Recent Advances in Nano-Engineered Thermochemical Energy Storage Materials: Morphologies, Characteristics, and Performance
Abstract
1. Introduction
2. Scope and Guidance
3. Overview of Thermochemical Heat Storage Materials
3.1. Sorption Thermochemical Heat Storage Materials
3.1.1. Liquid Sorption
3.1.2. Solid Sorption
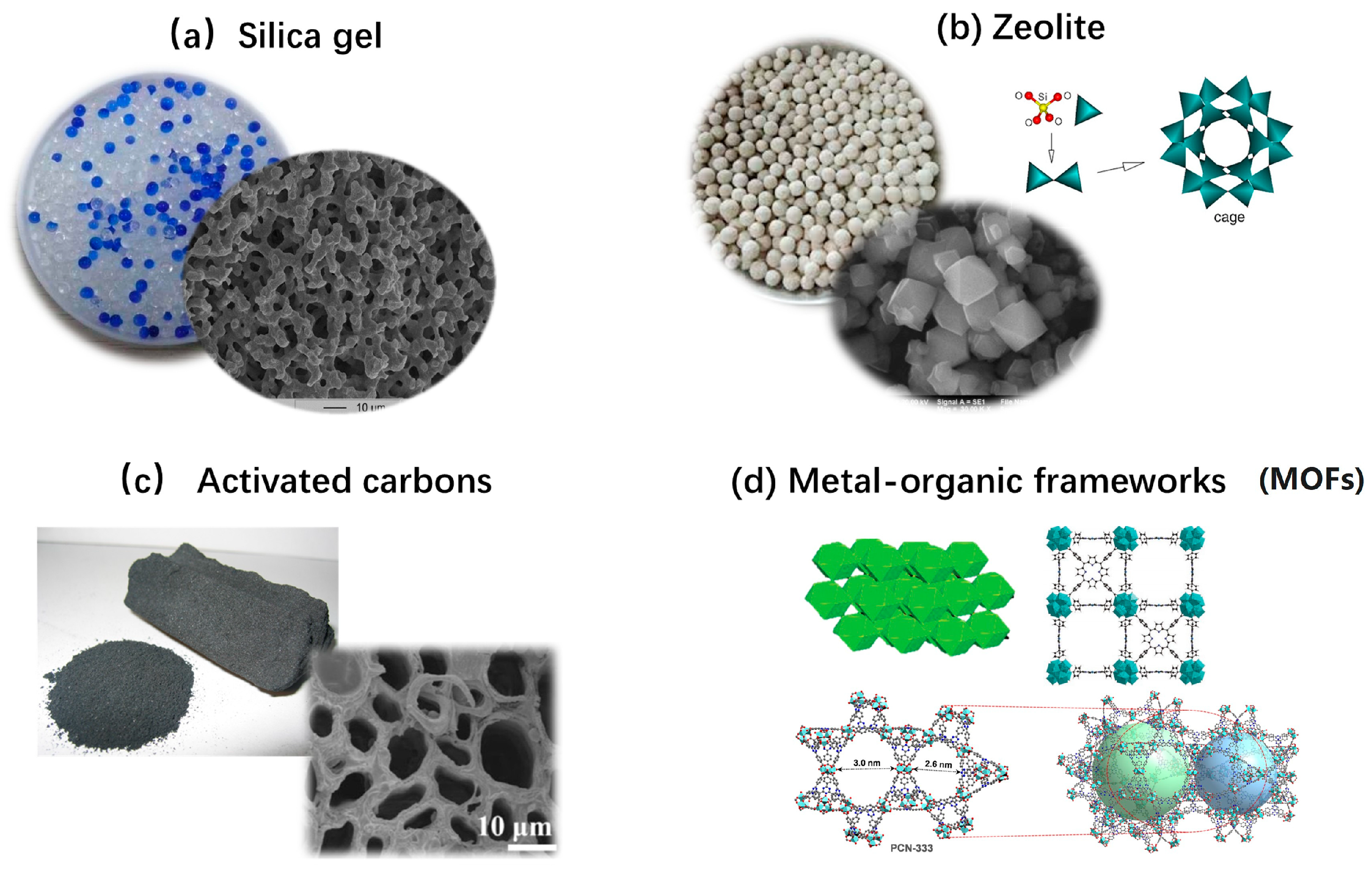
3.2. Reversible Reaction-Based Thermochemical Heat Storage Materials
3.2.1. Hydroxide System
3.2.2. Carbonate System
3.2.3. Redox System
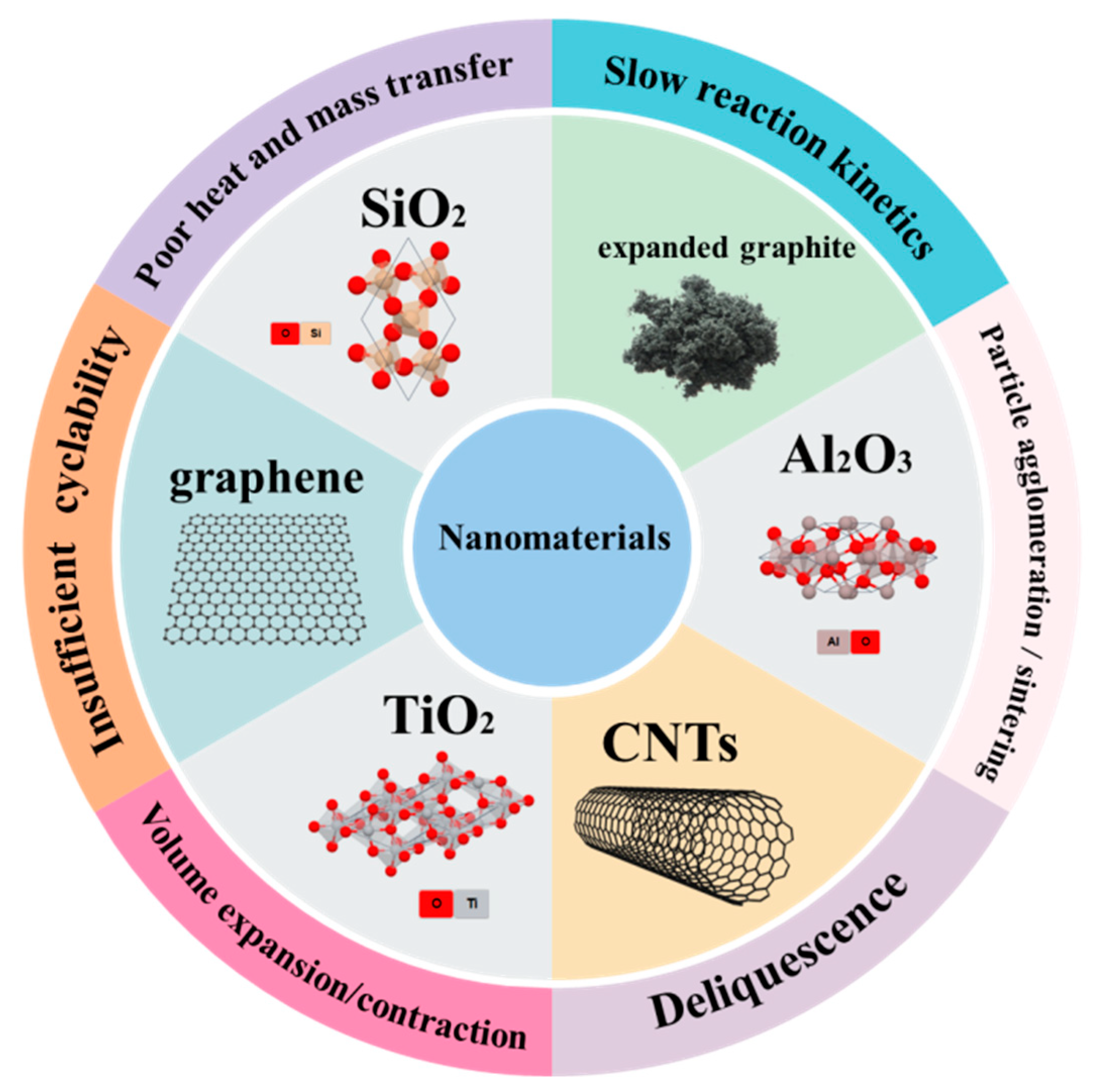
4. Fundamental Challenges of Pure Thermochemical Materials
- The slow reaction kinetics and poor heat and mass transfer of the pure TCMs, which are influenced by temperature, pressure, and material properties (e.g., intrinsic thermal conductivity, pore structure, thermal expansion [100]), restricting energy output and system efficiency.
- Insufficient cyclability is another critical issue, while commercial applications demand 3000–5000 cycles, most lab-tested materials fail after merely 20–100 cycles due to cumulative structural and chemical degradation.
- Particle agglomeration or sintering occurs over repeated cycles due to increased interparticle bonding, further decelerating mass transfer and long-term stability.
- Volume expansion/contraction during hydration–dehydration cycles is also an issue which induces mechanical stress, leading to structural fractures and performance degradation.
- Deliquescence presents an issue, especially for salt hydrate-based sorption TCMs, where hygroscopic salts absorb water vapour beyond intended levels, disrupting reaction equilibrium.
5. Nano-Engineered Composite Thermochemical Materials
5.1. Sorption-Based TCMs
5.2. Reversible Reaction-Based TCMs
5.2.1. Hydroxide System
5.2.2. Carbonate System
5.2.3. Redox System
5.3. Comparison Between Different Nano-Engineered Thermochemical Materials
6. Limitations and Implementation Challenges for Nano-Engineered Composite Thermochemical Materials
6.1. Material-Level Limitations
6.2. Pilot-Level Challenges
6.3. Commercialization Barriers
7. Concluding Remarks
- Sorption systems (e.g., salt hydrates) benefit from carbon-based nanomaterials (CNTs, graphene), which enhance both thermal conductivity and reaction kinetics, achieving energy densities up to 3935 kJ/kg with improved cycling stability.
- Hydroxides, redox, and carbonate systems leverage nanoparticle (e.g., SiO2, Al2O3, carbonaceous materials) doping and grain boundary engineering to mitigate sintering and agglomeration while enhancing reaction rates, thus enabling stable thermal cycles with minimal capacity loss. In addition, interfacial modification between reactants and nano-additives can further improve interfacial compatibility and dispersibility, contributing to the thermochemical storage performances.
- Nanoparticles can also serve as flowing agents to enhance the fluidization characteristics of bulk TCMs. This approach necessitates nanoparticle loadings as high as 30 wt.%, which presents significant limitations for practical thermochemical storage applications.
- Nanosized structure engineering presents another effective approach to improve thermochemical performance by increasing the reactants’ specific surface area and the associated reaction kinetics.
- The high surface reactivity of nanoparticles may lead to unintended chemical interactions with the TCMs, leading to side products that reduce the energy storage density and stability of thermal cycling. The chemical compatibility between the nanomaterials and the TCM should be carefully considered, especially at high temperature applications.
- Many nano-engineered TCMs rely on expensive nanomaterials (e.g., graphene) or complex synthesis methods (e.g., sol–gel, templated co-precipitation, CVD). Future work should prioritize low-cost nano-additives and scalable fabrication methods. In addition, emphasis should also be placed on optimizing nanoparticle loading levels to balance performance gains with cost-effectiveness.
- Most studies show material-level improvements; however, pilot-scale validation is still required for large-scale deployment. Future efforts must transition from gram-scale testing to integrated reactor demonstrations under realistic operating conditions to evaluate system-level performance, fluidization behaviour, heat transfer efficiency, and gas–solid interaction.
- Current studies lack comprehensive techno-economic assessments of nano-engineered TCES systems, particularly in evaluating their levelized cost of storage (LCOS). Such assessments are essential in the future to identify cost bottlenecks, optimize material and reactor designs, and establish a viable commercialization pathway that meets grid-scale storage cost targets.
Author Contributions
Funding
Conflicts of Interest
References
- Slorach, P.C.; Stamford, L. Net Zero in the Heating Sector: Technological Options and Environmental Sustainability from Now to 2050. Energy Convers. Manag. 2021, 230, 113838. [Google Scholar] [CrossRef]
- IRENA. Innovation Outlook: Thermal Energy Storage; IRENA: Abu Dhabi, United Arab Emirates, 2020; ISBN 978-92-9260-279-6. [Google Scholar]
- DE&E Ltd. Evidence Gathering: Thermal Energy Storage (TES) Technologies; Department for Business, Energy and Industrial Strategy: London, UK, 2016. [Google Scholar]
- Johansson, T.; Burnham, L. Renewable Energy: Sources for Fuels and Electricity; Island Press: Washington, DC, USA, 1993. [Google Scholar]
- Internationale Agentur für Erneuerbare Energien. Global Renewables Outlook Energy Transformation 2050; IRENA: Abu Dhabi, United Arab Emirates, 2020. [Google Scholar]
- Chen, X.; Wu, X.; Lee, K.Y. The Mutual Benefits of Renewables and Carbon Capture: Achieved by an Artificial Intelligent Scheduling Strategy. Energy Convers. Manag. 2021, 233, 113856. [Google Scholar] [CrossRef]
- Subashini, C.; Sivasubramanian, R.; Sundaram, M.M.; Priyadharsini, N. The Evolution of Allotropic Forms of Na2CoP2O7 Electrode and Its Role in Future Hybrid Energy Storage. J. Energy Storage 2025, 130, 117390. [Google Scholar] [CrossRef]
- Hasnain, S.M. Review on Sustainable Thermal Energy Storage Technologies, Part I: Heat Storage Materials and Techniques. Energy Convers. Manag. 1998, 39, 1127–1138. [Google Scholar] [CrossRef]
- Ortiz, C.; García-Luna, S.; Carro, A.; Carvajal, E.; Chacartegui, R. Techno-Economic Analysis of a Modular Thermochemical Battery for Electricity Storage Based on Calcium-Looping. Appl. Energy 2024, 367, 123366. [Google Scholar] [CrossRef]
- Nguyen, T.-T.; Martin, V.; Malmquist, A.; Silva, C.A.S. A Review on Technology Maturity of Small Scale Energy Storage Technologies. Renew. Energy Environ. Sustain. 2017, 2, 36. [Google Scholar] [CrossRef]
- Cavanagh, K.; Ward, J.K.; Behrens, S.; Bhatt, A.I.; Ratnam, E.L.; Oliver, E.; Hayward, J. Electrical Energy Storage: Technology Overview and Applications; CSIRO: Canberra, Australia, 2015. [Google Scholar]
- Grand View Research. Global Thermal Energy Storage Market Size & Outlook; Grand View Research: San Francisco, CA, USA, 2023. [Google Scholar]
- Santamaría Padilla, A.; Romero-Paredes Rubio, H. A Thermochemical Energy Storage Materials Review Based on Solid-Gas Reactions for Supercritical CO2 Solar Tower Power Plant with a Brayton Cycle. J. Energy Storage 2023, 73, 108906. [Google Scholar] [CrossRef]
- Carrillo, A.J.; González-Aguilar, J.; Romero, M.; Coronado, J.M. Solar Energy on Demand: A Review on High Temperature Thermochemical Heat Storage Systems and Materials. Chem. Rev. 2019, 119, 4777–4816. [Google Scholar] [CrossRef]
- Farulla, G.A.; Cellura, M.; Guarino, F.; Ferraro, M. A Review of Thermochemical Energy Storage Systems for Power Grid Support. Appl. Sci. 2020, 10, 3142. [Google Scholar] [CrossRef]
- Aydin, D.; Casey, S.P.; Riffat, S. The Latest Advancements on Thermochemical Heat Storage Systems. Renew. Sustain. Energy Rev. 2015, 41, 356–367. [Google Scholar] [CrossRef]
- Yang, H.; Wang, C.; Tong, L.; Yin, S.; Wang, L.; Ding, Y. Salt Hydrate Adsorption Material-Based Thermochemical Energy Storage for Space Heating Application: A Review. Energies 2023, 16, 2875. [Google Scholar] [CrossRef]
- Fopah-Lele, A.; Tamba, J.G. A Review on the Use of SrBr2·6H2O as a Potential Material for Low Temperature Energy Storage Systems and Building Applications. Sol. Energy Mater. Sol. Cells 2017, 164, 175–187. [Google Scholar] [CrossRef]
- Scapino, L.; Zondag, H.A.; Van Bael, J.; Diriken, J.; Rindt, C.C.M. Sorption Heat Storage for Long-Term Low-Temperature Applications: A Review on the Advancements at Material and Prototype Scale. Appl. Energy 2017, 190, 920–948. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Solé, A.; Barreneche, C. Review on Sorption Materials and Technologies for Heat Pumps and Thermal Energy Storage. Renew. Energy 2017, 110, 3–39. [Google Scholar] [CrossRef]
- Rammelberg, H.U.; Osterland, T.; Priehs, B.; Opel, O.; Ruck, W.K.L. Thermochemical Heat Storage Materials—Performance of Mixed Salt Hydrates. Sol. Energy 2016, 136, 571–589. [Google Scholar] [CrossRef]
- Clark, R.J.; Gholamibozanjani, G.; Woods, J.; Kaur, S.; Odukomaiya, A.; Al-Hallaj, S.; Farid, M. Experimental Screening of Salt Hydrates for Thermochemical Energy Storage for Building Heating Application. J. Energy Storage 2022, 51, 104415. [Google Scholar] [CrossRef]
- Kim, S.T.; Takasu, H.; Kato, Y. Chapter 6. Reversible Reaction-Based Thermochemical Energy Storage Materials. In Thermal Energy Storage: Materials, Devices, Systems and Applications; Royal Society of Chemistry: London, UK, 2021; pp. 107–120. [Google Scholar]
- André, L.; Abanades, S. Recent Advances in Thermochemical Energy Storage via Solid–Gas Reversible Reactions at High Temperature. Energies 2020, 13, 5859. [Google Scholar] [CrossRef]
- Carro, A.; Chacartegui, R.; Ortiz, C.; Becerra, J.A. Analysis of a Thermochemical Energy Storage System Based on the Reversible Ca(OH)2/CaO Reaction. Energy 2022, 261, 125064. [Google Scholar] [CrossRef]
- Wu, H.; Salles, F.; Zajac, J. A Critical Review of Solid Materials for Low-Temperature Thermochemical Storage of Solar Energy Based on Solid-Vapour Adsorption in View of Space Heating Uses. Molecules 2019, 24, 945. [Google Scholar] [CrossRef]
- Riazi, H.; Mesgari, S.; Ahmed, N.A.; Taylor, R.A. The Effect of Nanoparticle Morphology on the Specific Heat of Nanosalts. Int. J. Heat Mass Transf. 2016, 94, 254–261. [Google Scholar] [CrossRef]
- Ma, C.; Chen, L.; Cao, C.; Li, X. Nanoparticle-Induced Unusual Melting and Solidification Behaviours of Metals. Nat. Commun. 2017, 8, 14178. [Google Scholar] [CrossRef] [PubMed]
- Khodadadi, J.M.; Hosseinizadeh, S.F. Nanoparticle-Enhanced Phase Change Materials (NEPCM) with Great Potential for Improved Thermal Energy Storage. Int. Commun. Heat Mass Transf. 2007, 34, 534–543. [Google Scholar] [CrossRef]
- Serrano, A.; Caballero-Calero, O.; García, M.Á.; Lazić, S.; Carmona, N.; Castro, G.R.; Martín-González, M.; Fernández, J.F. Cold Sintering Process of ZnO Ceramics: Effect of the Nanoparticle/Microparticle Ratio. J. Eur. Ceram. Soc. 2020, 40, 5535–5542. [Google Scholar] [CrossRef]
- Choi, S.U.S.; Eastman, J.A. Enhancing Thermal Conductivity of Fluids with Nanoparticles. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, San Francisco, CA, USA, 12–17 November 1995; Volume 66, pp. 99–105. [Google Scholar] [CrossRef]
- Zhang, L.-D.; Chen, X.; Wu, Y.-T.; Lu, Y.-W.; Ma, C.-F. Effect of Nanoparticle Dispersion on Enhancing the Specific Heat Capacity of Quaternary Nitrate for Solar Thermal Energy Storage Application. Sol. Energy Mater. Sol. Cells 2016, 157, 808–813. [Google Scholar] [CrossRef]
- Hassan, N.; Minakshi, M.; Ruprecht, J.; Liew, W.Y.H.; Jiang, Z.T. A Binary Salt Mixture LiCl–LiOH for Thermal Energy Storage. Materials 2023, 16, 1434. [Google Scholar] [CrossRef]
- Hassan, N.; Minakshi, M.; Liew, W.Y.H.; Amri, A.; Jiang, Z.T. Thermal Characterization of Binary Calcium-Lithium Chloride Salts for Thermal Energy Storage at High Temperature. Energies 2023, 16, 4715. [Google Scholar] [CrossRef]
- Hassan, N.; Minakshi, M.; Hussain, M.; Hsien Liew, W.Y.; Mondinos, N.; Jiang, Z.-T. Organic and Inorganic Phase Change Materials in Thermal Energy Storage: A Review on Materials Perspectives and Insights with a Case Study. Sustain. Eng. 2025, 2, 38–75. [Google Scholar] [CrossRef]
- Thirumalaivasan, N.; Gopi, S.; Karthik, K.; Nangan, S.; Kanagaraj, K.; Rajendran, S. Nano-PCM Materials: Bridging the Gap in Energy Storage under Fluctuating Environmental Conditions. Process Saf. Environ. Prot. 2024, 189, 1003–1021. [Google Scholar] [CrossRef]
- Gao, H.; Wang, J.; Chen, X.; Wang, G.; Huang, X.; Li, A.; Dong, W. Nanoconfinement Effects on Thermal Properties of Nanoporous Shape-Stabilized Composite PCMs: A Review. Nano Energy 2018, 53, 769–797. [Google Scholar] [CrossRef]
- Paul, J.; Pandey, A.K.; Mishra, Y.N.; Said, Z.; Mishra, Y.K.; Ma, Z.; Jacob, J.; Kadirgama, K.; Samykano, M.; Tyagi, V.V. Nano-Enhanced Organic Form Stable PCMs for Medium Temperature Solar Thermal Energy Harvesting: Recent Progresses, Challenges, and Opportunities. Renew. Sustain. Energy Rev. 2022, 161, 112321. [Google Scholar] [CrossRef]
- Nematpour Keshteli, A.; Sheikholeslami, M. Nanoparticle Enhanced PCM Applications for Intensification of Thermal Performance in Building: A Review. J. Mol. Liq. 2019, 274, 516–533. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, R. Applied Sciences Synergistically-Enhanced Thermal Conductivity of Shape-Stabilized Phase Change Materials by Expanded Graphite and Carbon Nanotube. Appl. Sci. 2017, 7, 574. [Google Scholar] [CrossRef]
- Tao, Y.B.; Lin, C.H.; He, Y.L. Preparation and Thermal Properties Characterization of Carbonate Salt/Carbon Nanomaterial Composite Phase Change Material. Energy Convers. Manag. 2015, 97, 103–110. [Google Scholar] [CrossRef]
- Xing, M.; Yu, J.; Wang, R. Experimental Study on the Thermal Conductivity Enhancement of Water Based Nanofluids Using Different Types of Carbon Nanotubes. Int. J. Heat Mass Transf. 2015, 88, 609–616. [Google Scholar] [CrossRef]
- Yang, X.; Li, S.; Huang, H.; Li, J.; Kobayashi, N.; Kubota, M. Effect of Carbon Nanoadditives on Lithium Hydroxide Monohydrate-Based Composite Materials for Low Temperature Chemical Heat Storage. Energies 2017, 10, 644. [Google Scholar] [CrossRef]
- Mastronardo, E.; Bonaccorsi, L.; Kato, Y.; Piperopoulos, E.; Milone, C. Efficiency Improvement of Heat Storage Materials for MgO/H2O/Mg(OH)2 Chemical Heat Pumps. Appl. Energy 2016, 162, 31–39. [Google Scholar] [CrossRef]
- Mastronardo, E.; Bonaccorsi, L.; Kato, Y.; Piperopoulos, E.; Lanza, M.; Milone, C. Thermochemical Performance of Carbon Nanotubes Based Hybrid Materials for MgO/H2O/Mg(OH)2 Chemical Heat Pumps. Appl. Energy 2016, 181, 232–243. [Google Scholar] [CrossRef]
- Zamengo, M.; Ryu, J.; Kato, Y. Composite Block of Magnesium Hydroxide—Expanded Graphite for Chemical Heat Storage and Heat Pump. Appl. Therm. Eng. 2014, 69, 29–38. [Google Scholar] [CrossRef]
- Zamengo, M.; Ryu, J.; Kato, Y. Thermochemical Performance of Magnesium Hydroxide-Expanded Graphite Pellets for Chemical Heat Pump. Appl. Therm. Eng. 2014, 64, 339–347. [Google Scholar] [CrossRef]
- André, L.; Abanades, S.; Flamant, G. Screening of Thermochemical Systems Based on Solid-Gas Reversible Reactions for High Temperature Solar Thermal Energy Storage. Renew. Sustain. Energy Rev. 2016, 64, 703–715. [Google Scholar] [CrossRef]
- Jashari, A.; Afflerbach, S.; Afflerbach, K.; Krumm, W. Structural Stabilization of Granular Ca(OH)2 by Coating with Nanostructured Additives for Thermochemical Cycling in a Fixed Reaction Bed. Energy Convers. Manag. X 2023, 18, 100367. [Google Scholar] [CrossRef]
- Li, S.; Yang, X.; Li, X.; Qu, W.; Zhou, T.; Dong, T.; Deng, L.; Zhang, J.; Zhao, J. A High Energy Density 3D Nano-Carbon Based Magnesium Hydroxide Reversible Chemical Reaction Heat Storage Material Synthesis and Heat Transfer Performance Investigation. J. Energy Storage 2022, 50, 104260. [Google Scholar] [CrossRef]
- Han, X.; Wu, P.; Wang, L.; Xu, M.; Huai, X. Self-Assembled Micro-Nano Flower-like/Spherical Magnesium Hydroxide for Heat-Energy Storage. Mater. Lett. 2023, 334, 133723. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, N.; Kim, T.S.; Park, G.J.; Kwon, Y.; Yu, H.K. Mg(OH)2 Nano-Sheet Decorated MgO Micro-Beams by Electron Beam Irradiation for Thermochemical Heat Storage. Ceram. Int. 2019, 45, 18908–18913. [Google Scholar] [CrossRef]
- Desai, F.; Sunku Prasad, J.; Muthukumar, P.; Rahman, M.M. Thermochemical Energy Storage System for Cooling and Process Heating Applications: A Review. Energy Convers. Manag. 2021, 229, 113617. [Google Scholar] [CrossRef]
- Tatsidjodoung, P.; Le, N.; Es, P.; Luo, L. A Review of Potential Materials for Thermal Energy Storage in Building Applications. Renew. Sustain. Energy Rev. 2013, 18, 327–349. [Google Scholar] [CrossRef]
- Nie, B.; Liu, J.; He, N.; Liu, C.; Guo, H.; Ahmad, A.; Su, J. Chapter 5. Sorption-Based Thermochemical Energy Storage Materials. In Thermal Energy Storage: Materials, Devices, Systems and Applications; Royal Society of Chemistry: London, UK, 2021; pp. 91–106. [Google Scholar]
- Palacios, A.; Navarro, M.E.; Barreneche, C.; Ding, Y. Water Sorption-Based Thermochemical Storage Materials: A Review from Material Candidates to Manufacturing Routes. Front. Therm. Eng. 2022, 2, 1003863. [Google Scholar] [CrossRef]
- Lele, A.F. A Thermochemical Heat Storage System for Households: Combined Investigations of Thermal Transfers Coupled to Chemical Reactions; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Lahmidi, H.; Mauran, S.; Goetz, V. Definition, Test and Simulation of a Thermochemical Storage Process Adapted to Solar Thermal Systems. Sol. Energy 2006, 80, 883–893. [Google Scholar] [CrossRef]
- Mauran, S.; Lahmidi, H.; Goetz, V. Solar Heating and Cooling by a Thermochemical Process. First Experiments of a Prototype Storing 60 KW h by a Solid/Gas Reaction. Sol. Energy 2008, 82, 623–636. [Google Scholar] [CrossRef]
- Yu, N.; Wang, R.Z.; Lu, Z.S.; Wang, L.W.; Ishugah, T.F. Evaluation of a Three-Phase Sorption Cycle for Thermal Energy Storage. Energy 2014, 67, 468–478. [Google Scholar] [CrossRef]
- Solé, A.; Miró, L.; Barreneche, C.; Martorell, I.; Cabeza, L.F. Corrosion of Metals and Salt Hydrates Used for Thermochemical Energy Storage. Renew. Energy 2015, 75, 519–523. [Google Scholar] [CrossRef]
- Anderko, A.; Young, R.D. Model for Corrosion of Carbon Steel in Lithium Bromide Absorption Refrigeration Systems. Corrosion 2000, 56, 543–555. [Google Scholar] [CrossRef]
- Jarimi, H.; Aydin, D.; Yanan, Z.; Ozankaya, G.; Chen, X.; Riffat, S. Review on the Recent Progress of Thermochemical Materials and Processes for Solar Thermal Energy Storage and Industrial Waste Heat Recovery. Int. J. Low-Carbon Technol. 2019, 14, 44–69. [Google Scholar] [CrossRef]
- Tahat, M.A. Heat-Pump/Energy-Store Using Silica Gel and Water as a Working Pair. Appl. Energy 2001, 69, 19–27. [Google Scholar] [CrossRef]
- Yu, N.; Wang, R.Z.; Lu, Z.S.; Wang, L.W. Study on Consolidated Composite Sorbents Impregnated with LiCl for Thermal Energy Storage. Int. J. Heat Mass Transf. 2015, 84, 660–670. [Google Scholar] [CrossRef]
- Lu, Y.Z.; Wang, R.Z.; Zhang, M.; Jiangzhou, S. Adsorption Cold Storage System with Zeolite-Water Working Pair Used for Locomotive Air Conditioning. Energy Convers. Manag. 2003, 44, 1733–1743. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, J.; Xu, M.; Huang, C.; Wang, R.; Li, T.; Huai, X. Record Performance of Ultralow-Temperature-Driven Water-Based Sorption Refrigeration Enabled by Low-Cost Zeolite-like Porous Aluminophosphate. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Jänchen, J.; Ackermann, D.; Stach, H.; Brösicke, W. Studies of the Water Adsorption on Zeolites and Modified Mesoporous Materials for Seasonal Storage of Solar Heat. Sol. Energy 2004, 76, 339–344. [Google Scholar] [CrossRef]
- Rykl, D.; Pechar, F. Study Relating to Temperature Stability of the Natural Laumontite Zeolite. Cryst. Res. Technol. 1984, 19, 549–555. [Google Scholar] [CrossRef]
- Zbair, M.; Bennici, S. Survey Summary on Salts Hydrates and Composites Used in Thermochemical Sorption Heat Storage: A Review. Energies 2021, 14, 3105. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Tang, X.; Chang, Z.; Li, M. Hybrid Nanoparticle and Salt-Modified Aluminum Fumarate Metal-Organic Framework for Thermochemical Adsorption Heat Storage. Chem. Eng. J. 2025, 520, 165801. [Google Scholar] [CrossRef]
- Morris, R.V.; Ruff, S.W.; Gellert, R.; Ming, D.W.; Arvidson, R.E.; Clark, B.C.; Golden, D.C.; Siebach, K.; Klingelhöfer, G.; Schröder, C.; et al. Identification of Carbonate-Rich Outcrops on Mars by the Spirit Rover. Science 2010, 329, 421–424. [Google Scholar] [CrossRef]
- Jabbari-Hichri, A.; Bennici, S.; Auroux, A. CaCl2-Containing Composites as Thermochemical Heat Storage Materials. Sol. Energy Mater. Sol. Cells 2017, 172, 177–185. [Google Scholar] [CrossRef]
- Chen, H.J.; Cui, Q.; Tang, Y.; Chen, X.J.; Yao, H.Q. Attapulgite Based LiCl Composite Adsorbents for Cooling and Air Conditioning Applications. Appl. Therm. Eng. 2008, 28, 2187–2193. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Wang, R.Z.; Zhao, Y.J.; Li, T.X.; Riffat, S.B.; Wajid, N.M. Development and Thermochemical Characterizations of Vermiculite/SrBr2 Composite Sorbents for Low-Temperature Heat Storage. Energy 2016, 115, 120–128. [Google Scholar] [CrossRef]
- Tso, C.Y.; Chao, C.Y.H. Activated Carbon, Silica-Gel and Calcium Chloride Composite Adsorbents for Energy Efficient Solar Adsorption Cooling and Dehumidification Systems. Int. J. Refrig. 2012, 35, 1626–1638. [Google Scholar] [CrossRef]
- Whiting, G.T.; Grondin, D.; Stosic, D.; Bennici, S.; Auroux, A. Zeolite-MgCl2 Composites as Potential Long-Term Heat Storage Materials: Influence of Zeolite Properties on Heats of Water Sorption. Sol. Energy Mater. Sol. Cells 2014, 128, 289–295. [Google Scholar] [CrossRef]
- Whiting, G.; Grondin, D.; Bennici, S.; Auroux, A. Heats of Water Sorption Studies on Zeolite-MgSO4 Composites as Potential Thermochemical Heat Storage Materials. Sol. Energy Mater. Sol. Cells 2013, 112, 112–119. [Google Scholar] [CrossRef]
- Clark, R.J.; Farid, M. Experimental Investigation into Cascade Thermochemical Energy Storage System Using SrCl2-Cement and Zeolite-13X Materials. Appl. Energy 2022, 316, 119145. [Google Scholar] [CrossRef]
- Chao, J.; Xu, J.; Bai, Z.; Wang, P.; Wang, R.; Li, T. Integrated Heat and Cold Storage Enabled by High-Energy-Density Sorption Thermal Battery Based on Zeolite/MgCl2 Composite Sorbent. J. Energy Storage 2023, 64, 107155. [Google Scholar] [CrossRef]
- Shere, L.; Trivedi, S.; Roberts, S.; Sciacovelli, A.; Ding, Y. Synthesis and Characterization of Thermochemical Storage Material Combining Porous Zeolite and Inorganic Salts. Heat Transf. Eng. 2018, 40, 1176–1181. [Google Scholar] [CrossRef]
- Wang, D.; Yao, H.; Ye, J.; Gao, Y.; Cong, H.; Yu, B. Metal-Organic Frameworks (MOFs): Classification, Synthesis, Modification, and Biomedical Applications. Small 2024, 20, 2404350. [Google Scholar]
- Hua, W.; Yan, H.; Zhang, X.; Xu, X.; Zhang, L.; Shi, Y. Review of Salt Hydrates-Based Thermochemical Adsorption Thermal Storage Technologies. J. Energy Storage 2022, 56, 106158. [Google Scholar] [CrossRef]
- Kiyabu, S.; Girard, P.; Siegel, D.J. Discovery of Salt Hydrates for Thermal Energy Storage. J. Am. Chem. Soc. 2022, 144, 21617–21627. [Google Scholar] [CrossRef]
- Schmidt, M.; Szczukowski, C.; Roßkopf, C.; Linder, M.; Wörner, A. Experimental Results of a 10 KW High Temperature Thermochemical Storage Reactor Based on Calcium Hydroxide. Appl. Therm. Eng. 2014, 62, 553–559. [Google Scholar] [CrossRef]
- Schaube, F.; Koch, L.; Wörner, A.; Müller-Steinhagen, H. A Thermodynamic and Kinetic Study of the De- and Rehydration of Ca(OH)2 at High H2O Partial Pressures for Thermo-Chemical Heat Storage. Thermochim. Acta 2012, 538, 9–20. [Google Scholar] [CrossRef]
- Kato, Y.; Yamashita, N.; Kobayashi, K.; Yoshizawa, Y. Kinetic Study of the Hydration of Magnesium Oxide for a Chemical Heat Pump. Appl. Therm. Eng. 1996, 16, 853–862. [Google Scholar] [CrossRef]
- Müller, D.; Knoll, C.; Gravogl, G.; Artner, W.; Welch, J.M.; Eitenberger, E.; Friedbacher, G.; Schreiner, M.; Harasek, M.; Hradil, K.; et al. Tuning the Performance of MgO for Thermochemical Energy Storage by Dehydration—From Fundamentals to Phase Impurities. Appl. Energy 2019, 253, 113562. [Google Scholar] [CrossRef]
- Yan, T.; Wang, R.Z.; Li, T.X.; Wang, L.W.; Fred, I.T. A Review of Promising Candidate Reactions for Chemical Heat Storage. Renew. Sustain. Energy Rev. 2015, 43, 13–31. [Google Scholar] [CrossRef]
- L’vov, B.V.; Ugolkov, V.L. Peculiarities of CaCO3, SrCO3 and BaCO3 Decomposition in CO2 as a Proof of Their Primary Dissociative Evaporation. Thermochim. Acta 2004, 410, 47–55. [Google Scholar] [CrossRef]
- L’vov, B.V.; Ugolkov, V.L. Kinetics of Free-Surface Decomposition of Magnesium, Strontium and Barium Carbonates Analyzed Thermogravimetrically by the Third-Law Method. Thermochim. Acta 2004, 409, 13–18. [Google Scholar] [CrossRef]
- Fedunik-Hofman, L.; Bayon, A.; Donne, S.W. Kinetics of Solid-Gas Reactions and Their Application to Carbonate Looping Systems. Energies 2019, 12, 2981. [Google Scholar] [CrossRef]
- Durán-Martín, J.D.; Sánchez Jimenez, P.E.; Valverde, J.M.; Perejón, A.; Arcenegui-Troya, J.; García Triñanes, P.; Pérez Maqueda, L.A. Role of Particle Size on the Multicycle Calcium Looping Activity of Limestone for Thermochemical Energy Storage. J. Adv. Res. 2020, 22, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-W.; Feng, Y.-L.; Li, H.-R. Preparation of Basic Magnesium Carbonate and Its Thermal Decomposition Kinetics in Air. J. Cent. South Univ. Technol. 2011, 18, 1865–1870. [Google Scholar] [CrossRef]
- Hassanzadeh, A.; Abbasian, J. Regenerable MgO-Based Sorbents for High-Temperature CO2 Removal from Syngas: 1. Sorbent Development, Evaluation, and Reaction Modeling. Fuel 2010, 89, 1287–1297. [Google Scholar] [CrossRef]
- Miccio, F.; Doghieri, F.; Landi, E. Insights into High Temperature Sorbents for Carbon Dioxide. Chem. Eng. Trans. 2015, 43, 901–906. [Google Scholar] [CrossRef]
- Williamson, K.; Møller, K.T.; D’Angelo, A.M.; Humphries, T.D.; Paskevicius, M.; Buckley, C.E. Thermochemical Energy Storage in Barium Carbonate Enhanced by Iron(Iii) Oxide. Phys. Chem. Chem. Phys. 2023, 25, 7268–7277. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, C.; Doroodchi, E.; Nellore, R.; Moghtaderi, B. A Review on High-Temperature Thermochemical Energy Storage Based on Metal Oxides Redox Cycle. Energy Convers. Manag. 2018, 168, 421–453. [Google Scholar] [CrossRef]
- Neises, M.; Tescari, S.; de Oliveira, L.; Roeb, M.; Sattler, C.; Wong, B. Solar-Heated Rotary Kiln for Thermochemical Energy Storage. Sol. Energy 2012, 86, 3040–3048. [Google Scholar] [CrossRef]
- Chen, X.; Gao, L.; Dong, Z.; Zhu, L. Different Effects on Thermal Conductivity of Ca-Based Thermochemical Energy Storage Materials for a Concentrated Solar Power Plant. Chem. Eng. Sci. 2023, 269, 118501. [Google Scholar] [CrossRef]
- Deng, L.; Xiao, L.; Huang, H.; Li, J.; Chen, D.; Zhou, B.; Zhou, Y. Nano-Porous Carbon Supported Magnesium Hydroxide for Thermochemical Heat Storage Performance Enhancement. Energy Proc. 2022, 28, 1–4. [Google Scholar]
- Zhang, J.; Zhang, Y.; Huang, H.; Huang, K.; Deng, L.; Hu, H.; Chang, M.; Chen, D.; Wang, Y. Exploration of Highly Porous Biochar Derived from Dead Leaves to Immobilize LiOH·H2O Nanoparticles for Efficient Thermochemical Heat Storage Applications. Energy Technol. 2022, 10, 2101127. [Google Scholar] [CrossRef]
- Haruki, M.; Saito, K.; Takai, K.; Fujita, M.; Onishi, H.; Tada, Y. Thermal Conductivity and Reactivity of Mg(OH)2 and MgO/Expanded Graphite Composites with High Packing Density for Chemical Heat Storage. Thermochim. Acta 2019, 680, 178338. [Google Scholar] [CrossRef]
- Li, S.; Huang, H.; Li, J.; Kobayashi, N.; Osaka, Y.; He, Z.; Yuan, H. The Effect of 3D Carbon Nanoadditives on Lithium Hydroxide Monohydrate Based Composite Materials for Highly Efficient Low Temperature Thermochemical Heat Storage. RSC Adv. 2018, 8, 8199–8208. [Google Scholar] [CrossRef]
- Li, S.; Huang, H.; Yang, X.; Bai, Y.; Li, J.; Kobayashi, N.; Kubota, M. Hydrophilic Substance Assisted Low Temperature LiOH·H2O Based Composite Thermochemical Materials for Thermal Energy Storage. Appl. Therm. Eng. 2018, 128, 706–711. [Google Scholar] [CrossRef]
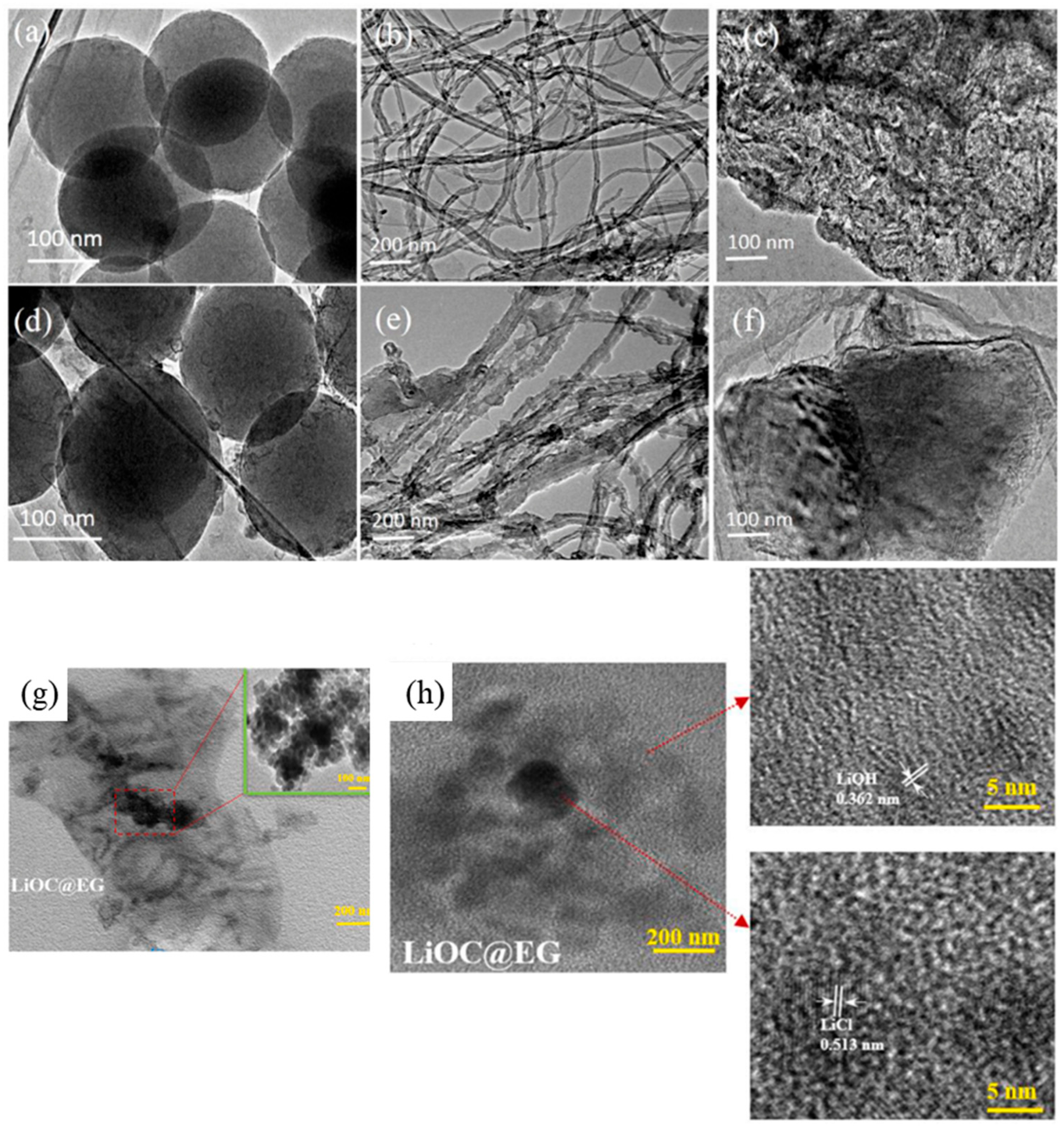
- Li, W.; Klemeš, J.J.; Wang, Q.; Zeng, M. Characterisation and Sorption Behaviour of LiOH-LiCl@EG Composite Sorbents for Thermochemical Energy Storage with Controllable Thermal Upgradeability. Chem. Eng. J. 2021, 421, 129586. [Google Scholar] [CrossRef]
- Ait Ousaleh, H.; Sair, S.; Mansouri, S.; Abboud, Y.; Zahouily, M.; Faik, A.; Bouari, A. El Enhanced Inorganic Salts Stability Using Bentonite Clay for High-Performance and Low-Cost Thermochemical Energy Storage. J. Energy Storage 2022, 49, 104140. [Google Scholar] [CrossRef]
- Tae Kim, S.; Ryu, J.; Kato, Y. Reactivity Enhancement of Chemical Materials Used in Packed Bed Reactor of Chemical Heat Pump. Prog. Nucl. Energy 2011, 53, 1027–1033. [Google Scholar] [CrossRef]
- Roßkopf, C.; Haas, M.; Faik, A.; Linder, M.; Wörner, A. Improving Powder Bed Properties for Thermochemical Storage by Adding Nanoparticles. Energy Convers. Manag. 2014, 86, 93–98. [Google Scholar] [CrossRef]
- Xu, M.; Huai, X.; Cai, J. Agglomeration Behavior of Calcium Hydroxide/Calcium Oxide as Thermochemical Heat Storage Material: A Reactive Molecular Dynamics Study. J. Phys. Chem. C 2017, 121, 3025–3033. [Google Scholar] [CrossRef]
- Schmidt, M.; Gollsch, M.; Giger, F.; Grün, M.; Linder, M. Development of a Moving Bed Pilot Plant for Thermochemical Energy Storage with CaO/Ca(OH)2. In AIP Conference Proceedings; American Institute of Physics Inc.: College Park, MD, USA, 2016; Volume 1734. [Google Scholar]
- Huang, C.; Xu, M.; Huai, X. Synthesis and Performances Evaluation of the Spindle-Shaped Calcium Hydroxide Nanomaterials for Thermochemical Energy Storage. J. Nanoparticle Res. 2019, 21, 262. [Google Scholar] [CrossRef]
- Roßkopf, C.; Afflerbach, S.; Schmidt, M.; Görtz, B.; Kowald, T.; Linder, M.; Trettin, R. Investigations of Nano Coated Calcium Hydroxide Cycled in a Thermochemical Heat Storage. Energy Convers. Manag. 2015, 97, 94–102. [Google Scholar] [CrossRef]
- Gollsch, M.; Afflerbach, S.; Angadi, B.V.; Linder, M. Investigation of Calcium Hydroxide Powder for Thermochemical Storage Modified with Nanostructured Flow Agents. Sol. Energy 2020, 201, 810–818. [Google Scholar] [CrossRef]
- Valverde, J.M.; Barea-López, M.; Perejón, A.; Sánchez-Jiménez, P.E.; Pérez-Maqueda, L.A. Effect of Thermal Pretreatment and Nanosilica Addition on Limestone Performance at Calcium-Looping Conditions for Thermochemical Energy Storage of Concentrated Solar Power. Energy Fuels 2017, 31, 4226–4236. [Google Scholar] [CrossRef]
- Khosa, A.A.; Zhao, C.Y. Heat Storage and Release Performance Analysis of CaCO3/CaO Thermal Energy Storage System after Doping Nano Silica. Sol. Energy 2019, 188, 619–630. [Google Scholar] [CrossRef]
- Tian, S.; Jiang, J.; Yan, F.; Li, K.; Chen, X. Synthesis of Highly Efficient CaO-Based, Self-Stabilizing CO2 Sorbents via Structure-Reforming of Steel Slag. Environ. Sci. Technol. 2015, 49, 7464–7472. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Jiang, J.; Yan, F.; Li, K.; Chen, X.; Manovic, V. Highly Efficient CO2 Capture with Simultaneous Iron and CaO Recycling for the Iron and Steel Industry. Green Chem. 2016, 18, 4022–4031. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Wei, J. Mn and Mg Synergistically Stabilized CaO as an Effective Thermochemical Material for Solar Energy Storage. Sol. Energy Mater. Sol. Cells 2023, 252, 112202. [Google Scholar] [CrossRef]
- Yu, C.T.; Kuo, H.T.; Chen, Y.M. Carbon Dioxide Removal Using Calcium Aluminate Carbonates on Titanic Oxide under Warm-Gas Conditions. Appl. Energy 2016, 162, 1122–1130. [Google Scholar] [CrossRef]
- Valverde, J.M.; Perejon, A.; Perez-Maqueda, L.A. Enhancement of Fast CO2 Capture by a Nano-SiO2/CaO Composite at Ca-Looping Conditions. Environ. Sci. Technol. 2012, 46, 6401–6408. [Google Scholar] [CrossRef]
- Khosa, A.A.; Shah, N.H.; Xinyue, H.A.N.; Husnain, N. Silica Dopant Effect on the Performance of Calcium Carbonate/Calcium Oxide Based Thermal Energy Storage System. Therm. Sci. 2024, 28, 837–850. [Google Scholar] [CrossRef]
- Han, R.; Gao, J.; Wei, S.; Su, Y.; Sun, F.; Zhao, G.; Qin, Y. Strongly Coupled Calcium Carbonate/Antioxidative Graphite Nanosheets Composites with High Cycling Stability for Thermochemical Energy Storage. Appl. Energy 2018, 231, 412–422. [Google Scholar] [CrossRef]
- Benitez-Guerrero, M.; Valverde, J.M.; Sanchez-Jimenez, P.E.; Perejon, A.; Perez-Maqueda, L.A. Calcium-Looping Performance of Mechanically Modified Al2O3-CaO Composites for Energy Storage and CO2 Capture. Chem. Eng. J. 2018, 334, 2343–2355. [Google Scholar] [CrossRef]
- Han, R.; Gao, J.; Wei, S.; Su, Y.; Su, C.; Li, J.; Liu, Q.; Qin, Y. High-Performance CaO-Based Composites Synthesized Using a Space-Confined Chemical Vapor Deposition Strategy for Thermochemical Energy Storage. Sol. Energy Mater. Sol. Cells 2020, 206, 110346. [Google Scholar] [CrossRef]
- Sánchez Jiménez, P.E.; Perejón, A.; Benítez Guerrero, M.; Valverde, J.M.; Ortiz, C.; Pérez Maqueda, L.A. High-Performance and Low-Cost Macroporous Calcium Oxide Based Materials for Thermochemical Energy Storage in Concentrated Solar Power Plants. Appl. Energy 2019, 235, 543–552. [Google Scholar] [CrossRef]
- Li, H.; Lin, J.; Wu, J.; Wang, J.; Wang, P.; Kang, G.; Huang, S.; Fu, M.; Wei, J.; Ding, Z.; et al. 3D Ordered Macroporous Mn, Zr-Doped CaCO3 Nanomaterials for Stable Thermochemical Energy Storage. Adv. Sci. 2024, 12, 2412082. [Google Scholar] [CrossRef]
- Bian, Z.; Li, Y.; Zhang, C.; Zhao, J.; Wang, Z.; Liu, W. CaO/Ca(OH)2 Heat Storage Performance of Hollow Nanostructured CaO-Based Material from Ca-Looping Cycles for CO2 Capture. Fuel Process. Technol. 2021, 217, 106834. [Google Scholar] [CrossRef]
- Portilla-Nieto, Y.; Vidal, K.; Hernaiz, M.; Aranzabe, E.; Doppiu, S.; Palomo del Barrio, E. Development and Stabilization of Co2.4Ni0.6O4 Material for Long-Term Thermochemical Energy Storage. J. Energy Storage 2022, 52, 104876. [Google Scholar] [CrossRef]
- Bielsa, D.; Zaki, A.; Arias, P.L.; Faik, A. Improving the Redox Performance of Mn2O3/Mn3O4 Pair by Si Doping to Be Used as Thermochemical Energy Storage for Concentrated Solar Power Plants. Sol. Energy 2020, 204, 144–154. [Google Scholar] [CrossRef]
- Zhou, J.; Xiang, D.; Zhu, P.; Deng, J.; Gu, C.; Xu, H.; Zhou, J.; Xiao, G. ZrO2-Doped Copper Oxide Long-Life Redox Material for Thermochemical Energy Storage. ACS Sustain. Chem. Eng. 2023, 11, 47–57. [Google Scholar] [CrossRef]




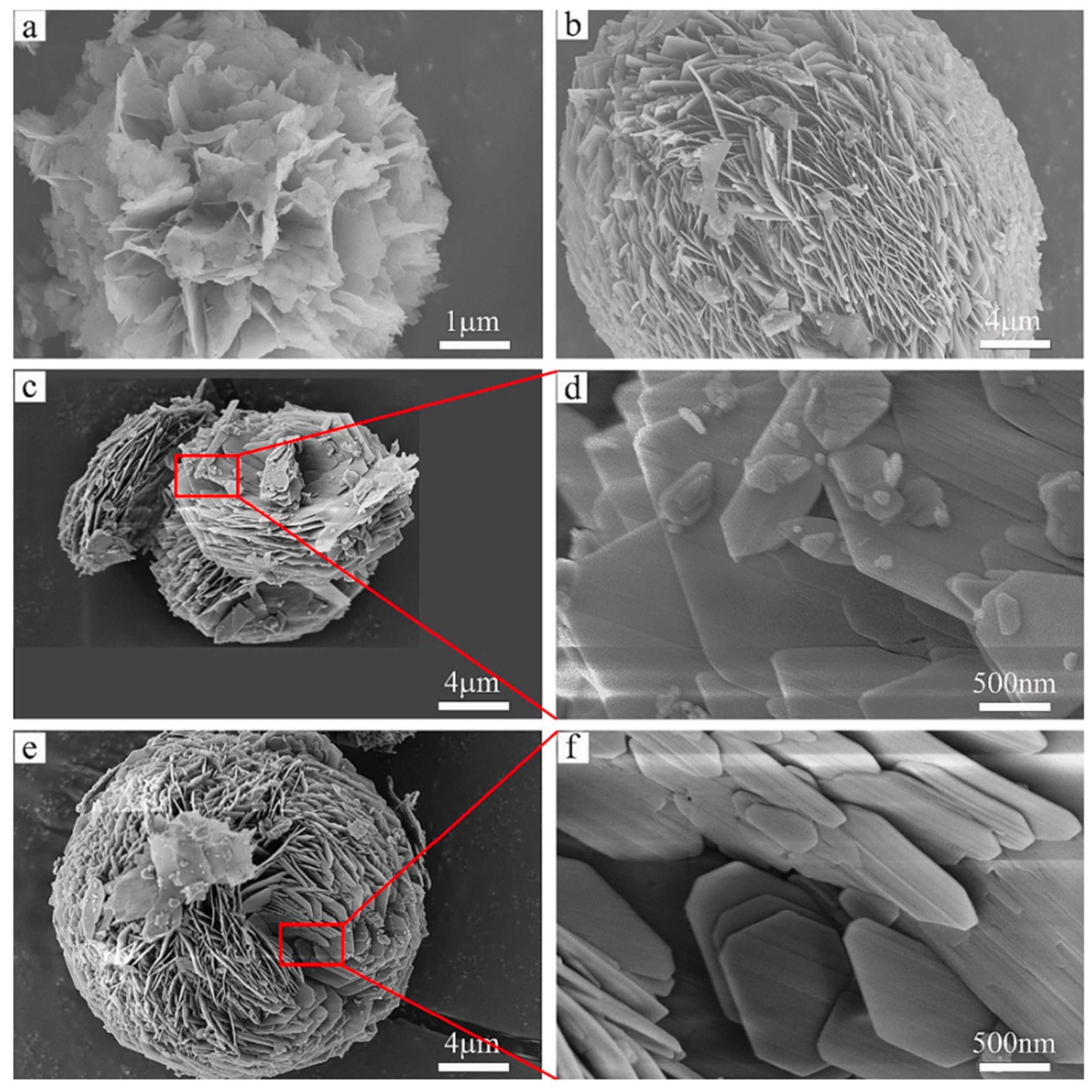

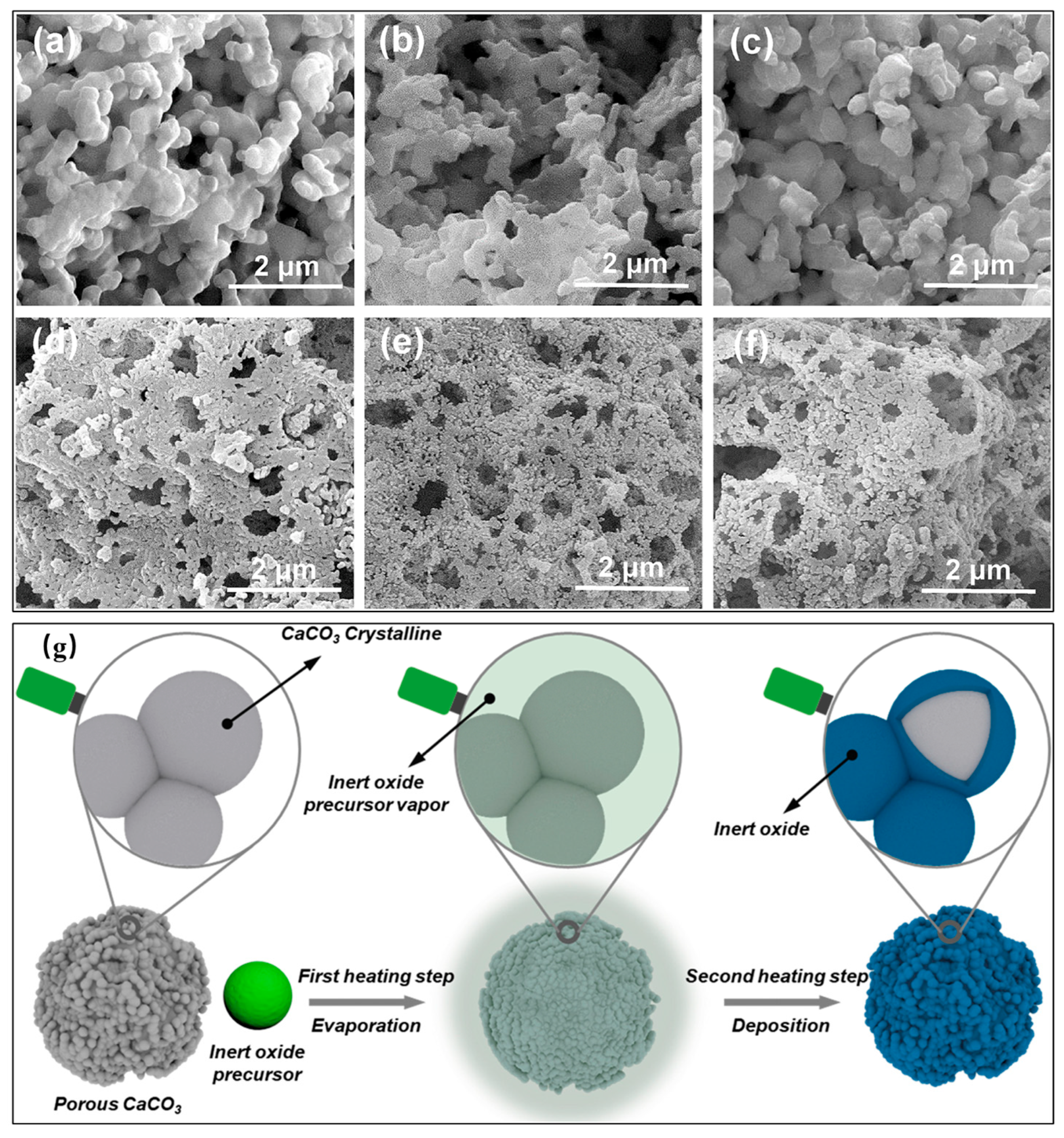
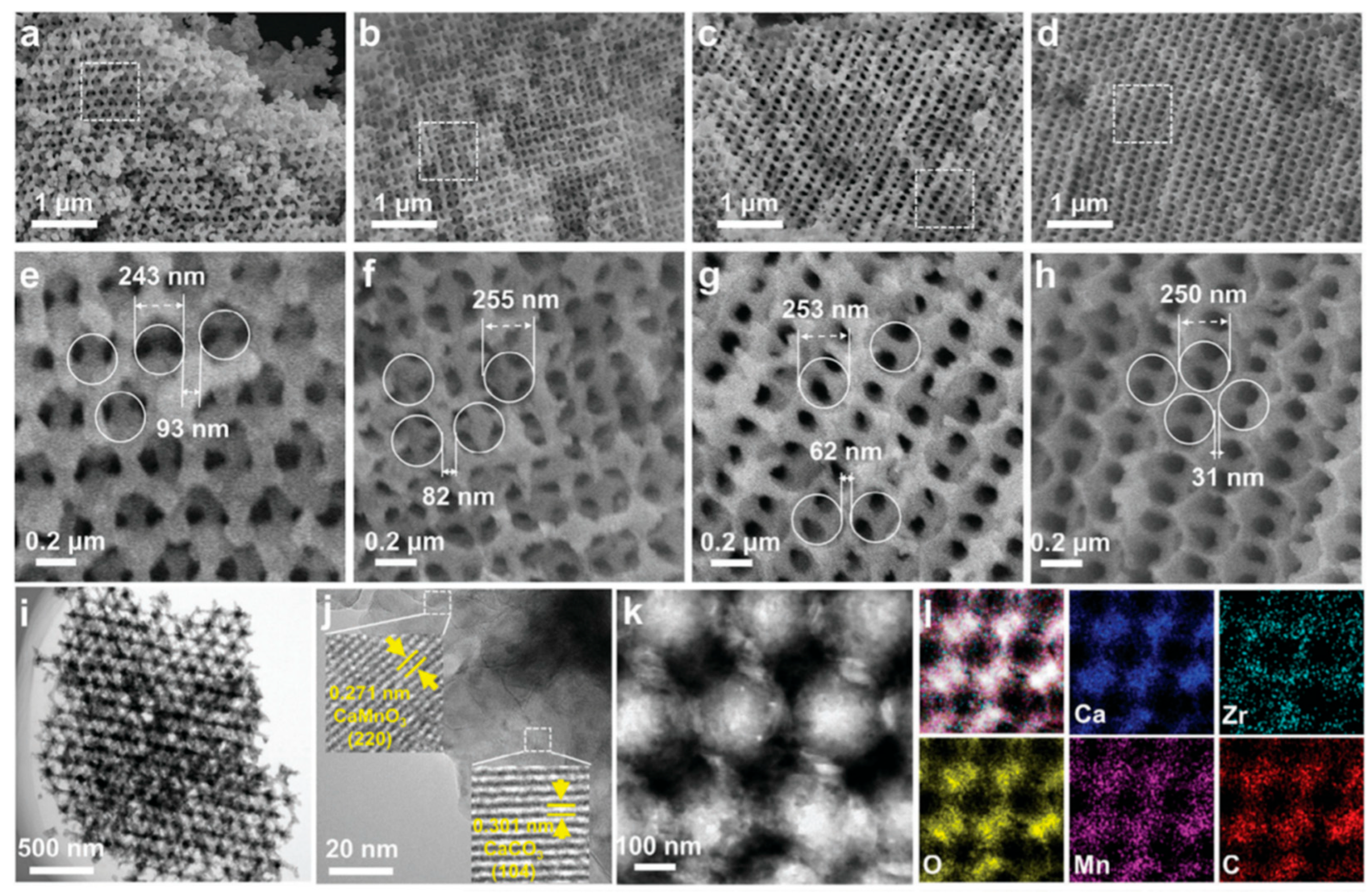

| Category | Solid Physical Adsorbents | Solid Chemical Adsorbents | Liquid Absorption |
|---|---|---|---|
| Mechanism | Physical interaction between water vapour and porous solids | Reversible chemical reactions forming salt hydrates | Concentration changes in liquid solutions |
| Materials | zeolites, silica gels, and activated carbon, MOF | CaCl2, MgSO4, MgCl2, MgSO4, SrBr2 | LiCl solution, LiBr solution |
| Energy Density (kwh/m3) | 50–220 | 800–1200 | 250–300 |
| charging temperature (°C) | 50–150 | 30–200 | 15–100 |
| Advantages |
|
|
|
| Challenges |
|
|
|
| Sorbent | DHR at 25 °C | Charging Temperature (°C) | Heat of Reaction (kwh/m3) | Price (EUR/MJ) |
|---|---|---|---|---|
| CaCl2·6H2O | 28–29% | 45~138 | 120~381 | 0.1 |
| LiCl·H2O | 11% | 66~87 | 253~400 | 9.11 |
| LiBr·2H2O | 7% | 40~90 | 252~313 | 55 |
| SrBr2·6H2O | 60% (30 °C) | 80 | 60~321 | 4.13 |
| CaSO4·2H2O | / | -- | 390 | / |
| MgCl2·6H2O | 30–35% | 130~150 | 556~695 | 0.09 |
| MgSO4·7H2O | 87–89% | 122~150 | 400~924 | 0.07 |
| Na2S·5H2O | 34% | 80~95 | 780 | / |
| Materials | Nano-Additives | Preparation Methods | Key Performance Metrics | Major Challenges | Scalability |
|---|---|---|---|---|---|
| Sorption-based system | Carbon additives (CNTs, graphene, activated carbon, Carbon nanospheres, EG) |
|
|
| Medium
|
| Hydroxide system | EG, nano-porous, CNTs, SiO2, Al2O3 |
|
|
| Medium to high
|
| Carbonate system | SiO2, Al2O3, graphite, TiO2, MgO, Mn and Zr doping |
|
|
| Low to Medium
|
| Redox system | SiO2, ZrO2 |
|
|
| Low
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Li, W.; Peng, B.; Huang, S.; Zhang, X. Recent Advances in Nano-Engineered Thermochemical Energy Storage Materials: Morphologies, Characteristics, and Performance. Nanomaterials 2025, 15, 1476. https://doi.org/10.3390/nano15191476
Jiang Z, Li W, Peng B, Huang S, Zhang X. Recent Advances in Nano-Engineered Thermochemical Energy Storage Materials: Morphologies, Characteristics, and Performance. Nanomaterials. 2025; 15(19):1476. https://doi.org/10.3390/nano15191476
Chicago/Turabian StyleJiang, Zhu, Wenye Li, Bohao Peng, Shifang Huang, and Xiaosong Zhang. 2025. "Recent Advances in Nano-Engineered Thermochemical Energy Storage Materials: Morphologies, Characteristics, and Performance" Nanomaterials 15, no. 19: 1476. https://doi.org/10.3390/nano15191476
APA StyleJiang, Z., Li, W., Peng, B., Huang, S., & Zhang, X. (2025). Recent Advances in Nano-Engineered Thermochemical Energy Storage Materials: Morphologies, Characteristics, and Performance. Nanomaterials, 15(19), 1476. https://doi.org/10.3390/nano15191476







