Experimental Evaluation of the Treatment Effect of High Viscosity Drilling Fluid and Floating Oil Using Ozone Fine Bubble Technology
Abstract
1. Introduction
2. Materials and Experiments
2.1. Experimental Materials
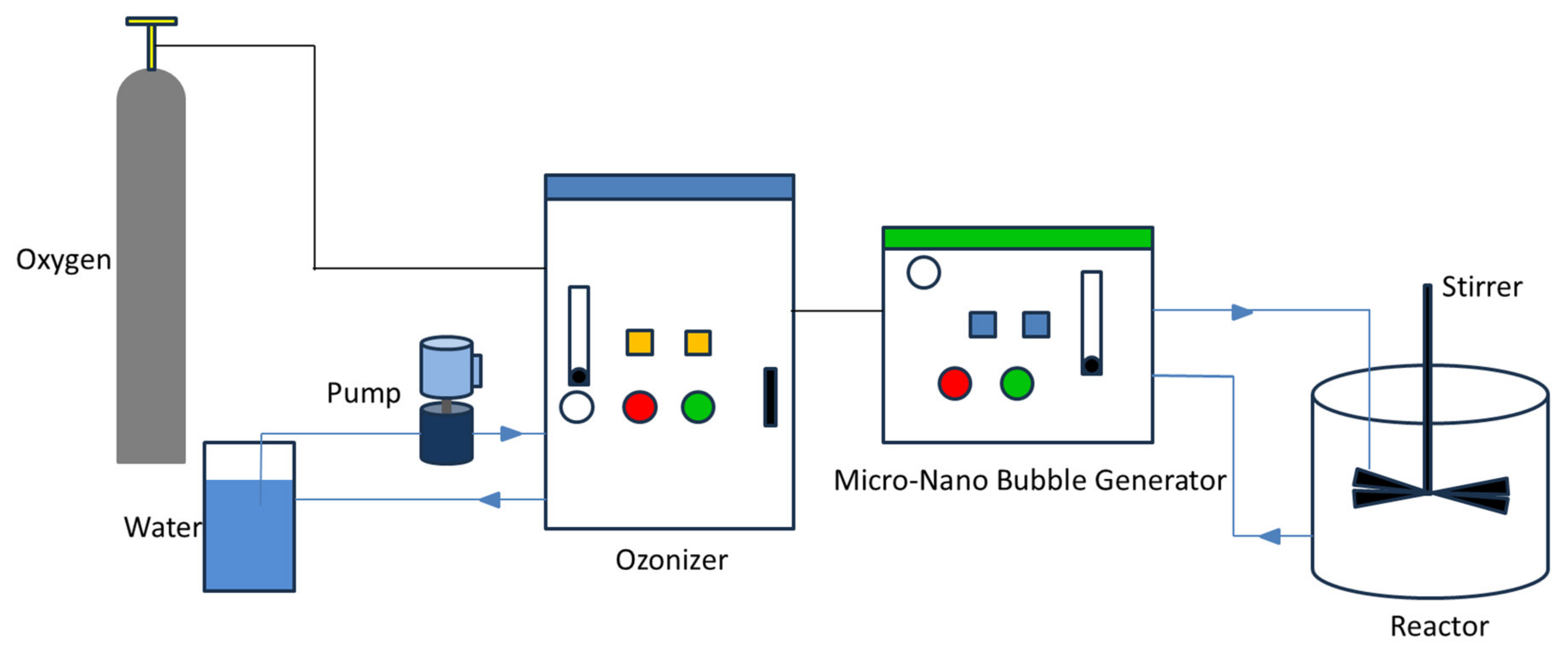
2.2. Experimental Equipment
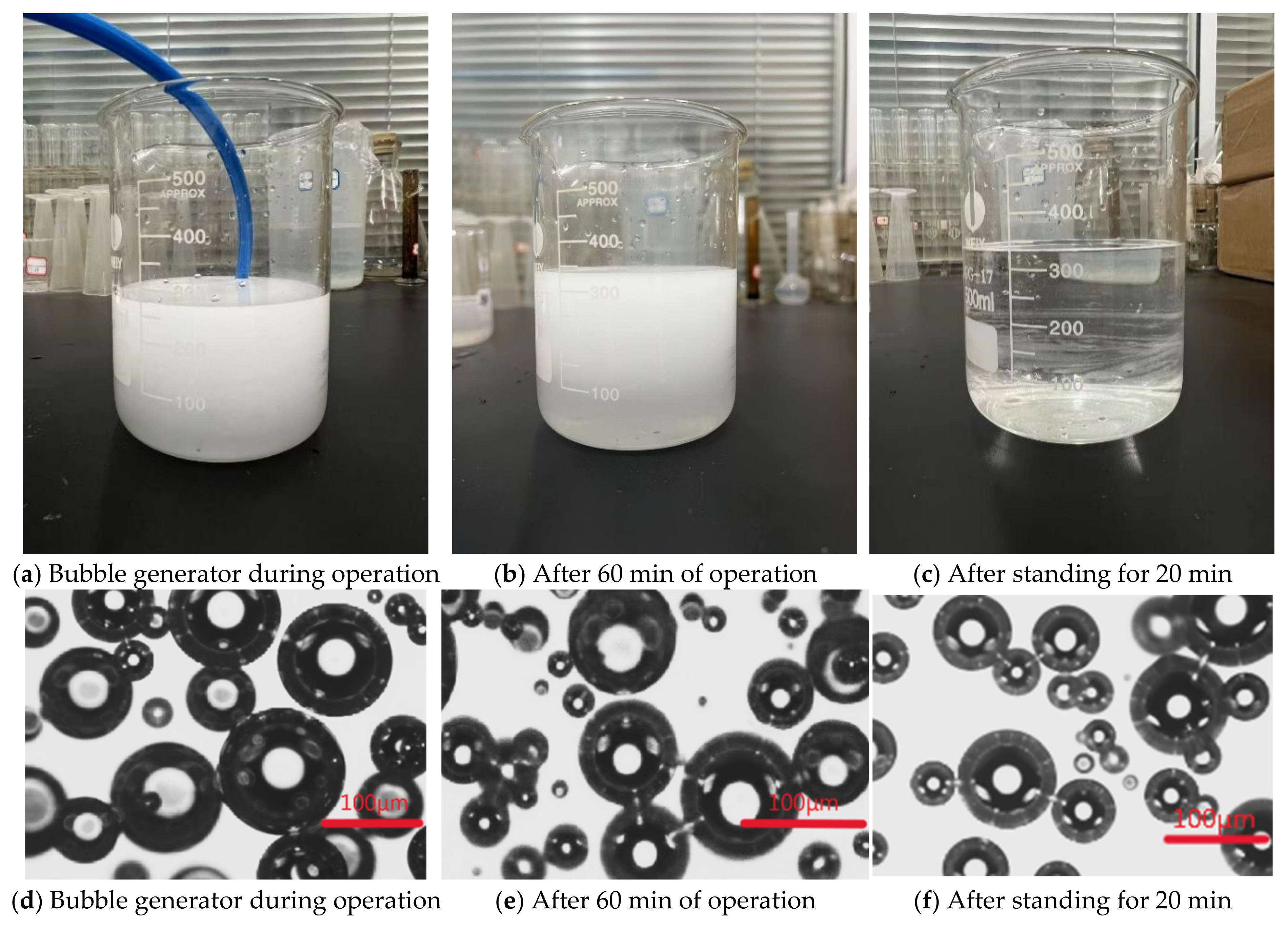
2.3. Experimental Methods
- (1)
- To validate the effectiveness of ozone fine bubble technology for treating high-viscosity drilling fluids, laboratory experiments were conducted on fluid samples. The evaluation focused on quantifying the viscosity reduction efficiency and assessing the feasibility for field applications.
- (2)
- To enhance residual oil removal, a combined process was implemented involving flocculation pretreatment followed by ozone fine bubble flotation. The efficacy of this treatment was determined through quantitative analysis of the residual oil content in the processed drilling fluid samples.
2.3.1. Viscosity Reduction Treatment of Original Drilling Fluid Using Ozone Fine Bubbles
2.3.2. Evaluation of Oil Removal from Oil-Containing Drilling Fluid Using Ozone Fine Bubbles
2.4. Detection Methods
3. Results and Discussion
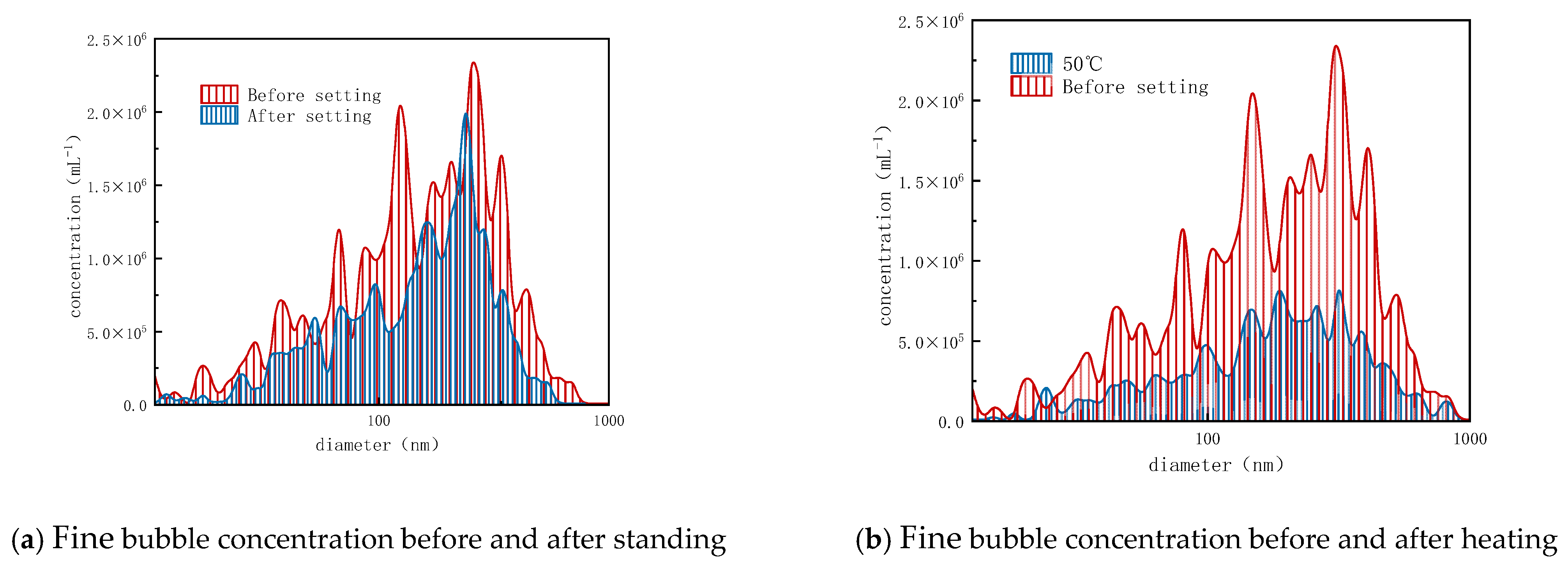
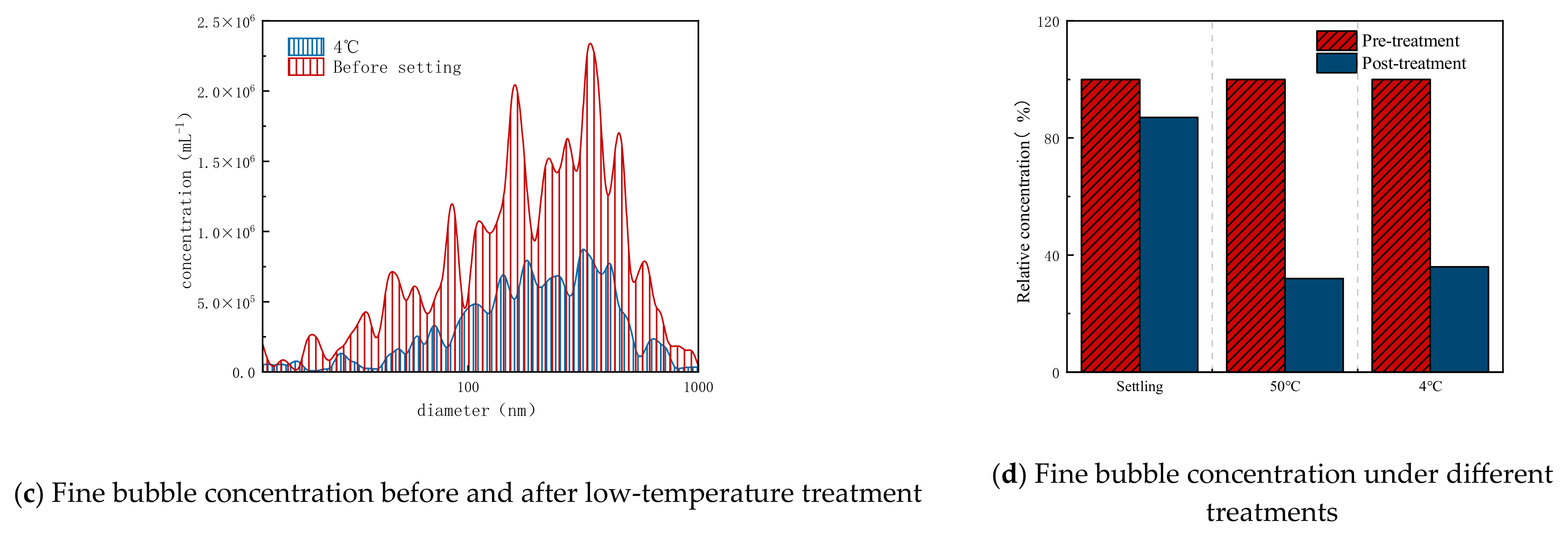
3.1. Evaluation of Viscosity Reduction Effect Using Ozone Fine Bubbles
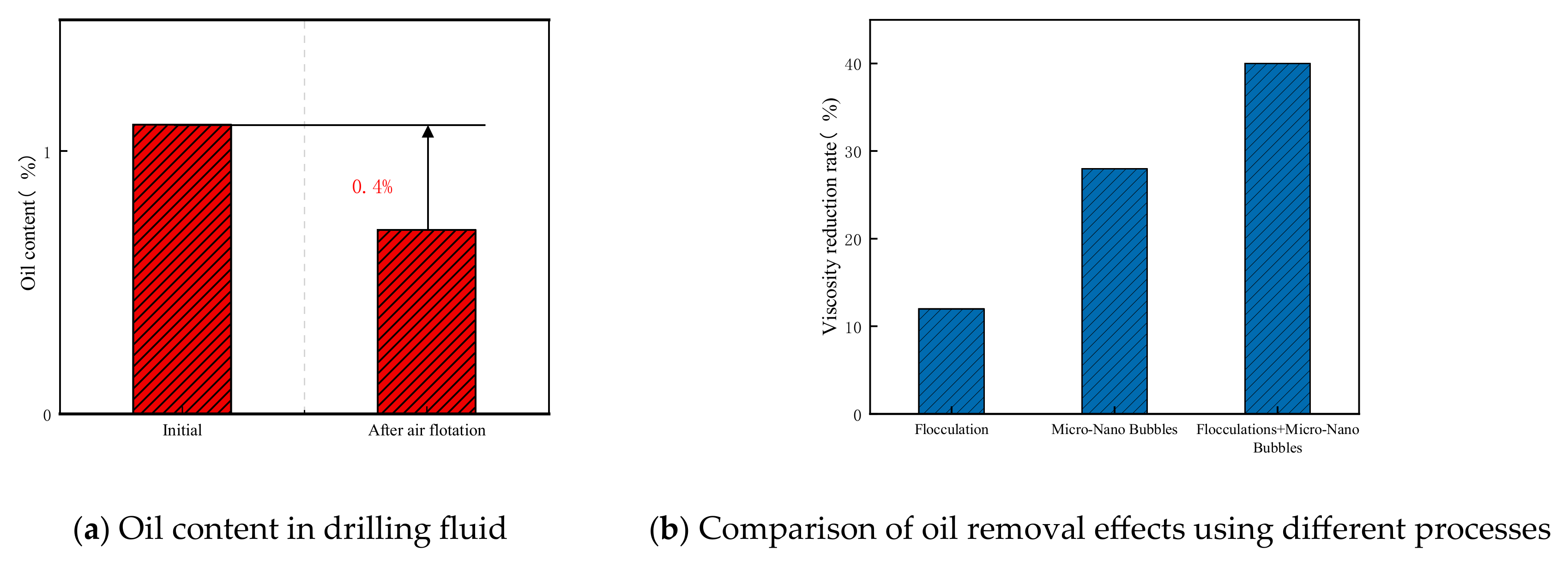
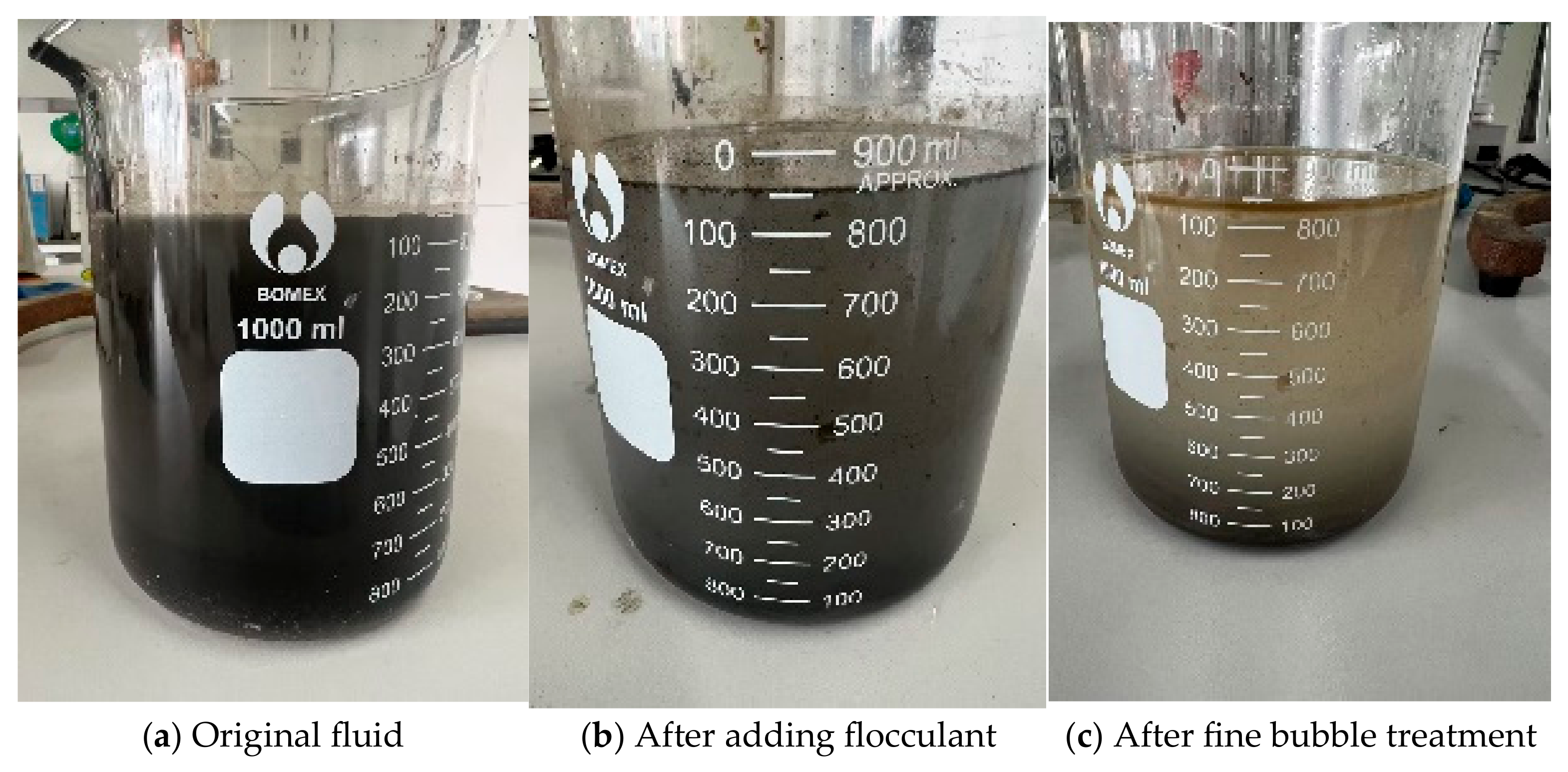
3.2. Evaluation of Oil Removal Effect Using Ozone Fine Bubbles
4. Conclusions
- (1)
- Ozone fine bubble technology demonstrates significant efficacy in reducing viscosity and removing oil content from high-viscosity drilling fluids.
- (2)
- Deep treatment using this technology achieved a 29% reduction in viscosity at an ozone dosage of 12.5 g, while the combination of flocculation and fine bubble treatment resulted in a 40% oil removal rate.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Khodja, M.; Khodja-Saber, M.; Canselier, J.P.; Cohaut, N.; Bergaya, F.J. Drilling fluid technology: Performances and environmental considerations. Prod. Serv. RD Final. Solut. 2010, 227–256. [Google Scholar] [CrossRef]
- Davoodi, S.; Al-Shargabi, M.; Wood, D.A.; Rukavishnikov, V.S.; Minaev, K.M. Synthetic polymers: A review of applications in drilling fluids. Pet. Sci. 2023, 21, 475–518. [Google Scholar] [CrossRef]
- Luo, T.; Li, J.; Xu, J.; Wang, J.; Zhang, L.; Yu, Z. The effects of organically modified lithium magnesium silicate on the rheological properties of water-based drilling fluids. Materials 2024, 17, 1564. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, H.; Mohammed, A.A.A.; Nasser, M.S.; Hussein, I.A.; El-Naas, M.H. Green drilling fluid additives for a sustainable hole-cleaning performance: A comprehensive review. Emergent Mater. 2024, 7, 387–402. [Google Scholar] [CrossRef]
- Oseh, J.O.; Mohd, N.M.N.A.; Gbadamosi, A.O.; Agi, A.; Blkoor, S.O.; Ismail, I.; Igwilo, K.C.; Igbafe, A.I. Polymer nanocomposites application in drilling fluids: A review. Geoenergy Sci. Eng. 2023, 222, 211416. [Google Scholar] [CrossRef]
- Caenn, R.; Chillingar, G.V. Drilling fluids: State of the art. J. Petrol. Sci. Eng. 1996, 14, 221–230. [Google Scholar] [CrossRef]
- Deville, J.P. Drilling fluids. In Fluid Chemistry, Drilling and Completion; Elsevier: Amsterdam, The Netherlands, 2022; pp. 115–185. [Google Scholar] [CrossRef]
- Qaidi, S.; Najm, H.M.; Abed, S.M.; Ahmed, H.U.; Al Dughaishi, H.; Al Lawati, J.; Sabri, M.M.; Alkhatib, F.; Milad, A. Fly ash-based geopolymer composites: A review of the compressive strength and microstructure analysis. Materials 2022, 15, 7098. [Google Scholar] [CrossRef]
- Qaidi, S.M.A.; Tayeh, B.A.; Zeyad, A.M.; De Azevedo, A.R.G.; Ahmed, H.U.; Emad, W. Recycling of mine tailings for the geopolymers production: A systematic review. Case Stud. Constr. Mater. 2022, 16, e00933. [Google Scholar] [CrossRef]
- Kumar, S.J. Synthesis and experimental evaluation of graft polymer nanocomposite in water based drilling fluid system for reactive unconventional reservoirs. J. Polym. Compos. 2017, 5, 34–41. [Google Scholar]
- Zhong, H.; He, Y.; Yang, E.; Bi, Y.; Yang, T.J. Engineering, Modeling of microflow during viscoelastic polymer flooding in heterogenous reservoirs of Daqing Oilfield. J. Petrol. Sci. Eng. 2022, 210, 110091. [Google Scholar] [CrossRef]
- Yang, J.; Sun, J.; Wang, R.; Qu, Y. Treatment of drilling fluid waste during oil and gas drilling: A review. Environ. Sci. Pollut. Res. 2023, 30, 19662–19682. [Google Scholar] [CrossRef]
- Ogunkunle, T.F.; Oni, B.A.; Afolabi, R.O.; Fadairo, A.S.; Ojo, T.; Adesina, O.J. Comparative analysis of the performance of hydrophobically associating polymers, xanthan and guar gum as mobility controlling agents in enhanced oil recovery application. J. King Saud Univ.-Eng. Sci. 2022, 34, 402–407. [Google Scholar] [CrossRef]
- Hamoodi, A.; Rahimy, A.A.; Khalid, A.W.J. The effect of proper selection of drilling fluid on drilling operation in Janbour field. Am. Sci. Res. J. Eng. Technol. Sci. 2018, 39, 224–234. [Google Scholar]
- Mi, S.; Hou, C.; Ge, W. Theoretical study on the stability of nanobubbles and its verification in molecular dynamics simulation. Particuology 2024, 87, 7. [Google Scholar] [CrossRef]
- Ahmed, N.; Alam, M.S.; Salam, M.A. Experimental analysis of drilling fluid prepared by mixing iron (III) oxide nanoparticles with a KCl–Glycol–PHPA polymer-based mud used in drilling operation. J. Petrol. Explor. Prod. Technol. 2020, 10, 3389–3397. [Google Scholar] [CrossRef]
- Feng, H.; Liu, M.; Zeng, W.; Chen, Y.; Wang, M.; Yuan, L.; Yu, Z. Feasibility of resource utilization of the refractory evaporation concentrate of gas field wastewater exhibiting high salinity: Application of UV/Fenton, desulfurization, distillation and crystallization process after pre-treatment. Environ. Res. 2022, 20, 112317. [Google Scholar] [CrossRef] [PubMed]
- Kaya, S.; Asci, Y. Heterogeneous Fenton process with Fe(III) based catalyst for treatment of textile industry wastewater. Indian J. Chem. Technol. 2022, 29, 166–173. [Google Scholar] [CrossRef]
- Pivokonsky, M.; Novotna, K.; Petricek, R.; Cermakova, L.; Prokopova, M.; Naceradska, J. Fundamental chemical aspects of coagulation in drinking water treatment–Back to basics. J. Water Process Eng. 2024, 57, 114–120. [Google Scholar] [CrossRef]
- Standard GB 8978-1996; Integrated wastewater discharge standard, State Bureau of Technical Supervision. China Standard Press: Beijing, China, 1996.
- Wang, R.Q.; Ji, Y.Z.; Lan, Q.Q. Experimental study on treatment of drilling engineering wastewater by ozone micro-nano bubble technology for oil and gas fields. J. Environ. Eng. Technol. 2024, 14, 1151–1157. [Google Scholar]
- Liu, T.; Zhang, B.; Li, W.; Li, B.; Han, Z.; Zhang, Y.; Ding, A.; Wang, S.; Ma, J. The catalytic oxidation process of atrazine by ozone microbubbles: Bubble formation, ozone mass transfer and hydroxyl radical generation. Chemosphere 2023, 325, 138361. [Google Scholar] [CrossRef]
- GB/T 6920-1986; Water Quality—Determination of pH Value—Glass Electrode Method. China Standard Press: Beijing, China, 1986.
- GB/T 29170-2012; Petroleum and Natural Gas Industries—Drilling Fluids—Laboratory Testing. China Standard Press: Beijing, China, 2012.
- GB/T 37894-2019; Evaluation Method for Flocculation Performance of Water Treatment Agents. China Standard Press: Beijing, China, 2019.
- Liang, J.; Fei, Y.; Yin, Y.; Han, Q.; Liu, Y.; Feng, L.; Zhang, L. Advancements in wastewater treatment: A comprehensive review of ozone microbubbles technology. Environ. Res. 2025, 266, 120469. [Google Scholar] [CrossRef]
- Krishnan, S.; Rawindran, H.; Sinnathambi, C.M.; Lim, J.W. Comparison of various advanced oxidation processes used in remediation of industrial wastewater laden with recalcitrant pollutants. IOP Conf. Ser. Mater. Sci. Eng. 2017, 206, 012089. [Google Scholar] [CrossRef]
- Wang, X.; Gong, X.; Zhang, Q.; Du, H. Degradation mechanism of Direct Pink 12B treated by iron-carbon micro-electrolysis and Fenton reaction. J. Environ. Sci. 2013, 25, S63–S68. [Google Scholar] [CrossRef]
- Lim, S.; Shi, J.L.; von Gunten, U.; McCurry, D.L. Ozonation of organic compounds in water and wastewater: A critical review. Water Res. 2022, 213, 118053. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Chiba, K.; Li, P. Free-radical generation from collapsing microbubbles in the absence of a dynamic stimulus. J. Phys. Chem. B 2007, 111, 1343–1347. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.B.; Kim, S.; Nguyen, T.S.; Mahmood, J.; Yavuz, C.T. Covalent organic framework membranes and water treatment. J. Am. Chem. Soc. 2024, 146, 3567–3584. [Google Scholar] [CrossRef] [PubMed]
- Yuan, N.; Wang, C.; Fan, W.; Liang, T.; Xi, J.; Li, Y.; Tong, X. Investigation into ozonation flotation for enhancing treatment of drilling wastewater in gas field operations. In Fifth International Conference on Green Energy, Environment and Sustainable Development (GEESD 2024), Mianyang, China, 26 September 2024; Volume 13279, pp. 884–891. [Google Scholar]
- Jiao, S.; Li, W.; Li, Z.; Gai, J.; Zou, L.; Su, Y. Hybrid physics-machine learning models for predicting rate of penetration in the Halahatang oil field, Tarim Basin. Sci. Rep. 2024, 14, 5957–5958. [Google Scholar] [CrossRef]
- Qiu, C.Y.; Song, X.Y.; Yang, Q.Y.; Jiang, C.L.; Zheng, C.S.; Zhang, H.Q. Research on recycling technology of drilling wastewater in Hangjin Banner area. Petrochem. Appl. 2024, 43, 49–54. [Google Scholar]
- Li, Y.; Tong, X.; Xi, J.; Li, Y.; Guo, X. Application of dissolved ozone flotation in the advanced treatment of drilling wastewater in gas field: Significance of ozone microbubble flocs. Water Air Soil Pollut. 2024, 235, 327–330. [Google Scholar] [CrossRef]
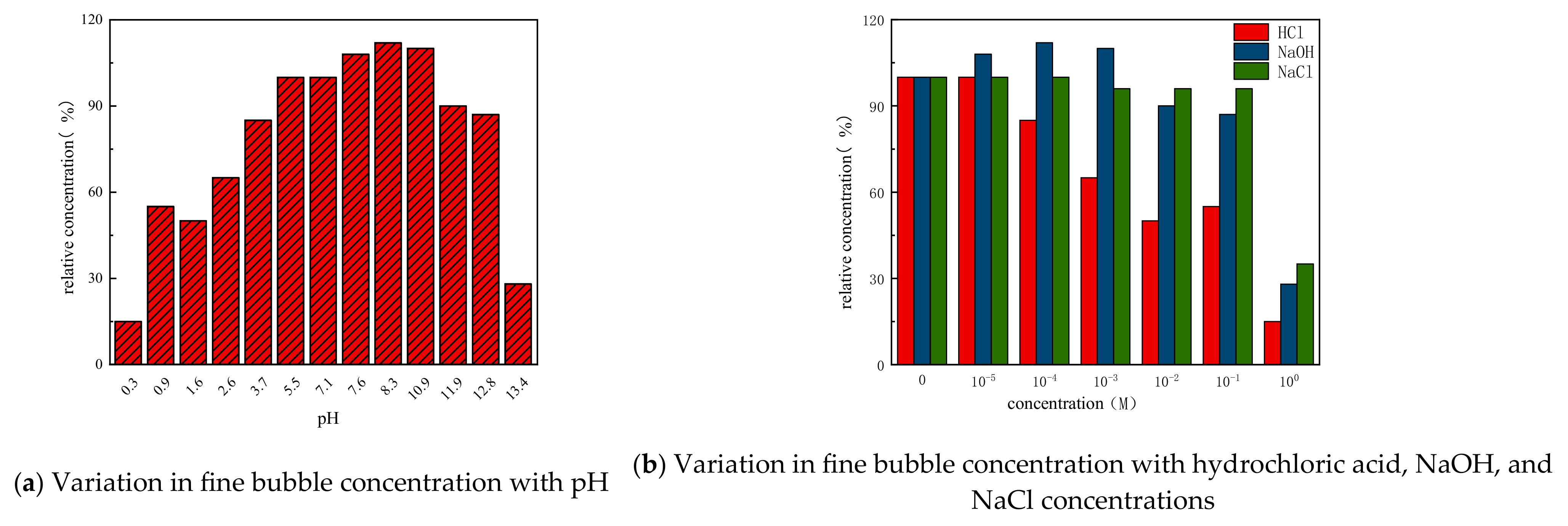
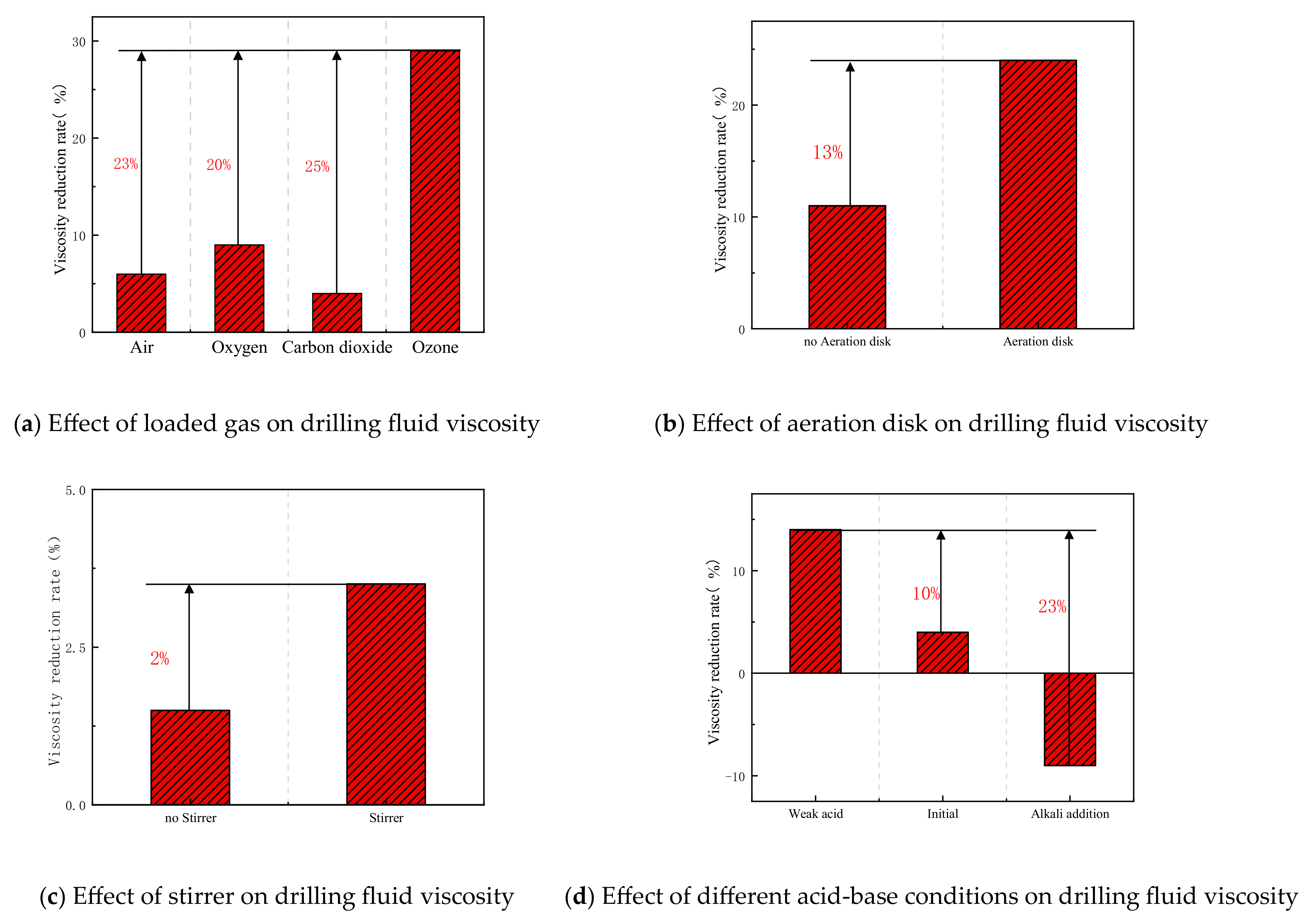
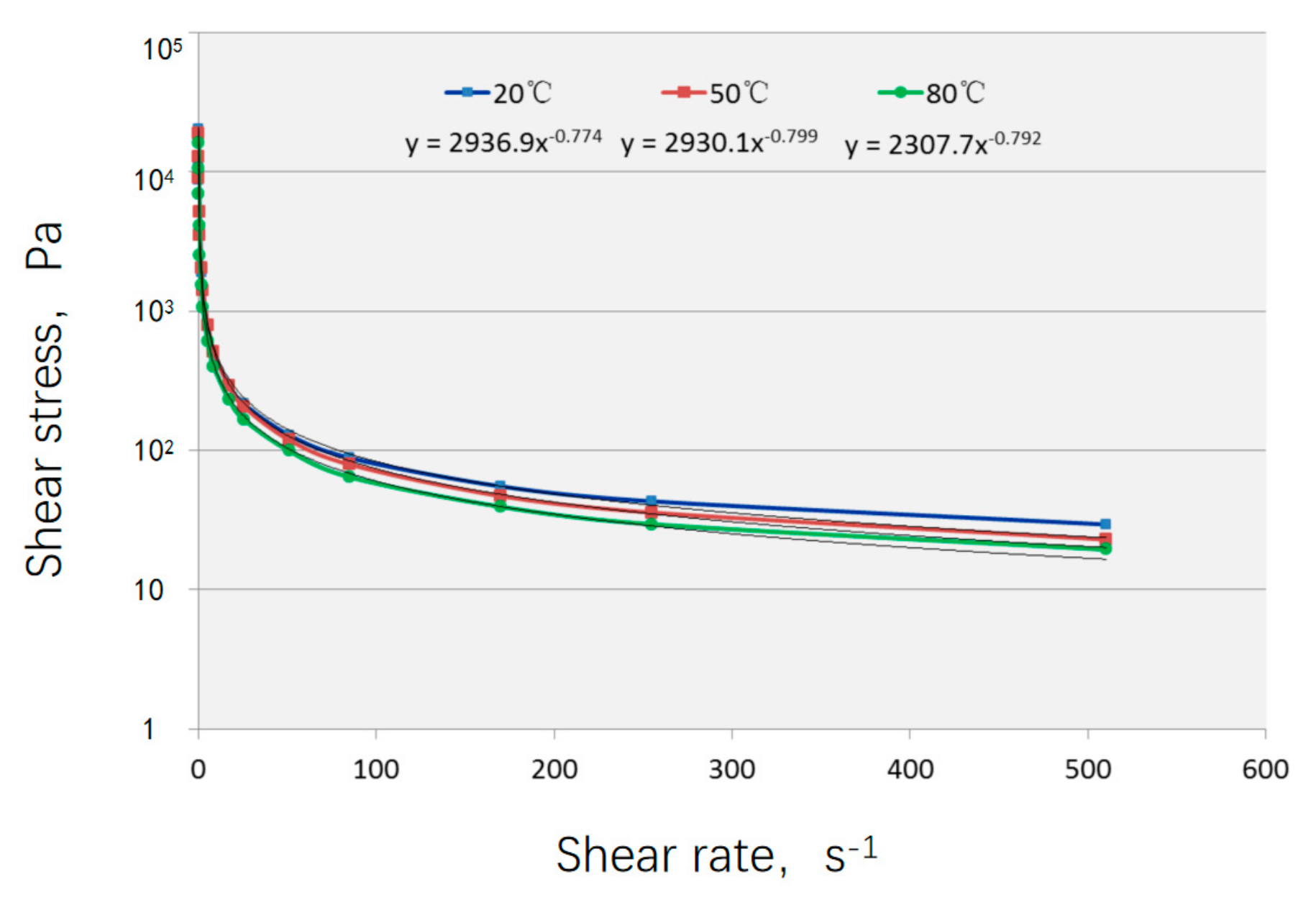

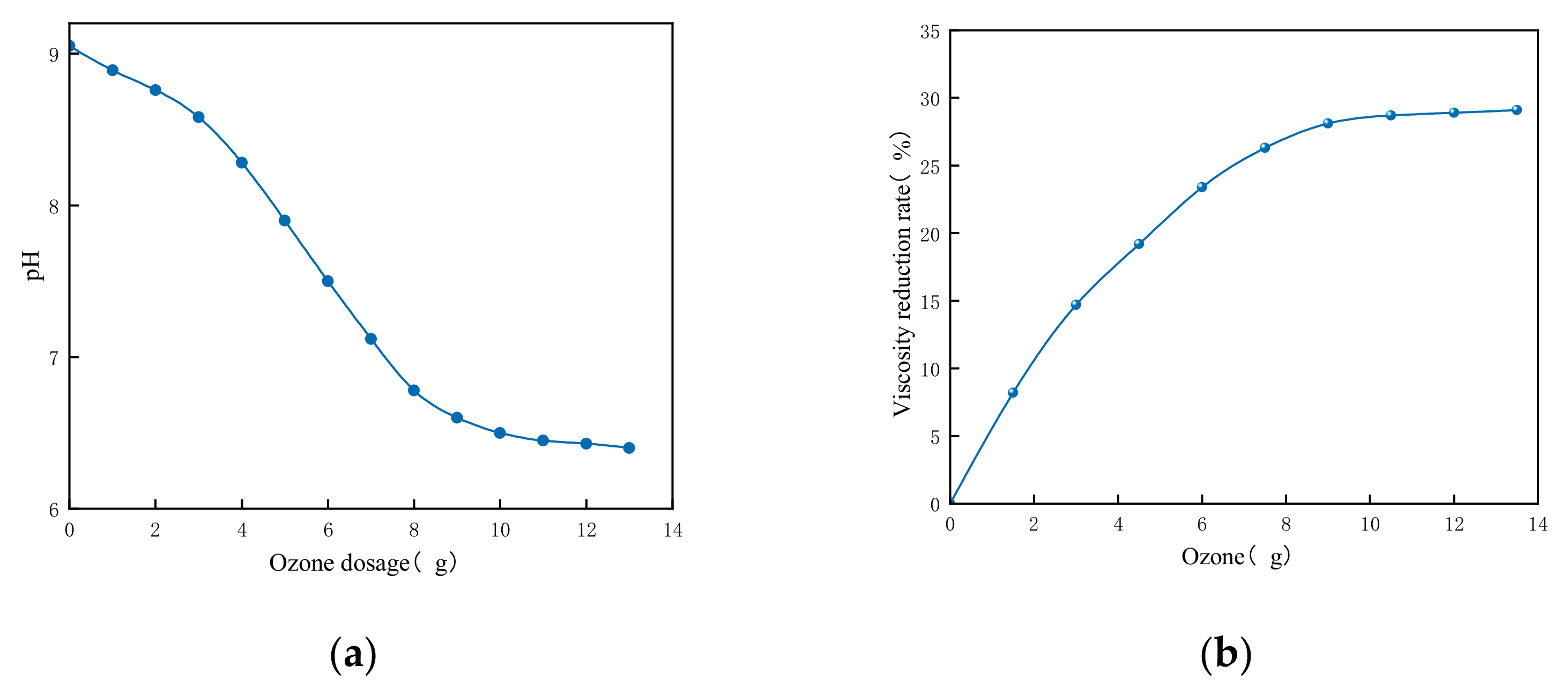
| Formula No. | pH | AV (mPa·s) | PV (mPa·s) | YP (Pa) |
|---|---|---|---|---|
| 1# | 8–10 | 24–27 | 8–22 | 10–24 |
| 2# | 8–10 | 26–30 | 10–21 | 10–25 |
| Instrument Name | Model | Manufacturer |
|---|---|---|
| Ultrapure Water System | YK-RO-B | Xiamen Shuhuoquan |
| Ozone Generator | ZJC-TF10 | Shanghai Zhongjing |
| Hot Rolling Oven | XGRL-5 | Qingdao Xinruide |
| High-Speed Stirrer | GJS-B12K | Qingdao Xinruide |
| Electronic Balance | WTB10002K | Changzhou Wantai |
| Six-Speed Viscometer | ZNN-D6 | Qingdao Xinruide |
| Electric Stirrer | D90-150 | Qingdao Xinruide |
| Magnetic Stirring Water Bath | HH-2J | Changzhou Langyue |
| Digital Constant Temperature Bath | HH-6 | Shanghai Lichen Bangxi |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Liu, L.; Liu, N.; Hu, F.; Zhang, L. Experimental Evaluation of the Treatment Effect of High Viscosity Drilling Fluid and Floating Oil Using Ozone Fine Bubble Technology. Nanomaterials 2025, 15, 1324. https://doi.org/10.3390/nano15171324
Guo X, Liu L, Liu N, Hu F, Zhang L. Experimental Evaluation of the Treatment Effect of High Viscosity Drilling Fluid and Floating Oil Using Ozone Fine Bubble Technology. Nanomaterials. 2025; 15(17):1324. https://doi.org/10.3390/nano15171324
Chicago/Turabian StyleGuo, Xiaoxuan, Lei Liu, Nannan Liu, Fulong Hu, and Lijuan Zhang. 2025. "Experimental Evaluation of the Treatment Effect of High Viscosity Drilling Fluid and Floating Oil Using Ozone Fine Bubble Technology" Nanomaterials 15, no. 17: 1324. https://doi.org/10.3390/nano15171324
APA StyleGuo, X., Liu, L., Liu, N., Hu, F., & Zhang, L. (2025). Experimental Evaluation of the Treatment Effect of High Viscosity Drilling Fluid and Floating Oil Using Ozone Fine Bubble Technology. Nanomaterials, 15(17), 1324. https://doi.org/10.3390/nano15171324






