Tunable Work Functions in Plasmonic Metals
Abstract
1. Introduction
2. Sample Fabrication
3. Work Functions of Silver Substrates
4. Work Functions of Gold Substrates
5. Discussion
5.1. Photopolymerization
5.2. Effect of BITh on the Work Functions of Ag and Au
5.3. Effect of Light Illumination
6. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Britannica, The Editors of Encyclopedia. “Electronic Work Function.” Encyclopedia Britannica. Available online: https://www.britannica.com/science/electronic-work-function (accessed on 8 August 2019).
- Hölzl, J.; Schulte, F.K. Work function of metals. In Solid Surface Physics; Springer: Berlin, Germany, 2006; pp. 1–150. [Google Scholar] [CrossRef]
- Pina-Galan, E.A. Mathematical analysis of the operation of a scanning Kelvin probe. arXiv 2019, arXiv:1907.07465. [Google Scholar] [CrossRef]
- Bermejo, J.; Colchero, J.; Palacios-Lidon, E. Kelvin Probe Microscopy Investigation of Poly-Octylthiophene Aggregates. Materials 2022, 15, 1212. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fuentes-Hernandez, C.; Shim, J.; Meyer, J.; Giordano, A.J.; Li, H.; Winget, P.; Papadopoulos, T.; Cheun, H.; Kim, J.; et al. A universal method to produce low–work function electrodes for organic electronics. Science 2012, 336, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Körber, C.; Wachau, A.; Säuberlich, F.; Gassenbauer, Y.; Harvey, S.P.; Proffit, D.E.; Mason, T.O. Transparent conducting oxides for photovoltaics: Manipulation of fermi level, work function and energy band alignment. Materials 2010, 3, 4892–4914. [Google Scholar] [CrossRef] [PubMed]
- Khabir, K.M.; Shahabuddin, M.; Noginova, N.; Noginov, M.A. Control of work functions of nanophotonic components. Sci. Rep. 2024, 14, 18044. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, J.A.; Liscio, A.; Schwartz, T.; Canaguier-Durand, A.; Genet, C.; Palermo, V.; Samorì, P.; Ebbesen, T.W. Tuning the work-function via strong coupling. Adv. Mater. 2013, 25, 2481–2485. [Google Scholar] [CrossRef] [PubMed]
- Potter, K.S.; Simmons, J.H. Optical Materials. Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- To-A-Ran, W.; Mastoi, N.R.; Ha, C.Y.; Song, Y.J.; Kim, Y.J. Kelvin Probe Force Microscopy and Electrochemical Atomic Force Microscopy Investigations of Lithium Nucleation and Growth: Influence of the Electrode Surface Potential. J. Phys. Chem. Lett. 2024, 15, 7265–7271. [Google Scholar] [CrossRef] [PubMed]
- Ambient Pressure Photoemission Spectroscopy System Manual: For Software Series 12 and Hardware Version APS04. KP Technology: Wick, Scotland, 2018.
- Baikie, I.D.; Grain, A.C.; Sutherland, J.; Law, J. Ambient pressure photoemission spectroscopy of metal surfaces. Appl. Surf. Sci. 2014, 323, 45–53. [Google Scholar] [CrossRef]
- Hutchison, T.S.; Baird, D.C. The Physics of Engineering Solids, 2nd ed.; John Wiley, and Sons, Inc: New York, NY, USA; London, UK; Sydney, Australia, 1968; p. 534. [Google Scholar]
- Hesami, L.; Yang, C.; Anwar, E.; Noginova, N.; Noginov, M.A. Effect of metal/dielectric substrates on photopolymerization of BITh thin films. Sci. Rep. 2022, 12, 19109. [Google Scholar] [CrossRef] [PubMed]
- Peters, V.N.; Prayakarao, S.; Koutsares, S.R.; Bonner, C.E.; Noginov, M.A. Control of physical and chemical processes with nonlocal metal–dielectric environments. ACS Photonics 2019, 6, 3039–3056. [Google Scholar] [CrossRef]
- Wu, L.; Huang, C.; Emery, B.P.; Sedgwick, A.C.; Bull, S.D.; He, X.-P.; Tian, H.; Yoon, J.; Sessler, J.L.; James, T.D. Förster resonance energy transfer (FRET)-based small-molecule sensors and imaging agents. Chem. Soc. Rev. 2020, 49, 5110–5139. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Zheng, Y.; Shen, X.; Wu, G.; Fields, K.; Hsu, W.-C.; Zhou, H.; Yang, Y.; Wudl, F. Single-crystal linear polymers through visible light–triggered topochemical quantitative polymerization. Science 2014, 343, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, S.; Leupold, E. Umwandlungen des Aethindiphtalids. I. Berichte Der Dtsch. Chem. Ges. 1898, 31, 1159–1174. [Google Scholar] [CrossRef]
- Available online: https://www.kelvinprobe.com/the-kelvin-probe/ (accessed on 1 September 2025).
- Matsumoto, A.; Odani, T.; Sada, K.; Miyata, M.; Tashiro, K. Intercalation of alkylamines into an organic polymer crystal. Nature 2000, 405, 328–330. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Nomura, S.; Uno, T.; Kubo, M.; Sada, K.; Miyata, M. Topochemical Polymerization of 7, 7, 8, 8-Tetrakis (methoxycarbonyl) quinodimethane. Angew. Chem. Int. Ed. 2002, 41, 4306–4309. [Google Scholar]
- Nomura, S.; Itoh, T.; Nakasho, H.; Uno, T.; Kubo, M.; Sada, K.; Inoue, K.; Miyata, M. Crystal structures and topochemical polymerizations of 7, 7, 8, 8-tetrakis (alkoxycarbonyl) quinodimethanes. J. Am. Chem. Soc. 2004, 126, 2035–2041. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Li, Y.; Pang, M.; Cheng, K.; Wang, Y.; Ma, Y.; Meng, J. A novel photochromic liquid crystal system based on biindenylidenedione derivatives. New J. Chem. 2007, 31, 543–548. [Google Scholar] [CrossRef]
- Peachey, N.M.; Eckhardt, C.J. Energetics of organic solid-state reactions: The topochemical principle and the mechanism of the oligomerization of the 2, 5-distyrylpyrazine molecular crystal. J. Am. Chem. Soc. 1993, 115, 3519–3526. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Goswami, D.K.; Okasinski, J.S.; Salaita, K.; Sun, P.; Bedzyk, M.J.; Mirkin, C.A. The controlled evolution of a polymer single crystal. Science 2005, 307, 1763–1766. [Google Scholar] [CrossRef] [PubMed]

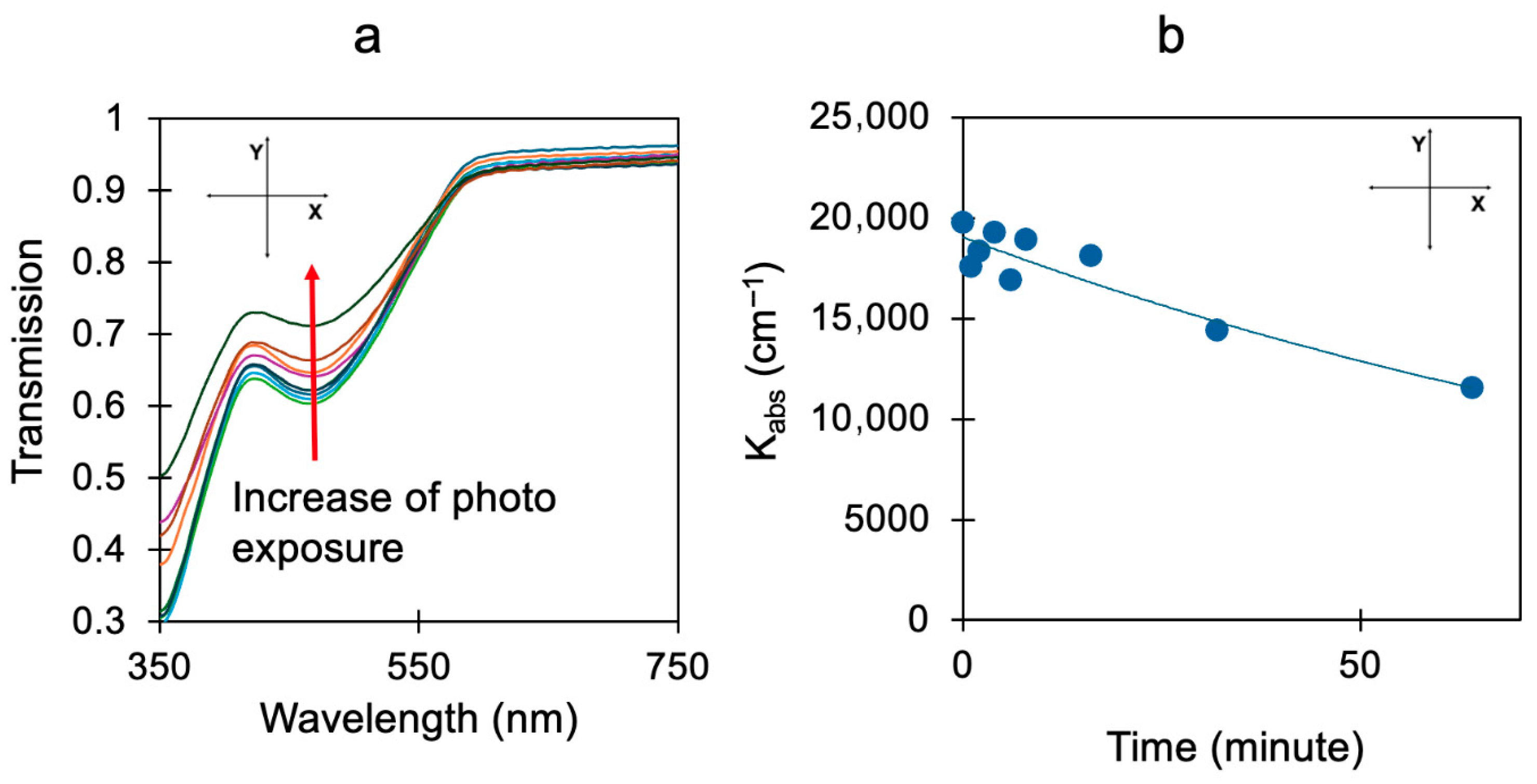

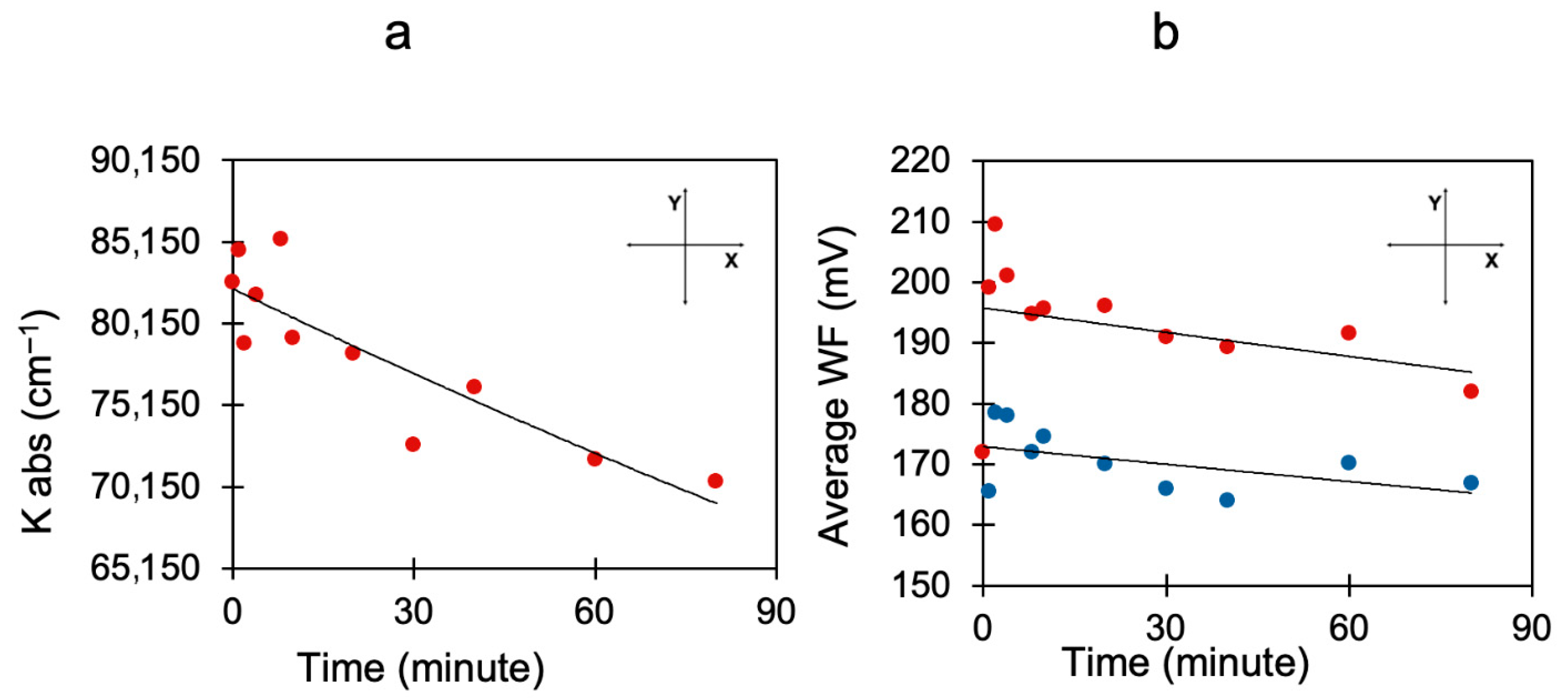

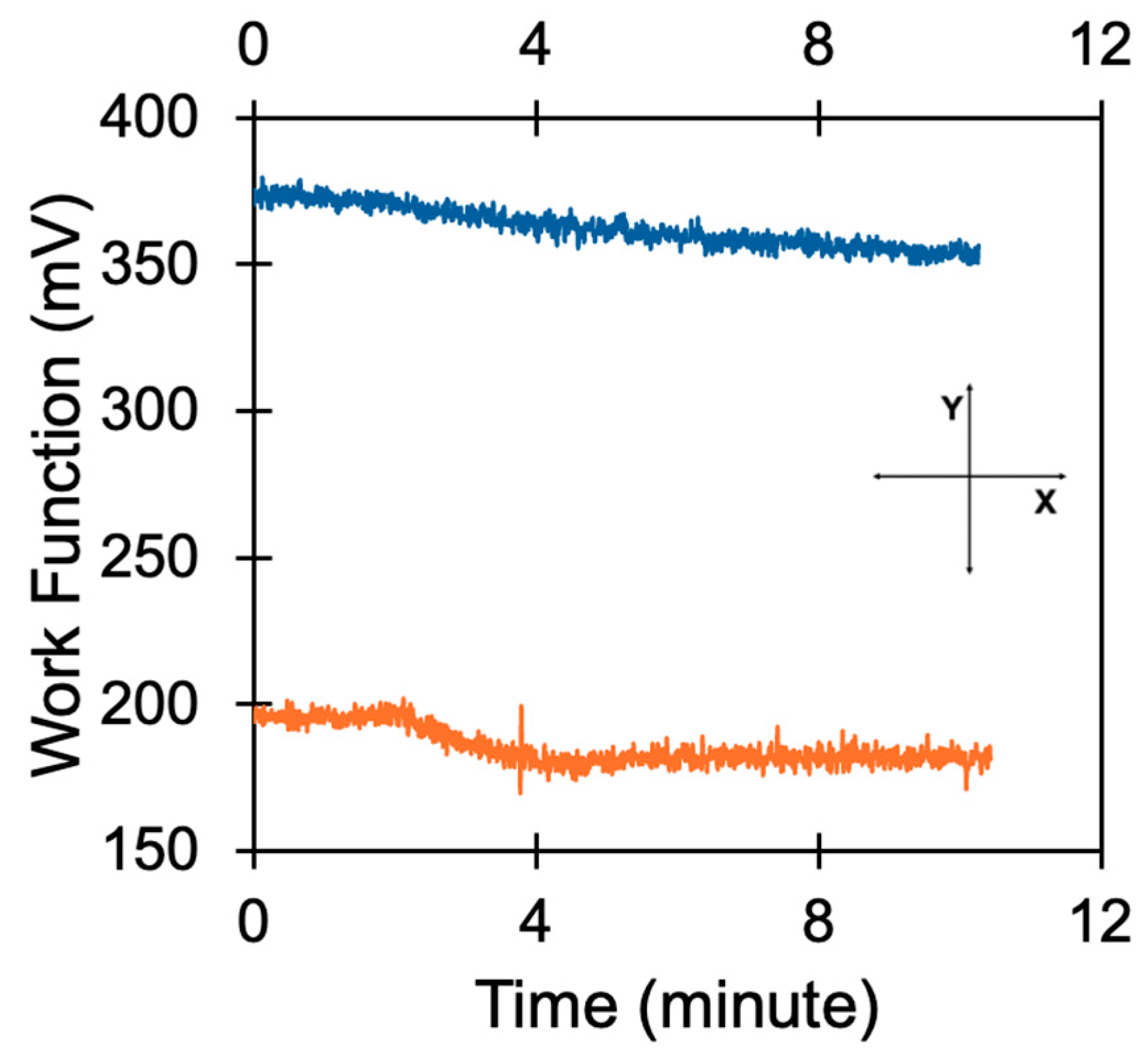

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khabir, K.M.; Hesami, L.; Martin, A.P.; Wilson, J.; Yang, C.; Noginov, M.A. Tunable Work Functions in Plasmonic Metals. Nanomaterials 2025, 15, 1483. https://doi.org/10.3390/nano15191483
Khabir KM, Hesami L, Martin AP, Wilson J, Yang C, Noginov MA. Tunable Work Functions in Plasmonic Metals. Nanomaterials. 2025; 15(19):1483. https://doi.org/10.3390/nano15191483
Chicago/Turabian StyleKhabir, Kanij Mehtanin, Leila Hesami, Anthony P. Martin, Jawuan Wilson, Chi Yang, and Mikhail A. Noginov. 2025. "Tunable Work Functions in Plasmonic Metals" Nanomaterials 15, no. 19: 1483. https://doi.org/10.3390/nano15191483
APA StyleKhabir, K. M., Hesami, L., Martin, A. P., Wilson, J., Yang, C., & Noginov, M. A. (2025). Tunable Work Functions in Plasmonic Metals. Nanomaterials, 15(19), 1483. https://doi.org/10.3390/nano15191483




