Abstract
Two-dimensional direct Z-scheme photocatalysts have emerged as highly promising photocatalysts for solar-driven water splitting owing to their effective separation of photogenerated carriers and strong redox abilities. This study focuses on the theoretical prediction of promising Z-scheme photocatalysts for solar-driven water splitting based on M2X/BiOY (M = Ag, Au; X = S, Se; Y = Cl, Br, I) heterojunctions using first-principles calculations. All M2X/BiOY heterojunctions possess staggered band alignments, Z-scheme carrier migration, and suitable band edges for overall water splitting. Optical absorption spectra indicate that these heterojunctions exhibit significantly extended solar absorption in the visible and near-infrared regions. Moreover, the interfacial built-in electric fields of (0.46–0.72 V/Å) point from M2X to BiOY, promote photogenerated carrier separation, and enhance redox overpotentials, thereby improving photocatalytic performance. These results suggest that M2X/BiOY heterojunctions are promising Z-scheme photocatalysts for solar-driven water splitting and are expected to be experimentally prepared and realized in the near future.
1. Introduction
Solar-driven photocatalytic water splitting for hydrogen production is a sustainable way to meet critical energy needs without harmful emissions [1,2,3]. Two-dimensional semiconductors have been widely investigated as photocatalysts for solar-driven water splitting due to their large specific surface areas, abundant active sites, and short carrier migration distances [4,5,6]. However, most monolayer photocatalysts face severe problems with photogenerated electron–hole recombination and mutual contradiction between broad optical absorption and strong redox capability [7,8,9,10,11,12,13]. To address these limitations, inspired by the natural photosynthesis of green plants, researchers proposed a direct Z-scheme photocatalytic system to overcome these drawbacks [14,15,16,17,18,19,20,21]. In the direct Z-scheme photocatalysts, electrons and holes are spatially separated with higher redox potentials, while solar absorptions extend significantly into the visible and near-infrared regions, ultimately enhancing the photocatalytic performance [22,23,24,25,26,27,28]. Therefore, constructing direct Z-scheme heterojunctions is a feasible strategy to improve the solar-driven photocatalytic water-splitting performance significantly.
Recently, 2D semiconducting group-11 chalcogenides (M2X; M = Cu, Ag, Au; X = S, Se, Te) with high carrier mobilities and wide bandgap ranges have been experimentally and theoretically reported [29,30,31,32,33,34]. Their band edges are suitable for half or overall redox reaction, indicating that they are promising to form direct Z-scheme photocatalysts for high-performance photocatalytic water splitting. Meanwhile, 2D bismuth oxyhalides (BiOY; Y = Cl, Br, I) are also widely investigated photocatalysts for water splitting [35,36,37,38,39]. Inspired by the potential applications of 2D group-11 chalcogenides and bismuth oxyhalides, further investigations in M2X/BiOY (M = Ag, Au; X = S, Se; Y = Cl, Br, I) heterojunctions via first-principles calculations are highly desired to provide references for experimental researchers to search for efficient solar-driven water-splitting photocatalysts more effectively.
In this study, we systematically investigate the photocatalytic properties of M2X/BiOY (M = Ag, Au; X = S, Se; Y = Cl, Br, I) heterojunctions via first-principles calculations. The results demonstrate that all M2X/BiOY heterojunctions exhibit staggered band alignments, which are favorable for the spatial separation of photogenerated carriers. An intrinsic electric field, Ein is present at the interfaces, directed from M2X towards BiOY, with field strengths ranging from 0.46 V/Å to 0.72 V/Å. On the driving force of the Ein, the carrier migration mechanisms of M2X/BiOY heterojunctions are all direct Z-schemes, which are beneficial for achieving high performance for overall water splitting owing to the effective separation of photogenerated carriers and the strong redox abilities. Furthermore, the optical absorbances of M2X/BiOY heterojunctions are enhanced in comparison to those of their isolated monolayers. And the first absorption peaks of Au2Se/BiOCl, Au2Se/BiOBr, and Au2Se/BiOI are in the infrared range of 1.31 eV, 1.40 eV, and 1.48 eV, respectively. The enhanced optical absorbances of M2X/BiOY heterojunctions further resulted in their better photocatalytic performances.
2. Materials and Methods
All calculations in this paper were based on the density functional theory (DFT) method in the Vienna ab initio simulation package (VASP) program package [40,41,42,43], version 5.4.4. The exchange-correlation potential was employed by the Perdew–Burke–Ernzerhof (PBE) of the Generalized Gradient Approximation (GGA) [44]. The cut-off energy for the plane-wave basis set was 600 eV. The conjugate gradient scheme was used for geometry optimizations, and the convergence criteria for the energy and ionic Hellmann-Feynman force were set to 10−5 eV/atom and 0.01 eV/Å, respectively. In all calculations, a vacuum layer of at least 15 Å along the z-direction was used to eliminate interlayer interactions. As a better description of the weak van der Waals forces between layered 2D materials, the DFT-D3 method with Becke–Johnson damping has been adopted [45,46,47]. The first Brillouin zone was sampled using a Γ-centered 6 × 6 × 1 grid for the geometrical optimization and calculations of the electronic properties. In addition, the Heyd–Scuseria–Ernzerhof (HSE06) [48] was adopted to compute the electronic and optical properties due to the fact that the PBE functional will underestimate the bandgap in semiconductors.
3. Results
The atomic and electronic structures of monolayer M2X (M = Ag, Au; X = S, Se) and BiOY (Y = Cl, Br, I) were first investigated, as illustrated in Figure 1. The unit cells of M2X and BiOY are both squares with the same space group of P4/nmm. The optimized lattice constants of Ag2S, Ag2Se, Au2Se, BiOCl, BiOBr, and BiOI are 5.84, 5.90, 5.82, 3.96, 3.98, and 4.03 Å, respectively. However, the PBE method usually overestimates the lattice constant [49,50,51], resulting in a deviation from the experimentally reported values. The band structures of each monolayer were calculated based on the HSE06 hybridization method. The results demonstrate that Ag2S, Ag2Se, and Au2Se are all direct bandgap semiconductors with valence band maximums (VBMs) and conduction band minimums (CBMs) at the Γ point. Their bandgaps are 2.59, 2.62, and 1.61 eV, respectively. BiOCl, BiOBr, and BiOI are indirect bandgap semiconductors with bandgaps of 3.76, 3.38, and 2.35 eV, respectively. Compared with previously reported bulk BiOX [52], the calculated band gap values in this study show certain differences due to quantum confinement effects [53], as illustrated in Table S1. Their VBMs and CBMs are located at the X-Γ line and Γ point, respectively.
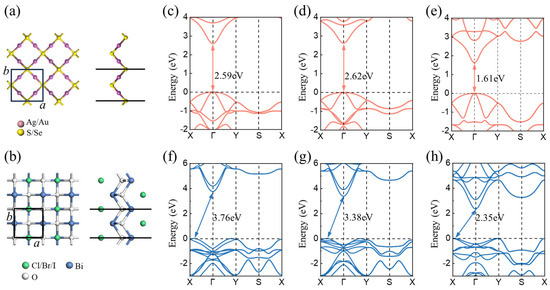
Figure 1.
Top and side views of monolayer (a) M2X (M = Ag, Au; X = S, Se) and (b) BiOY (Y = Cl, Br, I), respectively. The black squares represent the unit cells. Band structures of monolayer (c) Ag2S, (d) Ag2Se, (e) Au2Se, (f) BiOCl, (g) BiOBr, and (h) BiOI, respectively.
Due to the large differences in lattice constants between M2X and BiOY, M2X/BiOY heterojunctions were constructed by stacking 1 × 1 M2X on √2 × √2 BiOY to achieve a better lattice match, since strain induced by lattice mismatch will affect the stabilities and charge transfer dynamics of M2X/BiOY heterojunctions. Considering the stacking pattern has a tremendous influence on the stabilities and charge transfer dynamics of M2X/BiOY heterojunctions, the total energies, charge density differences, and projected band structures of Ag2S/BiOI with different stacking patterns (AA, AB, and AC) are compared in Figure 2, Figures S1 and S2. All Ag2S/BiOI with different stacking patterns are staggered band alignments. The charge density difference of the energetically favorable AC stacking is stronger than those of AA and AB stackings, indicating that the energetically favorable AC stacking is the most conducive to interlayer charge transfer in Ag2S/BiOI. Additionally, the lowest energies for Ag2Se/BiOI and Au2Se/BiOI are also observed in AC stacking. In the following, the AC stacking is used for all M2X/BiOY heterojunctions to further explore their structural, electronic, optical, and photocatalytic properties.
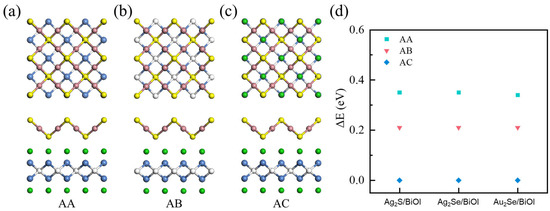
Figure 2.
Top and side views of Ag2S/BiOI heterojunctions with (a) AA, (b) AB, and (c) AC stackings, respectively. (d) Relative energies of AA, AB, and AC stackings for Ag2S/BiOI, Ag2Se/BiOI, and Au2Se/BiOI heterojunctions.
The structural stabilities of M2X/BiOY heterojunctions are judged by the binding energy (), whose calculation formula is as follows:
where , , and are the total energies of the heterostructures, isolated M2X, and BiOY monolayers, respectively. The Eb of M2X/BiOY heterojunctions ranges from −2.592 to −3.646 eV (see Table S2), whose negative values indicate these stable contacts are easy to form. Meanwhile, taking Ag2S/BiOI as an example, its thermodynamic stability is further evaluated by AIMD simulation and phonon dispersion calculation, as shown in Figure S3. A 3 × 3 × 1 supercell with 162 atoms is built to ensure the accuracy of the simulation. After 2 ps simulation at 300 K, the structure of Ag2S/BiOI is not significantly damaged. The integrity of the original structure during time evolution confirms its excellent thermal stability. Furthermore, the absence of any significant imaginary phonon modes indicates its dynamic stability. Since the non-layer bulk phases of M2X, it is suggested that they can be synthesized with molecular beam epitaxy (MBE) or chemical vapor deposition (CVD) methods, which have been extensively utilized for exploration of new 2D materials and heterostructures [54,55]. Considering the rapid development of experimental techniques for fabricating 2D materials and heterostructures in recent years, we are optimistic that these M2X/BiOY heterojunctions can be fabricated experimentally in the near future.
The electronic structures and band alignments of heterojunctions are important for their applications. In order to have a comprehensive understanding of the electronic properties of M2X/BiOY heterojunctions, we calculated the projected band structures of each heterojunction based on the HSE06 method, as shown in Figure 3a and Figure S4. All heterojunctions possess direct bandgaps with the CBMs and VBMs located at the Γ point. We also calculated the partial charge densities of these heterojunctions, as shown in Figure 3b and Figure S5. The CBMs and VBMs of M2X/BiOY heterojunctions are mainly contributed by BiOY and M2X, respectively, which suggests that M2X/BiOY heterojunctions are staggered band alignments. In these heterojunctions, electrons and holes are separated into different layers. Band edges of M2X/BiOY heterojunctions with VBMs set to 0 are summarized in Figure 3c. These M2X/BiOY heterojunctions are all staggered band alignments, which is beneficial to hinder photogenerated carrier recombination.
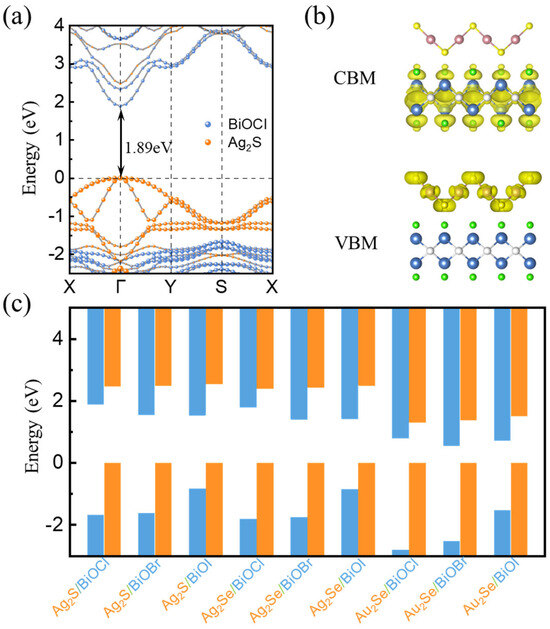
Figure 3.
(a) Projected band structure and (b) partial charge density of the Ag2S/BiOCl heterojunction, respectively. The yellow region represents the spatial distribution of the electronic states corresponding to the conduction band minimum (CBM) and valence band maximum (VBM). (c) Band edges of M2X/BiOY heterojunctions with VBMs set to 0.
When M2X and BiOY come into contact, internal built-in electric fields inevitably form. It has been well established that the interface electric field in staggered-band-alignment heterojunction plays a crucial role in facilitating the separation of photogenerated charge carriers in photocatalysts [56,57]. This interfacial electric field arises from charge redistribution at the interface, resulting from interlayer interactions. To investigate interfacial electric fields at the M2X/BiOY interface, the charge density difference along the z direction was calculated using the following formula:
where , , and represent the charge density of M2X/BiOY, isolated monolayer M2X, and BiOY, respectively. The planar average charge density difference of M2X/BiOY heterojunctions is presented in Figure 4a and Figure S6. The corresponding three-dimensional schematics of the charge density difference depict charge accumulation and depletion regions, marked in yellow and cyan, respectively. Notably, these results demonstrate significant charge transfer between the layers of the heterojunction. Taking Au2Se/BiOCl as an example, the electrons accumulate in BiOCl and are depleted in Au2Se. This charge redistribution results in the formation of an interlayer electric field directed from Au2Se to BiOCl. Similarly, other heterojunctions exhibit the same charge transfer behavior, indicating the presence of an electric field across the interfaces, directed from M2X to BiOY. Furthermore, we provide the Hartree potential difference to further confirm the direction of the electric field, as shown in Figure 4b and Figure S7. Here, ∆Φ represents the effect of the internal electric field, defined as the difference between the sum of the ∆φ values of isolated monolayers and that of the heterojunction. The ∆Φ of Au2Se/BiOCl is 0.41 eV, so the internal electric field is directed from Au2Se to BiOCl, in agreement with the charge density difference results.
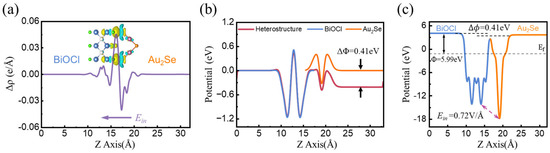
Figure 4.
Plane-averaged (a) charge density difference, (b) Hartree difference potential, and (c) electrostatic potential of Au2Se/BiOCl heterojunction, respectively.
Furthermore, the interfacial charge transfer can be further investigated using the work function , which is defined as follows:
where and represent the vacuum and Fermi energy levels, respectively. The work function values of Ag2S, Ag2Se, Au2Se, BiOCl, BiOBr, and BiOI are 5.97, 5.87, 5.02, 7.78, 7.57, and 6.54 eV, respectively. As shown in Figure S8, when M2X and BiOY form M2X/BiOY heterojunctions, electrons transfer from M2X (with the lower work function) to BiOY (with the higher work function) in order to align their Fermi levels, ultimately resulting in the formation of an internal electric field (Ein) at the interface, directed from M2X to BiOY. The plane-averaged electrostatic potential of the Au2Se/BiOCl heterojunction, as well as other systems, is shown in Figure 4c and Figure S9. Due to charge transfer, a difference of ∆Φ = 0.41 eV forms across the Au2Se/BiOCl heterojunction upon contact between the materials, corresponding to the ∆Φ value presented in Figure 4b. Meanwhile, a potential difference exists between Au2Se and BiOCl, corresponding to an intrinsic electric field Ein of 0.72 V/Å in quantitative terms.
Figure 5 summarizes the ∆Φ and Ein values for M2X/BiOY heterojunctions. The ∆Φ values of these heterojunctions range from 0.02 eV to 0.44 eV. As the radius of the Y atom increases, the ∆Φ of M2X/BiOY heterojunctions decreases. Notably, the ∆Φ values of Ag2S/BiOCl, Ag2Se/BiOCl, and Au2Se/BiOCl are significantly larger than those of the other systems. The Ein values of the M2X/BiOY heterojunctions range from 0.46 V/Å to 0.72 V/Å, which are comparable to those reported in 0.832 V/Å [58], 0.62 V/Å [59], and 0.422 V/Å [60]. A large Ein can facilitate the separation of photogenerated electron–hole pairs, thereby enhancing photocatalytic efficiency.
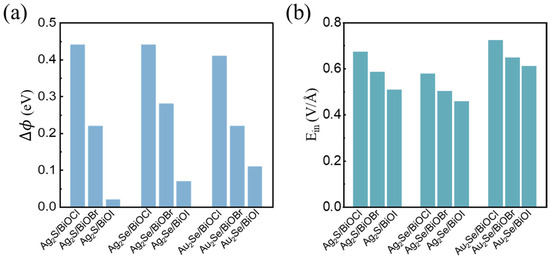
Figure 5.
(a) Potential differences (∆Φ) and (b) interfacial built-in electric fields (Ein) of M2X/BiOY heterojunctions.
Based on the Ein and band edge positions of M2X/BiOY heterojunctions, carrier migration pathways at their interfaces are further analyzed, as shown in Figure 6. All band edge positions and redox potentials are plotted relative to the vacuum level, with an aligned Fermi level for each heterojunction. Taking Ag2S/BiOCl as an example, the feasibility of photocatalytic water splitting was determined by comparing the CBM and VBM of each component material of the heterojunction with the redox potential of water splitting. Specifically, if electrons and holes migrate to the CBM of BiOCl and the VBM of Ag2S, respectively, the charges satisfy the type-II migration path (shown by the green dashed single arrows in Figure 6a). Here, the CBM of the system is below the reduction potential of water and cannot satisfy the potential requirement for the HER reaction. In contrast, if the electrons are retained in the CBM of Ag2S and the holes are located in the VBM of BiOCl, the band edge positions can satisfy the potential requirements for both HER and OER reactions, which is consistent with the direct Z-scheme charge transfer mechanism. Due to the driving force of the Ein, which points from Ag2S to BiOCl, photogenerated electrons at the CBM of BiOCl will combine with photogenerated holes at the VBM of Ag2S, which promotes the formation of a direct Z-scheme carrier migration path. Simultaneously, the Ein also restrains the migration of photogenerated electrons from the CBM of Ag2S to the CBM of BiOCl and the migration of photogenerated holes from the VBM of BiOCl to the VBM of Ag2S, thereby hindering carrier transfer along the type-II pathway. Owing to possessing a carrier migration mechanism of direct Z-scheme, HER and OER occur on the CBM of Ag2S and the VBM of BiOCl, respectively, where the higher CBM and the lower VBM result in larger overpotentials for HER χ(H2) and OER χ(O2), respectively. These increased overpotentials reflect stronger redox driving forces of the photogenerated carriers, thereby significantly enhancing the photocatalytic performance. Similar redox behavior is observed in other M2X/BiOY heterojunctions. Similarly, all M2X/BiOY heterojunctions are promising direct Z-scheme photocatalysts, with overpotentials for the HER χ(H2) and OER χ(O2) ranging from 0.48 to 0.65 eV and from 1.34 to 2.79 eV, respectively. Therefore, Z(S)-scheme M2X/BiOY heterojunctions are beneficial for achieving high performance for overall water splitting owing to the effective separation of photogenerated carriers and the strong redox abilities.
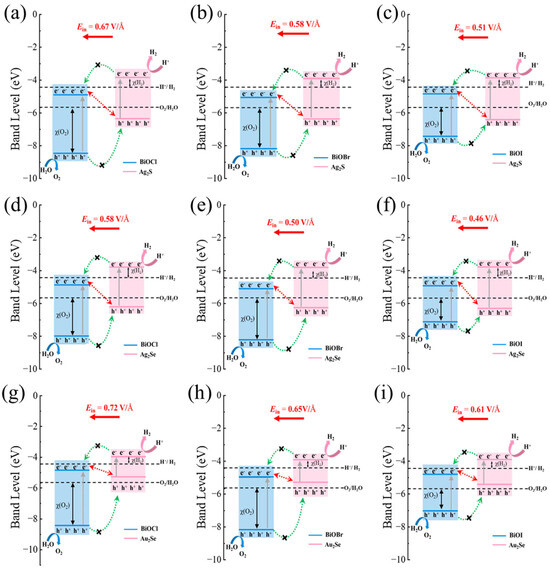
Figure 6.
Band edge positions and carrier migration mechanisms of (a) Ag2S/BiOCl, (b) Ag2S/BiOBr, (c) Ag2S/BiOI, (d) Ag2Se/BiOCl, (e) Ag2Se/BiOBr, (f) Ag2Se/BiOI, (g) Au2Se/BiOCl, (h) Au2Se/BiOBr, and (i) Au2Se/BiOI, respectively. The direct Z-scheme and type-II carrier migration pathways are presented by red dashed lines with double arrows and green dashed lines with single arrows, respectively. The χ(H2) and χ(O2) indicate the overpotential of the reduction and oxidation reactions at pH = 0, respectively.
The optical properties of photocatalysts are crucial for the performance of photocatalytic water splitting, as they determine the materials’ ability to effectively absorb and utilize visible light, which directly influences the efficiency of the photocatalytic reaction. Consequently, we calculated the optical absorbance of the M2X/BiOY heterojunction using the following equation:
where , ω, and c represent the unit cell thickness along the z-axis, the phonon frequency, and the speed of light, respectively. As shown in Figure 7. The optical absorbances of M2X/BiOY heterojunctions are enhanced in a wide range of visible and near-ultraviolet light, in comparison to those of their isolated monolayers. And the absorption edges of all heterojunctions show redshift. Notably, the first absorption peaks of Au2Se/BiOCl, Au2Se/BiOBr, and Au2Se/BiOI are observed in the near-infrared range, at 1.31 eV, 1.40 eV, and 1.48 eV, respectively. Besides the visible and ultraviolet regions, these heterojunctions also exhibit solar absorption in the near-infrared region. The enhanced optical absorbances of M2X/BiOY heterojunctions further resulted in their better photocatalytic performances.
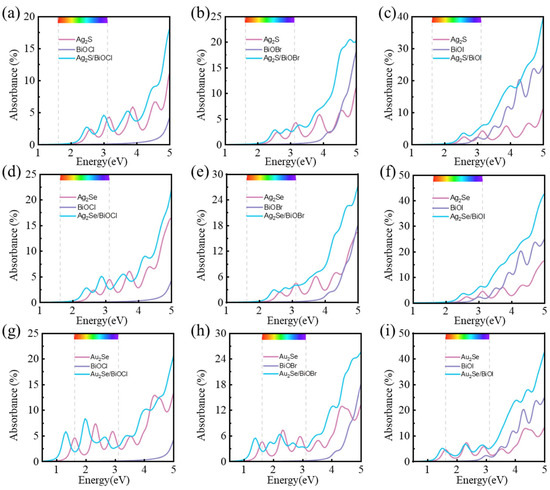
Figure 7.
Optical absorbances of (a) Ag2S/BiOCl, (b) Ag2S/BiOBr, (c) Ag2S/BiOI, (d) Ag2Se/BiOCl, (e) Ag2Se/BiOBr, (f) Ag2Se/BiOI, (g) Au2Se/BiOCl, (h) Au2Se/BiOBr, and (i) Au2Se/BiOI, respectively.
Furthermore, the activities of HER and OER reactions in Au2Se/BiOI are investigated. Through previous analysis, we know that the HER reaction occurs in Au2Se and the OER reaction occurs in BiOI, as shown in Figure 8a. Since 2D BiOX (X = Cl, Br, I) has been widely reported in the field of photocatalytic oxygen evolution [61,62,63,64,65,66], here we focus on the HER on the Au2Se side of Au2Se/BiOI, which is estimated by Gibbs free energies of the intermediate reactants (Figure 8b). Au2Se/BiOI exhibits a high value of 1.99 eV at pH = 0, which results in suppression of HER. To enhance catalytic efficiency, the modulation of single-atom vacancy is an effective method. We introduce the Se vacancy into Au2Se/BiOI via removing one Se atom from the Au2Se surface in a 2 × 2 supercell. The value of decreases significantly to 0.71 eV, indicating that Se atomic vacancy can substantially enhance its catalytic performance. These results suggest that Au2Se/BiOI is a promising photocatalyst for overall water splitting.
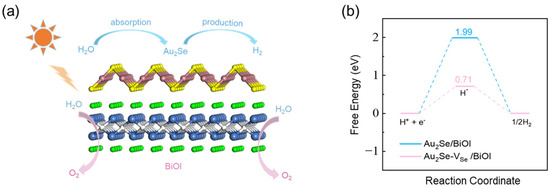
Figure 8.
(a) Schematic diagram of the photocatalytic hydrogen production mechanism in the heterojunction. (b) Gibbs free energy changes of HER processes. Pink and cyan lines represent of Au2Se surface with and without Se vacancy, respectively, at pH = 0.
4. Conclusions
In this study, we design a novel M2X/BiOY(M = Ag, Au; X = S, Se; and Y = Cl, Br, I) heterojunction and investigate its potential as an efficient Z-scheme photocatalyst for solar-driven overall water splitting. Using first-principles calculations, we systematically investigated the electronic structure, optical properties, and photocatalytic performance of these heterojunctions. The results show that all heterojunctions are staggered-band-alignment heterojunctions, which are favorable for the spatial separation of photogenerated carriers. Furthermore, we identified the presence of an internal electric field Ein at the heterojunction interface, pointing from M2X to BiOY, with electric field strengths between 0.46 V/Å and 0.72 V/Å. This electric field promotes the recombination of charge carriers with low oxidation–reduction capabilities while retaining the charge carriers with strong redox abilities. This internal field facilitates the recombination of charge carriers with lower redox potential while preserving those with higher redox potential. Consequently, the hydrogen evolution reaction (HER) occurs at the conduction band minimum (CBM) of M2X, while the oxygen evolution reaction (OER) takes place at the valence band maximum (VBM) of BiOY. This Z-scheme mechanism increases the overpotentials for both HER χ (H2) and OER χ (O2), enhancing the overall photocatalytic efficiency. Furthermore, compared to their respective isolated monolayers, M2X/BiOY heterojunctions exhibit significantly stronger optical absorption in the visible and near-ultraviolet light regions. Specifically, the first absorption peaks for Au2Se/BiOCl, Au2Se/BiOBr, and Au2Se/BiOI heterojunctions are located at 1.31 eV, 1.40 eV, and 1.48 eV, respectively, in the infrared region. These enhanced optical absorption properties further promote their photocatalytic performance. Finally, the Gibbs free energy of the hydrogen evolution reaction for the Au2Se/BiOI heterojunction. The results further indicate that M2X/BiOY heterojunctions hold significant promise as novel Z-scheme photocatalysts for water splitting applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano15110844/s1. Figure S1: Projected band structures of Ag2S/BiOI with (a) AA, (b) AB and (c) AC stackings, respectively; Figure S2: Charge density differences ∆ρ of Ag2S/BiOI with (a) AA, (b) AB and (c) AC stackings, respectively; Figure S3: (a) Top and side views of the snapshots of Ag2S/BiOI taken from ab initio molecular dynamic simulations carried out at 300 K for 2 ps. (b) Phonon dispersions of Ag2S/BiOI; Figure S4: Projected band structures of M2X/BiOY heterojunctions. The orange and blue colors represent the M2X monolayer and the BiOY monolayer, respectively; Figure S5: Partial charge densities of M2X/BiOY heterojunctions; Figure S6: Planar-averaged charge density differences along the z direction of M2X/BiOY hetero-junctions; Figure S7. Plane-averaged Hartree difference potentials along the z direction of M2X/BiOY heterojunctions; Figure S8: Fermi level change in M2X/BiOY before contact and after contact; Figure S9. Plane-averaged electrostatic potential along the z direction of M2X/BiOY heterojunctions. Ef represents the Fermi level and the black arrow indicates the difference between the Fermi level and the vacuum level. The vacuum energy level difference between the two sides of the heterojunction is represented by ∆Φ. Table S1: Comparison of Band Gaps between bulk and monolayer BiOX; Table S2: The binding energy (Eb) of M2X/BIOY heterojunctions.
Author Contributions
Q.D.: investigation, formal analysis, writing—original draft. L.G.: conceptualization, supervision, writing—review and editing, funding acquisition. W.G.: formal analysis. J.H.: formal analysis. C.Z.: supervision, funding acquisition. H.W.: supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (Grant Nos. 62301240 and 12265017); the Yunnan Fundamental Research Projects (Grant Nos. 202301AW070017, 202201BE070001-009, and 202201AV070003); the Yunnan Province Computational Physics and Applied Science, Technology Innovation Team; the Yunnan Ten Thousand Talents Plan Young & Elite Talents Project; and the Leading Metallurgical Energy-Saving & Emission-Reduction Team of Yunnan Province (No. 202405AS350028). Numerical computations were performed in the Hefei Advanced Computing Center.
Data Availability Statement
Data are contained within the article or Supplementary Materials. The data that support the findings of this study, including input files for the first-principles calculations, are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chen, S.; Takata, T.; Domen, K. Particulate photocatalysts for overall water splitting. Nat. Rev. Mater 2017, 2, 17050. [Google Scholar] [CrossRef]
- Licht, S.; Wang, B.; Mukerji, S.; Soga, T.; Umeno, M.; Tributsch, H. Over 18% solar energy conversion to generation of hydrogen fuel; theory and experiment for efficient solar water splitting. Int. J. Hydrogen Energy 2001, 26, 653–659. [Google Scholar] [CrossRef]
- Qi, M.-Y.; Conte, M.; Anpo, M.; Tang, Z.-R.; Xu, Y.-J. Cooperative coupling of oxidative organic synthesis and hydrogen production over semiconductor-based photocatalysts. Chem. Rev. 2021, 121, 13051–13085. [Google Scholar] [CrossRef]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef]
- Li, R.; Li, C. Chapter one-photocatalytic water splitting on semiconductor-based photocatalysts. In Advances in Catalysis; Academic Press: Cambridge, MA, USA, 2017; Volume 60, pp. 1–57. [Google Scholar]
- Shen, S.; Shi, J.; Guo, P.; Guo, L. Visible-light-driven photocatalytic water splitting on nanostructured semiconducting materials. Int. J. Nanotechnol. 2011, 8, 523–591. [Google Scholar] [CrossRef]
- Fan, Y.C.; Yang, B.; Song, X.H.; Shao, X.F.; Zhao, M.W. Direct Z-scheme photocatalytic overall water splitting on 2D CdS/InSe heterostructures. J. Phys. D Appl. Phys. 2018, 51, 395501. [Google Scholar] [CrossRef]
- Ge, M.; Yang, C.L.; Wang, M.S.; Ma, X.G. Photocatalytic hydrogen generation from overall water splitting with direct Z-scheme driven by two-dimensional InTe/Bismuthene heterostructure. Int. J. Hydrogen Energy 2023, 48, 138–146. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, P.; Zhang, X.; Shen, T.; Liu, J.; Ren, J.-C.; Wang, H.; Li, S.; Liu, W. Enhanced solar-to-hydrogen efficiency for photocatalytic water splitting based on a polarized heterostructure: The role of intrinsic dipoles in heterostructures. J. Mater. Chem. A 2021, 9, 14515–14523. [Google Scholar] [CrossRef]
- Wang, G.; Gong, L.; Li, Z.; Wang, B.; Zhang, W.; Yuan, B.; Zhou, T.; Long, X.; Kuang, A. A two-dimensional CdO/CdS heterostructure used for visible light photocatalysis. Phys. Chem. Chem. Phys. 2020, 22, 9587–9592. [Google Scholar] [CrossRef]
- Wang, G.Z.; Chang, J.L.; Tang, W.Y.; Xie, W.J.; Ang, Y.S. 2D materials and heterostructures for photocatalytic water-splitting: A theoretical perspective. J. Phys. D Appl. Phys. 2022, 55, 293002. [Google Scholar] [CrossRef]
- Yuan, Y.-P.; Ruan, L.-W.; Barber, J.; Joachim Loo, S.C.; Xue, C. Hetero-nanostructured suspended photocatalysts for solar-to-fuel conversion. Energy Environ. Sci. 2014, 7, 3934–3951. [Google Scholar] [CrossRef]
- Zhou, Z.; Niu, X.; Zhang, Y.; Wang, J. Janus MoSSe/WSeTe heterostructures: A direct Z-scheme photocatalyst for hydrogen evolution. J. Mater. Chem. A 2019, 7, 21835–21842. [Google Scholar] [CrossRef]
- Guo, H.-L.; Du, H.; Jiang, Y.-F.; Jiang, N.; Shen, C.; Zhou, X.; Liu, Y.; Xu, A.W. Artificial photosynthetic Z-scheme photocatalyst for hydrogen evolution with high quantum efficiency. J. Phys. Chem. C 2017, 121, 107–114. [Google Scholar] [CrossRef]
- Peng, Z.; Jiaguo, Y.; Mietek, J. All-solid-state Z-scheme photocatalytic systems. Adv. Mater. 2014, 26, 4920–4935. [Google Scholar]
- Tian, S.; Ding, Y.-F.; Cai, M.-Q.; Chen, L.; Au, C.-T.; Yin, S.-F. Enhanced photocatalytic activity of the direct Z-scheme black phosphorus/BiOX (X = Cl, Br, I) heterostructures. Phys. Chem. Chem. Phys. 2021, 23, 17894–17903. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, Z.; Nie, H.; Kong, B. The direct Z-scheme character and roles of S vacancy in BiOCl/Bi2S3-(001) heterostructures for superior photocatalytic activity: A hybrid density functional investigation. Phys. Chem. Chem. Phys. 2024, 26, 10723–10736. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.S.; Cao, J.X.; Yin, W.J.; Yang, L.W.; Wei, X.L. A 2D ZnSe/BiOX vertical heterostructure as a promising photocatalyst for water splitting: A first-principles study. J. Phys. D Appl. Phys. 2020, 53, 055108. [Google Scholar] [CrossRef]
- Yin, Q.-K.; Yang, C.-L.; Wang, M.-S.; Ma, X.-G. Two-dimensional heterostructures of AuSe/SnS for the photocatalytic hydrogen evolution reaction with a Z-scheme. J. Mater. Chem. C 2021, 9, 12231–12238. [Google Scholar] [CrossRef]
- Yu, J.; Wang, S.; Low, J.; Xiao, W. Enhanced photocatalytic performance of direct Z-scheme g-C3N4–TiO2 photocatalysts for the decomposition of formaldehyde in air. Phys. Chem. Chem. Phys. 2013, 15, 16883–16890. [Google Scholar] [CrossRef]
- Zhu, X.T.; Xu, Y.; Cao, Y.; Zou, D.F.; Sheng, W. Direct Z-scheme arsenene/HfS2 van der Waals heterojunction for overall photocatalytic water splitting: First-principles study. Appl. Surf. Sci. 2022, 574, 151650. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Z.; Fang, D.; Yang, D. Efficient photocatalytic hydrogen evolution of Z-scheme BiVO4/ZnIn2S4 heterostructure driven by visible light. Inorg. Chem. Commun. 2024, 169, 112971. [Google Scholar] [CrossRef]
- Low, J.; Dai, B.; Tong, T.; Jiang, C.; Yu, J. In situ irradiated X-ray photoelectron spectroscopy investigation on a direct Z-scheme TiO2/CdS composite film photocatalyst. Adv. Mater. 2019, 31, 1802981. [Google Scholar] [CrossRef] [PubMed]
- Low, J.; Jiang, C.; Cheng, B.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J. A review of direct Z-scheme photocatalysts. Small Methods 2017, 1, 1700080. [Google Scholar] [CrossRef]
- Wang, L.; Chen, R.; Zhang, Z.; Chen, X.; Ding, J.; Zhang, J.; Wan, H.; Guan, G. Constructing direct Z-scheme heterojunction g-C3N5/BiOBr for efficient photocatalytic CO2 reduction with H2O. J. Environ. Chem. Eng. 2023, 11, 109345. [Google Scholar] [CrossRef]
- Xie, K.-X.; Zhang, Y.; Qiang, Z.-B.; Ding, J.-X.; Nouguiza, H.; Chen, H.-X.; Duan, L.; Fan, J.-B.; Ni, L. A direct Z-scheme GeS/GeSe van der Waals heterojunction as a promising photocatalyst with high optical absorption, solar-to-hydrogen efficiency and catalytic activity for overall water splitting: First-principles prediction. Int. J. Hydrogen Energ. 2024, 51, 1381–1391. [Google Scholar] [CrossRef]
- Zhang, C.-F.; Yang, C.-L.; Wang, M.-S.; Ma, X.-G. Z-Scheme photocatalytic solar-energy-to-hydrogen conversion driven by the HfS2/SiSe heterostructure. J. Mater. Chem. C 2022, 10, 5474–5481. [Google Scholar] [CrossRef]
- Zhang, D.; Tan, G.; Wang, M.; Li, B.; Dang, M.; Wang, Y.; Zhang, B.; Ren, H.; Xia, A. The formation of direct Z-scheme Ag/BiOCl/AgIO3 heterojunction and its degradation stability. Appl. Surf. Sci. 2020, 530, 147228. [Google Scholar] [CrossRef]
- Cao, W.; Wang, Z.; Miao, L.; Shi, J.; Xiong, R. Thermoelectric Properties of Strained β-Cu2Se. ACS Appl. Mater. Interfaces 2021, 13, 34367–34373. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, Y.-F.; Du, S. Semiconducting M2X (M = Cu, Ag, Au; X = S, Se, Te) monolayers: A broad range of band gaps and high carrier mobilities. Nano Res. 2021, 14, 2826–2830. [Google Scholar] [CrossRef]
- Liu, H.; Gao, L.; Xue, Y.; Ye, Y.; Tian, Y.; Jiang, L.; He, S.; Ren, W.; Shai, X.; Wei, T.; et al. Two-dimensional semiconducting Ag2X (X = S, Se) with Janus-induced built-in electric fields and moderate band edges for overall water splitting. Appl. Surf. Sci. 2022, 597, 153707. [Google Scholar] [CrossRef]
- Qian, K.; Gao, L.; Chen, X.; Li, H.; Zhang, S.; Zhang, X.-L.; Zhu, S.; Yan, J.; Bao, D.; Cao, L.; et al. Air-stable monolayer Cu2Se exhibits a purely thermal structural phase transition. Adv. Mater. 2020, 32, 1908314. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Xu, W.W.; Lin, D.; Wang, J.; Zeng, X.C. Two-dimensional gold sulfide monolayers with direct band gap and ultrahigh electron mobility. J. Phys. Chem. Lett. 2019, 10, 3773–3778. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Gao, L.; Ren, W.; Shai, X.; Wei, T.; Zeng, C.; Wang, H. Prediction of 2D group-11 chalcogenides: Insights into novel auxetic M2X (M = Cu, Ag, Au; X = S, Se, Te) monolayers. Phys. Chem. Chem. Phys. 2023, 25, 32323–32329. [Google Scholar] [CrossRef]
- Gao, B.; Zhang, J.-R.; Chen, L.; Guo, J.; Shen, S.; Au, C.-T.; Yin, S.-F.; Cai, M.-Q. Density functional theory calculation on two-dimensional MoS2/BiOX (X = Cl, Br, I) van der Waals heterostructures for photocatalytic action. Appl. Surf. Sci. 2019, 492, 157–165. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, P.; Zhou, W.; Hong, J. Built-in electric field hindering photogenerated carrier recombination in polar bilayer SnO/BiOX (X = Cl, Br, I) for water splitting. J. Phys. Chem. C 2020, 124, 9696–9702. [Google Scholar] [CrossRef]
- Opoku, F.; Akoto, O.; Oppong, S.O.-B.; Adimado, A.A. Two-dimensional layered type-II MS2/BiOCl (M = Zr, Hf) van der Waals heterostructures: Promising photocatalysts for hydrogen generation. New J. Chem. 2021, 45, 20365–20373. [Google Scholar] [CrossRef]
- Pan, H.-X.; Feng, L.-P.; Zeng, W.; Zhang, Q.-C.; Zhang, X.-D.; Liu, Z.-T. Active sites in single-layer BiOX (X = Cl, Br, and I) catalysts for the hydrogen evolution reaction. Inorg. Chem. 2019, 58, 13195–13202. [Google Scholar] [CrossRef]
- Zhang, X.; Li, B.; Wang, J.; Yuan, Y.; Zhang, Q.; Gao, Z.; Liu, L.-M.; Chen, L. The stabilities and electronic structures of single-layer bismuth oxyhalides for photocatalytic water splitting. Phys. Chem. Chem. Phys. 2014, 16, 25854–25861. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal--amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Krukau, A.V.; Vydrov, O.A.; Izmaylov, A.F.; Scuseria, G.E. Influence of the exchange screening parameter on the performance of screened hybrid functionals. J. Chem. Phys. 2006, 125, 224106. [Google Scholar] [CrossRef]
- Haas, P.; Tran, F.; Blaha, P. Calculation of the lattice constant of solids with semilocal functionals. Phys. Rev. B 2009, 79, 085104. [Google Scholar] [CrossRef]
- Tran, F.; Laskowski, R.; Blaha, P.; Schwarz, K. Performance on molecules, surfaces, and solids of the Wu-Cohen GGA exchange-correlation energy functional. Phys. Rev. B 2007, 75, 115131. [Google Scholar] [CrossRef]
- Zhang, G.-X.; Reilly, A.M.; Tkatchenko, A.; Scheffler, M. Performance of various density-functional approximations for cohesive properties of 64 bulk solids. New J. Phys. 2018, 20, 063020. [Google Scholar] [CrossRef]
- Wang, G.; Luo, X.; Huang, Y.; Kuang, A.; Yuan, H.; Chen, H. BiOX/BiOY (X, Y = F, Cl, Br, I) superlattices for visible light photocatalysis applications. RSC Adv. 2016, 6, 91508–91516. [Google Scholar] [CrossRef]
- Choudhary, K.; Tavazza, F. Predicting anomalous quantum confinement effect in van der Waals materials. Phys. Rev. Mater. 2021, 5, 054602. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, B.; Zou, X.; Cheng, H.-M. Chemical vapor deposition growth and applications of two-dimensional materials and their heterostructures. Chem. Rev. 2018, 118, 6091–6133. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Y.-Y.; Guo, H.; Huang, L.; Lu, H.; Lin, X.; Wang, Y.-L.; Du, S.; Gao, H.-J. Epitaxial growth and physical properties of 2D materials beyond graphene: From monatomic materials to binary compounds. Chem. Soc. Rev. 2018, 47, 6073–6100. [Google Scholar] [CrossRef]
- Li, S.; Lyu, Y.; Zheng, J.; Sofer, Z.; Zhou, H. Boosting the built-in electric field in heterojunctions of 2D and 3D systems to accelerate the separation and transfer of photogenerated carriers for efficient photocatalysis. Flat Chem. 2024, 47, 100718. [Google Scholar] [CrossRef]
- Sun, R.; Yang, C.-L.; Wang, M.-S.; Ma, X.-G. High solar-to-hydrogen efficiency photocatalytic hydrogen evolution reaction with the HfSe2/InSe heterostructure. J. Power Sources 2022, 547, 232008. [Google Scholar] [CrossRef]
- Zhao, H.; Han, L.; Jia, B.; Chen, Y.; Guan, X.; Wu, L.; Lu, P. Type-II van der Waals heterostructures based on AsP and transition metal dichalcogenides: Great promise for applications in solar cell. Phys. Status Solidi RRL 2022, 16, 2200043. [Google Scholar] [CrossRef]
- Li, D.; Li, R.; Zeng, F.; Long, L.; Cai, S. Electronic structures and optoelectronic properties of self-powered black phosphorus/InSe heterojunction: A time-domain ab initio perspective. Appl. Surf. Sci. 2025, 681, 161524. [Google Scholar] [CrossRef]
- Ahammed, R.; Jena, N.; Rawat, A.; Mohanta, M.K.; Dimple; De Sarkar, A. Ultrahigh out-of-plane piezoelectricity meets giant rashba effect in 2D Janus monolayers and bilayers of group IV transition-metal trichalcogenides. J. Phys. Chem. C 2020, 124, 21250–21260. [Google Scholar] [CrossRef]
- Bai, S.; Li, X.-Y.; Kong, Q.; Long, R.; Wang, C.; Jiang, J.; Xiong, Y. Toward enhanced photocatalytic oxygen evolution: Synergetic utilization of plasmonic effect and schottky junction via Interfacing facet selection. Adv. Mater. 2015, 27, 3444–3452. [Google Scholar] [CrossRef]
- Di, J.; Chen, C.; Yang, S.-Z.; Ji, M.; Yan, C.; Gu, K.; Xia, J.; Li, H.; Li, S.; Liu, Z. Defect engineering in atomically-thin bismuth oxychloride towards photocatalytic oxygen evolution. J. Mater. Chem. A 2017, 5, 14144–14151. [Google Scholar] [CrossRef]
- Ji, M.; Chen, R.; Di, J.; Liu, Y.; Li, K.; Chen, Z.; Xia, J.; Li, H. Oxygen vacancies modulated Bi-rich bismuth oxyiodide microspheres with tunable valence band position to boost the photocatalytic activity. J. Colloid Interface Sci. 2019, 533, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shang, J.; Zhu, H.; Yang, Z.; Ai, Z.; Zhang, L. Oxygen vacancy structure associated photocatalytic water oxidation of BiOCl. ACS Catal. 2016, 6, 8276–8285. [Google Scholar] [CrossRef]
- Shi, M.; Li, G.; Li, J.; Jin, X.; Tao, X.; Zeng, B.; Pidko, E.A.; Li, R.; Li, C. Intrinsic facet-dependent reactivity of well-defined BiOBr nanosheets on photocatalytic water Splitting. Angew. Chem. 2020, 59, 6590–6595. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, L.; Wang, J.; Li, Q.; He, W.; Yin, J.J. Surface Structure-Dependent Molecular Oxygen Activation of BiOCl Single-Crystalline Nanosheets. J. Am. Chem. Soc. 2013, 135, 15750–15753. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).