Research Progress in the Application of Nanotechnology in Fracturing: A Review
Abstract
1. Introduction
2. Challenges of Fracturing Technology
2.1. Unconventional Reservoir Development
2.2. Conventional Fracturing Fluids
3. Nanotechnology Innovations in Fracturing
3.1. Unique Properties
3.2. Preparation and Dispersion
4. Applications
4.1. Nanomodified Fracturing Fluids
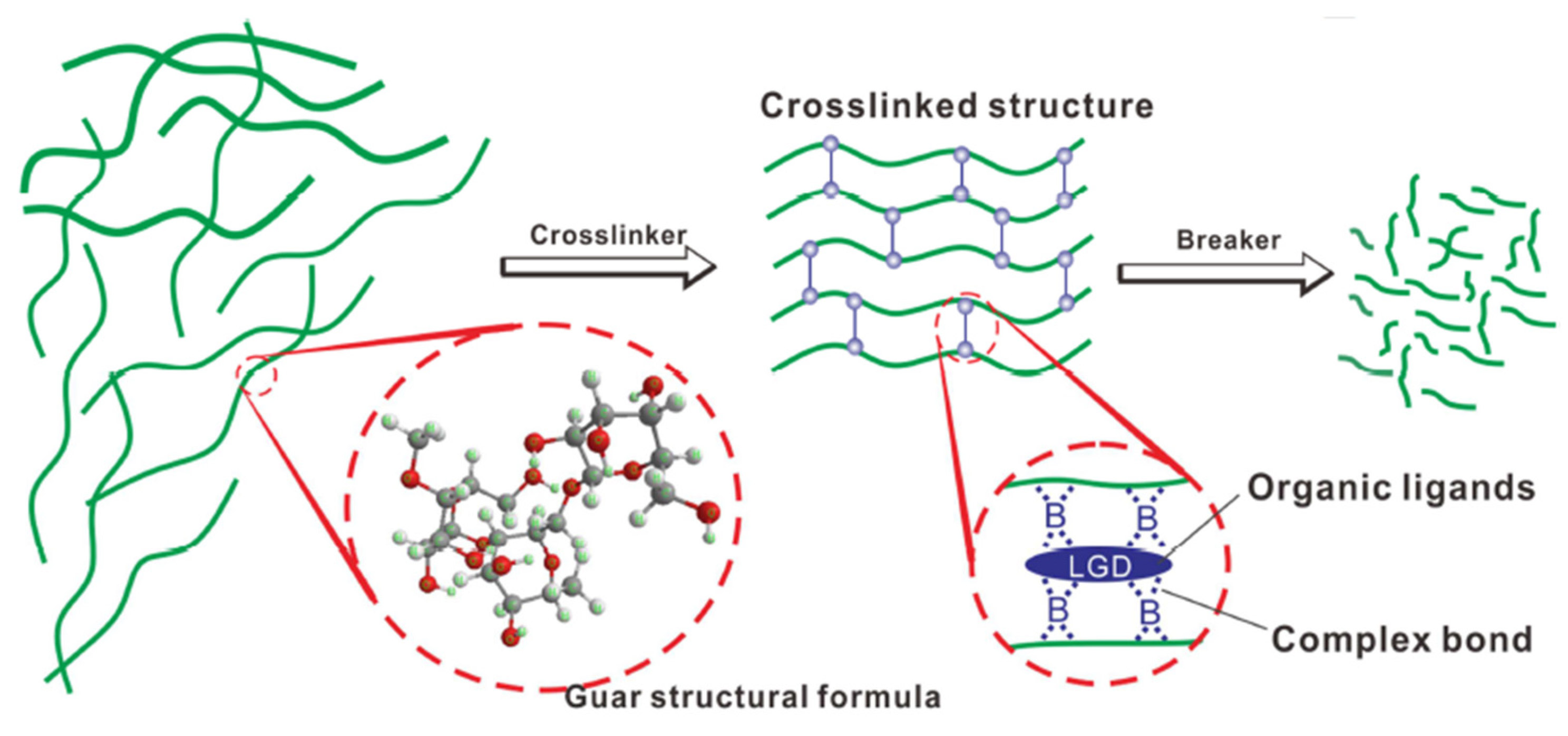
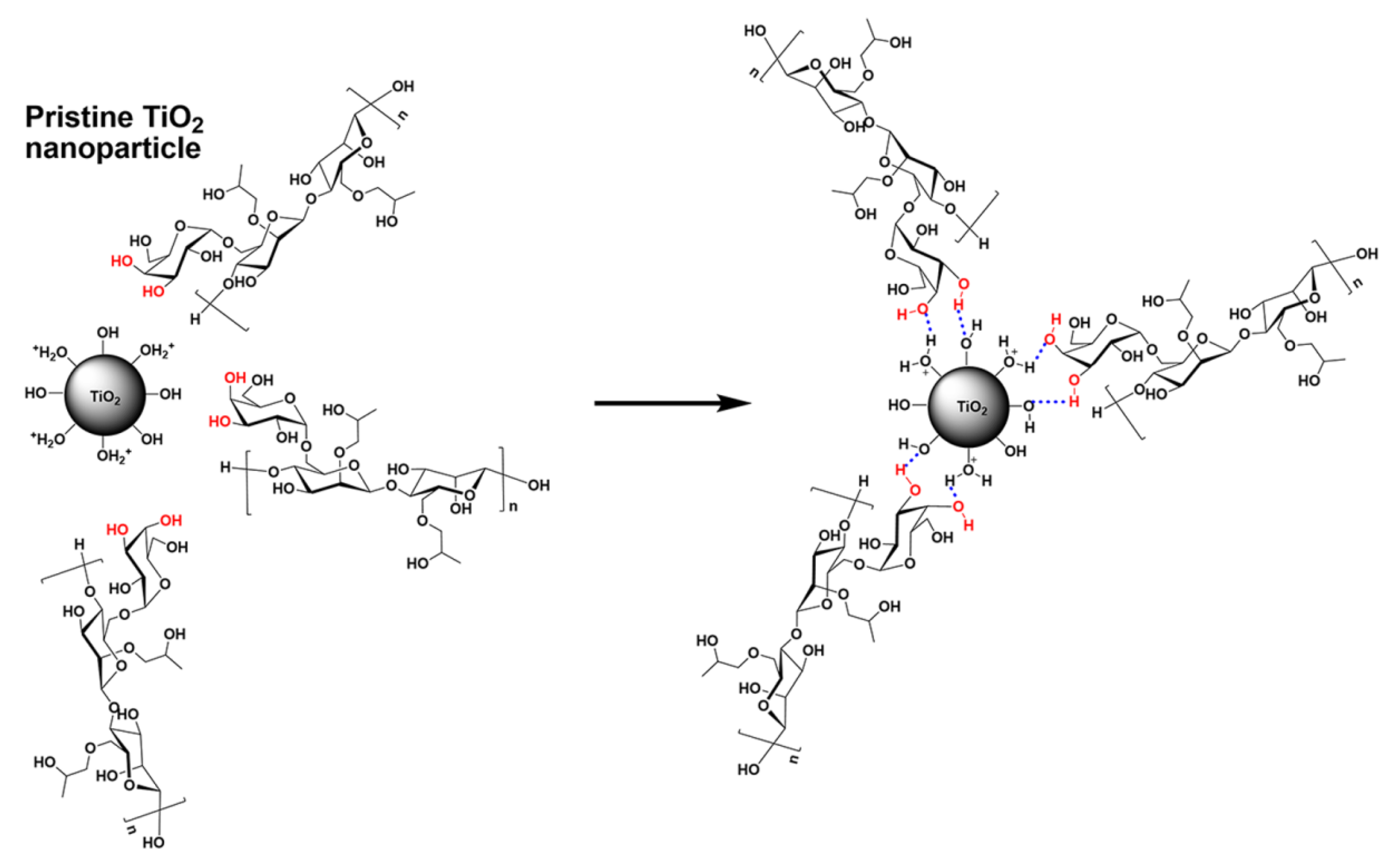
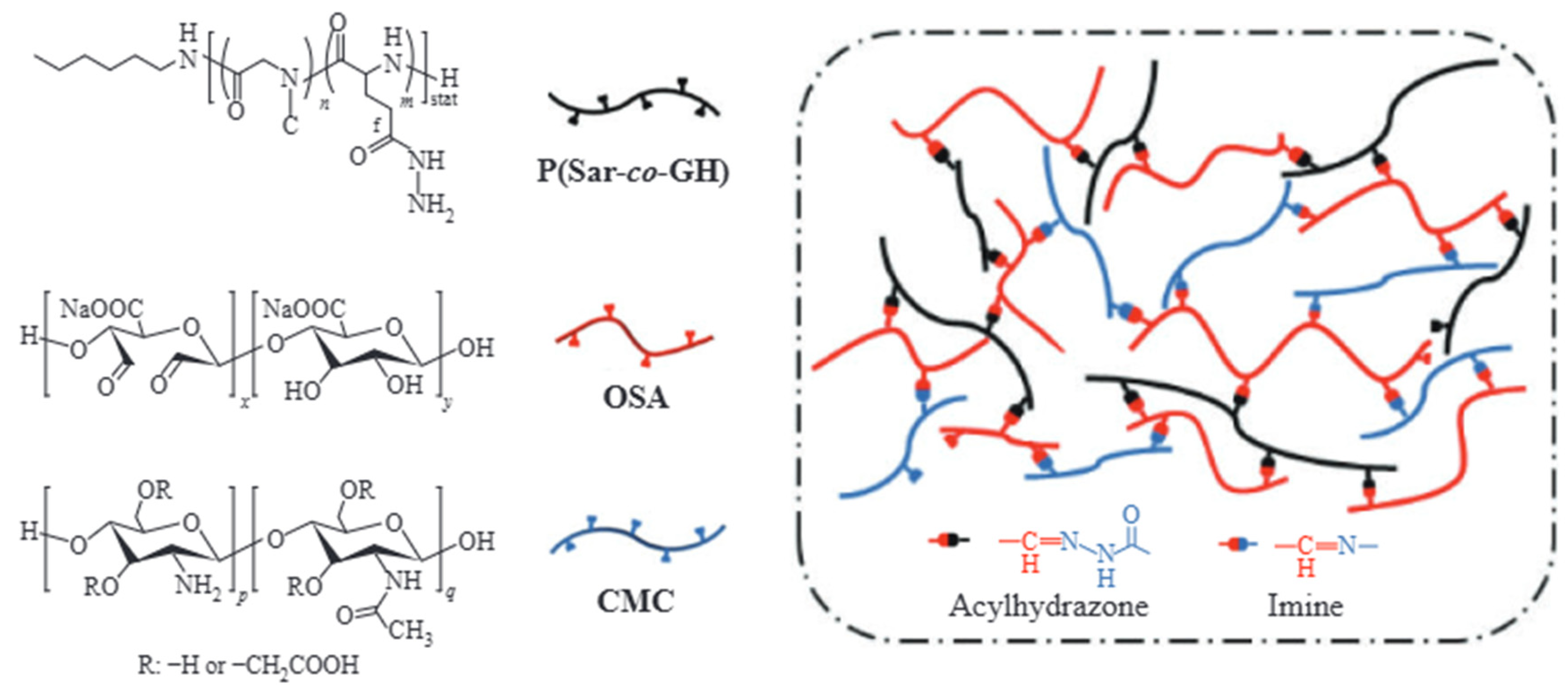
4.1.1. High-Temperature Resistant Crosslinking Systems
4.1.2. Filtrate Control and Clay Stabilization
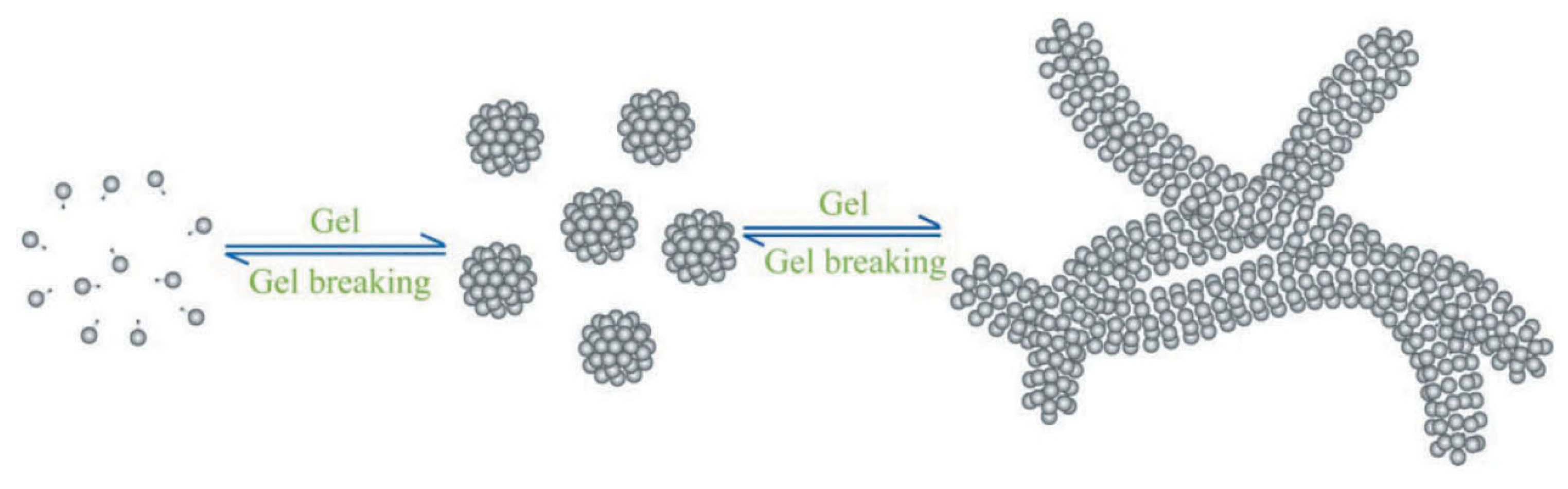
4.1.3. Smart Gel-Breaking and Rheology Modification
4.2. Nanoproppants for Enhanced Performance
4.2.1. High-Strength Composite Proppants
4.2.2. Wettability Modification and Conductivity Optimization
4.3. Waterless Fracturing and Environmental Sustainability
4.3.1. Nanoparticle-Stabilized Foam Systems
4.3.2. Liquid CO2/N2 Fracturing with Nanoparticle Additives
4.4. Nanotechnology for Monitoring and Multifunctional Integration
4.5. Challenges and Future Directions
4.5.1. Technical Hurdles
4.5.2. Future Research Priorities
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hou, Y.; Peng, Y.; Chen, Z.; Liu, Y.; Zhang, G.; Ma, Z.; Tian, W. Investigation on the Controlling Factors of Pressure Wave Propagation Behavior Induced by Pulsating Hydraulic Fracturing. SPE J. 2021, 26, 2716–2735. [Google Scholar] [CrossRef]
- Hofmann, H.; Babadagli, T.; Zimmermann, G. Hot water generation for oil sands processing from enhanced geothermal systems, Process simulation for different hydraulic fracturing scenarios. Appl. Energy 2014, 113, 524–547. [Google Scholar] [CrossRef]
- Rickards, A.R.; Brannon, H.D.; Wood, W.D. High Strength, Ultralightweight Proppant Lends New Dimensions to Hydraulic Fracturing Applications. SPE Prod. Oper. 2003, 21, 212–221. [Google Scholar] [CrossRef]
- Guo, K.; Li, H.; Yu, Z. In-situ heavy and extra-heavy oil recovery, A review. Fuel 2016, 185, 886–902. [Google Scholar] [CrossRef]
- Kuuskraa, V.A.; Stevens, S.H. How unconventional gas prospers without tax incentives. Oil Gas J. 1995, 93, 76–81. [Google Scholar]
- Wu, L.; Zhang, Y.; Di, S.; Tao, Z.; Liu, Z. Study of Fracture Propagation Mechanism of Horizontal Well Fracturing in Roof of Coal Seams. J. Energy Eng. 2024, 150, 8. [Google Scholar] [CrossRef]
- Nie, S.; Liu, K.; Zhong, X.; Wang, Y.; Yang, B.; Song, J. Research on Hydraulic Fracture Propagation Patterns in Multilayered Gas Hydrate Reservoirs Using a Three-Dimensional XFEM-Based Cohesive Zone Method. Energy Fuels 2024, 38, 5106–5123. [Google Scholar] [CrossRef]
- Li, J.; Luo, Z.; Zhang, N.; Zeng, X.; Jia, Y. Study on the Mechanism and Regulation Method of Longitudinal Penetration of Hydraulic Fractures in Multilayered Shale. SPE J. 2024, 29, 16. [Google Scholar] [CrossRef]
- Hanamertani, A.S.; Saraji, S.; Piri, M. The effects of in-situ emulsion formation and superficial velocity on foam performance in high-permeability porous media. Fuel 2021, 306, 121575. [Google Scholar] [CrossRef]
- Churchman, G.; Skjemstad, J.; Oades, J. Influence of clay minerals and organic matter on effects of sodicity on soils. Soil Res. 1993, 31, 779–800. [Google Scholar] [CrossRef]
- Wilson, M.J.; Wilson, L.; Patey, I. The influence of individual clay minerals on formation damage of reservoir sandstones, a critical review with some new insights. Clay Miner. 2016, 49, 147–164. [Google Scholar] [CrossRef]
- Barati, R.; Liang, J.-T. A review of fracturing fluid systems used for hydraulic fracturing of oil and gas wells. J. Appl. Polym. Sci. 2014, 131, 318–323. [Google Scholar] [CrossRef]
- Sun, J.; Schechter, D. Investigating the Effect of Improved Fracture Conductivity on Production Performance of Hydraulically Fractured Wells, Field-Case Studies and Numerical Simulations. J. Can. Pet. Technol. 2015, 54, 442–449. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, L.; Li, W.; Liu, H. Effect of Y(OH)3 microparticles on the electrochemical performance of alkaline zinc electrodes. J. Rare Earths 2009, 27, 980–985. [Google Scholar] [CrossRef]
- Aghaei, S.M.; Torres, I.; Calizo, I. Emergence of strong ferromagnetism in silicene nanoflakes via patterned hydrogenation and its potential application in spintronics. Comput. Mater. Sci. 2017, 138, 204–212. [Google Scholar] [CrossRef]
- Balbus, J.M.; Florini, K.; Denison, R.A.; Walsh, S.A. Protecting workers and the environment, An environmental NGO’s perspective on nanotechnology. J. Nanopart. Res. 2007, 9, 11–22. [Google Scholar] [CrossRef]
- von Gleich, A.; Steinfeldt, M.; Petschow, U. A suggested three-tiered approach to assessing the implications of nanotechnology and influencing its development. J. Clean. Prod. 2008, 16, 899–909. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, X. Multiple strategies to activate gold nanoparticles as antibiotics. Nanoscale 2013, 5, 8340–8350. [Google Scholar] [CrossRef]
- Yekeen, N.; Padmanabhan, E.; Idris, A.K.; Chauhan, P.S. Nanoparticles applications for hydraulic fracturing of unconventional reservoirs, A comprehensive review of recent advances and prospects. J. Pet. Sci. Eng. 2019, 174, 535–577. [Google Scholar] [CrossRef]
- Fu, C.; Liu, N. Waterless fluids in hydraulic fracturing—A Review. J. Nat. Gas Sci. Eng. 2019, 63, 102587. [Google Scholar] [CrossRef]
- Gurluk, M.R.; Nasr-El-Din, H.A.; Crews, J.B. Enhancing the performance of viscoelastic surfactant fluids using nanoparticles. In Proceedings of the SPE Annual Technical Conference and Exhibition, London, UK, 10–13 June 2013. SPE-164900-MS. [Google Scholar]
- Qiming, H.; Shimin, L.; Gang, W.; Bing, W.; Yongzhi, Z. Coalbed methane reservoir stimulation using guar-based fracturing fluid: A review. J. Nat. Gas Sci. Eng. 2019, 66, 107–125. [Google Scholar] [CrossRef]
- Ahmed, M.E.; Sultan, A.S.; Adebayo, A.R.; Babu, R.S.; Mahmoud, M.; Patil, S. Exploring the Impact of Chelating Agents on Viscoelastic Surfactant Flooding on Sandstone Cores Using Magnetic Resonance Imaging. Energy Fuels 2024, 38, 5022–5033. [Google Scholar] [CrossRef]
- Zhang, Z.; Pan, H.; Liu, P.; Zhao, M.; Li, X.; Zhang, Z. Boric acid incorporated on the surface of reactive nanosilica providing a nano-crosslinker with potential in guar gum fracturing fluid. J. Appl. Polym. Sci. 2017, 134, 45037. [Google Scholar] [CrossRef]
- Hurnaus, T.; Plank, J. Behavior of titania nanoparticles in cross-linking hydroxypropyl guar used in hydraulic fracturing fluids for oil recovery. Energy Fuels 2015, 29, 3601–3608. [Google Scholar] [CrossRef]
- Aderibigbe, A.; Cheng, K.; Heidari, Z.; Killough, J.; Fuss-Dezelic, T. Application of magnetic nanoparticles mixed with propping agents in enhancing near–wellbore fracture detection. J. Pet. Sci. Eng. 2016, 141, 133–143. [Google Scholar] [CrossRef]
- Emrani, A.S.; Ibrahim, A.F.; Nasr-El-Din, H.A. Mobility control using nanoparticle–stabilized CO2 foam as a hydraulic fracturing fluid. In Proceedings of the 79th EAGE Conference & Exhibition, Paris, France, 12–15 June 2017. SPE-185863-MS. [Google Scholar]
- Hao, C.; Amro, S.E.; Yunshen, C.; Jose, A.N.; Ali, M.A.; George, J.H.; Quoc, P.N.; Sibani, L.B.; Shenglai, Y.; Keith, P.J. Oil effect on CO2 foam stabilized by a switchable amine surfactant at high temperature and high salinity. Fuel 2018, 227, 247–255. [Google Scholar] [CrossRef]
- An, C.; Yan, B.; Alfi, M.; Killough, J.E. A new study of magnetic nanoparticle transport and quantifying magnetization analysis in fractured shale reservoir using numerical modeling. J. Nat. Gas Sci. Eng. 2016, 28, 502–521. [Google Scholar] [CrossRef]
- Rong, X.; Li, Y.; Zhang, L. Experimental study on the effect of nano-scale stabilizers on clay swelling inhibition. J. Nat. Gas Sci. Eng. 2017, 44, 424–431. [Google Scholar]
- Fakoya, M.; Shah, S. Enhancement of filtration properties in surfactant–based and polymeric fluids by nanoparticles. In Proceedings of the SPE Eastern Regional Meeting, Charleston, WV, USA, 21–23 October 2014. SPE-171029-MS. [Google Scholar]
- Lv, Q.; Li, Z.; Li, B.; Li, S.; Sun, Q. Study of nanoparticle–surfactant–stabilized foam as a fracturing fluid. Ind. Eng. Chem. Res. 2015, 54, 9468–9477. [Google Scholar] [CrossRef]
- Morrow, L.; Potter, D.K.; Barron, A.R. Detection of magnetic nanoparticles against proppant and shale reservoir rocks. J. Exp. Nanosci. 2015, 10, 1028–1041. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, Y.; Zou, C.; Dai, C.; Gao, M.; Li, Y.; Lv, W.; Jiang, J.; Wu, Y. Can More Nanoparticles Induce Larger Viscosities of Nanoparticle-Enhanced Wormlike Micellar System (NEWMS)? Materials 2017, 10, 1096. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Zhang, Y.; Gao, M.; Li, Y.; Lv, W.; Wang, X.; Wu, Y.; Zhao, M. The Study of a Novel Nanoparticle-Enhanced Wormlike Micellar System. Nanoscale Res. Lett. 2017, 12, 431. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Jiang, T.; Zhang, S. Novel Nanocomposite Fiber-Laden Viscoelastic Fracturing Fluid for Coal Bed Methane Reservoir Stimulation. J. Energy Resour. Technol. 2017, 139, 022906. [Google Scholar] [CrossRef]
- Yang, X.; Mao, J.; Chen, Z.; Chen, Y.; Zhao, J. Clean fracturing fluids for tight reservoirs, opportunities with viscoelastic surfactant. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 1881–1895. [Google Scholar] [CrossRef]
- Gupta, D.V.S. Unconventional fracturing fluids for tight gas reservoirs. In Proceedings of the SPE Hydraulic Fracturing Technology Conference, The Woodlands, TX, USA, 19–21 January 2009. SPE-119424-MS. [Google Scholar]
- Hanafy, A.; Najem, F.; Nasr–El–Din, H.A. Impact of Nanoparticles Shape on the VES Performance for High Temperature Applications. In Proceedings of the SPE Western Regional Meeting, Garden Grove, CA, USA, 22–26 April 2018. SPE-190099-MS. [Google Scholar]
- Baruah, A.; Pathak, A.K.; Ojha, K. Study on rheology and thermal stability of mixed (nonionic–anionic) surfactant based fracturing fluids. AIChE J. 2016, 62, 2177–2187. [Google Scholar] [CrossRef]
- Chauhan, G.; Ojha, K.; Baruah, A. Effects of nanoparticles and surfactant charge groups on the properties of VES gel. Braz. J. Chem. Eng. 2017, 34, 241–251. [Google Scholar] [CrossRef]
- Singh, R.; Mohanty, K.K. Synergy between nanoparticles and surfactants in stabilizing foams for oil recovery. Energy Fuels 2015, 29, 467–479. [Google Scholar] [CrossRef]
- Worthen, A.J.; Bagaria, H.G.; Chen, Y.; Bryant, S.L.; Huh, C.; Johnston, K.P. Nanoparticle stabilized carbon dioxide in water foams for enhanced oil recovery. In Proceedings of the SPE Annual Technical Conference and Exhibition, Tulsa, OK, USA, 14–18 April 2012. SPE-154285-MS. [Google Scholar]
- Al-Yousef, Z.A.; Almobarky, M.A.; Schechter, D.S. The effect of nanoparticle aggregation on surfactant foam stability. J. Colloid Interface Sci. 2018, 511, 365–373. [Google Scholar] [CrossRef]
- Ghobadi, S.; Sadighikia, S.; Papila, M.; Cebeci, F.Ç.; Gürsel, S.A. Graphene-reinforced Poly(vinyl alcohol) Electrospun Fibers as Building Blocks for High Performance Nanocomposites. RSC Adv. 2015, 5, 85009–85018. [Google Scholar] [CrossRef]
- Islam, A.; Islam, M.S.; Mondal, K.R.; Hossain, M.M. Load Forecasting Technique for System Expansion in Isolated Area Using Time Invariant Socio-Economic Factors of Identical Agglomerations. J. Energy Power Eng. 2013, 7, 1778–1785. [Google Scholar]
- Kaur, P.; Buttar, G.S.; Kaur, M.; Gil, M.; Sohu, R.S. Effect of foliar and split application of potassium on seed cotton yield and fibre quality of American cotton (Gossypium hirsutum). Indian J. Agric. Sci. 2011, 81, 838–842. [Google Scholar]
- Hughes, J.; Sharp, E.; Taylor, M.J.; Melton, L.; Hartley, G. Monitoring agricultural rodenticide use and secondary exposure of raptors in Scotland. Ecotoxicology 2013, 22, 974–984. [Google Scholar] [CrossRef]
- Duenckel, R.; Conway, M.W.; Eldred, B.; Vincent, M.C. Proppant Diagenesis-Integrated Analyses Provide New Insights Into Origin, Occurrence, and Implications for Proppant Performance. SPE Prod. Oper. 2012, 27, 131–144. [Google Scholar] [CrossRef]
- Sójka-Ledakowicz, J.; Chruściel, J.J.; Kudzin, M.H.; Łatwińska, M.; Kiwała, M. Antimicrobial Functionalization of Textile Materials with Copper Silicate. Fibres Text. East. Eur. 2016, 24, 151–156. [Google Scholar] [CrossRef]
- Yanping, T.; Yan, X. Preparation and properties of dual dynamic covalent bond crosslinked hydrogels. J. Funct. Polym. 2020, 33, 305. [Google Scholar]
- Irvine, E.B.; Reddy, S.T. Advancing antibody engineering through synthetic evolution and machine learning. J. Immunol. 2024, 212, 10. [Google Scholar] [CrossRef]
- Plescia, P.; Tempesta, E. Analysis of friction coefficients in a vibrating cup mill (ring mill) during grinding. Tribol. Int. 2017, 114, 458–468. [Google Scholar] [CrossRef]
- Di Toro, G.; Hirose, T.; Nielsen, S.; Pennacchioni, G.; Shimamoto, T. Natural and Experimental Evidence of Melt Lubrication of Faults During Earthquakes. Science 2006, 311, 647–649. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, L.; Lei, H.; Zhang, Q.; Zhao, W.; Liao, D.; Wang, D.; Xiong, Y.; Liu, L.; Liu, H.; Mei, Z. Research Progress in the Application of Nanotechnology in Fracturing: A Review. Nanomaterials 2025, 15, 1539. https://doi.org/10.3390/nano15201539
Liang L, Lei H, Zhang Q, Zhao W, Liao D, Wang D, Xiong Y, Liu L, Liu H, Mei Z. Research Progress in the Application of Nanotechnology in Fracturing: A Review. Nanomaterials. 2025; 15(20):1539. https://doi.org/10.3390/nano15201539
Chicago/Turabian StyleLiang, Lei, Huiru Lei, Qinwen Zhang, Wei Zhao, Dong Liao, Dong Wang, Yujia Xiong, Lang Liu, Hualin Liu, and Zilai Mei. 2025. "Research Progress in the Application of Nanotechnology in Fracturing: A Review" Nanomaterials 15, no. 20: 1539. https://doi.org/10.3390/nano15201539
APA StyleLiang, L., Lei, H., Zhang, Q., Zhao, W., Liao, D., Wang, D., Xiong, Y., Liu, L., Liu, H., & Mei, Z. (2025). Research Progress in the Application of Nanotechnology in Fracturing: A Review. Nanomaterials, 15(20), 1539. https://doi.org/10.3390/nano15201539




