One-Step Synthesized Folic Acid-Based Carbon Dots: A Biocompatible Nanomaterial for the Treatment of Bacterial Infections in Lung Pathologies
Abstract
1. Introduction
2. Materials and Methods
2.1. Microwave-Assisted Synthesis, Purification, and Yield
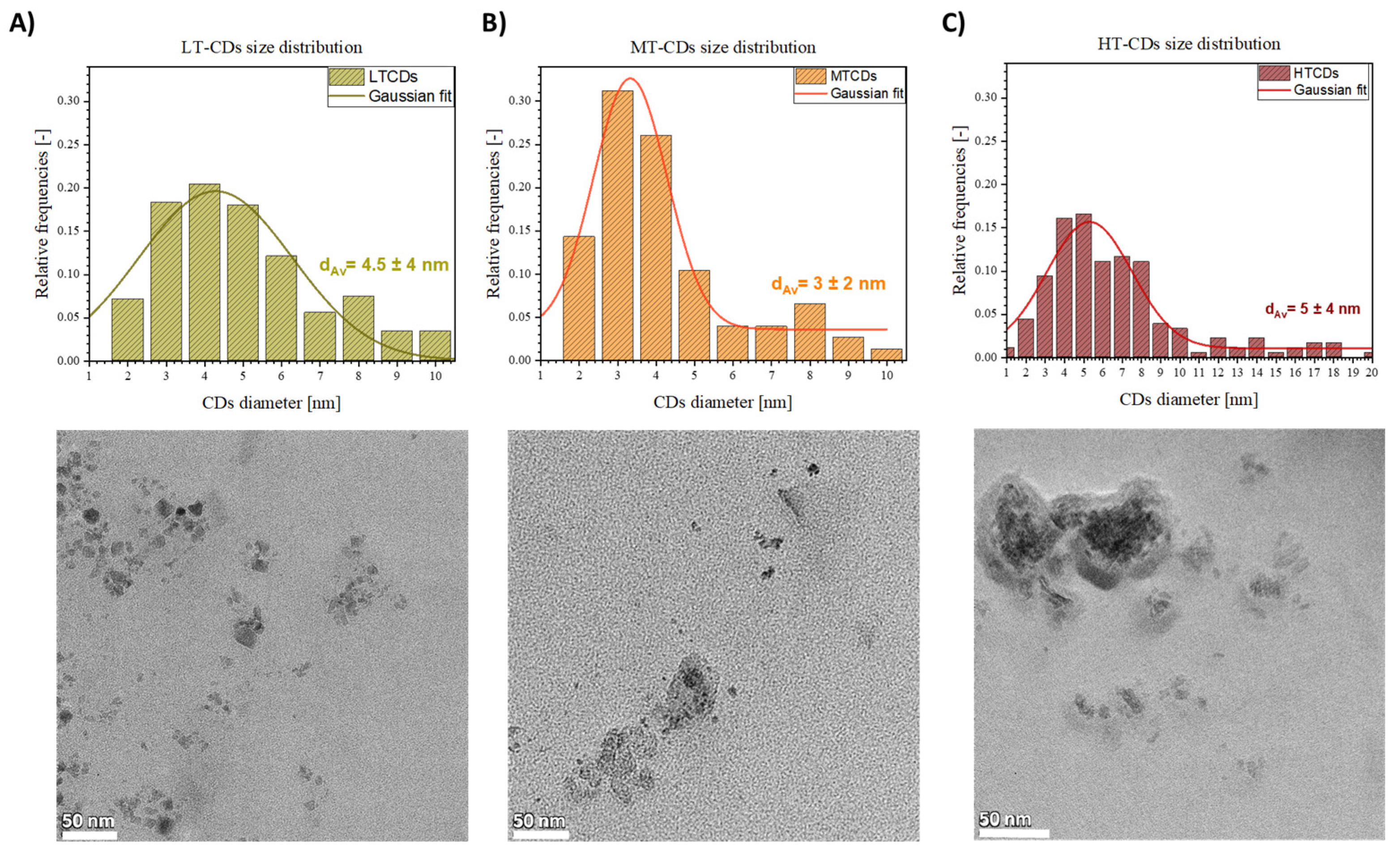
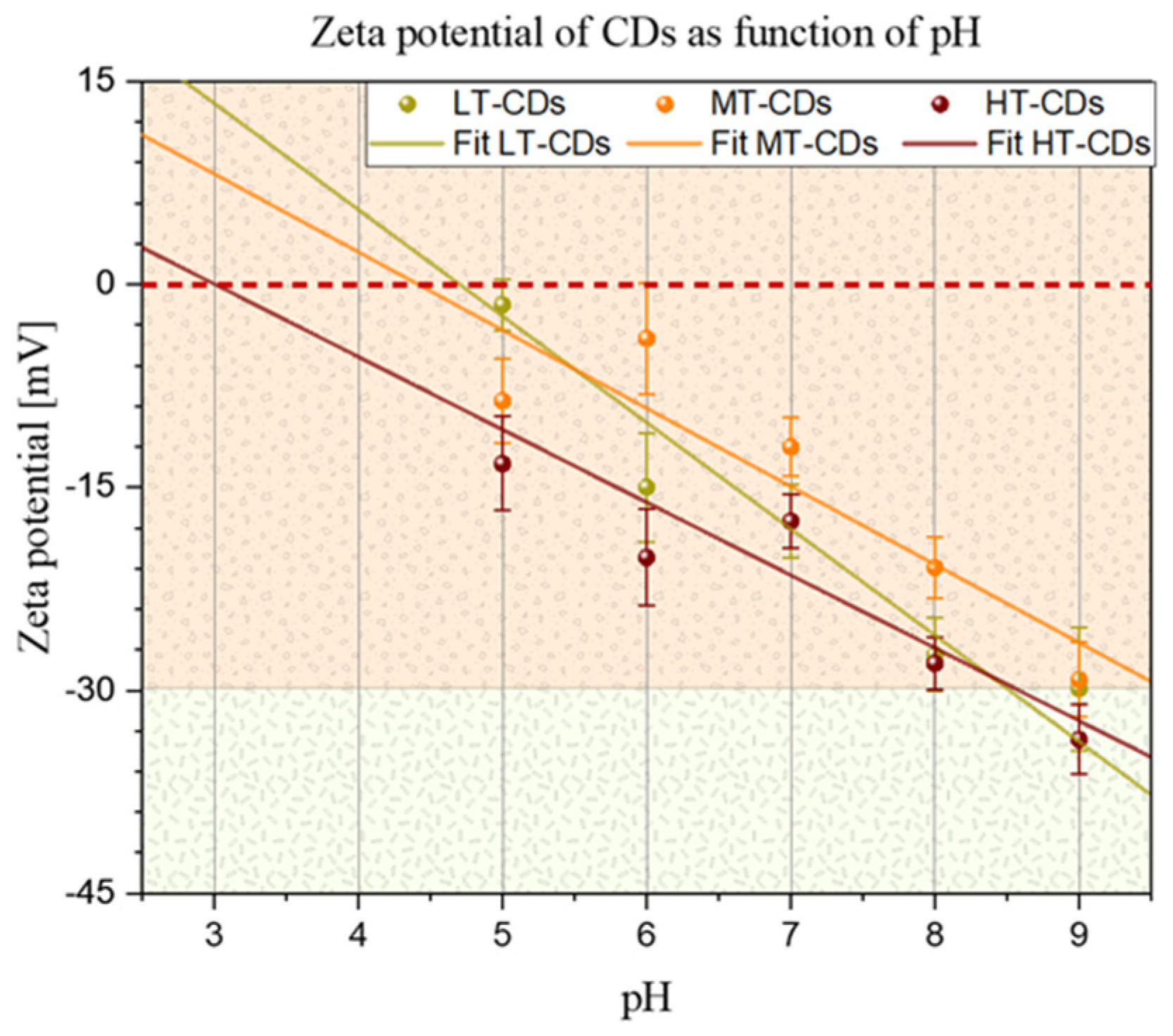
2.2. Dimensional Distribution and Zeta Potential at Different pHs
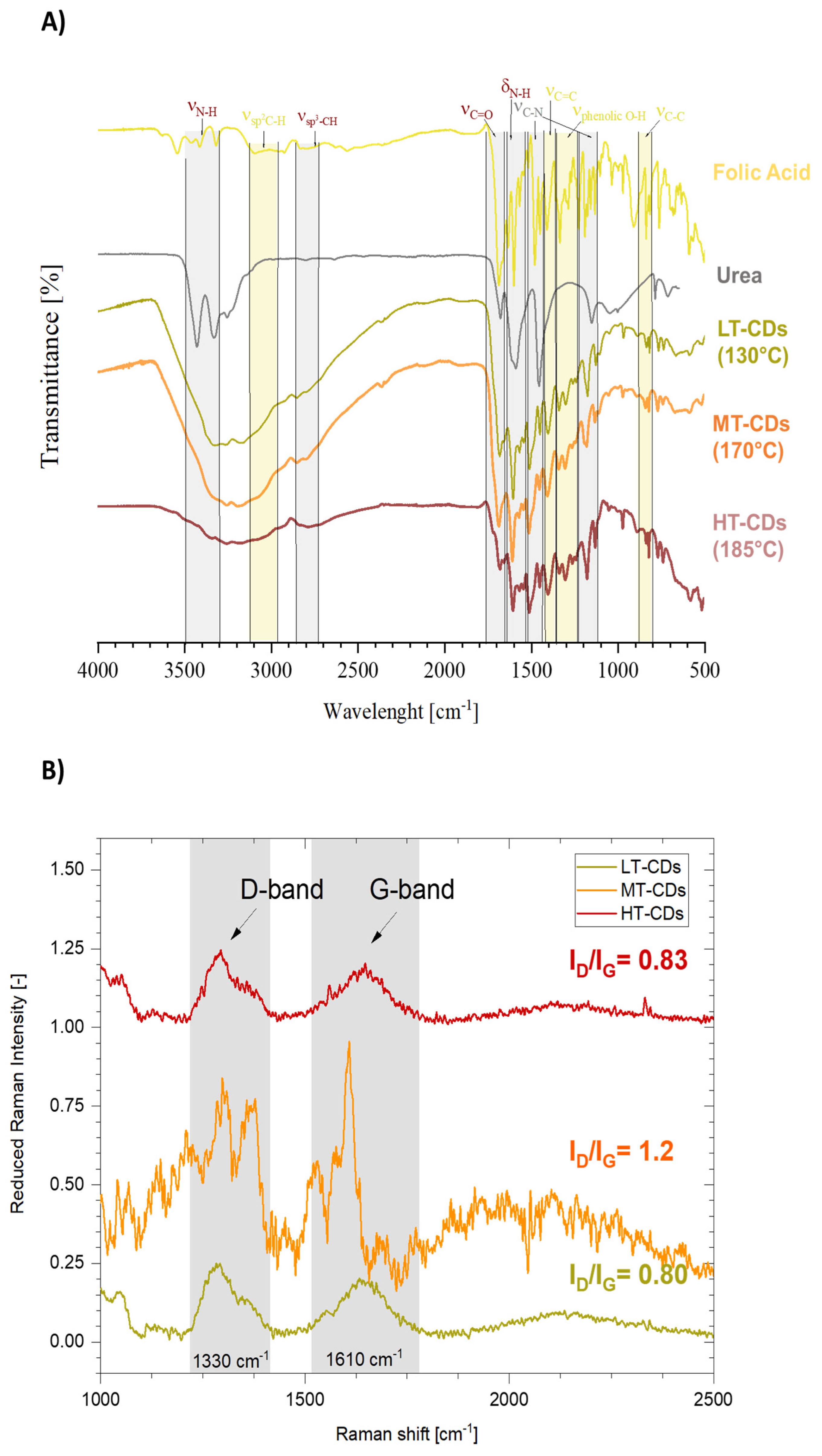
2.3. Fourier Transform Infrared (FT-IR) and Raman Spectroscopy
2.4. Thermogravimetric Analysis (TGA) and Derivative Thermogravimetry (DTG) of Precursors and FA-CDs
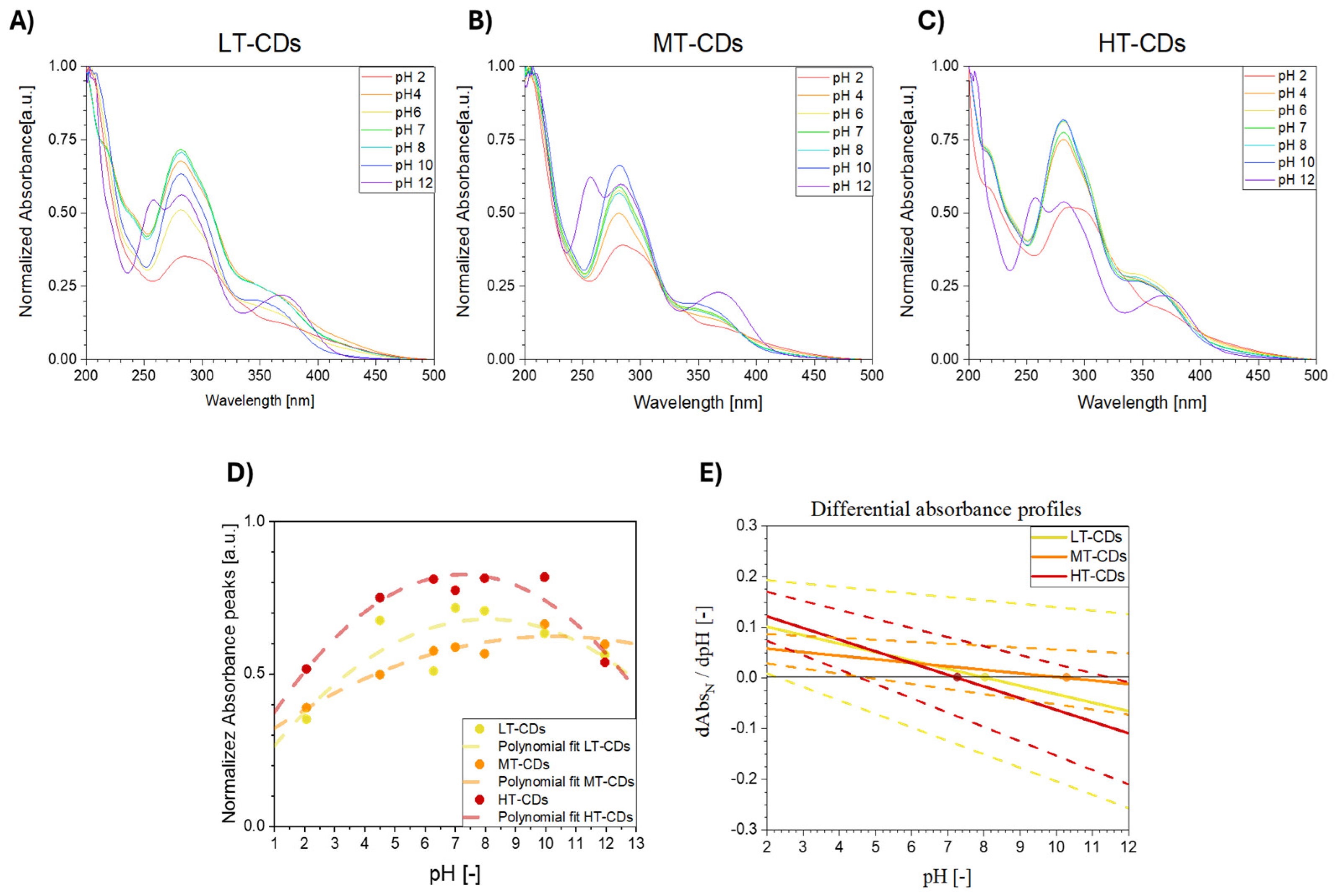
2.5. UV–Vis Absorbance as a Function of pH
2.6. Biological Properties
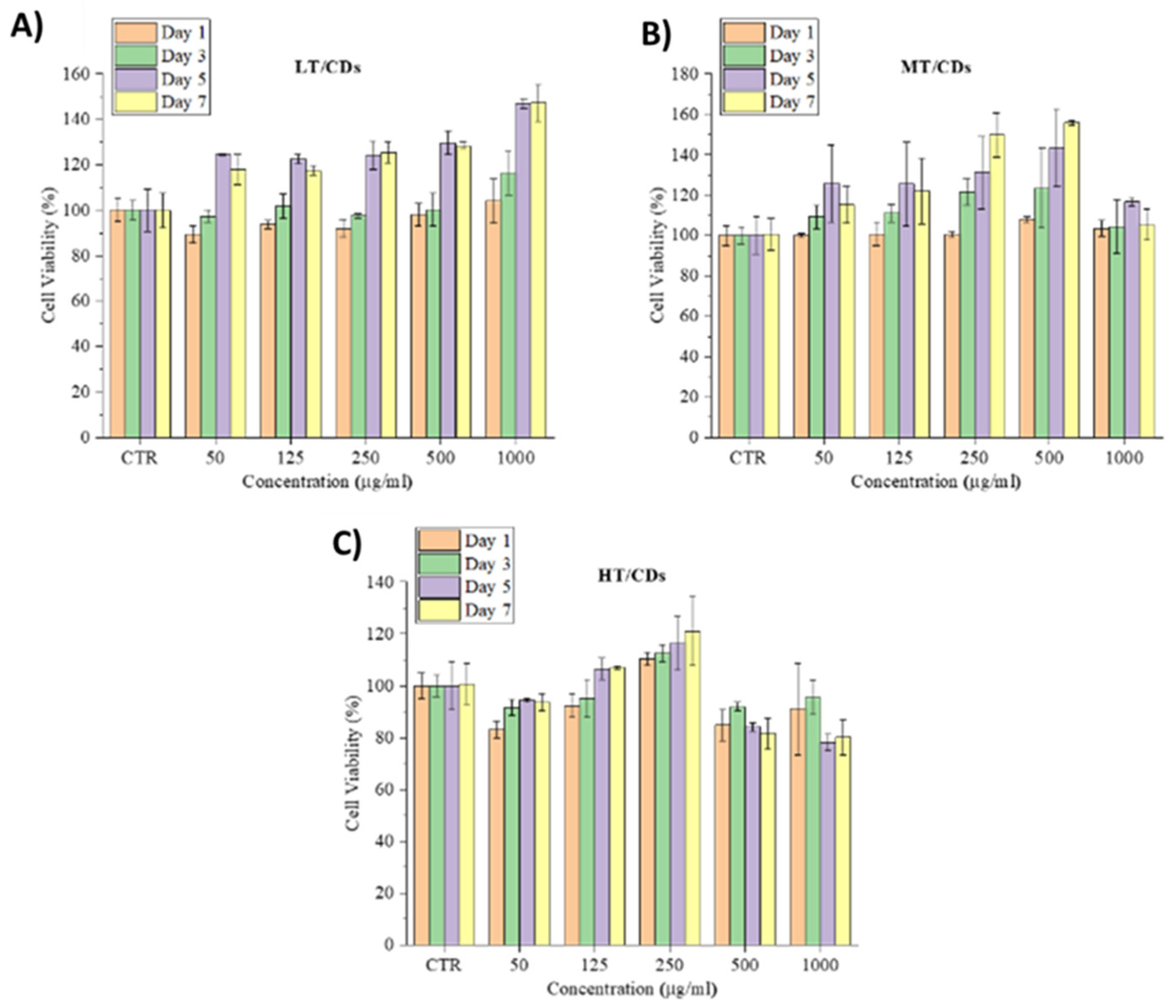
2.6.1. Cell Culture and Biocompatibility Assay
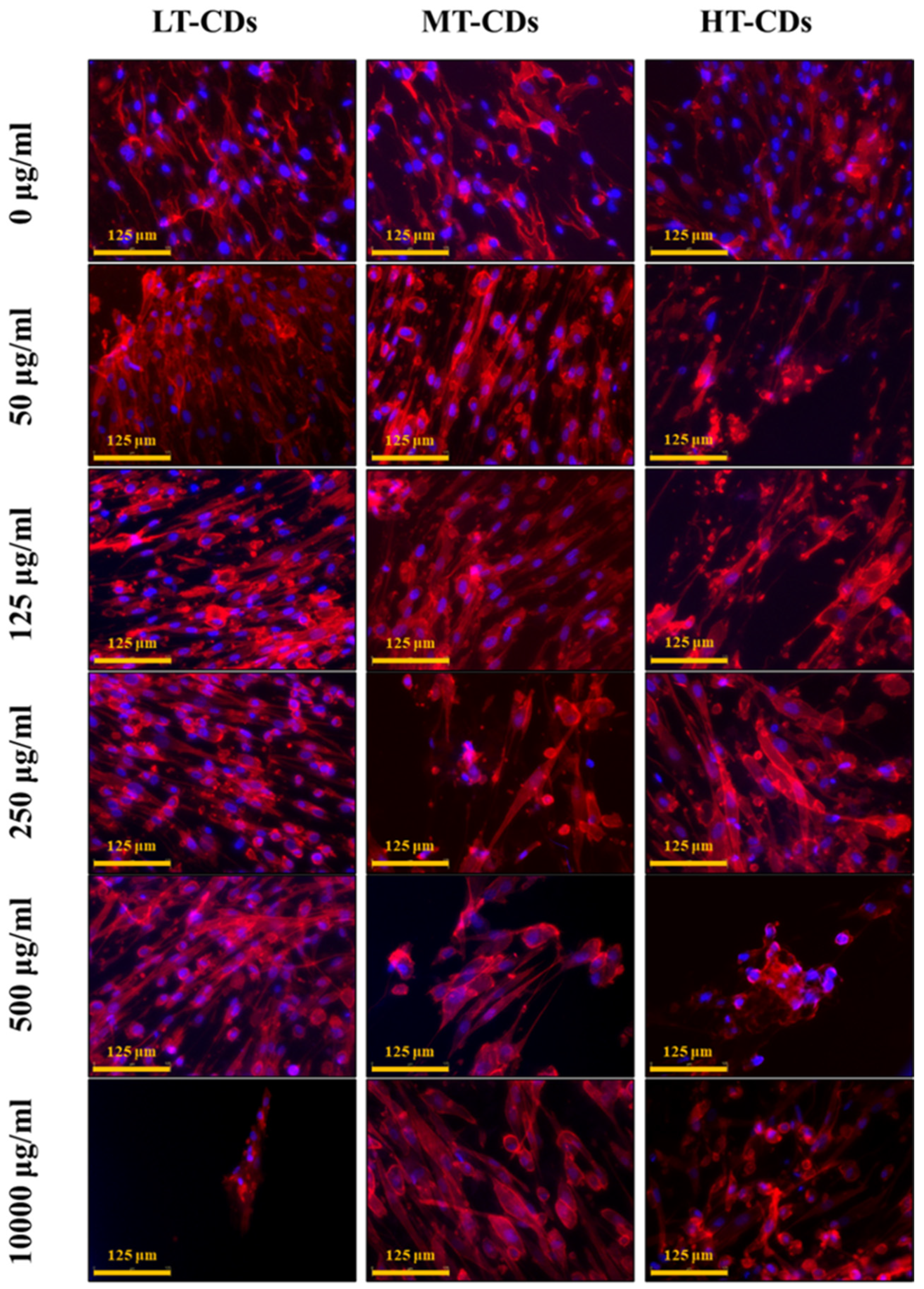
2.6.2. Morphology of HLF Cells
2.7. Antimicrobial Activity
2.8. ROS Generation by L-Ascorbic Acid Absorbance Reduction
2.9. FA-CDs Interaction with Mucin by UV–Vis Absorption and FTIR
3. Results and Discussion
3.1. Microwave-Assisted FA-CDs Synthesis
3.2. Dimensional Distribution and Zeta Potential as a Function of pH
3.3. Fourier Transform Infrared (FT-IR) and Raman Spectroscopy
3.4. TGA/DTG of Precursors and CDs
3.5. UV–Vis Absorbance at Different pH Levels
3.6. Viability and Morphology of HLFs
3.7. Antimicrobial Activity and Oxidase-like Activity by Monitoring ROS Generation
3.8. FA-CDs Interaction with Mucin by UV–Vis Absorption and FTIR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- D’Anna, S.E.; Maniscalco, M.; Cappello, F.; Carone, M.; Motta, A.; Balbi, B.; Ricciardolo, F.L.; Caramori, G.; Di Stefano, A. Bacterial and viral infections and related inflammatory responses in chronic obstructive pulmonary disease. Ann. Med. 2021, 53, 135–150. [Google Scholar] [CrossRef]
- Bagdonas, E.; Raudoniute, J.; Bruzauskaite, I.; Aldonyte, R. Novel aspects of pathogenesis and regeneration mechanisms in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2015, 10, 995–1013. [Google Scholar] [CrossRef] [PubMed]
- PRASAD, B. Chronic obstructive pulmonary disease (COPD). Int. J. Pharm. Res. Technol. (IJPRT) 2020, 10, 67–71. [Google Scholar]
- Mannino, D.M.; Homa, D.M.; Akinbami, L.J.; Ford, E.S.; Redd, S.C. Chronic obstructive pulmonary disease surveillance-United States, 1971–2000. MMWR Surveill. Summ. 2002, 51, 1–16. [Google Scholar]
- Sullivan, S.D.; Ramsey, S.D.; Lee, T.A. The economic burden of COPD. Chest 2000, 117, 5S–9S. [Google Scholar] [CrossRef]
- Confalonieri, M.; Salton, F.; Fabiano, F. Acute respiratory distress syndrome. Eur. Respir. Rev. 2017, 26, 160116. [Google Scholar] [CrossRef] [PubMed]
- Force, A.D.T.; Ranieri, V.; Rubenfeld, G.; Thompson, B.; Ferguson, N.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A. Acute respiratory distress syndrome. JAMA 2012, 307, 2526–2533. [Google Scholar] [PubMed]
- Miravitlles, M. Exacerbations of chronic obstructive pulmonary disease: When are bacteria important? Eur. Respir. J. 2002, 20, 9s–19s. [Google Scholar] [CrossRef]
- Pragman, A.A.; Lyu, T.; Baller, J.A.; Gould, T.J.; Kelly, R.F.; Reilly, C.S.; Isaacson, R.E.; Wendt, C.H. The lung tissue microbiota of mild and moderate chronic obstructive pulmonary disease. Microbiome 2018, 6, 7. [Google Scholar] [CrossRef]
- Kreitmann, L.; Monard, C.; Dauwalder, O.; Simon, M.; Argaud, L. Early bacterial co-infection in ARDS related to COVID-19. Intensive Care Med. 2020, 46, 1787–1789. [Google Scholar] [CrossRef]
- Hetta, H.F.; Ramadan, Y.N.; Al-Harbi, A.I.; Ahmed, E.A.; Battah, B.; Abd Ellah, N.H.; Zanetti, S.; Donadu, M.G. Nanotechnology as a promising approach to combat multidrug resistant bacteria: A comprehensive review and future perspectives. Biomedicines 2023, 11, 413. [Google Scholar] [CrossRef]
- Malmsten, M. Nanomaterials as antimicrobial agents. In Handbook of Nanomaterials Properties; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1053–1075. [Google Scholar]
- Naskar, A.; Kim, K.S. Nanomaterials as delivery vehicles and components of new strategies to combat bacterial infections: Advantages and limitations. Microorganisms 2019, 7, 356. [Google Scholar] [CrossRef]
- Roy, S.; Sarkhel, S.; Bisht, D.; Hanumantharao, S.N.; Rao, S.; Jaiswal, A. Antimicrobial mechanisms of biomaterials: From macro to nano. Biomater. Sci. 2022, 10, 4392–4423. [Google Scholar] [CrossRef]
- Ghirardello, M.; Ramos-Soriano, J.; Galan, M.C. Carbon dots as an emergent class of antimicrobial agents. Nanomaterials 2021, 11, 1877. [Google Scholar] [CrossRef]
- Li, P.; Sun, L.; Xue, S.; Qu, D.; An, L.; Wang, X.; Sun, Z. Recent advances of carbon dots as new antimicrobial agents. SmartMat 2022, 3, 226–248. [Google Scholar] [CrossRef]
- Dong, X.; Liang, W.; Meziani, M.J.; Sun, Y.-P.; Yang, L. Carbon dots as potent antimicrobial agents. Theranostics 2020, 10, 671. [Google Scholar] [CrossRef]
- Travlou, N.A.; Giannakoudakis, D.A.; Algarra, M.; Labella, A.M.; Rodríguez-Castellón, E.; Bandosz, T.J. S-and N-doped carbon quantum dots: Surface chemistry dependent antibacterial activity. Carbon 2018, 135, 104–111. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Stalikas, C. Antimicrobial properties of carbon quantum dots. In Nanotoxicity; Elsevier: Amsterdam, The Netherlands, 2020; pp. 301–315. [Google Scholar]
- Liu, H.; Li, Z.; Sun, Y.; Geng, X.; Hu, Y.; Meng, H.; Ge, J.; Qu, L. Synthesis of luminescent carbon dots with ultrahigh quantum yield and inherent folate receptor-positive cancer cell targetability. Sci. Rep. 2018, 8, 1086. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xu, R.; Yang, Q.; Tao, K.; Shan, D. Green one-step pyrolytic synthesis of folic acid-derived carbon dots for sensitive turn-on fluorescence detection of cysteine. Analyst 2024, 149, 5863–5870. [Google Scholar] [CrossRef] [PubMed]
- Patra, R.; Panda, P.K.; Hung, C.-Y.; Dash, P.; Yang, P.-C.; Hsieh, C.-T. Facile synthesis of yellow emissive heteroatom doped carbon quantum dots fluorescent probe with superior quantum yield for fast-track ionic detection in aqueous media. Mater. Res. Bull. 2024, 180, 113040. [Google Scholar] [CrossRef]
- Fahmi, M.Z.; Sholihah, N.F.; Wibrianto, A.; Sakti, S.C.; Firdaus, F.; Chang, J.Y. Simple and fast design of folic acid-based carbon dots as theranostic agent and its drug release aspect. Mater. Chem. Phys. 2021, 267, 124596. [Google Scholar] [CrossRef]
- Adhel, E.; Duong, N.-T.H.; Vu, T.H.; Taverna, D.; Ammar, S.; Serradji, N. Interaction between carbon dots from folic acid and their cellular receptor: A qualitative physicochemical approach. Phys. Chem. Chem. Phys. 2023, 25, 14324–14333. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, Q.; Qin, X.; Liu, Z.; Li, Z.; Zhong, X.; Xia, L.; He, J.; Fang, B. Carbon dots derived from folic acid attenuates osteoarthritis by protecting chondrocytes through NF-κB/MAPK pathway and reprogramming macrophages. J. Nanobiotechnol. 2022, 20, 469. [Google Scholar] [CrossRef] [PubMed]
- Kung, J.-C.; Tseng, I.-T.; Chien, C.-S.; Lin, S.-H.; Wang, C.-C.; Shih, C.-J. Microwave assisted synthesis of negative-charge carbon dots with potential antibacterial activity against multi-drug resistant bacteria. RSC Adv. 2020, 10, 41202–41208. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Adcock, A.F.; Dumra, S.; Collins, J.; Yang, L.; Bunker, C.E.; Qian, H.; Meziani, M.J.; Sun, Y.-P. Microwave-Assisted Carbonization Processing for Carbon Dot-like Nanomaterials with Antimicrobial Properties. Micro 2025, 5, 14. [Google Scholar] [CrossRef]
- Romero, M.P.; Alves, F.; Stringasci, M.D.; Buzzá, H.H.; Ciol, H.; Inada, N.M.; Bagnato, V.S. One-pot microwave-assisted synthesis of carbon dots and in vivo and in vitro antimicrobial photodynamic applications. Front. Microbiol. 2021, 12, 662149. [Google Scholar] [CrossRef]
- Serag, E.; Helal, M.; El Nemr, A. Curcumin loaded onto folic acid carbon dots as a potent drug delivery system for antibacterial and anticancer applications. J. Clust. Sci. 2024, 35, 519–532. [Google Scholar] [CrossRef]
- Kazeminava, F.; Javanbakht, S.; Nouri, M.; Gholizadeh, P.; Nezhad-Mokhtari, P.; Ganbarov, K.; Tanomand, A.; Kafil, H.S. Gentamicin-loaded chitosan/folic acid-based carbon quantum dots nanocomposite hydrogel films as potential antimicrobial wound dressing. J. Biol. Eng. 2022, 16, 36. [Google Scholar] [CrossRef]
- Xu, Q.; Li, K.; Wang, P. pH-Sensitive Silver-Containing Carbon Dots Based on Folic Acid. Materials 2022, 15, 1880. [Google Scholar] [CrossRef]
- Li, S.; Zhang, H.; Bao, Y.; Zhang, H.; Wang, J.; Liu, M.; Yan, R.; Wang, Z.; Wu, X.; Jin, Y. Immunoantitumor activity and oxygenation effect based on iron–copper-doped folic acid carbon dots. ACS Appl. Mater. Interfaces 2024, 16, 16653–16668. [Google Scholar] [CrossRef]
- Guo, B.; Liu, G.; Hu, C.; Lei, B.; Liu, Y. The structural characteristics and mechanisms of antimicrobial carbon dots: A mini review. Mater. Adv. 2022, 3, 7726–7741. [Google Scholar] [CrossRef]
- Guan, W.; Gu, W.; Ye, L.; Guo, C.; Su, S.; Xu, P.; Xue, M. Microwave-assisted polyol synthesis of carbon nitride dots from folic acid for cell imaging. Int. J. Nanomed. 2014, 9, 5071–5078. [Google Scholar] [CrossRef]
- Burns, D.B.; Zydney, A.L. Buffer effects on the zeta potential of ultrafiltration membranes. J. Membr. Sci. 2000, 172, 39–48. [Google Scholar] [CrossRef]
- Della Sala, F.; Longobardo, G.; Lista, G.; Messina, F.; Borzacchiello, A. Effect of hyaluronic acid and mesenchymal stem cells secretome combination in promoting alveolar regeneration. Int. J. Mol. Sci. 2023, 24, 3642. [Google Scholar] [CrossRef]
- Park, K.; Sadeghi, K.; Panda, P.K.; Seo, J.; Seo, J. Ethylene vinyl acetate/low-density polyethylene/oyster shell powder composite films: Preparation, characterization, and antimicrobial properties for biomedical applications. J. Taiwan Inst. Chem. Eng. 2022, 134, 104301. [Google Scholar] [CrossRef]
- Guaresti, O.; Maiz–Fernández, S.; Palomares, T.; Alonso–Varona, A.; Eceiza, A.; Pérez–Álvarez, L.; Gabilondo, N. Dual charged folate labelled chitosan nanogels with enhanced mucoadhesion capacity for targeted drug delivery. Eur. Polym. J. 2020, 134, 109847. [Google Scholar] [CrossRef]
- de Medeiros, T.V.; Manioudakis, J.; Noun, F.; Macairan, J.-R.; Victoria, F.; Naccache, R. Microwave-assisted synthesis of carbon dots and their applications. J. Mater. Chem. C 2019, 7, 7175–7195. [Google Scholar] [CrossRef]
- Kappe, C.O. Controlled microwave heating in modern organic synthesis. Angew. Chem. Int. Ed. 2004, 43, 6250–6284. [Google Scholar] [CrossRef]
- Geng, T.; Liu, C.; Xiao, G.; Lu, S.; Zou, B. Advances in the application of high pressure in carbon dots. Mater. Chem. Front. 2019, 3, 2617–2626. [Google Scholar] [CrossRef]
- Du, X.; Zhang, M.; Ma, Y.; Wang, X.; Liu, Y.; Huang, H.; Kang, Z. Size-dependent antibacterial of carbon dots by selective absorption and differential oxidative stress of bacteria. J. Colloid Interface Sci. 2023, 634, 44–53. [Google Scholar] [CrossRef]
- Zhao, W.B.; Liu, K.K.; Wang, Y.; Li, F.K.; Guo, R.; Song, S.Y.; Shan, C.X. Antibacterial carbon dots: Mechanisms, design, and applications. Adv. Healthc. Mater. 2023, 12, 2300324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Feng, X.; Zhang, F.; Yang, Y.; Liu, X. Effect of reaction temperature on structure and fluorescence properties of nitrogen-doped carbon dots. Appl. Surf. Sci. 2016, 387, 1236–1246. [Google Scholar] [CrossRef]
- Santos, N.; Santana, P.A.; Osorio-Roman, I.; Jara-Gutiérrez, C.; Villena, J.; Ahumada, M. Effect of temperature on the carbonization process of cationic carbon dots: A physicochemical and in vitro study. RSC Adv. 2025, 15, 12814–12824. [Google Scholar] [CrossRef]
- Demirci, S.; McNally, A.B.; Ayyala, R.S.; Lawson, L.B.; Sahiner, N. Synthesis and characterization of nitrogen-doped carbon dots as fluorescent nanoprobes with antimicrobial properties and skin permeability. J. Drug Deliv. Sci. Technol. 2020, 59, 101889. [Google Scholar] [CrossRef]
- Koç, O.K.; Üzer, A.; Apak, R. High quantum yield nitrogen-doped carbon quantum dot-based fluorescent probes for selective sensing of 2, 4, 6-trinitrotoluene. ACS Appl. Nano Mater. 2022, 5, 5868–5881. [Google Scholar] [CrossRef]
- Lazar, P.; Mach, R.; Otyepka, M. Spectroscopic fingerprints of graphitic, pyrrolic, pyridinic, and chemisorbed nitrogen in N-doped graphene. J. Phys. Chem. C 2019, 123, 10695–10702. [Google Scholar] [CrossRef]
- Fawaz, W.; Hasian, J.; Alghoraibi, I. Synthesis and physicochemical characterization of carbon quantum dots produced from folic acid. Sci. Rep. 2023, 13, 18641. [Google Scholar] [CrossRef] [PubMed]
- Carbonaro, C.M.; Chiriu, D.; Stagi, L.; Casula, M.F.; Thakkar, S.V.; Malfatti, L.; Suzuki, K.; Ricci, P.C.; Corpino, R. Carbon dots in water and mesoporous matrix: Chasing the origin of their photoluminescence. J. Phys. Chem. C 2018, 122, 25638–25650. [Google Scholar] [CrossRef]
- Carbonaro, C.M.; Corpino, R.; Salis, M.; Mocci, F.; Thakkar, S.V.; Olla, C.; Ricci, P.C. On the emission properties of carbon dots: Reviewing data and discussing models. C 2019, 5, 60. [Google Scholar] [CrossRef]
- Dong, Y.; Pang, H.; Yang, H.B.; Guo, C.; Shao, J.; Chi, Y.; Li, C.M.; Yu, T. Carbon-based dots co-doped with nitrogen and sulfur for high quantum yield and excitation-independent emission. Angew. Chem. Int. Ed. 2013, 52, 7800–7804. [Google Scholar] [CrossRef]
- Vinci, J.C.; Ferrer, I.M.; Guterry, N.W.; Colón, V.M.; Destino, J.F.; Bright, F.V.; Colón, L.A. Spectroscopic characteristics of carbon dots (C-dots) derived from carbon fibers and conversion to sulfur-bridged C-dots nanosheets. Appl. Spectrosc. 2015, 69, 1082–1090. [Google Scholar] [CrossRef]
- Wu, Q.; Jia, Y.; Liu, Q.; Mao, X.; Guo, Q.; Yan, X.; Zhao, J.; Liu, F.; Du, A.; Yao, X. Ultra-dense carbon defects as highly active sites for oxygen reduction catalysis. Chem 2022, 8, 2715–2733. [Google Scholar] [CrossRef]
- Ibarra-Prieto, H.D.; Garcia-Garcia, A.; Aguilera-Granja, F.; Navarro-Ibarra, D.C.; Rivero-Espejel, I. One-Pot, Optimized Microwave-Assisted Synthesis of Difunctionalized and B–N Co-Doped Carbon Dots: Structural Characterization. Nanomaterials 2023, 13, 2753. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, M.; Wu, Z.; Chen, D.; Singh, A.; Sonar, P.; Thiel, D.; Li, Q. Composition and concentration-dependent photoluminescence of nitrogen-doped carbon dots. Adv. Powder Technol. 2022, 33, 103560. [Google Scholar] [CrossRef]
- Nguyen, K.G.; Huš, M.; Baragau, I.A.; Bowen, J.; Heil, T.; Nicolaev, A.; Abramiuc, L.E.; Sapelkin, A.; Sajjad, M.T.; Kellici, S. Engineering nitrogen-doped carbon quantum dots: Tailoring optical and chemical properties through selection of nitrogen precursors. Small 2024, 20, 2310587. [Google Scholar] [CrossRef]
- Schaber, P.M.; Colson, J.; Higgins, S.; Thielen, D.; Anspach, B.; Brauer, J. Thermal decomposition (pyrolysis) of urea in an open reaction vessel. Thermochim. Acta 2004, 424, 131–142. [Google Scholar] [CrossRef]
- Vora, A.; Riga, A.; Dollimore, D.; Alexander, K. Thermal stability of folic acid in the solid-state. J. Therm. Anal. Calorim. 2004, 75, 709–717. [Google Scholar] [CrossRef]
- Ngidi, N.P.; Ollengo, M.A.; Nyamori, V.O. Effect of doping temperatures and nitrogen precursors on the physicochemical, optical, and electrical conductivity properties of nitrogen-doped reduced graphene oxide. Materials 2019, 12, 3376. [Google Scholar] [CrossRef] [PubMed]
- Szapoczka, W.K.; Olla, C.; Carucci, C.; Truskewycz, A.L.; Skodvin, T.; Salis, A.; Carbonaro, C.M.; Holst, B.; Thomas, P.J. Ratiometric Fluorescent pH Sensing with Carbon Dots: Fluorescence Mapping across pH Levels for Potential Underwater Applications. Nanomaterials 2024, 14, 1434. [Google Scholar] [CrossRef]
- Khmeleva, M.Y.E.; Laptinskiy, K.A.; Kasyanova, P.S.; Tomskaya, A.E.E.; Dolenko, T.Y.A.D. Dependence of photoluminescence of carbon dots with different surface functionalization on hydrogen factor of water. Opt. Spectrosc. 2022, 130, 882–889. [Google Scholar] [CrossRef]
- Ren, J.; Weber, F.; Weigert, F.; Wang, Y.; Choudhury, S.; Xiao, J.; Lauermann, I.; Resch-Genger, U.; Bande, A.; Petit, T. Influence of surface chemistry on optical, chemical and electronic properties of blue luminescent carbon dots. Nanoscale 2019, 11, 2056–2064. [Google Scholar] [CrossRef]
- Yang, M.; Li, B.; Zhong, K.; Lu, Y. Photoluminescence properties of N-doped carbon dots prepared in different solvents and applications in pH sensing. J. Mater. Sci. 2018, 53, 2424–2433. [Google Scholar] [CrossRef]
- Wang, S.; Cole, I.S.; Li, Q. The toxicity of graphene quantum dots. RSC Adv. 2016, 6, 89867–89878. [Google Scholar] [CrossRef]
- Knoblauch, R.; Geddes, C.D. Carbon nanodots in photodynamic antimicrobial therapy: A review. Materials 2020, 13, 4004. [Google Scholar] [CrossRef]
- Yu, M.; Guo, X.; Lu, H.; Li, P.; Huang, R.; Xu, C.; Gong, X.; Xiao, Y.; Xing, X. Carbon dots derived from folic acid as an ultra-succinct smart antimicrobial nanosystem for selective killing of S. aureus and biofilm eradication. Carbon 2022, 199, 395–406. [Google Scholar] [CrossRef]
- Zajac, M.; Dreano, E.; Edwards, A.; Planelles, G.; Sermet-Gaudelus, I. Airway surface liquid pH regulation in airway epithelium current understandings and gaps in knowledge. Int. J. Mol. Sci. 2021, 22, 3384. [Google Scholar] [CrossRef]
- Kong, B.; Yang, T.; Cheng, F.; Qian, Y.; Li, C.; Zhan, L.; Li, Y.; Zou, H.; Huang, C. Carbon dots as nanocatalytic medicine for anti-inflammation therapy. J. Colloid Interface Sci. 2022, 611, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, S.; Li, Y.; Liu, J. Facile synthesis of iron and nitrogen co-doped carbon dot nanozyme as highly efficient peroxidase mimics for visualized detection of metabolites. Molecules 2023, 28, 6064. [Google Scholar] [CrossRef] [PubMed]
- Nie, R.; Zhang, J.; Jia, Q.; Li, Y.; Tao, W.; Qin, G.; Liu, X.; Tao, Y.; Zhang, Y.; Li, P. Structurally oriented carbon dots as ROS nanomodulators for dynamic chronic inflammation and infection elimination. ACS Nano 2024, 18, 22055–22070. [Google Scholar] [CrossRef]
- Gao, W.; He, J.; Chen, L.; Meng, X.; Ma, Y.; Cheng, L.; Tu, K.; Gao, X.; Liu, C.; Zhang, M. Deciphering the catalytic mechanism of superoxide dismutase activity of carbon dot nanozyme. Nat. Commun. 2023, 14, 160. [Google Scholar] [CrossRef]
- Dong, W.; Xu, L.; Chen, M.; Jiang, T.; Su, L.; Ma, J.; Chen, C.P.; Zhang, G. Co-, N-doped carbon dot nanozymes based on an untriggered ROS generation approach for anti-biofilm activities and in vivo anti-bacterial treatment. J. Mater. Chem. B 2024, 12, 1052–1063. [Google Scholar] [CrossRef]
- Ng, A.W.; Bidani, A.; Heming, T.A. Innate host defense of the lung: Effects of lung-lining fluid pH. Lung 2004, 182, 297–317. [Google Scholar] [CrossRef]
- Steinmann, E. La secretion bronchique et le pH. Bronches 1956, 6, 126–129. [Google Scholar]
- England, R.; Homer, J.; Knight, L.; Ell, S. Nasal pH measurement: A reliable and repeatable parameter. Clin. Otolaryngol. Allied Sci. 1999, 24, 67–68. [Google Scholar] [CrossRef]
- Adler, K.; Wooten, O.; Philippoff, W.; Lerner, E.; Dulfano, M. Physical properties of sputum: III. Rheologic variability and intrinsic relationships. Am. Rev. Respir. Dis. 1972, 106, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Uthaiwat, P.; Priprem, A.; Puthongking, P.; Daduang, J.; Nukulkit, C.; Chio-Srichan, S.; Boonsiri, P.; Thapphasaraphong, S. Characteristic evaluation of gel formulation containing niosomes of melatonin or its derivative and mucoadhesive properties using ATR-FTIR spectroscopy. Polymers 2021, 13, 1142. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Bansil, R.; Bhaskar, K.R.; Turner, B.S.; LaMont, J.T.; Niu, N.; Afdhal, N.H. pH-dependent conformational change of gastric mucin leads to sol-gel transition. Biophys. J. 1999, 76, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
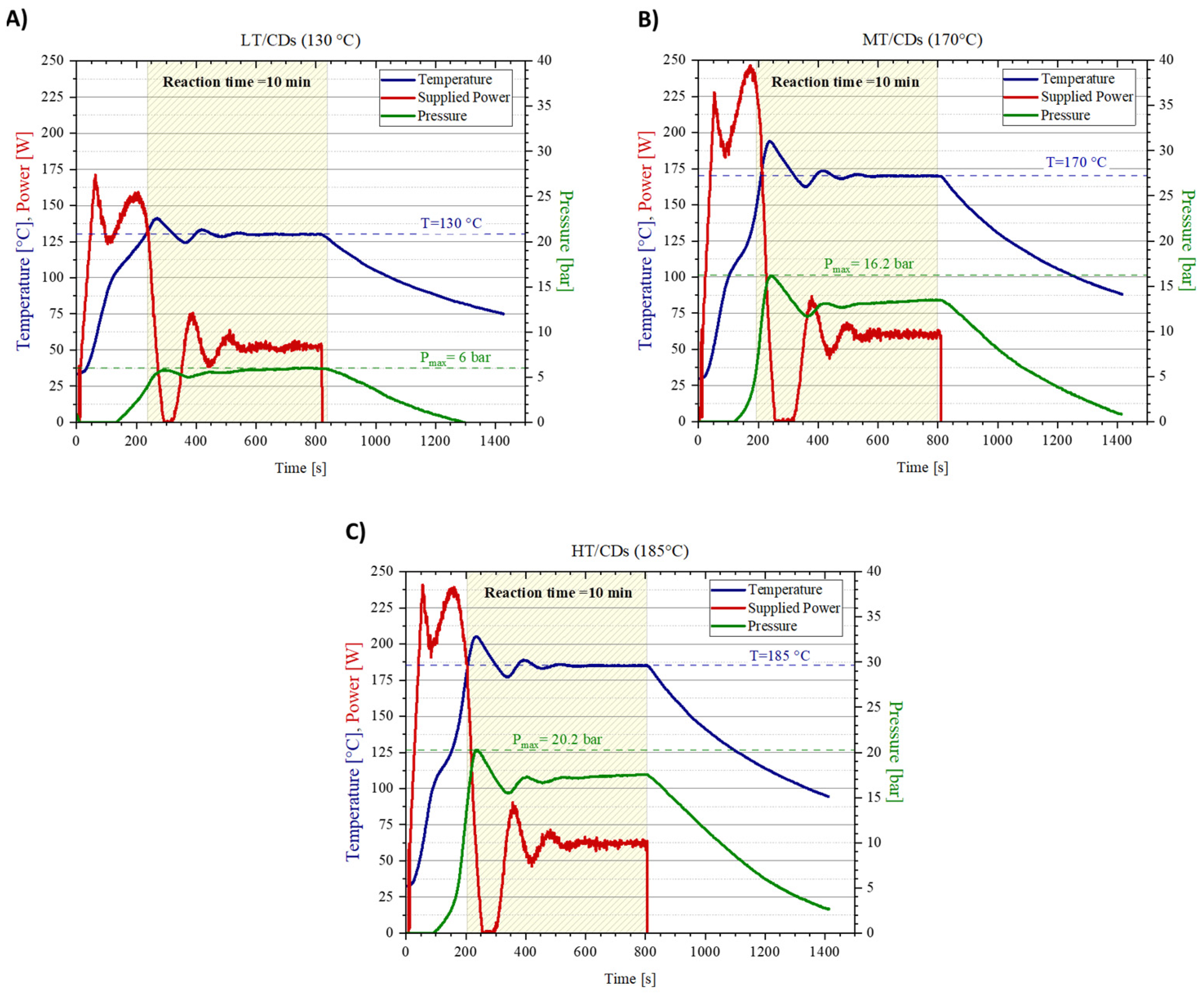



| Sample | Reaction Temperature [°C] | Reaction Time [min] | Precursors Solution pH |
|---|---|---|---|
| LT-CDs | 130 | 10 | pH = 7 (Water) |
| MT-CDs | 170 | 10 | pH = 7 (Water) |
| HT-CDs | 185 | 10 | pH = 7 (Water) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longobardo, G.; Della Sala, F.; Marino, G.; Barretta, M.; Forte, M.; Paradiso, R.; Borriello, G.; Borzacchiello, A. One-Step Synthesized Folic Acid-Based Carbon Dots: A Biocompatible Nanomaterial for the Treatment of Bacterial Infections in Lung Pathologies. Nanomaterials 2025, 15, 1657. https://doi.org/10.3390/nano15211657
Longobardo G, Della Sala F, Marino G, Barretta M, Forte M, Paradiso R, Borriello G, Borzacchiello A. One-Step Synthesized Folic Acid-Based Carbon Dots: A Biocompatible Nanomaterial for the Treatment of Bacterial Infections in Lung Pathologies. Nanomaterials. 2025; 15(21):1657. https://doi.org/10.3390/nano15211657
Chicago/Turabian StyleLongobardo, Gennaro, Francesca Della Sala, Giuseppe Marino, Marco Barretta, Mario Forte, Rubina Paradiso, Giorgia Borriello, and Assunta Borzacchiello. 2025. "One-Step Synthesized Folic Acid-Based Carbon Dots: A Biocompatible Nanomaterial for the Treatment of Bacterial Infections in Lung Pathologies" Nanomaterials 15, no. 21: 1657. https://doi.org/10.3390/nano15211657
APA StyleLongobardo, G., Della Sala, F., Marino, G., Barretta, M., Forte, M., Paradiso, R., Borriello, G., & Borzacchiello, A. (2025). One-Step Synthesized Folic Acid-Based Carbon Dots: A Biocompatible Nanomaterial for the Treatment of Bacterial Infections in Lung Pathologies. Nanomaterials, 15(21), 1657. https://doi.org/10.3390/nano15211657







