Effect of Synthesis Conditions on Graphene Directly Grown on SiO2: Structural Features and Charge Carrier Mobility
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Synthesis Conditions Effects on Graphene Structure
3.2. Raman Spectroscopy and AFM Results
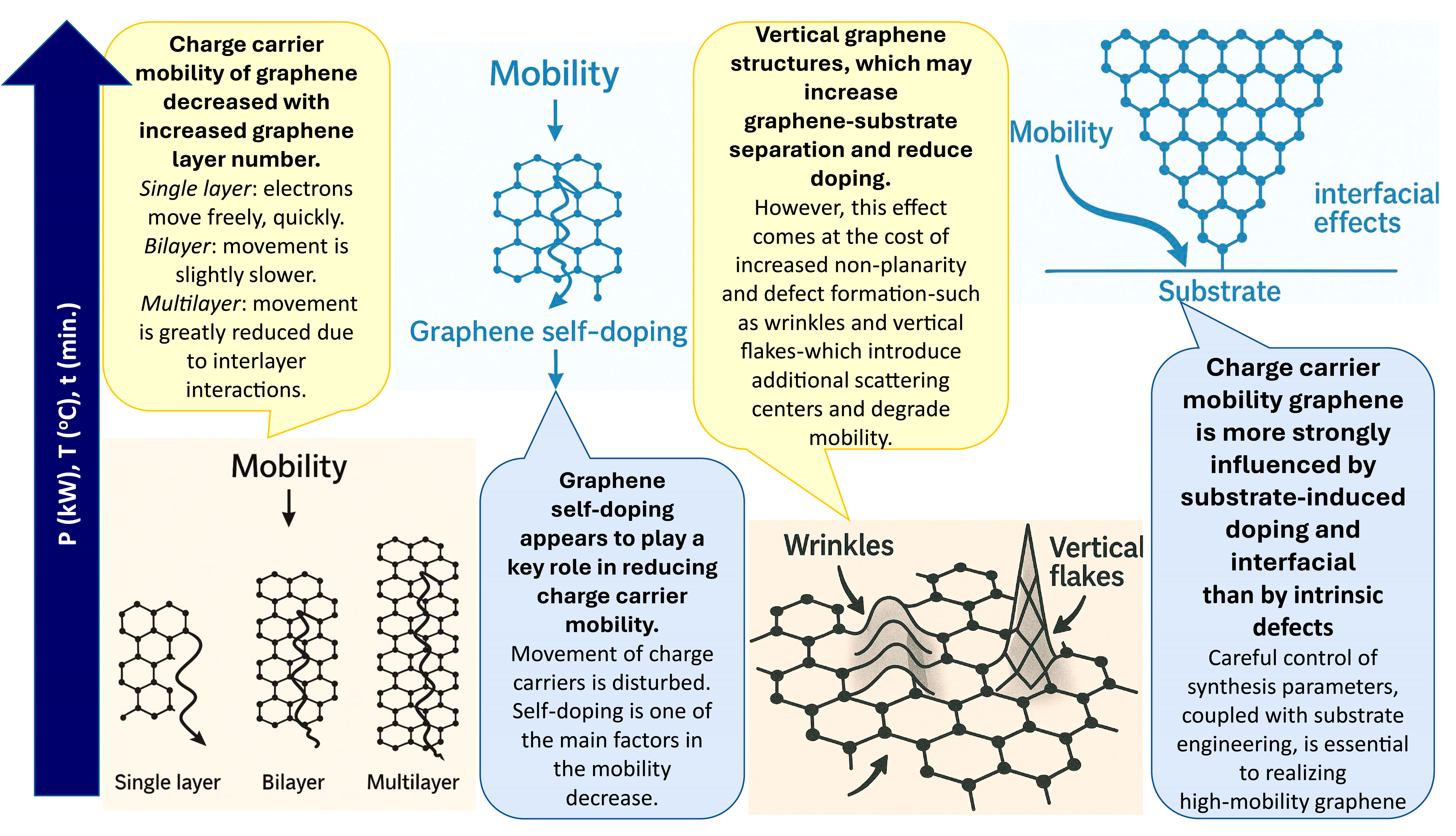
3.3. Synthesis Conditions Effects on Graphene Electrical Properties
3.4. Charge Carrier Mobility in Graphene and Raman Scattering Spectra Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Du, J.; Tong, B.; Yuan, S.; Dai, N.; Liu, R.; Zhang, D.; Cheng, H.M.; Ren, W. Advances in flexible optoelectronics based on chemical vapor deposition-grown graphene. Adv. Funct. Mater. 2022, 32, 2203115. [Google Scholar] [CrossRef]
- Huang, K.; Yu, X.; Cong, J.; Yang, D. Progress of graphene–silicon heterojunction photovoltaic devices. Adv. Mater. Interfaces 2018, 5, 1801520. [Google Scholar] [CrossRef]
- Li, X.; Tao, L.; Chen, Z.; Fang, H.; Li, X.; Wang, X.; Xu, J.-B.; Zhu, H. Graphene and related two-dimensional materials: Structure-property relationships for electronics and optoelectronics. Appl. Phys. Rev. 2017, 4, 021306. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Sun, Z.; Hasan, T.; Ferrari, A.C. Graphene photonics and optoelectronics. Nat. Photonics 2010, 4, 611–622. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Graphene on the pilot line. Nat. Mater. 2021, 20, 573. [CrossRef] [PubMed]
- Colmiais, I.; Silva, V.; Borme, J.; Alpuim, P.; Mendes, P.M. Towards RF graphene devices: A review. FlatChem 2022, 35, 100409. [Google Scholar] [CrossRef]
- Liu, Z.; Geng, M.; Chen, H.; Zhang, A.-Q.; Lu, W.B. A Perspective on the Recent Progress of Graphene in Microwave Applications: Problems, Challenges and Opportunities. IEEE Microw. Mag. 2023, 24, 40–53. [Google Scholar] [CrossRef]
- Lukosius, M.; Lukose, R.; Lisker, M.; Luongo, G.; Elviretti, M.; Mai, A.; Wenger, C. Graphene Research in 200 mm CMOS Pilot Line. In Proceedings of the 2022 45th Jubilee International Convention on Information, Communication and Electronic Technology (MIPRO), Opatija, Croatia, 23–27 May 2022; pp. 113–117. [Google Scholar]
- Szunerits, S.; Rodrigues, T.; Bagale, R.; Happy, H.; Boukherroub, R.; Knoll, W. Graphene-based field-effect transistors for biosensing: Where is the field heading to? Anal. Bioanal. Chem. 2023, 416, 2137–2150. [Google Scholar] [CrossRef]
- Wang, Z.; Yi, K.; Lin, Q.; Yang, L.; Chen, X.; Chen, H.; Liu, Y.; Wei, D. Free radical sensors based on inner-cutting graphene field-effect transistors. Nat. Commun. 2019, 10, 1544. [Google Scholar] [CrossRef]
- Yeh, C.-H.; Lain, Y.-W.; Chiu, Y.-C.; Liao, C.; Moyano, D.R.; Hsu, S.S.H.; Chiu, P.-W. Gigahertz flexible graphene transistors for microwave integrated circuits. ACS Nano 2014, 8, 7663–7670. [Google Scholar] [CrossRef]
- Liang, X.; Sperling, B.A.; Calizo, I.G.; Cheng, G.; Hacker, C.A.; Zhang, Q.; Obeng, Y.S.; Yan, K.; Peng, H.; Li, Q.; et al. Toward clean and crackless transfer of graphene. ACS Nano 2011, 5, 9144–9153. [Google Scholar] [CrossRef]
- Lupina, G.; Kitzmann, J.; Costina, I.; Lukosius, M.; Wenger, C.; Wolff, A.; Vaziri, S.; Östling, M.; Pasternak, I.; Krajewska, A.; et al. Residual metallic contamination of transferred chemical vapor deposited graphene. ACS Nano 2015, 9, 4776–4785. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Shen, Z.-g. A review on mechanical exfoliation for the scalable production of graphene. J. Mater. Chem. 2015, 3, 11700–11715. [Google Scholar] [CrossRef]
- Li, M.; Liu, D.; Wei, D.; Song, X.; Wei, D.; Wee, A.T.S. Controllable Synthesis of Graphene by Plasma-Enhanced Chemical Vapor Deposition and Its Related Applications. Adv. Sci. 2016, 3, 1600003. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Bi, H.; Wan, D.; Huang, F.; Xie, X.; Jiang, M. Direct PECVD growth of vertically erected graphene walls on dielectric substrates as excellent multifunctional electrodes. J. Mater. Chem. 2013, 1, 770–775. [Google Scholar] [CrossRef]
- Rehman, M.A.; Roy, S.B.; Akhtar, I.; Bhopal, M.F.; Choi, W.; Nazir, G.; Khan, M.F.; Kumar, S.; Eom, J.; Chun, S.-H.; et al. Thickness-dependent efficiency of directly grown graphene based solar cells. Carbon 2019, 148, 187–195. [Google Scholar] [CrossRef]
- Chen, J.; Wen, Y.; Guo, Y.; Wu, B.; Huang, L.; Xue, Y.; Geng, D.; Wang, D.; Yu, G.; Liu, Y. Oxygen-aided synthesis of polycrystalline graphene on silicon dioxide substrates. J. Am. Chem. Soc. 2011, 133, 17548–17551. [Google Scholar] [CrossRef]
- Luo, J.; Wang, J.; Xia, F.; Huang, X. Catalyst-free synthesis of few-layer graphene films on silicon dioxide/Si substrates using ethylene glycol by chemical vapor deposition. Mater. Res. Express 2018, 6, 035602. [Google Scholar] [CrossRef]
- Du, L.; Yang, L.; Hu, Z.; Zhang, J.; Huang, C.; Sun, L.; Wang, L.; Wei, D.; Chen, G.; Lu, W. Thickness-controlled direct growth of nanographene and nanographite film on non-catalytic substrates. Nanotechnology 2018, 29, 215711. [Google Scholar] [CrossRef]
- Liu, Q.; Gong, Y.; Wang, T.; Chan, W.L.; Wu, J.Z. Metal-catalyst-free and controllable growth of high-quality monolayer and AB-stacked bilayer graphene on silicon dioxide. Carbon 2016, 96, 203–211. [Google Scholar] [CrossRef]
- Hopfe, V.; Spitzl, R.; Dani, I.; Maeder, G.; Roch, L.; Rogler, D.; Leupolt, B.; Schoeneich, B. Remote microwave PECVD for continuous, wide-area coating under atmospheric pressure. Chem. Vap. Depos. 2005, 11, 497–509. [Google Scholar] [CrossRef]
- Engemann, J.; Walter, M. Modelling of microwave plasma sources: Potential and applications. Plasma Phys. Control. Fusion 1999, 41, B259. [Google Scholar] [CrossRef]
- Sung-Spitzl, H. Device for the Production of Homogenous Microwave Plasma. U.S. Patent No. 6,543,380, 8 April 2003. [Google Scholar]
- Spitzl, R.; Aschermann, B.; Walter, M. Microwave Plasma Generator with the Short Cross-Sectional Side of the Resonator Parallel to the Chamber Axis. U.S. Patent No. 6,198,224, 6 March 2001. [Google Scholar]
- Meškinis, Š.; Lazauskas, A.; Jankauskas, Š.; Guobienė, A.; Gudaitis, R. Advancing Graphene Synthesis: Low-Temperature Growth and Hydrogenation Mechanisms Using Plasma-Enhanced Chemical Vapor Deposition. Molecules 2024, 30, 33. [Google Scholar] [CrossRef]
- Meškinis, Š.; Vasiliauskas, A.; Guobienė, A.; Talaikis, M.; Niaura, G.; Gudaitis, R. The direct growth of planar and vertical graphene on Si(100) via microwave plasma chemical vapor deposition: Synthesis conditions effects. RSC Adv. 2022, 12, 18759–18772. [Google Scholar] [CrossRef]
- Zhong, H.; Zhang, Z.; Xu, H.; Qiu, C.; Peng, L.-M. Comparison of mobility extraction methods based on field-effect measurements for graphene. AIP Adv. 2015, 5, 057136. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, R.; Zhao, Y.; Li, Z.; Zhang, W.; Tian, J. Independently tunable bifunctional terahertz metasurface based on double-layer graphene. Opt. Mater. 2022, 132, 112793. [Google Scholar] [CrossRef]
- Kumar, B.; Min, K.; Bashirzadeh, M.; Farimani, A.B.; Bae, M.-H.; Estrada, D.; Kim, Y.D.; Yasaei, P.; Park, Y.D.; Pop, E.; et al. The role of external defects in chemical sensing of graphene field-effect transistors. Nano Lett. 2013, 13, 1962–1968. [Google Scholar] [CrossRef]
- Khan, A.; Islam, S.M.; Ahmed, S.; Kumar, R.R.; Habib, M.R.; Huang, K.-F.; Hu, M.; Yu, X.; Yang, D. Direct CVD Growth of Graphene on Technologically Important Dielectric and Semiconducting Substrates. Adv. Sci. 2018, 5, 1800050. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-s.; Lin, Y.-H.; Hwang, J.-Y.; Chang, R.; Chattopadhyay, S.; Chen, C.-J.; Chen, P.; Chiang, H.-P.; Tsai, T.-r.; Chen, L.C.; et al. Imaging layer number and stacking order through formulating Raman fingerprints obtained from hexagonal single crystals of few layer graphene. Nanotechnology 2013, 24, 015702. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Ren, L.; Gao, S.; Shi, L. Histogram method for reliable thickness measurements of graphene films using atomic force microscopy (AFM). J. Mater. Sci. Technol. 2017, 33, 815–820. [Google Scholar] [CrossRef]
- Casiraghi, C.; Pisana, S.; Novoselov, K.S.; Geim, A.K.; Ferrari, A.C. Raman Fingerprint of Charged Impurities in Graphene. Appl. Phys. Lett. 2007, 91, 233108. [Google Scholar] [CrossRef]
- Lee, Y.G.; Lim, S.K.; Kang, C.G.; Kim, Y.J.; Choi, D.H.; Chung, H.-J.; Choi, R.; Lee, B.H. Origin of the channel width dependent field effect mobility of graphene field effect transistors. Microelectron. Eng. 2016, 163, 55–59. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Kuo, C.-C.; Chen, L.C.; Chen, K.-H. Correlating defect density with carrier mobility in large-scaled graphene films: Raman spectral signatures for the estimation of defect density. Nanotechnology 2010, 21, 465705. [Google Scholar] [CrossRef]
- Scarfe, S.; Cui, W.-w.; Luican-Mayer, A.; Ménard, J.-M. Systematic THz study of the substrate effect in limiting the mobility of graphene. Sci. Rep. 2021, 11, 8729. [Google Scholar] [CrossRef]
- Lin, Z.; Ye, X.; Han, J.; Chen, Q.; Fan, P.; Zhang, H.; Xie, D.; Zhu, H.; Zhong, M. Precise Control of the Number of Layers of Graphene by Picosecond Laser Thinning. Sci. Rep. 2015, 5, 11662. [Google Scholar] [CrossRef]
- Simionescu, O.-G.; Avram, A.; Adiaconiţă, B.; Preda, P.; Parvulescu, C.C.; Năstase, F.; Chiriac, E.; Avram, M. Field-Effect Transistors Based on Single-Layer Graphene and Graphene-Derived Materials. Micromachines 2023, 14, 1096. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Zhao, X.; Qiao, Z.; Jung, J.; Zhu, Y.; Lu, Y.; Zhang, L.L.; MacDonald, A.H.; Ruoff, R.S. Capacitance of carbon-based electrical double-layer capacitors. Nat. Commun. 2014, 5, 3317. [Google Scholar] [CrossRef] [PubMed]
- Gosling, J.H.; Makarovsky, O.; Wang, F.; Cottam, N.D.; Greenaway, M.T.; Patané, A.; Wildman, R.D.; Tuck, C.J.; Turyanska, L.; Fromhold, T.M. Universal mobility characteristics of graphene originating from charge scattering by ionised impurities. Commun. Phys. 2021, 4, 30. [Google Scholar] [CrossRef]
- Lee, J.E.; Ahn, G.; Shim, J.; Lee, Y.S.; Ryu, S. Optical separation of mechanical strain from charge doping in graphene. Nat. Commun. 2012, 3, 1024. [Google Scholar] [CrossRef]
- Bo, Z.; Yang, Y.; Chen, J.; Yu, K.; Yan, J.H.; Cen, K. Plasma-enhanced chemical vapor deposition synthesis of vertically oriented graphene nanosheets. Nanoscale 2013, 5, 5180–5204. [Google Scholar] [CrossRef]
- Soin, N.; Sinha, S.; O’Kane, C.; McLaughlin, J.A.; Lim, T.H.; Hetherington, C. Exploring the fundamental effects of deposition time on the microstructure of graphene nanoflakes by Raman scattering and X-ray diffraction. CrystEngComm 2011, 13, 312–318. [Google Scholar] [CrossRef]
- Mamiyev, Z.; Balayeva, N.O.; Ghosal, C.; Zahn, D.R.; Tegenkamp, C. Confinement induced strain effects in epitaxial graphene. Carbon 2025, 234, 120002. [Google Scholar] [CrossRef]
- Limbu, T.B.; Hernández, J.C.; Mendoza, F.; Katiyar, R.K.; Razink, J.J.; Makarov, V.I.; Weiner, B.R.; Morell, G. A Novel Approach to the Layer-Number-Controlled and Grain-Size-Controlled Growth of High Quality Graphene for Nanoelectronics. ACS Appl. Nano Mater. 2018, 1, 1502–1512. [Google Scholar] [CrossRef]
- Peng, H.; Schröter, N.B.; Yin, J.; Wang, H.; Chung, T.F.; Yang, H.; Ekahana, S.; Liu, Z.; Jiang, J.; Yang, L. Substrate doping effect and unusually large angle van Hove singularity evolution in twisted bi-and multilayer graphene. Adv. Mater. 2017, 29, 1606741. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yang, R.; Cheng, M.; Yang, W.; Xie, G.; Chen, P.; Shi, D.; Zhang, G. Defect-enhanced coupling between graphene and SiO2 substrate. Appl. Phys. Lett. 2014, 105, 063113. [Google Scholar] [CrossRef]
- Nagashio, K.; Nishimura, T.; Kita, K.; Toriumi, A. Mobility Variations in Mono- and Multi-Layer Graphene Films. Appl. Phys. Express 2008, 2, 025003. [Google Scholar] [CrossRef]
- Dorgan, V.E.; Bae, M.-H.; Pop, E. Mobility and Saturation Velocity in Graphene on SiO2. Appl. Phys. Lett. 2010, 97, 082112. [Google Scholar] [CrossRef]
- Chaitoglou, S.; Bertran, E. Effect of temperature on graphene grown by chemical vapor deposition. J. Mater. Sci. 2017, 52, 8348–8356. [Google Scholar] [CrossRef]
- Santhosh, N.M.; Filipič, G.; Tatarova, E.S.; Baranov, O.O.; Kondo, H.; Sekine, M.; Hori, M.; Ostrikov, K.K.; Cvelbar, U. Oriented Carbon Nanostructures by Plasma Processing: Recent Advances and Future Challenges. Micromachines 2018, 9, 565. [Google Scholar] [CrossRef]
- Baranov, O.O.; Levchenko, I.; Xu, S.; Lim, J.W.M.; Cvelbar, U.; Bazaka, K. Formation of vertically oriented graphenes: What are the key drivers of growth? 2D Mater. 2018, 5, 044002. [Google Scholar] [CrossRef]
- Kumar, K.; Kim, Y.-S.; Yang, E.H. The influence of thermal annealing to remove polymeric residue on the electronic doping and morphological characteristics of graphene. Carbon 2013, 65, 35–45. [Google Scholar] [CrossRef]
- Ni, G.; Zheng, Y.; Bae, S.; Kim, H.R.; Pachoud, A.J.; Kim, Y.S.; Tan, C.; Im, D.; Ahn, J.-H.; Hong, B.H.; et al. Quasi-periodic nanoripples in graphene grown by chemical vapor deposition and its impact on charge transport. ACS Nano 2012, 6, 1158–1164. [Google Scholar] [CrossRef]
- Dean, C.R.; Young, A.F.; Meric, I.; Lee, C.H.; Wang, L.; Sorgenfrei, S.; Watanabe, K.; Taniguchi, T.; Kim, P.; Shepard, K.L.; et al. Boron nitride substrates for high-quality graphene electronics. Nat. Nanotechnol. 2010, 5, 722–726. [Google Scholar] [CrossRef]
- Kang, Y.-J.; Kang, J.; Chang, K.J. Electronic structure of graphene and doping effect on SiO2. Phys. Rev. B 2008, 78, 115404. [Google Scholar] [CrossRef]
- Zhao, W.; Tan, P.H.; Liu, J.; Ferrari, A.C. Intercalation of Few-Layer Graphite Flakes with FeCl3: Raman Determination of Fermi Level, Layer by Layer Decoupling, and Stability. J. Am. Chem. Soc. 2011, 133, 5941–5946. [Google Scholar] [CrossRef]
- Szirmai, P.; Márkus, B.G.; Chacón-Torres, J.C.; Eckerlein, P.; Edelthalhammer, K.; Englert, J.M.; Mundloch, U.; Hirsch, A.; Hauke, F.; Náfrádi, B.; et al. Characterizing the maximum number of layers in chemically exfoliated graphene. Sci. Rep. 2019, 9, 19480. [Google Scholar] [CrossRef] [PubMed]
- Childres, I.; Jauregui, L.A.; Tian, J.; Chen, Y.P. Effect of oxygen plasma etching on graphene studied using Raman spectroscopy and electronic transport measurements. New J. Phys. 2011, 13, Article 025008. [Google Scholar] [CrossRef]
- Sakavičius, A.; Astromskas, G.; Bukauskas, V.; Kamarauskas, M.; Lukša, A.; Nargelienė, V.; Niaura, G.; Ignatjev, I.; Treideris, M.; Šetkus, A. Long distance distortions in the graphene near the edge of planar metal contacts. Thin Solid Films 2020, 698, 137850. [Google Scholar] [CrossRef]
- Kim, S.; Ryu, S. Thickness-dependent native strain in graphene membranes visualized by Raman spectroscopy. Carbon 2016, 100, 283–290. [Google Scholar] [CrossRef]
- Armano, A.; Buscarino, G.; Cannas, M.; Gelardi, F.M.; Giannazzo, F.; Schilirò, E.; Agnello, S. Monolayer graphene doping and strain dynamics induced by thermal treatments in controlled atmosphere. Carbon 2018, 127, 270–279. [Google Scholar] [CrossRef]
- Neumann, C.; Reichardt, S.; Venezuela, P.; Drögeler, M.; Banszerus, L.; Schmitz, M.; Watanabe, K.; Taniguchi, T.; Mauri, F.; Beschoten, B.; et al. Raman spectroscopy as probe of nanometre-scale strain variations in graphene. Nat. Commun. 2015, 6, 8429. [Google Scholar] [CrossRef] [PubMed]
- Lee, U.; Han, Y.; Lee, S.; Kim, J.S.; Lee, Y.H.; Kim, U.J.; Son, H. Time Evolution Studies on Strain and Doping of Graphene Grown on a Copper Substrate Using Raman Spectroscopy. ACS Nano 2020, 14, 919–926. [Google Scholar] [CrossRef]
- Wu, J.-B.; Lin, M.-L.; Cong, X.; Liu, H.-N.; Tan, P.-H. Raman spectroscopy of graphene-based materials and its applications in related devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef]
- Zeng, Y.; Lo, C.-L.; Zhang, S.; Chen, Z.; Marconnet, A. Dynamically tunable thermal transport in polycrystalline graphene by strain engineering. Carbon 2020, 158, 63–68. [Google Scholar] [CrossRef]
- Mohiuddin, T.M.G.; Lombardo, A.; Nair, R.R.; Bonetti, A.; Savini, G.; Jalil, R.; Bonini, N.; Basko, D.M.; Galiotis, C.; Marzari, N.; et al. Uniaxial Strain in Graphene by Raman Spectroscopy: G peak splitting, Gruneisen Parameters and Sample Orientation. Phys. Rev. B. 2009, 79, 205433. [Google Scholar] [CrossRef]
- Ni, Z.H.; Yu, T.; Lu, Y.H.; Wang, Y.Y.; Feng, Y.P.; Shen, Z.X. Uniaxial Strain on Graphene: Raman Spectroscopy Study and Band-Gap Opening. ACS Nano 2008, 2, 2301–2305. [Google Scholar] [CrossRef]
- Chugh, S.; Mehta, R.; Lu, N.; Dios, F.D.; Kim, M.J.; Chen, Z. Comparison of graphene growth on arbitrary non-catalytic substrates using low-temperature PECVD. Carbon 2015, 93, 393–399. [Google Scholar] [CrossRef]
- Eckmann, A.; Felten, A.; Mishchenko, A.; Britnell, L.; Krupke, R.; Novoselov, K.S.; Casiraghi, C. Probing the Nature of Defects in Graphene by Raman Spectroscopy. Nano Lett. 2012, 12, 3925–3930. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.M.; Sanchez Rodrigues, V.; Freires, F.G.M. Use of Partial Least Squares Structural Equation Modeling (PLS-SEM) to Improve Plastic Waste Management. Appl. Sci. 2024, 14, 628. [Google Scholar] [CrossRef]
- Di Leo, G.; Sardanelli, F. Statistical significance: P value, 0.05 threshold, and applications to radiomics—reasons for a conservative approach. Eur. Radiol. Exp. 2020, 4, 18. [Google Scholar] [CrossRef] [PubMed]
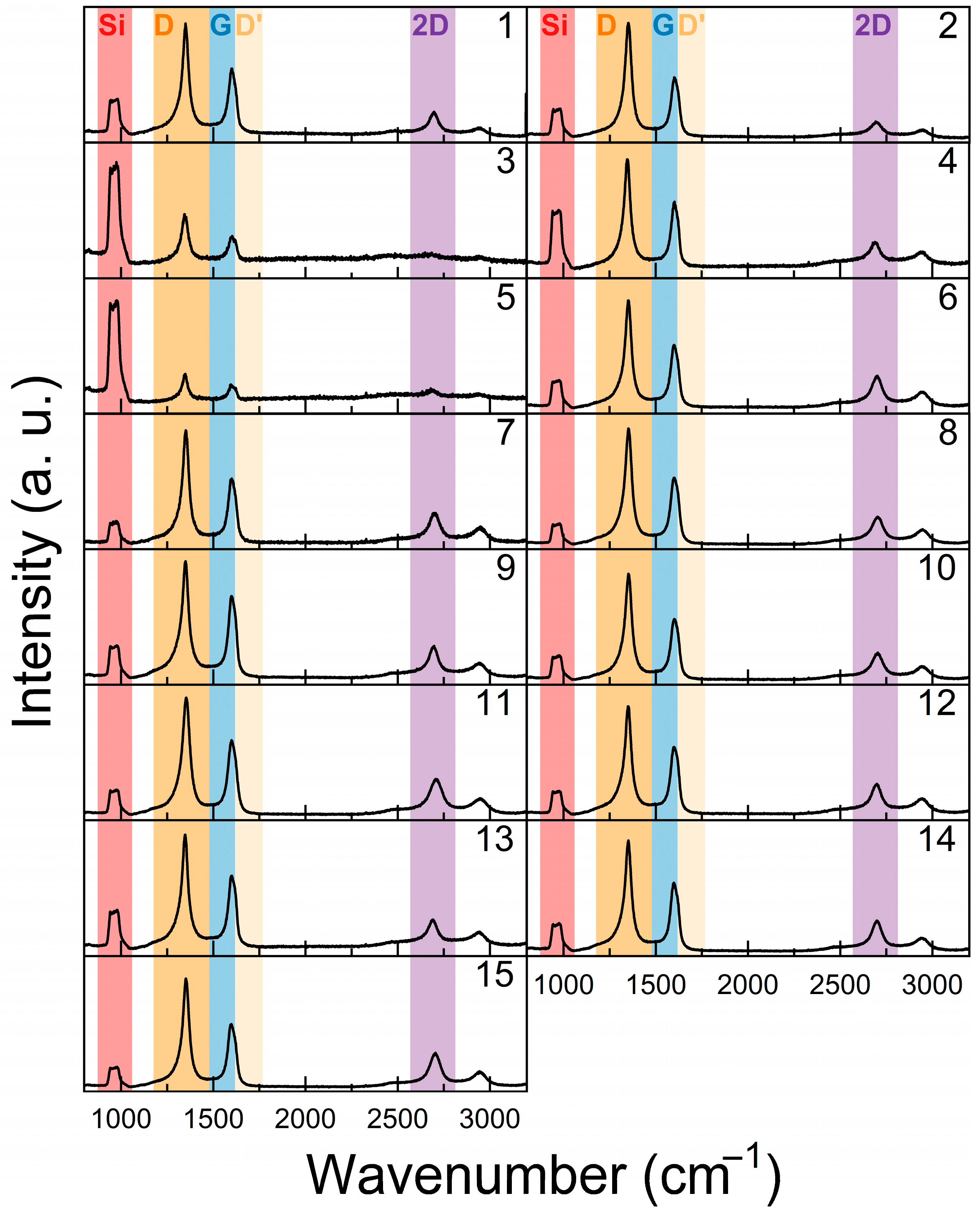
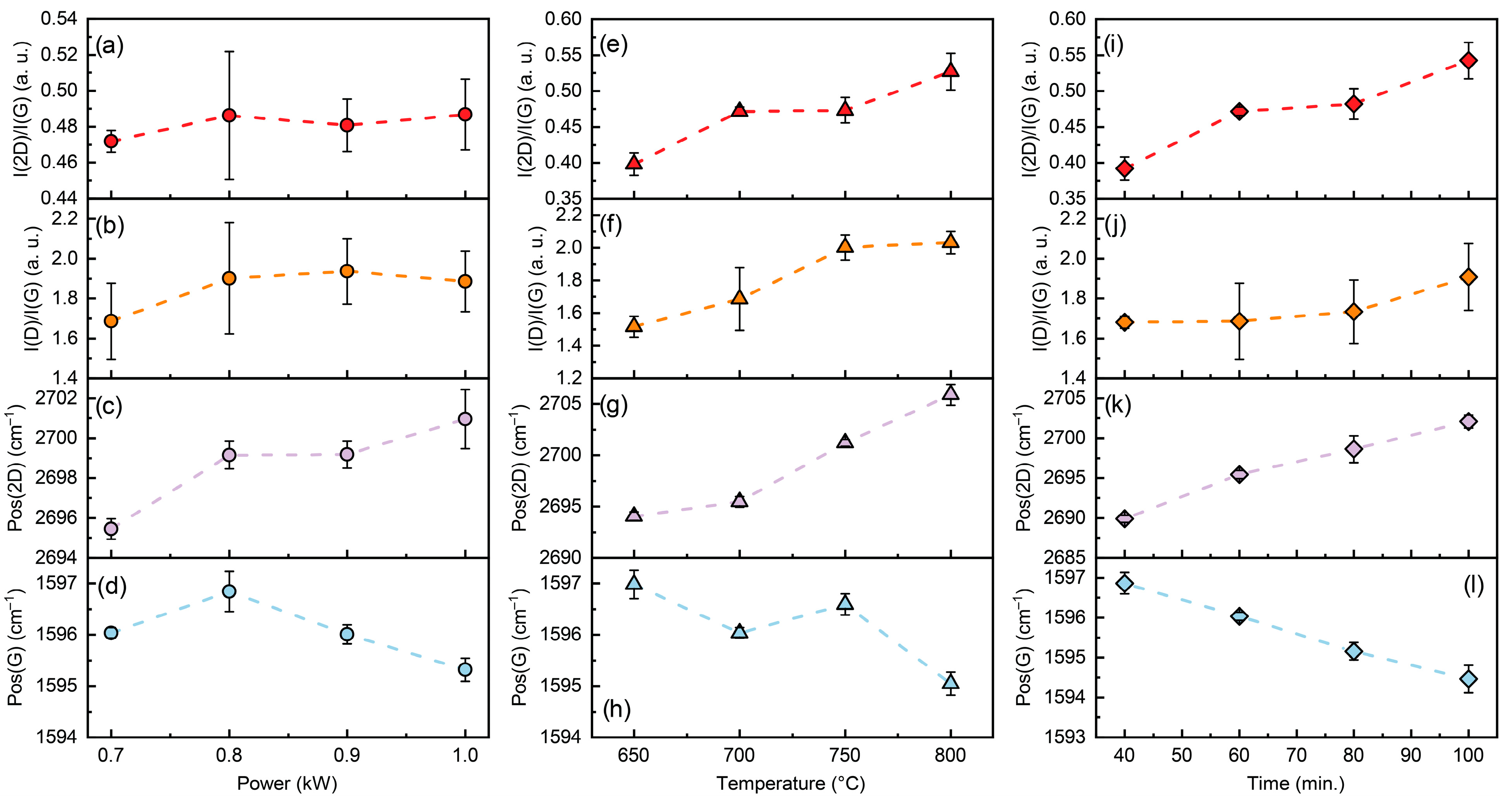
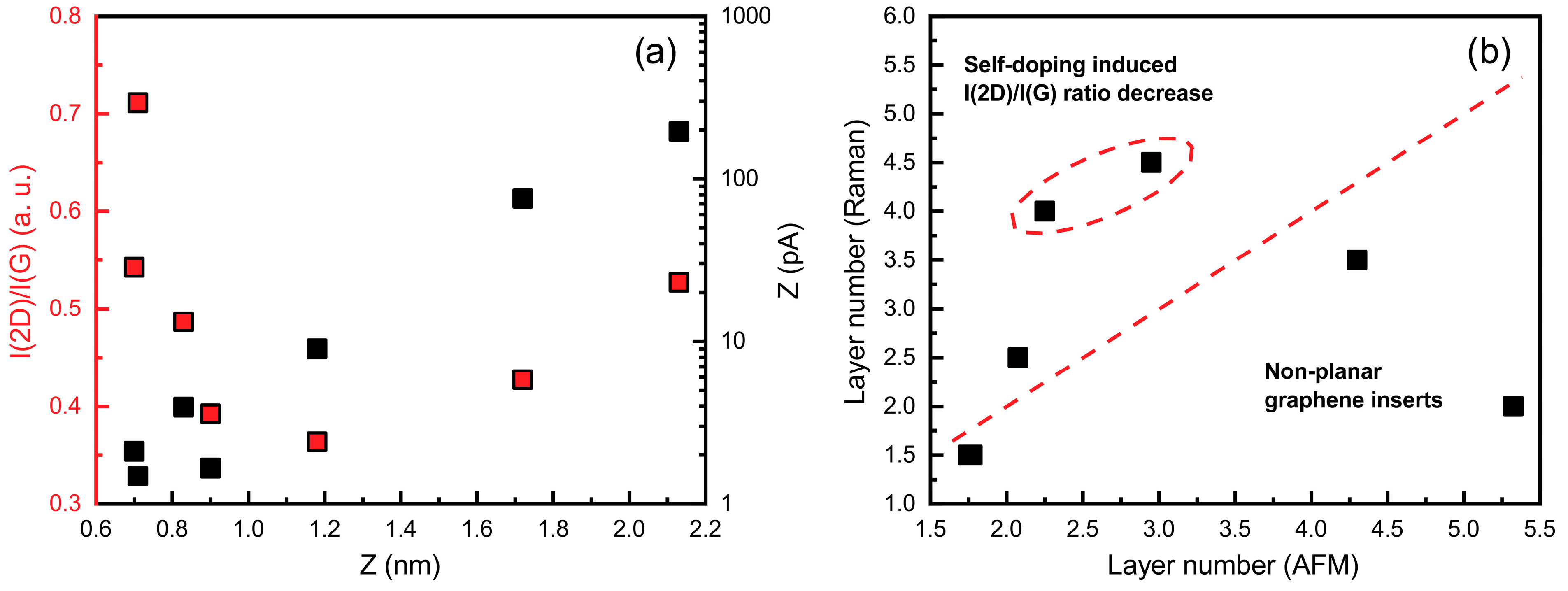


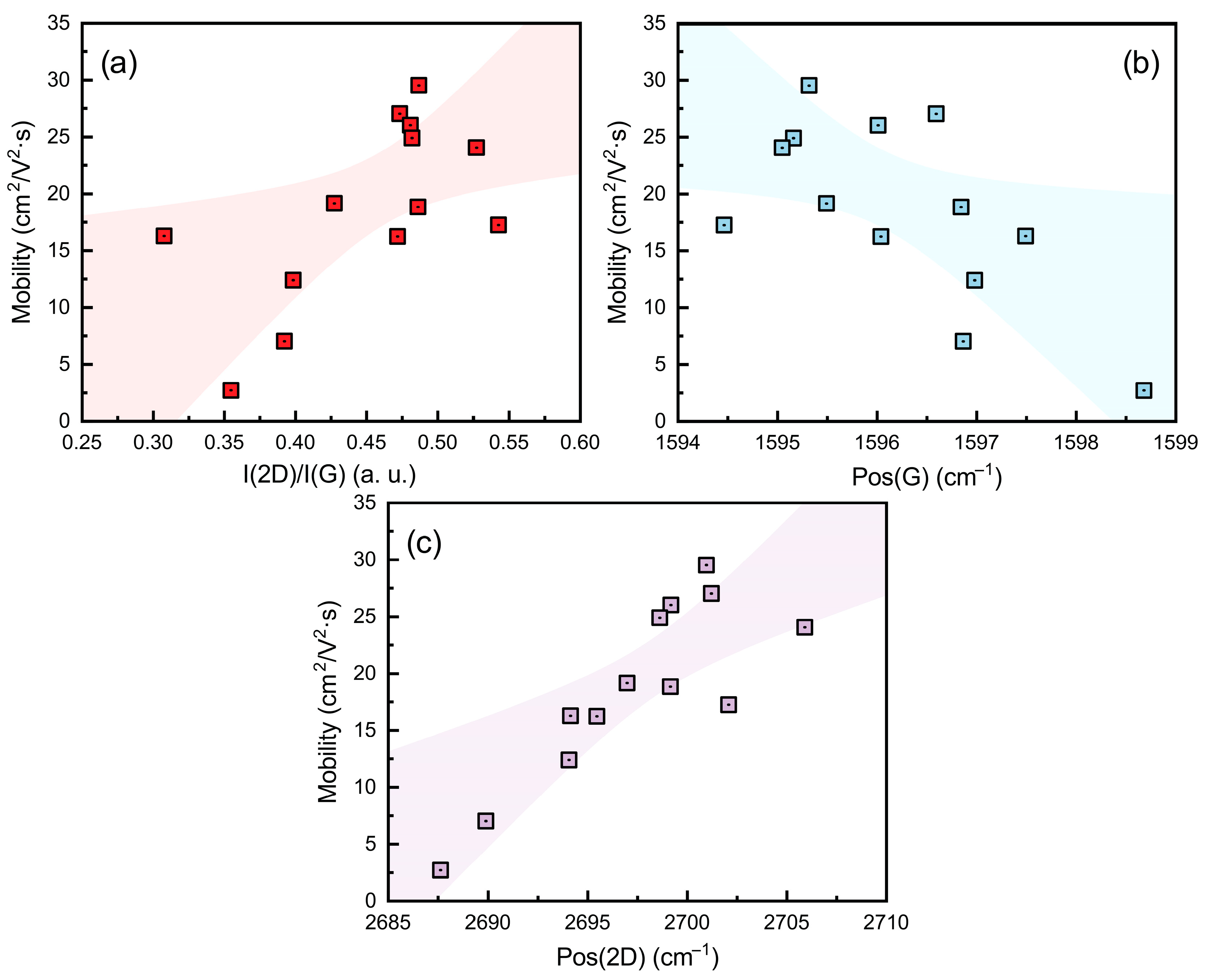

| No. | T (°C) | P (kW) | H2 (sccm) | CH4 (sccm) | p (mBar) | t (min) |
|---|---|---|---|---|---|---|
| 1 | 700 | 0.7 | 75 | 25 | 10 | 60 |
| 2 | 700 | 0.7 | 75 | 25 | 20 | 60 |
| 3 | 700 | 0.7 | 75 | 25 | 30 | 60 |
| 4 | 700 | 0.7 | 75 | 25 | 25 | 60 |
| 5 | 700 | 0.7 | 75 | 25 | 5 | 60 |
| 6 | 700 | 0.8 | 75 | 25 | 10 | 60 |
| 7 | 700 | 0.9 | 75 | 25 | 10 | 60 |
| 8 | 700 | 1 | 75 | 25 | 10 | 60 |
| 9 | 650 | 0.7 | 75 | 25 | 10 | 60 |
| 10 | 750 | 0.7 | 75 | 25 | 10 | 60 |
| 11 | 800 | 0.7 | 75 | 25 | 10 | 60 |
| 12 | 700 | 0.7 | 150 | 50 | 10 | 60 |
| 13 | 700 | 0.7 | 75 | 25 | 10 | 40 |
| 14 | 700 | 0.7 | 75 | 25 | 10 | 80 |
| 15 | 700 | 0.7 | 75 | 25 | 10 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meškinis, Š.; Jankauskas, Š.; Kamarauskas, L.; Vasiliauskas, A.; Guobienė, A.; Lazauskas, A.; Gudaitis, R. Effect of Synthesis Conditions on Graphene Directly Grown on SiO2: Structural Features and Charge Carrier Mobility. Nanomaterials 2025, 15, 1315. https://doi.org/10.3390/nano15171315
Meškinis Š, Jankauskas Š, Kamarauskas L, Vasiliauskas A, Guobienė A, Lazauskas A, Gudaitis R. Effect of Synthesis Conditions on Graphene Directly Grown on SiO2: Structural Features and Charge Carrier Mobility. Nanomaterials. 2025; 15(17):1315. https://doi.org/10.3390/nano15171315
Chicago/Turabian StyleMeškinis, Šarūnas, Šarūnas Jankauskas, Lukas Kamarauskas, Andrius Vasiliauskas, Asta Guobienė, Algirdas Lazauskas, and Rimantas Gudaitis. 2025. "Effect of Synthesis Conditions on Graphene Directly Grown on SiO2: Structural Features and Charge Carrier Mobility" Nanomaterials 15, no. 17: 1315. https://doi.org/10.3390/nano15171315
APA StyleMeškinis, Š., Jankauskas, Š., Kamarauskas, L., Vasiliauskas, A., Guobienė, A., Lazauskas, A., & Gudaitis, R. (2025). Effect of Synthesis Conditions on Graphene Directly Grown on SiO2: Structural Features and Charge Carrier Mobility. Nanomaterials, 15(17), 1315. https://doi.org/10.3390/nano15171315








