Abstract
Recently, two-dimensional metal–organic framework (2D MOF) hybrids are attracting much attention in supercapacitors. However, their performance is limited by the insufficient utilization of active sites and poor conductivity. Notably, the full utilization of active sites highly depends on the fast transport and diffusion of ions. Here, a Cu-MOF@GO-COOH hybrid was constructed, with GO-COOH as the substrate, to promote electron transfer, and Meso-Cu-MOF@GO-COOH was further obtained by introducing mesopores inside nanosheets to optimize the transportation paths for ions. The GO-COOH substrate improves the capacitance by enhancing the surface capacitive behavior, while the mesopores improve the charge-storage capacity by enhancing the diffusive behavior. The as-obtained Meso-Cu-MOF@GO-COOH exhibits a higher capacitance of 292.5 F g−1 compared with Cu-MOF@GO-COOH (193.7 F g−1) and 2D Cu-MOF (141.4 F g−1) at a current density of 1 A g−1. Moreover, the prepared Cu-MOF@GO-COOH//AC device delivers a capacitance of 63 F g−1 (0.5 A g−1), an energy density of 27.7 Wh kg−1, and a power density of 496.8 W kg−1, showing a great potential for practical applications.
1. Introduction
Benefiting from their high-power density, rapid charge–discharge process, greenness, and long lifetime, supercapacitors are gaining great attention as next-generation energy storage devices. To enhance their electrochemical performance, various materials, including traditional materials (carbon-based, conducting polymers, and metal oxides) and novel nanomaterials (quantum dots, transition metal dichalcogenides, Mxenes, etc.), have been developed as supercapacitor electrodes [1,2]. Metal–organic frameworks (MOFs) are a novel porous material, formed via the coordination reaction between metal ions and organic ligands. Benefiting from their diverse pore architecture, high surface area, and tunable functionality, MOFs are being intensively investigated in energy storage [3,4,5,6,7,8], detection [9,10,11,12,13], sensing [14,15,16,17,18], and adsorption [19], etc. Notably, owing to the highly accessible active sites, two-dimensional MOFs (2D MOFs), which are prone to producing capacitance, are drawing much attention in the field of supercapacitor electrodes [20,21,22,23,24,25].
However, the electrochemical performance of 2D MOFs is restricted by their unsatisfactory conductivity, stacking problems, and insufficient utilization of active sites, limiting their application as energy-storing electrodes. To overcome these shortcomings, some conductive materials, such as graphene-based and Ti3C2Tx substrates, are preferred to construct MOF-based hybrids [26,27,28]. By combining the merits of 2D MOFs with those of conductive materials, the electrochemical performances of MOF hybrids have been certainly enhanced [29,30,31].
To further shorten the gap between the practical and theoretical performance of 2D MOFs, maximizing the utilization of active sites is imperative. Notably, the hierarchically porous architecture has been proved to be an effective strategy to enhance electrode performance by promoting ion transport and diffusion [32,33,34,35,36]. By promoting the accessibility of ions to micropores and small-sized mesopores, the utilization of active sites is improved.
In this paper, to optimize the transport paths and to promote the movement of ions directly through 2D MOF nanosheets rather than around them, a soft template was used to produce mesopores inside MOF nanosheets, thus providing convenient channels for ion transport. Moreover, as a low-cost and environmentally friendly material, Cu-based MOFs present great potential in developing high-performance electrodes [37].
As an amphiphilic block copolymer, polystyrene-b-poly(ethylene oxide) presents a prominent merit, in that it has adjusting pore sizes, in which changing lengths of hydrophobic chain segments lead to various pores [38,39]. Here, a polystyrene-b-poly(ethylene oxide) (PS102-b-PEO114) was chosen as the soft template to produce mesopores inside MOF nanosheets. Benefiting from the ample carboxyl groups on the surface of GO-COOH, hydrogen bonds can be formed between GO-COOH and the micelles to promote micelles to arrange on the GO-COOH surface and to further guide the growth of Cu-MOF. After the micelles were removed, mesopores were left inside MOF nanosheets to obtain hierarchical Meso-Cu-MOFs@GO-COOH, in which new transport channels are provided for ions and their diffusion ability is enhanced, improving the effective utilization of active sites inside MOFs.
2. Experimental Methods
2.1. Synthesis of Materials
The synthetic methods of 2D Cu-MOF, GO-COOH, and BCP@GO-COOH are described in the Supplementary Materials Section.
Synthesis of Cu-MOF@GO-COOH. Firstly, 20 mg of Cu(OAc)2•H2O (>98%) was dissolved in a mixed solvent containing 2 mL of N, N-dimethylformamide (DMF, 99.5%), and 4 mL of ethanol absolute (EtOH, 99.7%). Next, 20 mg of H2BDC-NH2 (98.6%) and 10 mg of GO-COOH were added to another solvent containing 10 mL of DMF and 5 mL of EtOH to form a mixture. The copper–salt solution was slowly added to the mixture and stirred continuously for 30 min. After that, the product was collected, washed with DMF and EtOH, and dried at 60 °C for 24 h, to obtain Cu-MOF@GO-COOH.
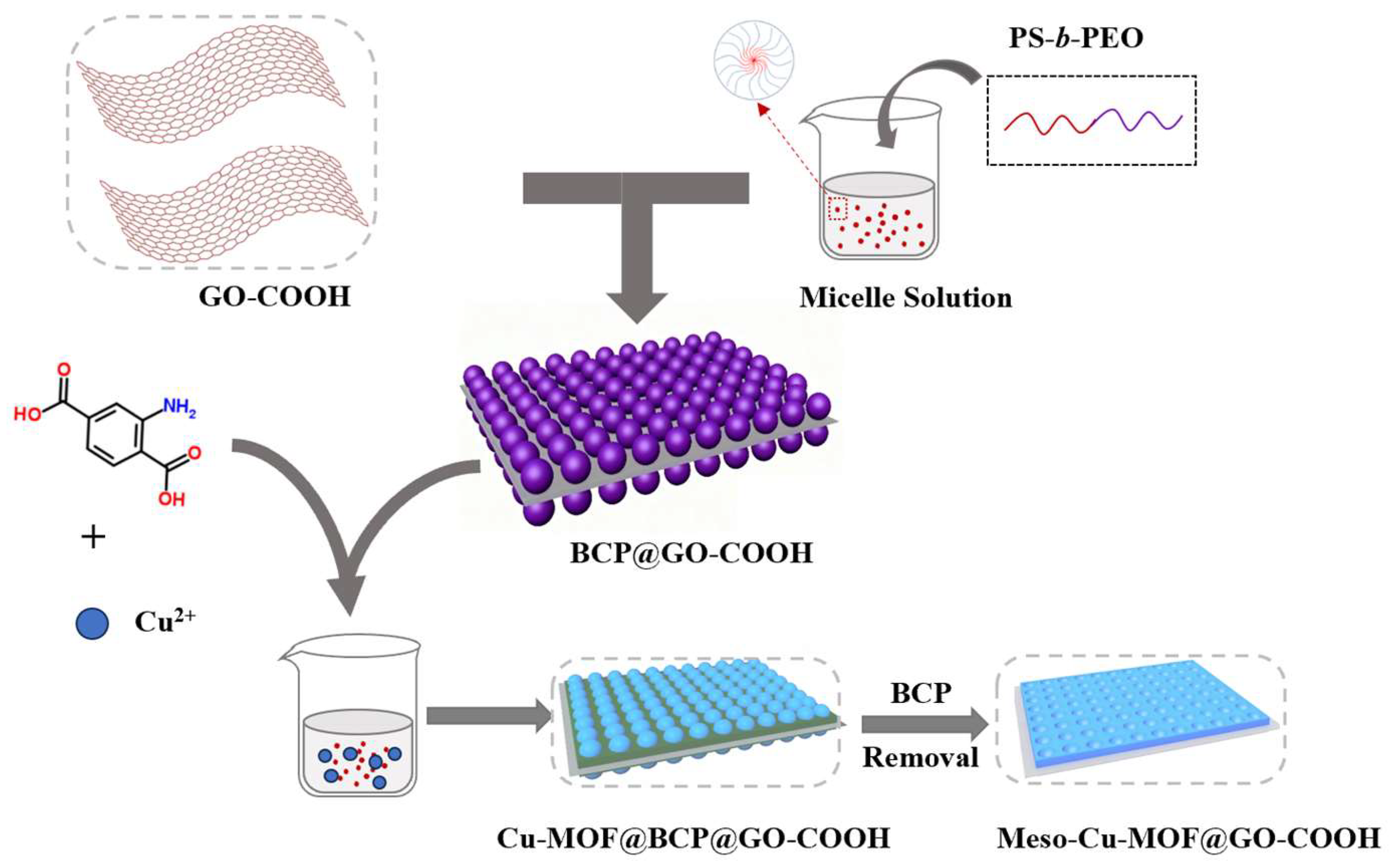
Synthesis of Meso-Cu-MOF@GO-COOH. Firstly, 20 mg of Cu(OAc)2•H2O was dissolved in a mixture containing 2 mL of DMF and 4 mL of EtOH. Next, 20 mg of H2BDC-NH2 was added to the BCP@GO-COOH solution and stirred continuously for 60 min to form a mixture. Subsequently, the copper–salt solution was slowly added to the mixture and stirred continuously for 30 min. After that, the product was collected, and then washed with DMF, tetrahydrofuran (THF, 99.5%), and EtOH. At last, the as-obtained product was dried at 60 °C for 24 h to obtain Meso-Cu-MOF@GO-COOH (Figure 1).
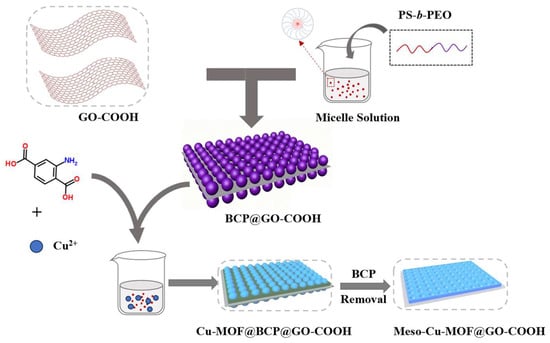
Figure 1.
Schematic illustration of preparing Meso-Cu-MOF@GO-COOH.
Notably, the Cu-MOF@BCP@GO-GOOH can be obtained by washing with just DMF and EtOH, without THF.
2.2. Structural Analysis
Scanning electron microscopy (SEM, JEOL JXA-840A) and transmission electron microscopy (TEM, JEOL JEM-2100 PLUS) were used to analyze the surface morphology of samples. Fourier transforms infrared (FTIR) and X-ray diffraction (XRD) were performed to investigate the elemental composition of materials. The specific surface area and pore characteristic of samples were analyzed via a physisorption analyzer (ASAP 2020M).
2.3. Electrochemical Characterization
The electrochemical performances of samples were tested on an electrochemical workstation (CHI760E), including cyclic voltammetry (CV), galvanostatic charge–discharge (GCD), and electrochemical impedance spectroscopy (EIS). Using a three-electrode system to estimate the performance of single electrode, in which a working electrode (the as-prepared materials), counter electrode (Pt plate), and reference electrode (Ag/AgCl) were used. Additionally, 3 M KOH aqueous solution was used as the electrolyte.
The specific capacitance of electrode was calculated based on the following formula [40]:
in which I, Δt, and ΔV correspond to the current, discharge time, and voltage, respectively, while m is the mass of active materials.
C (F g−1) = I ∫ (1/m × V(t)) dt = I Δt/m ΔV
An asymmetric supercapacitor (ASC) was prepared using Meso-Cu-MOF@GO-COOH and activated carbon (AC) as electrodes. The preparation process of working electrodes is displayed in the Supplementary Materials. The loading mass of the active material was 1–1.5 mg cm−2. To maximize the performance of device, the optimal mass ratio of two electrodes was calculated according to the following formula:
in which m, C, and ΔV correspond to the mass, specific capacitance, and voltage of the positive and negative electrodes, respectively. Moreover, the capacitance value of the as-assembled ASC device can also by estimated according to Equation (1), except, in that case, m would refer to the mass of two electrodes. The energy density (E) and power density (P) of the ASC device are calculated via the following formulas [41]:
in which E is the energy density, P is the power density, Δt is the discharge time, and ΔV is the potential window.
3. Results and Discussion
3.1. Structural Characteristics
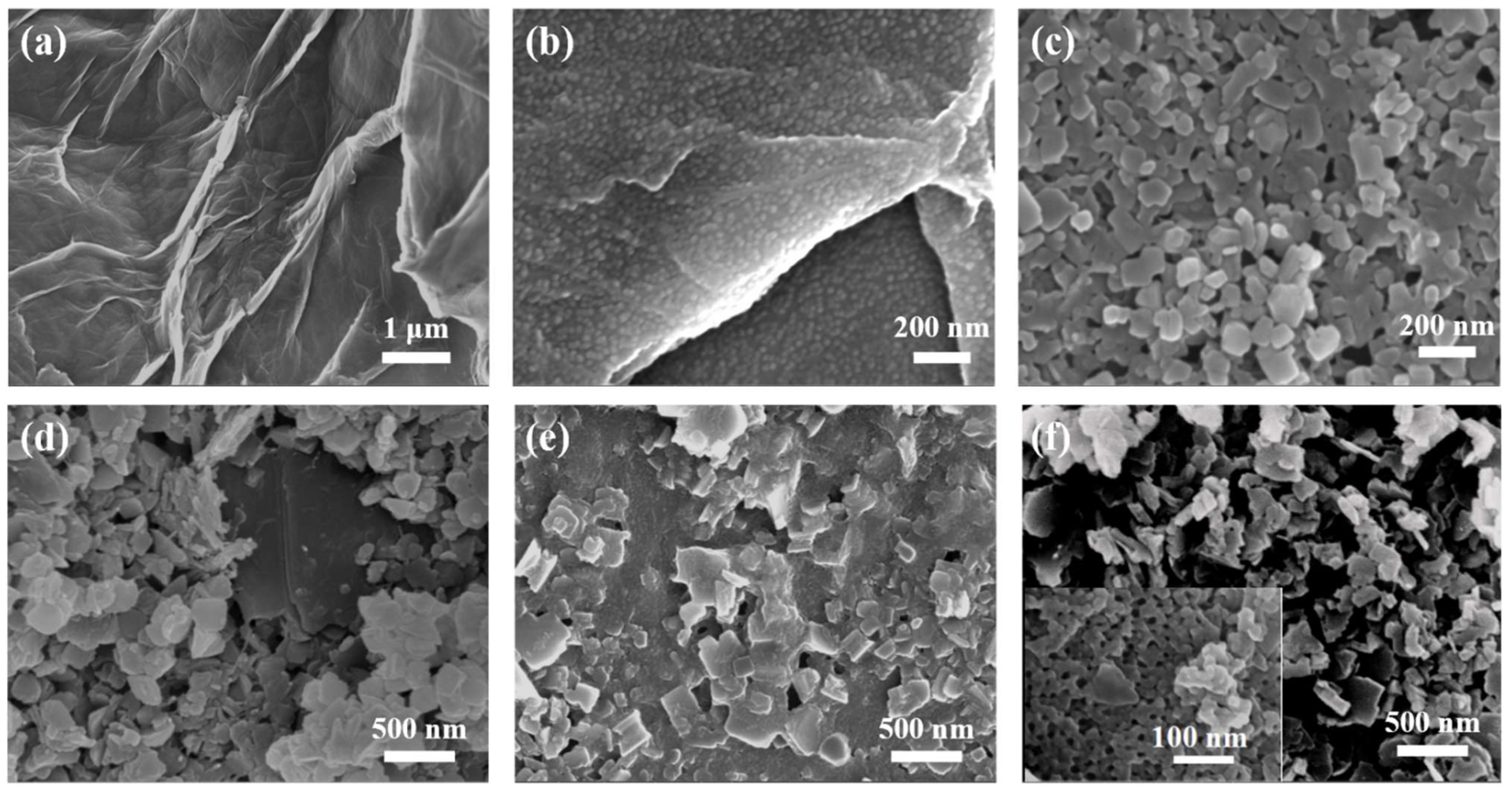
The microstructure of GO-COOH is shown in Figure 2a; many wrinkles can be noticed. Furthermore, when using GO-COOH as the substrate, promoted by the hydrogen bonding force, the soft template (PS102-b-PEO114) self-assembled into uniform spherical micelles (Figure 2b), and covered the surface of GO-COOH substrate. Notably, when the length of the hydrophobic chain segment in PSn-b-PEO114 (n = 70, 90, 102 and 150) was changed, micelles with different sizes were obtained (Figure S1), by which the sizes of mesopores can be regulated. Figure 2c displays the morphology of pristine 2D Cu-MOF, in which uniform nanosheets with a size of 100~200 nm can be seen. For the Cu-MOF@GO-COOH sample, the size of the nanosheets (Figure 2d) is somewhat larger than that of the pristine 2D Cu-MOF, presenting an average size of about 300 nm, which may be related to the induced growth originating from the functional groups on the GO-COOH surface (Figure S2). When using BCP@GO-COOH as the substrate to prepare 2D Cu-MOF, large numbers of spherical micelles could still be identified (Figure 2e). During the preparation of MOF nanosheets, the coordination force could be produced between the hydrophilic segments of micelles and Cu2+, forming the nuclear and further guiding the growth of MOF nanosheets, and, simultaneously, wrapping the micelles. THF was used to remove the BCP micelles, leaving abundant pores inside the MOF nanosheets (Figure 2f).
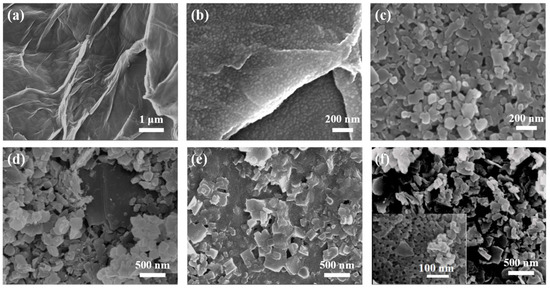
Figure 2.
SEM images of samples: (a) GO-COOH, (b) BCP@GO-COOH, (c) 2D Cu-MOF, (d) Cu-MOF@GO-COOH, (e) Cu-MOF@BCP@GO-COOH, and (f) Meso-Cu-MOF@GO-COOH (the insert is high-resolution SEM image).
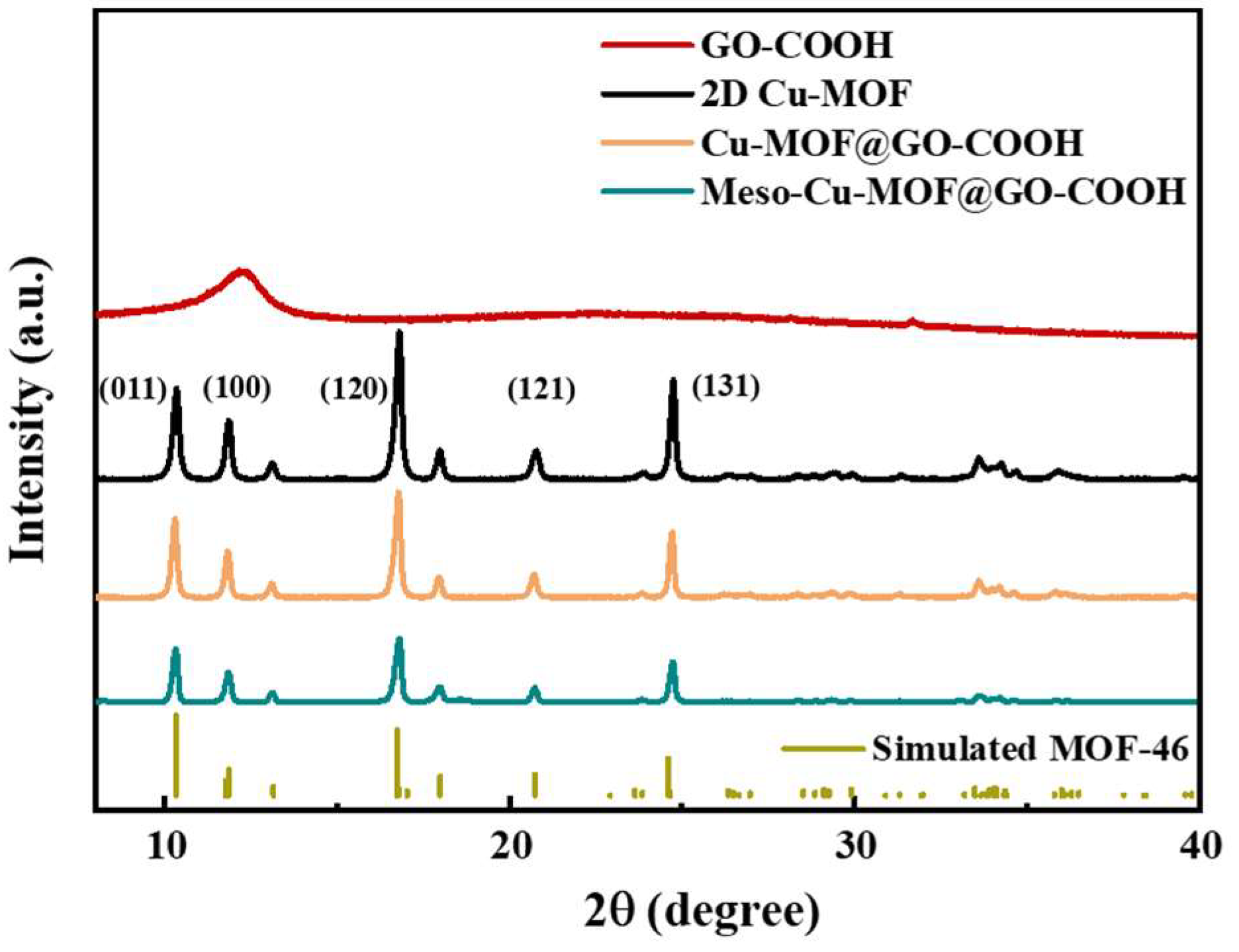
To further understand the crystal structure of different samples, XRD analysis was performed. As shown in Figure 3, the peaks at 10.3, 11.8, 16.7, 20.8, and 24.8° correspond to the (001), (100), (120), (121), and (131) crystal planes, respectively, which is basically consistent with those of the simulated MOF-46 crystals, proving the success synthesis of 2D Cu-MOF [42,43]. After combining 2D Cu-MOF with GO-COOH, though the corresponding peak intensity decreased slightly, the peak position of Cu-MOF@GO-COOH showed no obvious difference with that of the 2D Cu-MOF. Similarly, compared with Cu-MOF@GO-COOH, Meso-Cu-MOF@GO-COOH presented a very similar peak position except for a slight decrease in peak intensity, which suggests that the crystal structure of Cu-MOF was not obviously changed during the introduction of the mesoporous structure using a soft template.
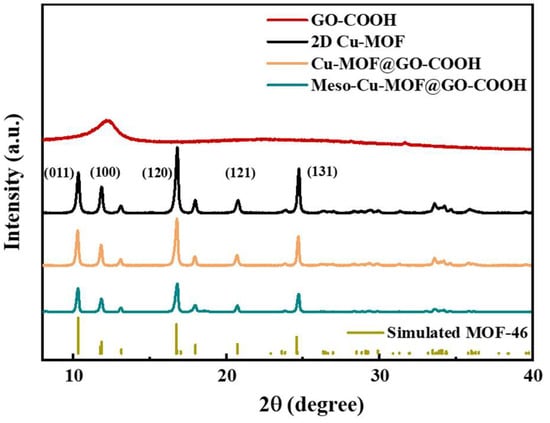
Figure 3.
XRD patterns of samples.
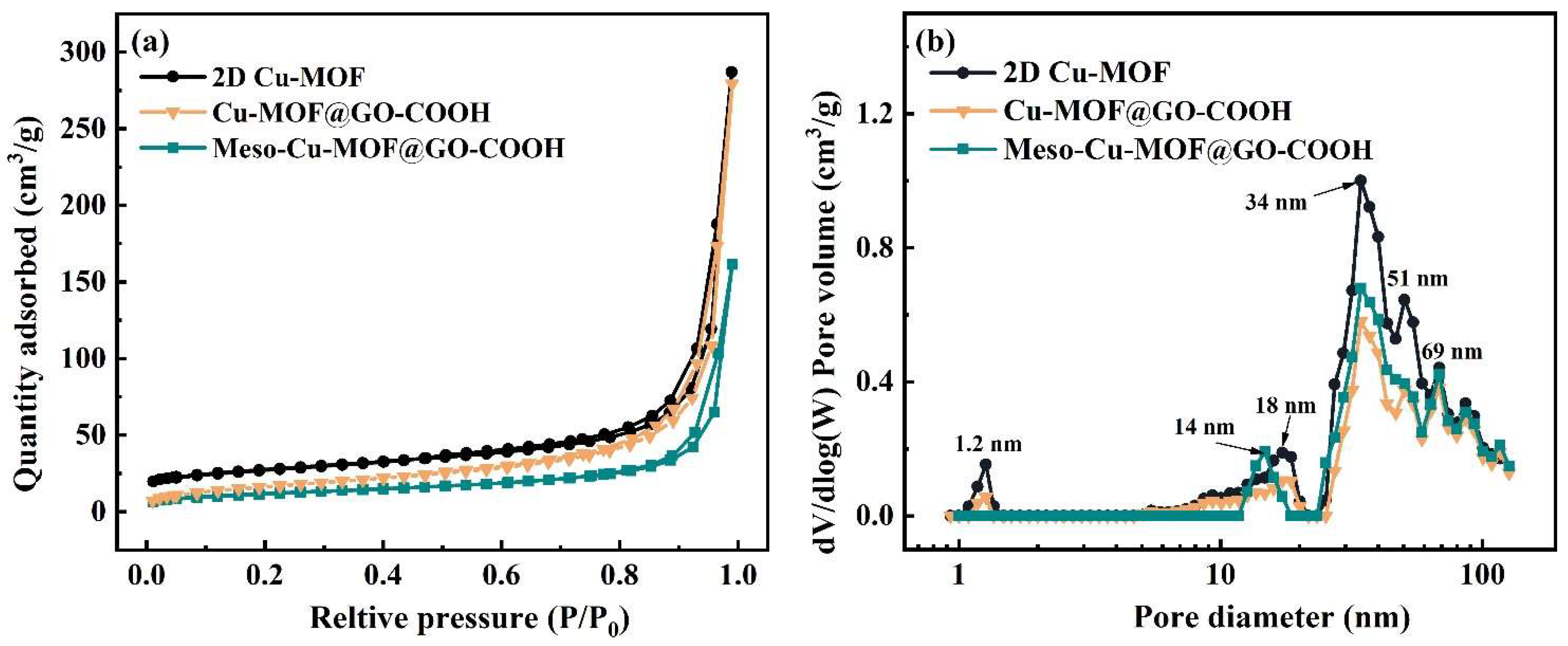
The pore characteristics of samples are displayed in Figure 4. The materials exhibit type I isotherms as well as obvious hysteresis loops, indicating the architecture of hierarchical pores. The type I isotherm shape in low pressure suggests the presence of micropores, while the obvious H3-type hysteresis loop in higher pressure suggests the presence of large-sized slit pores caused by the accumulation of 2D Cu-MOF nanosheets. As displayed in Figure 4b, similar pore distribution, including micropore (1.2 nm), mesopores (18 and 34 nm), as well as macropores (51 and 69 nm), can be noticed. The proportion of the micropores (1.2 nm) decreased greatly while a new mesopore (14 nm) was formed in Meso-Cu-MOF@GO-COOH. Upon the removal of the BCP micelles, a new mesopore was produced inside the 2D Cu-MOF nanosheets (Figure 2f), which could provide interpenetrated channels for ion transport and subsequently make it easier to access the active sites in MOF.
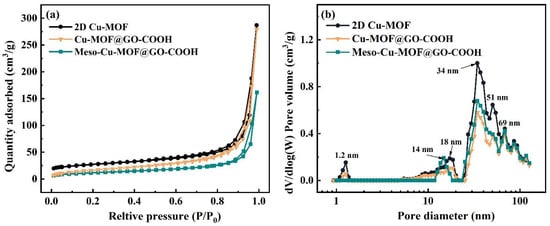
Figure 4.
(a) N2 adsorption–desorption isotherms and (b) corresponding BJH pore distribution plots of samples.
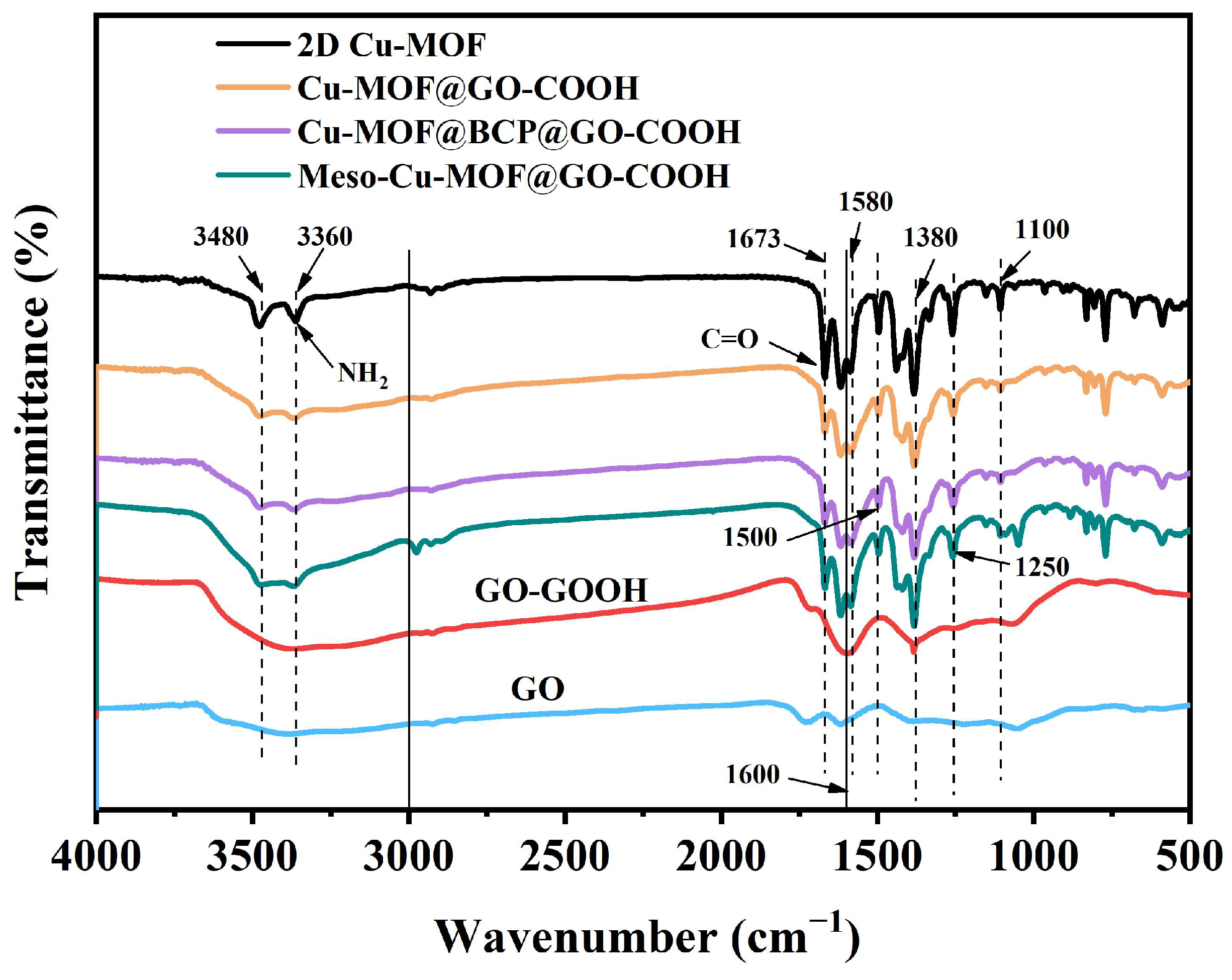
The infrared spectra of samples are shown in Figure 5. Obviously, for GO-COOH, the broad peak at 3666~3245 cm−1, corresponding to the stretching vibration of -OH, was strengthened compared with GO, which is associated with the introduction of -COOH [44,45,46,47]. For 2D Cu-MOF and its hybrids, the two peaks at 3364 and 3486 cm−1 correspond to the symmetric and asymmetric stretching vibrations of the N-H groups in H2BDC-NH2, respectively. The peak of C=O in 2D Cu-MOF presented a blue shift to 1669 cm−1 compared with that in GO and GO-COOH, which is related to the coordination with Cu2+ ions, also proving the success synthesis of 2D Cu-MOF. Because the characteristic functional groups of PS102-b-PEO114 are similar to those of H2BDC-NH2, the characteristic peaks of Cu-MOF@BCP@GO-COOH presented no obvious difference to those of Cu-MOF@GO-COOH and Meso-Cu-MOF@GO-COOH. However, in the above-mentioned SEM analysis (Figure 2), the difference in the microstructure of the as-prepared samples has been clearly displayed.
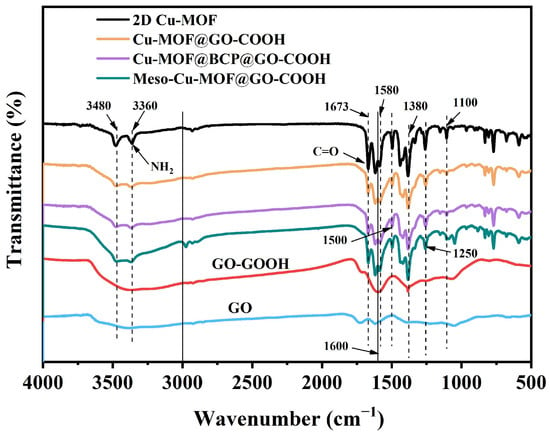
Figure 5.
FTIR spectra of samples.
3.2. Electrochemical Performance of 2D MOF Hybrid Electrodes
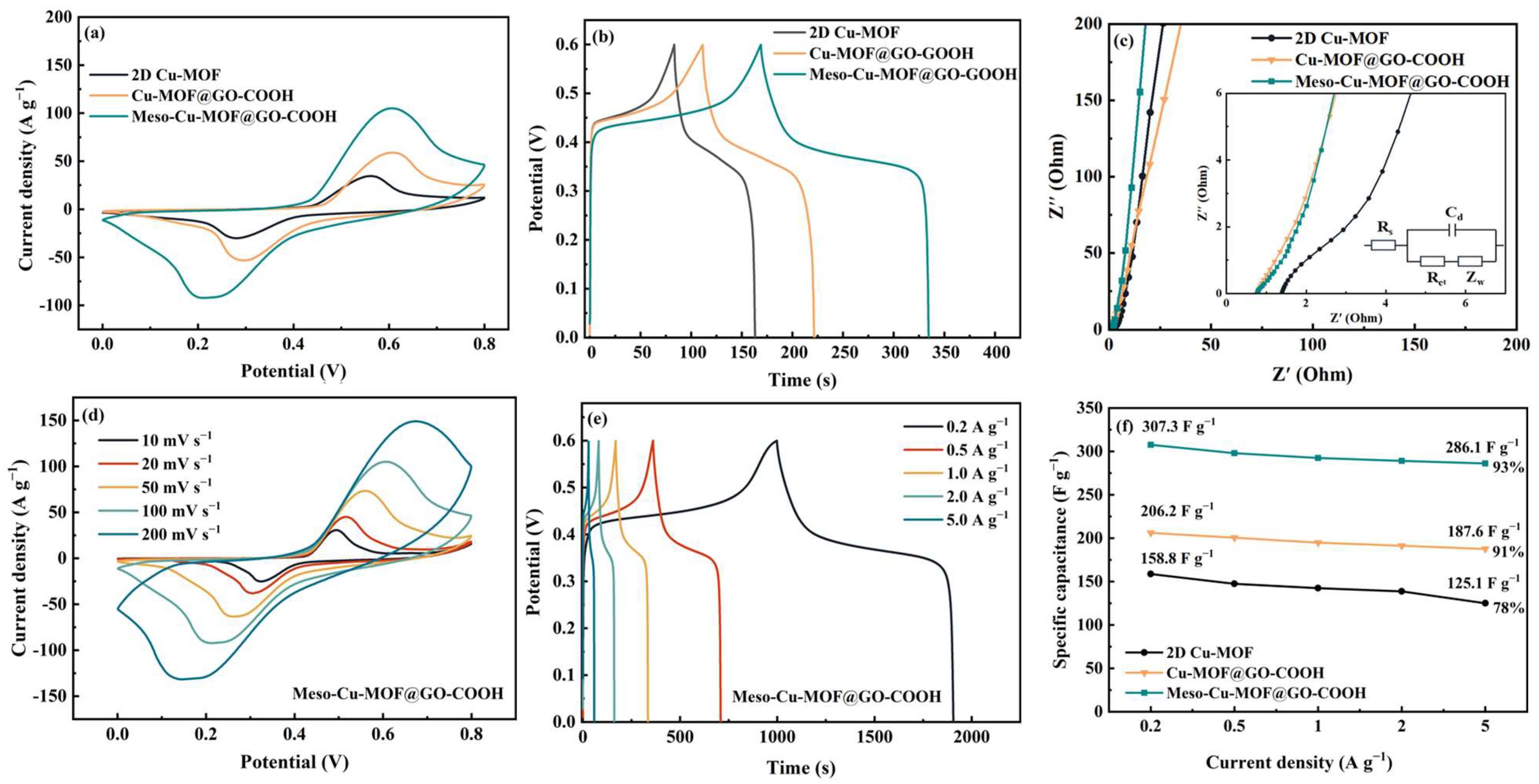
The electrochemical performances of the prepared materials are shown in Figure 6. According to the CV and GCD curves, the energy storage process of the hybrids consists of pseudocapacitive and double-layer behaviors. The enclosed region in CV curves (Figure 6a) reveals the ability of the samples to store charges. Furthermore, at a current density of 1 A g−1 (Figure 6b), the capacitance values of 2D Cu-MOF, Cu-MOF@GO-COOH, and Meso-Cu-MOF@GO-COOH were calculated to be 141.4, 193.7, and 292.5 F g−1 (1 A g−1), respectively, which can be explained by the Nyquist plots and equivalent circuit in Figure 6c. Clearly, the two hybrids exhibit a smaller intercept at the real axis compared with 2D Cu-MOF, implying lower internal resistance (Rs) owed to the introduction of GO-COOH substrate. The pi-pi interaction between 2D Cu-MOF and GO-COOH nanosheets could enhance carrier transport, which is conducive to improving conductivity. Moreover, in the low-frequency region, Meso-Cu-MOF@GO-COOH presents a steeper slope of the straight line than those of the other samples, suggesting faster ion diffusion rates [48,49,50], which is attributed to the convenient channels for ion transport stemming from the interpenetrated holes inside MOF nanosheets. For the equivalent circuit, Rs means the electrolyte resistance, Cd presents the double-layer capacitance, Rct is the polarization resistance stemmed from charge transfer, and Zw is the Warburg resistance associated with ion diffusion. For Meso-Cu-MOF@GO-COOH, with the increased scan rates, the shapes of the CV curves present no obvious change (Figure 6d), suggesting fast electron transport inside the active material [51]. According to the GCD curves (Figure 6e), the specific capacitance values of Meso-Cu-MOF@GO-COOH were calculated to be 307.3 and 286.1 F g−1 as the current density increased from 0.2 to 5 A g−1, presenting a 93% capacitance retention (Figure 6f). The as-obtained Meso-Cu-MOF@GO-COOH presents enhanced performance compared with some reported Cu-MOF electrodes (Cu3(HHTP)2, 110–114 F g−1 at 0.04–0.05 A g−1 [52]; Cu@BTC, 228 F g−1 at 1.5 A g−1 [53]; and Cu-MOF, 37.91 F g−1 at 0.8 A g−1 [54], etc.).
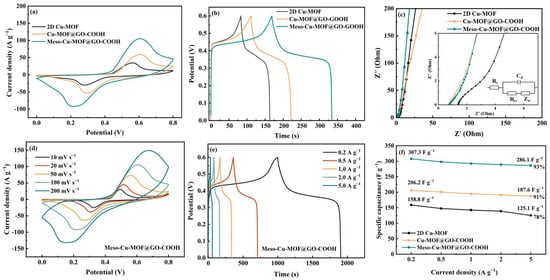
Figure 6.
Electrochemical performances of samples: (a) CV curves at a scan rate of 100 mV s−1, (b) GCD curves at a current density of 1 A g−1, and (c) Nyquist plots of samples. (d) CV curves at various scan rates, (e) GCD curves at various current densities, and (f) the specific capacitance as a function of current density of samples.
To further investigate the cycling stability of the Meso-Cu-MOF@GO-COOH hybrid, 2000 cycles were performed at a current density of 2 A g−1 and the results are shown in Figure S3, delivering an 82% capacitance retention.
3.3. Energy Storage Mechanism
By integrating GO-COOH into 2D MOF to construct 2D MOF hybrids and subsequently producing pores inside MOF nanosheets, Cu-MOF@GO-COOH and Meso-Cu-MOF@GO-COOH were endowed with enhanced capacitive properties. To further investigate the energy storage mechanism, the b-value model [55] and Dunn method [56] were used to reveal the kinetic behaviors. The peak current (i) and scan rate (v) can be described in the following formula in the b-value model:
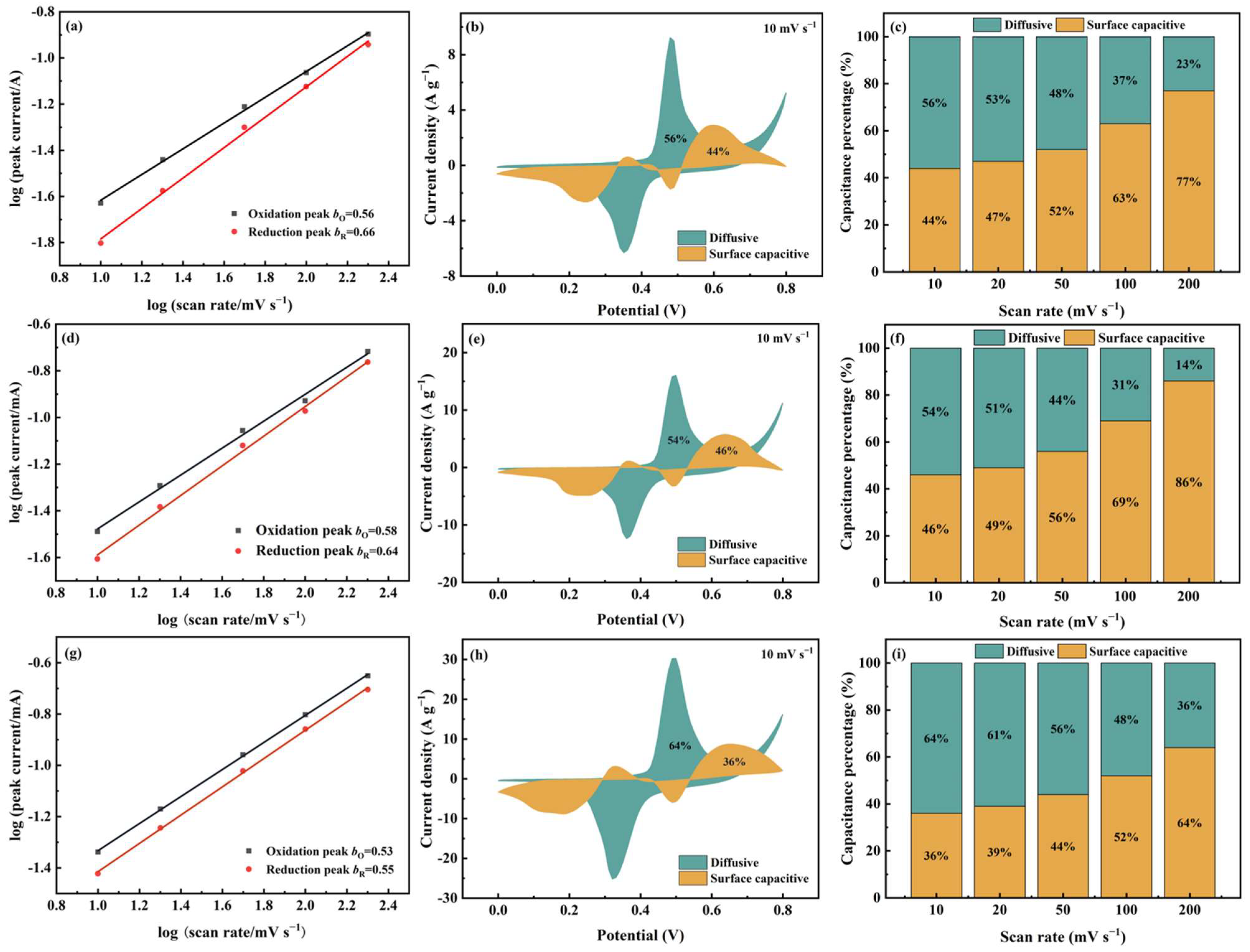
Here, b is a variable with a range of 0.5~1.0, and the 0.5 value corresponds to the diffusive-controlled behavior, while the 1.0 corresponds to the surface capacitive behavior.
As shown in Figure 7a,d, for Cu-MOF@GO-COOH, the b-values of both oxidation and the reduction peaks are close to those of 2D Cu-MOF, implying that the two samples present similar charge-storage behaviors. Differently, the b-values of Meso-Cu-MOF@GO-COOH are closer to the critical value of 0.5, indicating that the diffusive-controlled behavior is enhanced, which can be confirmed by the Dunn method’s results. The contributions of the two charge-storage behaviors can be identified via the Dunn model [57,58]:
where k1v and k1v0.5 correspond to the surface capacitive current and diffusive current, respectively, by which the charge-storage process of electrode materials can be investigated. Clearly, at a scan rate of 10 mV s−1 (Figure 7b,e,h), the surface capacitive ratios of 2D Cu-MOF, Cu-MOF@GO-COOH, and Meso-Cu-MOF@GO-COOH are 44%, 46%, and 36%, respectively. For 2D Cu-MOF and Cu-MOF@GO-COOH, the surface capacitive percentages increased greatly with the increased scan rates, obtaining 77% and 86%, respectively, at a scan rate of 200 mV s−1. Utilizing GO-COOH to construct 2D MOF hybrids accelerates the charge transfer, thus lowering the internal resistance (Figure 6c), and thereby enhancing the surface capacitive behavior, which may be the main reason for the improved capacitance value of Cu-MOF@GO-COOH. Notably, for Meso-Cu-MOF@GO-COOH, even when the scan rate reached 50 mV s−1, the ratio of diffusive current was still higher than that of surface capacitive current, implying the dominant diffusion-controlled behavior. Owing to the interpenetrated pores, ions could diffuse adequately inside Meso-Cu-MOF@GO-COOH, allowing the active sites to be utilized more fully and conducting sufficient redox reactions inside MOF nanosheets. Thus, benefiting from the enhanced diffusion behavior, the electrochemical performance of Meso-Cu-MOF@GO-COOH was further improved.
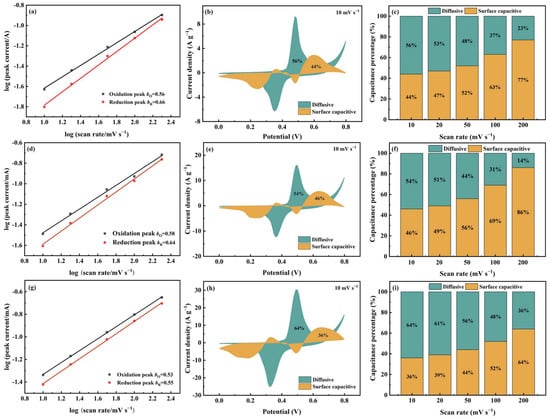
Figure 7.
The b-value and Dunn model analysis of (a–c) 2D Cu-MOF, (d–f) Cu-MOF@GO-COOH, and (g–i) Meso-Cu-MOF@GO-COOH. (a,d,g) Plots of log(i) against log(v), (b,e,h) surface capacitive and diffusive contributions at a scan rate of 10 mV s−1, and (c,f,i) capacitance contribution ratios at different scan rates.
3.4. Electrochemical Performance of ASC Device
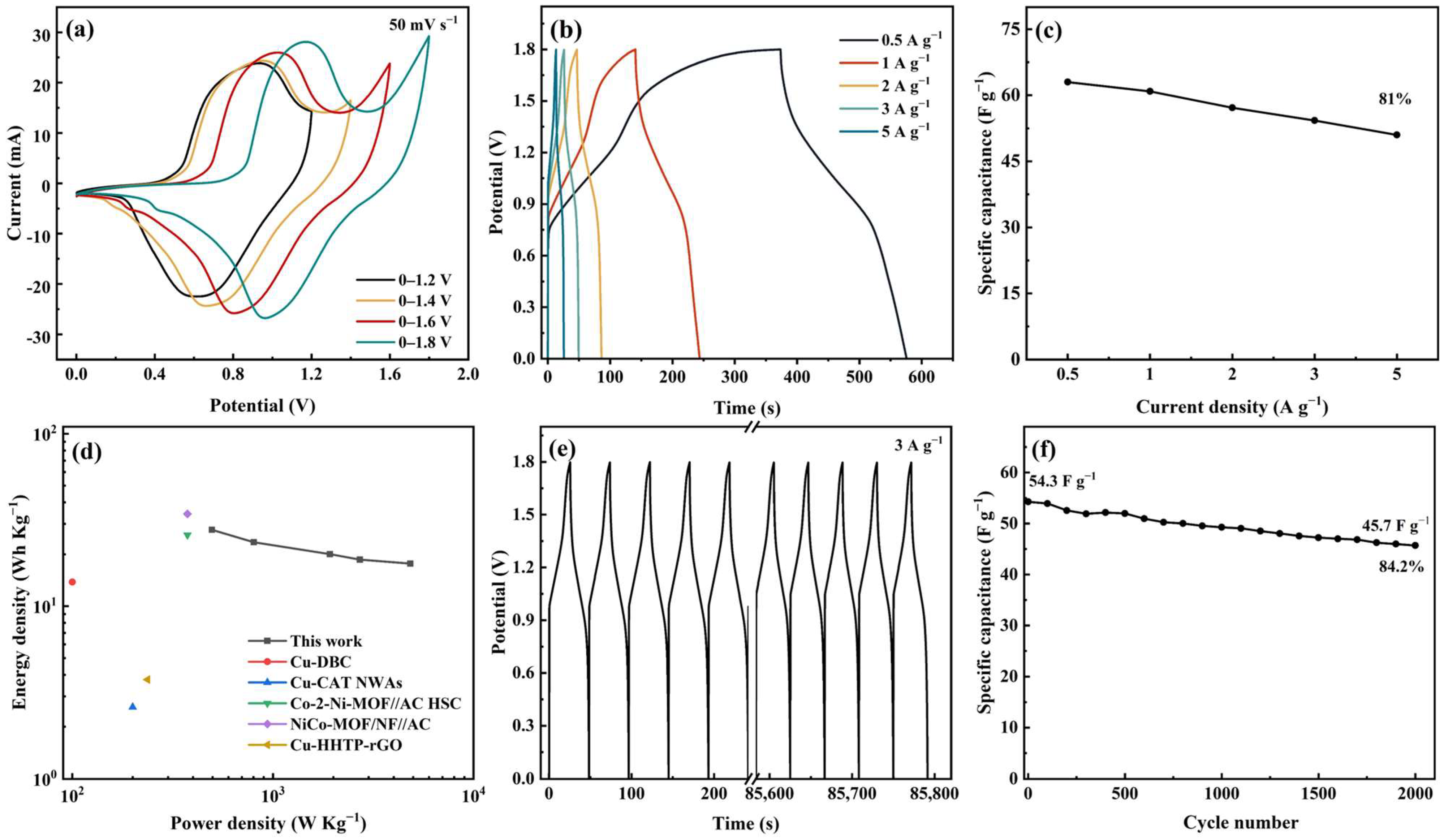
An ASC device was prepared using Meso-Cu-MOF@GO-COOH and activated carbon (AC) as the positive and negative electrodes, respectively. Combining the capacitance values of AC, calculated via the GCD curves, with the potential ranges of two electrodes (Figure S4), the optimal mass ratio (m+/m−) of 1.2 was chosen to prepare the ASC device. Furthermore, CV tests under various voltage windows showed that the curve shape presented no deformation even at 1.8 V (Figure 8a), exhibiting a broad working voltage window. With the scan rate was increased to 100 mV s−1 (Figure S4), no obvious changes can be noticed, indicating fast charge transfer and ion transport.
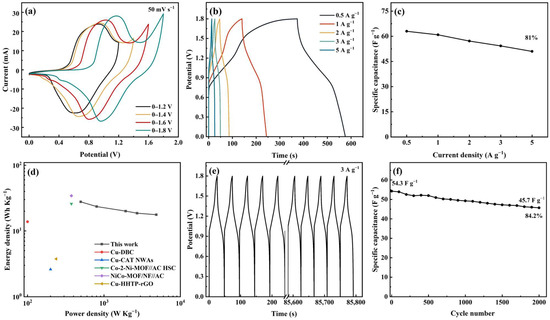
Figure 8.
Electrochemical performances of the ASC device. (a) CV curves at different potential windows, (b) GCD curves at different current densities, (c) specific capacitance under various current densities, (d) Ragone plots, (e) cycling GCD curves, and (f) cycling performance.
The capacitance values corresponding to 0.5, 1, 2, 3, and 5 A g−1 were calculated to be 63, 60.9, 57.2, 54.3, and 50.9 F g−1, respectively, and the 81% capacitance retention rate shows a relatively good performance rate (Figure 8b,c). The device delivers a maximum energy density of 27.7 Wh kg−1 at a power density of 496.8 W kg−1, which are figures comparable to or surpassing many of the recently reported MOF devices mentioned in Figure 8d [51,59,60,61,62]. Moreover, the cycle performance was investigated at a current density of 3 A g−1, delivering a capacitance retention of 84.2% after 2000 cycles (Figure 8e,f).
4. Conclusions
Here, a hierarchical Meso-Cu-MOFs@GO-COOH was synthesized and used as supercapacitor electrode. The introduction of GO-COOH accelerates electron transfer, lowers the internal resistance of the hybrid, and thus enhances the charge storage capacity by promoting the surface capacitive behavior. Additionally, the interpenetrated mesopores inside MOF sheets optimize the diffusion paths of ions, improving the energy storage capacitance via enhancing the diffusive behavior. The Meso-Cu-MOFs@GO-COOH exhibited an increased capacitance of 292.5 F g−1 (1 A g−1), which is 1.5 and 2.1 times as high as that of Cu-MOFs@GO-COOH and 2D Cu-MOF, respectively. When the current density increased from 0.2 to 5 A g−1, an excellent capacitance retention of 93% was obtained. Furthermore, the assembled Cu-MOF@GO-COOH//AC supercapacitor exhibited a capacitance of 63 F g−1 (0.5 A g−1), an energy density of 27.7 Wh kg−1, and a power density of 496.8 W kg−1, showing its practical potential in energy storage applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano15211628/s1, Figure S1: SEM of BCP@GO-COOH samples. (a) PS70-BCP@GO-COOH, (b) PS90-BCP@GO-COOH, (c) PS102-BCP@GO-COOH and (d) PS150-BCP@GO-COOH; Figure S2: TEM image of Cu-MOF@GO-COOH; Figure S3: Cycling test of Meso-Cu-MOF@GO-COOH: (a) GCD curves at a current density of 2 A g-1 and (b) cycling capacitance; Figure S4: (a) GCD curves of AC electrode, (b) CV curves of AC and Meso-Cu-MOF@GO-COOH, and (c) CV curves of Meso-Cu-MOF@GO-COOH at different scan rates.
Author Contributions
Methodology, P.Y., Y.N. and M.Z.; validation, Y.Z. and W.L.; writing—original draft preparation, M.Y.; writing—review and editing, S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (52073126, 52173020).
Data Availability Statement
The original contributions presented in this study are included in the article and Supplementary Material. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We are thankful to the National Natural Science Foundation of China (52073126, 52173020).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bhaduri, A.; Kim, C.; Ha, T. Recent Developments in Materials Design for Advanced Supercapacitors. Energy Environ. Mater. 2025, 8, e70070. [Google Scholar] [CrossRef]
- Tsyganov, A.; Vikulova, M.; Zotov, I.; Korotaev, E.; Plugin, I.; Sysoev, V.; Kirilenko, D.; Rabchinskii, M.; Asoyan, A.; Gorokhovsky, A.; et al. Application of W1.33CTz MXenes obtained by hydrothermal etching as an additive to enhance the electrochemical energy storage properties of binder-free Ti3C2Tx MXene films. Dalton Trans. 2025, 54, 8547–8558. [Google Scholar] [CrossRef]
- Xia, W.; Mahmood, A.; Zou, R.; Xu, Q. Metal-organic frameworks and their derived nanostructures for electrochemical energy storage and conversion. Energy Environ. Sci. 2015, 8, 1837–1866. [Google Scholar] [CrossRef]
- Qiu, T.; Liang, Z.; Guo, W.; Tabassum, H.; Gao, S.; Zou, R. Metal–Organic Framework-Based Materials for Energy Conversion and Storage. ACS Energy Lett. 2020, 5, 520–532. [Google Scholar] [CrossRef]
- Mohamed, A.M.; Sayed, D.M.; Allam, N.K. Optimized Fabrication of Bimetallic ZnCo Metal-Organic Framework at NiCo-Layered Double Hydroxides for Multiple Storage and Capability Synergy All-Solid-State Supercapacitors. ACS Appl. Mater. Interfaces 2023, 15, 16755–16767. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Chen, L.; Wang, X.; Lu, D.; Zhou, W.; Shen, D.; Yang, Q.; Xiao, S.; Li, Y. Ni/Co-MOF@aminated MXene hierarchical electrodes for high-stability supercapacitors. Chem. Eng. J. 2023, 451, 138687. [Google Scholar] [CrossRef]
- Li, S.; Wang, R.; Xie, M.; Xu, Y.; Chen, J.; Jiao, Y. Construction of trifunctional electrode material based on Pt-Coordinated Ce-Based metal organic framework. J. Colloid Interface Sci. 2022, 611, 378–389. [Google Scholar] [CrossRef]
- Wang, D.-G.; Liang, Z.; Gao, S.; Qu, C.; Zou, R. Metal-organic framework-based materials for hybrid supercapacitor application. Coord. Chem. Rev. 2020, 404, 213093. [Google Scholar] [CrossRef]
- Liang, N.; Shi, B.; Hu, X.; Li, W.; Huang, X.; Li, Z.; Zhang, X.; Zou, X.; Shi, J. A ternary heterostructure aptasensor based on metal-organic framework and polydopamine nanoparticles for fluorescent detection of sulfamethazine. Food Chem. 2024, 460, 140570. [Google Scholar] [CrossRef]
- Xu, S.; Shao, D.; Wang, J.; Zheng, X.; Yang, Z.; Wang, A.; Chen, Z.; Gao, Y. Pre-ligand-induced porous MOF as a peroxidase mimic for electrochemical analysis of deoxynivalenol (DON). Food Chem. 2025, 480, 143860. [Google Scholar] [CrossRef]
- Liang, N.; Shi, B.; Hu, X.; Shi, Y.; Wang, T.; Huang, X.; Li, Z.; Zhang, X.; Zou, X.; Shi, J. Simultaneous adsorption and fluorescent sensing of ampicillin based on a trimetallic metal-organic framework. Food Chem. 2025, 472, 142891. [Google Scholar] [CrossRef]
- Guan, H.; Chen, Y.; Wang, D.; Liu, Q.; Zhong, J.; Zhang, Z.; Lü, D. The novel nanozyme-based electrochemical-driven electrochromic visual biosensor based on PEDOT: PSS/RGO conductive film for rapid detection of nitrite in food samples. Food Chem. 2025, 481, 143971. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Wu, X.; Xue, S.; Yin, L.; Gao, S.; Zhang, Y.; Wang, C.; Wang, Y.; El-Seedi, H.R.; Zou, X.; et al. Magnetic metal-organic frameworks-based ratiometric SERS aptasensor for sensitive detection of patulin in apples. Food Chem. 2025, 466, 142200. [Google Scholar] [CrossRef]
- Shi, B.; Zhang, X.; Li, W.; Liang, N.; Hu, X.; Xiao, J.; Wang, D.; Zou, X.; Shi, J. An intrinsic dual-emitting fluorescence sensing toward tetracycline with self-calibration model based on luminescent lanthanide-functionalized metal-organic frameworks. Food Chem. 2023, 400, 133995. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Hu, X.; Zhang, X.; Li, W.; Guo, Z.; Huang, X.; Li, Z.; Zhang, R.; Shen, T.; Zou, X.; et al. Ratiometric sensing for ultratrace tetracycline using electrochemically active metal–organic frameworks as response signals. J. Agric. Food Chem. 2023, 71, 7584–7592. [Google Scholar] [CrossRef]
- Chen, X.; Xu, J.; Li, Y.; Zhang, L.; Bi, N.; Gou, J.; Zhu, T.; Jia, L. A novel intelligently integrated MOF-based ratio fluorescence sensor for ultra-sensitive monitoring of TC in water and food samples. Food Chem. 2023, 405, 134899. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Jayan, H.; Gao, S.; Zhou, R.; Yosri, N.; Zou, X.; Guo, Z. Recent and emerging trends of metal-organic frameworks (MOFs)-based sensors for detecting food contaminants: A critical and comprehensive review. Food Chem. 2024, 448, 139051. [Google Scholar] [CrossRef]
- Marimuthu, M.; Arumugam, S.S.; Sabarinathan, D.; Li, H.; Chen, Q. Metal organic framework based fluorescence sensor for detection of antibiotics. Trends Food Sci. Technol. 2021, 116, 1002–1028. [Google Scholar] [CrossRef]
- Li, S.; Dai, Y.; Ye, P.; Liu, F.; Li, L.; Zhang, D.; Zhu, M. Hierarchical porous MOF/CTF hybrid frameworks used as protection against acidic harmful gases. Chem. Eng. J. 2024, 491, 152035. [Google Scholar] [CrossRef]
- Zhao, W.; Peng, J.; Wang, W.; Liu, S.; Zhao, Q.; Huang, W. Ultrathin two-dimensional metal-organic framework nanosheets for functional electronic devices. Coord. Chem. Rev. 2018, 377, 44–63. [Google Scholar] [CrossRef]
- Muzaffar, A.; Ahamed, M.B.; Deshmukh, K.; Thirumalai, J. A review on recent advances in hybrid supercapacitors: Design, fabrication and applications. Renew. Sustain. Energy Rev. 2019, 101, 123–145. [Google Scholar] [CrossRef]
- Liu, Q.; Guo, Z.; Wang, C.; Guo, S.; Xu, Z.; Hu, C.; Liu, Y.; Wang, Y.; He, J.; Wong, W. A Cobalt-Based Metal-Organic Framework Nanosheet as the Electrode for High-Performance Asymmetric Supercapacitor. Adv. Sci. 2023, 10, e2207545. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Yan, S.; Liu, Y.; Tian, Z.; Li, D.; Chen, Y.; Guo, L.; Wang, Y. Significantly enhanced electrochemical performance of 2D Ni-MOF by carbon quantum dot for high-performance supercapacitors. Electrochim. Acta 2022, 422, 140560. [Google Scholar] [CrossRef]
- Xu, X.; Lu, H.; Xu, D.; Zhou, P.; Ying, Y.; Li, L.; Liu, Y. Oxygen-rich vacancies CuCoLDH with 1D/2D nanoarray structure for high performance asymmetric supercapacitor. Appl. Surf. Sci. 2023, 614, 156174. [Google Scholar] [CrossRef]
- Jia, H.; Lu, S.; Shin, S.H.R.; Sushko, M.L.; Tao, X.; Hummel, M.; Thallapally, P.K.; Liu, J.; Gu, Z. In situ anodic electrodeposition of two-dimensional conductive metal-organic framework@nickel foam for high-performance flexible supercapacitor. J. Power Sources 2022, 526, 231163. [Google Scholar] [CrossRef]
- Bai, W.; Li, S.; Ma, J.; Cao, W.; Zheng, J. Ultrathin 2D metal–organic framework (nanosheets and nanofilms)-based xD–2D hybrid nanostructures as biomimetic enzymes and supercapacitors. J. Mater. Chem. A 2019, 7, 9086–9098. [Google Scholar] [CrossRef]
- Pomerantseva, E.; Gogotsi, Y. Two-dimensional heterostructures for energy storage. Nat. Energy 2017, 2, 17089. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, F.; Sun, Y.; Yu, K.; Guo, W.; Qu, F. A 2D/2D NiCo-MOF/Ti3C2 heterostructure for the simultaneous detection of acetaminophen, dopamine and uric acid by differential pulse voltammetry. Dalton Trans. 2021, 50, 16593–16600. [Google Scholar] [CrossRef]
- Ehrnst, Y.; Ahmed, H.; Komljenovic, R.; Massahud, E.; Shepelin, N.A.; Sherrell, P.C.; Ellis, A.V.; Rezk, A.R.; Yeo, L.Y. Acoustotemplating: Rapid Synthesis of Freestanding Quasi-2D MOF/Graphene Oxide Heterostructures for Supercapacitor Applications. J. Mater. Chem. A 2022, 10, 7058–7072. [Google Scholar] [CrossRef]
- Rajak, R.; Saraf, M.; Mobin, S.M. Robust heterostructures of a bimetallic sodium–zinc metal–organic framework and reduced graphene oxide for high-performance supercapacitors. J. Mater. Chem. A 2019, 7, 1725–1736. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, S.; Lu, W.; Lei, D.; Tian, Y.; Guo, M.; Mi, P.; Qu, N.; Zhao, Y. MXenes induced formation of Ni-MOF microbelts for high-performance Supercapacitors. J. Colloid Interface Sci. 2021, 592, 95–102. [Google Scholar] [CrossRef]
- Shao, S.; Zhang, L.; Liu, W.; Zhang, Y.; Shen, X.; Nie, Y.; Yang, W.; Liu, H.; Li, S.; Li, S. Synthesis of Hierarchical Porous MOFs via Ligand Thermolysis for High-Performance Supercapacitor. J. Inorg. Organomet. Polym. Mater. 2022, 32, 4412–4421. [Google Scholar] [CrossRef]
- Li, S.; Yang, K.; Ye, P.; Ma, K.; Zhang, Z.; Huang, Q. Three-dimensional porous carbon/Co3O4 composites derived from graphene/Co-MOF for high performance supercapacitor electrodes. Appl. Surf. Sci. 2020, 503, 144090. [Google Scholar] [CrossRef]
- Li, S.; Yang, K.; Ye, P.; Jiang, H.; Zhang, Z.; Huang, Q.; Wang, L. Hierarchical interpenetrating rHGO-decorated NiCo2O4 nanowires architectures for high-performance supercapacitors. Appl. Surf. Sci. 2019, 473, 326–333. [Google Scholar] [CrossRef]
- Lan, K.; Wei, Q.; Wang, R.; Xia, Y.; Tan, S.; Wang, Y.; Elzatahry, A.; Feng, P.; Mai, L.; Zhao, D. Two-Dimensional Mesoporous Heterostructure Delivering Superior Pseudocapacitive Sodium Storage via Bottom-Up Monomicelle Assembly. J. Am. Chem. Soc. 2019, 141, 16755–16762. [Google Scholar] [CrossRef]
- Babu, R.S.; Vinodh, R.; de Barros, A.; Samyn, L.; Prasanna, K.; Maier, M.; Alves, C.; Kim, H.-J. Asymmetric supercapacitor based on carbon nanofibers as the anode and two-dimensional copper cobalt oxide nanosheets as the cathode. Chem. Eng. J. 2019, 366, 390–403. [Google Scholar] [CrossRef]
- Ma, Y.; Gao, G.; Su, H.; Rong, H.; Lai, L.; Liu, Q. A Cu4 cluster-based MOF as a supercapacitor electrode material with ultrahigh capacitance. Ionics 2021, 27, 1699–1707. [Google Scholar] [CrossRef]
- Liu, S.; Gordiichuk, P.; Wu, Z.-S.; Liu, Z.; Wei, W.; Wagner, M.; Mohamed-Noriega, N.; Wu, D.; Mai, Y.; Herrmann, A.; et al. Patterning two-dimensional free-standing surfaces with mesoporous conducting polymers. Nat. Commun. 2015, 6, 8817. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhou, X.; Ma, J.; Yang, X.; Deng, Y. Recent advances in amphiphilic block copolymer templated mesoporous metal-based materials: Assembly engineering and applications. Chem. Soc. Rev. 2020, 49, 1173–1208. [Google Scholar] [CrossRef]
- Hang, X.; Xue, Y.; Cheng, Y.; Du, M.; Du, L.; Pang, H. From Co-MOF to CoNi-MOF to Ni-MOF: A Facile Synthesis of 1D Micro-/Nanomaterials. Inorg. Chem. 2021, 60, 13168–13176. [Google Scholar] [CrossRef]
- Liu, F.; Ye, P.; Cheng, Q.; Zhang, D.; Nie, Y.; Shen, X.; Zhu, M.; Xu, H.; Li, S. By introducing multiple hydrogen bonds endows MOF electrodes with an enhanced structural stability. Inorg. Chem. 2024, 63, 14630–14640. [Google Scholar] [CrossRef]
- Nicks, J.; Zhang, J.; Foster, J.A. Tandem catalysis by ultrathin metal–organic nanosheets formed through post-synthetic functionalisation of a layered framework. Chem. Commun. 2019, 55, 8788–8791. [Google Scholar] [CrossRef] [PubMed]
- Rad, M.; Dehghanpour, S. ZnO as an efficient nucleating agent and morphology template for rapid, facile and scalable synthesis of MOF-46 and ZnO@MOF-46 with selective sensing properties and enhanced photocatalytic ability. RSC Adv. 2016, 6, 61784–61793. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, J.; Xiong, N.; Bai, Y.; Yilihamu, A.; Ma, Q.; Yang, S.; Wu, D.; Yang, S.-T. Carboxylation as an effective approach to improve the adsorption performance of graphene materials for Cu2+ removal. Sci. Total. Environ. 2019, 682, 591–600. [Google Scholar] [CrossRef]
- Cui, M.; Park, S.-J.; Kim, S. Carboxylated group effect of graphene oxide on capacitance performance of Zr-based metal organic framework electrodes. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1939–1945. [Google Scholar] [CrossRef]
- Yuan, Y.; Gao, X.; Wei, Y.; Wang, X.; Wang, J.; Zhang, Y.; Gao, C. Enhanced desalination performance of carboxyl functionalized graphene oxide nanofiltration membranes. Desalination 2017, 405, 29–39. [Google Scholar] [CrossRef]
- Guo, S.; Raya, J.; Ji, D.; Nishina, Y.; Ménard-Moyon, C.; Bianco, A. Is carboxylation an efficient method for graphene oxide functionalization? Nanoscale Adv. 2020, 2, 4085–4092. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ding, R.; Shi, W.; Xu, Q.; Liu, E. Ternary Ni-Co-F nanocrystal-based supercapacitors. Chem.-Eur. J. 2017, 23, 6896–6904. [Google Scholar] [CrossRef]
- Kong, M.; Wang, Z.; Wang, W.; Ma, M.; Liu, D.; Hao, S.; Kong, R.; Du, G.; Asiri, A.M.; Yao, Y.; et al. NiCoP nanoarray: A superior pseudocapacitor electrode with high areal capacitance. Chem.-Eur. J. 2017, 23, 4435–4441. [Google Scholar] [CrossRef]
- Cheng, H.; Xiao, R.; Bian, H.; Li, Z.; Zhan, Y.; Tsang, C.K.; Chung, C.; Lu, Z.; Li, Y.Y. Periodic porous silicon thin films with interconnected channels as durable anode materials for lithium ion batteries. Mater. Chem. Phys. 2014, 144, 25–30. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, Q.; Xiong, Y.; Cheng, D.; Zeng, Y.; Bu, Y. Fabrication of 3D Co-doped Ni-based MOF hierarchical micro-flowers as a high-performance electrode material for supercapacitors. Appl. Surf. Sci. 2019, 483, 1158–1165. [Google Scholar] [CrossRef]
- Gittins, J.W.; Balhatchet, C.J.; Chen, Y.; Liu, C.; Madden, D.G.; Britto, S.; Golomb, M.J.; Walsh, A.; Fairen-Jimenez, D.; Dutton, S.E.; et al. Insights into the electric double-layer capacitance of two-dimensional electrically conductive metal–organic frameworks. J. Mater. Chem. A 2021, 9, 16006–16015. [Google Scholar] [CrossRef]
- Ramachandran, R.; Zhao, C.; Luo, D.; Wang, K.; Wang, F. Synthesis of copper benzene-1, 3, 5-tricarboxylate metal organic frameworks with mixed phases as the electrode material for supercapacitor applications. Appl. Surf. Sci. 2018, 460, 33–39. [Google Scholar] [CrossRef]
- Rajak, R.; Saraf, M.; Kumar, P.; Natarajan, K.; Mobin, S.M. Construction of a Cu-based metal–organic framework by employing a mixed-ligand strategy and its facile conversion into nanofibrous CuO for electrochemical energy storage applications. Inorg. Chem. 2021, 60, 16986–16995. [Google Scholar] [CrossRef]
- Zou, J.; Xie, D.; Xu, J.; Song, X.; Zeng, X.; Wang, H.; Zhao, F. Rational design of honeycomb Ni-Co LDH/graphene composite for remarkable supercapacitor via ultrafast microwave synthesis. Appl. Surf. Sci. 2022, 571, 151322. [Google Scholar] [CrossRef]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (anatase) nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, X.; Wang, Z.; Wang, X.; Li, Z.; Wang, L.; Zhou, C.; Chen, D.; Luo, Y. Yolk–shell structured nickel cobalt sulfide and carbon nanotube composite for high-performance hybrid supercapacitors. Energy Fuels 2021, 35, 5342–5351. [Google Scholar] [CrossRef]
- Mao, F.; Li, Y.; Zou, Z.; Huang, B.; Yang, J.; Yao, J. Zn2+ storage performance and structural change of orthorhombic V2O5 nanowires as the cathode material for rechargeable aqueous zinc-ion batteries. Electrochim. Acta 2021, 397, 139255. [Google Scholar] [CrossRef]
- Ninawe, P.; Gupta, K.; Ballav, N. Chemically integrating a 2D metal–organic framework with 2D functionalized graphene. Inorg. Chem. 2021, 60, 19079–19085. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ding, K.; Tian, H.; Yao, M.; Nath, B.; Deng, W.; Wang, Y.; Xu, G. Conductive metal–organic framework nanowire array electrodes for high-performance solid-state supercapacitors. Adv. Funct. Mater. 2017, 27, 1702067. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Y.; Xie, Z.; Li, Y.; Liu, Y.; Sun, J.; Ma, Y.; Terasaki, O.; Chen, L. Inside cover: Conjugated copper–catecholate framework electrodes for efficient energy storage. Angew. Chem. Int. Ed. 2020, 59, 962. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, Q.; Zeng, Y.; Cheng, D.; Xiong, Y.; Bu, Y. Rational construction of triangle-like nickel-cobalt bimetallic metal-organic framework nanosheets arrays as battery-type electrodes for hybrid supercapacitors. J. Colloid Interface Sci. 2019, 555, 42–52. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).