The Air Stability of Sodium Layered Oxide NaTMO2 (100) Surface Investigated via DFT Calculations
Abstract
1. Introduction
2. Computation Details
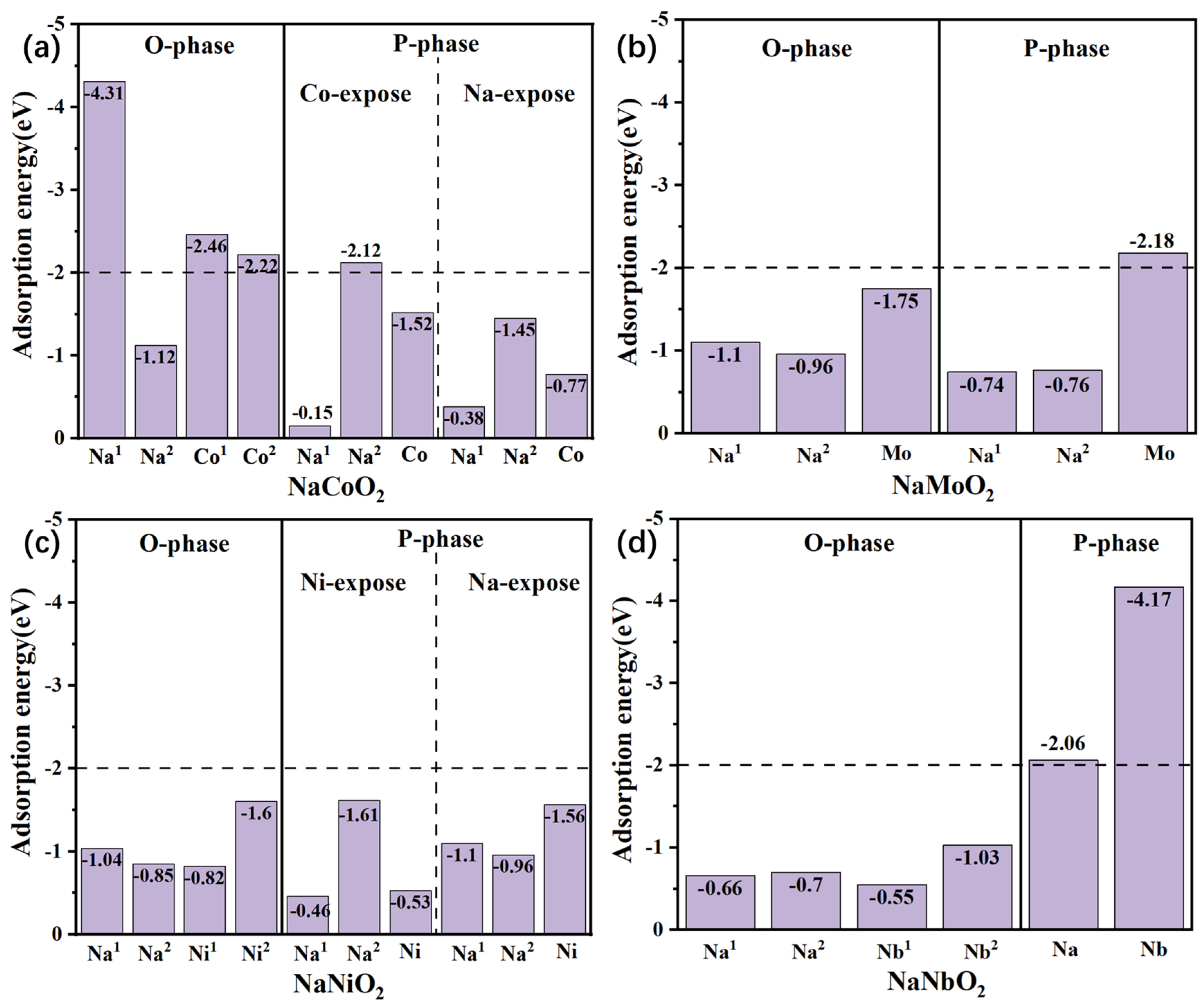
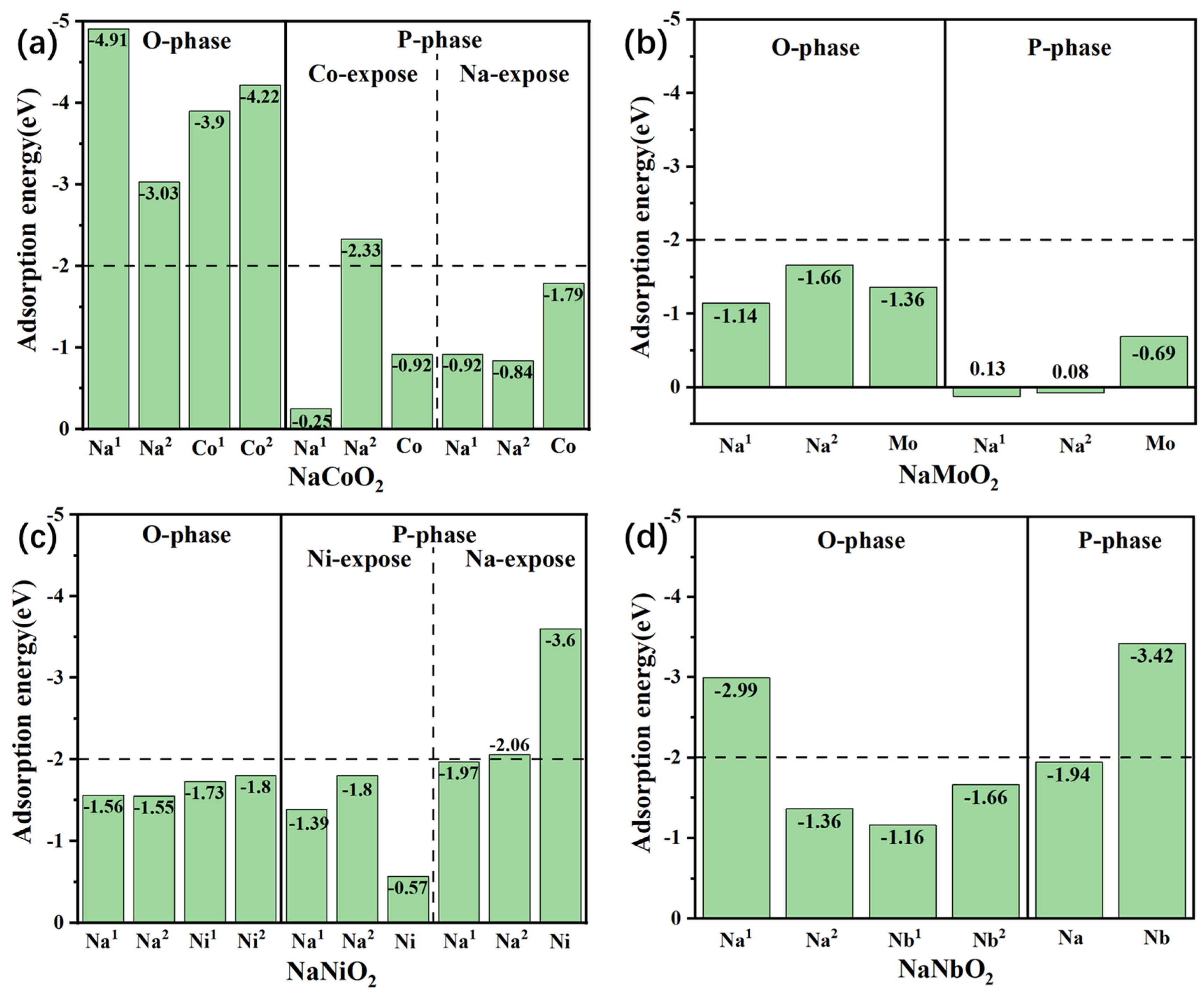
3. CO2 and H2O Adsorption Energy
4. Discussion
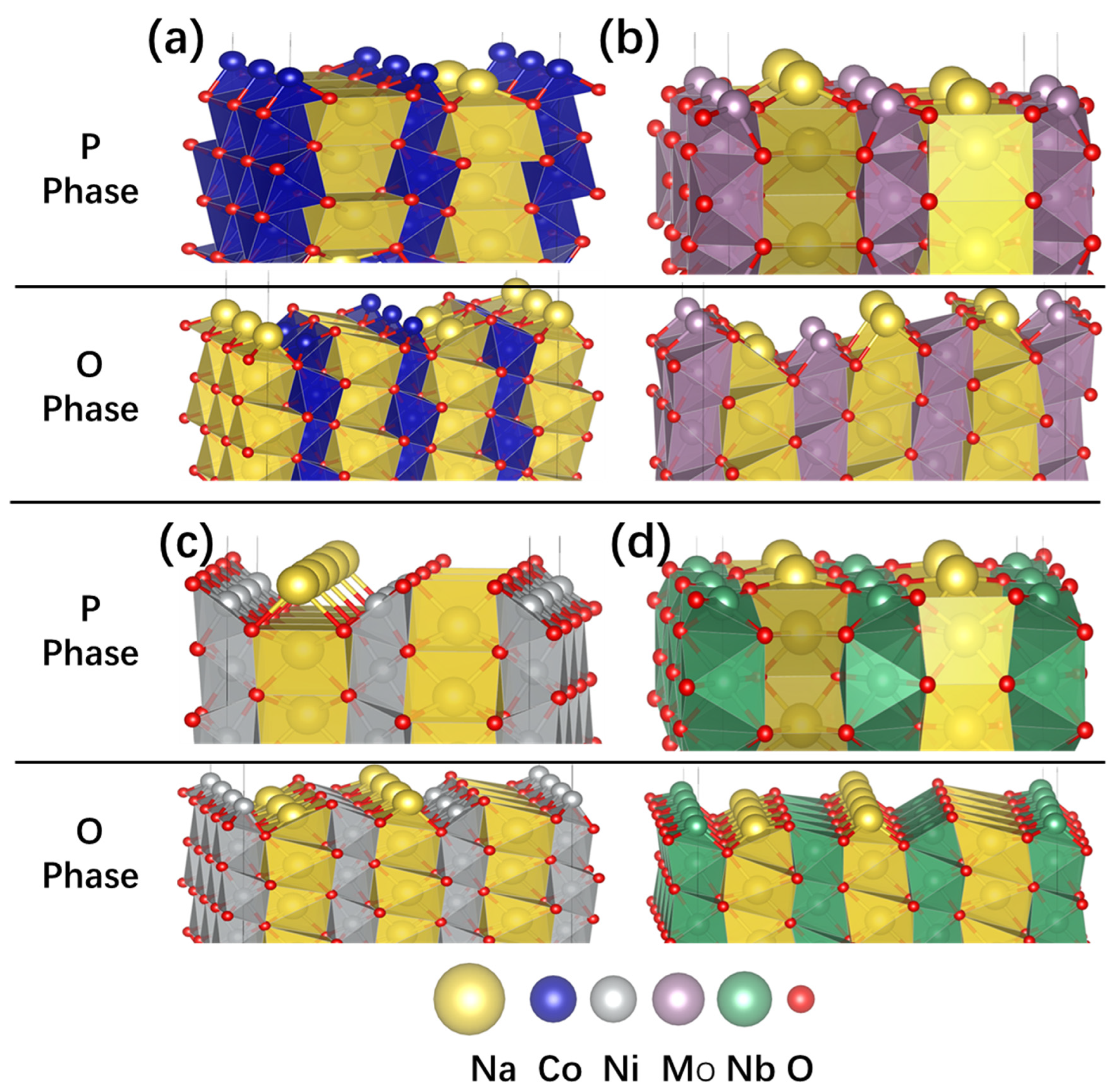
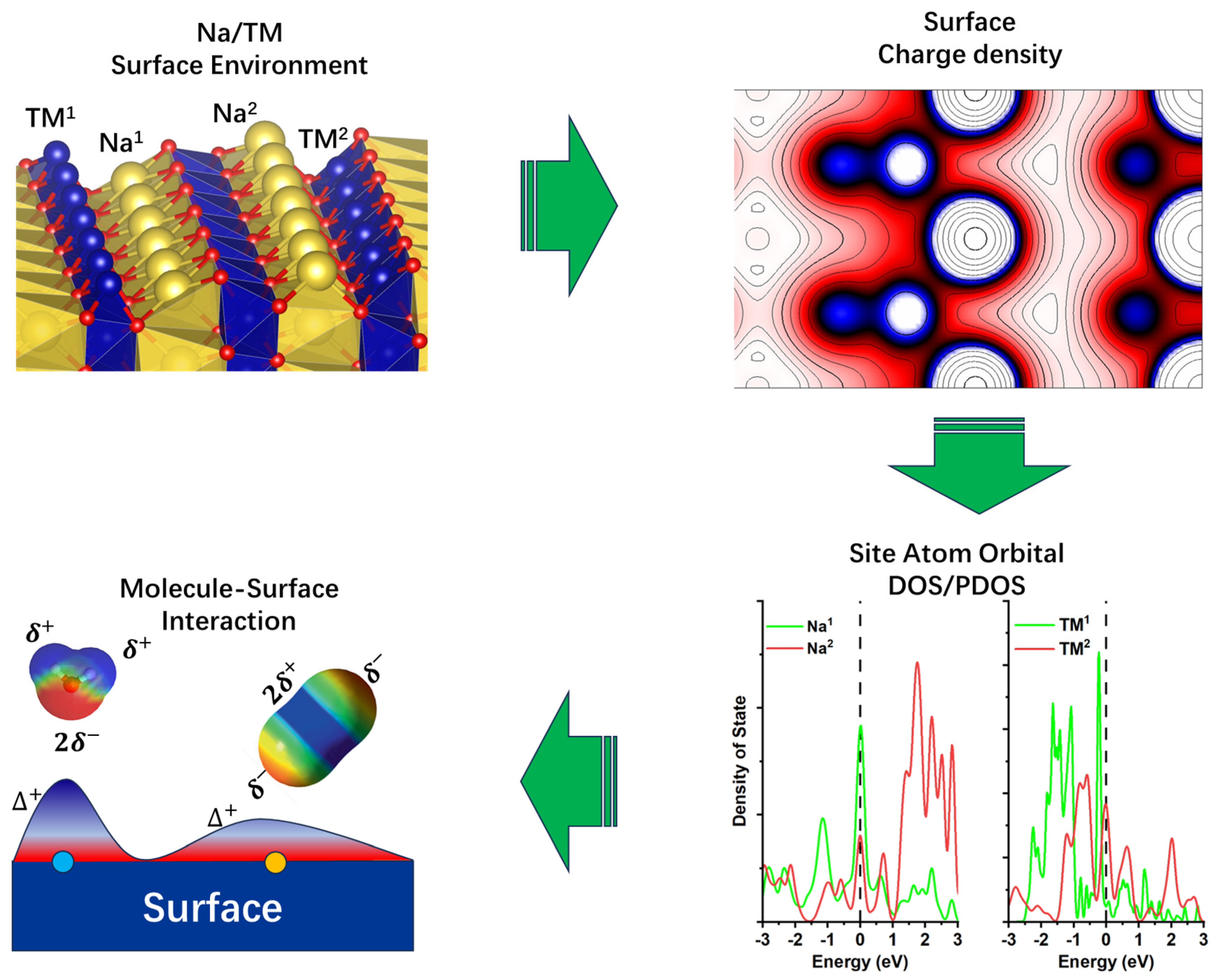
5. Surface Atomic Environment
6. Electron Density Distribution
7. Projected Density of State (PDOS)
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, C.; Zhang, L.; Zhang, Z.; Zhao, R.; Zhao, D.; Ma, R.; Yin, L. Layered materials for supercapacitors and batteries: Applications and challenges. Prog. Mater. Sci. 2021, 118, 100763. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, Z.; Chen, M.; Zou, C.; Jin, H.; Wang, S.; Chou, S.-L.; Liu, Y.; Dou, S.-X. The Cathode Choice for Commercialization of Sodium-Ion Batteries: Layered Transition Metal Oxides versus Prussian Blue Analogs. Adv. Funct. Mater. 2020, 30, 1909530. [Google Scholar] [CrossRef]
- Yang, Y.; Okonkwo, E.G.; Huang, G.; Xu, S.; Sun, W.; He, Y. On the sustainability of lithium ion battery industry—A review and perspective. Energy Storage Mater. 2021, 36, 186–212. [Google Scholar] [CrossRef]
- Zhang, M.; Meng, Y.S. Sodium-Ion Batteries Paving the Way for Grid Energy Storage. ECS Meet. Abstr. 2021, MA2021-02, 230. [Google Scholar] [CrossRef]
- Chayambuka, K.; Mulder, G.; Danilov, D.L.; Notten, P.H.L. From Li-Ion Batteries Toward Na-Ion Chemistries: Challenges and Opportunities. Adv. Energy Mater. 2020, 10, 2001310. [Google Scholar] [CrossRef]
- Li, Y.; Chen, M.; Liu, B.; Zhang, Y.; Xia, X. Heteroatom Doping: An Effective Way to Boost Sodium Ion Storage. Adv. Energy Mater. 2020, 10, 2000927. [Google Scholar] [CrossRef]
- Yang, C.; Xin, S.; Mai, L.; You, Y. Materials Design for High-Safety Sodium-Ion Battery. Adv. Energy Mater. 2021, 11, 2000974. [Google Scholar] [CrossRef]
- Jin, T.; Li, H.X.; Zhu, K.J.; Wang, P.-F.; Liu, P.; Jiao, L.F. Polyanion-type cathode materials for sodium-ion batteries. Chem. Soc. Rev. 2020, 49, 2342–2377. [Google Scholar] [CrossRef]
- Liu, Y.F.; Han, K.; Peng, D.N.; Kong, L.Y.; Su, Y.; Li, H.W.; Hu, H.Y.; Li, J.Y.; Wang, H.R.; Fu, Z.Q.; et al. Layered oxide cathodes for sodium-ion batteries: From air stability, interface chemistry to phase transition. InfoMat 2023, 5, e12422. [Google Scholar] [CrossRef]
- Wang, P.F.; You, Y.; Yin, Y.X.; Guo, Y.G. Layered Oxide Cathodes for Sodium-Ion Batteries: Phase Transition, Air Stability, and Performance. Adv. Energy Mater. 2018, 8, 1701912. [Google Scholar] [CrossRef]
- Guo, S.; Liu, P.; Yu, H.; Zhu, Y.; Chen, M. A Layered P2- and O3-Type Composite as a High-Energy Cathode for Rechargeable Sodium-Ion Batteries. Angew. Chem. 2015, 127, 5992–5997. [Google Scholar] [CrossRef]
- Mu, L.Q.; Xu, S.Y.; Li, Y.M.; Hu, Y.-S.; Li, H.; Chen, L.Q.; Huang, X.J. Prototype Sodium-Ion Batteries Using an Air-Stable and Co/Ni-Free O-3-Layered Metal Oxide Cathode. Adv. Mater. 2015, 27, 6928–6933. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.N.; Hu, Z.; Chen, M.Z.; Zou, C.; Jin, H.L.; Wang, S.; Chou, S.-L.; Dou, S.-X. Recent Progress of Layered Transition Metal Oxide Cathodes for Sodium-Ion Batteries. Small 2019, 15, 1805381. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Cai, H.; Chen, Q.; Kong, X.; Pan, H.; Hu, Y.S. Origin of Air-Stability for Transition Metal Oxide Cathodes in Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 5338–5345. [Google Scholar] [CrossRef]
- Joshi, A.; Chakrabarty, S.; Akella, S.H.; Saha, A.; Mukherjee, A.; Schmerling, B.; Ejgenberg, M.; Sharma, R.; Noked, M. High-Entropy Co-Free O3-Type Layered Oxyfluoride: A Promising Air-Stable Cathode for Sodium-Ion Batteries. Adv. Mater. 2023, 35, 2304440. [Google Scholar] [CrossRef]
- Huang, Z.-X.; Li, K.; Cao, J.-M.; Zhang, K.-Y.; Liu, H.-H.; Guo, J.-Z.; Liu, Y.; Wang, T.; Dai, D.; Zhang, X.-Y.; et al. New Insights into Anionic Redox in P2-Type Oxide Cathodes for Sodium-Ion Batteries. Nano Lett. 2024, 24, 13615–13623. [Google Scholar] [CrossRef]
- Lamb, J.; Manthiram, A. Surface-Modified Na(Ni0.3Fe0.4Mn0.3)O2 Cathodes with Enhanced Cycle Life and Air Stability for Sodium-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 11735–11742. [Google Scholar] [CrossRef]
- Wei, S.-B.; He, Y.-J.; Tang, Y.; Fu, H.-W.; Zhou, J.; Liang, S.-Q.; Cao, X.-X. A Ca-substituted air-stable layered oxide cathode material with facilitated phase transitions for high-performance Na-ion batteries. Rare Met. 2024, 43, 5701–5711. [Google Scholar] [CrossRef]
- Zhang, L.; Deshmukh, J.; Hijazi, H.; Ye, Z.; Johnson, M.B.; George, A.; Dahn, J.R.; Metzger, M. Impact of Calcium on Air Stability of Na[Ni1/3Fe1/3Mn1/3]O2 Positive Electrode Material for Sodium-ion Batteries. J. Electrochem. Soc. 2023, 170, 070514. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, Q.; Zhao, S.; Feng, W.; Liu, Y.; Yang, X.; Wang, Z.; Zhao, Y. Investigation on the Air Stability of P2-Layered Transition Metal Oxides by Nb Doping in Sodium Ion Batteries. Batteries 2023, 9, 183. [Google Scholar] [CrossRef]
- Yin, W.; Huang, Z.; Zhang, T.; Yang, T.; Ji, H.; Zhou, Y.; Shi, S.; Zhang, Y. P2-type layered oxide cathode with honeycomb-ordered superstructure for sodium-ion batteries. Energy Storage Mater. 2024, 69, 103424. [Google Scholar] [CrossRef]
- Liu, Y.-F.; Hu, H.-Y.; Li, J.-Y.; Wang, H.; Zhao, Y.; Wang, J.; Wu, Y.-B.; Li, Y.-J.; Zhang, G.-Y.; Sun, Q.-Q.; et al. An air-stable single-crystal layered oxide cathode based on multifunctional structural modulation for high-energy-density sodium-ion batteries. Sci. China Chem. 2024, 67, 4242–4250. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Q.; Chen, M.; Yang, Y.; Zhao, J. Unveiling the Origin of Air Stability in Polyanion and Layered-Oxide Cathode Materials for Sodium-Ion Batteries and Their Practical Application Considerations. Adv. Funct. Mater. 2024, 34, 2308257. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Z.; Xu, S.; Mu, L.; Gu, L.; Hu, Y.S.; Li, H.; Chen, L. Air-Stable Copper-Based P2-Na7/9Cu2/9Fe1/9Mn2/3O2 as a New Positive Electrode Material for Sodium-Ion Batteries. Adv. Sci. 2015, 2, 1500031. [Google Scholar] [CrossRef]
- Chen, T.R.; Sheng, T.; Wu, Z.G.; Li, J.T.; Wang, E.H.; Wu, C.J.; Li, H.T.; Guo, X.D.; Zhong, B.H.; Huang, L.; et al. Cu2+ Dual-Doped Layer-Tunnel Hybrid Na0.6Mn1−xCuxO2 as a Cathode of Sodium-Ion Battery with Enhanced Structure Stability, Electrochemical Property, and Air Stability. Acs Appl. Mater. Interfaces 2018, 10, 10147. [Google Scholar] [CrossRef]
- Zhou, Y.N.; Wang, P.F.; Zhang, X.D.; Huang, L.B.; Guo, Y.G. Air-Stable and High-Voltage Layered P3-Type Cathode for Sodium-Ion Full Battery. ACS Appl. Mater. Interfaces 2019, 11, 24184–24191. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.; Liu, X.; Qiu, J.; Zhang, D.; Xiao, Z.; Xie, J.; Ren, F.; Wang, J.; Li, Y.; Ortiz, G.F.; et al. Engineering Na+-layer spacings to stabilize Mn-based layered cathodes for sodium-ion batteries. Nat. Commun. 2021, 12, 4903. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Y.; Shen, X.; Li, Y.; Kong, Q.; Lee, S.; Wang, Z.; Yu, R.; Hu, Y.; Chen, L. Anti-P-2 structured Na0.5NbO2 and its negative strain effect. Energy Environ. Sci. 2015, 8, 2753–2759. [Google Scholar] [CrossRef]
- Roth, H.-F.; Meyer, G.; Hu, Z.W.; Kaindl, G. Synthesis, structure, and X-ray absorption spectra of LixNbO2 and NaxNbO2 (x ≤ 1). Z. Für Anorg. Und Allg. Chem. 1993, 619, 1369–1373. [Google Scholar] [CrossRef]
- Vitoux, L.; Guignard, M.; Penin, N.; Carlier, D.; Darriet, J.; Delmas, C. NaMoO2: A Layered Oxide with Molybdenum Clusters. Inorg. Chem. 2020, 59, 4015–4023. [Google Scholar] [CrossRef]
- Vitoux, L.; Guignard, M.; Suchomel, M.R.; Pramudita, J.C.; Sharma, N.; Delmas, C. The NaxMoO2 phase diagram (1/2 ≤ x < 1): An electrochemical devil’s staircase. Chem. Mater. 2017, 29, 7243–7254. [Google Scholar]
- Zheng, Y.M.; Huang, X.B.; Meng, X.M.; Xu, S.D.; Chen, L.; Liu, S.B.; Zhang, D. Copper and Zirconium Codoped O3-Type Sodium Iron and Manganese Oxide as the Cobalt/Nickel-Free High-Capacity and Air-Stable Cathode for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 45528–45537. [Google Scholar] [CrossRef]
- Stansby, J.H.; Sharma, N.; Goonetilleke, D. Probing the charged state of layered positive electrodes in sodium-ion batteries: Reaction pathways, stability and opportunities. J. Mater. Chem. A 2020, 8, 24833–24867. [Google Scholar] [CrossRef]
- Brugnetti, G.; Triolo, C.; Massaro, A.; Ostroman, I.; Pianta, N.; Ferrara, C.; Sheptyakov, D.; Muñoz-García, A.B.; Pavone, M.; Santangelo, S.; et al. Structural Evolution of Air-Exposed Layered Oxide Cathodes for Sodium-Ion Batteries: An Example of Ni-doped NaxMnO2. Chem. Mater. 2023, 35, 8440–8454. [Google Scholar] [CrossRef]
- Zuo, W.; Innocenti, A.; Zarrabeitia, M.; Bresser, D.; Yang, Y.; Passerini, S. Layered oxide cathodes for sodium-ion batteries: Storage mechanism, electrochemistry, and techno-economics. Acc. Chem. Res. 2023, 56, 284–296. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef]
- Hafner, J. Ab-initio simulations of materials using VASP: Density-functional theory and beyond. J. Comput. Chem. 2008, 29, 2044–2078. [Google Scholar] [CrossRef]
- Furness, J.W.; Kaplan, A.D.; Ning, J.; Perdew, J.P.; Sun, J. Accurate and numerically efficient r2SCAN meta-generalized gradient approximation. J. Phys. Chem. Lett. 2020, 11, 8208–8215. [Google Scholar] [CrossRef]
- Tran, R.; Xu, Z.; Radhakrishnan, B.; Winston, D.; Sun, W.; Persson, K.A.; Ong, S.P. Surface energies of elemental crystals. Sci. Data 2016, 3, 160080. [Google Scholar] [CrossRef]
- Abrantes, P.; Kort-Kamp, W.; Rosa, F.; Farina, C.; Pinheiro, F.; Cysne, T.P. Controlling electric and magnetic Purcell effects in phosphorene via strain engineering. Phys. Rev. B 2023, 108, 155427. [Google Scholar] [CrossRef]
- Jiang, Y.-L.; Liu, H.-Q.; Yi, D.-Q.; Lin, G.-Y.; Xun, D.; Zhang, R.-Q.; Sun, Y.-D.; Liu, S.-Q. Microstructure evolution and recrystallization behavior of cold-rolled Zr-1Sn-0.3 Nb-0.3 Fe-0.1 Cr alloy during annealing. Trans. Nonferrous Met. Soc. China 2018, 28, 651–661. [Google Scholar] [CrossRef]
- Boyarkin, O.V.; Koshelev, M.A.; Aseev, O.; Maksyutenko, P.; Rizzo, T.R.; Zobov, N.F.; Lodi, L.; Tennyson, J.; Polyansky, O.L. Accurate bond dissociation energy of water determined by triple-resonance vibrational spectroscopy and ab initio calculations. Chem. Phys. Lett. 2013, 568, 14–20. [Google Scholar] [CrossRef]
- Fukuda, T.; Maekawa, T.; Hasumura, T.; Rantonen, N.; Ishii, K.; Nakajima, Y.; Hanajiri, T.; Yoshida, Y.; Whitby, R.; Mikhalovsky, S. Dissociation of carbon dioxide and creation of carbon particles and films at room temperature. New J. Phys. 2007, 9, 321. [Google Scholar] [CrossRef]
- Schmid, M.; Parkinson, G.S.; Diebold, U. Analysis of Temperature-Programmed Desorption via Equilibrium Thermodynamics. ACS Phys. Chem. Au 2023, 3(1), 44–62. [Google Scholar] [CrossRef]
- Lei, Y.; Li, X.; Liu, L.; Ceder, G. Synthesis and stoichiometry of different layered sodium cobalt oxides. Chem. Mater. 2014, 26, 5288–5296. [Google Scholar] [CrossRef]
- Pai, W.W.; Huang, S.H.; Meng, Y.S.; Chao, Y.C.; Lin, C.H.; Liu, H.L.; Chou, F.C. Sodium Trimer Ordering on a NaxCoO2 Surface. Phys. Rev. Lett. 2008, 100, 206404. [Google Scholar] [CrossRef]
- Pillay, D.; Johannes, M.; Mazin, I. Electronic Structure of the NaxCoO2 Surface. Phys. Rev. Lett. 2008, 101, 246808. [Google Scholar] [CrossRef]
- Grépin, E.; Moiseev, I.A.; Abakumov, A.M.; Tarascon, J.-M.; Mariyappan, S. Rational selection of sodium layered oxides for high performance Na-ion batteries: P2 vs O3 vs P2-O3 intergrowths. J. Electrochem. Soc. 2023, 170, 080510. [Google Scholar] [CrossRef]
- Feng, J.; Fang, D.; Yang, Z.; Zhong, J.; Zheng, C.; Wei, Z.; Li, J. A novel P2/O3 composite cathode toward synergistic electrochemical optimization for sodium ion batteries. J. Power Sources 2023, 553, 232292. [Google Scholar] [CrossRef]
- Leybo, D.; Etim, U.J.; Monai, M.; Bare, S.R.; Zhong, Z.; Vogt, C. Metal–support interactions in metal oxide-supported atomic, cluster, and nanoparticle catalysis. Chem. Soc. Rev. 2024, 53, 10450–10490. [Google Scholar] [CrossRef] [PubMed]
- Hammer, B.; Nørskov, J.K. Theoretical surface science and catalysis—Calculations and concepts. In Advances in Catalysis; Academic Press: Cambridge, MA, USA, 2000; Volume 45, pp. 71–129. [Google Scholar]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; et al. Commentary: The Materials Project: A materials genome approach to accelerating materials innovation. APL Mater. 2013, 1, 011002. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Yang, Y.; Qian, Y.; Luo, Z.; Li, H.; Chen, L.; Cao, X.; Wei, S.; Zhou, B.; Zhang, Z.; Chen, S.; et al. Water induced ultrathin Mo2C nanosheets with high-density grain boundaries for enhanced hydrogen evolution. Nat. Commun. 2022, 13, 7225. [Google Scholar] [CrossRef]
- Chiter, F.; Costa, D.; Pébère, N.; Marcus, P.; Lacaze-Dufaure, C. Insight at the atomic scale of corrosion inhibition: DFT study of 8-hydroxyquinoline on oxidized aluminum surfaces. Phys. Chem. Chem. Phys. 2023, 25, 4284–4296. [Google Scholar] [CrossRef]
- Weng, H.; Xu, G.; Zhang, H.; Zhang, S.-C.; Dai, X.; Fang, Z. Half-metallic surface states and topological superconductivity in NaCoO 2 from first principles. Phys. Rev. B 2011, 84, 060408. [Google Scholar] [CrossRef]
- Ning, C.G.; Hajgató, B.; Huang, Y.R.; Zhang, S.F.; Liu, K.; Luo, Z.H.; Knippenberg, S.; Deng, J.K.; Deleuze, M.S. High resolution electron momentum spectroscopy of the valence orbitals of water. Chem. Phys. 2008, 343, 19–30. [Google Scholar] [CrossRef]
- Ishida, H. Theory of the alkali-metal chemisorption on metal surfaces. II. Phys. Rev. B 1990, 42, 10899. [Google Scholar] [CrossRef]
- Sun, Z.; Song, Z.; Yin, W.-J. Going beyond the d-band center to describe CO2 activation on single-atom alloys. Adv. Energy Sustain. Res. 2022, 3, 2100152. [Google Scholar] [CrossRef]
- Sun, X.; Sun, L.; Li, G.; Tuo, Y.; Ye, C.; Yang, J.; Low, J.; Yu, X.; Bitter, J.H.; Lei, Y.; et al. Phosphorus Tailors the d-Band Center of Copper Atomic Sites for Efficient CO2 Photoreduction under Visible-Light Irradiation. Angew. Chem. 2022, 134, e202207677. [Google Scholar] [CrossRef]
- Stojković, M.; Pašti, I.A. Strain Engineering for Tuning the Photocatalytic Activity of Metal-Organic Frameworks-Theoretical Study of the UiO-66 Case. Catalysts 2021, 11, 264. [Google Scholar] [CrossRef]
- Zhao, Z.-J.; Gong, J. Catalyst design via descriptors. Nat. Nanotechnol. 2022, 17, 563–564. [Google Scholar] [CrossRef]
- Muruganantham, R.; Liu, W.-R.; Lin, C.-H.; Rudysh, M.; Piasecki, M. Design of meso/macro porous 2D Mn-vanadate as potential novel anode materials for sodium-ion storage. J. Energy Storage 2019, 26, 100915. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Xue, Q.; Li, S.; Wang, X.; Hou, Y.; Sun, C.; Wang, C.; Sheng, G.; Sheng, P.; Bai, H.; et al. The Air Stability of Sodium Layered Oxide NaTMO2 (100) Surface Investigated via DFT Calculations. Nanomaterials 2025, 15, 1067. https://doi.org/10.3390/nano15141067
Li H, Xue Q, Li S, Wang X, Hou Y, Sun C, Wang C, Sheng G, Sheng P, Bai H, et al. The Air Stability of Sodium Layered Oxide NaTMO2 (100) Surface Investigated via DFT Calculations. Nanomaterials. 2025; 15(14):1067. https://doi.org/10.3390/nano15141067
Chicago/Turabian StyleLi, Hui, Qing Xue, Shengyi Li, Xuechun Wang, Yijie Hou, Chang Sun, Cun Wang, Guozheng Sheng, Peng Sheng, Huitao Bai, and et al. 2025. "The Air Stability of Sodium Layered Oxide NaTMO2 (100) Surface Investigated via DFT Calculations" Nanomaterials 15, no. 14: 1067. https://doi.org/10.3390/nano15141067
APA StyleLi, H., Xue, Q., Li, S., Wang, X., Hou, Y., Sun, C., Wang, C., Sheng, G., Sheng, P., Bai, H., Xu, L., & Qian, Y. (2025). The Air Stability of Sodium Layered Oxide NaTMO2 (100) Surface Investigated via DFT Calculations. Nanomaterials, 15(14), 1067. https://doi.org/10.3390/nano15141067





