Strategic Design of Ethanol Oxidation Catalysts: From Active Metal Selection to Mechanistic Insights and Performance Engineering
Abstract
1. Introduction
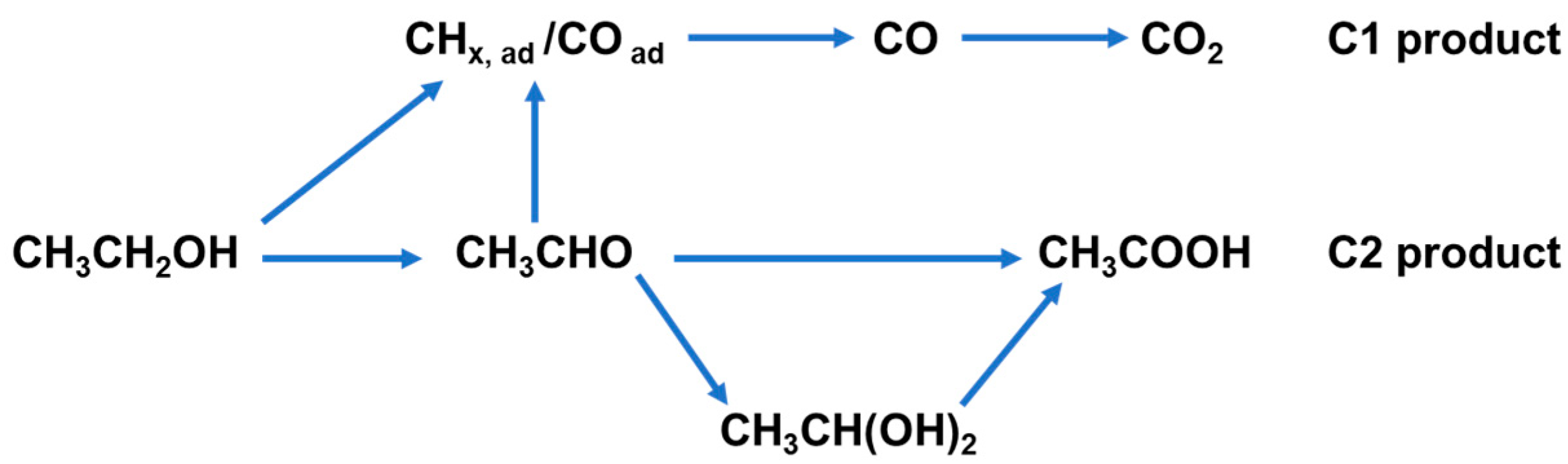
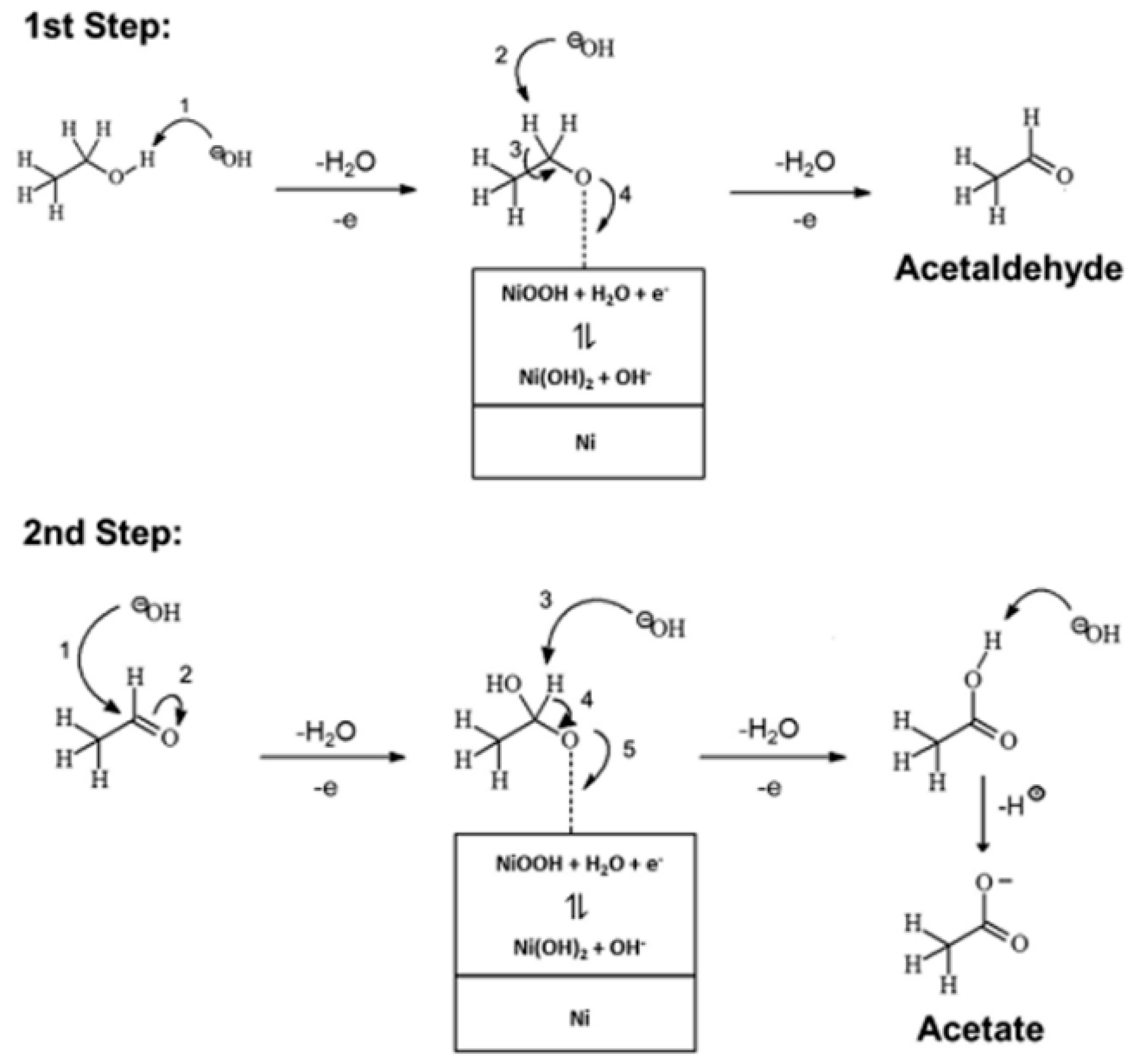
2. Reaction Mechanism for EOR
3. Characterization Techniques and Evaluation Method of EOR Catalysts
3.1. Physicochemical Characterization
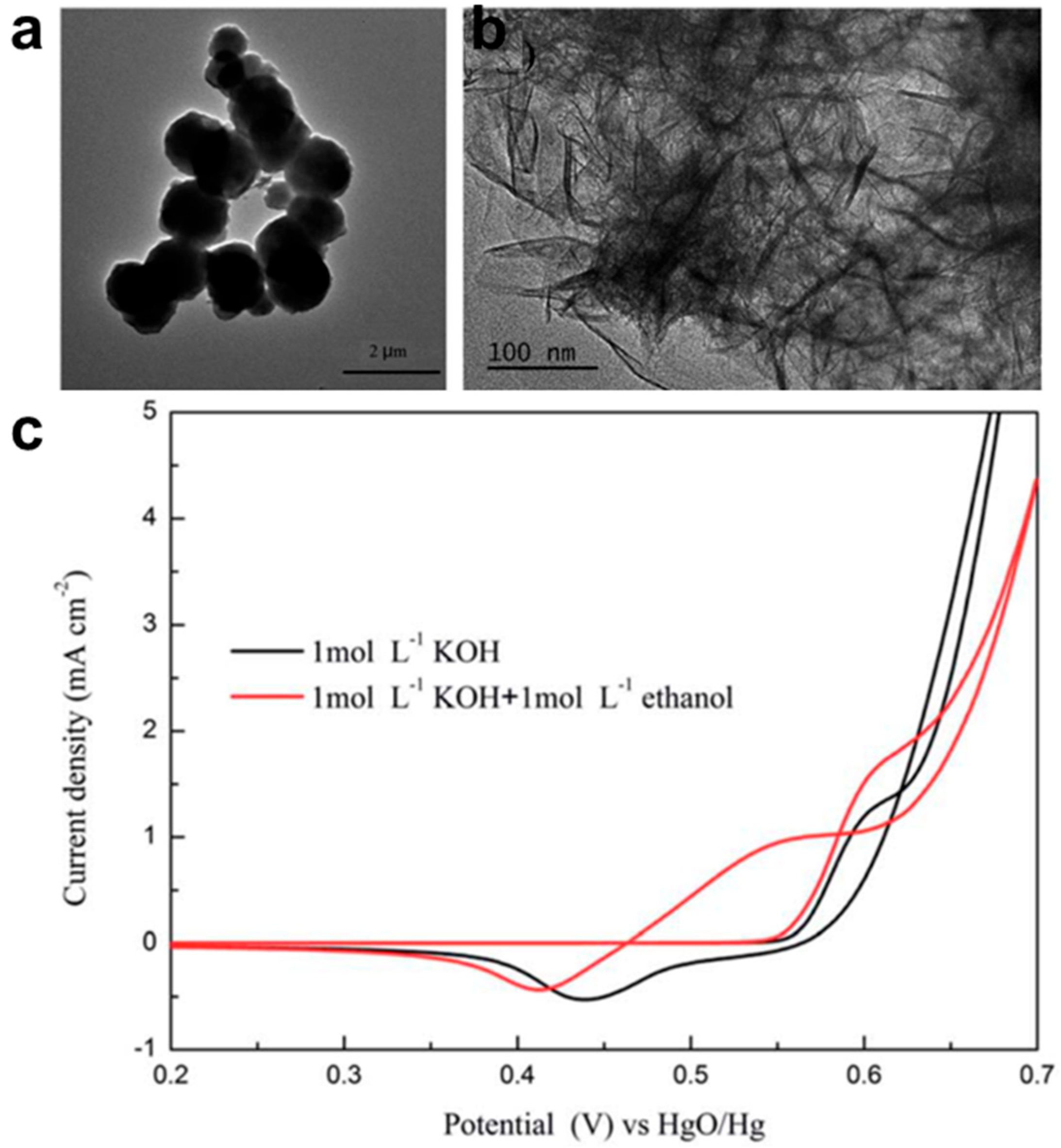
3.1.1. Structural and Morphological Analysis
3.1.2. Surface Composition and Electronic States
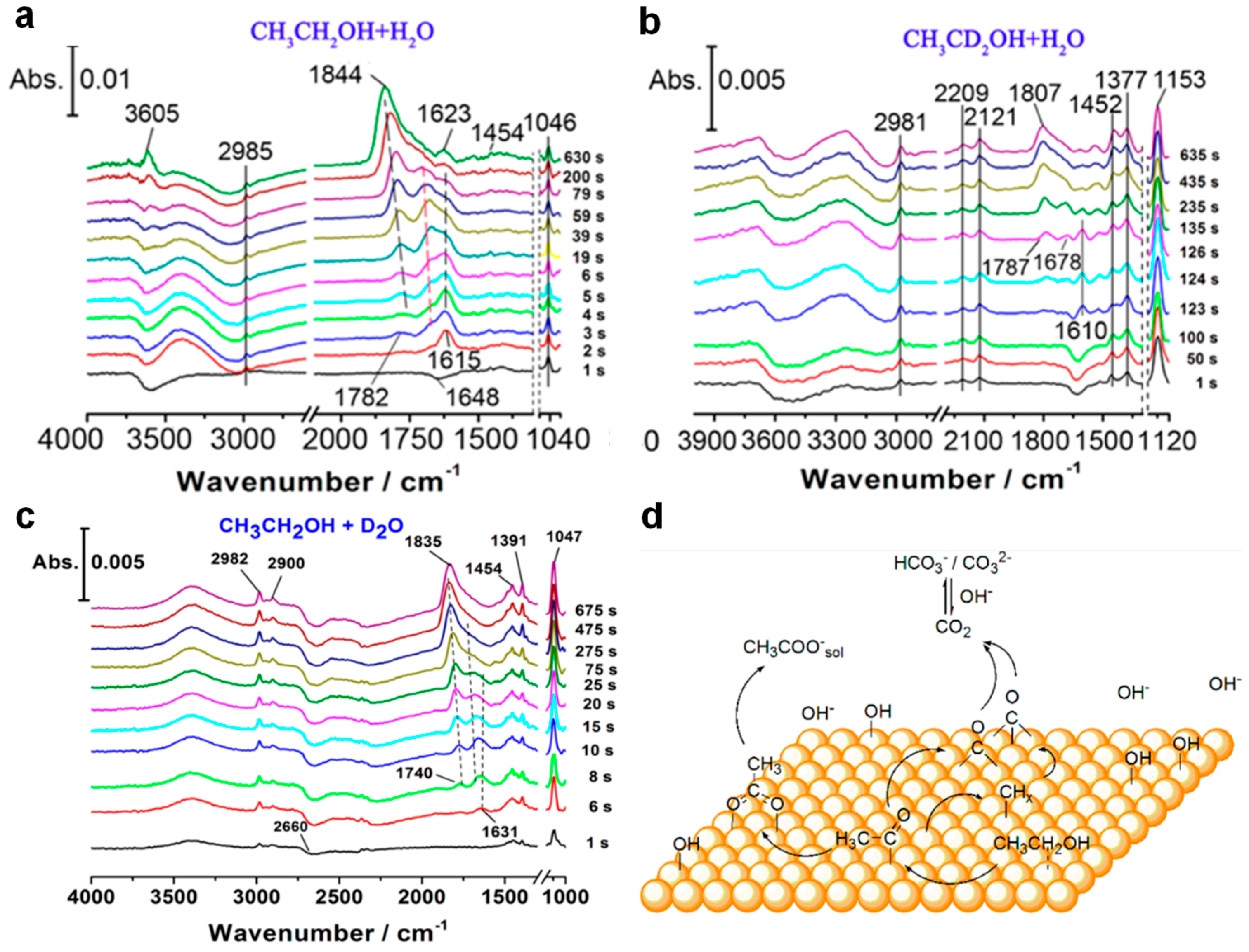
3.1.3. In Situ/Operando Characterization
3.2. Electrochemical Performance Evaluation of EOR Catalysts
3.2.1. Activity
3.2.2. Selectivity
3.2.3. Stability
3.2.4. MEA Testing in DEFCs
4. Electrocatalysts for EOR
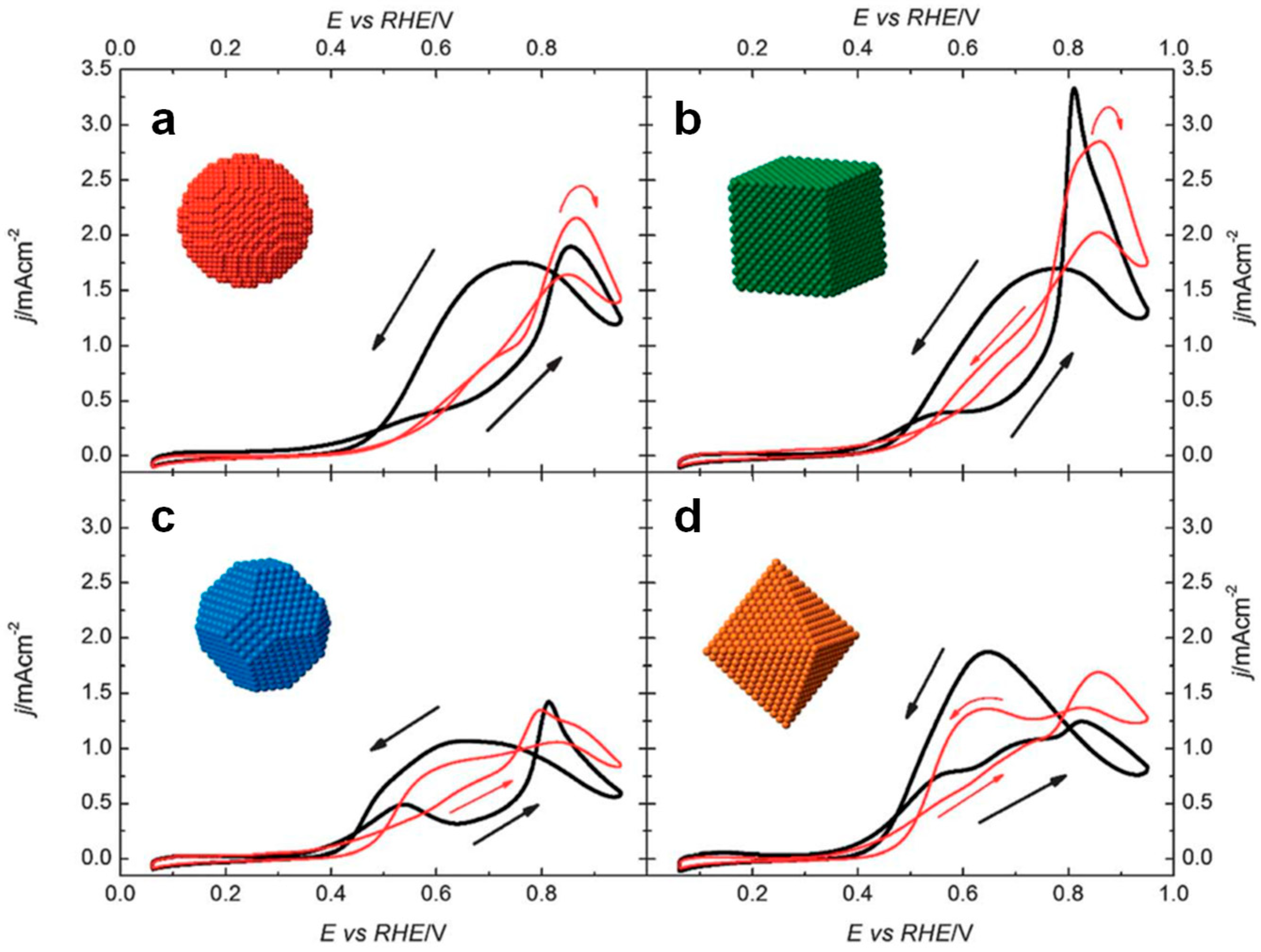
4.1. Pt-Based Catalysts
4.2. Pd-Based Catalysts
4.3. Rh-Based Catalysts
4.4. Au-Based Catalysts
4.5. Non-Noble Metal Catalysts
4.6. Comparative Mechanistic Insights in EOR Catalysts
5. Recent Advances in Electrocatalyst Design for EOR
6. Outlook and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Perry, M.L.; Fuller, T.F. A historical perspective of fuel cell technology in the 20th century. J. Electroanal. Chem. 2002, 149, S59–S67. [Google Scholar] [CrossRef]
- Joon, K. Fuel cells—A 21st century power system. J. Power Sources 1998, 71, 12–18. [Google Scholar] [CrossRef]
- Nicoletti, G.; Arcuri, N.; Nicoletti, G.; Bruno, R. A technical and environmental comparison between hydrogen and some fossil fuels. Energy Convers. Manag. 2015, 89, 205–213. [Google Scholar] [CrossRef]
- Abruña, H.D. Energy in the age of sustainability. ACS Publ. 2013, 90, 1411–1413. [Google Scholar] [CrossRef]
- Goldemberg, J. Ethanol for a sustainable energy future. Science 2007, 315, 808–810. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Kamarudin, M.Z.F.; Kamarudin, S.K.; Masdar, M.S.; Daud, W.R.W. Direct ethanol fuel cells. Int. J. Hydrogen Energy 2013, 38, 9438–9453. [Google Scholar] [CrossRef]
- Antolini, E.; Gonzalez, E.R. Alkaline direct alcohol fuel cells. J. Power Sources 2010, 195, 3431–3450. [Google Scholar] [CrossRef]
- Gottesfeld, S.; Dekel, D.R.; Page, M.; Bae, C.; Yan, Y.; Zelenay, P.; Kim, Y.S. Anion exchange membrane fuel cells: Current status and remaining challenges. J. Power Sources 2018, 375, 170–184. [Google Scholar] [CrossRef]
- Kim, I.; Han, O.H.; Chae, S.A.; Paik, Y.; Kwon, S.H.; Lee, K.S.; Sung, Y.E.; Kim, H. Catalytic reactions in direct ethanol fuel cells. Angew. Chem. 2011, 50, 2270–2274. [Google Scholar] [CrossRef]
- Camara, G.A.; Iwasita, T. Parallel pathways of ethanol oxidation: The effect of ethanol concentration. J. Electroanal. Chem. 2005, 578, 315–321. [Google Scholar] [CrossRef]
- Akhairi, M.A.F.; Kamarudin, S.K. Catalysts in direct ethanol fuel cell (DEFC): An overview. Int. J. Hydrogen Energy 2016, 41, 4214–4228. [Google Scholar] [CrossRef]
- Antolini, E. Catalysts for direct ethanol fuel cells. J. Power Sources 2007, 170, 1–12. [Google Scholar] [CrossRef]
- Badwal, S.P.S.; Giddey, S.; Kulkarni, A.; Goel, J.; Basu, S. Direct ethanol fuel cells for transport and stationary applications—A comprehensive review. Appl. Energy 2015, 145, 80–103. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, G.; Jiang, L.; Xin, Q.; Sun, S.G.; Jiang, Y.X.; Chen, S.P.; Jusys, Z.; Behm, R.J. Adsorption and oxidation of ethanol on colloid-based Pt/C, PtRu/C and Pt3Sn/C catalysts: In situ FTIR spectroscopy and on-line DEMS studies. Phys. Chem. Chem. Phys. 2007, 9, 2686–2696. [Google Scholar] [CrossRef]
- Wang, H.; Jusys, Z.; Behm, R. Ethanol electro-oxidation on carbon-supported Pt, PtRu and Pt3Sn catalysts: A quantitative DEMS study. J. Power Sources 2006, 154, 351–359. [Google Scholar] [CrossRef]
- Sheng, T.; Qiu, C.; Lin, X.; Lin, W.F.; Sun, S.G. Insights into ethanol electro-oxidation over solvated Pt (100): Origin of selectivity and kinetics revealed by DFT. Appl. Surf. Sci. 2020, 533, 147505. [Google Scholar] [CrossRef]
- Monyoncho, E.A.; Steinmann, S.N.; Michel, C.; Baranova, E.A.; Woo, T.K.; Sautet, P. Ethanol electro-oxidation on palladium revisited using polarization modulation infrared reflection absorption spectroscopy (PM-IRRAS) and density functional theory (DFT): Why is it difficult to break the C–C bond? ACS Catal. 2016, 6, 4894–4906. [Google Scholar] [CrossRef]
- Shao, M.H.; Adzic, R.R. Electrooxidation of ethanol on a Pt electrode in acid solutions: In situ ATR-SEIRAS study. Electrochim. Acta 2005, 50, 2415–2422. [Google Scholar] [CrossRef]
- Christensen, P.A.; Jones, S.W.M.; Hamnett, A. In situ FTIR studies of ethanol oxidation at polycrystalline Pt in alkaline solution. J. Phys. Chem. C 2016, 116, 24681–24689. [Google Scholar] [CrossRef]
- Zhang, B.W.; Yang, H.L.; Wang, Y.X.; Dou, S.X.; Liu, H.K. A comprehensive review on controlling surface composition of Pt-based bimetallic electrocatalysts. Adv. Energy Mater. 2018, 8, 1703597. [Google Scholar] [CrossRef]
- Wang, H.F.; Liu, Z.P. Comprehensive mechanism and structure-sensitivity of ethanol oxidation on platinum: New transition-state searching method for resolving the complex reaction network. J. Am. Chem. Soc. 2008, 130, 10996. [Google Scholar] [PubMed]
- Busó-Rogero, C.; Grozovski, V.; Vidal-Iglesias, F.J.; Solla-Gullón, J.; Herrero, E.; Feliu, J.M. Surface structure and anion effects in the oxidation of ethanol on platinum nanoparticles. J. Mater. Chem. A 2013, 1, 7068–7076. [Google Scholar] [CrossRef]
- Bai, J.; Xiao, X.; Xue, Y.Y.; Jiang, J.X.; Zeng, J.H.; Li, X.F.; Chen, Y. Bimetallic platinum–rhodium alloy nanodendrites as highly active electrocatalyst for the ethanol oxidation reaction. ACS Appl. Mater. Interfaces 2018, 10, 19755–19763. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, Y.; Ren, F.; Song, T.; Du, Y. Tiny Ir doping of sub-one-nanometer PtMn nanowires: Highly active and stable catalysts for alcohol electrooxidation. Nanoscale 2020, 12, 12098–12105. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, M.; Li, Y.; Ye, C.; Chen, J.; Ye, J.; Zhang, Q.; Li, J.; Zhou, Z.; Fu, X.Z. p–d orbital hybridization induced by a monodispersed Ga site on a Pt3Mn nanocatalyst boosts ethanol electrooxidation. Angew. Chem. 2022, 134, e202115735. [Google Scholar] [CrossRef]
- Zhao, F.; Yuan, Q.; Luo, B.; Li, C.; Yang, F.; Yang, X.; Zhou, Z. Surface composition-tunable octahedral PtCu nanoalloys advance the electrocatalytic performance on methanol and ethanol oxidation. Sci. China Mater. 2019, 62, 1877–1887. [Google Scholar] [CrossRef]
- Dessal, C.; Len, T.; Morfin, F.; Rousset, J.L.; Aouine, M.; Afanasiev, P.; Piccolo, L. Dynamics of single Pt atoms on alumina during CO oxidation monitored by operando X-ray and infrared spectroscopies. ACS Catal. 2019, 9, 5752–5759. [Google Scholar] [CrossRef]
- Nie, Y.; Qi, X.; Wu, R.; Yang, R.; Wang, H.; Deng, M.; Zhang, S.; Lu, S.; Gu, Z.; Liu, X. Structurally ordered PtFe intermetallic nanocatalysts toward efficient electrocatalysis of methanol oxidation. Appl. Surf. Sci. 2021, 569, 151004. [Google Scholar] [CrossRef]
- Xia, B.Y.; Wu, H.B.; Wang, X.; Lou, X.W. One-pot synthesis of cubic PtCu3 nanocages with enhanced electrocatalytic activity for the methanol oxidation reaction. J. Am. Chem. Soc. 2012, 134, 13934–13937. [Google Scholar] [CrossRef]
- Saleem, F.; Zhang, Z.; Xu, B.; Xu, X.; He, P.; Wang, X. Ultrathin Pt-Cu nanosheets and nanocones. J. Am. Chem. Soc. 2013, 135, 18304–18307. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, T.; Bukhvalov, D.; Han, F.; Wang, C.; Yang, X. Metal-support interaction promoted multifunctional electroca talysis on PtCo/NC with ultralow Pt loading for oxygen reduction reaction and zinc-air battery. Appl. Catal. B Environ. 2023, 337, 122976. [Google Scholar] [CrossRef]
- Mondal, A.; De, A.; Datta, J. Selective methodology for developing PtCo NPs and performance screening for energy efficient electro-catalysis in direct ethanol fuel cell. Int. J. Hydrogen Energy 2019, 44, 10996–11011. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Liu, G.; Ling, C.; Chen, B.; Huang, J.; Liu, X.; Li, B.; Wang, A.L.; Hu, Z.; et al. Crystal phase-controlled growth of PtCu and PtCo alloys on 4H Au nanoribbons for electrocatalytic ethanol oxidation reaction. Nano Res. 2020, 13, 1970–1975. [Google Scholar] [CrossRef]
- Matin, M.A.; Kumar, A.; Saad, M.A.H.S.; Al-Marri, M.J.; Suslov, S. Zn-enriched PtZn nanoparticle electrocatalysts synthesized by solution combustion for ethanol oxidation reaction in an alkaline medium. MRS Commun. 2018, 8, 411–419. [Google Scholar] [CrossRef]
- Zhang, B.W.; Lai, W.H.; Sheng, T.; Qu, X.M.; Wang, Y.X.; Ren, L.; Zhang, L.; Du, Y.; Jiang, Y.X.; Sun, S.G.; et al. Ordered platinum–bismuth intermetallic clusters with Pt-skin for a highly efficient electrochemical ethanol oxidation reaction. J. Mater. Chem. A 2019, 7, 5214–5220. [Google Scholar] [CrossRef]
- Han, S.; Ma, Y.; Yun, Q.; Wang, A.L.; Zhu, Q.; Zhang, H.; He, C.; Xia, J.; Meng, X.; Gao, L.; et al. The synergy of tensile strain and ligand effect in PtBi nanorings for boosting electrocatalytic alcohol oxidation. Adv. Funct. Mater. 2022, 32, 2208760. [Google Scholar] [CrossRef]
- Peng, H.; Ren, J.; Wang, Y.; Xiong, Y.; Wang, Q.; Li, Q.; Zhao, X.; Zhan, L.; Zheng, L.; Tang, Y.; et al. One-stone, two birds: Alloying effect and surface defects induced by Pt on Cu2−xSe nanowires to boost C-C bond cleavage for electrocatalytic ethanol oxidation. Nano Energy 2021, 88, 106307. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.; Zhang, Y.; Chen, X.; Ye, J.; Chen, J.; Lyu, Z.; Chen, X.; Kuang, Q.; Xie, S.; et al. Edge enrichment of ultrathin 2d PdPtCu trimetallic nanostructures effectuates top-ranked ethanol electrooxidation. Nano Lett. 2020, 20, 5458–5464. [Google Scholar] [CrossRef]
- Queiroz, M.A.R.; Ribeiro, J. Catalysts of PtSn/C modified with Ru and Ta for electrooxidation of ethanol. Catalysts 2019, 9, 277. [Google Scholar] [CrossRef]
- Chen, J.Y.; Lim, S.C.; Kuo, C.H.; Tuan, H.Y. Sub-1 nm PtSn ultrathin sheet as an extraordinary electrocatalyst for methanol and ethanol oxidation reactions. J. Colloid Interface Sci. 2019, 545, 54–62. [Google Scholar] [CrossRef]
- Xiao, S.; Wang, L.; Chen, L.; Li, Y.; Shen, K. Tandem upgrading of bio-furans to benzene, toluene, and p-xylene by Pt1Sn1 intermetallic coupling ordered mesoporous SnO2 catalyst. Adv. Mater. 2025, 37, 2415295. [Google Scholar] [CrossRef]
- Pushkarev, A.S.; Pushkareva, I.V.; Ivanova, N.A.; Preez, S.P.D.; Bessarabov, D.; Chumakov, R.G.; Stankevich, V.G.; Fateev, V.N.; Evdokimov, A.A.; Grigoriev, S.A. Pt/C and Pt/SnOx/C catalysts for ethanol electrooxidation: Rotating disk electrode study. Catalysts 2019, 9, 271. [Google Scholar] [CrossRef]
- Tran, L.T.; Nguyen, Q.M.; Nguyen, M.D.; Le, H.N.T.; Nguyen, T.T.; Vu, T.H.T. Preparation and electrocatalytic characteristics of the Pt-based anode catalysts for ethanol oxidation in acid and alkaline media. Int. J. Hydrogen Energy 2018, 43, 20563–20572. [Google Scholar] [CrossRef]
- Fang, X.; Wang, L.; Shen, P.K.; Cui, G.; Bianchini, C. An in situ Fourier transform infrared spectroelectrochemical study on ethanol electrooxidation on Pd in alkaline solution. J. Power Sources 2010, 195, 1375–1378. [Google Scholar] [CrossRef]
- Liang, Z.X.; Zhao, T.S.; Xu, J.B.; Zhu, L.D. Mechanism study of the ethanol oxidation reaction on palladium in alkaline media. Electrochim. Acta 2009, 54, 2203–2208. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Wang, Q.; Lin, J.L.; Tian, N.; Sun, S.G. In situ FTIR spectroscopic studies of electrooxidation of ethanol on Pd electrode in alkaline media. Electrochim. Acta 2010, 55, 7995–7999. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, S.; Cai, W.B. Recent advances on electro-oxidation of ethanol on Pt- and Pd-based catalysts: From reaction mechanisms to catalytic materials. Catalysts 2015, 5, 1507–1534. [Google Scholar] [CrossRef]
- Ma, L.; Chu, D.; Chen, R. Comparison of ethanol electro-oxidation on Pt/C and Pd/C catalysts in alkaline media. Int. J. Hydrogen Energy 2012, 37, 11185–11194. [Google Scholar] [CrossRef]
- Antolini, E. Palladium in fuel cell catalysis. Energy Environ. Sci. 2009, 2, 915–931. [Google Scholar] [CrossRef]
- Wang, H.; Jiao, S.; Liu, S.; Zhang, H.; Xu, Y.; Li, X.; Wang, Z.; Wang, L. PdNi/Ni nanotubes assembled by mesoporous nanoparticles for efficient alkaline ethanol oxidation reaction. Chem. Eur. J. 2021, 17, 14472–14477. [Google Scholar] [CrossRef]
- Torrero, J.; Montiel, M.; Pea, M.A.; Ocón, P.; Rojas, S. Insights on the electrooxidation of ethanol with Pd-based catalysts in alkaline electrolyte. Int. J. Hydrogen Energy 2019, 44, 31995–32002. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Ren, J.; Li, Q.X.; Zhou, Z.Y.; Sun, S.G.; Cai, W.B. Electrocatalysis of ethanol on a Pd electrode in alkaline media: An in situ attenuated total reflection surface-enhanced infrared absorption spectroscopy study. ACS Catal. 2014, 4, 798–803. [Google Scholar] [CrossRef]
- Bambagioni, V.; Bianchini, C.; Filippi, J.; Oberhauser, W.; Marchionni, A.; Vizza, F.; Psaro, R.; Sordelli, L.; Foresti, M.L.; Innocenti, M. Ethanol oxidation on electrocatalysts obtained by spontaneous deposition of palladium onto nickel-zinc materials. ChemSusChem 2009, 2, 99–112. [Google Scholar] [CrossRef]
- Mao, H.; Huang, T.; Yu, A.S. Facile synthesis of trimetallic Cu1Au0.15Pd1.5/C catalyst for ethanol oxidation with superior activity and stability. J. Mater. Chem. A 2014, 2, 16378–16380. [Google Scholar] [CrossRef]
- Kumar, K.S.; Haridoss, P.; Seshadri, S. Synthesis and characterization of electrodeposited Ni–Pd alloy electrodes for methanol oxidation. Surf. Coat. Technol. 2008, 202, 1764–1770. [Google Scholar] [CrossRef]
- Hosseini-Sarvari, M.; Khanivar, A.; Moeini, F. Magnetically recoverable nano Pd/Fe3O4/ZnO catalyst: Preparation, characterization, and application for the synthesis of 2-oxazolines and benzoxazoles. J. Mater. Sci. 2015, 50, 3065–3074. [Google Scholar] [CrossRef]
- Wang, A.L.; He, X.J.; Lu, X.F.; Xu, H.; Tong, Y.X.; Li, G.R. Palladium–cobalt nanotube arrays supported on carbon fiber cloth as high-performance flexible electrocatalysts for ethanol oxidation. Angew. Chem. 2015, 54, 3669–3673. [Google Scholar] [CrossRef]
- Begum, H.; Ahmed, M.S.; Lee, D.W.; Kim, Y.B. Carbon nanotubes-based PdM bimetallic catalysts through N4-system for efficient ethanol oxidation and hydrogen evolution reaction. Sci. Rep. 2019, 9, 11051. [Google Scholar] [CrossRef]
- Jiang, K.; Wang, P.; Guo, S.; Zhang, X.; Shen, X.; Lu, G.; Su, D.; Huang, X. Ordered PdCu-Based nanoparticles as bifunctional oxygen-reduction and ethanol-oxidation electrocatalysts. Angew. Chem. 2016, 128, 9176–9181. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, J.; Zhang, Y.; Liu, Y.; Han, X.; Zhong, C.; Hu, W.; Deng, Y. NiO-induced synthesis of PdNi bimetallic hollow nanocrystals with enhanced electrocatalytic activities toward ethanol and formic acid oxidation. Nano Energy 2017, 42, 353–362. [Google Scholar] [CrossRef]
- Chang, J.; Wang, G.; Wang, M.; Wang, Q.; Li, B.; Zhou, H.; Zhu, Y.; Zhang, W.; Omer, M.; Orlovskaya, N.; et al. Improving Pd–N–C fuel cell electrocatalysts through fluorination-driven rearrangements of local coordination environment. Nat. Energy 2021, 6, 1144–1153. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, J.; Zhang, L.; Wei, D.; Zhu, W.; Zhuang, Z. Amorphous palladium-based alloy nanoparticles as highly active electrocatalysts for ethanol oxidation. Chem. Commun. 2022, 58, 4488–4491. [Google Scholar] [CrossRef]
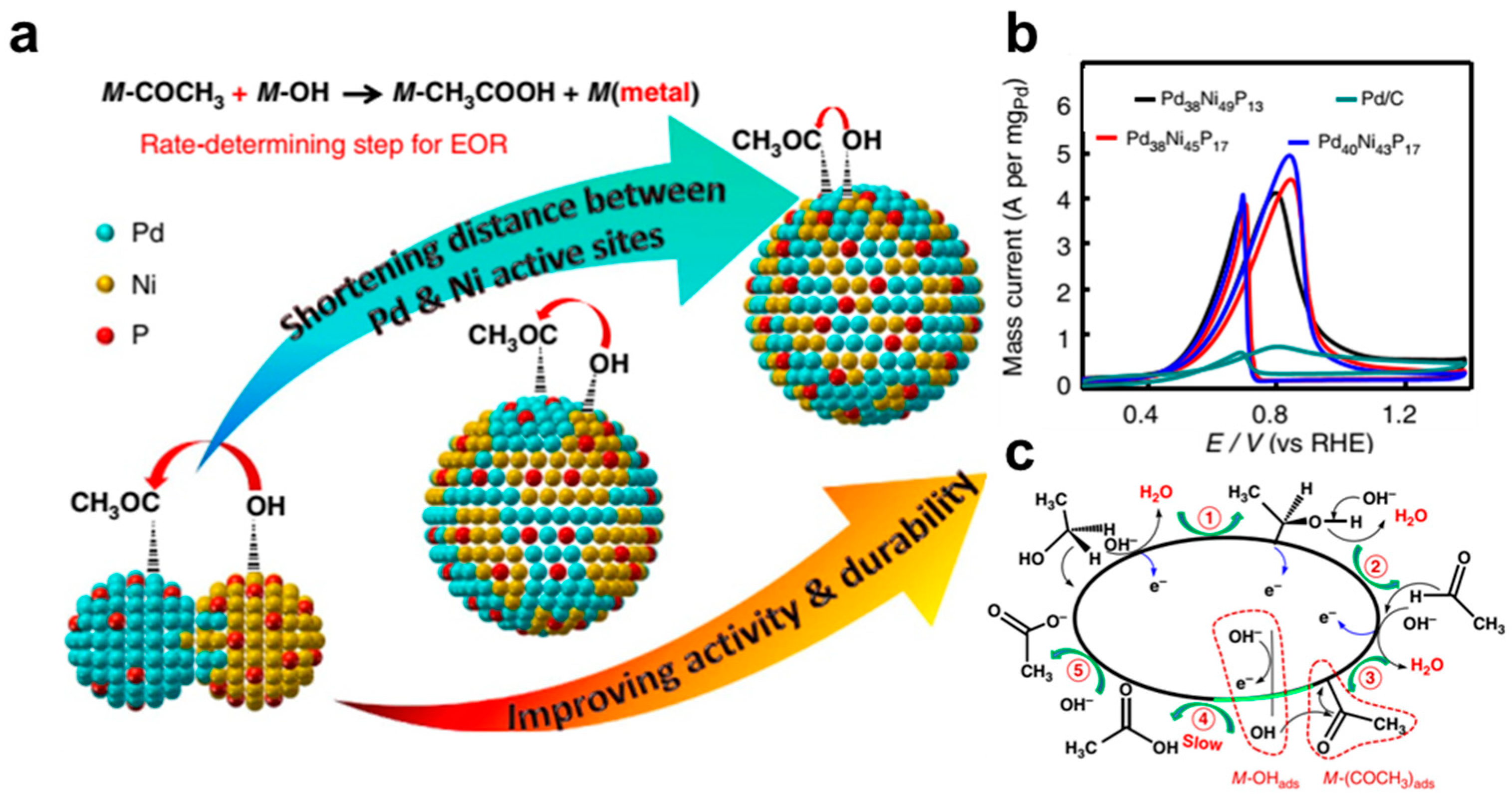
- Chen, L.; Lu, L.; Zhu, H.; Chen, Y.; Huang, Y.; Li, Y.; Wang, L. Improved ethanol electrooxidation performance by shortening Pd–Ni active site distance in Pd–Ni–P nanocatalysts. Nat. Commun. 2017, 8, 14136. [Google Scholar] [CrossRef]
- Moreira, T.F.M.; Kokoh, K.B.; Napporn, T.W.; Olivi, P.; Morais, C. Insights on the C2 and C3 electroconversion in alkaline medium on Rh/C catalyst: In situ FTIR spectroscopic and chromatographic studies. Electrochim. Acta 2022, 422, 140507. [Google Scholar] [CrossRef]
- Zhu, C.; Zhao, Z.G.; Yang, Y.Y. Real-time electrochemical ATR-SEIRAS investigation of CO adsorption and oxidation on Rh electrode in 0.1 M NaOH and 0.1 M H2SO4. J. Electroanal. Chem. 2019, 840, 462–467. [Google Scholar] [CrossRef]
- Delpeuch, A.B.; Chatenet, M.; Rau, M.S.; Cremers, C. Influence of H-and OH-adsorbates on the ethanol oxidation reaction–a DEMS study. Phys. Chem. Chem. Phys. 2015, 17, 10881–10893. [Google Scholar] [CrossRef]
- Mei, Y.; Che, F.; Deskins, N.A. Modeling interfacial electric fields and the ethanol oxidation reaction at electrode surfaces. Phys. Chem. Chem. Phys. 2024, 26, 27544–27560. [Google Scholar] [CrossRef]
- Zhu, C.; Lan, B.; Wei, R.L.; Wang, C.N.; Yang, Y.Y. Potential-dependent selectivity of ethanol complete oxidation on Rh electrode in alkaline media: A synergistic study of electrochemical ATR-SEIRAS and IRAS. ACS Catal. 2019, 9, 4046–4053. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, J.; Fan, Q.; Jiang, Y.; Zhu, Y.; Li, H.; Cao, Z.; Kuang, Q.; Cheng, J.; Zheng, J.; et al. Cyclic penta-twinned rhodium nanobranches as superior catalysts for ethanol electro-oxidation. J. Am. Chem. Soc. 2018, 140, 11232–11240. [Google Scholar] [CrossRef]
- Li, H.; Fan, Q.; Ye, J.; Cao, Z.; Ma, Z.; Jiang, Y.; Zhang, J.; Cheng, J.; Xie, Z.; Zheng, L. Excavated Rh nanobranches boost ethanol electro-oxidation. Mater. Today Energy 2019, 11, 120–127. [Google Scholar] [CrossRef]
- Zhai, Y.N.; Li, Y.; Zhu, J.Y.; Jiang, Y.C.; Li, S.N.; Chen, Y. The electrocatalytic performance of carbon ball supported RhCo alloy nanocrystals for the methanol oxidation reaction in alkaline media. J. Power Sources 2017, 371, 129–135. [Google Scholar] [CrossRef]
- Shen, T.; Chen, S.; Wang, S.; Huang, X.; Song, M.; Zhao, X.; Hu, J.; Wang, D. Optimizing dual-intermediates adsorption on Rh-based intermetallics for formic acid electrooxidation. Appl. Catal. B Environ. Energy 2023, 333, 122766. [Google Scholar]
- Huang, M.; Lan, B.; Jiang, X.L.; Yang, Y.Y.; Jia, W.S. Interfacial engineering Pb skin-layer at Rh surface to promote ethanol electrooxidation into CO2 in alkaline media. Int. J. Hydrogen Energy 2022, 47, 34932–34942. [Google Scholar] [CrossRef]
- Li, H.; Ye, J.; Li, X.; Zhang, J.; Zhu, Y.; Zhou, Z.; Xue, Y.; Jiang, Y.; Xie, Z.; Zheng, L. Excavated RhNi alloy nanobranches enable superior CO-tolerance and CO2 selectivity at low potentials toward ethanol electro-oxidation. J. Mater. Chem. A 2019, 7, 26266–26271. [Google Scholar] [CrossRef]
- Li, L.; Chu, M.; Song, R.; Liu, S.; Ren, G.; Xu, Y.; Wang, L.; Xu, Q.; Shao, Q.; Lu, J.; et al. CO spillover on ultrathin bimetallic Rh/Rh-M nanosheets. Chem. Catal. 2022, 2, 1709–1719. [Google Scholar] [CrossRef]
- Bai, S.; Xu, Y.; Cao, K.; Huang, X. Selective ethanol oxidation reaction at the Rh–SnO2 interface. Adv. Mater. 2020, 33, 2005767. [Google Scholar] [CrossRef]
- Lan, B.; Huang, M.; Wei, R.L.; Wang, C.N.; Wang, Q.L.; Yang, Y.Y. Ethanol electrooxidation on rhodium–lead catalysts in alkaline media: High mass activity, long-term durability, and considerable CO2 selectivity. Small 2020, 16, 2004380. [Google Scholar] [CrossRef]
- Lan, B.; Wang, Q.L.; Ma, Z.X.; Wu, Y.J.; Jiang, X.L.; Jia, W.S.; Zhou, C.X.; Yang, Y.Y. Efficient electrochemical ethanol-to-CO2 conversion at rhodium and bismuth hydroxide interfaces. Appl. Catal. B Environ. 2022, 300, 120728. [Google Scholar] [CrossRef]
- Zhu, F.; Tu, K.; Huang, L.; Qu, X.; Zhang, J.; Liao, H.; Zhou, Z.; Jiang, Y.; Sun, S. High selectivity PtRh/RGO catalysts for ethanol electro-oxidation at low potentials: Enhancing the efficiency of CO2 from alcoholic groups. Electrochim. Acta 2018, 292, 208–216. [Google Scholar]
- Erini, N.; Beermann, V.; Gocyla, M.; Gliech, M.; Heggen, M.; Dunin-Borkowski, R.E.; Strasser, P. The effect of surface site ensembles on the activity and selectivity of ethanol electrooxidation by octahedral PtNiRh nanoparticles. Angew. Chem. 2017, 129, 6633–6638. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Fu, G.; Chen, Y.; Sun, D.; Lee, J.M.; Tang, Y. Porous PdRh nanobowls: Facile synthesis and activity for alkaline ethanol oxidation. Nanoscale 2019, 11, 2974–2980. [Google Scholar] [CrossRef]
- Yahikozawa, K.; Nishimura, K.; Kumazawa, M.; Tateishi, N.; Takasu, Y. Electrocatalytic properties of ultrafine gold particles supported onto glassy carbon substrates toward formaldehyde oxidation in alkaline media. Chem. Inf. 1992, 37, 453–455. [Google Scholar] [CrossRef]
- Nishimura, K.; Kunimatsu, K.; Enyo, M. Electrocatalysis on Pd + Au alloy electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1989, 260, 167–179. [Google Scholar]
- Vyas, A.N.; Saratale, G.D.; Sartale, S.D. Recent developments in nickel based electrocatalysts for ethanol electrooxidation. Int. J. Hydrogen Energy 2020, 45, 5928–5947. [Google Scholar]
- Cao, X.; Li, C.; Peng, D.; Lu, Y.; Huang, K.; Wu, J.; Zhao, C.; Huang, Y. Highly strained Au nanoparticles for improved electrocatalysis of ethanol oxidation reaction. J. Phys. Chem. Lett. 2020, 11, 3005–3013. [Google Scholar] [CrossRef]
- Zhang, A.; Chen, Y.; Yang, Z.; Ma, S.; Huang, Y.; Richter, G.; Schu, P.; Zhong, C.; Wang, Z. Enhanced electrocatalytic activities toward the ethanol oxidation of nanoporous gold prepared via solid-phase reaction. ACS Appl. Energy Mater. 2019, 3, 336–343. [Google Scholar] [CrossRef]
- Zhu, L.; Zhao, T.; Xu, J.; Liang, Z. Preparation and characterization of carbon-supported sub-monolayer palladium decorated gold nanoparticles for the electro-oxidation of ethanol in alkaline media. J. Power Sources 2009, 187, 80–84. [Google Scholar]
- Chen, Y.; Fan, Z.; Luo, Z.; Liu, X.; Lai, Z.; Li, B.; Zong, Y.; Gu, L.; Zhang, H. High-yield synthesis of crystal-phase-heterostructured 4H/fcc Au@ Pd core–shell nanorods for electrocatalytic ethanol oxidation. Adv. Mater. 2017, 29, 1701331. [Google Scholar]
- Dutta, A.; Datta, J. Outstanding catalyst performance of PdAuNi nanoparticles for the anodic reaction in an alkaline direct ethanol (with anion-exchange membrane) fuel cell. J. Phys. Chem. C 2012, 116, 25677–25688. [Google Scholar] [CrossRef]
- Tan, W.; Deng, J.; Xie, S.; Yang, H.; Jiang, Y.; Guo, G.; Dai, H. Ce0.6Zr0.3Y0.1O2 nanorod supported gold and palladium alloy nanoparticles: High-performance catalysts for toluene oxidation. Nanoscale 2015, 7, 8510–8523. [Google Scholar] [CrossRef]
- Xu, Q.; Lei, W.; Li, X.; Qi, X.; Yu, J.; Liu, G.; Wang, J.; Zhang, P. Efficient removal of formaldehyde by nanosized gold on well-defined CeO2 nanorods at room temperature. Environ. Sci. Technol. 2014, 48, 9702–9708. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Liu, P.; Hrbek, J.; Evans, J.; Pérez, M. Water gas shift reaction on cu and Au nanoparticles supported on CeO2(111) and ZnO(000$\bar 1$): Intrinsic activity and importance of support interactions. Angew. Chem. 2007, 46, 1329–1332. [Google Scholar] [CrossRef]
- Fei, Z.Y.; Sun, B.; Zhao, L.; Ji, W.J.; Au, C.T. Strong morphological effect of Mn3O4 nanocrystallites on the catalytic activity of Mn3O4 and Au/Mn3O4 in benzene combustion. Chem. Eur. J. 2013, 19, 6480–6487. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, J.; Liu, G. Enhancement of ethanol electrooxidation on plasmonic Au/TiO2 nanotube arrays. Electrochem. Commun. 2011, 13, 1260–1263. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Zhai, C.; Ren, F.; Zhu, M.; Yang, P.; Du, Y. Three-dimensional Au0.5/reduced graphene oxide/Au0.5/reduced graphene oxide/carbon fiber electrode and its high catalytic performance toward ethanol electrooxidation in alkaline media. J. Mater. Chem. A 2015, 3, 4389–4398. [Google Scholar] [CrossRef]
- Chen, G.F.; Luo, Y.; Ding, L.X.; Wang, H. Low-voltage electrolytic hydrogen production derived from efficient water and ethanol oxidation on fluorine-modified FeOOH anode. ACS Catal. 2018, 8, 526–530. [Google Scholar] [CrossRef]
- Dai, L.; Qin, Q.; Zhao, X.; Xu, C.; Hu, C.; Mo, S.; Wang, Y.O.; Lin, S.; Tang, Z.; Zheng, N. Electrochemical partial reforming of ethanol into ethyl acetate using ultrathin Co3O4 nanosheets as a highly selective anode catalyst. ACS Cent. Sci. 2016, 2, 538–544. [Google Scholar] [CrossRef]
- Zhou, M.; Jin, B.; Kong, W.; Chen, A.; Chen, Y.; Zhang, X.; Lu, F.; Wang, X.; Zeng, X. Dual polarization of Ni sites at VOx− Ni3N interface boosts ethanol oxidation reaction. Adv. Sci. 2024, 11, 2407473. [Google Scholar] [CrossRef]
- Wolfart, F.; Dubal, D.P.; Vidotti, M.; Gómez-Romero, P. Hybrid core–shell nanostructured electrodes made of polypyrrole nanotubes coated with Ni(OH)2 nanoflakes for high energy-density supercapacitors. RSC Adv. 2016, 6, 15062–15070. [Google Scholar] [CrossRef]
- Hong, Y.; Kim, H.J.; Lee, H.J.; Kim, J. Sang-Il Choi S-Il Ni(OH)2 decorated Pt-Cu octahedra for ethanol electrooxidation reaction. Front. Chem. 2019, 7, 608. [Google Scholar] [CrossRef]
- Barbosa, A.F.B.; Oliveira, V.L.; van Drunen, J.; Tremiliosi-Filho, G. Ethanol electro-oxidation reaction using a polycrystalline nickel electrode in alkaline media: Temperature influence and reaction mechanism. J. Electroanal. Chem. 2015, 746, 31–38. [Google Scholar] [CrossRef]
- Lidasan, J.J.B.; del Rosario, J.A.D.; Ocon, J.D. Ethanol electrooxidation on phase- and morphology-controlled Ni(OH)2 microspheres. Catalysts 2020, 10, 740. [Google Scholar] [CrossRef]
- Xu, C.; Hu, Y.; Rong, J.; Liu, Y. Ni hollow spheres as catalysts for methanol and ethanol electrooxidation. Electrochem. Commun. 2007, 9, 2009–2012. [Google Scholar] [CrossRef]
- Shan, J.; Janvelyan, N.; Li, H.; Liu, J.; Egle, T.M.; Ye, J.; Biener, M.M.; Biener, J.; Friend, C.M.; Flytzani-Stephanopoulos, M. Selective non-oxidative dehydrogenation of ethanol to acetaldehyde and hydrogen on highly dilute NiCu alloys. Appl. Catal. B Environ. 2017, 205, 541–550. [Google Scholar] [CrossRef]
- Hou, Y.C.; Ding, M.W.; Liu, S.K.; Wu, S.K.; Lin, Y.-C. Ni-substituted LaMnO3 perovskites for ethanol oxidation. RSC Adv. 2014, 4, 5329–5338. [Google Scholar] [CrossRef]
- Zhao, Y.; He, X.; Chen, R.; Liu, Q.; Liu, J.; Yu, J.; Li, J.; Zhang, H.; Dong, H.; Zhang, M. A flexible all-solid-state asymmetric supercapacitors based on hierarchical carbon cloth@CoMoO4@NiCo layered double hydroxide core-shell heterostructures. Chem. Eng. J. 2018, 352, 29–38. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, Y.; Qi, T. Hierarchical Ni–Fe layered double hydroxide/MnO2 sphere architecture as an efficient noble metal-free electrocatalyst for ethanol electro-oxidation in alkaline solution. RSC Adv. 2015, 5, 83314–83319. [Google Scholar] [CrossRef]
- Hassan, H.B.; Hamid, Z.A. Electrodeposited Ni–Cr2O3 nanocomposite anodes for ethanol electrooxidation. Int. J. Hydrogen Energy 2011, 36, 5117–5127. [Google Scholar] [CrossRef]
- Xu, Z.; Ye, Y.; Chen, Z.; Chen, Z.; Chen, J.; Xie, F.; Jin, Y.; Wang, N.; Meng, H. Enhanced ethanol oxidation reaction of CoSeO3 by Ni-doping for water electrolysis and an innovative zinc–ethanol–air battery. Chem. Commun. 2023, 59, 8392–8395. [Google Scholar] [CrossRef]
- Wang, M.; Ding, R.; Xiao, Y.; Wang, H.; Wang, L.; Chen, C.M.; Mu, Y.; Wu, G.-P.; Lv, B. CoP/RGO-Pd hybrids with heterointerfaces as highly active catalysts for ethanol electrooxidation. ACS Appl. Mater. Interfaces 2020, 12, 28903–28914. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, T.; Fan, J.; Liu, E.; Zhao, B.; Sun, T. NiFe LDH/MoS2/Ni3S2 pn/Mott-Schottky heterojunction for efficient hydrogen generation coupled with electrochemical oxidation of organic molecules. J. Alloys Compd. 2024, 970, 172710. [Google Scholar] [CrossRef]
- El-Jemni, M.A.; Hassan, H.H.; El Rehim, S.S.A.; Abdel-Samad, H.S. Nano-nickel doped carbon xerogel: Synthesis, characterization and catalytic performance towards ethanol fuel electro-oxidation. Chem. Select. 2023, 8, e202300809. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, W.; Fang, J.; Xu, Z.; Xue, Y.; Liu, D.; Sui, R.; Lv, Q.; Liu, X.; Wang, Y.; et al. Defective Ni3S2 nanowires as highly active electrocatalysts for ethanol oxidative upgrading. Nano Res. 2022, 15, 2987–2993. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Song, C.; Qin, Y.; Lu, L.; Zhu, W.; Zhuang, Z. Nickel chalcogenides as selective ethanol oxidation electro-catalysts and their structure–performance relationships. Chem. Commun. 2022, 58, 2496–2499. [Google Scholar] [CrossRef]







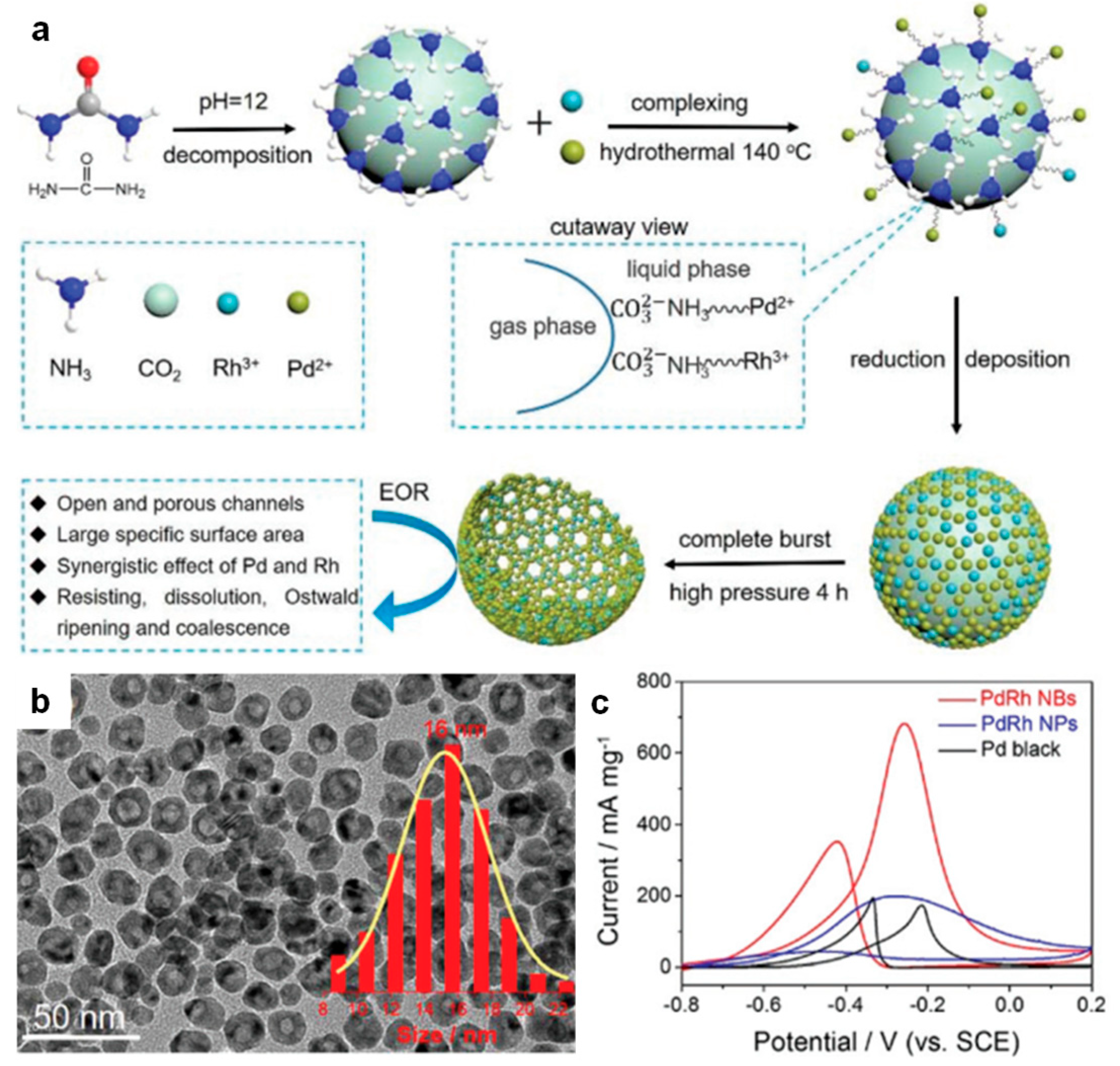
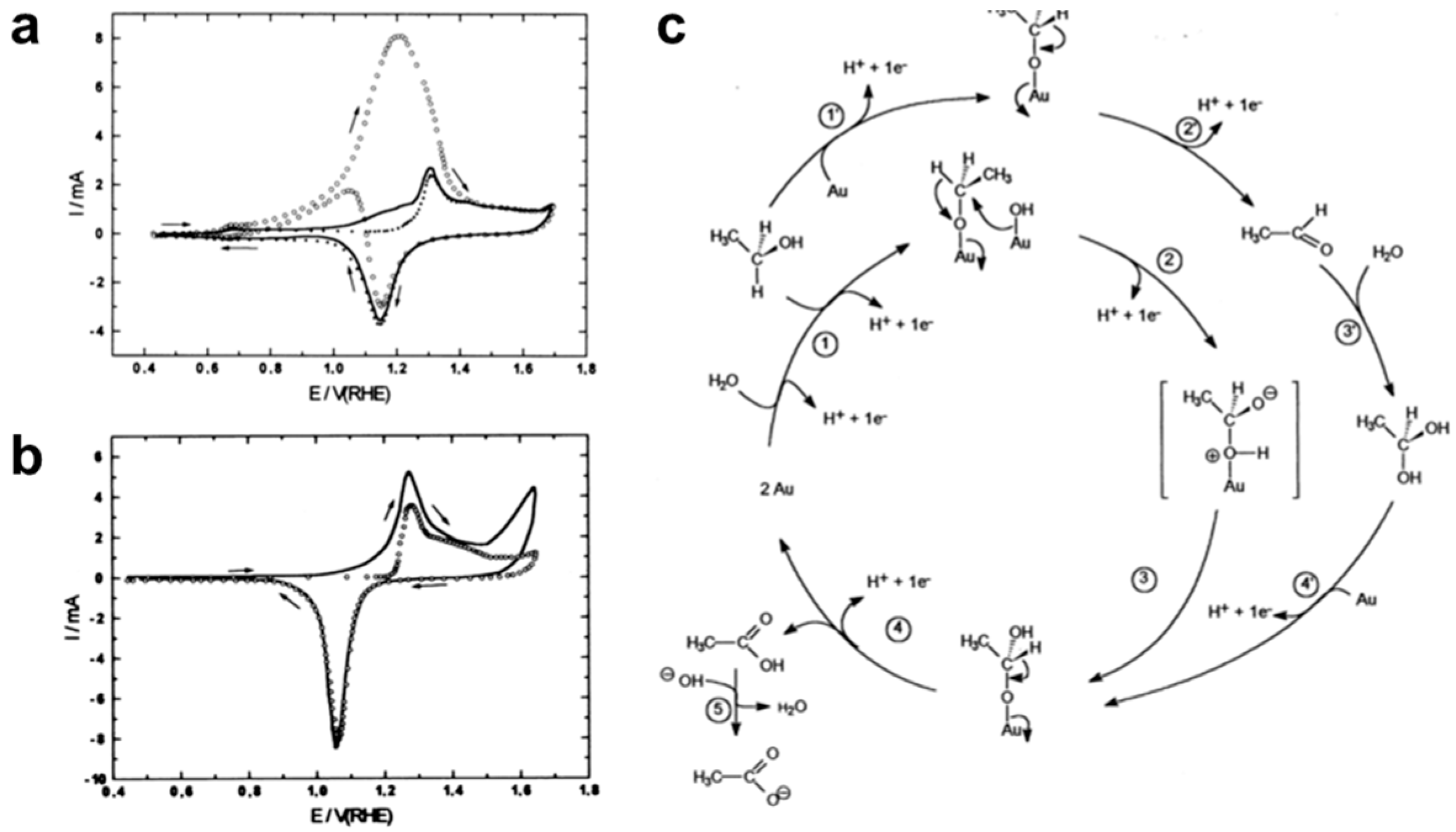
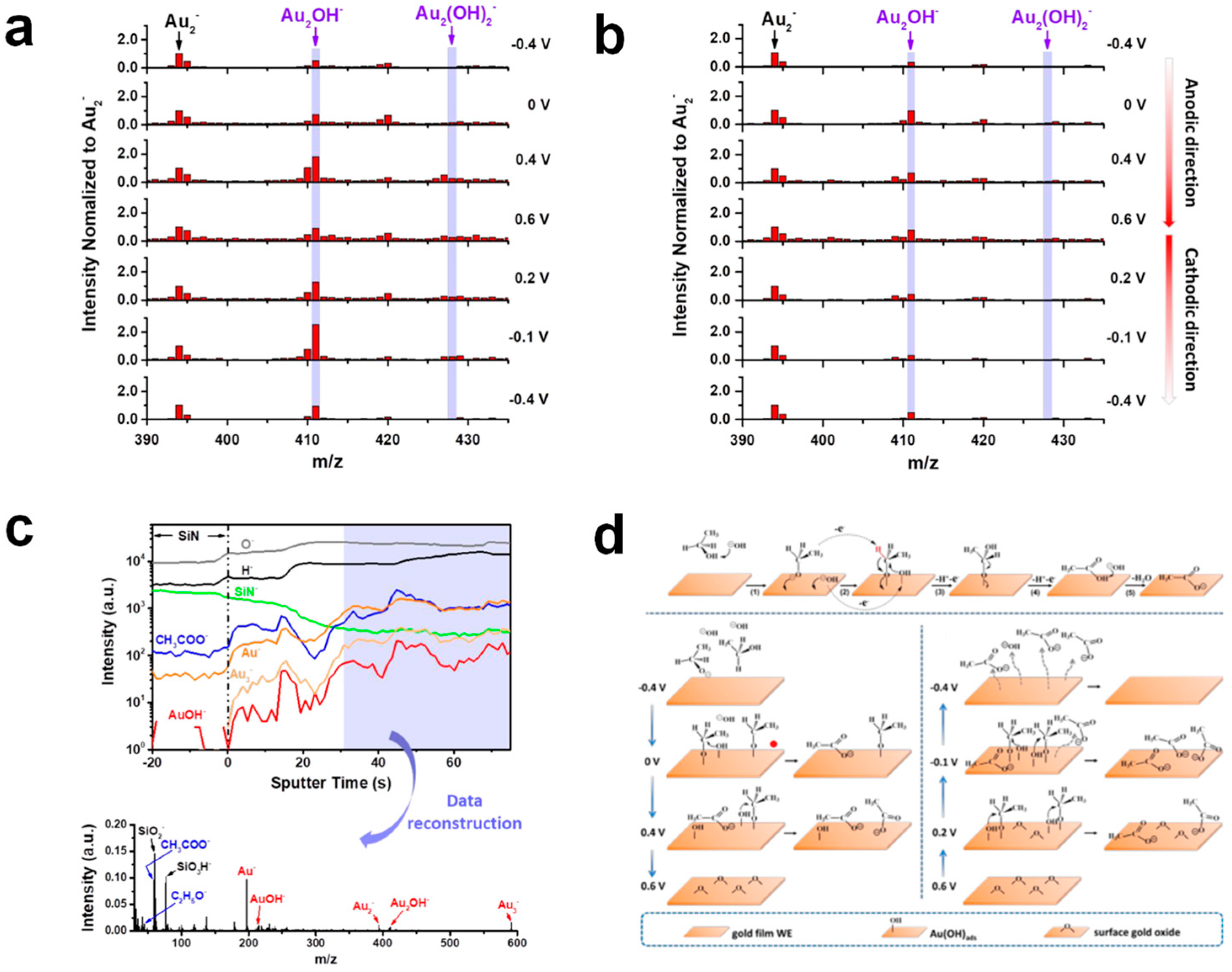

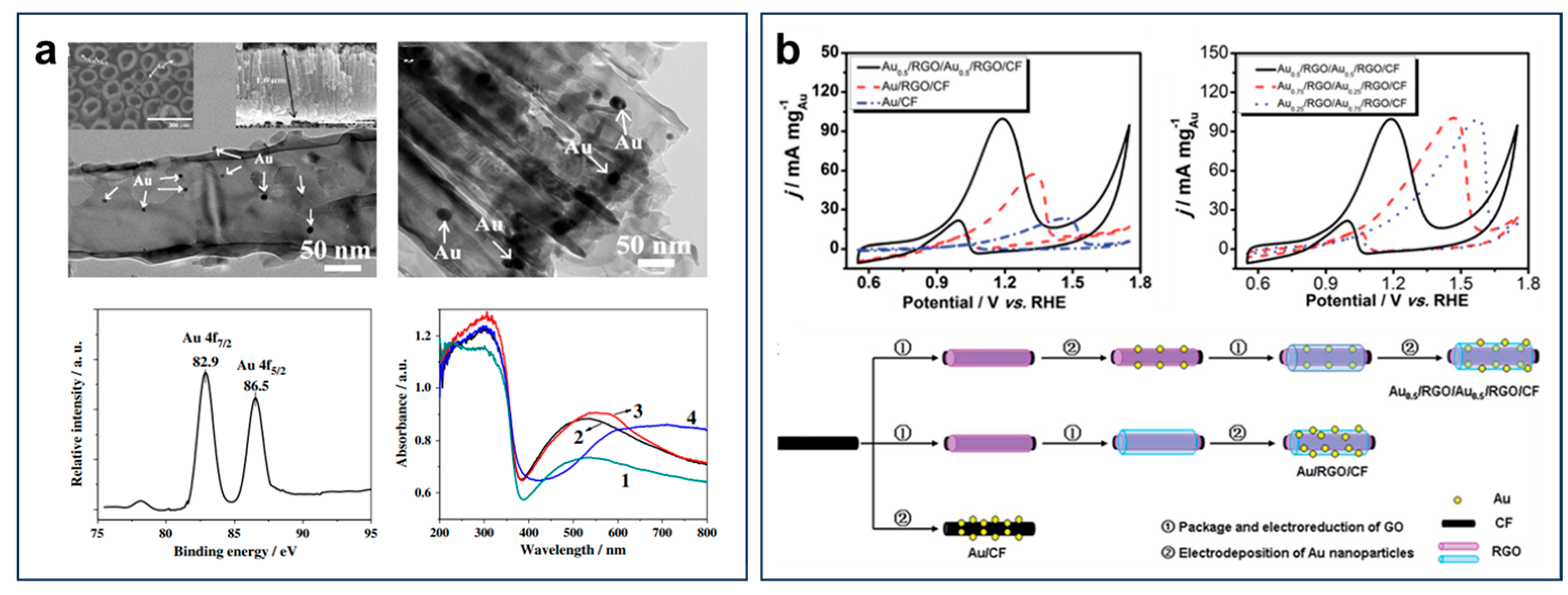
| Catalyst Composition | Electrolyte | Activity | Product Selectivity | Stability Test | References |
|---|---|---|---|---|---|
| Pt/C | 0.1 M HClO4 + 0.5 M EtOH | 260 mA·mg−1metal | main C2 | 190 mA·mg−1 (after 500 CVs) | [25] |
| Pt1Rh1 ANDs | 1.0 M KOH + 1.0 M EtOH | 462.1 mA·mg−1metal | main C2 | 400.6 mA·mg−1 (after 1000 CVs) | [24] |
| Pt74Mn21Ir5 | 0.1 M HClO4 + 0.5 M EtOH | 1020 mA·mg−1metal | main C2 | 870 mA·mg−1 (after 500 CVs) | [25] |
| Pt59Cu41 | 0.5 M KOH + 0.5 M EtOH | 5580 mA·mg−1metal | main C2 | 2.44 mA cm−2 (after 3600 s) | [27] |
| 4H-Au@4H-PtCu | 1.0 M KOH + 1.0 M EtOH | 4220 mA·mg−1metal | main C2 | ~20 mA·mg−1 (at 0.7 V, after 3000 s) | [34] |
| PtZn | 1.0 M KOH + 2.0 M EtOH | 1029.7 mA·mg−1metal | main C2 | ~7.5 mA·mg−1 (at 0.7 V, after 2 h) | [35] |
| PtBi@Pt | 1.0 M KOH + 1.0 M EtOH | 9010 mA·mg−1metal | main C2 | 7080 mA·mg−1 (after 5000 CVs) | [36] |
| PtCu/Cu2− xSe | 1.0 M KOH + 1.0 M EtOH | 5030 mA·mg−1metal | main C2 | 250 mA·mg−1 (at 0.7 V, after 2 h) | [38] |
| PtSn | 0.2 M H2SO4 + 0.2 M EtOH | 673.6 mA·mg−1metal | main C2 | 497.8 mA·mg−1 (after 5000 CVs) | [41] |
| Cu1Au0.15Pd1.5 | 0.5 M KOH + 0.5 M EtOH | 1250 mA·mg−1metal | main C2 | 812.5 mA·mg−1 (after 8000 CVs) | [55] |
| PdCo NTAs | 1.0 M KOH + 1.0 M EtOH | 1562.1 mA·mg−1metal | main C2 | 200 mA·mg−1 (after 550 s) | [58] |
| PdMn-N4 | 1.0 M NaOH + 2.0 M EtOH | 3740 mA·mg−1metal | main C2 | 3553 mA·mg−1 (after 8000 CVs) | [59] |
| PdCu | 1.0 M NaOH + 1.0 M EtOH | 10,590 mA·mg−1metal | main C2 | 7070 mA·mg−1 (after 250 CVs) | [60] |
| NiO-PdNi | 1.0 M KOH + 1.0 M EtOH | 1201.5 mA·mg−1metal | main C2 | 0.045 mA·mg−1 (after 30,000 s) | [61] |
| Pd/S&F–C | 1.0 M KOH + 1.0 M EtOH | 22,000 mA·mg−1metal | main C2 | 19,800 mA·mg−1 (after 10,000 CVs) | [62] |
| a-PdP0.1 | 1.0 M KOH + 1.0 M EtOH | 4851 mA·mg−1metal | main C2 | 220 mA·mg−1 (after 3600 s) | [63] |
| Pd–Ni–P | 1.0 M NaOH + 1.0 M EtOH | 4950 mA·mg−1metal | main C2 | 215.4 mA·mg−1 (after 2000 s) | [64] |
| CPT Rh NBs | 1.0 M NaOH + 1.0 M EtOH | 185.3 mA·mg−1metal | 14.5% C1 | 122.3 mA·mg−1 (after 1200 s) | [70] |
| Rh nanobranches | 1.0 M NaOH + 1.0 M EtOH | 79.1 mA·mg−1metal | 15.8% C1 | / | [71] |
| Pb@Rh | 1.0 M NaOH + 1.0 M EtOH | 1454 mA·mg−1metal | 21% C1 | 828.8 mAmg−1 (after 20,000 s) | [74] |
| Rh85Ni15 | 1.0 M NaOH + 1.0 M EtOH | 159 mA·mg−1metal | 16% C1 | ~0.7 mA cm−2 (after 1000 s) | [75] |
| Rh–Pb | 1.0 M NaOH + 1.0 M EtOH | 2636 mA·mg−1metal | 20% C1 | 57% retention (after 10,000 s) | [78] |
| Rh-Bi(OH)3 | 1.0 M NaOH + 1.0 M EtOH | 3500 mA·mg−1metal | 26.2% C1 | 25% retention (after 4 h) | [79] |
| PdRh NBs | 1.0 M KOH + 1.0 M EtOH | 682 mA·mg−1metal | main C2 | / | [82] |
| nanoporous Au | 1.0 M KOH + 1.0 M EtOH | 308 mA·mg−1metal | main C2 | 268.3 mA·mg−1 (after 800 CVs) | [87] |
| Au/C | 0.1 M KOH + 1.0 M EtOH | 140 mA·mg−1metal | main C2 | 65.0 mA·mg−1 (after 2000 s) | [87] |
| Pd@Au | 1.0 M KOH + 1.0 M EtOH | ~800 mA·mg−1metal | main C2 | 2 mA (after 3600 s) | [88] |
| Au0.5/RGO/Au0.5 | 1.0 M KOH + 1.0 M EtOH | 100.5 mA·cm−2 | main C2 | ~0.4 mA·mg−1 (after 4000 s) | [96] |
| α-Ni(OH)2 | 1.0 M KOH + 1.0 M EtOH | 40 mA·cm−2 | main C2 | / | [103] |
| Ni HS | 1.0 M KOH + 1.0 M EtOH | 17 mA·cm−2 | main C2 | / | [104] |
| Ni–Fe LDH@MnO2 | 1.0 M KOH + 1.0 M EtOH | ~5 mA·cm−2 | main C2 | / | [108] |
| Ni-Cr2O3 | 1.0 M NaOH + 2.0 M EtOH | ~300 mA·cm−2 | main C2 | / | [109] |
| Ni-6/CX/G | 1.0 M NaOH + 2.0 M EtOH | 5925 mA·cm−2 | main C2 | / | [113] |
| Ni3S2 NWs | 1.0 M KOH + 1.0 M EtOH | 106 mA·cm−2 | main C2 | / | [114] |
| NiSe2 NWs | 1.0 M KOH + 1.0 M EtOH | 70.2 mA·cm−2 | main C2 | / | [115] |
| Catalyst System | Strengths | Weaknesses |
|---|---|---|
| Pt | Benchmark activity in acidic media Strong ability to activate ethanol and intermediates | Severe CO poisoning Low CO2 selectivity |
| Pd | High intrinsic activity in alkaline media Favorable adsorption of ethanol and intermediates | Low CO2 selectivity Surface easily oxidized and deactivated |
| Rh | Strong ability to cleave C–C bond Relatively low initial potential | Extremely high cost and scarcity Lower overall activity compared to Pt/Pd |
| Au | Excellent resistance to CO poisoning Stable under alkaline conditions | Low CO2 selectivity Poor activity in acidic media |
| Ni, Co, etc. | Abundant and low-cost High activity in alkaline media | Low CO2 selectivity Surface easily oxidized and corroded Limited activity in acidic electrolytes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Lv, Q.; Zheng, D.; Zhou, C.; Chen, S.; Zhang, K.; Han, S.; Huang, H.-Z.; Zhang, Y.; Chen, L. Strategic Design of Ethanol Oxidation Catalysts: From Active Metal Selection to Mechanistic Insights and Performance Engineering. Nanomaterials 2025, 15, 1477. https://doi.org/10.3390/nano15191477
Liu D, Lv Q, Zheng D, Zhou C, Chen S, Zhang K, Han S, Huang H-Z, Zhang Y, Chen L. Strategic Design of Ethanol Oxidation Catalysts: From Active Metal Selection to Mechanistic Insights and Performance Engineering. Nanomaterials. 2025; 15(19):1477. https://doi.org/10.3390/nano15191477
Chicago/Turabian StyleLiu, Di, Qingqing Lv, Dahai Zheng, Chenhui Zhou, Shuchang Chen, Kaiyang Zhang, Suqin Han, Hui-Zi Huang, Yufeng Zhang, and Liwei Chen. 2025. "Strategic Design of Ethanol Oxidation Catalysts: From Active Metal Selection to Mechanistic Insights and Performance Engineering" Nanomaterials 15, no. 19: 1477. https://doi.org/10.3390/nano15191477
APA StyleLiu, D., Lv, Q., Zheng, D., Zhou, C., Chen, S., Zhang, K., Han, S., Huang, H.-Z., Zhang, Y., & Chen, L. (2025). Strategic Design of Ethanol Oxidation Catalysts: From Active Metal Selection to Mechanistic Insights and Performance Engineering. Nanomaterials, 15(19), 1477. https://doi.org/10.3390/nano15191477







