β-Ga2O3 Thin Films via an Inorganic Sol–Gel Spin Coating: Preparation and Characterization
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Precursor Characterization
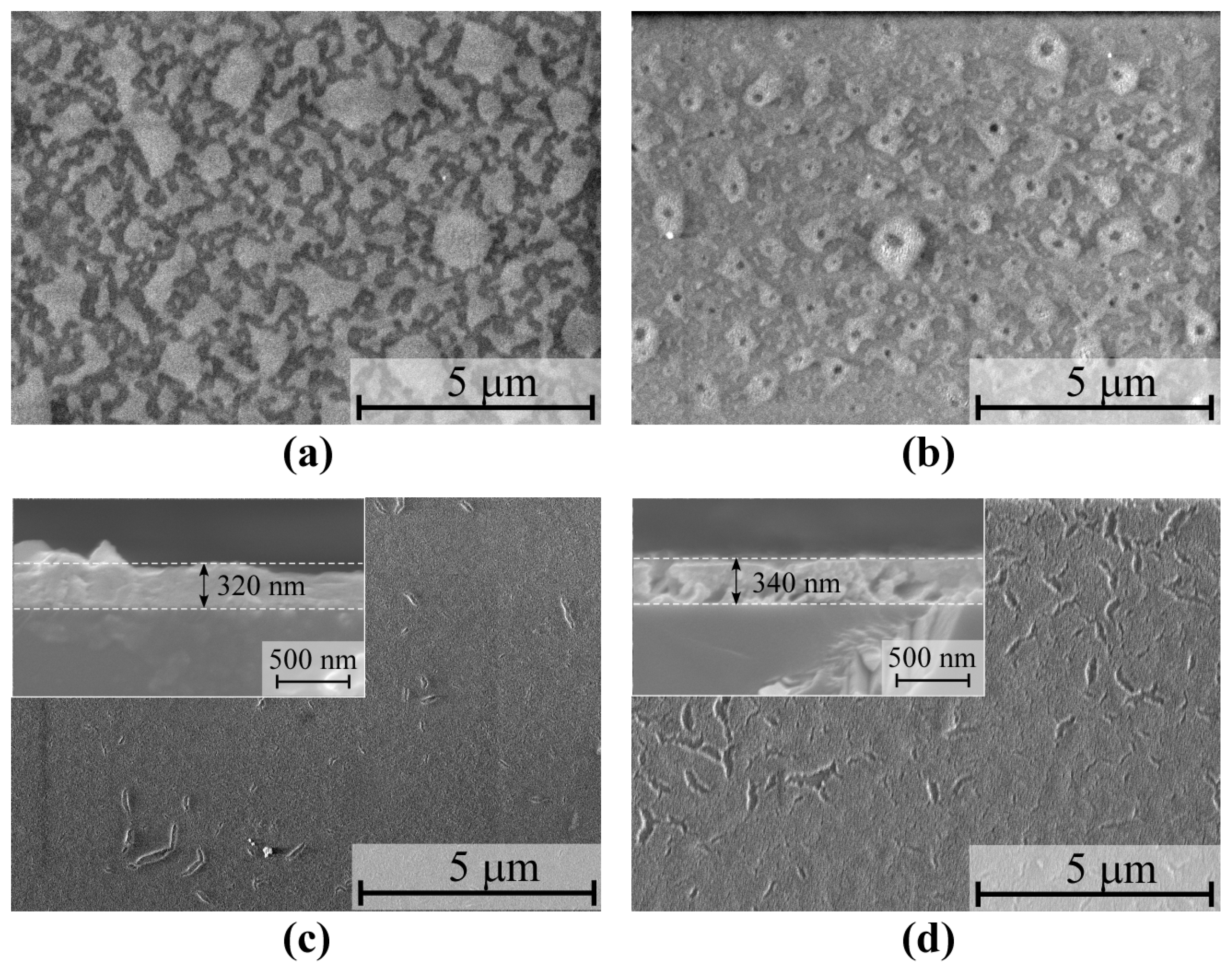
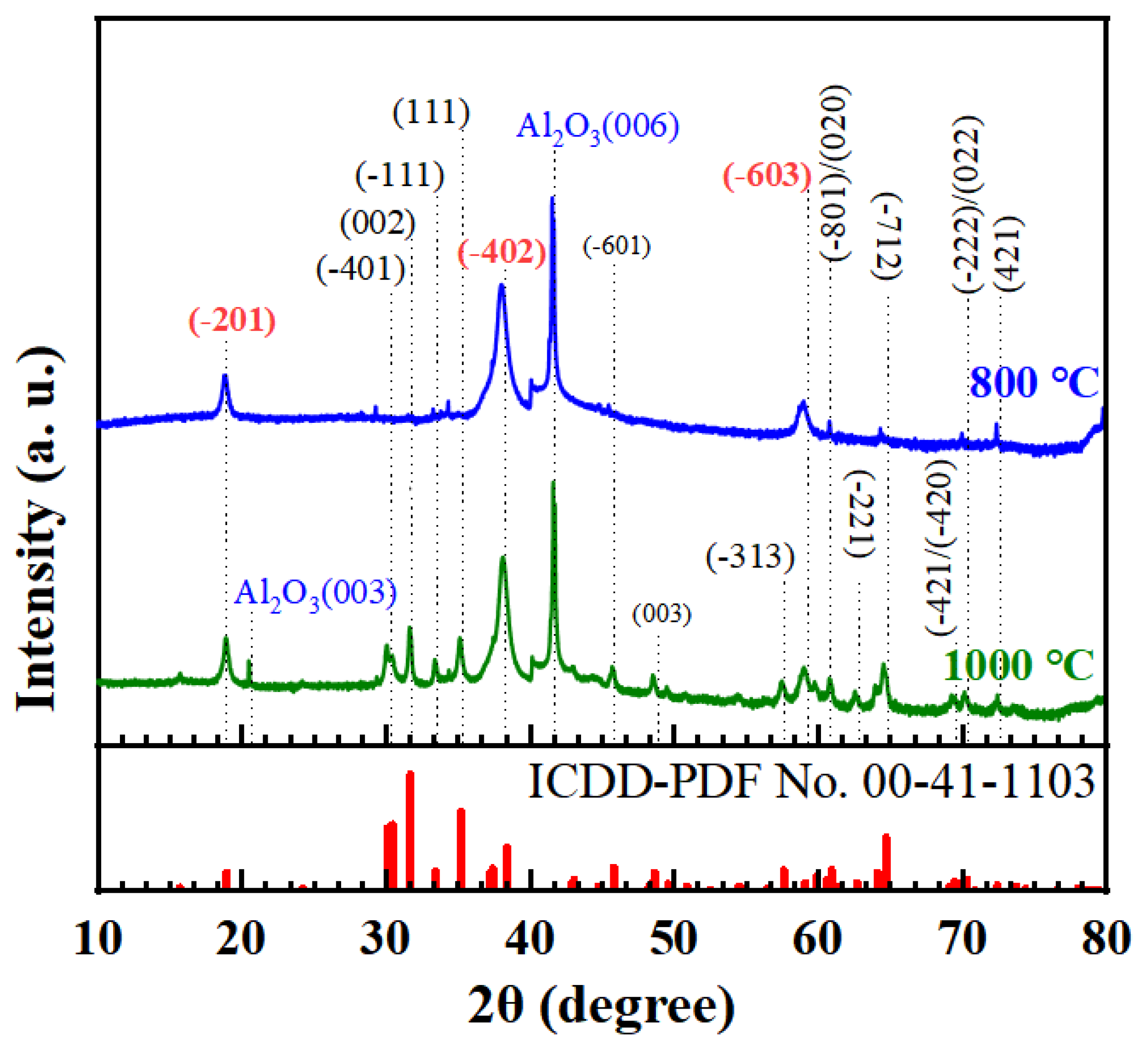
3.2. Analysis of the Surface Morphologies and Crystal Structure of the β-Ga2O3 Films on (0001) Sapphire
3.3. Transmittance and Bandgap Study Using Ultraviolet–Visible–Near-Infrared Spectroscopy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Higashiwaki, M.; Sasaki, K.; Murakami, H.; Kumagai, Y.; Koukitu, A.; Kuramata, A.; Masui, T.; Yamakoshi, S. Recent Progress in Ga2O3 Power Devices. Semicond. Sci. Technol. 2016, 31, 034001. [Google Scholar] [CrossRef]
- Higashiwaki, M.; Kaplar, R.; Pernot, J.; Zhao, H. Ultrawide Bandgap Semiconductors. Appl. Phys. Lett. 2021, 118, 200401. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, J.; Zhang, X.; Huang, Y.; Xu, X.; Xue, Y.; Zou, J.; Tang, C. Single-Crystalline Spherical β-Ga2O3 Particles: Synthesis, N-Doping and Photoluminescence Properties. J. Lumin. 2013, 140, 30–37. [Google Scholar] [CrossRef]
- Villora, E.G.; Shimamura, K.; Yoshikawa, Y.; Aoki, K.; Ichinose, N. Large-Size β-Ga2O3 Single Crystals and Wafers. J. Cryst. Growth 2004, 270, 420–426. [Google Scholar] [CrossRef]
- Chen, X.; Mu, W.; Xu, Y.; Fu, B.; Jia, Z.; Ren, F.F.; Gu, S.; Zhang, R.; Zheng, Y.; Tao, X.; et al. Highly Narrow-Band Polarization-Sensitive Solar-Blind Photodetectors Based on β-Ga2O3 Single Crystals. ACS Appl. Mater. Interfaces 2019, 11, 7131–7137. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.F.; Xia, C.; Sai, Q.; Cui, H.; Pan, M.; Qi, H. Growth and Fundamentals of Bulk β-Ga2O3 Single Crystals. J. Semicond. 2019, 40, 011801. [Google Scholar] [CrossRef]
- Feng, Z.; Anhar Uddin Bhuiyan, A.F.M.; Karim, M.R.; Zhao, H. MOCVD Homoepitaxy of Si-Doped (010) β-Ga2O3 Thin Films with Superior Transport Properties. Appl. Phys. Lett. 2019, 114, 250601. [Google Scholar] [CrossRef]
- Cheah, L.B.; Osman, R.A.M.; Poopalan, P. Ga2O3 Thin Films by Sol-Gel Method Its Optical Properties. AIP Conf. Proc. 2020, 2203, 020028. [Google Scholar]
- Shen, H.; Yin, Y.; Tian, K.; Baskaran, K.; Duan, L.; Zhao, X.; Tiwari, A. Growth and Characterization of β-Ga2O3 Thin Films by Sol-Gel Method for Fast-Response Solar-Blind Ultraviolet Photodetectors. J. Alloys Compd. 2018, 766, 601–608. [Google Scholar] [CrossRef]
- Rafique, S.; Han, L.; Tadjer, M.J.; Freitas, J.A.; Mahadik, N.A.; Zhao, H. Homoepitaxial Growth of β-Ga2O3 Thin Films by Low Pressure Chemical Vapor Deposition. Appl. Phys. Lett. 2016, 108, 182105. [Google Scholar] [CrossRef]
- Ghose, S.; Rahman, S.; Hong, L.; Rojas-Ramirez, J.S.; Jin, H.; Park, K.; Klie, R.; Droopad, R. Growth and Characterization of β-Ga2O3 Thin Films by Molecular Beam Epitaxy for Deep-UV Photodetectors. J. Appl. Phys. 2017, 122, 095302. [Google Scholar] [CrossRef]
- Feng, Z.; Karim, M.R.; Zhao, H. Low Pressure Chemical Vapor Deposition of β-Ga2O3 Thin Films: Dependence on Growth Parameters. APL Mater. 2019, 7, 022514. [Google Scholar] [CrossRef]
- Liu, L.L.; Li, M.K.; Yu, D.Q.; Zhang, J.; Zhang, H.; Qian, C.; Yang, Z. Fabrication and Characteristics of N-Doped β-Ga2O3 Nanowires. Appl. Phys. A Mater. Sci. Process. 2010, 98, 831–835. [Google Scholar] [CrossRef]
- Feng, P.; Zhang, J.Y.; Li, Q.H.; Wang, T.H. Individual β-Ga2O3 Nanowires as Solar-Blind Photodetectors. Appl. Phys. Lett. 2006, 88, 153107. [Google Scholar] [CrossRef]
- Li, X.; Liu, D.; Mo, X.; Li, K. Nanorod β-Ga2O3 Semiconductor Modified Activated Carbon as Catalyst for Improving Power Generation of Microbial Fuel Cell. J. Solid State Electrochem. 2019, 23, 2843–2852. [Google Scholar] [CrossRef]
- Kumar, S.; Dhara, S.; Agarwal, R.; Singh, R. Study of Photoconduction Properties of CVD Grown β-Ga2O3 Nanowires. J. Alloys Compd. 2016, 683, 143–148. [Google Scholar] [CrossRef]
- Gopal, R.; Goyal, A.; Saini, A.; Nagar, M.; Sharma, N.; Gupta, D.K.; Dhayal, V. Sol- Gel Synthesis of Ga2O3 Nanorods and Effect of Precursor Chemistry on Their Structural and Morphological Properties. Ceram. Int. 2018, 44, 19099–19105. [Google Scholar] [CrossRef]
- Girija, K.; Thirumalairajan, S.; Avadhani, G.S.; Mangalaraj, D.; Ponpandian, N.; Viswanathan, C. Synthesis, Morphology, Optical and Photocatalytic Performance of Nanostructured β-Ga2O3. Mater. Res. Bull. 2013, 48, 2296–2303. [Google Scholar] [CrossRef]
- Miao, Y.; Liang, B.; Tian, Y.; Xiong, T.; Sun, S.; Chen, C. Epitaxial Growth of β-Ga2O3 Nanowires from Horizontal to Obliquely Upward Evolution. Vacuum 2021, 192, 110444. [Google Scholar] [CrossRef]
- Alema, F.; Zhang, Y.; Osinsky, A.; Orishchin, N.; Valente, N.; Mauze, A.; Speck, J.S. Low 1014 cm−3 Free Carrier Concentration in Epitaxial β-Ga2O3 Grown by MOCVD. APL Mater. 2020, 8, 021110. [Google Scholar] [CrossRef]
- Feng, Z.; Bhuiyan, A.F.M.A.U.; Kalarickal, N.K.; Rajan, S.; Zhao, H. Mg Acceptor Doping in MOCVD (010) β-Ga2O3. Appl. Phys. Lett. 2020, 117, 222106. [Google Scholar] [CrossRef]
- Nandi, A.; Cherns, D.; Sanyal, I.; Kuball, M. Epitaxial Growth of (−201) β-Ga2O3 on (001) Diamond Substrates. Cryst. Growth Des. 2023, 23, 8290–8295. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xia, X.; Liang, H.; Abbas, Q.; Liu, Y.; Du, G. Growth Pressure Controlled Nucleation Epitaxy of Pure Phase ϵ- And β-Ga2O3 Films on Al2O3 via Metal-Organic Chemical Vapor Deposition. Cryst. Growth Des. 2018, 18, 1147–1154. [Google Scholar] [CrossRef]
- Horng, R.H.; Wuu, D.S.; Liu, P.L.; Sood, A.; Tarntair, F.G.; Chen, Y.H.; Pratap, S.J.; Hsiao, C.L. Growth Mechanism and Characteristics of β-Ga2O3 Heteroepitaxailly Grown on Sapphire by Metalorganic Chemical Vapor Deposition. Mater. Today Adv. 2022, 16, 100320. [Google Scholar] [CrossRef]
- Pozina, G.; Hsu, C.W.; Abrikossova, N.; Kaliteevski, M.A.; Hemmingsson, C. Development of β-Ga2O3 Layers Growth on Sapphire Substrates Employing Modeling of Precursors Ratio in Halide Vapor Phase Epitaxy Reactor. Sci. Rep. 2020, 10, 22261. [Google Scholar] [CrossRef]
- Son, H.; Choi, Y.J.; Hong, S.K.; Park, J.H.; Jeon, D.W. Reduction of Dislocations in α-Ga2O3 Epilayers Grown by Halide Vapor-Phase Epitaxy on a Conical Frustum-Patterned Sapphire Substrate. IUCrJ 2021, 8, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Nakahata, H.; Murakami, H.; Kumagai, Y. Temperature Dependence of Ga2O3 Growth by Halide Vapor Phase Epitaxy on Sapphire and β-Ga2O3 Substrates. Appl. Phys. Lett. 2020, 117, 222101. [Google Scholar] [CrossRef]
- Mazzolini, P.; Falkenstein, A.; Galazka, Z.; Martin, M.; Bierwagen, O. Offcut-Related Step-Flow and Growth Rate Enhancement during (100) β-Ga2O3homoepitaxy by Metal-Exchange Catalyzed Molecular Beam Epitaxy (MEXCAT-MBE). Appl. Phys. Lett. 2020, 117, 222105. [Google Scholar] [CrossRef]
- Itoh, T.; Mauze, A.; Zhang, Y.; Spe, J.S. Epitaxial Growth of β-Ga2O3 on (110) Substrate by Plasma-Assisted Molecular Beam Epitaxy. Appl. Phys. Lett. 2020, 117, 152105. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Z.; Karim, M.R.; Zhao, H. High-Temperature Low-Pressure Chemical Vapor Deposition of β-Ga2O3. J. Vac. Sci. Technol. A Vac. Surf. Film. 2020, 38, 050806. [Google Scholar] [CrossRef]
- Jiao, Y.; Jiang, Q.; Meng, J.; Zhao, J.; Yin, Z.; Gao, H.; Zhang, J.; Deng, J.; Zhang, X. Growth and Characteristics of β-Ga2O3 Thin Films on Sapphire (0001) by Low Pressure Chemical Vapour Deposition. Vacuum 2021, 189, 110253. [Google Scholar] [CrossRef]
- Rafique, S.; Han, L.; Zhao, H. Synthesis of Wide Bandgap Ga2O3 (Eg ∼ 4.6-4.7 eV) Thin Films on Sapphire by Low Pressure Chemical Vapor Deposition. Phys. Status Solidi Appl. Mater. Sci. 2016, 213, 1002–1009. [Google Scholar] [CrossRef]
- Joshi, G.; Chauhan, Y.S.; Verma, A. Temperature Dependence of β-Ga2O3 Heteroepitaxy on c-Plane Sapphire using Low Pressure Chemical Vapor Deposition. J. Alloys Compd. 2021, 883, 160799. [Google Scholar] [CrossRef]
- An, Y.; Dai, L.; Wu, Y.; Wu, B.; Zhao, Y.; Liu, T.; Hao, H.; Li, Z.; Niu, G.; Zhang, J.; et al. Epitaxial Growth of β-Ga2O3 Thin Films on Ga2O3 and Al2O3 Substrates by Using Pulsed Laser Deposition. J. Adv. Dielectr. 2019, 9, 1950032. [Google Scholar] [CrossRef]
- Lu, Y.M.; Li, C.; Chen, X.H.; Han, S.; Cao, P.J.; Jia, F.; Zeng, Y.X.; Liu, X.K.; Xu, W.Y.; Liu, W.J.; et al. Preparation of Ga2O3 Thin Film Solar-Blind Photodetectors Based on Mixed-Phase Structure by Pulsed Laser Deposition. Chin. Phys. B 2019, 28, 018504. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, J.; Li, Q.; Qu, C.; Zhang, L.; Li, T. Structural and Optical Properties of N-Doped β-Ga2O3 Films Deposited by RF Magnetron Sputtering. Phys. B Condens. Matter 2011, 406, 3079–3082. [Google Scholar] [CrossRef]
- Akazawa, H. Formation of Various Phases of Gallium Oxide Films Depending on Substrate Planes and Deposition Gases. Vacuum 2016, 123, 8–16. [Google Scholar] [CrossRef]
- Kobayashi, E.; Boccard, M.; Jeangros, Q.; Rodkey, N.; Vresilovic, D.; Hessler-Wyser, A.; Döbeli, M.; Franta, D.; De Wolf, S.; Morales-Masis, M.; et al. Amorphous Gallium Oxide Grown by Low-Temperature PECVD. J. Vac. Sci. Technol. A Vacuum, Surfaces, Film. 2018, 36, 021518. [Google Scholar] [CrossRef]
- Hu, H.; Wu, C.; Zhao, N.; Zhu, Z.; Li, P.; Wang, S.; Tang, W.; Guo, D. Epitaxial Growth and Solar-Blind Photoelectric Characteristic of Ga2O3 Film on Various Oriented Sapphire Substrates by Plasma-Enhanced Chemical Vapor Deposition. Phys. Status Solidi Appl. Mater. Sci. 2021, 218, 2100076. [Google Scholar] [CrossRef]
- Zhu, Y.; Xiu, X.; Cheng, F.; Li, Y.; Xie, Z.; Tao, T.; Chen, P.; Liu, B.; Zhang, R.; Zheng, Y.D. Growth and Nitridation of β-Ga2O3 Thin Films by Sol-Gel Spin-Coating Epitaxy with Post-Annealing Process. J. Sol-Gel Sci. Technol. 2021, 100, 183–191. [Google Scholar] [CrossRef]
- Kokubun, Y.; Miura, K.; Endo, F.; Nakagomi, S. Sol-Gel Prepared β-Ga2O3 Thin Films for Ultraviolet Photodetectors. Appl. Phys. Lett. 2007, 90, 031912. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Y.; Xiu, X.; Sun, X.; Xie, Z.; Tao, T.; Chen, P.; Liu, B.; Ye, J.; Zhang, R.; et al. Preparation of β-Ga2O3films on off-Angled Sapphire Substrates and Solar-Blind Ultraviolet Photodetectors. J. Phys. D Appl. Phys. 2022, 55, 424001. [Google Scholar] [CrossRef]
- Wheeler, V.D.; Nepal, N.; Boris, D.R.; Qadri, S.B.; Nyakiti, L.O.; Lang, A.; Koehler, A.; Foster, G.; Walton, S.G.; Eddy, C.R.; et al. Phase Control of Crystalline Ga2O3 Films by Plasma-Enhanced Atomic Layer Deposition. Chem. Mater. 2020, 32, 1140–1152. [Google Scholar] [CrossRef]
- Alves Machado Filho, M.; Hsiao, C.-L.; dos Santos, R.B.; Hultman, L.; Birch, J.; Gueorguiev, G.K. Self-Induced Core–Shell InAlN Nanorods: Formation and Stability Unraveled by Ab Initio Simulations. ACS Nanosci. Au 2023, 3, 84–93. [Google Scholar] [CrossRef]
- Filho, M.A.M.; Farmer, W.; Hsiao, C.-L.; dos Santos, R.B.; Hultman, L.; Birch, J.; Ankit, K.; Gueorguiev, G.K. Density Functional Theory-Fed Phase Field Model for Semiconductor Nanostructures: The Case of Self-Induced Core–Shell InAlN Nanorods. Cryst. Growth Des. 2024, 24, 4717–4727. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi 1966, 15, 627–637. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Niu, D.; Yang, J.; Zhang, X.; Zhu, J.; Li, W. β-Ga2O3 Thin Films via an Inorganic Sol–Gel Spin Coating: Preparation and Characterization. Nanomaterials 2025, 15, 277. https://doi.org/10.3390/nano15040277
Zhang H, Niu D, Yang J, Zhang X, Zhu J, Li W. β-Ga2O3 Thin Films via an Inorganic Sol–Gel Spin Coating: Preparation and Characterization. Nanomaterials. 2025; 15(4):277. https://doi.org/10.3390/nano15040277
Chicago/Turabian StyleZhang, Hai, Dingyuan Niu, Junbiao Yang, Xiaoyang Zhang, Jun Zhu, and Wencai Li. 2025. "β-Ga2O3 Thin Films via an Inorganic Sol–Gel Spin Coating: Preparation and Characterization" Nanomaterials 15, no. 4: 277. https://doi.org/10.3390/nano15040277
APA StyleZhang, H., Niu, D., Yang, J., Zhang, X., Zhu, J., & Li, W. (2025). β-Ga2O3 Thin Films via an Inorganic Sol–Gel Spin Coating: Preparation and Characterization. Nanomaterials, 15(4), 277. https://doi.org/10.3390/nano15040277





