z-scheme Sn3O4/WO3 heterostructure photocatalyst for enhanced Congo red degradation and Cr(VI) reduction performance. J. Photochem. Photobiol. A Chem. 2024, 460, 116122. [Google Scholar] [CrossRef]
- Shi, D.; Guo, J.; Ma, Y.; Zhang, B.; Zhang, Y. One-pot solvothermal synthesis of magnetic high-performance Zn2+ doped CoFe2O4 visible light photocatalyst. J. Alloys Compd. 2025, 1028, 180699. [Google Scholar] [CrossRef]
- Saeed, M.; Asghar, H.; Khan, I.; Akram, N.; Usman, M. Usman, Synthesis of TiO2-g-C3N4 for efficient photocatalytic degradation of Congo Red dye. Catal. Today 2024, 447, 115154. [Google Scholar] [CrossRef]
- Khan, A.; Chen, H.-Y.; Rusly, C. Visible-light-driven removal of mixed dye pollutants by a novel ZnO/CNT/GO ternary nanocomposite: Synergistic degradation of Congo red and methylene blue. Environ. Res. 2025, 283, 122156. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, Y.; Xiao, T.; Zhu, X.; Jiao, Y.; Yu, Q.; Qi, Z. Construction of flower-like BiOI/Bi2O2CO3 p-n heterojunction for photocatalytic degradation of Congo Red dye. J. Mol. Struct. 2025, 1336, 142076. [Google Scholar] [CrossRef]
- Wahba, M.A.; Abdelkader, M.M.G.; El-Mossalamy, E. Tailoring optical, dielectric, and magnetic properties of highly efficient visible-light LaFeO3 photocatalysts via Ca/Ba mono and Co-doping. Mater. Chem. Phys. 2025, 341, 130916. [Google Scholar] [CrossRef]
- Kumar, K.; Rath, C. Mn doping induced band gap narrowing and enhanced photocatalytic degradation in GdCoO3 perovskite. Vacuum 2025, 242, 114749. [Google Scholar] [CrossRef]
- Chandel, A.; Choudhary, R.; Sharma, P.; Kaur, G.; Pandey, O. Atmosphere-dependent synthesis of BiVO4 and its photocatalytic performance on methylene blue dye degradation. Chem. Phys. 2025, 598, 112798. [Google Scholar] [CrossRef]
- Rao, M.S.; Chaudhary, Y.; Jaihindh, D.P.; Lin, Y.-F.; Rakesh, B.; Sankaran, K.J. ZnO-graphene nanohybrids for photocatalytic degradation of methylene blue dye. Diam. Relat. Mater. 2025, 159, 112791. [Google Scholar] [CrossRef]
- Sharma, Y.; Anand, V.; Dhiman, V.; Heera, P. Biogenic CuO-ZnO nanocomposites synthesized from Jatropha curcas for methylene blue dye degradation. JCIS Open 2025, 19, 100146. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, Y.; Yan, J.; Duan, Z.; Han, H.; Jiang, D. Enhanced BiVO4 photocatalyst anchored on construction waste bricks powder for efficient formaldehyde and methylene blue degradation. Constr. Build. Mater. 2025, 469, 140536. [Google Scholar] [CrossRef]
- Anandkumar, M.; Kannan, P.; Sudarsan, S.; Trofimov, E. High-entropy oxide (CeGdHfPrZr)O2 nanoparticles as reusable photocatalyst for wastewater remediation. Surf. Interfaces 2024, 51, 104815. [Google Scholar] [CrossRef]
- Trasatti, S. The absolute electrode potential: An explanatory note (Recommendations 1986). Pure Appl. Chem. 1986, 58, 955–966. [Google Scholar] [CrossRef]
- Subramanian, Y.; Dhanasekaran, A.; Omeiza, L.A.; Zaini, J.H.; Irvine, J.T.; Azad, A.K. Influence of various sacrificial reagents on congo red degradation and H2O2 production based on heteroanionic titanium oxycarbide photocatalyst and its mechanism. Ionics 2023, 29, 2435–2447. [Google Scholar] [CrossRef]
- Xie, X.; Liu, M.; Wang, C.; Chen, L.; Xu, J.; Cheng, Y.; Dong, H.; Lu, F.; Wang, W.-H.; Liu, H.; et al. Efficient photo-degradation of dyes using CuWO4 nanoparticles with electron sacrificial agents: A combination of experimental and theoretical exploration. RSC Adv. 2015, 6, 953–959. [Google Scholar] [CrossRef]
- Teja, Y.N.; Prakash, R.M.; Murali, A.; Sakar, M. Chapter 5—Defective photocatalysts. In Photocatalytic Systems by Design; Sakar, M., Balakrishna, R.G., Do, T.-O., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 131–163. [Google Scholar]
- Hu, Y.; Anandkumar, M.; Joardar, J.; Wang, X.; Deshpande, A.S.; Reddy, K.M. Effective band gap engineering in multi-principal oxides (CeGdLa-Zr/Hf)Ox by temperature-induced oxygen vacancies. Sci. Rep. 2023, 13, 2362. [Google Scholar] [CrossRef]

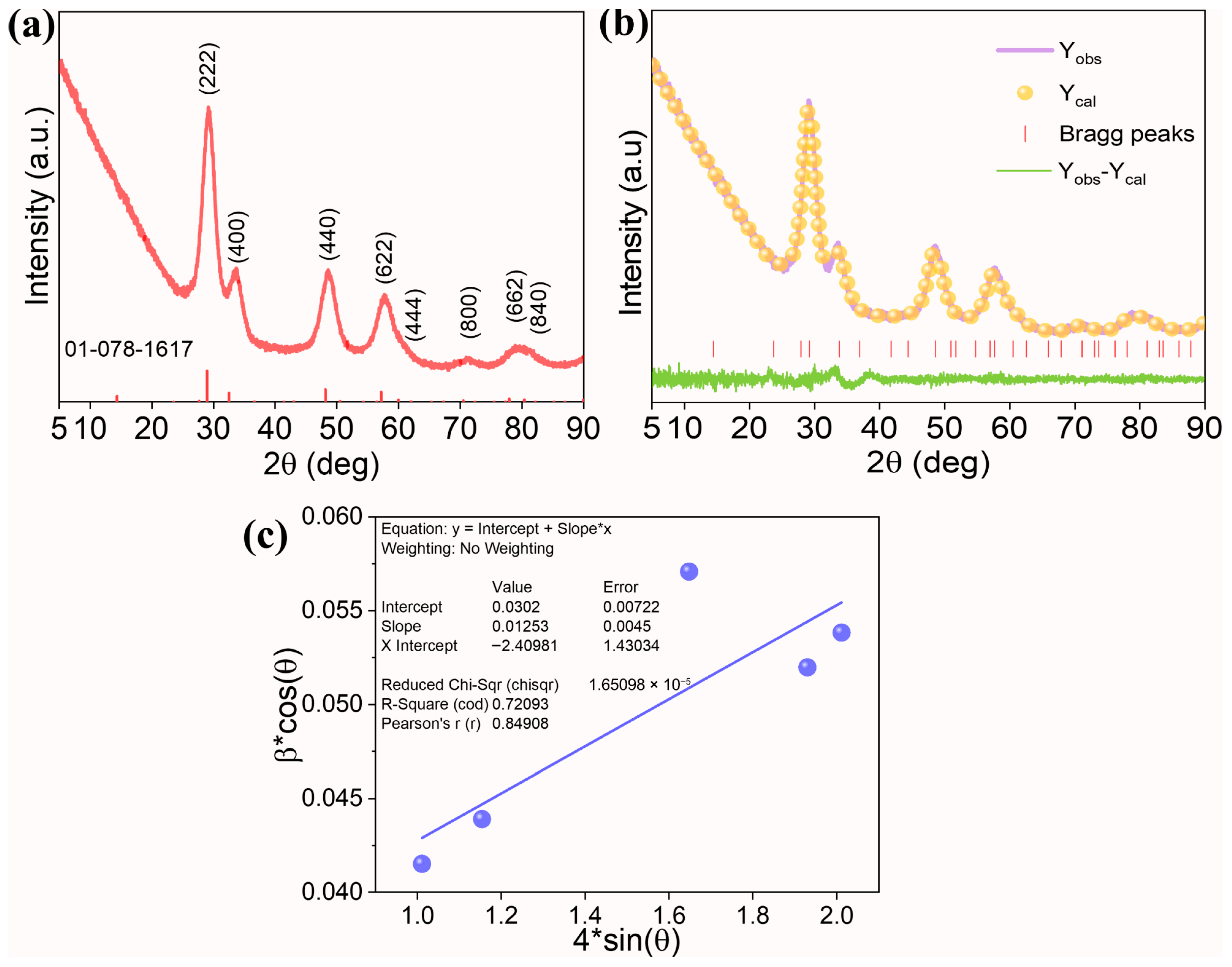
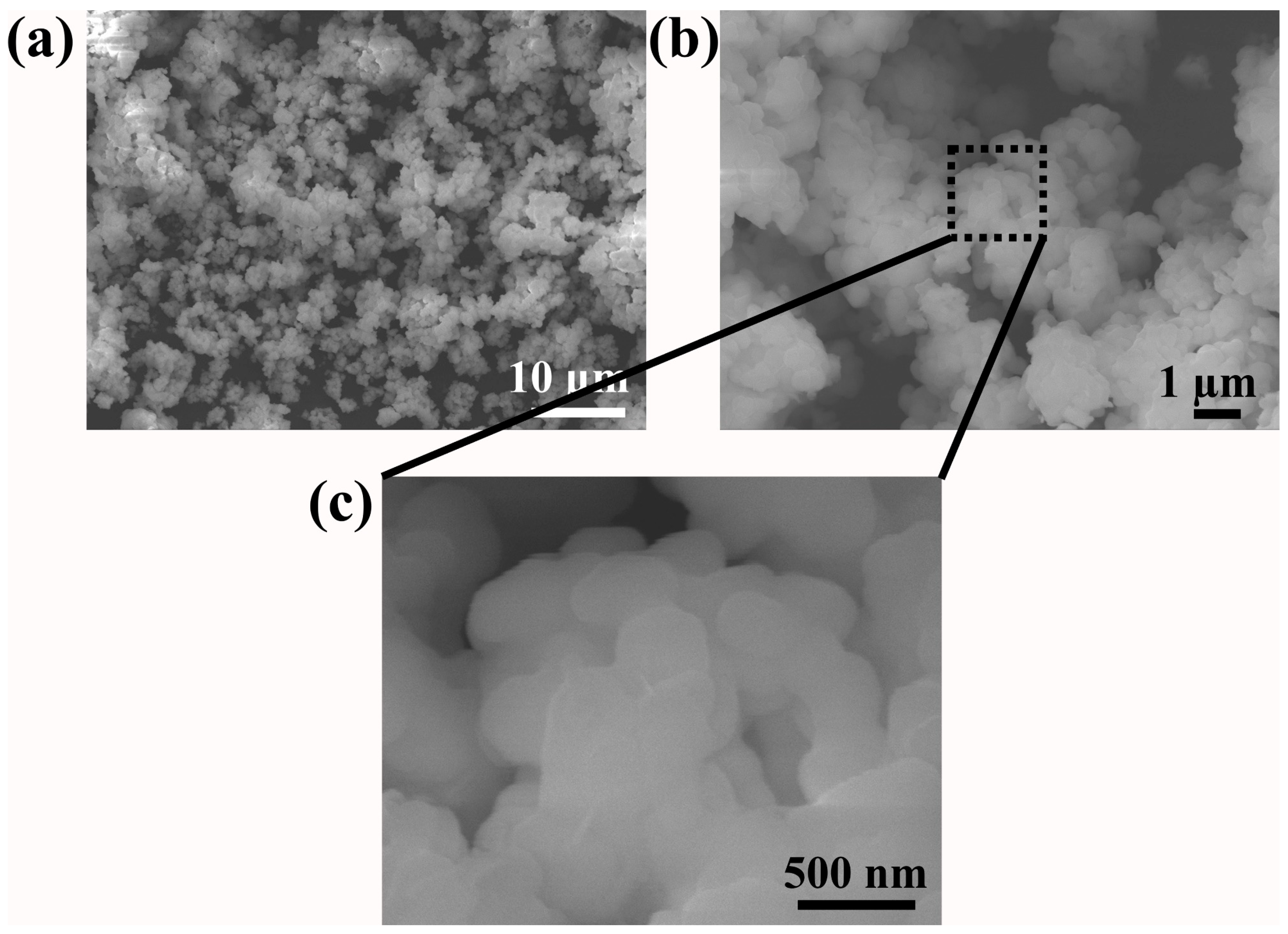
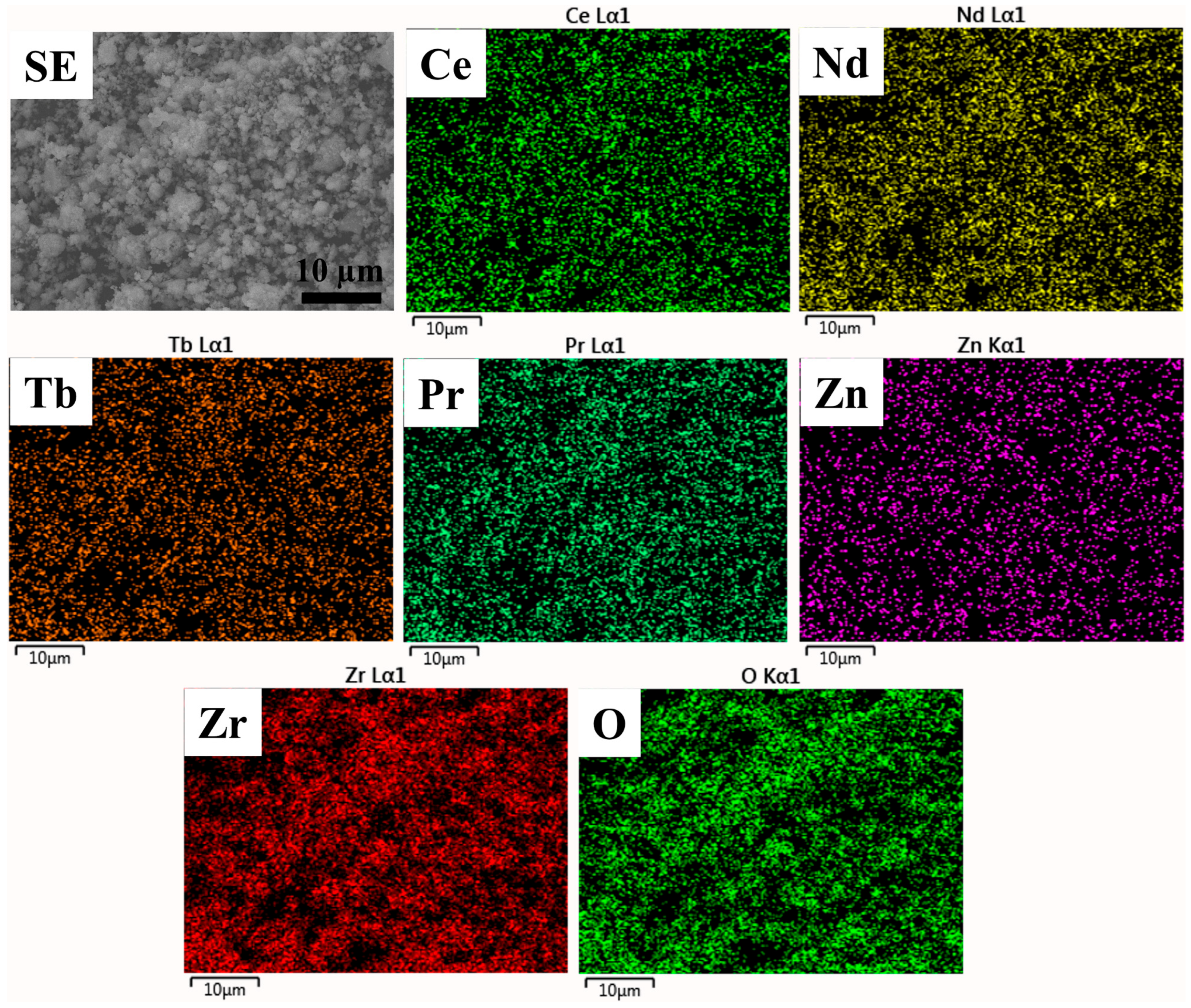
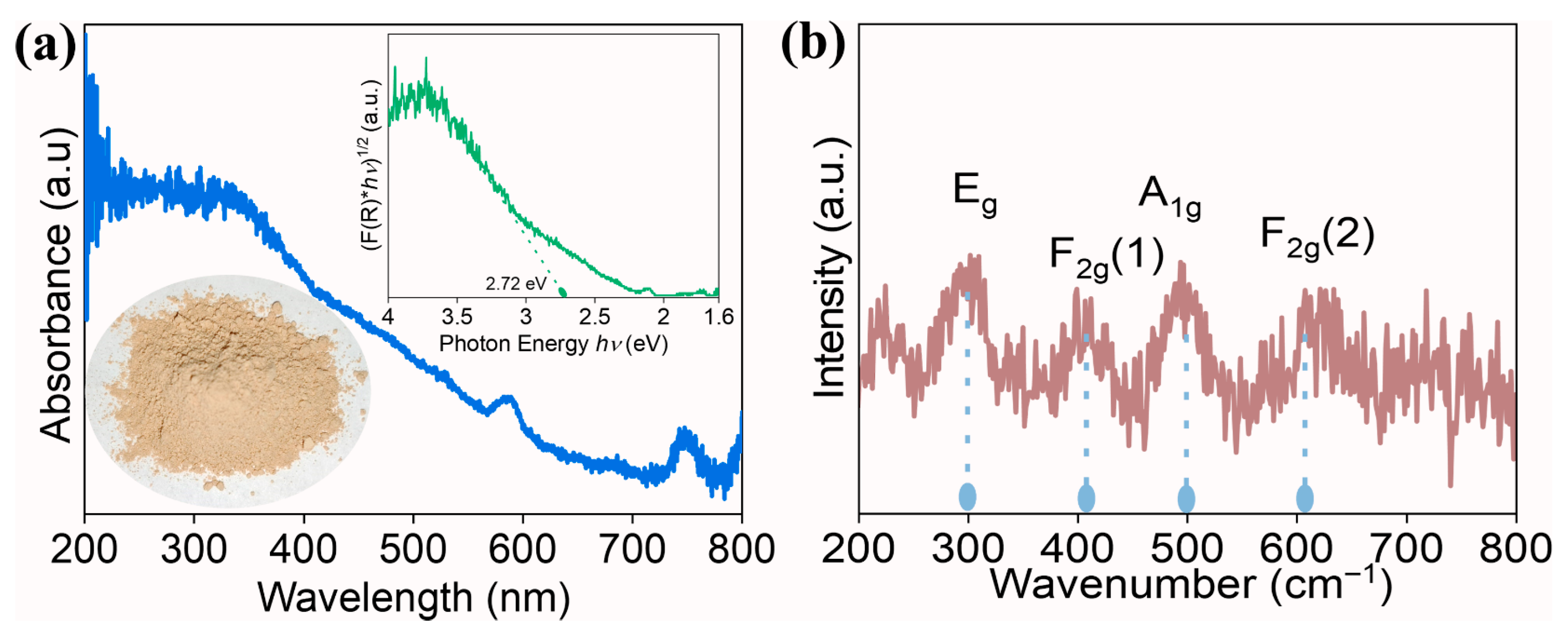


| Sample | Rp (%) | Rwp (%) | Rexp (%) | χ2 | Lattice Parameter (Å) |
|---|---|---|---|---|---|
| (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 | 10.1 | 9.34 | 7.93 | 1.39 | 10.594 |
| Site | Element (Atomic %) | ||||||
|---|---|---|---|---|---|---|---|
| Ce | Pr | Nd | Tb | Zn | Zr | O | |
| Site 1 | 2.48 | 2.16 | 2.75 | 2.22 | 2.33 | 12.55 | 75.52 |
| Site 2 | 3.6 | 3.19 | 3.52 | 2.49 | 3.11 | 13.36 | 70.73 |
| Site 3 | 2.69 | 2.59 | 2.99 | 2.19 | 2.26 | 11.9 | 75.39 |
| Site 4 | 2.68 | 2.23 | 2.63 | 2.05 | 2.38 | 11.73 | 76.29 |
| Site 5 | 2.69 | 2.75 | 3.43 | 2.51 | 2.62 | 12.87 | 73.14 |
| Mean | 2.82 | 2.58 | 3.06 | 2.29 | 2.54 | 12.48 | 74.21 |
| S.D. | 0.39 | 0.37 | 0.35 | 0.17 | 0.30 | 0.60 | 2.03 |
| Pollutant | Cr(VI) | Congo Red | Methylene Blue |
|---|---|---|---|
| Rate Constant k (min−1) | 0.0506 | 0.0183 | 0.0128 |
| Photocatalyst | Dye | Irradiation Source | Dye/Cr(VI) Concentration | Photocatalyst Concentration | Irradiation Duration (min) | k min−1 | Reference |
|---|---|---|---|---|---|---|---|
| ZnO-QDs/In2O3 nanofibers | Cr(VI) | Visible light | 50 µM/L | 500 mg/L | 120 | 0.018 | [6] |
| Zn QDs/ZnWO4 | 400 W metal halide lamp | 20 mg/L | 100 mg/L | 40 | 0.0599 | [8] | |
| SW-2 | 500 W Xe lamp | 50 mg/L | 1 g/L | 40 | 0.075 | [35] | |
| Bi2S3/Bi | 300 W xenon lamp | 20 mg/L | 500 mg/L | 30 | 0.0246 | [4] | |
| Ti–60Nb | UVC germicidal lamp (TUV 54 W, Philips; 254 nm) | 20 ppm | - | 120 | 0.0167 | [2] | |
| 4 % Zn-CoFe2O4 | Visible light | 20 mg/L | 333 mg/L | 135 | 0.025 | [36] | |
| (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 | UV-LED light | 50 ppm | 500 mg/L | 120 | 0.0506 | This work | |
| TiO2-g-C3N4-10 | CR | Sunlight | 50 mg/L | 50 mg/L | 160 | 0.0259 | [37] |
| ZnO/CNT/GO | LED light | 10 ppm | 600 mg/L | 60 | 0.0253 | [38] | |
| 0.5-BiOI/BOC | Xenon lamp | 100 mg/L | 30 mg/L | 60 | 0.0207 | [39] | |
| BiOI/GO | 100 W tungsten iodide lamp | 20 mg/L | 200 mg/L | 120 | 0.02151 | [10] | |
| C2B2LF | Sunlight | 10 mg/L | 1000 mg/L | 120 | 0.0093 | [40] | |
| GdCo0.80Mn0.20O3 | 200 W xenon lamp | 10 ppm | 600 mg/L | 120 | 0.0079 | [41] | |
| (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 | UV-LED light | 50 ppm | 500 mg/L | 120 | 0.0183 | This work | |
| BV3 | MB | Visible light | 1 mg/L | 80 mg/L | 120 | 0.00592 | [42] |
| ZG nanohybrids | UV–visible light | 1 ppm | - | 130 | 0.048 | [43] | |
| JC-Cu0.05Zn0.95O | 250 W mercury vapor lamp | 10 mg/L | 1000 mg/L | 120 | 0.01831 | [44] | |
| Mo–Bi2WO6/WO3/Biochar | 500 W halogen lamp | 6.4 mg/L | 600 mg/L | 30 | 0.02816 | [5] | |
| 1 wt% Ca-doped TiO2 | UV-visible irradiation | 20 ppm | 800 mg/L | 180 | 0.0087 | [11] | |
| BVO/CWB-11 | 300 W xenon lamp | 20 mg/L | 1000 mg/L | 120 | 0.02732 | [45] | |
| (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 | UV-LED light | 50 ppm | 500 mg/L | 120 | 0.0128 | This work |
| Pollutant | Degradation/Reduction (%) | ||
|---|---|---|---|
| Cr(VI) | Congo Red | Methylene Blue | |
| Cycle 1 | 98.8 | 91.3 | 79.3 |
| Cycle 2 | 91.1 | 87.1 | 75.1 |
| Cycle 3 | 90.1 | 84.6 | 71.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anandkumar, M.; Sudarsan, S.; Naganaboina, V.R.; Bandari, N.K.; Litvinyuk, K.S.; Singh, S.G.; Trofimov, E.A. High-Entropy (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 Zirconate Pyrochlore: A Promising Photocatalyst for Diverse Environmental Applications. Nanomaterials 2025, 15, 1668. https://doi.org/10.3390/nano15211668
Anandkumar M, Sudarsan S, Naganaboina VR, Bandari NK, Litvinyuk KS, Singh SG, Trofimov EA. High-Entropy (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 Zirconate Pyrochlore: A Promising Photocatalyst for Diverse Environmental Applications. Nanomaterials. 2025; 15(21):1668. https://doi.org/10.3390/nano15211668
Chicago/Turabian StyleAnandkumar, Mariappan, Shanmugavel Sudarsan, Venkata Ramesh Naganaboina, Naveen Kumar Bandari, Ksenia Sergeevna Litvinyuk, Shiv Govind Singh, and Evgeny Alekseevich Trofimov. 2025. "High-Entropy (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 Zirconate Pyrochlore: A Promising Photocatalyst for Diverse Environmental Applications" Nanomaterials 15, no. 21: 1668. https://doi.org/10.3390/nano15211668
APA StyleAnandkumar, M., Sudarsan, S., Naganaboina, V. R., Bandari, N. K., Litvinyuk, K. S., Singh, S. G., & Trofimov, E. A. (2025). High-Entropy (Ce0.2Pr0.2Zn0.2Nd0.2Tb0.2)2Zr2O7 Zirconate Pyrochlore: A Promising Photocatalyst for Diverse Environmental Applications. Nanomaterials, 15(21), 1668. https://doi.org/10.3390/nano15211668








