Ultrasound-Mediated Membrane Modulation for Biomedical Applications
Abstract
1. Introduction
2. Approaches of Ultrasound-Mediated Membrane Modulation
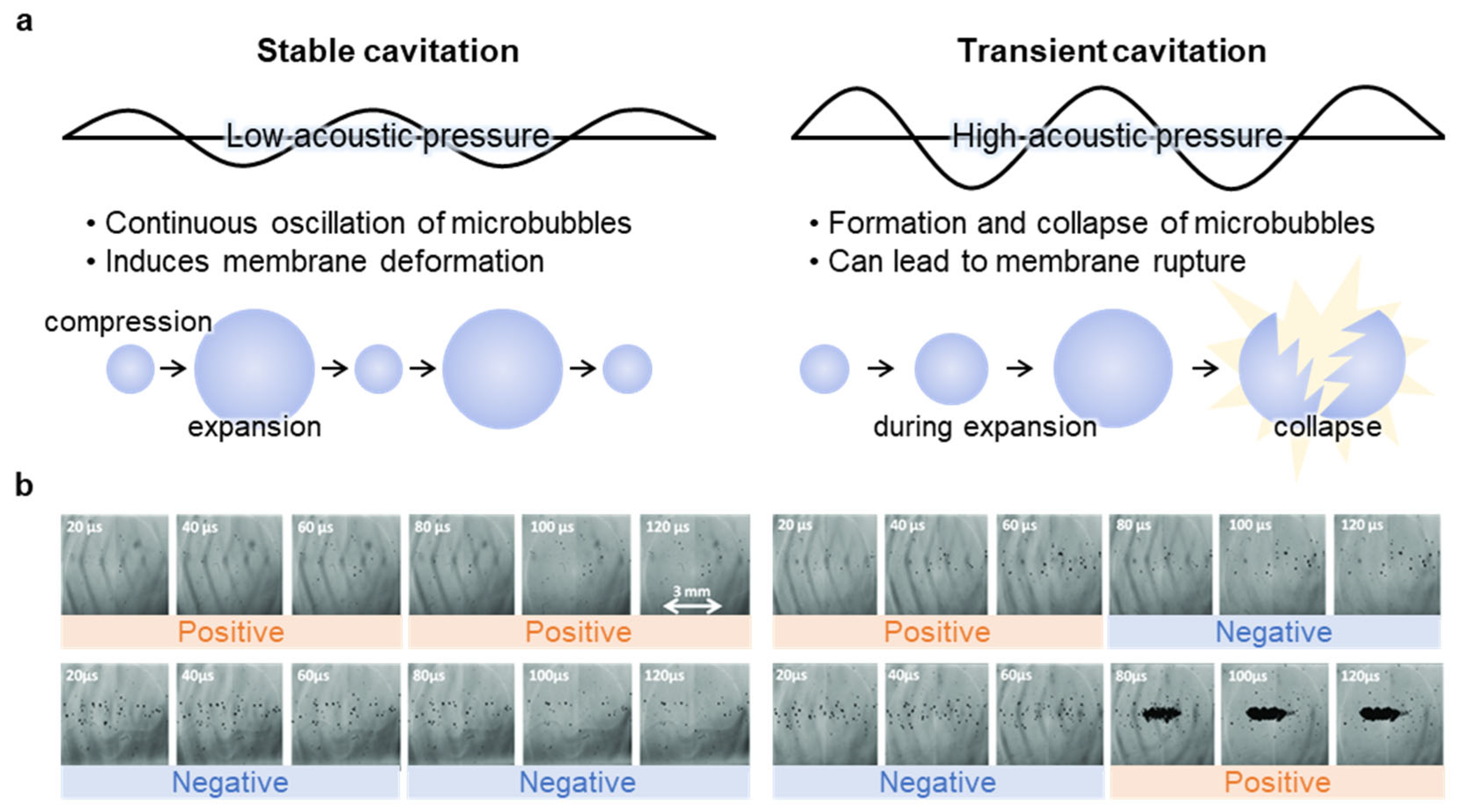
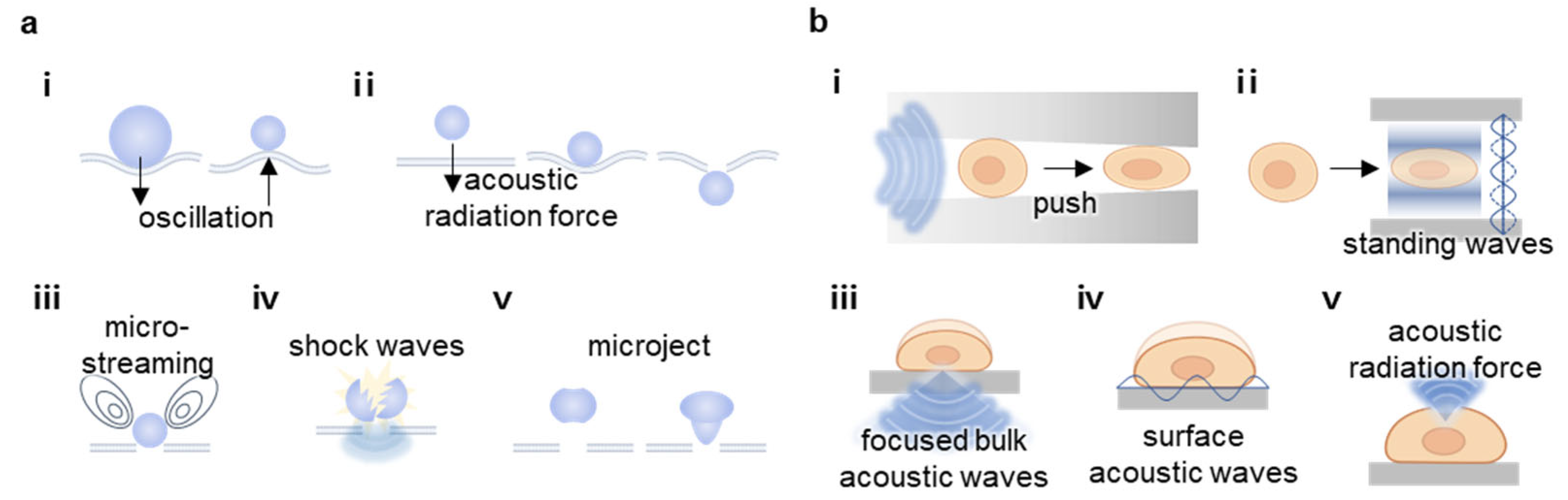
2.1. Cavitation
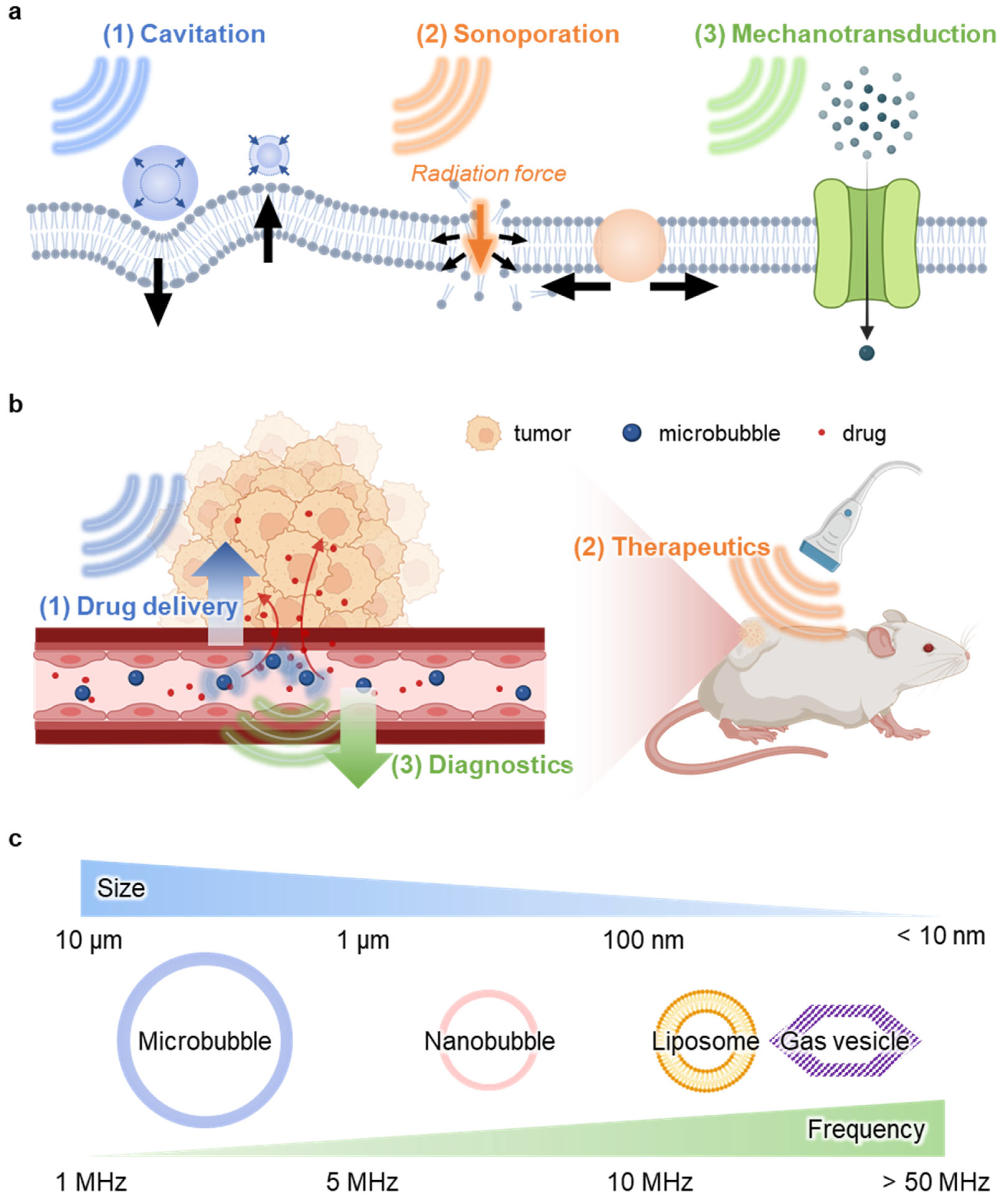
2.2. Sonoporation
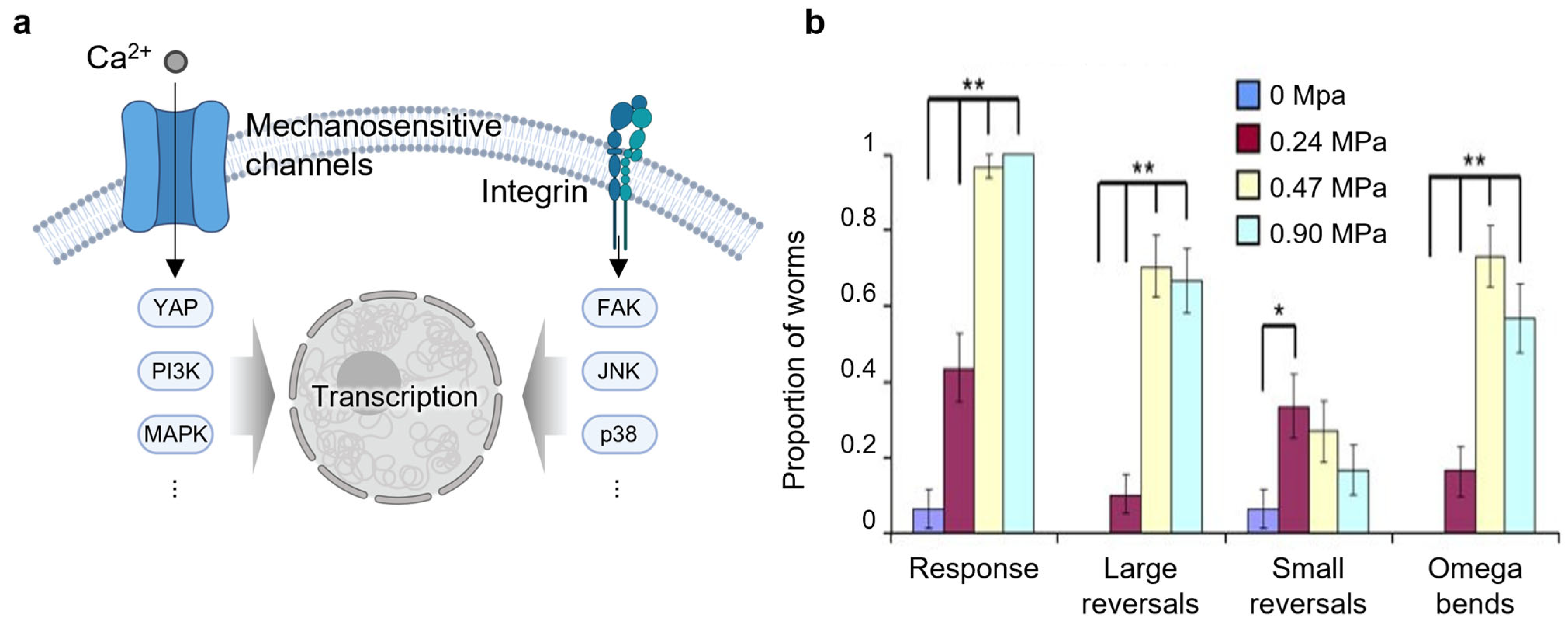
2.3. Mechanotransduction
3. Biomedical Applications of Ultrasound-Mediated Membrane Modulation
3.1. Drug Delivery
3.1.1. Tumor Treatment
3.1.2. Blood–Brain Barrier Opening
3.1.3. Gene Delivery
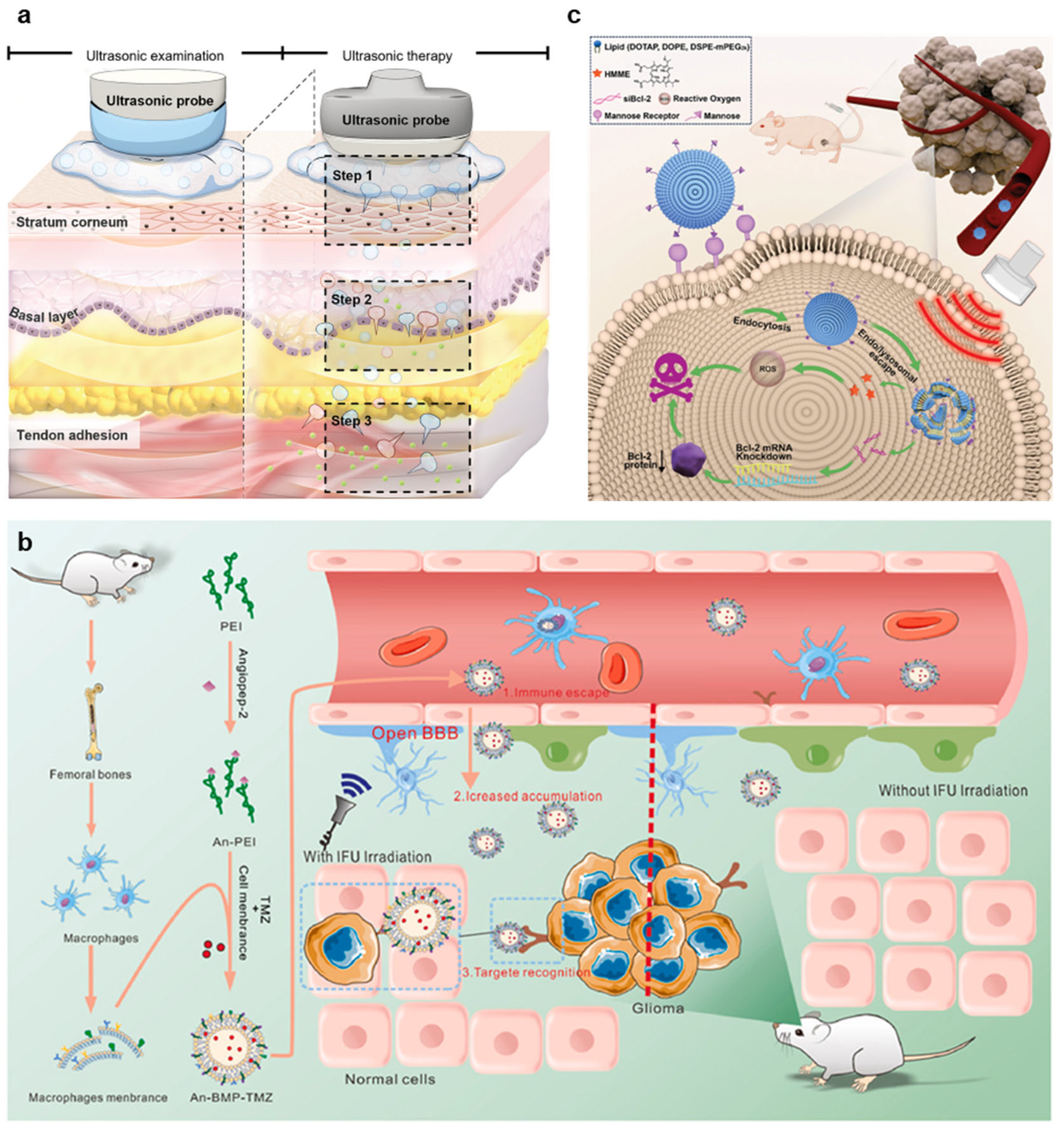
3.2. Therapeutics
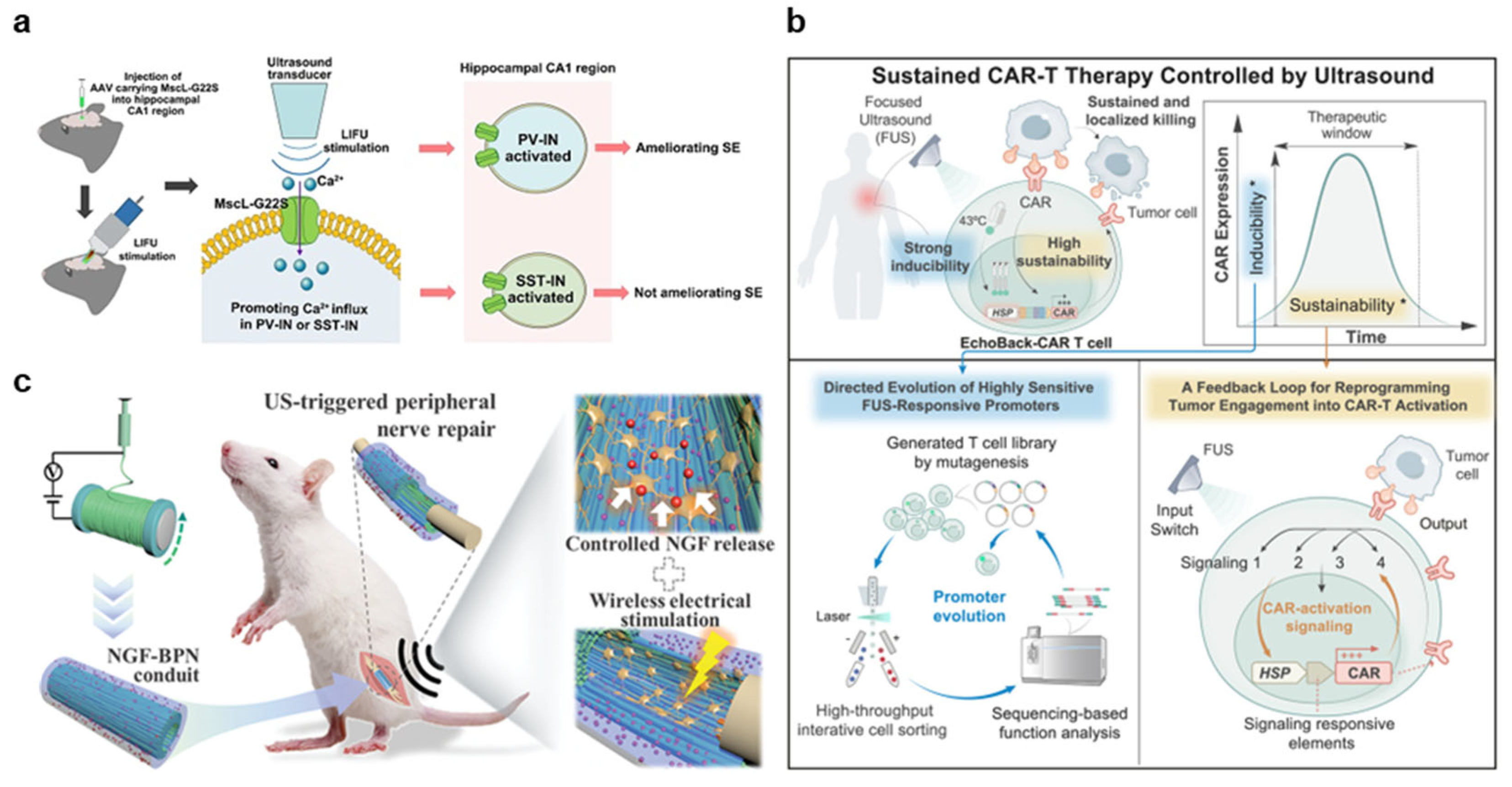
3.2.1. Neuromodulation
3.2.2. Immunotherapy
3.2.3. Regeneration
3.3. Diagnostics
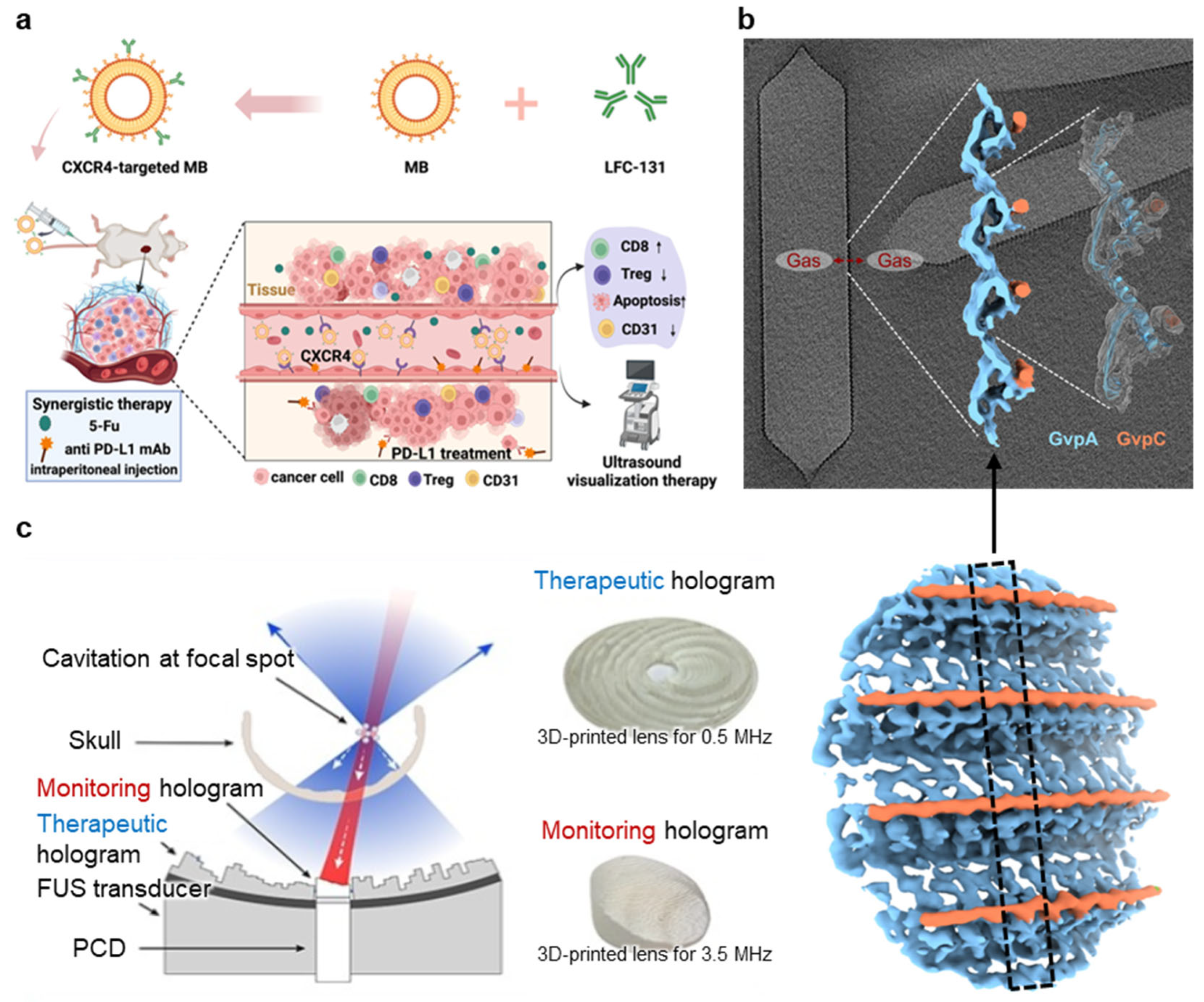
3.3.1. Targeted Microbubble
3.3.2. Gas Vesicle (GV)
3.3.3. Passive Cavitation Imaging (PCI)
4. Challenges and Future Perspectives
5. Limitations of Ultrasound-Mediated Membrane Modulation
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Branton, D. Membrane structure. Annu. Rev. Plant Physiol. 1969, 20, 209–238. [Google Scholar] [CrossRef]
- Zalba, S.; Ten Hagen, T.L. Cell membrane modulation as adjuvant in cancer therapy. Cancer Treat. Rev. 2017, 52, 48–57. [Google Scholar] [CrossRef]
- Cho, W.; Stahelin, R.V. Membrane-protein interactions in cell signaling and membrane trafficking. Annu. Rev. Biophys. Biomol. Struct. 2005, 34, 119–151. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Droogmans, G. Ion channels and their functional role in vascular endothelium. Physiol. Rev. 2001, 81, 1415–1459. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Liu, W.; Cao, H.; Xiao, G. Molecular mechanosensors in osteocytes. Bone Res. 2020, 8, 23. [Google Scholar] [CrossRef]
- Rojas, M.; Donahue, J.P.; Tan, Z.; Lin, Y.Z. Genetic engineering of proteins with cell membrane permeability. Nat. Biotechnol. 1998, 16, 370–375. [Google Scholar] [CrossRef]
- Sheetz, M.P.; Dai, J. Modulation of membrane dynamics and cell motility by membrane tension. Trends Cell Biol. 1996, 6, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.P.; Langer, R.; Jensen, K.F. Intracellular Delivery by Membrane Disruption: Mechanisms, Strategies, and Concepts. Chem. Rev. 2018, 118, 7409–7531. [Google Scholar] [CrossRef]
- Xiong, R.H.; Samal, S.K.; Demeester, J.; Skirtach, A.G.; De Smedt, S.C.; Braeckmans, K. Laser-assisted photoporation: Fundamentals, technological advances and applications. Adv. Phys.-X 2016, 1, 596–620. [Google Scholar] [CrossRef]
- Fajrial, A.K.; He, Q.Q.; Wirusanti, N.I.; Slansky, J.E.; Ding, X. A review of emerging physical transfection methods for CRISPR/Cas9-mediated gene editing. Theranostics 2020, 10, 5532–5549. [Google Scholar] [CrossRef]
- Popescu, G.; Ikeda, T.; Goda, K.; Best-Popescu, C.A.; Laposata, M.; Manley, S.; Dasari, R.R.; Badizadegan, K.; Feld, M.S. Optical measurement of cell membrane tension. Phys. Rev. Lett. 2006, 97, 218101. [Google Scholar] [CrossRef]
- Akinlaja, J.; Sachs, F. The breakdown of cell membranes by electrical and mechanical stress. Biophys. J. 1998, 75, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Rich, J.; Tian, Z.; Huang, T.J. Sonoporation: Past, Present, and Future. Adv. Mater. Technol. 2022, 7, 2100885. [Google Scholar] [CrossRef] [PubMed]
- Izadifar, Z.; Babyn, P.; Chapman, D. Ultrasound Cavitation/Microbubble Detection and Medical Applications. J. Med. Biol. Eng. 2019, 39, 259–276. [Google Scholar] [CrossRef]
- Hou, X.; Liu, L.; Sun, L. Precise modulation of cell activity using sono-responsive nano-transducers. Biomaterials 2025, 314, 122857. [Google Scholar] [CrossRef]
- Hernot, S.; Klibanov, A.L. Microbubbles in ultrasound-triggered drug and gene delivery. Adv. Drug Deliv. Rev. 2008, 60, 1153–1166. [Google Scholar] [CrossRef]
- Unga, J.; Hashida, M. Ultrasound induced cancer immunotherapy. Adv. Drug Deliv. Rev. 2014, 72, 144–153. [Google Scholar] [CrossRef]
- Shapiro, M.G.; Goodwill, P.W.; Neogy, A.; Yin, M.; Foster, F.S.; Schaffer, D.V.; Conolly, S.M. Biogenic gas nanostructures as ultrasonic molecular reporters. Nat. Nanotechnol. 2014, 9, 311–316. [Google Scholar] [CrossRef]
- Wu, P.; Wang, X.; Lin, W.; Bai, L. Acoustic characterization of cavitation intensity: A review. Ultrason. Sonochem. 2022, 82, 105878. [Google Scholar] [CrossRef]
- Church, C.C. Spontaneous homogeneous nucleation, inertial cavitation and the safety of diagnostic ultrasound. Ultrasound Med. Biol. 2002, 28, 1349–1364. [Google Scholar] [CrossRef]
- Wu, P.; Bai, L.; Lin, W.; Wang, X. Mechanism and dynamics of hydrodynamic-acoustic cavitation (HAC). Ultrason. Sonochem. 2018, 49, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, K.; Cui, J.; Ye, J.Y.; Deng, C.X. Controlled permeation of cell membrane by single bubble acoustic cavitation. J. Control. Release 2012, 157, 103–111. [Google Scholar] [CrossRef]
- Peruzzi, G.; Sinibaldi, G.; Silvani, G.; Ruocco, G.; Casciola, C.M. Perspectives on cavitation enhanced endothelial layer permeability. Colloids Surf. B Biointerfaces 2018, 168, 83–93. [Google Scholar] [CrossRef]
- Vignon, F.; Shi, W.T.; Powers, J.E.; Everbach, E.C.; Liu, J.; Gao, S.; Xie, F.; Porter, T.R. Microbubble cavitation imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2013, 60, 661–670. [Google Scholar] [CrossRef]
- Vanhille, C. Numerical simulations of stable cavitation bubble generation and primary Bjerknes forces in a three-dimensional nonlinear phased array focused ultrasound field. Ultrason. Sonochem. 2020, 63, 104972. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Liu, X.; Wang, Y.; Zhang, W.; Zhou, W.; Cai, F.; Li, F.; Wu, J.; Xu, L.; Niu, L.; et al. Sonoporation of Cells by a Parallel Stable Cavitation Microbubble Array. Adv. Sci. Weinh. 2019, 6, 1900557. [Google Scholar] [CrossRef]
- Meijering, B.D.; Juffermans, L.J.; van Wamel, A.; Henning, R.H.; Zuhorn, I.S.; Emmer, M.; Versteilen, A.M.; Paulus, W.J.; van Gilst, W.H.; Kooiman, K.; et al. Ultrasound and microbubble-targeted delivery of macromolecules is regulated by induction of endocytosis and pore formation. Circ. Res. 2009, 104, 679–687. [Google Scholar] [CrossRef]
- Richardson, E.S.; Pitt, W.G.; Woodbury, D.J. The role of cavitation in liposome formation. Biophys. J. 2007, 93, 4100–4107. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.C.; Lim, J.; Chien, A.; Chen, C.C.; Wang, J.L. Activation of Mechanosensitive Ion Channels by Ultrasound. Ultrasound Med. Biol. 2022, 48, 1981–1994. [Google Scholar] [CrossRef]
- Cochran, S.A.; Prausnitz, M.R. Sonoluminescence as an indicator of cell membrane disruption by acoustic cavitation. Ultrasound Med. Biol. 2001, 27, 841–850. [Google Scholar] [CrossRef]
- Forbes, M.M.; Steinberg, R.L.; O’Brien, W.D., Jr. Examination of inertial cavitation of Optison in producing sonoporation of chinese hamster ovary cells. Ultrasound Med. Biol. 2008, 34, 2009–2018. [Google Scholar] [CrossRef] [PubMed]
- Rychak, J.J.; Klibanov, A.L.; Hossack, J.A. Acoustic radiation force enhances targeted delivery of ultrasound contrast microbubbles: In vitro verification. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2005, 52, 421–433. [Google Scholar] [CrossRef]
- Liu, H.L.; Hua, M.Y.; Chen, P.Y.; Chu, P.C.; Pan, C.H.; Yang, H.W.; Huang, C.Y.; Wang, J.J.; Yen, T.C.; Wei, K.C. Blood-brain barrier disruption with focused ultrasound enhances delivery of chemotherapeutic drugs for glioblastoma treatment. Radiology 2010, 255, 415–425. [Google Scholar] [CrossRef]
- Suzuki, R.; Oda, Y.; Utoguchi, N.; Maruyama, K. Progress in the development of ultrasound-mediated gene delivery systems utilizing nano- and microbubbles. J. Control. Release 2011, 149, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Hall, T.L.; Vlaisavljevich, E.; Lee, F.T., Jr. Histotripsy: The first noninvasive, non-ionizing, non-thermal ablation technique based on ultrasound. Int. J. Hyperth. 2021, 38, 561–575. [Google Scholar] [CrossRef]
- Yildirim, A.; Shi, D.; Roy, S.; Blum, N.T.; Chattaraj, R.; Cha, J.N.; Goodwin, A.P. Nanoparticle-Mediated Acoustic Cavitation Enables High Intensity Focused Ultrasound Ablation Without Tissue Heating. ACS Appl. Mater. Interfaces 2018, 10, 36786–36795. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.Y.; Liu, S.; Chen, Z.G.; Zou, J.Z.; Wu, F. Cavitation enhances coagulated size during pulsed high-intensity focussed ultrasound ablation in an isolated liver perfusion system. Int. J. Hyperth. 2017, 33, 343–353. [Google Scholar] [CrossRef][Green Version]
- Barger, J.E. Thresholds of Acoustic Cavitation in Water. J. Acoust. Soc. Am. 1964, 36, 1008–1009. [Google Scholar] [CrossRef]
- Yoshizawa, S.; Takagi, R.; Umemura, S. Enhancement of High-Intensity Focused Ultrasound Heating by Short-Pulse Generated Cavitation. Appl. Sci. 2017, 7, 288. [Google Scholar] [CrossRef]
- Ye, L.Z.; Chuai, S.; Zhu, X.J.; Wang, D. Experimental Study on Ultrasonic Cavitation Intensity Based on Fluorescence Analysis. Chin. J. Mech. Eng. 2023, 36, 103. [Google Scholar] [CrossRef]
- Brotchie, A.; Grieser, F.; Ashokkumar, M. Effect of power and frequency on bubble-size distributions in acoustic cavitation. Phys. Rev. Lett. 2009, 102, 084302. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, I.; Mikhailova, N. An Analysis of Acoustic Cavitation Thresholds of Water Based on the Incubation Time Criterion Approach. Fluids 2021, 6, 134. [Google Scholar] [CrossRef]
- Sulheim, E.; Hanson, I.; Snipstad, S.; Vikedal, K.; Morch, Y.; Boucher, Y.; Davies, C.D. Sonopermeation with Nanoparticle-Stabilized Microbubbles Reduces Solid Stress and Improves Nanomedicine Delivery to Tumors. Adv. Ther. 2021, 4, 2100147. [Google Scholar] [CrossRef]
- Yu, H.; Lin, Z.; Xu, L.; Liu, D.; Shen, Y. Theoretical study of microbubble dynamics in sonoporation. Ultrasonics 2015, 61, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Helfield, B.; Chen, X.; Watkins, S.C.; Villanueva, F.S. Biophysical insight into mechanisms of sonoporation. Proc. Natl. Acad. Sci. USA 2016, 113, 9983–9988. [Google Scholar] [CrossRef]
- Zhou, Y.; Cui, J.; Deng, C.X. Dynamics of sonoporation correlated with acoustic cavitation activities. Biophys. J. 2008, 94, L51–L53. [Google Scholar] [CrossRef]
- Fan, Z.; Chen, D.; Deng, C.X. Characterization of the dynamic activities of a population of microbubbles driven by pulsed ultrasound exposure in sonoporation. Ultrasound Med. Biol. 2014, 40, 1260–1272. [Google Scholar] [CrossRef]
- Yoon, S.; Wang, P.; Peng, Q.; Wang, Y.; Shung, K.K. Acoustic-transfection for genomic manipulation of single-cells using high frequency ultrasound. Sci. Rep. 2017, 7, 5275. [Google Scholar] [CrossRef] [PubMed]
- Ibsen, S.; Tong, A.; Schutt, C.; Esener, S.; Chalasani, S.H. Sonogenetics is a non-invasive approach to activating neurons in Caenorhabditis elegans. Nat. Commun. 2015, 6, 8264. [Google Scholar] [CrossRef]
- Zhu, J.; Xian, Q.; Hou, X.; Wong, K.F.; Zhu, T.; Chen, Z.; He, D.; Kala, S.; Murugappan, S.; Jing, J.; et al. The mechanosensitive ion channel Piezo1 contributes to ultrasound neuromodulation. Proc. Natl. Acad. Sci. USA 2023, 120, e2300291120. [Google Scholar] [CrossRef]
- Inoue, S.; Li, C.; Hatakeyama, J.; Jiang, H.; Kuroki, H.; Moriyama, H. Higher-intensity ultrasound accelerates fracture healing via mechanosensitive ion channel Piezo1. Bone 2023, 177, 116916. [Google Scholar] [CrossRef] [PubMed]
- Xian, Q.; Qiu, Z.; Murugappan, S.; Kala, S.; Wong, K.F.; Li, D.; Li, G.; Jiang, Y.; Wu, Y.; Su, M.; et al. Modulation of deep neural circuits with sonogenetics. Proc. Natl. Acad. Sci. USA 2023, 120, e2220575120. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.S.; Yoon, C.W.; Wang, Q.; Moon, S.; Koo, K.M.; Jung, H.; Chen, R.; Jiang, L.; Lu, G.; Fernandez, A.; et al. Focused Ultrasound Stimulates ER Localized Mechanosensitive PANNEXIN-1 to Mediate Intracellular Calcium Release in Invasive Cancer Cells. Front. Cell Dev. Biol. 2020, 8, 504. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.W.; Jung, H.; Goo, K.; Moon, S.; Koo, K.M.; Lee, N.S.; Weitz, A.C.; Shung, K.K. Low-Intensity Ultrasound Modulates Ca(2+) Dynamics in Human Mesenchymal Stem Cells via Connexin 43 Hemichannel. Ann. Biomed. Eng. 2018, 46, 48–59. [Google Scholar] [CrossRef]
- Sato, M.; Nagata, K.; Kuroda, S.; Horiuchi, S.; Nakamura, T.; Karima, M.; Inubushi, T.; Tanaka, E. Low-intensity pulsed ultrasound activates integrin-mediated mechanotransduction pathway in synovial cells. Ann. Biomed. Eng. 2014, 42, 2156–2163. [Google Scholar] [CrossRef]
- Cheng, K.; Xia, P.; Lin, Q.; Shen, S.; Gao, M.; Ren, S.; Li, X. Effects of low-intensity pulsed ultrasound on integrin-FAK-PI3K/Akt mechanochemical transduction in rabbit osteoarthritis chondrocytes. Ultrasound Med. Biol. 2014, 40, 1609–1618. [Google Scholar] [CrossRef]
- Han, X.; Wang, F.; Shen, J.; Chen, S.; Xiao, P.; Zhu, Y.; Yi, W.; Zhao, Z.; Cai, Z.; Cui, W.; et al. Ultrasound Nanobubble Coupling Agent for Effective Noninvasive Deep-Layer Drug Delivery. Adv. Mater. 2024, 36, e2306993. [Google Scholar] [CrossRef]
- Cui, R.; Zhou, J.; Yang, W.; Chen, Y.; Chen, L.; Tan, L.; Zhang, F.; Liu, G.; Yu, J. Ultrasound-Triggered Nanogel Boosts Chemotherapy and Immunomodulation in Colorectal Cancer. ACS Appl. Mater. Interfaces 2025, 17, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Nittayacharn, P.; Abenojar, E.; Cooley, M.B.; Berg, F.M.; Counil, C.; Sojahrood, A.J.; Khan, M.S.; Yang, C.; Berndl, E.; Golczak, M.; et al. Efficient ultrasound-mediated drug delivery to orthotopic liver tumors—Direct comparison of doxorubicin-loaded nanobubbles and microbubbles. J. Control. Release 2024, 367, 135–147. [Google Scholar] [CrossRef]
- Li, B.; Zhong, H.H.; Wei, H.Y.; Chen, G.J.; Lin, M.Z.; Huang, S.C.; Zhang, Q.Y.; Xing, C.F.; Li, T.; Huang, J.S.; et al. Ultrasound-irradiated bindable microbomb opens the blood-brain barrier to enhance glioma therapy. Nano Today 2024, 56, 102312. [Google Scholar] [CrossRef]
- Huang, X.; Gao, L.; Ge, W.; Li, S.; Liu, Y.; Fan, X.; Tu, S.; Wang, F. An ultrasound-activated piezoelectric sonosensitizer enhances mitochondrial depolarization for effective treatment of orthotopic glioma. Acta Biomater. 2024, 190, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhou, W.; Yang, H.; Cao, L.; Li, M.; Yin, P.; Zhou, Y. Molecular Modeling of Shockwave-Mediated Blood-Brain Barrier Opening for Targeted Drug Delivery. ACS Appl. Mater. Interfaces 2024, 16, 20212–20220. [Google Scholar] [CrossRef]
- Lawton, S.M.; Manson, M.A.; Fan, M.N.; Chao, T.Y.; Chen, C.Y.; Kim, P.; Campbell, C.; Cai, X.; Vander Kooi, A.; Miao, C.H. Ultrasound-mediated gene delivery specifically targets liver sinusoidal endothelial cells for sustained FVIII expression in hemophilia A mice. Mol. Ther. 2024, 32, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Sotoudehbagha, P.; Flores, A.C.; Hartmann, T.; Pattilachan, T.; Razavi, M. Bone-targeted ultrasound-responsive nanobubbles for siRNA delivery to treat osteoporosis in mice. Biomater. Adv. 2025, 166, 214078. [Google Scholar] [CrossRef] [PubMed]
- Li, H.R.; Harb, M.; Heath, J.E.; Trippett, J.S.; Shapiro, M.G.; Szablowski, J.O. Engineering viral vectors for acoustically targeted gene delivery. Nat. Commun. 2024, 15, 4924. [Google Scholar] [CrossRef]
- Tu, L.; Liao, Z.; Luo, Z.; Wu, Y.L.; Herrmann, A.; Huo, S. Ultrasound-controlled drug release and drug activation for cancer therapy. In Exploration; John Wiley & Sons: Hoboken, NJ, USA, 2021; p. 20210023. [Google Scholar]
- Husseini, G.A.; Pitt, W.G. Ultrasonic-activated micellar drug delivery for cancer treatment. J. Pharm. Sci. 2009, 98, 795–811. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Hynynen, K.; Lipsman, N. Applications of focused ultrasound in the brain: From thermoablation to drug delivery. Nat. Rev. Neurol. 2021, 17, 7–22. [Google Scholar] [CrossRef]
- Al Refaai, K.A.; AlSawaftah, N.A.; Abuwatfa, W.; Husseini, G.A. Drug Release via Ultrasound-Activated Nanocarriers for Cancer Treatment: A Review. Pharmaceutics 2024, 16, 1383. [Google Scholar] [CrossRef]
- Kim, S.; Im, S.; Park, E.-Y.; Lee, J.; Kim, C.; Kim, T.-i.; Kim, W.J. Drug-loaded titanium dioxide nanoparticle coated with tumor targeting polymer as a sonodynamic chemotherapeutic agent for anti-cancer therapy. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102110. [Google Scholar] [CrossRef]
- Kang, Y.; Kim, J.; Park, J.; Lee, Y.M.; Saravanakumar, G.; Park, K.M.; Choi, W.; Kim, K.; Lee, E.; Kim, C. Tumor vasodilation by N-Heterocyclic carbene-based nitric oxide delivery triggered by high-intensity focused ultrasound and enhanced drug homing to tumor sites for anti-cancer therapy. Biomaterials 2019, 217, 119297. [Google Scholar] [CrossRef]
- Gorick, C.M.; Breza, V.R.; Nowak, K.M.; Cheng, V.W.T.; Fisher, D.G.; Debski, A.C.; Hoch, M.R.; Demir, Z.E.F.; Tran, N.M.; Schwartz, M.R.; et al. Applications of focused ultrasound-mediated blood-brain barrier opening. Adv. Drug Deliv. Rev. 2022, 191, 114583. [Google Scholar] [CrossRef] [PubMed]
- Gasca-Salas, C.; Fernandez-Rodriguez, B.; Pineda-Pardo, J.A.; Rodriguez-Rojas, R.; Obeso, I.; Hernandez-Fernandez, F.; Del Alamo, M.; Mata, D.; Guida, P.; Ordas-Bandera, C.; et al. Blood-brain barrier opening with focused ultrasound in Parkinson’s disease dementia. Nat. Commun. 2021, 12, 779. [Google Scholar] [CrossRef] [PubMed]
- Sheikov, N.; McDannold, N.; Jolesz, F.; Zhang, Y.Z.; Tam, K.; Hynynen, K. Brain arterioles show more active vesicular transport of blood-borne tracer molecules than capillaries and venules after focused ultrasound-evoked opening of the blood-brain barrier. Ultrasound Med. Biol. 2006, 32, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Rezai, A.R.; Ranjan, M.; Haut, M.W.; Carpenter, J.; D’Haese, P.F.; Mehta, R.I.; Najib, U.; Wang, P.; Claassen, D.O.; Chazen, J.L.; et al. Focused ultrasound-mediated blood-brain barrier opening in Alzheimer’s disease: Long-term safety, imaging, and cognitive outcomes. J. Neurosurg. 2023, 139, 275–283. [Google Scholar] [CrossRef]
- Lin, J.; Lin, Z.; Liu, L.; Lin, W.; Xie, X.; Zhang, X. Enhancing glioma-specific drug delivery through self-assembly of macrophage membrane and targeted polymer assisted by low-frequency ultrasound irradiation. Mater. Today Bio 2024, 26, 101067. [Google Scholar] [CrossRef] [PubMed]
- Sirsi, S.R.; Borden, M.A. Advances in ultrasound mediated gene therapy using microbubble contrast agents. Theranostics 2012, 2, 1208–1222. [Google Scholar] [CrossRef]
- Li, Y.S.; Davidson, E.; Reid, C.N.; McHale, A.P. Optimising ultrasound-mediated gene transfer (sonoporation) in vitro and prolonged expression of a transgene in vivo: Potential applications for gene therapy of cancer. Cancer Lett. 2009, 273, 62–69. [Google Scholar] [CrossRef]
- Chang, E.L.; Ting, C.Y.; Hsu, P.H.; Lin, Y.C.; Liao, E.C.; Huang, C.Y.; Chang, Y.C.; Chan, H.L.; Chiang, C.S.; Liu, H.L.; et al. Angiogenesis-targeting microbubbles combined with ultrasound-mediated gene therapy in brain tumors. J. Control. Release 2017, 255, 164–175. [Google Scholar] [CrossRef]
- Wang, G.; Lu, H.; Pan, Y.; Qi, Y.; Huang, Y. Ultrasound-Sensitive Targeted Liposomes as a Gene Delivery System for the Synergistic Treatment of Hepatocellular Carcinoma. Small 2024, 20, e2406182. [Google Scholar] [CrossRef]
- Xu, K.; Yang, Y.; Hu, Z.; Yue, Y.; Gong, Y.; Cui, J.; Culver, J.P.; Bruchas, M.R.; Chen, H. TRPV1-mediated sonogenetic neuromodulation of motor cortex in freely moving mice. J. Neural Eng. 2023, 20, 016055. [Google Scholar] [CrossRef]
- Phan, T.N.; Fan, C.H.; Wang, H.C.; Liu, H.L.; Lin, Y.C.; Yeh, C.K. Modulation of GABAergic neurons in acute epilepsy using sonogenetics. J. Control. Release 2025, 377, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Tan, D.; Wang, Y.; Gong, C.; Yuan, J.; Yang, X.; Wen, Y.; Ban, Y.; Liang, M.; Hu, Y.; et al. Targeted sonogenetic modulation of GABAergic interneurons in the hippocampal CA1 region in status epilepticus. Theranostics 2024, 14, 6373–6391. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, Y.; Yang, L.; Gong, Y.; Chukwu, C.; Ye, D.; Yue, Y.; Yuan, J.; Kravitz, A.V.; Chen, H. Airy-beam holographic sonogenetics for advancing neuromodulation precision and flexibility. Proc. Natl. Acad. Sci. USA 2024, 121, e2402200121. [Google Scholar] [CrossRef]
- Liu, L.; He, P.; Wang, Y.; Ma, F.; Li, D.; Bai, Z.; Qu, Y.; Zhu, L.; Yoon, C.W.; Yu, X.; et al. Engineering sonogenetic EchoBack-CAR T cells. Cell 2025, 188, 2621–2636.E20. [Google Scholar] [CrossRef]
- Sun, S.; Huang, X.; Yang, N.; Lei, H.; Pei, Z.; Han, Z.; Liu, L.; Gong, F.; Yu, Q.; Li, J.; et al. Fluorinated Titanium Oxide (TiO2-xFx) Nanospindles as Ultrasound-Triggered Pyroptosis Inducers to Boost Sonodynamic Immunotherapy. ACS Nano 2024, 18, 19756–19770. [Google Scholar] [CrossRef]
- Wang, X.G.; Shen, M.; Sun, Y.Y.; Tang, Q.Y.; Du, L.; Yang, S.; Zou, H.B.; Zhao, X.; Chen, X.J.; Li, H.S.; et al. Ultrasound-triggered cascade reaction via MnO2-CpG nanoparticles for boosting pyroptosis-mediated cancer immunotherapy. Nano Today 2024, 57, 102394. [Google Scholar] [CrossRef]
- Xu, D.; Fu, S.; Zhang, H.; Lu, W.; Xie, J.; Li, J.; Wang, H.; Zhao, Y.; Chai, R. Ultrasound-Responsive Aligned Piezoelectric Nanofibers Derived Hydrogel Conduits for Peripheral Nerve Regeneration. Adv. Mater. 2024, 36, e2307896. [Google Scholar] [CrossRef] [PubMed]
- Ricotti, L.; Cafarelli, A.; Manferdini, C.; Trucco, D.; Vannozzi, L.; Gabusi, E.; Fontana, F.; Dolzani, P.; Saleh, Y.; Lenzi, E.; et al. Ultrasound Stimulation of Piezoelectric Nanocomposite Hydrogels Boosts Chondrogenic Differentiation in Vitro, in Both a Normal and Inflammatory Milieu. ACS Nano 2024, 18, 2047–2065. [Google Scholar] [CrossRef]
- Wang, A.A.; Ma, X.B.; Yang, Y.F.; Shi, G.L.; Han, L.W.; Hu, X.T.; Shi, R.; Yan, J.; Guo, Q.Y.; Zhao, Y.T. Biophysical-driven piezoelectric and aligned nanofibrous scaffold promotes bone regeneration by re-establishing physiological electrical microenvironment. Nano Res. 2024, 17, 7376–7393. [Google Scholar] [CrossRef]
- Zhang, F.; Lv, M.; Wang, S.; Li, M.; Wang, Y.; Hu, C.; Hu, W.; Wang, X.; Wang, X.; Liu, Z.; et al. Ultrasound-triggered biomimetic ultrashort peptide nanofiber hydrogels promote bone regeneration by modulating macrophage and the osteogenic immune microenvironment. Bioact. Mater. 2024, 31, 231–246. [Google Scholar] [CrossRef]
- Jiang, T.; Yu, F.; Zhou, Y.; Li, R.; Zheng, M.; Jiang, Y.; Li, Z.; Pan, J.; Ouyang, N. Synergistic effect of ultrasound and reinforced electrical environment by bioinspired periosteum for enhanced osteogenesis via immunomodulation of macrophage polarization through Piezo1. Mater. Today Bio 2024, 27, 101147. [Google Scholar] [CrossRef]
- Zhou, S.; Xiao, C.; Fan, L.; Yang, J.; Ge, R.; Cai, M.; Yuan, K.; Li, C.; Crawford, R.W.; Xiao, Y.; et al. Injectable ultrasound-powered bone-adhesive nanocomposite hydrogel for electrically accelerated irregular bone defect healing. J. Nanobiotechnology 2024, 22, 54. [Google Scholar] [CrossRef]
- Dunham, C.L.; Frank, J.A. Ultrasound Pressure-Dependent Cytokine and Immune Cell Response Lost in Aged Muscle. Ultrasound Med. Biol. 2024, 50, 494–501. [Google Scholar] [CrossRef]
- Feng, R.; Sheng, H.; Lian, Y. Advances in using ultrasound to regulate the nervous system. Neurol. Sci. 2024, 45, 2997–3006. [Google Scholar] [CrossRef]
- Hahmann, J.; Ishaqat, A.; Lammers, T.; Herrmann, A. Sonogenetics for Monitoring and Modulating Biomolecular Function by Ultrasound. Angew. Chem. Int. Ed. Engl. 2024, 63, e202317112. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Choi, M.H.; Zhu, J.; Zhu, T.; Yang, J.; Li, N.; Chen, Z.; Xian, Q.; Hou, X.; He, D.; et al. Sonogenetics: Recent advances and future directions. Brain Stimul. 2022, 15, 1308–1317. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-S.; Fan, C.-H.; Hsu, N.; Chiu, N.-H.; Wu, C.-Y.; Chang, C.-Y.; Wu, B.-H.; Hong, S.-R.; Chang, Y.-C.; Yan-Tang Wu, A. Sonogenetic modulation of cellular activities using an engineered auditory-sensing protein. Nano Lett. 2019, 20, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.H.; Wei, K.C.; Chiu, N.H.; Liao, E.C.; Wang, H.C.; Wu, R.Y.; Ho, Y.J.; Chan, H.L.; Wang, T.A.; Huang, Y.Z.; et al. Sonogenetic-Based Neuromodulation for the Amelioration of Parkinson’s Disease. Nano Lett. 2021, 21, 5967–5976. [Google Scholar] [CrossRef]
- Zhi, W.; Li, Y.; Wang, L.; Hu, X. Advancing Neuroscience and Therapy: Insights into Genetic and Non-Genetic Neuromodulation Approaches. Cells 2025, 14, 122. [Google Scholar] [CrossRef]
- Meng, Z.; Zhang, Y.; She, J.; Zhou, X.; Xu, J.; Han, X.; Wang, C.; Zhu, M.; Liu, Z. Ultrasound-Mediated Remotely Controlled Nanovaccine Delivery for Tumor Vaccination and Individualized Cancer Immunotherapy. Nano Lett. 2021, 21, 1228–1237. [Google Scholar] [CrossRef]
- Li, X.; Khorsandi, S.; Wang, Y.; Santelli, J.; Huntoon, K.; Nguyen, N.; Yang, M.; Lee, D.; Lu, Y.; Gao, R.; et al. Cancer immunotherapy based on image-guided STING activation by nucleotide nanocomplex-decorated ultrasound microbubbles. Nat. Nanotechnol. 2022, 17, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Tang, Q.; Sun, L.; Zhang, J.; Zhang, L.; Xu, M.; Chen, J.; Gong, M.; Liang, X. Ultrasound-mediated immune regulation in tumor immunotherapy. Mater. Today Adv. 2022, 14, 100248. [Google Scholar] [CrossRef]
- Ho, Y.J.; Li, J.P.; Fan, C.H.; Liu, H.L.; Yeh, C.K. Ultrasound in tumor immunotherapy: Current status and future developments. J. Control. Release 2020, 323, 12–23. [Google Scholar] [CrossRef]
- de Lucas, B.; Perez, L.M.; Bernal, A.; Galvez, B.G. Ultrasound Therapy: Experiences and Perspectives for Regenerative Medicine. Genes 2020, 11, 1086. [Google Scholar] [CrossRef]
- Bansal, K.; Jha, C.K.; Bhatia, D.; Shekhar, H. Ultrasound-Enabled Therapeutic Delivery and Regenerative Medicine: Physical and Biological Perspectives. ACS Biomater. Sci. Eng. 2021, 7, 4371–4387. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Nakamura, T.; Fujihara, S.; Tanaka, E. Ultrasound modulates the inflammatory response and promotes muscle regeneration in injured muscles. Ann. Biomed. Eng. 2013, 41, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Luo, Z.; Sun, Y.; He, Z.; Qi, B.; Chen, Y.; Wang, J.; Li, C.; Lin, W.; Han, Z.; et al. Low-intensity pulsed ultrasound promotes skeletal muscle regeneration via modulating the inflammatory immune microenvironment. Int. J. Biol. Sci. 2023, 19, 1123–1145. [Google Scholar] [CrossRef]
- Claes, L.; Willie, B. The enhancement of bone regeneration by ultrasound. Prog. Biophys. Mol. Biol. 2007, 93, 384–398. [Google Scholar] [CrossRef]
- Maddi, A.; Hai, H.; Ong, S.T.; Sharp, L.; Harris, M.; Meghji, S. Long wave ultrasound may enhance bone regeneration by altering OPG/RANKL ratio in human osteoblast-like cells. Bone 2006, 39, 283–288. [Google Scholar] [CrossRef]
- Acheta, J.; Stephens, S.B.Z.; Belin, S.; Poitelon, Y. Therapeutic Low-Intensity Ultrasound for Peripheral Nerve Regeneration—A Schwann Cell Perspective. Front. Cell. Neurosci. 2021, 15, 812588. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, Y.; Tang, J.; Peng, J.; Wang, Y.; Guo, Q.; Guo, Z.; Li, P.; Xiao, B.; Zhang, J. Low-intensity pulsed ultrasound treatment improved the rate of autograft peripheral nerve regeneration in rat. Sci. Rep. 2016, 6, 22773. [Google Scholar] [CrossRef] [PubMed]
- Kawai, H.; Ito, A.; Kawaguchi, A.; Nagai-Tanima, M.; Nakahara, R.; Xu, S.; Kuroki, H. Ultrasound therapy for a week promotes regeneration and reduces pro-inflammatory macrophages in a rat sciatic nerve autograft model. Sci. Rep. 2023, 13, 11494. [Google Scholar] [CrossRef] [PubMed]
- Krut, Z.; Gazit, D.; Gazit, Z.; Pelled, G. Applications of Ultrasound-Mediated Gene Delivery in Regenerative Medicine. Bioengineering 2022, 9, 190. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Sun, J.; Wei, H.; Hao, J.; Liu, W.; Wang, X. Ultrasound-Targeted Microbubble Destruction: Modulation in the Tumor Microenvironment and Application in Tumor Immunotherapy. Front. Immunol. 2022, 13, 937344. [Google Scholar] [CrossRef]
- Castillo, J.I.; Navarro-Becerra, J.A.; Angelini, I.; Kokoshinskiy, M.; Borden, M.A. Frequency-Selective Microbubble Targeting In Vitro: A Step Toward Multicolor Ultrasound Molecular Imaging. ACS Appl. Bio Mater. 2025, 8, 2128–2140. [Google Scholar] [CrossRef]
- Xiong, X.; Zhou, H.; Xu, X.; Fu, Q.; Wan, Y.; Cao, Y.; Tang, R.; Li, F.; Zhang, J.; Li, P. Ultrasound Molecular Imaging Enhances High-Intensity Focused Ultrasound Ablation on Liver Cancer With B7-H3-Targeted Microbubbles. Cancer Med. 2024, 13, e70341. [Google Scholar] [CrossRef]
- Qiu, Y.J.; Cao, J.Y.; Liao, J.H.; Duan, Y.; Chen, S.; Cheng, R.; Huang, Y.L.; Lu, X.Y.; Cheng, J.; Wang, W.P.; et al. CXCR4-targeted ultrasound microbubbles for imaging and enhanced chemotherapy/Immunotherapy in liver cancer. Acta Biomater. 2025, 197, 416–430. [Google Scholar] [CrossRef]
- Wang, D.; Xing, C.; Liang, Y.; Wang, C.; Zhao, P.; Liang, X.; Li, Q.; Yuan, L. Ultrasound Imaging of Tumor Vascular CD93 with MMRN2 Modified Microbubbles for Immune Microenvironment Prediction. Adv. Mater. 2024, 36, e2310421. [Google Scholar] [CrossRef]
- Dutka, P.; Metskas, L.A.; Hurt, R.C.; Salahshoor, H.; Wang, T.Y.; Malounda, D.; Lu, G.J.; Chou, T.F.; Shapiro, M.G.; Jensen, G.J. Structure of Anabaena flos-aquae gas vesicles revealed by cryo-ET. Structure 2023, 31, 518–528.E6. [Google Scholar] [CrossRef]
- Garrute, F.V.; Pacheco, A.B.F.; Lu, G.J.; Machado, J.C. A Bioengineered Cathepsin B-sensitive Gas Vesicle Nanosystem That Responds With Increased Gray-level Intensity of Ultrasound Biomicroscopic Images. Ultrasound Med. Biol. 2025, 51, 120–127. [Google Scholar] [CrossRef]
- Li, J.; Cui, Y.; Jiang, X.; Chi, X.; Li, H.; Ma, X.; Tang, Y.; Huang, D.; Liu, Z. Surface-engineered bio-manufactured gas vesicles for multimodal imaging of glioma. J. Nanobiotechnology 2025, 23, 116. [Google Scholar] [CrossRef]
- Shen, Q.; Li, Z.; Wang, Y.; Meyer, M.D.; De Guzman, M.T.; Lim, J.C.; Xiao, H.; Bouchard, R.R.; Lu, G.J. 50-nm Gas-Filled Protein Nanostructures to Enable the Access of Lymphatic Cells by Ultrasound Technologies. Adv. Mater. 2024, 36, e2307123. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Warbal, P.; Basterrechea, K.F.; Bader, K.; Shekhar, H. Enhancing Passive Cavitation Imaging Using pth Root Compression Delay, Sum, and Integrate Beamforming: In Vitro and in Vivo Studies. IEEE Trans. Biomed. Eng. 2025, 1–12. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, Y.; Zhang, G.; Xue, H.; Ding, B.; Tu, J.; Zhang, D.; Guo, X. 2D spatiotemporal passive cavitation imaging and evaluation during ultrasound thrombolysis based on diagnostic ultrasound platform. Ultrason. Sonochem. 2024, 110, 107051. [Google Scholar] [CrossRef] [PubMed]
- Lachambre, C.; Basarab, A.; Bera, J.C.; Nicolas, B.; Varray, F.; Gilles, B. An Inverse Method Using Cross-Spectral Matrix Fitting for Passive Cavitation Imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2024, 71, 995–1005. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, G.; Zhang, Q.; Luo, L.; Ding, B.; Guo, X.; Zhang, D.; Tu, J. Real-time passive cavitation mapping and B-mode fusion imaging via hybrid adaptive beamformer with modified diagnostic ultrasound platform. Ultrasonics 2024, 142, 107375. [Google Scholar] [CrossRef]
- Li, D.; Wang, N.; Li, M.; Mishra, A.; Tang, Y.; Vu, T.; Xiang, G.; Chen, J.; Lipkin, M.; Zhong, P.; et al. Three-Dimensional Super-Resolution Passive Cavitation Mapping in Laser Lithotripsy. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2024, 71, 1690–1700. [Google Scholar] [CrossRef] [PubMed]
- Lamothe, N.; Andrés, D.; Carrión, A.; Camarena, F.; Pineda-Pardo, J.A.; Jiménez, N. Monitoring holograms for therapeutic ultrasound using passive cavitation beamforming. Appl. Acoust. 2024, 224, 110144. [Google Scholar] [CrossRef]
- Klibanov, A.L. Ultrasound molecular imaging with targeted microbubble contrast agents. J. Nucl. Cardiol. 2007, 14, 876–884. [Google Scholar] [CrossRef]
- Lindner, J.R. Molecular imaging with contrast ultrasound and targeted microbubbles. J. Nucl. Cardiol. 2004, 11, 215–221. [Google Scholar] [CrossRef]
- Dayton, P.A.; Ferrara, K.W. Targeted imaging using ultrasound. J. Magn. Reason. Imaging 2002, 16, 362–377. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.R.; Hu, X.; Zhang, H.; Tlaxca, J.; Decleves, A.E.; Houghtaling, R.; Sharma, K.; Lawrence, M.; Ferrara, K.W.; Rychak, J.J. Ultrasound molecular imaging of tumor angiogenesis with an integrin targeted microbubble contrast agent. Investig. Radiol. 2011, 46, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.S.; Sennoga, C.A.; McConnell, E.; Eckersley, R.; Tang, M.X.; Nourshargh, S.; Seddon, J.M.; Haskard, D.O.; Nihoyannopoulos, P. A Targeting Microbubble for Ultrasound Molecular Imaging. PLoS ONE 2015, 10, e0129681. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abou-Elkacem, L.; Wang, H.; Chowdhury, S.M.; Kimura, R.H.; Bachawal, S.V.; Gambhir, S.S.; Tian, L.; Willmann, J.K. Thy1-Targeted Microbubbles for Ultrasound Molecular Imaging of Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. 2018, 24, 1574–1585. [Google Scholar] [CrossRef]
- Wang, S.; Hossack, J.A.; Klibanov, A.L. Targeting of microbubbles: Contrast agents for ultrasound molecular imaging. J. Drug Target. 2018, 26, 420–434. [Google Scholar] [CrossRef]
- Unnikrishnan, S.; Du, Z.; Diakova, G.B.; Klibanov, A.L. Formation of microbubbles for targeted ultrasound contrast imaging: Practical translation considerations. Langmuir 2018, 35, 10034–10041. [Google Scholar] [CrossRef]
- Jugniot, N.; Bam, R.; Meuillet, E.J.; Unger, E.C.; Paulmurugan, R. Current status of targeted microbubbles in diagnostic molecular imaging of pancreatic cancer. Bioeng. Transl. Med. 2021, 6, e10183. [Google Scholar] [CrossRef]
- Lakshmanan, A.; Lu, G.J.; Farhadi, A.; Nety, S.P.; Kunth, M.; Lee-Gosselin, A.; Maresca, D.; Bourdeau, R.W.; Yin, M.; Yan, J.; et al. Preparation of biogenic gas vesicle nanostructures for use as contrast agents for ultrasound and MRI. Nat. Protoc. 2017, 12, 2050–2080. [Google Scholar] [CrossRef]
- Bourdeau, R.W.; Lee-Gosselin, A.; Lakshmanan, A.; Farhadi, A.; Kumar, S.R.; Nety, S.P.; Shapiro, M.G. Acoustic reporter genes for noninvasive imaging of microorganisms in mammalian hosts. Nature 2018, 553, 86–90. [Google Scholar] [CrossRef]
- Sawyer, D.P.; Bar-Zion, A.; Farhadi, A.; Shivaei, S.; Ling, B.; Lee-Gosselin, A.; Shapiro, M.G. Ultrasensitive ultrasound imaging of gene expression with signal unmixing. Nat. Methods 2021, 18, 945–952. [Google Scholar] [CrossRef]
- Kim, S.; Zhang, S.; Yoon, S. Multiplexed Ultrasound Imaging Using Spectral Analysis on Gas Vesicles. Adv. Healthc. Mater. 2022, 11, e2200568. [Google Scholar] [CrossRef]
- Farhadi, A.; Ho, G.H.; Sawyer, D.P.; Bourdeau, R.W.; Shapiro, M.G. Ultrasound imaging of gene expression in mammalian cells. Science 2019, 365, 1469–1475. [Google Scholar] [CrossRef]
- Pfeifer, F. Recent Advances in the Study of Gas Vesicle Proteins and Application of Gas Vesicles in Biomedical Research. Life 2022, 12, 1455. [Google Scholar] [CrossRef] [PubMed]
- Salgaonkar, V.A.; Datta, S.; Holland, C.K.; Mast, T.D. Passive cavitation imaging with ultrasound arrays. J. Acoust. Soc. Am. 2009, 126, 3071–3083. [Google Scholar] [CrossRef]
- Haworth, K.J.; Bader, K.B.; Rich, K.T.; Holland, C.K.; Mast, T.D. Quantitative Frequency-Domain Passive Cavitation Imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2017, 64, 177–191. [Google Scholar] [CrossRef]
- Xu, S.; Ye, D.; Wan, L.; Shentu, Y.; Yue, Y.; Wan, M.; Chen, H. Correlation Between Brain Tissue Damage and Inertial Cavitation Dose Quantified Using Passive Cavitation Imaging. Ultrasound Med. Biol. 2019, 45, 2758–2766. [Google Scholar] [CrossRef] [PubMed]
- Haworth, K.J.; Raymond, J.L.; Radhakrishnan, K.; Moody, M.R.; Huang, S.L.; Peng, T.; Shekhar, H.; Klegerman, M.E.; Kim, H.; McPherson, D.D.; et al. Trans-Stent B-Mode Ultrasound and Passive Cavitation Imaging. Ultrasound Med. Biol. 2016, 42, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Bader, K.B.; Haworth, K.J.; Maxwell, A.D.; Holland, C.K. Post Hoc Analysis of Passive Cavitation Imaging for Classification of Histotripsy-Induced Liquefaction in Vitro. IEEE Trans. Med. Imaging 2018, 37, 106–115. [Google Scholar] [CrossRef]
- Suarez Escudero, D.; Goudot, G.; Vion, M.; Tanter, M.; Pernot, M. 2D and 3D real-time passive cavitation imaging of pulsed cavitation ultrasound therapy in moving tissues. Phys. Med. Biol. 2018, 63, 235028. [Google Scholar] [CrossRef]
- Yoo, J.; Ahn, J.; Ha, H.; Claud Jonas, J.; Kim, C.; Ham Kim, H. Single-Beam Acoustic Tweezers for Cell Biology: Molecular to In Vivo Level. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2024, 71, 1269–1288. [Google Scholar] [CrossRef]
- Yoo, J.; Kim, J.; Lee, J.; Kim, H.H. Red blood cell trapping using single-beam acoustic tweezers in the Rayleigh regime. iScience 2023, 26, 108178. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Kim, H.; Kim, Y.; Lim, H.G.; Kim, H.H. Collapse pressure measurement of single hollow glass microsphere using single-beam acoustic tweezer. Ultrason. Sonochem. 2022, 82, 105844. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Choi, S.; Knieling, F.; Clingman, B.; Bohndiek, S.; Wang, L.V.; Kim, C. Clinical translation of photoacoustic imaging. Nat. Rev. Bioeng. 2025, 3, 193–212. [Google Scholar] [CrossRef]
- Choi, S.; Kim, J.; Jeon, H.; Kim, C.; Park, E.-Y. Advancements in photoacoustic detection techniques for biomedical imaging. npj Acoustics 2025, 1, 1. [Google Scholar] [CrossRef]
- Yang, M.; Nah, Y.; Oh, D.; Li, X.; Kim, H.; Park, S.; Lim, J.; Chen, A.; Kim, C.; Kim, W.J.; et al. Transferrin-Mediated Nanophotosensitizer to Enhance Phototherapy with Photoacoustic Imaging Under Hypoxia. CCS Chem. 2025, 7, 1127–1141. [Google Scholar] [CrossRef]
- Lee, E.S.; Choi, S.; Lee, J.; Shin, J.M.; Kim, J.; Wi, J.S.; Lee, T.G.; Kim, Y.H.; Kim, C.; Na, H.K. Au/Fe/Au trilayer nanodiscs as theranostic agents for magnet-guided photothermal, chemodynamic therapy and ferroptosis with photoacoustic imaging. Chem. Eng. J. 2025, 505, 159137. [Google Scholar] [CrossRef]
- Choi, H.; Choi, W.; Kim, J.; Kong, W.H.; Kim, K.S.; Kim, C.; Hahn, S.K. Multifunctional nanodroplets encapsulating naphthalocyanine and perfluorohexane for bimodal image-guided therapy. Biomacromolecules 2019, 20, 3767–3777. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Ninjbadgar, T.; Kang, M.; Kim, C.; Kim, J. Recent advances in photoacoustic agents for theranostic applications. Nanomaterials 2023, 13, 695. [Google Scholar] [CrossRef]
- Zhang, H.K.; Yan, P.; Kang, J.; Abou, D.S.; Le, H.N.; Jha, A.K.; Thorek, D.L.; Kang, J.U.; Rahmim, A.; Wong, D.F.; et al. Listening to membrane potential: Photoacoustic voltage-sensitive dye recording. J. Biomed. Opt. 2017, 22, 45006. [Google Scholar] [CrossRef]
- Kim, J.; Choi, S.; Kim, C.; Kim, J.; Park, B. Review on Photoacoustic Monitoring after Drug Delivery: From Label-Free Biomarkers to Pharmacokinetics Agents. Pharmaceutics 2024, 16, 1240. [Google Scholar] [CrossRef]
- Park, E.-Y.; Oh, D.; Park, S.; Kim, W.; Kim, C. New contrast agents for photoacoustic imaging and theranostics: Recent 5-year overview on phthalocyanine/naphthalocyanine-based nanoparticles. APL Bioeng. 2021, 5, 031510. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Heo, D.; Cho, S.; Ha, M.; Park, J.; Ahn, J.; Kim, M.; Kim, D.; Jung, D.H.; Kim, H.H.; et al. Enhanced dual-mode imaging: Superior photoacoustic and ultrasound endoscopy in live pigs using a transparent ultrasound transducer. Sci. Adv. 2024, 10, eadq9960. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Misra, S.; Hwang, D.G.; Yoon, C.; Ahn, J.; Kim, D.; Jang, J.; Kim, C. Unsupervised inter-domain transformation for virtually stained high-resolution mid-infrared photoacoustic microscopy using explainable deep learning. Nat. Commun. 2024, 15, 10892. [Google Scholar] [CrossRef]
- Yoo, J.; Oh, D.; Kim, C.; Kim, H.H.; Um, J.Y. Switchable preamplifier for dual modal photoacoustic and ultrasound imaging. Biomed. Opt. Express 2023, 14, 89–105. [Google Scholar] [CrossRef]
- Barulin, A.; Barulina, E.; Oh, D.K.; Jo, Y.; Park, H.; Park, S.; Kye, H.; Kim, J.; Yoo, J.; Kim, J.; et al. Axially multifocal metalens for 3D volumetric photoacoustic imaging of neuromelanin in live brain organoid. Sci. Adv. 2025, 11, eadr0654. [Google Scholar] [CrossRef]
- Park, B.; Han, M.; Kim, H.; Yoo, J.; Oh, D.K.; Moon, S.; Ahn, J.; Lim, H.G.; Kim, I.; Kim, H.H.; et al. Shear-Force Photoacoustic Microscopy: Toward Super-resolution Near-Field Imaging. Laser Photonics Rev. 2022, 16, 2200296. [Google Scholar] [CrossRef]
- Yang, J.; Choi, S.; Kim, J.; Lee, J.; Kim, W.J.; Kim, C. Multiplane Spectroscopic Whole-Body Photoacoustic Computed Tomography of Small Animals In Vivo. Laser Photonics Rev. 2025, 19, 2400672. [Google Scholar] [CrossRef]
- Park, S.; Park, G.; Kim, J.; Choi, W.; Jeong, U.; Kim, C. Bi 2 Se 3 nanoplates for contrast-enhanced photoacoustic imaging at 1064 nm. Nanoscale 2018, 10, 20548–20558. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Park, E.; Park, J.; Perleberg, B.; Jeon, S.; Ahn, J.; Ha, M.; Kim, H.H.; Kim, J.Y.; Jung, C.K. An ultraviolet-transparent ultrasound transducer enables high-resolution label-free photoacoustic histopathology. Laser Photonics Rev. 2024, 18, 2300652. [Google Scholar] [CrossRef]
- Park, B.; Oh, D.; Kim, J.; Kim, C. Functional photoacoustic imaging: From nano-and micro-to macro-scale. Nano Converg. 2023, 10, 29. [Google Scholar] [CrossRef]
- Choi, W.; Park, B.; Choi, S.; Oh, D.; Kim, J.; Kim, C. Recent advances in contrast-enhanced photoacoustic imaging: Overcoming the physical and practical challenges. Chem. Rev. 2023, 123, 7379–7419. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.G.N.; Kang, J.H.; Kim, W.; Lim, J.; Kang, S.J.; You, J.Y.; Hoang, Q.T.; Kim, W.J.; Rhee, W.J.; Kim, C. Engineered extracellular vesicle-based sonotheranostics for dual stimuli-sensitive drug release and photoacoustic imaging-guided chemo-sonodynamic cancer therapy. Theranostics 2022, 12, 1247. [Google Scholar]
- Wang, Z.; Li, J.; Wu, R. Time-delay- and time-reversal-based robust Capon beamformers for ultrasound imaging. IEEE Trans. Med. Imaging 2005, 24, 1308–1322. [Google Scholar] [CrossRef] [PubMed]
- Mornstein, V. Cavitation-induced risks associated with contrast agents used in ultrasonography. Eur. J. Ultrasound 1997, 5, 101–111. [Google Scholar] [CrossRef]
- Brennen, C.E. Cavitation in medicine. Interface Focus 2015, 5, 20150022. [Google Scholar] [CrossRef]
- Konofagou, E.E. Optimization of the ultrasound-induced blood-brain barrier opening. Theranostics 2012, 2, 1223–1237. [Google Scholar] [CrossRef]
- Kim, J.; Choi, W.; Park, E.Y.; Kang, Y.; Lee, K.J.; Kim, H.H.; Kim, W.J.; Kim, C. Real-Time Photoacoustic Thermometry Combined With Clinical Ultrasound Imaging and High-Intensity Focused Ultrasound. IEEE Trans. Biomed. Eng. 2019, 66, 3330–3338. [Google Scholar] [CrossRef]
- Liu, D.; Ebbini, E.S. Real-time 2-D temperature imaging using ultrasound. IEEE Trans. Biomed. Eng. 2010, 57, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Arnal, B.; Baranger, J.; Demene, C.; Tanter, M.; Pernot, M. In vivo real-time cavitation imaging in moving organs. Phys. Med. Biol. 2017, 62, 843–857. [Google Scholar] [CrossRef]
- Munoz, F.; Meaney, A.; Gross, A.; Liu, K.; Pouliopoulos, A.N.; Liu, D.; Konofagou, E.E.; Ferrera, V.P. Long term study of motivational and cognitive effects of low-intensity focused ultrasound neuromodulation in the dorsal striatum of nonhuman primates. Brain Stimul. 2022, 15, 360–372. [Google Scholar] [CrossRef] [PubMed]
| Application | Strength | Approach | Reference |
|---|---|---|---|
| Tumor treatment |
| Cavitation and sonoporation | Han et al., 2024 [57] Cui et al., 2024 [58] Nittayacharn et al., 2024 [59] |
| BBB opening |
| Cavitation and sonoporation | Li et al., 2024 [60] Huang et al., 2024 [61] Zhou et al., 2024 [62] |
| Gene delivery |
| Sonoporation | Lawton et al., 2024 [63] Sotoudehbagha et al., 2025 [64] Li et al., 2024 [65] |
| Application | Strength | Approach | Reference |
|---|---|---|---|
| Neuromodulation |
| Mechanotransduction | Xu et al., 2023 [81] Phan et al., 2025 [82] Xu et al., 2024 [83] Hu et al., 2024 [84] |
| Immunotherapy |
| Mechanotransduction | Liu et al., 2025 [85] |
| Cavitation | Sun et al., 2024 [86] Wang et al., 2024 [87] | ||
| Regeneration |
| Mechanotransduction | Xu et al., 2024 [88] Ricotti et al., 2024 [89] Wang et al., 2024 [90] Zhang et al., 2024 [91] Jiang et al., 2024 [92] Zhou et al., 2024 [93] Dunham et al., 2024 [94] |
| Application | Strength | Approach | Reference |
|---|---|---|---|
| Targeted microbubble |
| Cavitation (stable) | Castillo et al., 2025 [116] Xiong et al., 2024 [117] Qiu et al., 2025 [118] Wang et al., 2024 [119] |
| Gas vesicle |
| Cavitation (stable) | Dutka et al., 2023 [120] Garrute et al., 2025 [121] Li et al., 2025 [122] Shen et al., 2024 [123] |
| Passive cavitation imaging |
| Cavitation (stable) | Singh et al., 2025 [124] Zhang et al., 2024 [125] Lachambre et al., 2024 [126] Zhu et al., 2024 [127] Li et al., 2024 [128] Lamothe et al., 2024 [129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, J.; Heo, D.; Hwang, Y.; Kim, C.; Park, B. Ultrasound-Mediated Membrane Modulation for Biomedical Applications. Nanomaterials 2025, 15, 884. https://doi.org/10.3390/nano15120884
Yoo J, Heo D, Hwang Y, Kim C, Park B. Ultrasound-Mediated Membrane Modulation for Biomedical Applications. Nanomaterials. 2025; 15(12):884. https://doi.org/10.3390/nano15120884
Chicago/Turabian StyleYoo, Jinhee, Dasom Heo, Yunhee Hwang, Chulhong Kim, and Byullee Park. 2025. "Ultrasound-Mediated Membrane Modulation for Biomedical Applications" Nanomaterials 15, no. 12: 884. https://doi.org/10.3390/nano15120884
APA StyleYoo, J., Heo, D., Hwang, Y., Kim, C., & Park, B. (2025). Ultrasound-Mediated Membrane Modulation for Biomedical Applications. Nanomaterials, 15(12), 884. https://doi.org/10.3390/nano15120884








