Recent Advances in the Application of VO2 for Electrochemical Energy Storage
Abstract
1. Introduction
2. Crystalline Structure and Synthesis of VO2
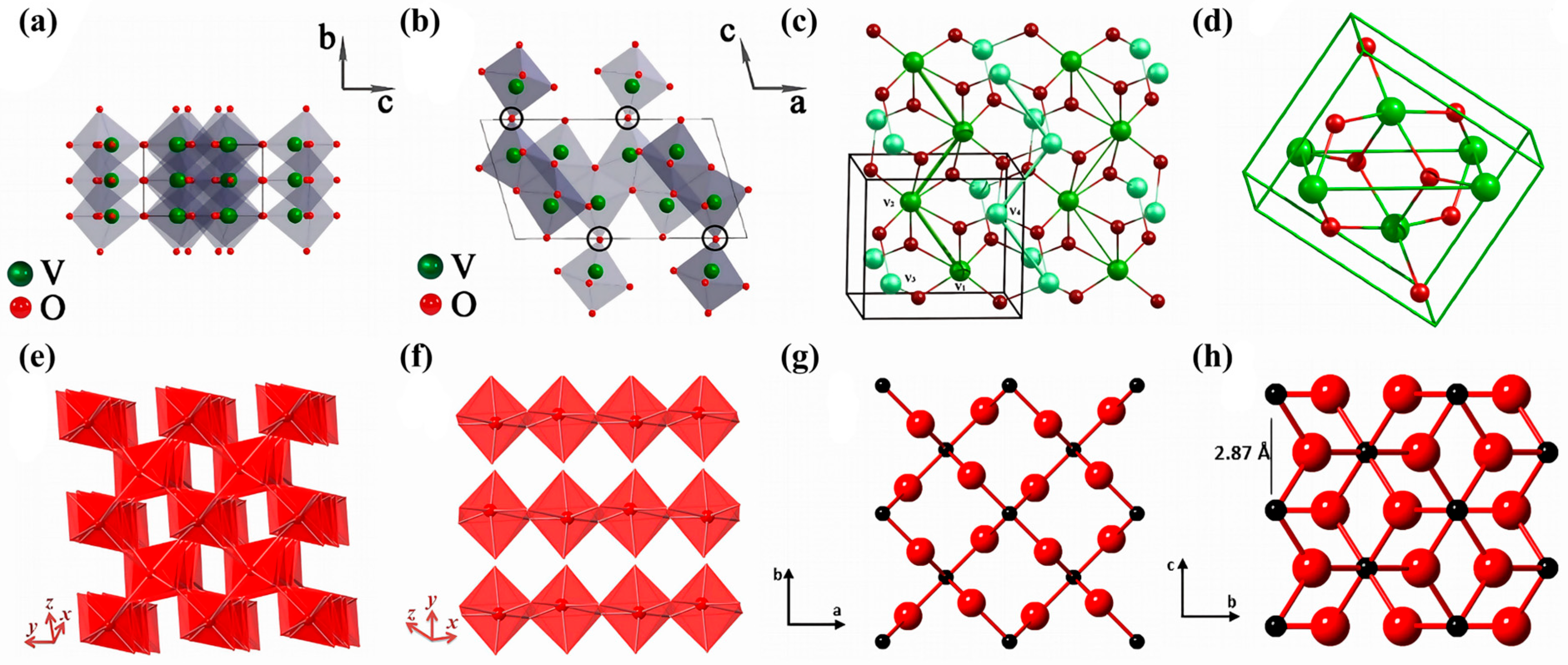
2.1. Crystalline Structure of VO2
2.2. Synthetic Method of VO2
2.2.1. Hydrothermal Methods
2.2.2. Pyrolysis Methods
2.2.3. Other Methods
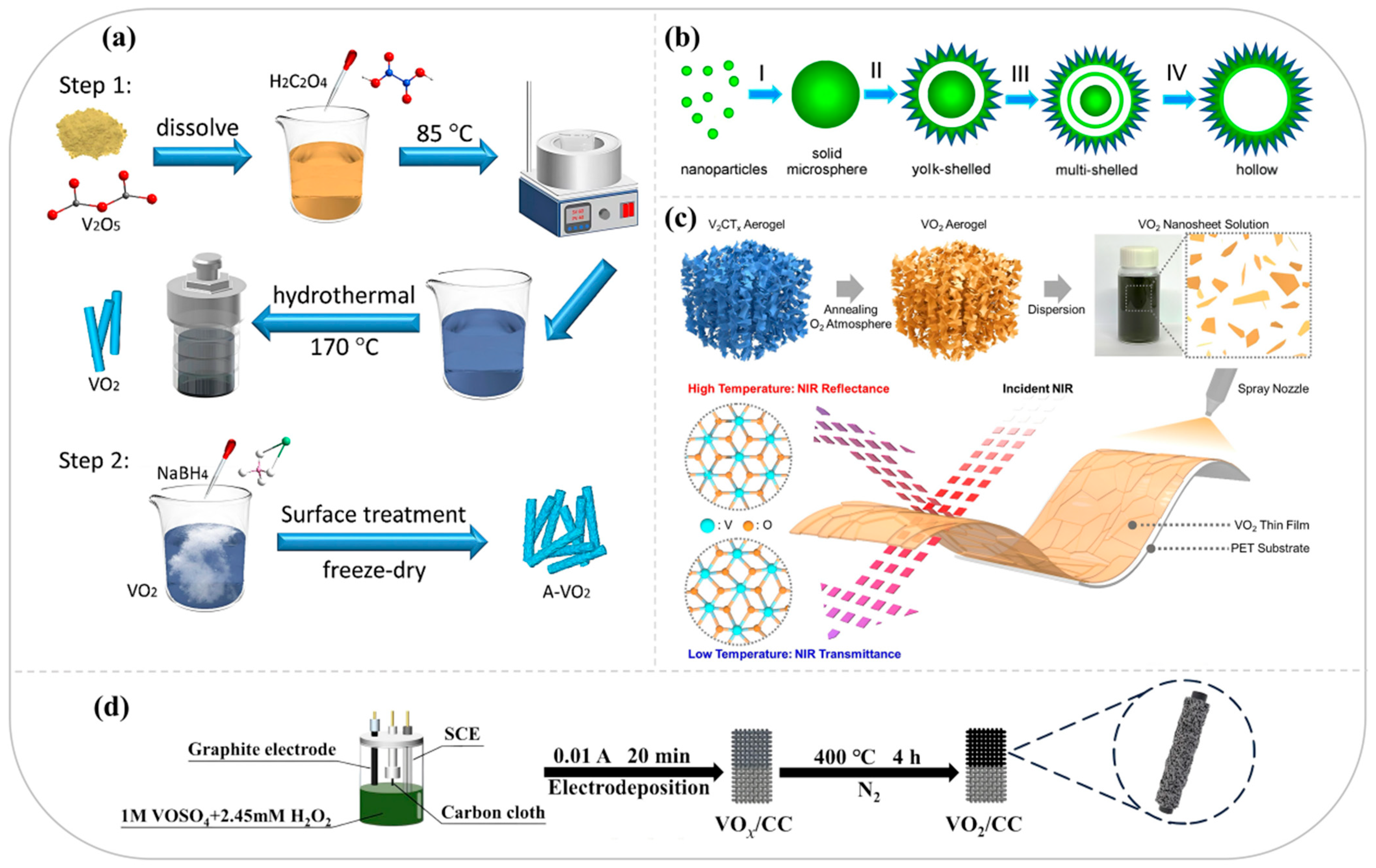
3. Application of VO2 in Electrochemical Energy Storage
3.1. Lithium-Ion Batteries
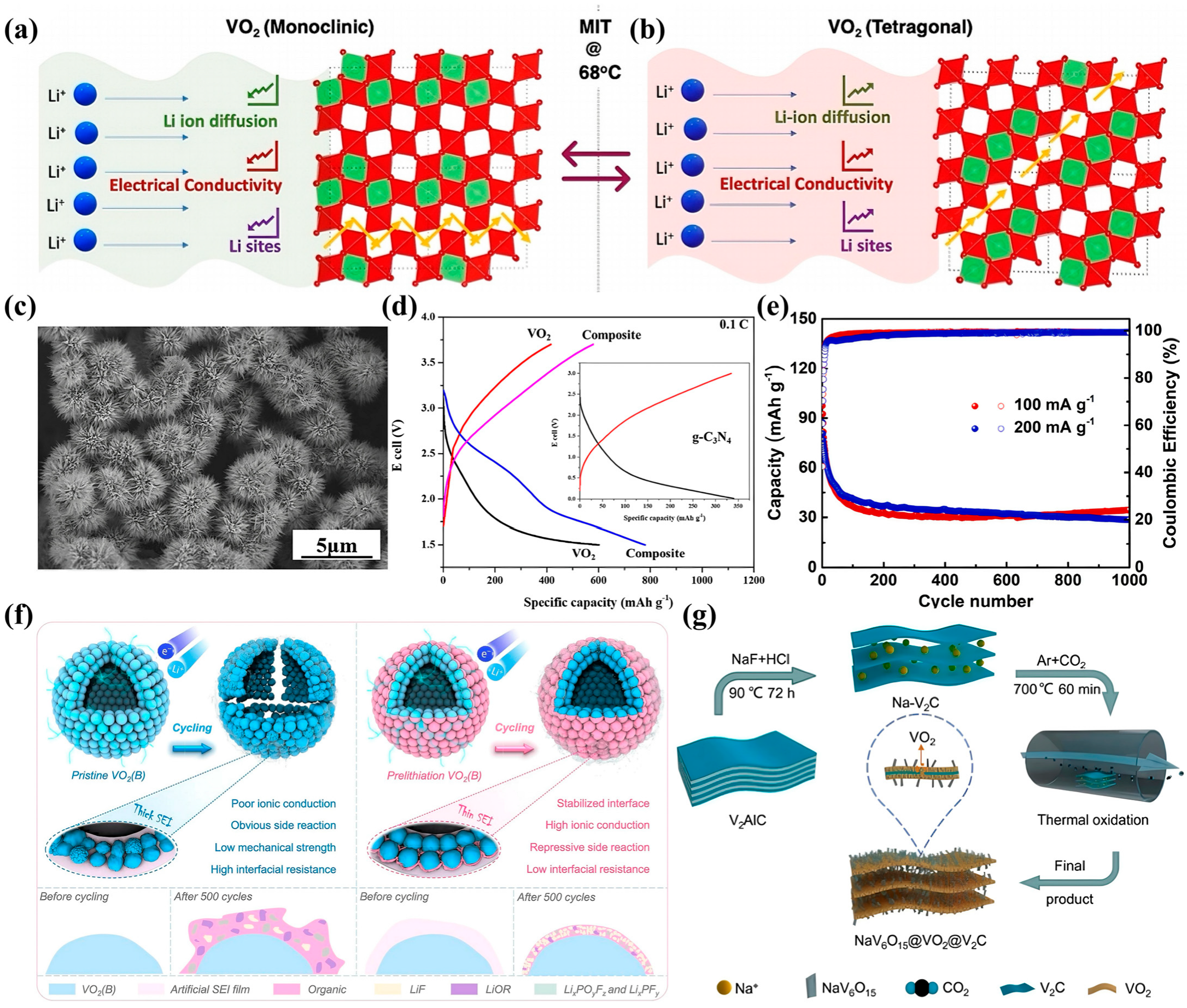
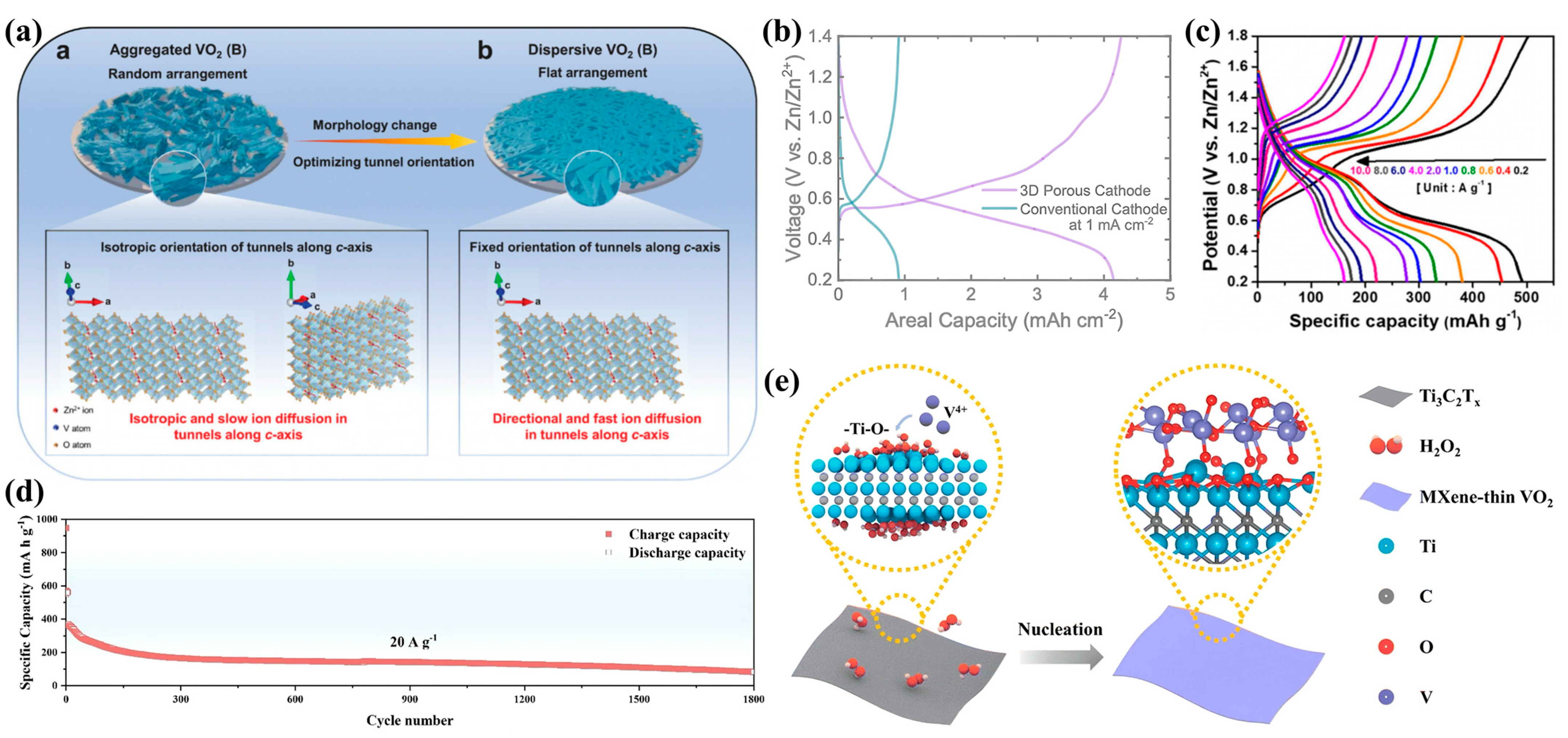
3.2. Zinc-Ion Batteries
| Materials | Cell Type | Voltage Range (V) | Capacity (mA h g−1) | Capacity Retention (Cycles) | Ref. |
|---|---|---|---|---|---|
| VO2-D | half-cell | 0.2–1.5 | 420.8 (0.1 A g−1) | 84.3 % (5 A g−1, 5000) | [50] |
| VO2 (B) | half-cell | 0.2–1.6 | 447 (0.2 A g−1) | 71.3 % (2 A g−1, 1000) | [51] |
| D-VO2 | half-cell | 0.2–1.6 | 332 (0.1 A g−1) | 46.2 % (20 A g−1, 1800) | [53] |
| VO2 | half-cell | 0.3–1.3 | 317 (0.5 A g−1) | 81.0 % (10 A g−1, 2000) | [59] |
| H-VO2@GO | half-cell | 0.2–1.5 | 400.1 (0.5 A g−1) | 96.1 % (10 A g−1, 1500) | [56] |
| pp-fibers@VO2 (B) | half-cell | 0.2–1.8 | 491 (0.2 A g−1) | 80.17 % (1 A g−1, 20,000) | [52] |
| CrVO | half-cell | 0.2–1.3 | 312.8 (0.1 A g−1) | 90.39 % (57 A g−1, 2000) | [60] |
| Ov-CoVO | half-cell | 0.3–1.4 | 475 (0.2 A g−1) | 97.5 % (5 A g−1, 3000) | [57] |
| MnVO | half-cell | 0.3–1.4 | 209.6 (0.1 A g−1) | 80.7 % (5 A g−1, 10,000) | [61] |
| Mg-VO2 | half-cell | 0.2–1.5 | 385.7 (0.1 A g−1) | 70.5 % (2 A g−1, 800) | [62] |
| Ce-VO2 | half-cell | 0.2–1.4 | 371.4 (0.1 A g−1) | 85.0 % (5 A g−1, 2000) | [63] |
| Ov-VO2@CNF | half-cell | 0.2–1.4 | 450 (0.1 A g−1) | 85.0 % (5 A g−1, 2000) | [64] |
| V2O3/VO2@S/N-C | half-cell | 0.2–1.6 | 257.8 (1 A g−1) | 81.8 % (200 A g−1, 150,000) | [65] |
| V6O13/VO2 | half-cell | 0.2–1.6 | 498.3 (0.2 A g−1) | 96.8 % (10 A g−1, 5000) | [66] |
| VO2/V2C@CNF | half-cell | 0.2–1.7 | 549 (0.1 A g−1) | 87.0 % (10 A g−1, 5000) | [55] |
| MXene-VO2−x | half-cell | 0.2–1.6 | 487.9 (0.2 A g−1) | 98.6 % (20 A g−1, 30,000) | [54] |
| Mo-VO2 | half-cell | 0.4–1.5 | 409.3 (0.1 A g−1) | 89.5 % (2 A g−1, 1000) | [67] |
| VO@NDA | half-cell | 0.4–1.6 | 241 (10 A g−1) | 97.45 % (10 A g−1, 15,000) | [58] |
3.3. Photoassisted Batteries
3.4. Other Batteries and Supercapacitors
4. Summary and Outlook
- Material design:
- 2.
- Electrode construction:
- 3.
- Research methodology:
- 4.
- Industrialization:
Author Contributions
Funding
Conflicts of Interest
References
- Tang, L.; Peng, H.; Kang, J.; Chen, H.; Zhang, M.; Liu, Y.; Kim, D.H.; Liu, Y.; Lin, Z. Zn-Based batteries for sustainable energy storage: Strategies and mechanisms. Chem. Soc. Rev. 2024, 53, 4877–4925. [Google Scholar] [CrossRef]
- Huang, J.; Xie, Y.; You, Y.; Yuan, J.; Xu, Q.; Xie, H.; Chen, Y. Rational Design of Electrode Materials for Advanced Supercapacitors: From Lab Research to Commercialization. Adv. Funct. Mater. 2023, 33, 2213095. [Google Scholar] [CrossRef]
- Guo, A.; Wang, Z.; Chen, L.; Liu, W.; Zhang, K.; Cao, L.; Liang, B.; Luo, D. A Comprehensive Review of the Mechanism and Modification Strategies of V2O5 Cathodes for Aqueous Zinc-Ion Batteries. ACS Nano 2024, 18, 27261–27286. [Google Scholar] [CrossRef]
- Liu, J.; Gao, X.; Zhang, Y.; Zhang, N.; Shao, Z.; Sun, R.; Zhou, J.; Li, D.; Xiang, X.; Feng, W. Boosted energy capacity and cycling stability of photo-assisted zinc-ion Batteries by K+ intercalated hydrated vanadium oxide-based photocathodes. J. Energy Storage 2025, 130, 117461. [Google Scholar] [CrossRef]
- Zhanadilov, O.; Kim, H.J.; Konarov, A.; Jeong, J.; Park, J.-H.; Chung, K.Y.; Bakenov, Z.; Yashiro, H.; Myung, S.-T. Layered manganese oxide cathode boosting high-capacity and long-term cyclability in aqueous Zinc-Ion batteries. Energy Storage Mater. 2024, 67, 103283. [Google Scholar] [CrossRef]
- Gao, X.; Tian, D.; Shi, Z.; Zhang, N.; Sun, R.; Liu, J.; Tsai, H.; Xiang, X.; Feng, W. An Efficient MnO2 Photocathode with an Excellent SnO2 Electron Transport Layer for Photo-accelerated Zinc Ion Batteries. Small 2024, 20, 2405627. [Google Scholar] [CrossRef]
- Tian, D.; Gao, X.; Wang, J.; Sun, R.; Liu, J.; Cao, J.; Feng, W. An optimized MnO2 photocathode by doping engineering for high capacity and stability photo-assisted zinc-ion batteries. J. Mater. Chem. A 2025, 13, 22057–22065. [Google Scholar] [CrossRef]
- Yi, P.; Li, Z.; Ma, L.; Feng, B.; Liu, Z.; Liu, Y.; Lu, W.; Cao, S.; Fang, H.; Ye, M.; et al. Eco-friendly High-performance Symmetric all-COF/Graphene Aqueous Zinc-ion Batteries. Adv. Mater. 2024, 36, 2414379. [Google Scholar] [CrossRef]
- Cho, B.; Huh, S.; Kim, S.H.; Yu, S.; Bae, J.; Yoo, J.; Yu, S. Long cycle-life aqueous Zn battery enabled by facile carbon nanotube coating on Cu current collector. Carbon Energy 2024, 6, e441. [Google Scholar] [CrossRef]
- Yuan, W.; Weng, J.; Ding, M.; Jiang, H.-M.; Fan, Z.; Zhao, Z.; Zhang, P.; Xu, L.-P.; Zhou, P. Novel covalent organic framework/carbon nanotube composites with multiple redox-active sites for high-performance Na storage. Energy Storage Mater. 2024, 65, 103142. [Google Scholar] [CrossRef]
- Li, Y.X.; Feng, Y.S.; Li, L.X.; Yin, X.; Cao, F.F.; Ye, H. Green synthesis and applications of MXene for lithium–sulfur batteries. Energy Storage Mater. 2024, 67, 103257. [Google Scholar] [CrossRef]
- Zhang, G.; Yu, H.; Li, X.; Zhang, X.; Hou, C.; Sun, S.; Du, Y.; Guo, Z.; Dang, F. Construction of MnS/MoS2 heterostructure on two-dimensional MoS2 surface to regulate the reaction pathways for high-performance Li-O2 batteries. J. Energy Chem. 2024, 93, 443–452. [Google Scholar] [CrossRef]
- Housel, L.M.; Quilty, C.D.; Abraham, A.; Tang, C.R.; McCarthy, A.H.; Renderos, G.D.; Liu, P.; Takeuchi, E.S.; Marschilok, A.C.; Takeuchi, K.J. Investigation of Conductivity and Ionic Transport of VO2 (M) and VO2 (R) via Electrochemical Study. Chem. Mater. 2018, 30, 7535–7544. [Google Scholar] [CrossRef]
- Li, W.; Huang, J.; Cao, L.; Li, X.; Chen, S.; Feng, L. Polycrystalline VO2 (M) with well-dispersed crystalline zones for enhanced electroactivity of Lithium-ion batteries. J. Alloys Compd. 2020, 812, 152122. [Google Scholar] [CrossRef]
- Shvets, P.; Shabanov, A.; Maksimova, K.; Goikhman, A. Micro-Raman mapping of VO2 (T) microcrystals orientation. Vib. Spectrosc. 2022, 118, 103328. [Google Scholar] [CrossRef]
- Oka, Y.; Sato, S.; Yao, T.; Yamamoto, N. Crystal Structures and Transition Mechanism of VO2 (A). J. Solid State Chem. 1998, 141, 594–598. [Google Scholar] [CrossRef]
- Cheng, B.; Zhang, H.; Li, Q.; Liu, J.; Liu, B. Morphology Tuned Pressure Induced Amorphization in VO2 (B) Nanobelts. Inorganics 2022, 10, 122. [Google Scholar] [CrossRef]
- Hagrman, D.; Zubieta, J.; Warren, C.J.; Meyer, L.M.; Treacy, M.M.J.; Haushalter, R.C. A New Polymorph of VO2 Prepared by Soft Chemical Methods. J. Solid State Chem. 1998, 138, 178–182. [Google Scholar] [CrossRef]
- Qu, B.Y.; Liu, L.; Xie, Y.; Pan, B.C. Theoretical study of the new compound VO2 (D). Phys. Lett. A 2011, 375, 3474–3477. [Google Scholar] [CrossRef]
- Wu, C.; Hu, Z.; Wang, W.; Zhang, M.; Yang, J.; Xie, Y. Synthetic paramontroseite VO2 with good aqueous Lithium-ion battery performance. Chem. Commun. 2008, 7, 3891–3893. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Lv, T.-T.; Wang, H.; Guo, X.-T.; Liu, C.-S.; Pang, H. Nsutite-type VO2 microcrystals as highly durable cathode materials for aqueous zinc-Ion batteries. Chem. Eng. J. 2021, 417, 128408. [Google Scholar] [CrossRef]
- Khan, Z.; Singh, P.; Ansari, S.A.; Manippady, S.R.; Jaiswal, A.; Saxena, M. VO2 Nanostructures for Batteries and Supercapacitors: A Review. Small 2021, 17, 2006651. [Google Scholar] [CrossRef]
- Wan, B.; Wang, Y.; Chen, X.; Zhan, C.; Jiang, H.; Liu, J.-H.; Gao, Y.; Jiang, X.; Cao, X.; Zhang, H.; et al. Critical issues and optimization strategies of vanadium dioxide-based cathodes towardds high-performance aqueous Zn-ion batteries. Chem. Sci. 2025, 16, 8217–8239. [Google Scholar] [CrossRef]
- Hou, J.-F. Disorder/order-heterophase VO2 for enhanced lithium storage performance in lithium-ion capacitors. J. Energy Storage 2024, 81, 110433. [Google Scholar] [CrossRef]
- Pan, A.; Wu, H.B.; Yu, L.; Lou, X.W. (David). Template-free Synthesis of VO2 Hollow Microspheres with Various Interiors and Their Conversion into V2O5 for Lithium-ion Batteries. Angew. Chem. Int. Ed. 2013, 52, 2226–2230. [Google Scholar] [CrossRef]
- Zheng, C.; Zhang, X.; Zhang, J.; Liao, K. Preparation and Characterization of VO2 Nanopowders. J. Solid State Chem. 2001, 156, 274–280. [Google Scholar] [CrossRef]
- Jung, D.; Kim, U.; Cho, W. Fabrication of pure monoclinic VO2 nanoporous nanorods via a mild pyrolysis process. Ceram. Int. 2018, 44, 6973–6979. [Google Scholar] [CrossRef]
- Lai, X.; Li, Y.; Yang, Y.; Zhou, Z.; Wang, C. Vanadium dioxide (IV) (B) nanostructures prepared via electrodeposition for zinc-Ion storage in electrochemical energy storage devices. J. Energy Storage 2024, 102, 114157. [Google Scholar] [CrossRef]
- Xiang, W.; Le Drogoff, B.; Koch, D.; Margot, J.; Chaker, M. High-quality VO2 films synthesized on polymer substrates using room-temperature pulsed laser deposition and annealing. Ceram. Int. 2024, 50, 838–846. [Google Scholar] [CrossRef]
- Kim, J.; Haeng Lee, K.; Seo, D.-B.; Jang, H.; Kang, S.; Yim, S.; Sook Lee, S.; An, K.-S. Two-dimensional VO2 nanosheet converted from MXene for flexible thermochromic smart windows. Chem. Eng. J. 2023, 477, 147014. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, D.; Liu, Y.; Jin, J.; Yang, Y.; Chen, T.; Guan, H.; Fan, P.; Lv, W. Phase transition analysis of thermochromic VO2 thin films by temperature-dependent Raman scattering and ellipsometry. Appl. Surf. Sci. 2018, 456, 545–551. [Google Scholar] [CrossRef]
- Wu, H.; Yu, Y.; Yu, Y.; Shi, J.; Morikawa, H.; Zhu, C. A facile method for continuous production of temperature-adaptive hyperthermal management core-sheath polyurethane nanofiber yarns based on vanadium dioxide toward commercialization. J. Energy Storage 2024, 86, 111311. [Google Scholar] [CrossRef]
- Chen, Z.; Tang, Y.; Ji, A.; Zhang, L.; Gao, Y. Large-Scale Preparation of Durable VO2 Nanocomposite Coatings. ACS Appl. Nano Mater. 2021, 4, 4048–4054. [Google Scholar] [CrossRef]
- Man, P.; Zhang, Q.; Sun, J.; Guo, J.; Wang, X.; Zhou, Z.; He, B.; Li, Q.; Xie, L.; Zhao, J.; et al. Hierarchically structured VO2@PPy core-shell nanowire arrays grown on carbon nanotube fibers as advanced cathodes for high-performance wearable asymmetric supercapacitors. Carbon 2018, 139, 21–28. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Zhong, Y.; Yu, L.; Deng, Y.; Huang, C.; Liu, X. Direct fabrication of organic carbon coated VO2 (B) (VO2 (B)@C) core-shell structured nanobelts by one step hydrothermal route and its formation mechanism. Appl. Surf. Sci. 2012, 263, 124–131. [Google Scholar] [CrossRef]
- Lin, S.; Wang, Y.; Chen, Y.; Cai, Z.; Xiao, J.; Muhmood, T.; Hu, X. Dendrite-Free and Ultra-Long-Life Lithium Metal Anode Enabled via a Three-Dimensional Ordered Porous Nanostructure. ACS Appl. Mater. Interfaces 2021, 13, 41744–41752. [Google Scholar] [CrossRef]
- Bai, M.-X.; He, Z.-H.; Hou, J.-F.; Gao, J.-F.; Kong, L.-B. Solvothermally prepared hydrated VO2 (B) for aqueous zinc ion batteries with high capacity and excellent rate capability. J. Alloys Compd. 2023, 936, 168218. [Google Scholar] [CrossRef]
- Chen, X.; Wang, L.; Li, H.; Cheng, F.; Chen, J. Porous V2O5 nanofibers as cathode materials for rechargeable aqueous zinc-ion batteries. J. Energy Chem. 2019, 38, 20–25. [Google Scholar] [CrossRef]
- Selvam, T.; Dhinasekaran, D.; Subramanian, B.; Rajendran, A.R. Layered Structures of Enriched V5+ States of Vanadium Oxide as a Hybrid Cathode Material for Long-Cyclable Aqueous Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2023, 15, 30350–30359. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Qin, G.; Luo, K.; Lu, J.; Li, Y.; Liang, G.; Huang, Z.; Zhou, J.; Hultman, L.; et al. Halogenated Ti3C2 MXenes with Electrochemically Active Terminals for High-Performance Zinc Ion Batteries. ACS Nano 2021, 15, 1077–1085. [Google Scholar] [CrossRef]
- Shao, F.; Huang, Y.; Wang, X.; Li, Z.; Huang, X.; Huang, W.; Dong, L.; Kang, F.; Liu, W.; Xu, C. MoS2 with high 1T phase content enables fast reversible zinc-ion storage via pseudocapacitance. Chem. Eng. J. 2022, 448, 137688. [Google Scholar] [CrossRef]
- Lin, S.; Chen, Y.; Wang, Y.; Cai, Z.; Xiao, J.; Muhmood, T.; Hu, X. Three-Dimensional Ordered Porous Nanostructures for Lithium-Selenium Battery Cathodes That Confer Superior Energy-Storage Performance. ACS Appl. Mater. Interfaces 2021, 13, 9955–9964. [Google Scholar] [CrossRef]
- Xiao, J.; Cai, Z.; Muhmood, T.; Hu, X.; Lin, S.; Hu, X. Tailoring Ordered Porous Carbon Embedded with Cu Clusters for High-energy and Long-lasting Phosphorus Anode. Small 2022, 18, 2106930. [Google Scholar] [CrossRef] [PubMed]
- Castro-Pardo, S.; Puthirath, A.B.; Fan, S.; Saju, S.; Yang, G.; Nanda, J.; Vajtai, R.; Tang, M.; Ajayan, P.M. VO2 phase change electrodes in Li-ion batteries. J. Mater. Chem. A 2024, 12, 2738–2747. [Google Scholar] [CrossRef]
- Liang, F.; Zou, Z.; Zhang, S.; Chen, M.; Yu, F.; Jia, S.; Nong, J. Ultrathin VO2 (B) nanosheets assembled into 3D micro/nano structured flower-like microspheres for high-performance cathode materials of Li-ion batteries. J. Energy Storage 2024, 77, 109787. [Google Scholar] [CrossRef]
- Jang, W.Y.; Reddy, C.V.; Wang, R.; Choi, J.; Son, J.; Kakarla, R.R.; Aminabhavi, T.M.; Shim, J. Synthesis of 1D/2D VO2 (B) nanowire/g-C3N4 hybrid architectures as cathode materials for high-performance Li-ion batteries. Chem. Eng. J. 2023, 476, 146786. [Google Scholar] [CrossRef]
- Yan, B.; Xu, C.; Liu, L.; Wang, F.; Xiao, W.; Zhang, L.; Yang, X.; Li, X.; Wang, R. NonNon-destructive chemical prelithiation of VO2 (B) anodes constructing a multifunctional interphase layer for advanced Li-Ion batteries. Chem. Eng. J. 2024, 489, 151185. [Google Scholar] [CrossRef]
- Ma, Y.; Zhu, D.; Zhou, H.; Tang, Y.; Hu, C.; Meng, X.; Jin, X.; Xu, T.; Cao, X. VO2 (D) submicron-spherical hierarchical structures as novel anode materials for aqueous lithium-ion batteries. J. Alloys Compd. 2023, 930, 167472. [Google Scholar] [CrossRef]
- Tan, L.; Wu, J.; Guan, Y.; Jin, Y.; Xu, Z.; Zhu, H.; Zhang, Q.; Li, X.; Dong, Z.; Cong, Y. Dual conductive confinement effects on enhancing Li-ion storage of NaV6O15@VO2 (M)@V2C heterojunction. J. Alloys Compd. 2023, 964, 171242. [Google Scholar] [CrossRef]
- He, Q.; Hu, T.; Wu, Q.; Wang, C.; Han, X.; Chen, Z.; Zhu, Y.; Chen, J.; Zhang, Y.; Shi, L.; et al. Tunnel-Oriented VO2 (B) Cathode for High-Rate Aqueous Zinc-Ion Batteries. Adv. Mater. 2024, 36, 2400888. [Google Scholar] [CrossRef]
- Pinnock, I.P.; Fan, Y.; Zhu, Y.; Narayan, B.; Wang, T.; Parkin, I.P.; Deka Boruah, B. Advancing high capacity 3D VO2 (B) cathodes for improved zinc-ion battery performance. J. Mater. Chem. A 2025, 13, 1372–1383. [Google Scholar] [CrossRef]
- Yeon, J.S.; Park, S.K.; Kim, S.; Mohite, S.V.; Il Kim, W.; Jang, G.; Jang, H.; Bae, J.; Lee, S.M.; Hong, W.G.; et al. Self-supported VO2 on polydopamine-derived pyroprotein-based fibers for ultrastable and flexible aqueous zinc-ion batteries. Carbon Energy 2024, 6, e469. [Google Scholar] [CrossRef]
- Liu, X.; Meng, L.; Xu, Z.; Wu, J.; Wang, K.; Zhang, L.; Yu, C.; Li, L. Engineering vanadium vacancies to accelerate ion kinetics for high performance zinc ion battery. J. Colloid Interface Sci. 2025, 684, 439–448. [Google Scholar] [CrossRef]
- Fang, Y.; Qi, C.; Bao, W.; Xu, F.; Sun, W.; Liu, B.; Yu, X.; Wang, L.; Jiang, W.; Qiu, P.; et al. Asymmetric orbital hybridization at the MXene-VO2-x interface stabilizes oxygen vacancies for enhanced reversibility in aqueous zinc-ion batteries. Energy Environ. Sci. 2025, 18, 367–377. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, Z.-H.; Yang, Z.-Q.; Yu, Y.-X.; Yang, Y. In situ synthesis of VO2 containing high-valent vanadium via surface oxidation of V2C Mxene for robust near-interface reactions in aqueous zinc-ion batteries. Chem. Eng. J. 2025, 511, 162146. [Google Scholar] [CrossRef]
- Li, Y.; Liao, X.; Xie, B.; Li, Y.; Zheng, Q.; Lin, D. Hollow VO2 microspheres anchored on graphene as advanced cathodes for aqueous zinc-ion batteries. J. Colloid Interface Sci. 2024, 662, 404–412. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, P.; Wang, X.; Chang, M.; Yu, Y.; You, J.; Hu, F.; Wu, Y.; Zhu, K. Co-Substitution Engineering Boosting the Kinetics and Stablity of VO2 for Zn Ion Batteries. Adv. Funct. Mater. 2024, 34, 2407925. [Google Scholar] [CrossRef]
- Song, Z.; Zhao, Y.; Wang, H.; Zhou, A.; Jin, X.; Huang, Y.; Li, L.; Wu, F.; Chen, R. Dual mechanism with graded energy storage in long-term aqueous zinc-Ion batteries achieved using a polymer/vanadium dioxide cathode. Energy Environ. Sci. 2024, 17, 6666–6675. [Google Scholar] [CrossRef]
- Wang, W.; Feng, C.; Lei, L.; Yang, X.; Li, X.; Ma, L.; Zhang, M.; Fan, H. Regulating Crystal Orientation in VO2 for Aqueous Zinc Batteries with Enhanced Pseudocapacitance. ACS Appl. Mater. Interfaces 2024, 16, 10009–10018. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhai, X.; Wu, Y.; Wang, X.; Zhang, L.; Shang, C.; Zhang, H.; Zhao, C.; Shang, J.; Liu, D. Chromium-doped tunnel-structured VO2 (B) nanorods as high-capacity and stable cathode materials for aqueous zinc-ion batteries. J. Energy Storage 2025, 114, 115826. [Google Scholar] [CrossRef]
- Deng, S.; Li, H.; Chen, B.; Xu, Z.; Jiang, Y.; Li, C.; Xiao, W.; Yan, X. High performance of Mn-doped VO2 cathode for aqueous zinc-ion batteries: An insight into Zn2+ storage mechanism. Chem. Eng. J. 2023, 452, 139115. [Google Scholar] [CrossRef]
- Wang, N.; Liu, H.; Sun, M.; Ren, X.; Hu, L.; Li, Z.; Yao, X.; Gong, Y.; Jia, C. Achieving Wide-Temperature-Range Sustainable Zinc-Ion Batteries via Magnesium-Doped Cathodes and Gel Electrolytes. ACS Sustain. Chem. Eng. 2024, 12, 3527–3537. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Liu, J.; Ding, N.-W.; Zhang, Q.; Lei, S.; Sydorov, D. Synergistically Electronic and Structural Regulation of VO2 by Ce Doping Toward High-Performance Zinc-Ion Batteries. ACS Sustain. Chem. Eng. 2025, 13, 5091–5100. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, Z.-H.; Yang, C.-Q.; Guo, X.-Z.; Yu, Y.-X.; Yang, Y. Rational Regulation of Optimal Oxygen Vacancy Concentrations on VO2 for Superior Aqueous Zinc-Ion Battery Cathodes. ACS Appl. Mater. Interfaces 2024, 16, 40903–40913. [Google Scholar] [CrossRef]
- Chen, L.; Chen, Z.; Wu, Z.; Zeng, H.; Chen, Y.; Yang, H.; Qian, W.; Huang, J. V2O3/VO2@S/N-C nanofibers with excellent cycling stability and superior rate capability in aqueous zinc ion batteries. J. Energy Chem. 2024, 99, 83–91. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X. High durable aqueous zinc ion batteries by synergistic effect of V6O13/VO2 electrode materials. J. Energy Chem. 2023, 87, 334–341. [Google Scholar] [CrossRef]
- Zhang, D.; Yue, Y.; Yang, C.; Limphirat, W.; Zhang, X.; Qin, J.; Cao, J. Kinetics-Boosted and Dissolution-Suppressed Molybdenum-Doped vanadium dioxide for Long-Life Zinc-Ion batteries. Chem. Eng. J. 2025, 506, 160160. [Google Scholar] [CrossRef]
- Deka Boruah, B.; Mathieson, A.; Park, S.K.; Zhang, X.; Wen, B.; Tan, L.; Boies, A.; De Volder, M. Vanadium Dioxide Cathodes for High-Rate Photo-rechargeable Zinc-Ion Batteries. Adv. Energy Mater. 2021, 11, 2100115. [Google Scholar] [CrossRef]
- Deka Boruah, B.; De Volder, M. Vanadium dioxide-zinc oxide stacked photocathodes for photo-rechargeable zinc-ion batteries. J. Mater. Chem. A 2021, 9, 23199–23205. [Google Scholar] [CrossRef]
- Ding, Z.; Jiang, T.; Zhao, W.; Luo, Z.; San, H.; Li, S.; Li, X.; Zhang, L. Photo-rechargeable zinc-ion battery using highly ordered and vertically oriented C@VO2/ZnO microrod arrays. Energy Storage Mater. 2024, 71, 103646. [Google Scholar] [CrossRef]
- Roy, R.; Mahendra, G.; Singh, A.K. Photo-assisted self-chargeable aqueous Zn-ion energy storage device. Chem. Eng. J. 2024, 500, 156870. [Google Scholar] [CrossRef]
- Yang, H.; Wu, S.; Badalge, A.M.; Li, X.; Wang, Q.; Zhong, Y.L.; Zhu, T. Controlled oxidation of V2O5/VO2 hollow nanospheres as photocathodes for photo-rechargeable zinc ion batteries with an ultrahigh capacity enhancement. J. Mater. Chem. A 2025, 13, 12216–12225. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Zhang, W.; Chao, F.; Li, J.; Kong, Q.; Qiao, F.; Zhang, L.; Huang, M.; An, Q. Promising VO2 (B)/rGO Heterojunction Cathode for Building High-Capacity and Long-Lifespan Ca-Ion Batteries. Adv. Funct. Mater. 2024, 34, 2314761. [Google Scholar] [CrossRef]
- Pang, Y.; Xie, M.; Lu, X.; Wan, Z.; Zhong, Z.; Muhammad, W.; Huan, X.L.; Niu, Y.; Zhang, Z.; Lv, W. Electronic Metal-Support Interactions in Atomically Dispersed Fe(III)-VO2 Nanoribbons for High-performance Lithium-Sulfur Batteries. Adv. Funct. Mater. 2024, 34, 2308849. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, H.; Li, Z. Orbital-Modulated Cu-Doped VO2 Nanoflowers via Glucose-Assisted Synthesis: Structural Optimization and Electronic Coupling Engineering for High-Capacity Aqueous Aluminum Ion Batteries. Small 2025, 21, e2503861. [Google Scholar] [CrossRef]
- Chen, Q.; Tang, Z.; Li, H.; Liang, W.; Zeng, Y.; Zhang, J.; Hou, G.; Tang, Y. Cobalt Ion-Stabilized VO2 for Aqueous Ammonium Ion Hybrid Supercapacitors. ACS Appl. Mater. Interfaces 2024, 16, 18824–18832. [Google Scholar] [CrossRef]
- Zhu, Z.; Xia, Q.; Wang, L.; Hu, Q.; Chang, Y.; Shinde, N.M. In situ grown VO2/V2C MXene and its supercapacitor applications. J. Energy Storage 2024, 88, 111484. [Google Scholar] [CrossRef]
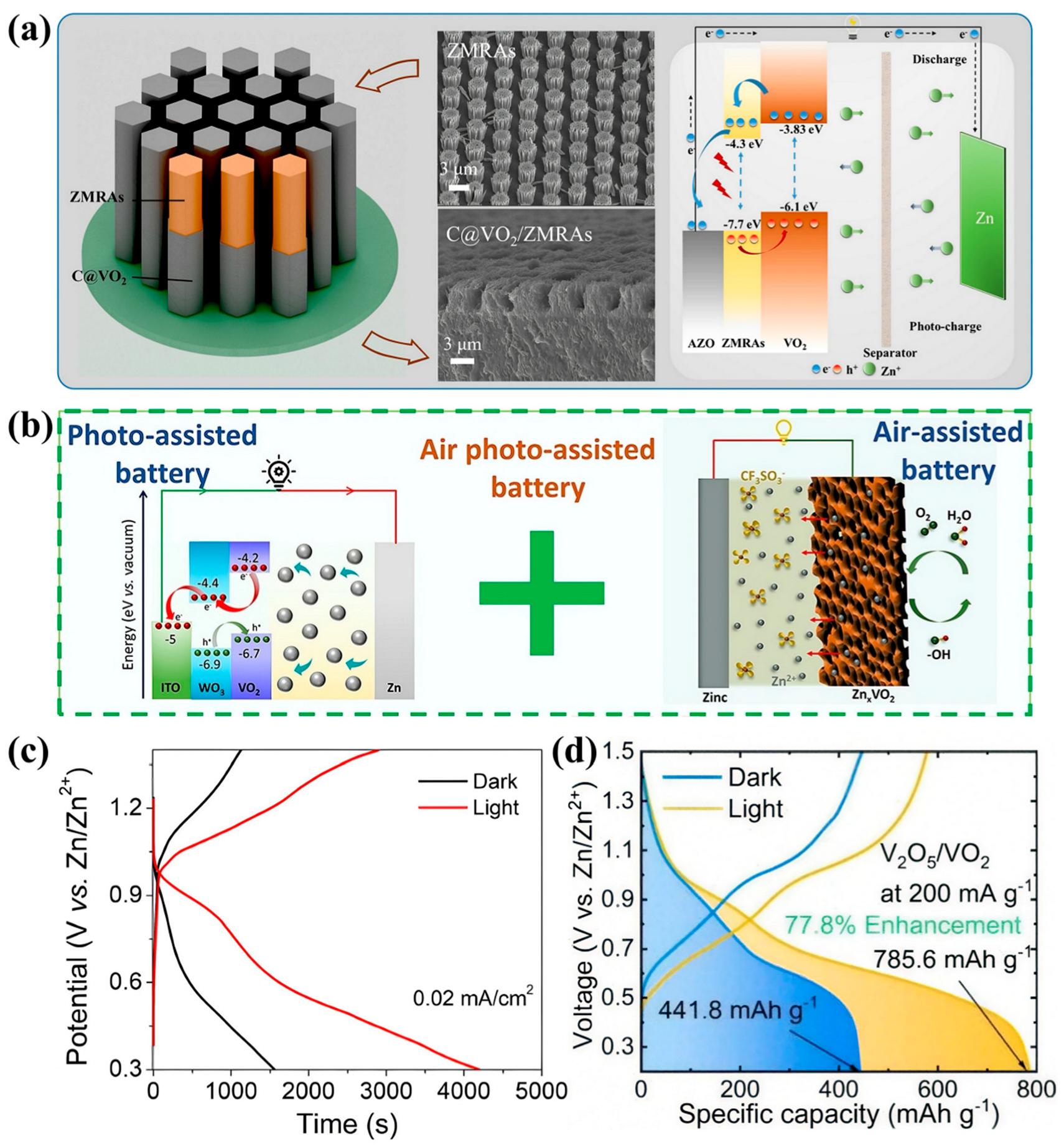
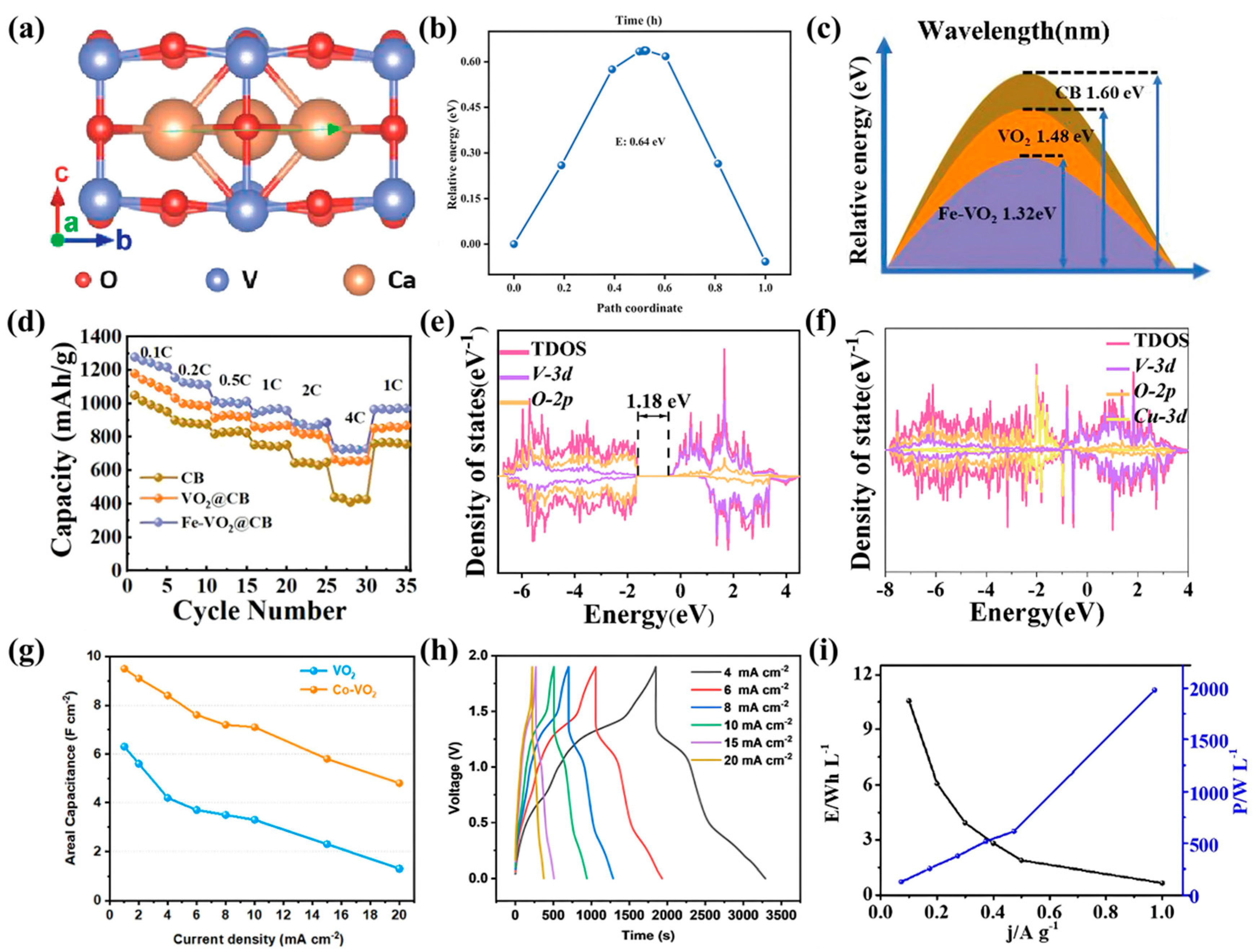
| Materials | Cost | Capacity (mA h g−1) | Stability (Cycles) | Scalability | Typical Applications | Ref. |
|---|---|---|---|---|---|---|
| VO2 | Moderate | 400.2 (0.5 A g−1) | 84.3% (5 A g−1, 6000) | Moderate | Zn-ion, Li-ion, photoassisted batteries | [37] |
| V2O5 | Low | 319 (0.02 A g−1) | 81% (2 A g−1, 500) | High | Zn-ion, Li-ion, photoassisted batteries | [38] |
| V6O13 | Moderate | 394.2 (0.1 A g−1) | 94% (2 A g−1, 100) | Moderate | Zn-ion, Li-ion batteries | [39] |
| Ti3C2I2 | High | 181 (0.25 A g−1) | 80% (4 A g−1, 700) | Low | Supercapacitors, Li-ion, Na-ion batteries | [40] |
| MoS2 | Moderate | 156 (0.1 A g−1) | 97.3% (1 A g−1, 500) | Moderate | Li-ion, Na-ion, Zn-ion batteries | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Gao, X.; Liu, J.; Zhou, J.; Wang, J.; Li, D.; Zhao, S.; Feng, W. Recent Advances in the Application of VO2 for Electrochemical Energy Storage. Nanomaterials 2025, 15, 1167. https://doi.org/10.3390/nano15151167
He Y, Gao X, Liu J, Zhou J, Wang J, Li D, Zhao S, Feng W. Recent Advances in the Application of VO2 for Electrochemical Energy Storage. Nanomaterials. 2025; 15(15):1167. https://doi.org/10.3390/nano15151167
Chicago/Turabian StyleHe, Yuxin, Xinyu Gao, Jiaming Liu, Junxin Zhou, Jiayu Wang, Dan Li, Sha Zhao, and Wei Feng. 2025. "Recent Advances in the Application of VO2 for Electrochemical Energy Storage" Nanomaterials 15, no. 15: 1167. https://doi.org/10.3390/nano15151167
APA StyleHe, Y., Gao, X., Liu, J., Zhou, J., Wang, J., Li, D., Zhao, S., & Feng, W. (2025). Recent Advances in the Application of VO2 for Electrochemical Energy Storage. Nanomaterials, 15(15), 1167. https://doi.org/10.3390/nano15151167








