Phase-Dependent Photocatalytic Activity of Nb2O5 Nanomaterials for Rhodamine B Degradation: The Role of Surface Chemistry and Crystal Structure
Abstract
1. Introduction
2. Materials and Methods
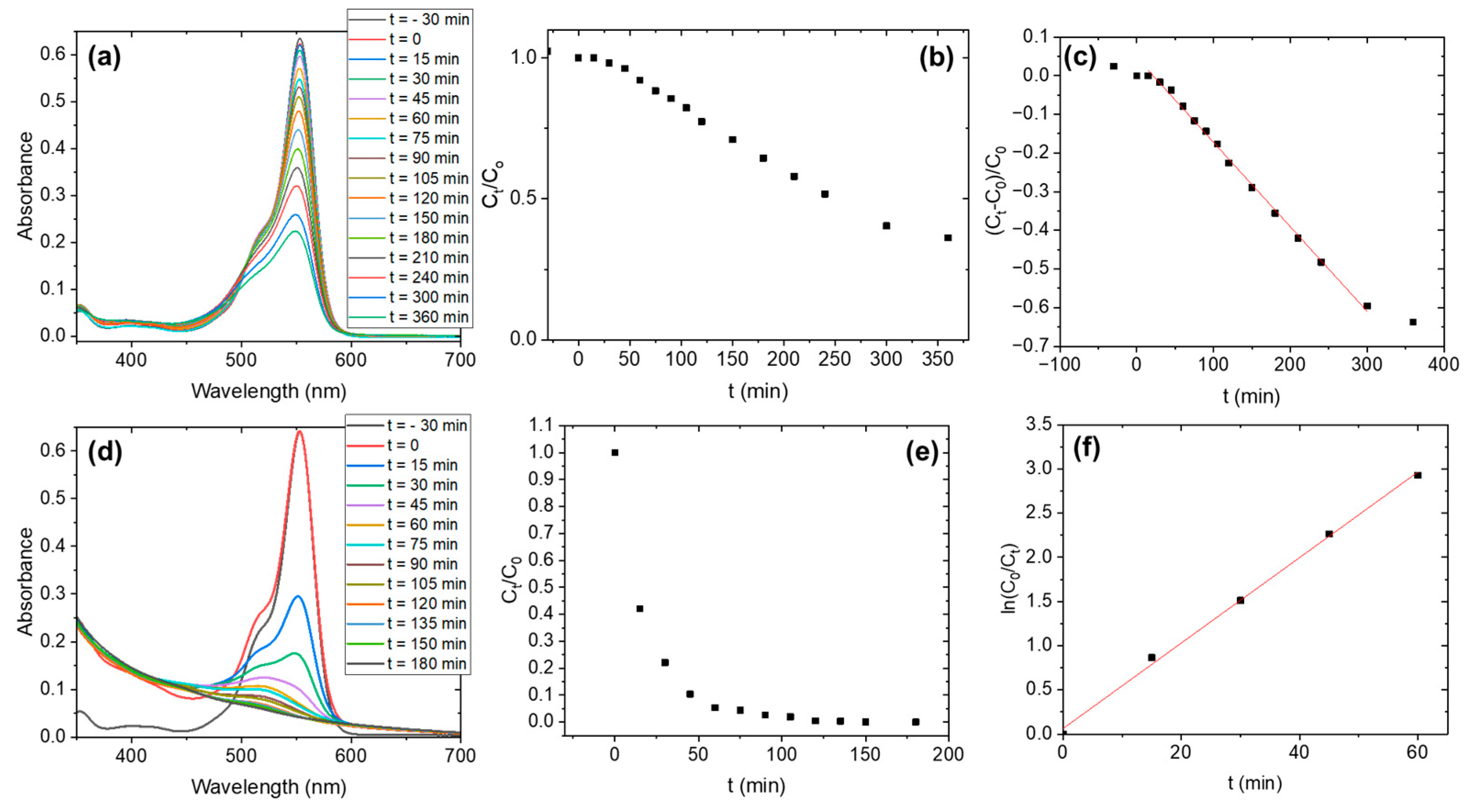
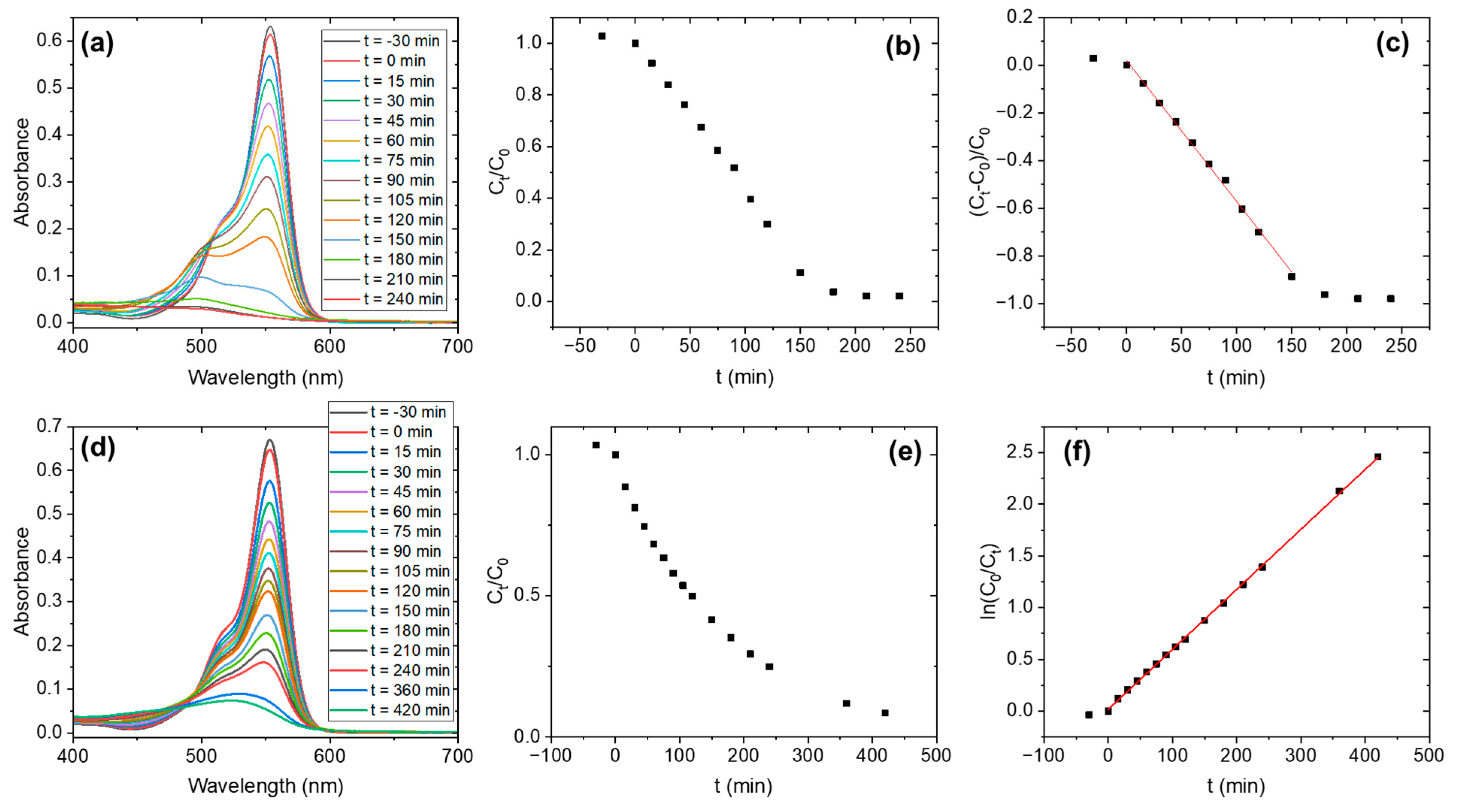
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, P.; Duraipandy, N.; Manikantan Syamala, K.; Rajendran, N. Antibacterial Effects, Biocompatibility and Electrochemical Behavior of Zinc Incorporated Niobium Oxide Coating on 316L SS for Biomedical Applications. Appl. Surf. Sci. 2018, 427, 1166–1181. [Google Scholar] [CrossRef]
- Zitter, H.; Plenk, H., Jr. The Electrochemical Behavior of Metallic Implant Materials as an Indicator of Their Biocompatibility. J. Biomed. Mater. Res. 1987, 21, 881–896. [Google Scholar] [CrossRef] [PubMed]
- Canepa, P.; Ghiara, G.; Spotorno, R.; Canepa, M.; Cavalleri, O. Structural vs. Electrochemical Investigation of Niobium Oxide Layers Anodically Grown in a Ca and P Containing Electrolyte. J. Alloys Compd. 2021, 851, 156937. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, J.; Cheng, Z.; Wu, M.; Guo, Z.; Zhang, H. Design, Perspective, and Challenge of Niobium-Based Anode Materials for High-Energy Alkali Metal-Ion Batteries. Adv. Funct. Mater. 2024, 34, 2405392. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, X.; Ye, L.; Chi Edman Tsang, S. Nanostructured Nb2O5 Catalysts. Nano Rev. 2012, 3, 17631. [Google Scholar] [CrossRef]
- Nico, C.; Monteiro, T.; Graça, M.P.F. Niobium Oxides and Niobates Physical Properties: Review and Prospects. Prog. Mater. Sci. 2016, 80, 1–37. [Google Scholar] [CrossRef]
- Tanabe, K. Catalytic Application of Niobium Compounds. Catal. Today 2003, 78, 65–77. [Google Scholar] [CrossRef]
- Brauer, G. Die Oxyde Des Niobs. Z. Anorg. Allg. Chem. 1941, 248, 1–31. [Google Scholar] [CrossRef]
- Schäfer, H.; Gruehn, R.; Schulte, F. The Modifications of Niobium Pentoxide. Angew. Chem. Int. Ed. Engl. 1966, 5, 40–52. [Google Scholar] [CrossRef]
- Kreissl, H.T.; Li, M.M.J.; Peng, Y.K.; Nakagawa, K.; Hooper, T.J.N.; Hanna, J.V.; Shepherd, A.; Wu, T.S.; Soo, Y.L.; Tsang, S.C.E. Structural Studies of Bulk to Nanosize Niobium Oxides with Correlation to Their Acidity. J. Am. Chem. Soc. 2017, 139, 12670–12680. [Google Scholar] [CrossRef]
- Peng, X.; Bao, Z.; Zhang, S.; Li, Y.; Ding, L.; Shi, H.; Liu, J.; Zhong, X.; Li, X.; Wang, J. Modulation of Lewis and Brønsted Acid Centers with Oxygen Vacancies for Nb2O5 Electrocatalysts: Towards Highly Efficient Simultaneously Electrochemical Ozone and Hydrogen Peroxide Production. Chem. Eng. Sci. 2023, 271, 118573. [Google Scholar] [CrossRef]
- Skrodczky, K.; Antunes, M.M.; Han, X.; Santangelo, S.; Scholz, G.; Valente, A.A.; Pinna, N.; Russo, P.A. Niobium Pentoxide Nanomaterials with Distorted Structures as Efficient Acid Catalysts. Commun. Chem. 2019, 2, 129. [Google Scholar] [CrossRef]
- Ücker, C.L.; Riemke, F.C.; de Andrade Neto, N.F.; Santiago, A.d.A.G.; Siebeneichler, T.J.; Carreño, N.L.V.; Moreira, M.L.; Raubach, C.W.; Cava, S. Influence of Nb2O5 Crystal Structure on Photocatalytic Efficiency. Chem. Phys. Lett. 2021, 764, 138271. [Google Scholar] [CrossRef]
- Jehng, J.M.; Wachs, I.E. Structural Chemistry and Raman Spectra of Niobium Oxides. Chem. Mater. 1991, 3, 100–107. [Google Scholar] [CrossRef]
- Falk, G.; Borlaf, M.; López-Muñoz, M.J.; Fariñas, J.C.; Rodrigues Neto, J.B.; Moreno, R. Microwave-Assisted Synthesis of Nb2O5 for Photocatalytic Application of Nanopowders and Thin Films. J. Mater. Res. 2017, 32, 3271–3278. [Google Scholar] [CrossRef]
- Ücker, C.L.; Gularte, L.T.; Fernandes, C.D.; Goetzke, V.; Moreira, E.C.; Raubach, C.W.; Moreira, M.L.; Cava, S.S. Investigation of the Properties of Niobium Pentoxide for Use in Dye-Sensitized Solar Cells. J. Am. Ceram. Soc. 2019, 102, 1884–1892. [Google Scholar] [CrossRef]
- Silva, R.R.M.; Oliveira, J.A.; Ruotolo, L.A.M.; Faria, A.L.A.; Ribeiro, C.; Nogueira, F.G.E. Unveiling the Role of Peroxo Groups in Nb2O5 Photocatalytic Efficiency under Visible Light. Mater. Lett. 2020, 273, 127915. [Google Scholar] [CrossRef]
- Filho, J.B.G.; Almeida, L.D.; Victória, H.F.V.; Gomes, G.H.M.; Krambrock, K.; Robles-Azocar, P.A.; Pereira, M.C.; Oliveira, L.C.A. Niobium Oxides: The Key Role of Hydroxylated Surface on Photocatalytic Driven C–C Reductive Coupling of Acetophenone. J. Catal. 2024, 436, 115580. [Google Scholar] [CrossRef]
- Ziolek, M.; Sobczak, I.; Decyk, P.; Sobańska, K.; Pietrzyk, P.; Sojka, Z. Search for Reactive Intermediates in Catalytic Oxidation with Hydrogen Peroxide over Amorphous Niobium(V) and Tantalum(V) Oxides. Appl. Catal. B 2015, 164, 288–296. [Google Scholar] [CrossRef]
- Prado, N.T.; Nogueria, F.G.E.; Nogueira, A.E.; Nunes, C.A.; Diniz, R.; Oliveira, L.C.A. Modified Niobia as a New Catalyst for Selective Production of Dimethoxymethane from Methanol. Energy Fuels 2010, 24, 4793–4796. [Google Scholar] [CrossRef]
- Wolski, L.; Walkowiak, A.; Ziolek, M. Photo-Assisted Activation of H2O2 over Nb2O5—The Role of Active Oxygen Species on Niobia Surface in Photocatalytic Discoloration of Rhodamine B. Mater. Res. Bull. 2019, 118, 110530. [Google Scholar] [CrossRef]
- Wolski, L.; Wolska, J.; Frankowski, M. Recent Advances in H2O2 Activation on Niobia-Based Catalysts and Their Reactivity in Oxidative Degradation of Organic Pollutants. Inorg. Chem. Commun. 2024, 168, 112992. [Google Scholar] [CrossRef]
- Djurišić, A.B.; He, Y.; Ng, A.M.C. Visible-Light Photocatalysts: Prospects and Challenges. APL Mater. 2020, 8, 030903. [Google Scholar] [CrossRef]
- Dollimore, D. The Thermal Decomposition of Oxalates. A Review. Thermochim. Acta 1987, 117, 331–363. [Google Scholar] [CrossRef]
- Lebarbier, V.; Houalla, M.; Onfroy, T. New Insights into the Development of Brønsted Acidity of Niobic Acid. Catal. Today 2012, 192, 123–129. [Google Scholar] [CrossRef]
- Sotillo, B.; López, F.A.; Alcaraz, L.; Fernández, P. Characterization of Nb22O54 Microrods Grown from Niobium Oxide Powders Recovered from Mine Tailings. Ceram. Int. 2021, 47, 13859–13864. [Google Scholar] [CrossRef]
- Murayama, T.; Chen, J.; Hirata, J.; Matsumoto, K.; Ueda, W. Hydrothermal Synthesis of Octahedra-Based Layered Niobium Oxide and Its Catalytic Activity as a Solid Acid. Catal. Sci. Technol. 2014, 4, 4250–4257. [Google Scholar] [CrossRef]
- Graça, M.P.F.; Meireles, A.; Nico, C.; Valente, M.A. Nb2O5 Nanosize Powders Prepared by Sol–Gel–Structure, Morphology and Dielectric Properties. J. Alloys Compd. 2013, 553, 177–182. [Google Scholar] [CrossRef]
- Sotillo, B.; Ariza, R.; Fernández, P.; Solis, J. Ultrafast-Laser Powder Bed Fusion of Oxygen-Deficient Nb2O5 Ceramics with Highly Improved Electrical Properties. Mater. Des. 2022, 224, 111346. [Google Scholar] [CrossRef]
- Ikeya, T.; Senna, M. Change in the Structure of Niobium Pentoxide Due to Mechanical and Thermal Treatments. J. Non Cryst. Solids 1988, 105, 243–250. [Google Scholar] [CrossRef]
- Rathnasamy, R.; Thangasamy, P.; Aravindhan, V.; Sathyanarayanan, P.; Alagan, V. Facile One-Pot Solvothermal-Assisted Synthesis of Uniform Sphere-like Nb2O5 Nanostructures for Photocatalytic Applications. Res. Chem. Intermed. 2019, 45, 3571–3584. [Google Scholar] [CrossRef]
- De Oliveira Cantão, F.; De Carvalho Melo, W.; Oliveira, L.C.A.; Passos, A.R.; Da Silva, A.C. Utilization of Sn/Nb2O5 Composite for the Removal of Methylene Blue. Quim. Nova 2010, 33, 528–531. [Google Scholar] [CrossRef]
- Sharma, N.; Gautam, R.; Saini, K.; Li, H.; Saravanamurugan, S. Surface Hydroxyl Species Enabled Nb2O5 for the Photocatalytic Oxidation of 5-Hydroxymethylfurfural. ChemPhotoChem 2025, e202500004. [Google Scholar] [CrossRef]
- Bruziquesi, C.G.O.; Filho, J.B.G.; Victoria, H.F.V.; Krambrock, K.; Mansur, H.S.; Mansur, A.A.P.; Chagas, P.; Silva, A.C.; Oliveira, L.C.A. Synthesis of a Highly Active Nb2O5 for 1,2-Cyclohexanediol Production. Catal. Commun. 2022, 171, 106511. [Google Scholar] [CrossRef]
- Oliveira, L.C.A.; Portilho, M.F.; Silva, A.C.; Taroco, H.A.; Souza, P.P. Modified Niobia as a Bifunctional Catalyst for Simultaneous Dehydration and Oxidation of Glycerol. Appl. Catal. B 2012, 117–118, 29–35. [Google Scholar] [CrossRef]
- Rani, R.A.; Zoolfakar, A.S.; O’Mullane, A.P.; Austin, M.W.; Kalantar-Zadeh, K. Thin Films and Nanostructures of Niobium Pentoxide: Fundamental Properties, Synthesis Methods and Applications. J. Mater. Chem. A Mater. 2014, 2, 15683–15703. [Google Scholar] [CrossRef]
- da Cruz, J.A.; Volnistem, E.A.; Ferreira, R.F.; Freitas, D.B.; Sales, A.J.M.; Costa, L.C.; Graça, M.P.F. Structural Characterization of Brazilian Niobium Pentoxide and Treatment to Obtain the Single Phase (H-Nb2O5). Therm. Sci. Eng. Prog. 2021, 25, 101015. [Google Scholar] [CrossRef]
- Li, T.; Nam, G.; Liu, K.; Wang, J.-H.H.; Zhao, B.; Ding, Y.; Soule, L.; Avdeev, M.; Luo, Z.; Zhang, W.; et al. A Niobium Oxide with a Shear Structure and Planar Defects for High-Power Lithium Ion Batteries. Energy Environ. Sci. 2022, 15, 254–264. [Google Scholar] [CrossRef]
- Kominami, H.; Oki, K.; Kohno, M.; Onoue, S.; Kera, Y.; Ohtani, B. Novel Solvothermal Synthesis of Niobium (V) Oxide Powders and Their Photocatalytic Activity in Aqueous Suspensions. J. Mater. Chem. 2001, 11, 604–609. [Google Scholar] [CrossRef]
- Ziolek, M.; Sobczak, I.; Decyk, P.; Wolski, L. The Ability of Nb2O5 and Ta2O5 to Generate Active Oxygen in Contact with Hydrogen Peroxide. Catal. Commun. 2013, 37, 85–91. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Rytwo, G.; Zelkind, A.L. Evaluation of Kinetic Pseudo-Order in the Photocatalytic Degradation of Ofloxacin. Catalysts 2022, 12, 24. [Google Scholar] [CrossRef]
- Tran, H.D.; Nguyen, D.Q.; Do, P.T.; Tran, U.N.P. Kinetics of Photocatalytic Degradation of Organic Compounds: A Mini-Review and New Approach. RSC Adv. 2023, 13, 16915–16925. [Google Scholar] [CrossRef]
- Li, X.; Anwer, S.; Guan, Q.; Li, B.; Chan, V.; Palmisano, G.; Zheng, L. Surface Diffusion Induced Degradation Enhancement and Zero-Order Kinetics in Edge-Connected MoS2/Au/TiO2 Z-Scheme Photocatalytic System. Chem. Eng. Sci. 2024, 284, 119501. [Google Scholar] [CrossRef]
- Zhang, P.; Peng, C.; Li, H.; Huang, J.; Wang, Y.; Yu, Y.; Ding, S.; Liu, S.; Zhao, Y. Wavelength-Dependent Generation of Reactive Species in the Photodegradation Process over Pure and C-Doped Nb2O5. Sep. Purif. Technol. 2022, 286, 120406. [Google Scholar] [CrossRef]
- Patra, R.; Dash, P.; Panda, P.K.; Yang, P.C. A Breakthrough in Photocatalytic Wastewater Treatment: The Incredible Potential of g-C3N4/Titanate Perovskite-Based Nanocomposites. Nanomaterials 2023, 13, 2173. [Google Scholar] [CrossRef]
- Ücker, C.L.; Goetzke, V.; Almeida, S.R.; Moreira, E.C.; Ferrer, M.M.; Jardim, P.L.G.; Moreira, M.L.; Raubach, C.W.; Cava, S. Photocatalytic Degradation of Rhodamine B Using Nb2O5 Synthesized with Different Niobium Precursors: Factorial Design of Experiments. Ceram. Int. 2021, 47, 20570–20578. [Google Scholar] [CrossRef]
- Ücker, C.L.; Rodrigues, F.S.M.; Riemke, F.C.; Morisso, F.D.P.; Teodoro, M.D.; Mastelaro, V.R.; Ferrer, M.M.; Raubach, C.W.; da Silva Cava, S. Surface Modification of T-Nb2O5 with Low-Crystallinity Nb2O5 to Enhance Photocatalytic Degradation of Rhodamine B. Ceram. Int. 2023, 49, 34333–34338. [Google Scholar] [CrossRef]
- Hashemzadeh, F.; Rahimi, R.; Gaffarinejad, A.; Jalalat, V.; Safapour, S. Photocatalytic Treatment of Wastewater Containing Rhodamine B Dye via Nb2O5 Nanoparticles: Effect of Operational Key Parameters. Desalinat. Water Treat. 2015, 56, 181–193. [Google Scholar] [CrossRef]
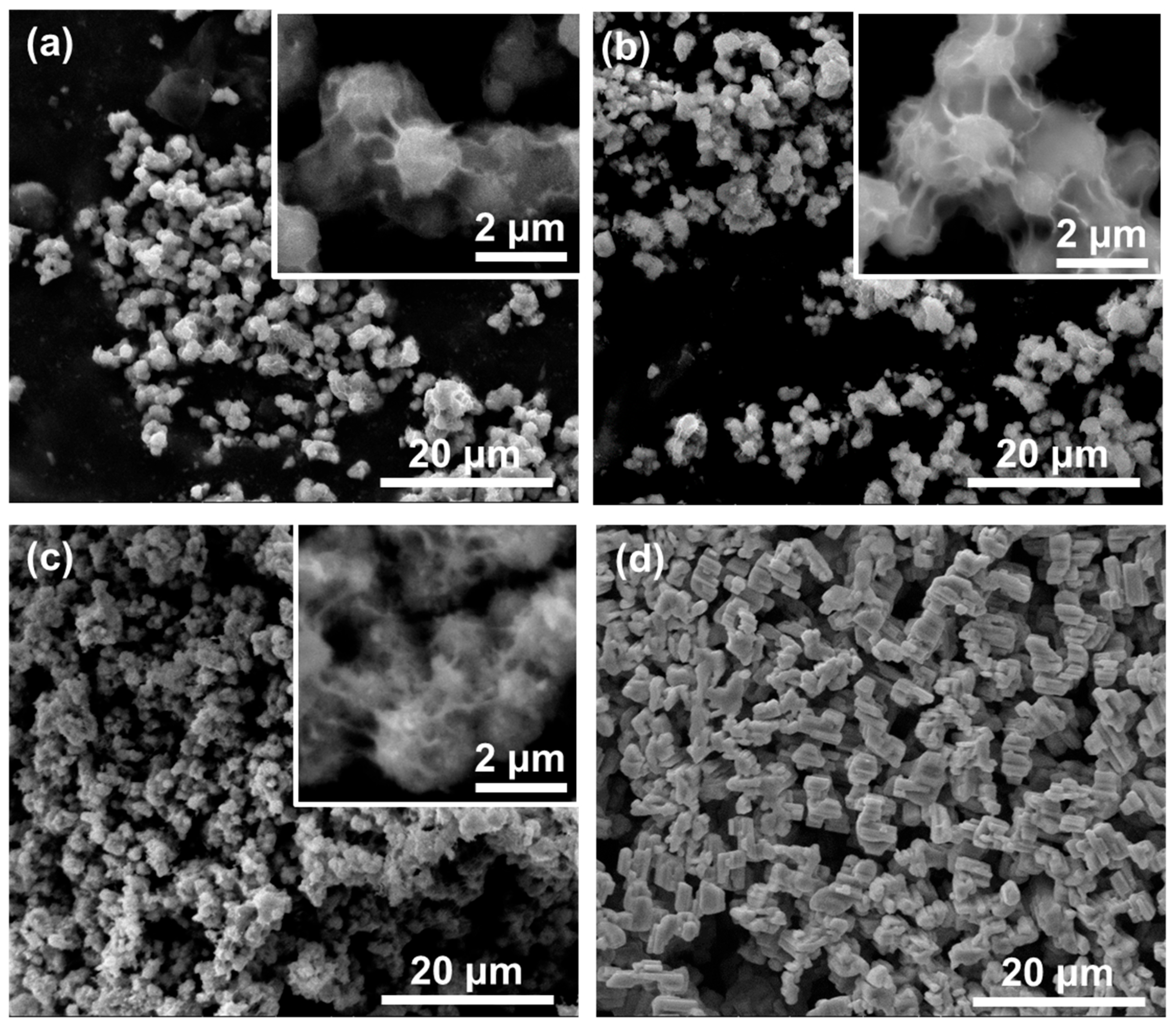
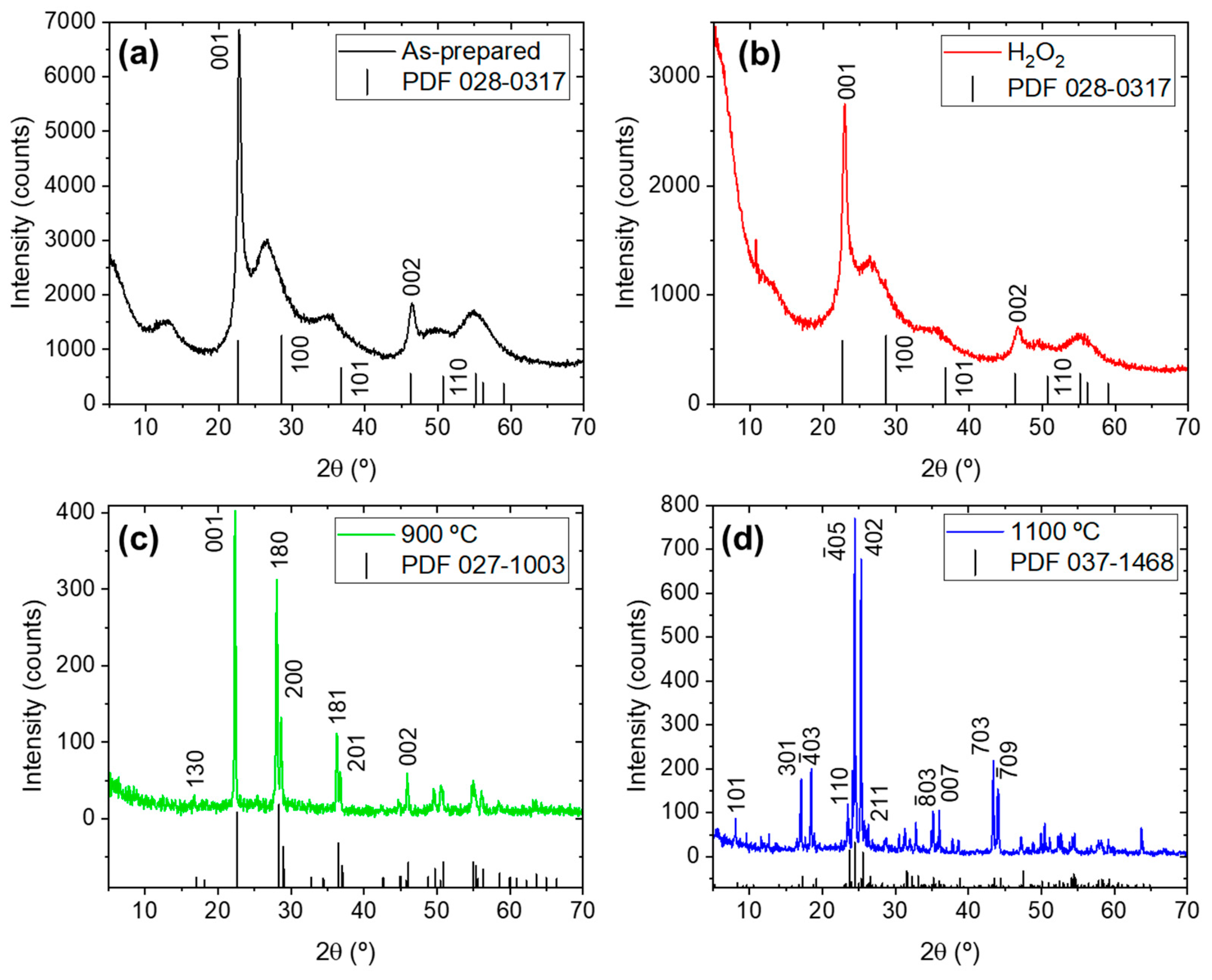
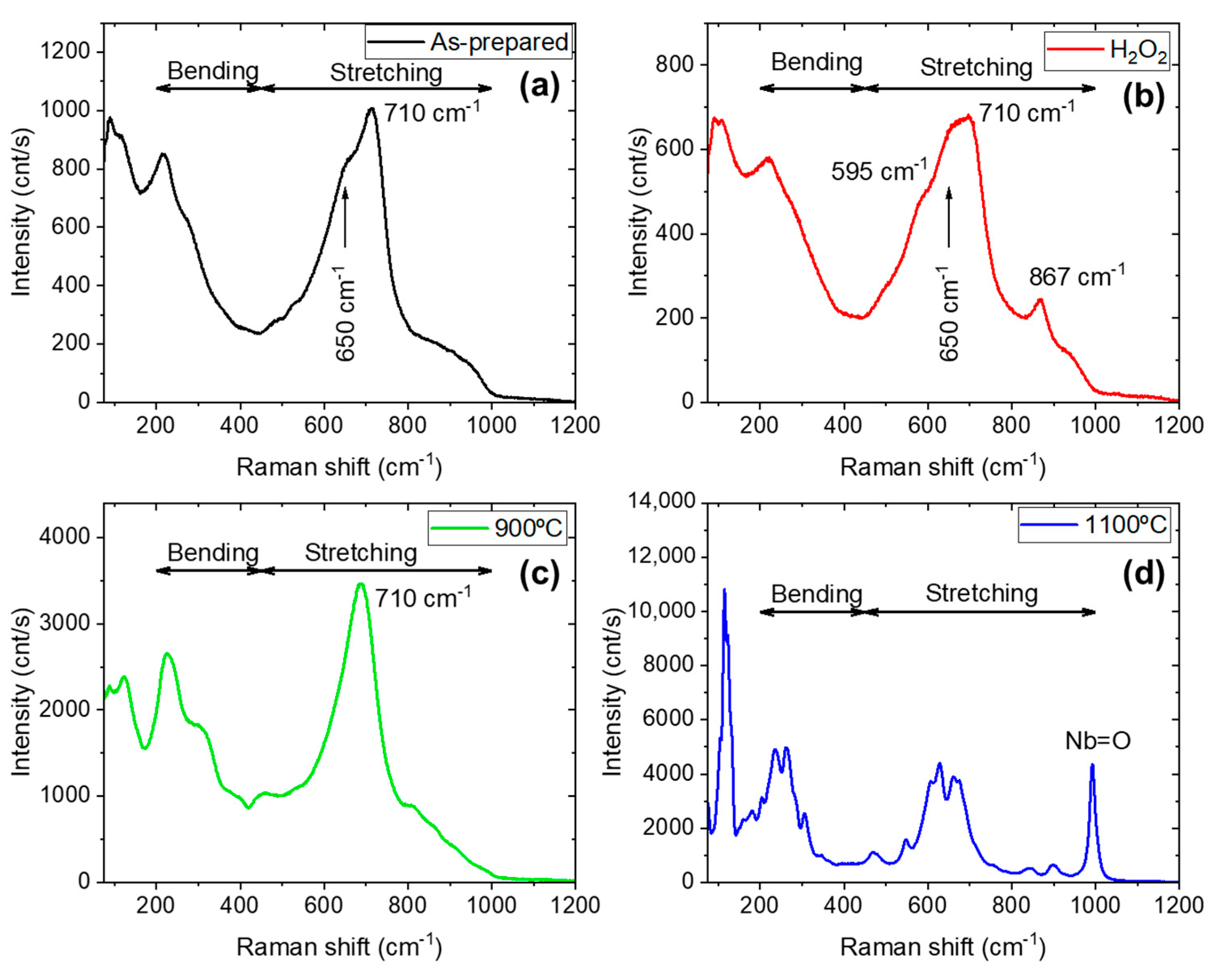
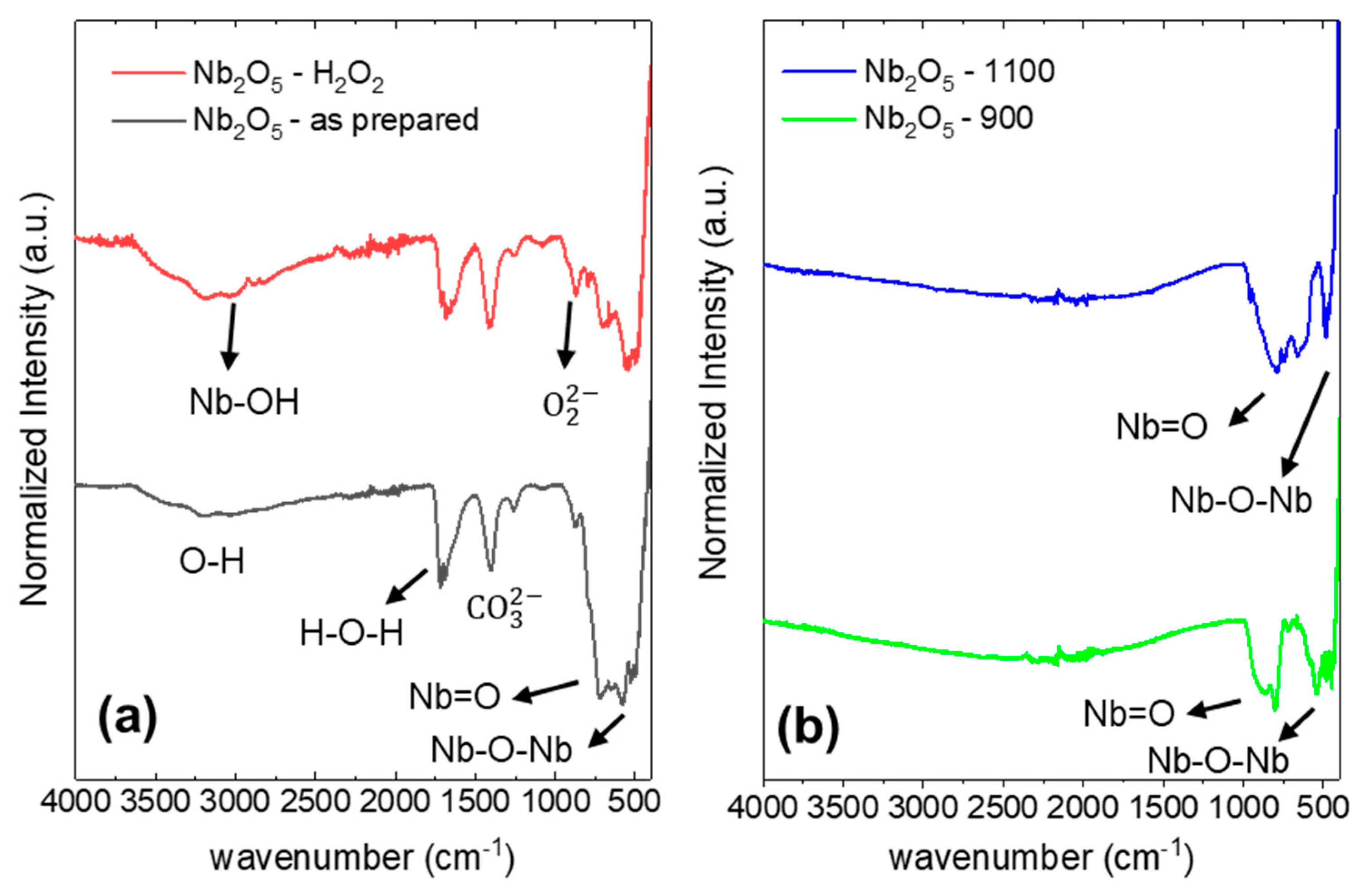
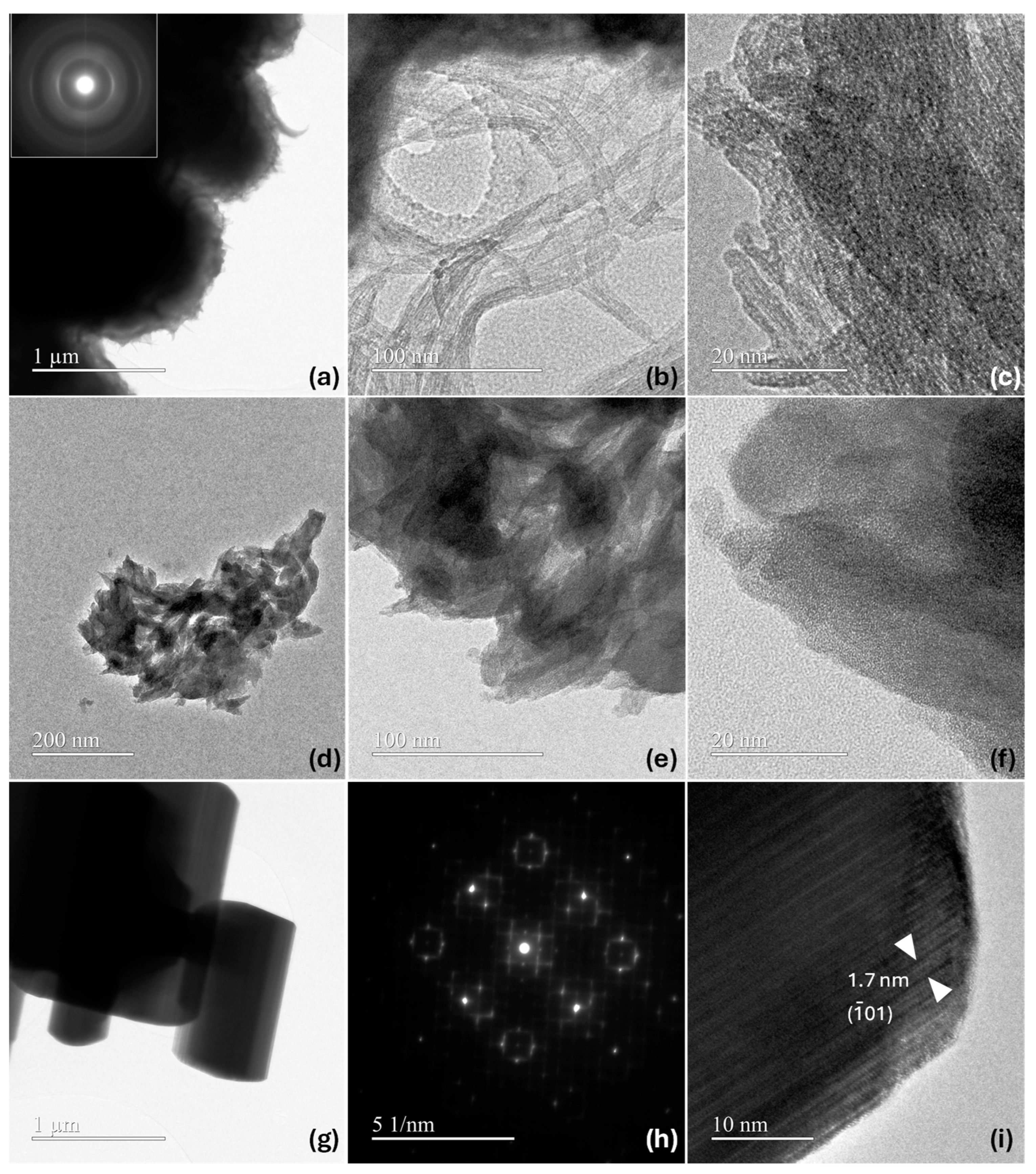
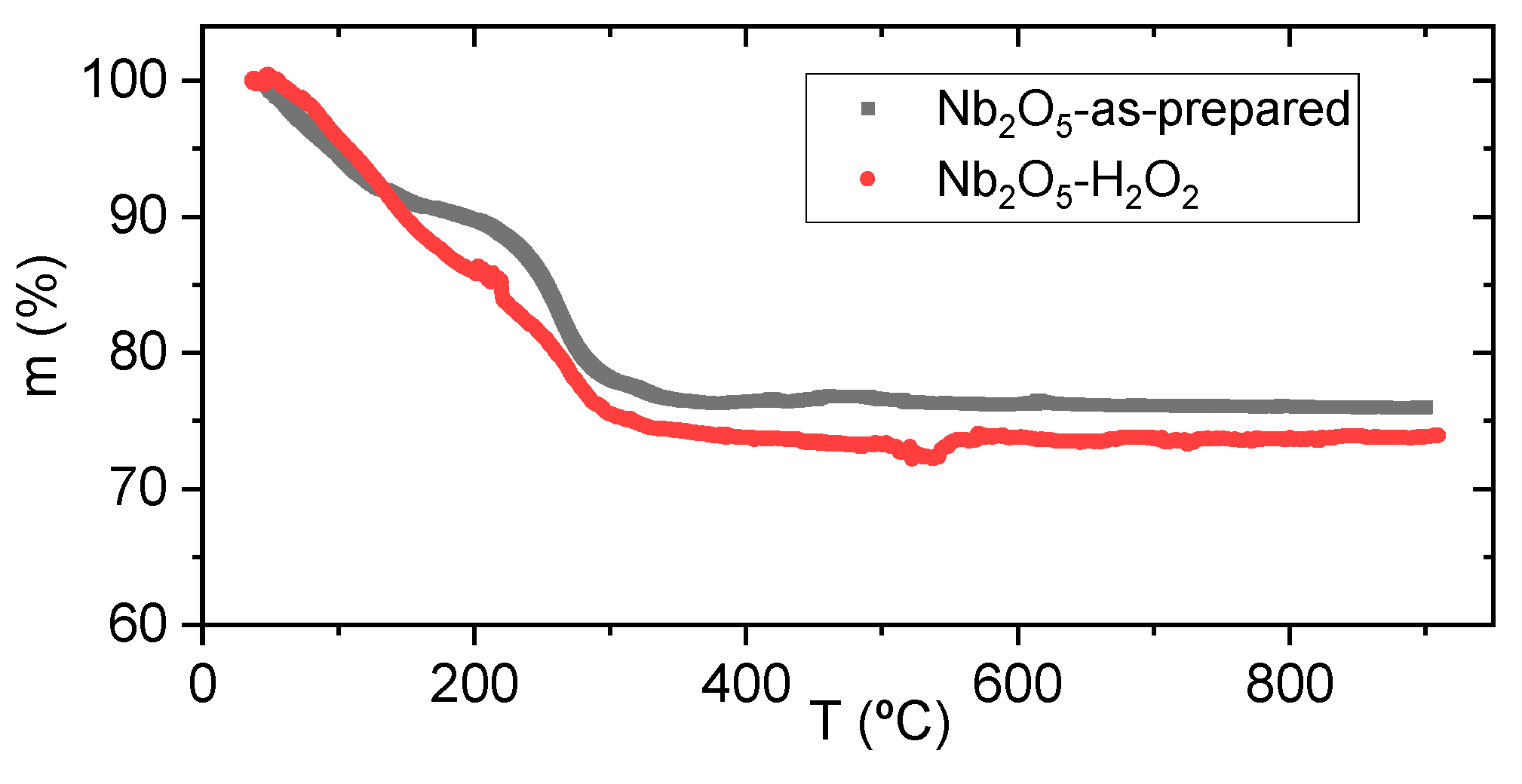
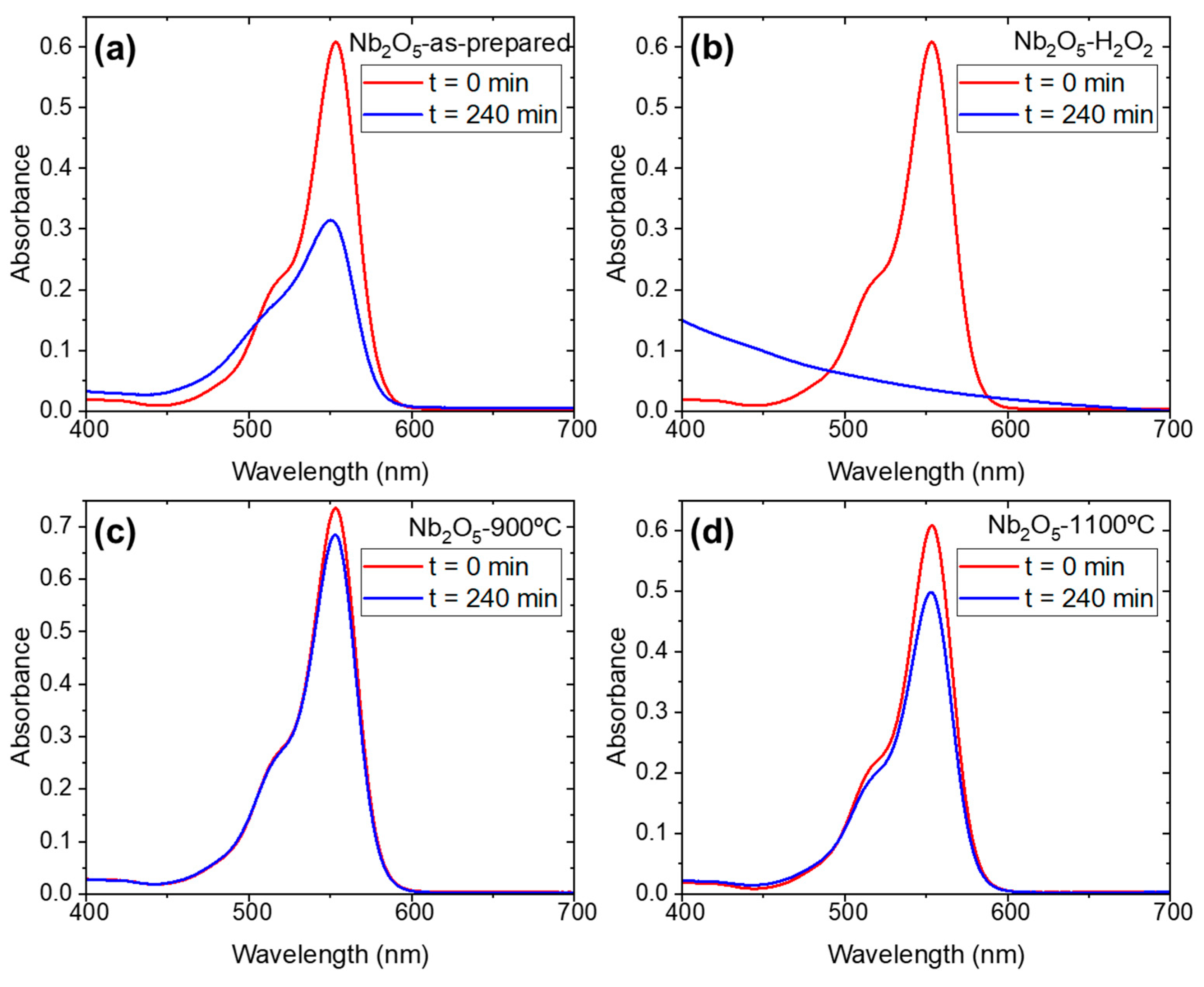
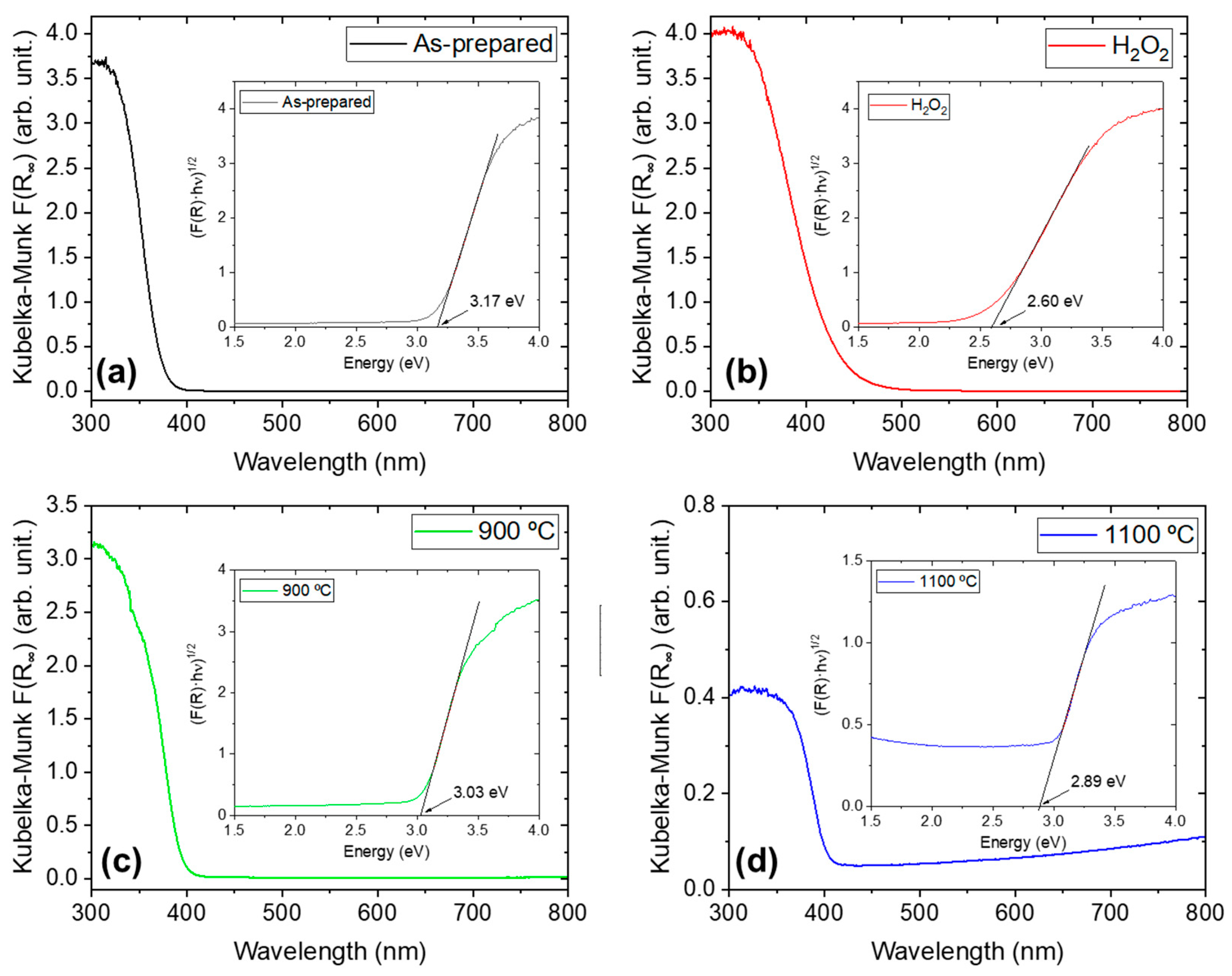
| Sample Name | Preparation |
|---|---|
| Nb2O5-as-prepared | As prepared white powders from solvothermal synthesis |
| Nb2O5-H2O2 | As prepared powders treated with H2O2 |
| Nb2O5-900 | As prepared powders thermal treated at 900 °C |
| Nb2O5-1100 | As prepared powders thermal treated at 1100 °C |
| Sample | Irradiation | Order | (min−1) | t1/2 (min) | tfinal (min) |
|---|---|---|---|---|---|
| Nb2O5-as-prepared | UV | 0 | 0.00219·C0 | 228.3 | - |
| Nb2O5-H2O2 | UV | 1 | 0.04838 | 14.3 | ~75 |
| Nb2O5-as-prepared | Vis | 0 | 0.00591·C0 | 84.6 | ~180 |
| Nb2O5-H2O2 | Vis | 1 | 0.0058 | 119.5 | - |
| Material | Illumination | Catalyst Dosage | RhB | Performance | Reference |
|---|---|---|---|---|---|
| Nb2O5 micro-coils of nanofibers in H2O2 | 365 nm + 405 nm 25 W × 2 | 0.4 g/L | 2.5 ppm | 100%, 75 min | This work |
| Nb2O5 micro-coils of nanofibers | 420–750 nm 35 W × 2 | 0.4 g/L | 2.5 ppm | 100%, 180 min | This work |
| Nb2O5 nanoparticles | 254 nm (UVC) 15 W × 6 | 1 g/L | 1 × 105 M | 99%, 60 min | [47] |
| Nb2O5 particles (T + low crystallinity) | 254 nm (UVC) 15 W × 5 | 1 g/L | 1 × 105 M | 100%, 60 min | [48] |
| Nb2O5 particles (low crystallinity) | 254 nm (UVC) 15 W × 5 | 1 g/L | 1 × 105 M | 100%, 90 min | [13] |
| T-Nb2O5 nanoparticles | UV Hg lamp 400 W | 0.5 g/L +H2O2 | 10 mg/L | 99%, 2 min | [49] |
| Nb2O5 nanorods | 365 nm 8 W | 1 g/L +H2O2 | 15 mg/L | 92.5%, 30 min | [21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvo-Villoslada, A.; Álvarez-Serrano, I.; López, M.L.; Fernández, P.; Sotillo, B. Phase-Dependent Photocatalytic Activity of Nb2O5 Nanomaterials for Rhodamine B Degradation: The Role of Surface Chemistry and Crystal Structure. Nanomaterials 2025, 15, 846. https://doi.org/10.3390/nano15110846
Calvo-Villoslada A, Álvarez-Serrano I, López ML, Fernández P, Sotillo B. Phase-Dependent Photocatalytic Activity of Nb2O5 Nanomaterials for Rhodamine B Degradation: The Role of Surface Chemistry and Crystal Structure. Nanomaterials. 2025; 15(11):846. https://doi.org/10.3390/nano15110846
Chicago/Turabian StyleCalvo-Villoslada, Aarón, Inmaculada Álvarez-Serrano, María Luisa López, Paloma Fernández, and Belén Sotillo. 2025. "Phase-Dependent Photocatalytic Activity of Nb2O5 Nanomaterials for Rhodamine B Degradation: The Role of Surface Chemistry and Crystal Structure" Nanomaterials 15, no. 11: 846. https://doi.org/10.3390/nano15110846
APA StyleCalvo-Villoslada, A., Álvarez-Serrano, I., López, M. L., Fernández, P., & Sotillo, B. (2025). Phase-Dependent Photocatalytic Activity of Nb2O5 Nanomaterials for Rhodamine B Degradation: The Role of Surface Chemistry and Crystal Structure. Nanomaterials, 15(11), 846. https://doi.org/10.3390/nano15110846







