Enhancing Water Splitting Performance via NiFeP-CoP on Cobalt Foam: Synergistic Effects and Structural Optimization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Growing CoOOH Nanosheets on Co Foam (CoOOH/CF)
2.3. Preparation of CoP Nanosheets on Co Foam (CoP/CF)
2.4. Fabrication of NiFeP on CoP/CF Nanoplates (NiFeP-CoP/CF)
2.5. Characterization
2.6. Electrochemical Measurements
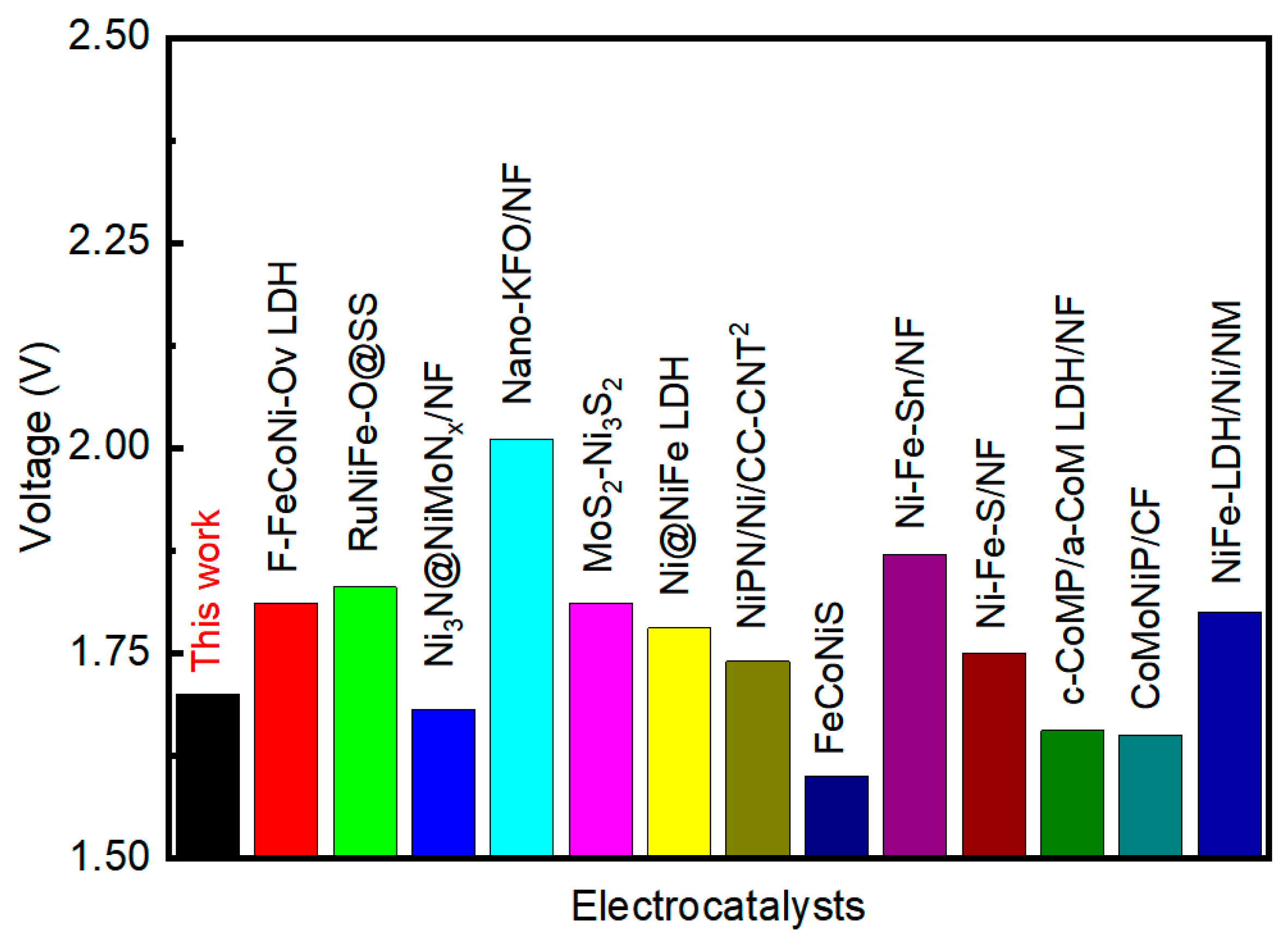
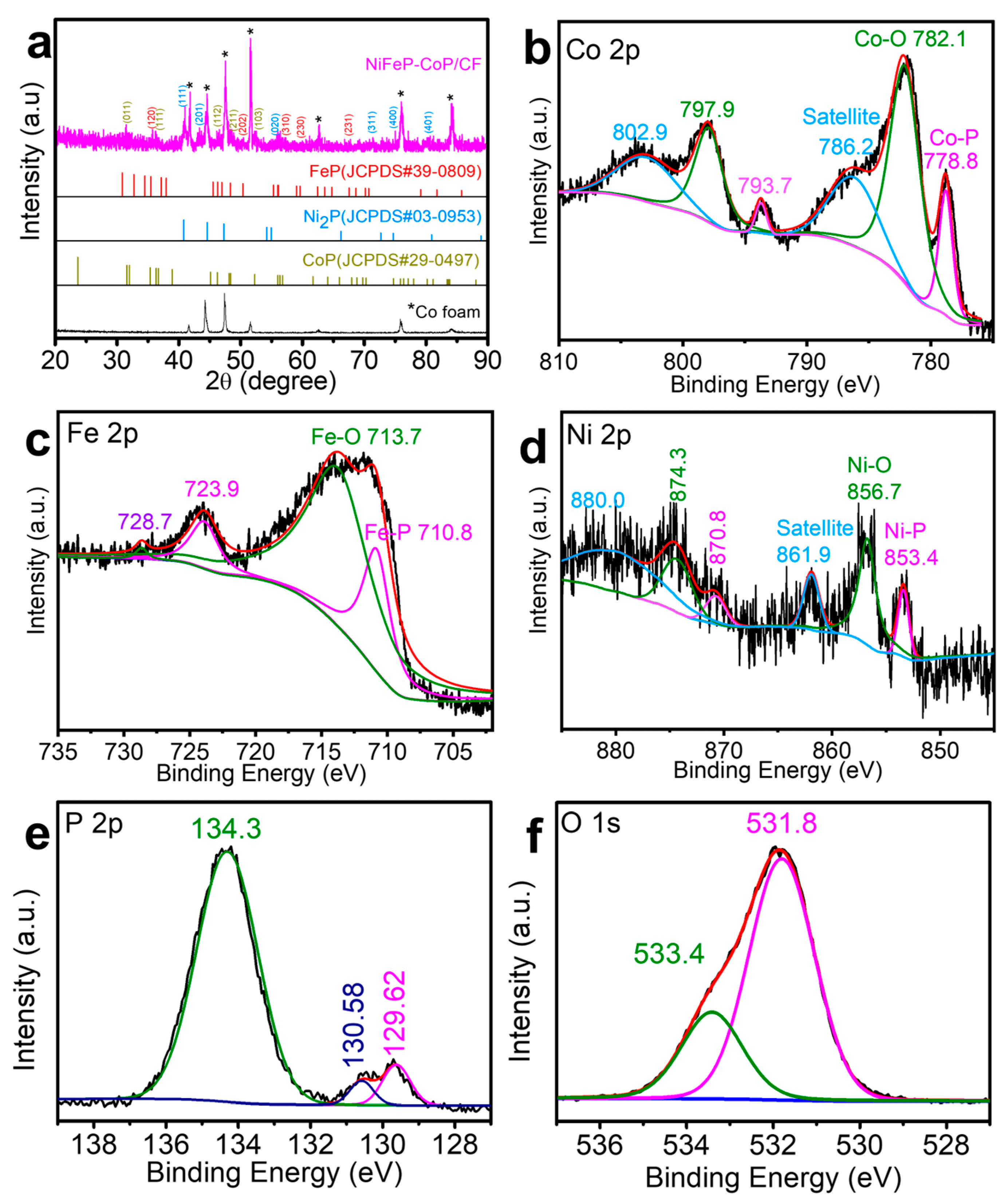
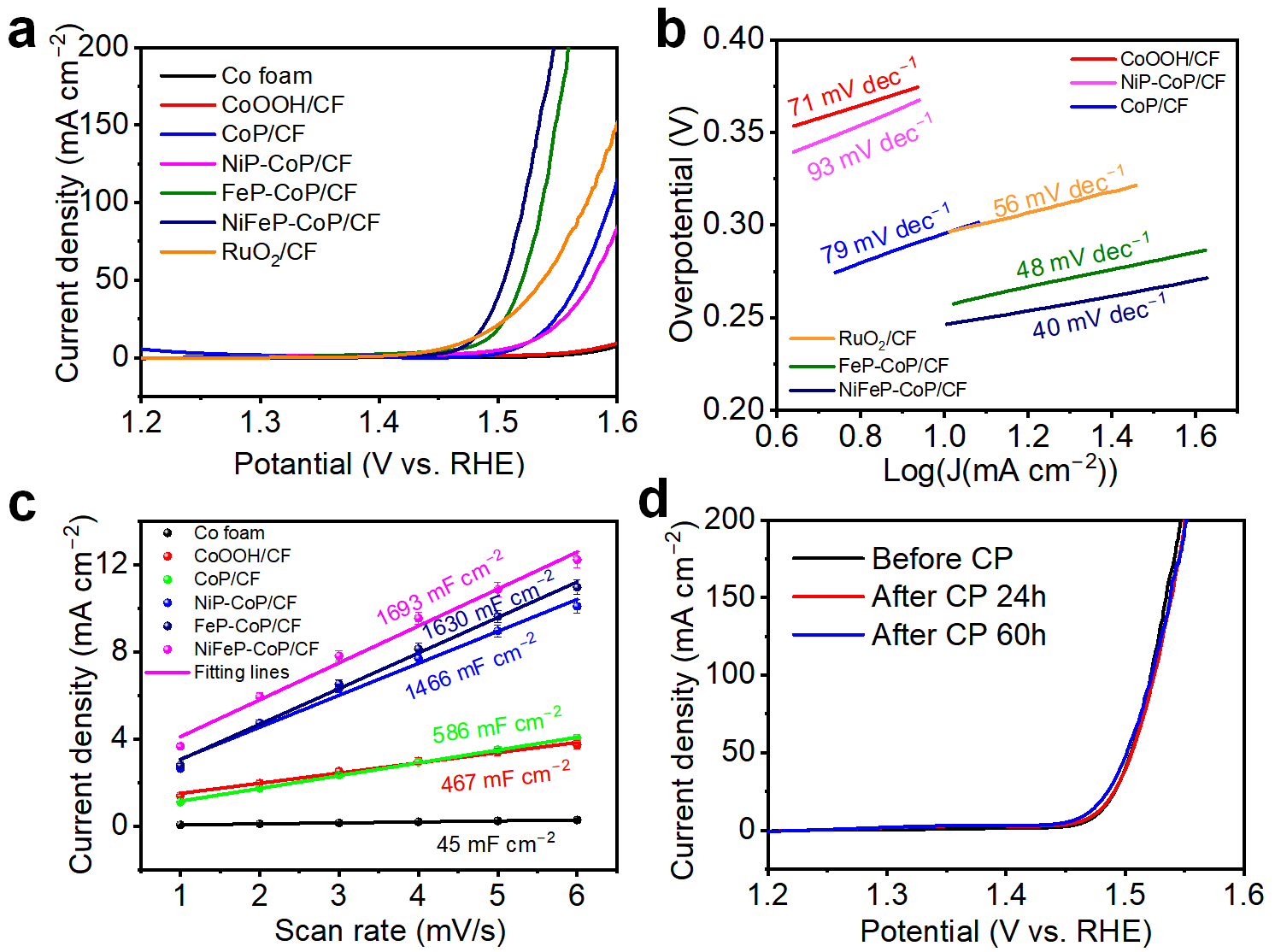
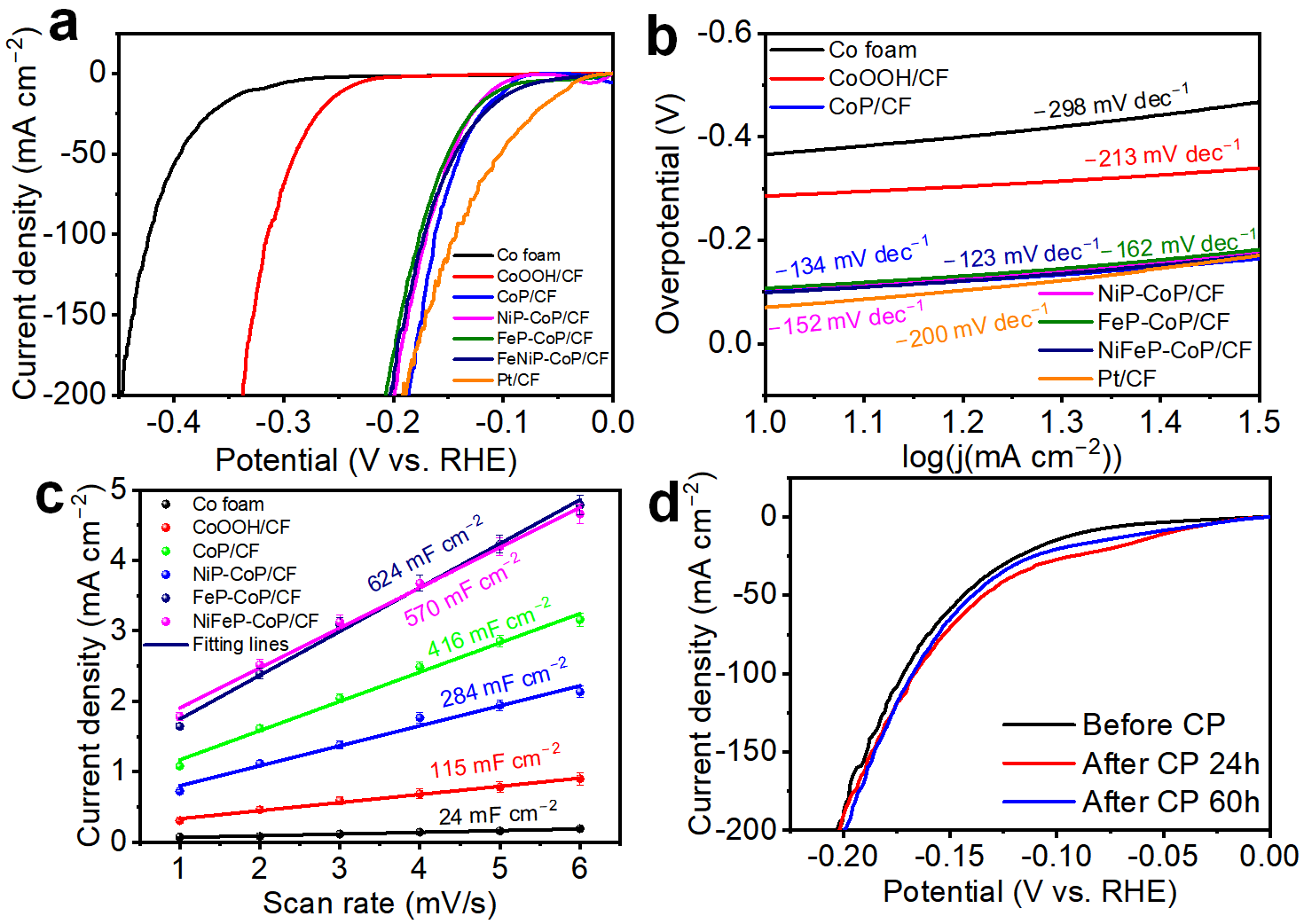
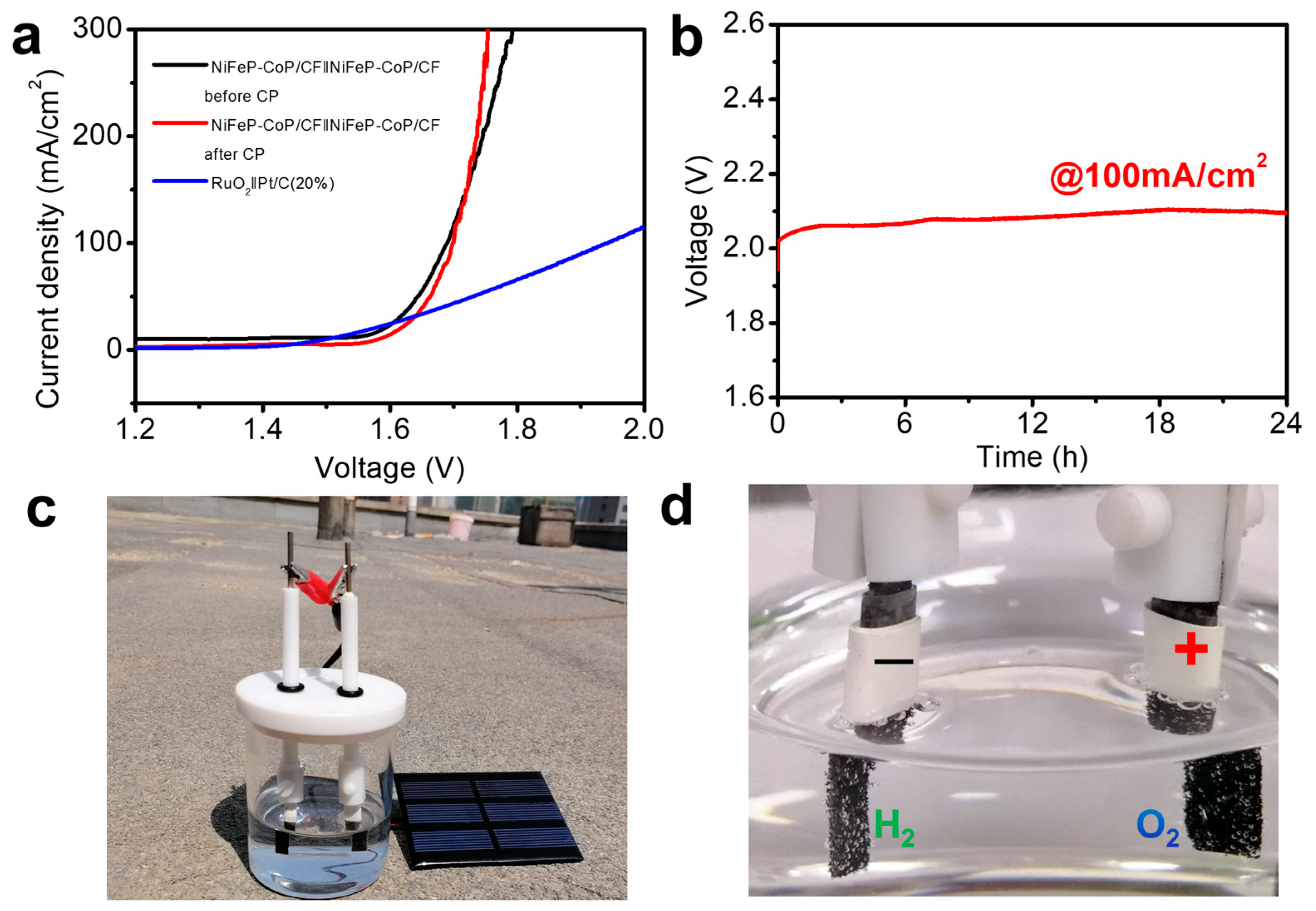
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tong, W.; Forster, M.; Dionigi, F.; Dresp, S.; Erami, R.S.; Strasser, P.; Cowan, A.J.; Farràs, P. Electrolysis of Low-Grade and Saline Surface Water. Nat. Energy 2020, 5, 367–377. [Google Scholar] [CrossRef]
- Chu, S.; Majumdar, A. Opportunities and Challenges for a Sustainable Energy Future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The Role of Hydrogen and Fuel Cells in the Global Energy System. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Y. Noble Metal-Free Hydrogen Evolution Catalysts for Water Splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef]
- Tachibana, Y.; Vayssieres, L.; Durrant, J.R. Artificial Photosynthesis for Solar Water-Splitting. Nat. Photonics 2012, 6, 511–518. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, L.; Gong, J. Recent Progress Made in the Mechanism Comprehension and Design of Electrocatalysts for Alkaline Water Splitting. Energy Environ. Sci. 2019, 12, 2620–2645. [Google Scholar] [CrossRef]
- Zeng, K.; Zhang, D. Recent Progress in Alkaline Water Electrolysis for Hydrogen Production and Applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Yu, S.; Yu, X.; Yang, H.; Li, F.; Li, S.; Kang, Y.S.; Zheng, J.Y. Mechanism, Modification and Stability of Tungsten Oxide-Based Electrocatalysts for Water Splitting: A Review. J. Energy Chem. 2024, 99, 23–49. [Google Scholar] [CrossRef]
- Wang, J.; Cui, W.; Liu, Q.; Xing, Z.; Asiri, A.M.; Sun, X. Recent Progress in Cobalt-Based Heterogeneous Catalysts for Electrochemical Water Splitting. Adv. Mater. 2016, 28, 215–230. [Google Scholar] [CrossRef]
- Guo, F.; Macdonald, T.J.; Sobrido, A.J.; Liu, L.; Feng, J.; He, G. Recent Advances in Ultralow-Pt-Loading Electrocatalysts for the Efficient Hydrogen Evolution. Adv. Sci. 2023, 10, 2301098. [Google Scholar] [CrossRef]
- Fan, K.; Chen, H.; Ji, Y.; Huang, H.; Claesson, P.M.; Daniel, Q.; Philippe, B.; Rensmo, H.; Li, F.; Luo, Y.; et al. Nickel-Vanadium Monolayer Double Hydroxide for Efficient Electrochemical Water Oxidation. Nat. Commun. 2016, 7, 11981. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Design of Electrocatalysts for Oxygen- and Hydrogen-Involving Energy Conversion Reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, F.; Zhang, X.; Lin, Y.; Zhang, L.; Chan, T.S.; Zhang, Q.; Chen, L. Engineering Lattice Distortion in Ruthenium Oxide Enables Robust Acidic Water Oxidation via Direct O–O Coupling. Adv. Mater. 2025, 2500449. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Guo, F.; Wei, Q.; Lai, F.; Chen, R.; Guo, J.; Gong, M.; Zhang, S.; Wang, Z.; Zhong, J.; et al. Engineering the Metal/Oxide Interfacial O-Filling Effect to Tailor Oxygen Spillover for Efficient Acidic Water Oxidation. Adv. Funct. Mater. 2025, 35, 2421354. [Google Scholar] [CrossRef]
- Reier, T.; Oezaslan, M.; Strasser, P. Electrocatalytic Oxygen Evolution Reaction (OER) on Ru, Ir, and Pt Catalysts: A Comparative Study of Nanoparticles and Bulk Materials. ACS Catal. 2012, 2, 1765–1772. [Google Scholar] [CrossRef]
- Yu, F.; Yu, L.; Mishra, I.K.; Yu, Y.; Ren, Z.F.; Zhou, H.Q. Recent Developments in Earth-Abundant and Non-Noble Electrocatalysts for Water Electrolysis. Mater. Today Phys. 2018, 7, 121–138. [Google Scholar] [CrossRef]
- Qiu, Z.; Tai, C.W.; Niklasson, G.A.; Edvinsson, T. Direct Observation of Active Catalyst Surface Phases and the Effect of Dynamic Self-Optimization in NiFe-Layered Double Hydroxides for Alkaline Water Splitting. Energy Environ. Sci. 2019, 12, 572–581. [Google Scholar] [CrossRef]
- Gao, R.; Yan, D. Recent Development of Ni/Fe-Based Micro/Nanostructures toward Photo/Electrochemical Water Oxidation. Adv. Energy Mater. 2020, 10, 1900954. [Google Scholar] [CrossRef]
- Menezes, P.W.; Indra, A.; Zaharieva, I.; Walter, C.; Loos, S.; Hoffmann, S.; Schlögl, R.; Dau, H.; Driess, M. Helical Cobalt Borophosphates to Master Durable Overall Water-Splitting. Energy Environ. Sci. 2019, 12, 988–999. [Google Scholar] [CrossRef]
- Li, S.; Liu, T.; Yu, S.; Yu, X.; Yang, H.; Wang, C.; Zheng, J.Y. Anchoring Platinum Clusters in CoP@CoNi Layered Double Hydroxide to Prepare High-Performance and Stable Electrodes for Efficient Water Splitting at High Current Density. J. Colloid. Interface Sci. 2025, 684, 717–728. [Google Scholar] [CrossRef]
- Sha, Q.; Wang, S.; Yan, L.; Feng, Y.; Zhang, Z.; Li, S.; Guo, X.; Li, T.; Li, H.; Zhuang, Z.; et al. 10,000-h-Stable Intermittent Alkaline Seawater Electrolysis. Nature 2025, 639, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Su, S.; Li, J.; Ping, D.; Li, Y.; He, M.; Yu, X.; Wei, Z.; Liu, Y.; Li, S.; et al. High-Performance Bimetallic Electrocatalysts for Hydrogen Evolution Reaction Using N-Doped Graphene-Supported N-Co6Mo6C. Nanomaterials 2024, 14, 1422. [Google Scholar] [CrossRef]
- Milikić, J.; Nastasić, A.; Rakočević, L.; Radinović, K.; Stojadinović, S.; Stanković, D.; Šljukić, B. FeM/RGO (M = Ni and Cu) as Bifunctional Oxygen Electrode. Fuel 2024, 368, 131654. [Google Scholar] [CrossRef]
- Milikić, J.; Nastasić, A.; Knežević, S.; Rakočević, L.; Stojadinović, S.; Stanković, D.; Šljukić, B. Efficient Nano-Size ZnM/RGO (M = Ni, Cu, and Fe) Electrocatalysts for Oxygen Electrode Reactions in Alkaline Media. Int. J. Hydrogen Energy 2025, 97, 247–258. [Google Scholar] [CrossRef]
- Cheng, G.; Kou, T.; Zhang, J.; Si, C.; Gao, H.; Zhang, Z. O22-/O- Functionalized Oxygen-Deficient Co3O4 Nanorods as High Performance Supercapacitor Electrodes and Electrocatalysts towards Water Splitting. Nano Energy 2017, 38, 155–166. [Google Scholar] [CrossRef]
- Huang, J.; Clark, A.H.; Hales, N.; Crossley, K.; Guehl, J.; Skoupy, R.; Schmidt, T.J.; Fabbri, E. Oxidation of Interfacial Cobalt Controls the PH Dependence of the Oxygen Evolution Reaction. Nat. Chem. 2025, 17, 856–864. [Google Scholar] [CrossRef]
- Bergmann, A.; Jones, T.E.; Martinez Moreno, E.; Teschner, D.; Chernev, P.; Gliech, M.; Reier, T.; Dau, H.; Strasser, P. Unified Structural Motifs of the Catalytically Active State of Co(Oxyhydr)Oxides during the Electrochemical Oxygen Evolution Reaction. Nat. Catal. 2018, 1, 711–719. [Google Scholar] [CrossRef]
- Zhong, H.-X.; Wang, J.; Zhang, Q.; Meng, F.; Bao, D.; Liu, T.; Yang, X.-Y.; Chang, Z.-W.; Yan, J.-M.; Zhang, X.-B. In Situ Coupling FeM (M = Ni, Co) with Nitrogen-Doped Porous Carbon toward Highly Efficient Trifunctional Electrocatalyst for Overall Water Splitting and Rechargeable Zn-Air Battery. Adv. Sustain. Syst. 2017, 1, 1700020. [Google Scholar] [CrossRef]
- Balogun, M.S.; Huang, Y.; Qiu, W.; Yang, H.; Ji, H.; Tong, Y. Updates on the Development of Nanostructured Transition Metal Nitrides for Electrochemical Energy Storage and Water Splitting. Mater. Today 2017, 20, 425–451. [Google Scholar] [CrossRef]
- Wu, D.; Wei, Y.; Ren, X.; Ji, X.; Liu, Y.; Guo, X.; Liu, Z.; Asiri, A.M.; Wei, Q.; Sun, X. Co(OH)2 Nanoparticle-Encapsulating Conductive Nanowires Array: Room-Temperature Electrochemical Preparation for High-Performance Water Oxidation Electrocatalysis. Adv. Mater. 2018, 30, 1705366. [Google Scholar] [CrossRef]
- Hou, Y.; Qiu, M.; Nam, G.; Kim, M.; Zhang, T.; Cho, J.; Yuan, C.; Feng, X. Integrated Hierarchical Cobalt Sulfide/Nickel Selenide Hybrid Nanosheets as An Efficient 3D Electrode for Electrochemical and Photoelectrochemical Water Splitting. Nano Lett. 2017, 17, 4202–4209. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Tang, J.; Wang, Z.; Kang, Y.M.; Bando, Y.; Yamauchi, Y. Elaborately Assembled Core-Shell Structured Metal Sulfides as a Bifunctional Catalyst for Highly Efficient Electrochemical Overall Water Splitting. Nano Energy 2018, 47, 494–502. [Google Scholar] [CrossRef]
- Hu, E.; Feng, Y.; Nai, J.; Zhao, D.; Hu, Y.; Lou, X.W. Construction of Hierarchical Ni-Co-P Hollow Nanobricks with Oriented Nanosheets for Efficient Overall Water Splitting. Energy Environ. Sci. 2018, 11, 872–880. [Google Scholar] [CrossRef]
- Xue, Z.H.; Su, H.; Yu, Q.Y.; Zhang, B.; Wang, H.H.; Li, X.H.; Chen, J.S. Janus Co/CoP Nanoparticles as Efficient Mott–Schottky Electrocatalysts for Overall Water Splitting in Wide PH Range. Adv. Energy Mater. 2017, 7, 1602355. [Google Scholar] [CrossRef]
- Tabassum, H.; Guo, W.; Meng, W.; Mahmood, A.; Zhao, R.; Wang, Q.; Zou, R. Metal–Organic Frameworks Derived Cobalt Phosphide Architecture Encapsulated into B/N Co-Doped Graphene Nanotubes for All PH Value Electrochemical Hydrogen Evolution. Adv. Energy Mater. 2017, 7, 1601671. [Google Scholar] [CrossRef]
- Miao, M.; Zhang, L.; Zheng, J.; Ma, W.; Li, H.; Jiang, S.; Yu, X.; Huang, Y.; Gao, J.; Han, G. Urchin-like CoP Nanomaterial as an Electrocatalyst for Efficient Hydrogen Evolution Reaction. Int. J. Energy Res. 2021, 45, 4735–4745. [Google Scholar] [CrossRef]
- Yu, X.; Shi, W.; Wei, J.; Liu, T.; Li, Y.; He, M.; Wei, Z.; Ping, D.; Sun, P.; Zheng, J.Y.; et al. Green Fabrication of Ultrafine N-MoxC/CoP Hybrids for Boosting Electrocatalytic Water Reduction. Nanotechnology 2024, 6, 065704. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yu, X.; Yu, S.; Yang, H.; Sun, Q.; Wang, C.; Li, S.; Zheng, J.Y. Robust CoP@NiFe LDH/Ni Heterostructured Electrodes for Efficient Overall Water Splitting with High Current Density. J. Alloys Compd. 2024, 973, 172886. [Google Scholar] [CrossRef]
- Tan, Y.; Feng, J.; Kang, L.; Liu, L.; Zhao, F.; Zhao, S.; Brett, D.J.L.; Shearing, P.R.; He, G.; Parkin, I.P. Hybrid Ni2P/CoP Nanosheets as Efficient and Robust Electrocatalysts for Domestic Wastewater Splitting. Energy Environ. Mater. 2023, 6, e12398. [Google Scholar] [CrossRef]
- Liang, Q.; Jin, H.; Wang, Z.; Xiong, Y.; Yuan, S.; Zeng, X.; He, D.; Mu, S. Metal-Organic Frameworks Derived Reverse-Encapsulation Co-NC@Mo2C Complex for Efficient Overall Water Splitting. Nano Energy 2019, 57, 746–752. [Google Scholar] [CrossRef]
- Li, X.; Hao, X.; Abudula, A.; Guan, G. Nanostructured Catalysts for Electrochemical Water Splitting: Current State and Prospects. J. Mater. Chem. A Mater. 2016, 4, 11973–12000. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhong, H.; Meng, F.; Bao, D.; Zhang, X.; Wei, X. Three-Dimensional Interconnected Ni(Fe)OxHy Nanosheets on Stainless Steel Mesh as a Robust Integrated Oxygen Evolution Electrode. Nano Res. 2018, 11, 1294–1300. [Google Scholar] [CrossRef]
- Chen, S.; Duan, J.; Jaroniec, M.; Qiao, S.Z. Three-Dimensional N-Doped Graphene Hydrogel/NiCo Double Hydroxide Electrocatalysts for Highly Efficient Oxygen Evolution. Angew. Chem. Int. Ed. 2013, 52, 13567–13570. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.Y.; Cao, J.L.; Jaroniec, M.; Qiao, S.Z. Interacting Carbon Nitride and Titanium Carbide Nanosheets for High-Performance Oxygen Evolution. Angew. Chem. 2016, 128, 1150–1154. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, H.; Qin, Y.; Zhang, X. An Efficient Three-Dimensional Oxygen Evolution Electrode. Angew. Chem. 2013, 125, 5356–5361. [Google Scholar] [CrossRef]
- Chen, P.; Xu, K.; Fang, Z.; Tong, Y.; Wu, J.; Lu, X.; Peng, X.; Ding, H.; Wu, C.; Xie, Y. Metallic Co4N Porous Nanowire Arrays Activated by Surface Oxidation as Electrocatalysts for the Oxygen Evolution Reaction. Angew. Chem. 2015, 127, 14923–14927. [Google Scholar] [CrossRef]
- Qin, F.; Zhao, Z.; Alam, M.K.; Ni, Y.; Robles-Hernandez, F.; Yu, L.; Chen, S.; Ren, Z.; Wang, Z.; Bao, J. Trimetallic NiFeMo for Overall Electrochemical Water Splitting with a Low Cell Voltage. ACS Energy Lett. 2018, 3, 546–554. [Google Scholar] [CrossRef]
- Xu, Q.; Jiang, H.; Zhang, H.; Hu, Y.; Li, C. Heterogeneous Interface Engineered Atomic Configuration on Ultrathin Ni(OH)2/Ni3S2 Nanoforests for Efficient Water Splitting. Appl. Catal. B 2019, 242, 60–66. [Google Scholar] [CrossRef]
- Tso, S.; Li, W.S.; Wu, B.H.; Chen, L.J. Enhanced H2 Production in Water Splitting with CdS-ZnO Core-Shell Nanowires. Nano Energy 2018, 43, 270–277. [Google Scholar] [CrossRef]
- Zhao, M.; Du, J.; Lei, H.; Pei, L.; Gong, Z.; Wang, X.; Bao, H. Enhanced Electrocatalytic Activity of FeNi Alloy Quantum Dot-Decorated Cobalt Carbonate Hydroxide Nanosword Arrays for Effective Overall Water Splitting. Nanoscale 2022, 14, 3191–3199. [Google Scholar] [CrossRef]
- Wang, C.; Jiu, H.; Zhang, L.; Song, W.; Zhang, Y.; Wei, H.; Xu, Q.; Qin, Y.; Che, S.; Guo, Z. Heterostructured ZnCo2O4–CoOOH Nanosheets on Ni Foam for a High Performance Bifunctional Alkaline Water Splitting Catalyst. Dalton Trans. 2022, 51, 10061–10068. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, J.; Zhang, X. Fe and Cu Dual-Doped Ni3S4 Nanoarrays with Less Low-Valence Ni Species for Boosting Water Oxidation Reaction. Dalton Trans. 2022, 51, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Nakate, U.T.; Bhuyan, P.; Chen, J.; Tran, D.T.; Park, S. Mo/Co Doped 1T-VS2 Nanostructures as a Superior Bifunctional Electrocatalyst for Overall Water Splitting in Alkaline Media. J. Mater. Chem. A 2022, 10, 9067–9079. [Google Scholar] [CrossRef]
- Sha, W.; Song, Y.; Liu, P.; Wang, J.; Xu, B.; Feng, X.; Guo, J. Constructing Multiple Heterostructures on Nickel Oxide Using Rare-Earth Oxide and Nickel as Efficient Bifunctional Electrocatalysts for Overall Water Splitting. ChemCatChem 2022, 14, e202101975. [Google Scholar] [CrossRef]
- Bai, X.-J.; Chen, H.; Li, Y.-N.; Shao, L.; Ma, J.-C.; Li, L.-L.; Chen, J.-Y.; Wang, T.-Q.; Zhang, X.-M.; Zhang, L.-Y.; et al. CoNi-Based Metal–Organic Framework Nanoarrays Supported on Carbon Cloth as Bifunctional Electrocatalysts for Efficient Water-Splitting. New J. Chem. 2020, 44, 1694–1698. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, X.; Sun, Y.; Qi, K.; Jin, Z.; Wei, S.; Li, W.; Zhang, L.; Zheng, W. Nanoporous Sulfur-Doped Copper Oxide (Cu2OxS1–x) for Overall Water Splitting. ACS Appl. Mater. Interfaces 2018, 10, 745–752. [Google Scholar] [CrossRef]
- Wei, Z.; Hu, X.; Ning, S.; Kang, X.; Chen, S. Supported Heterostructured MoC/Mo2C Nanoribbons and Nanoflowers as Highly Active Electrocatalysts for Hydrogen Evolution Reaction. ACS Sustain. Chem. Eng. 2019, 7, 8458–8465. [Google Scholar] [CrossRef]
- Karthick, K.; Anantharaj, S.; Patchaiammal, S.; Jagadeesan, S.N.; Kumar, P.; Ede, S.R.; Pattanayak, D.K.; Kundu, S. Advanced Cu3Sn and Selenized Cu3Sn@Cu Foam as Electrocatalysts for Water Oxidation under Alkaline and Near-Neutral Conditions. Inorg. Chem. 2019, 58, 9490–9499. [Google Scholar] [CrossRef]
- Xiong, D.; Zhang, Q.; Thalluri, S.M.; Xu, J.; Li, W.; Fu, X.; Liu, L. One-Step Fabrication of Monolithic Electrodes Comprising Co9S8 Particles Supported on Cobalt Foam for Efficient and Durable Oxygen Evolution Reaction. Chem. A Eur. J. 2017, 23, 8749–8755. [Google Scholar] [CrossRef]
- Xiong, D.; Zhang, Q.; Li, W.; Li, J.; Fu, X.; Cerqueira, M.F.; Alpuim, P.; Liu, L. Atomic-Layer-Deposited Ultrafine MoS2 Nanocrystals on Cobalt Foam for Efficient and Stable Electrochemical Oxygen Evolution. Nanoscale 2017, 9, 2711–2717. [Google Scholar] [CrossRef]
- Li, Y.; Wei, B.; Yu, Z.; Bondarchuk, O.; Araujo, A.; Amorim, I.; Zhang, N.; Xu, J.; Neves, I.C.; Liu, L. Bifunctional Porous Cobalt Phosphide Foam for High-Current-Density Alkaline Water Electrolysis with 4000-h Long Stability. ACS Sustain. Chem. Eng. 2020, 8, 10193–10200. [Google Scholar] [CrossRef]
- Xu, X.; Pan, Y.; Ge, L.; Chen, Y.; Mao, X.; Guan, D.; Li, M.; Zhong, Y.; Hu, Z.; Peterson, V.K.; et al. High-Performance Perovskite Composite Electrocatalysts Enabled by Controllable Interface Engineering. Small 2021, 17, 2101573. [Google Scholar] [CrossRef] [PubMed]
- Abdelghafar, F.; Xu, X.; Guan, D.; Lin, Z.; Hu, Z.; Ni, M.; Huang, H.; Bhatelia, T.; Jiang, S.P.; Shao, Z. New Nanocomposites Derived from Cation-Nonstoichiometric Bax(Co, Fe, Zr, Y)O3−δ as Efficient Electrocatalysts for Water Oxidation in Alkaline Solution. ACS Mater. Lett. 2024, 6, 2985–2994. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Song, G.; Hong, J.; Van, T.K.; Pawar, A.U.; Kim, D.Y.; Kim, C.W.; Haider, Z.; Kang, Y.S. Facile Fabrication of WO3 Nanoplates Thin Films with Dominant Crystal Facet of (002) for Water Splitting. Cryst. Growth Des. 2014, 14, 6057–6066. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Pawar, A.U.; Kang, Y.S. Preparation of C3N4 Thin Films for Photo-/Electrocatalytic CO2 Reduction to Produce Liquid Hydrocarbons. Catalysts 2022, 12, 1399. [Google Scholar] [CrossRef]
- Jing, C.; Liu, X.D.; Li, K.; Liu, X.; Dong, B.; Dong, F.; Zhang, Y. The Pseudocapacitance Mechanism of Graphene/CoAl LDH and Its Derivatives: Are All the Modifications Beneficial? J. Energy Chem. 2021, 52, 218–227. [Google Scholar] [CrossRef]
- Yu, H.; Qi, L.; Hu, Y.; Qu, Y.; Yan, P.; Isimjan, T.T.; Yang, X. Nanowire-Structured FeP-CoP Arrays as Highly Active and Stable Bifunctional Electrocatalyst Synergistically Promoting High-Current Overall Water Splitting. J. Colloid. Interface Sci. 2021, 600, 811–819. [Google Scholar] [CrossRef]
- Zeng, L.; Sun, K.; Wang, X.; Liu, Y.; Pan, Y.; Liu, Z.; Cao, D.; Song, Y.; Liu, S.; Liu, C. Three-Dimensional-Networked Ni2P/Ni3S2 Heteronanoflake Arrays for Highly Enhanced Electrochemical Overall-Water-Splitting Activity. Nano Energy 2018, 51, 26–36. [Google Scholar] [CrossRef]
- Jiang, X.; Li, Y.; He, M.; Zhou, L.; Zheng, Q.; Xie, F.; Jie, W.; Lin, D. Construction of NiFeP/CoP Nanosheets/Nanowires Hierarchical Array as Advanced Electrocatalysts for Water Oxidation. Int. J. Hydrogen Energy 2019, 44, 19986–19994. [Google Scholar] [CrossRef]
- Yang, J.; Guo, D.; Zhao, S.; Lin, Y.; Yang, R.; Xu, D.; Shi, N.; Zhang, X.; Lu, L.; Lan, Y.-Q.; et al. Cobalt Phosphides Nanocrystals Encapsulated by P-Doped Carbon and Married with P-Doped Graphene for Overall Water Splitting. Small 2019, 15, 1804546. [Google Scholar] [CrossRef]
- Jeon, Y.; Na, H.; Hwang, H.; Park, J.; Hwang, H.; Shul, Y.G. Accelerated Life-Time Test Protocols for Polymer Electrolyte Membrane Fuel Cells Operated at High Temperature. Int. J. Hydrogen Energy 2015, 40, 3057–3067. [Google Scholar] [CrossRef]
- Louie, M.W.; Bell, A.T. An Investigation of Thin-Film Ni-Fe Oxide Catalysts for the Electrochemical Evolution of Oxygen. J. Am. Chem. Soc. 2013, 135, 12329–12337. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, H.; Li, S.; Wang, R.; Sun, X.; Zhou, M.; Zhou, J.; Lou, X.W.; Xie, Y. Defect-Rich MoS2 Ultrathin Nanosheets with Additional Active Edge Sites for Enhanced Electrocatalytic Hydrogen Evolution. Adv. Mater. 2013, 25, 5807–5813. [Google Scholar] [CrossRef]
- Zhai, Z.; Yan, W.; Zhang, J. Layered FeCoNi Double Hydroxides with Tailored Surface Electronic Configurations Induced by Oxygen and Unsaturated Metal Vacancies for Boosting the Overall Water Splitting Process. Nanoscale 2022, 14, 4156–4169. [Google Scholar] [CrossRef]
- Kim, M.; Ha, J.; Kim, Y.-T.; Choi, J. Trace Amounts of Ru-Doped Ni–Fe Oxide Bone-like Structures via Single-Step Anodization: A Flexible and Bifunctional Electrode for Efficient Overall Water Splitting. J. Mater. Chem. A 2021, 9, 12041–12050. [Google Scholar] [CrossRef]
- Chen, P.; Feng, D.; Li, K.; Tong, Y. Hierarchically Structured Nickel/Molybdenum Nitride Heterojunctions as Superior Bifunctional Electrodes for Overall Water Splitting. Dalton Trans. 2022, 51, 16990–16999. [Google Scholar] [CrossRef]
- Jian, J.; Chen, W.; Zeng, D.; Chang, L.; Zhang, R.; Jiang, M.; Yu, G.; Huang, X.; Yuan, H.; Feng, S. Metal-Ionic-Conductor Potassium Ferrite Nanocrystals with Intrinsic Superhydrophilic Surfaces for Electrocatalytic Water Splitting at Ultrahigh Current Densities. J. Mater. Chem. A 2021, 9, 7586–7593. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Y.; Mu, Z.; Wang, Y.; Ali, U.; Jing, S.; Xing, S. Urea-Assisted Enhanced Electrocatalytic Activity of MoS2–Ni3S2 for Overall Water Splitting. Inorg. Chem. Front. 2020, 7, 3588–3597. [Google Scholar] [CrossRef]
- Cai, Z.; Bu, X.; Wang, P.; Su, W.; Wei, R.; Ho, J.C.; Yang, J.; Wang, X. Simple and Cost Effective Fabrication of 3D Porous Core–Shell Ni Nanochains@NiFe Layered Double Hydroxide Nanosheet Bifunctional Electrocatalysts for Overall Water Splitting. J. Mater. Chem. A 2019, 7, 21722–21729. [Google Scholar] [CrossRef]
- Liu, M.; Wang, E.; Zhao, Z. NiPN/Ni Nanoparticle-Decorated Carbon Nanotube Forest as an Efficient Bifunctional Electrocatalyst for Overall Water Splitting in an Alkaline Electrolyte. ACS Appl. Nano Mater. 2022, 5, 5335–5345. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, L.-W.; Liu, H.; Wang, J.-J. Electronic Structure Regulation and Polysulfide Bonding of Co-Doped (Ni, Fe)1+xS Enable Highly Efficient and Stable Electrocatalytic Overall Water Splitting. Chem. Eng. J. 2022, 441, 136121. [Google Scholar] [CrossRef]
- You, S.; Wu, Y.; Wang, Y.; He, Z.; Yin, L.; Zhang, Y.; Sun, Z.; Zhang, Z. Pulse-Electrodeposited Ni–Fe–Sn Films Supported on Ni Foam as an Excellent Bifunctional Electrocatalyst for Overall Water Splitting. Int. J. Hydrogen Energy 2022, 47, 29315–29326. [Google Scholar] [CrossRef]
- Pan, Z.; Yaseen, M.; Kang Shen, P.; Zhan, Y. Designing Highly Efficient 3D Porous Ni-Fe Sulfide Nanosheets Based Catalyst for the Overall Water Splitting through Component Regulation. J. Colloid Interface Sci. 2022, 616, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Ren, J.; Li, X.; Li, G.; Jiao, L.; Xu, C. Engineering Crystalline CoMP-Decorated (M = Mn, Fe, Ni, Cu, Zn) Amorphous CoM LDH for High-Rate Alkaline Water Splitting. Chem. Eng. J. 2022, 441, 136031. [Google Scholar] [CrossRef]
- Jin, J.; Chen, F.; Feng, Y.; Zhou, J.; Lei, W.; Gao, F. Co-Ni-Mo Phosphides Hierarchical Nanoarrays as Bifunctional Electrocatalysts for Excellent Overall Water Splitting. Fuel 2023, 332, 126131. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, H.; Lai, C.; Guo, Z.; Hu, J.; Ji, S.; Lei, L. Hierarchical Heterogeneous NiFe-LDH/Ni/NM Nanosheets Grown in Situ for Stably Overall Water Splitting at Large Current Densities. J. Electroanal. Chem. 2022, 919, 116527. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; Yang, Y.; Zhao, M.; Zhao, H.; Liu, S.; Zheng, J. Enhancing Water Splitting Performance via NiFeP-CoP on Cobalt Foam: Synergistic Effects and Structural Optimization. Nanomaterials 2025, 15, 883. https://doi.org/10.3390/nano15120883
Zhu S, Yang Y, Zhao M, Zhao H, Liu S, Zheng J. Enhancing Water Splitting Performance via NiFeP-CoP on Cobalt Foam: Synergistic Effects and Structural Optimization. Nanomaterials. 2025; 15(12):883. https://doi.org/10.3390/nano15120883
Chicago/Turabian StyleZhu, Shihu, Yingxing Yang, Mengyao Zhao, Hui Zhao, Siyuan Liu, and Jinyou Zheng. 2025. "Enhancing Water Splitting Performance via NiFeP-CoP on Cobalt Foam: Synergistic Effects and Structural Optimization" Nanomaterials 15, no. 12: 883. https://doi.org/10.3390/nano15120883
APA StyleZhu, S., Yang, Y., Zhao, M., Zhao, H., Liu, S., & Zheng, J. (2025). Enhancing Water Splitting Performance via NiFeP-CoP on Cobalt Foam: Synergistic Effects and Structural Optimization. Nanomaterials, 15(12), 883. https://doi.org/10.3390/nano15120883