Enhancing Stability and Emissions in Metal Halide Perovskite Nanocrystals Through Mn2⁺ Doping
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Mn-Doped MAPbBr3 NCs
2.3. Surface Passivation
2.4. Preparation of NC Superlattice
2.5. Characterizations
3. Results and Discussion
3.1. Structural Characterization of Mn-Doped MAPbBr3 NCs
3.2. Optical Characterization of Mn-Doped MAPbBr3 NCs
3.3. Optical Stability of Mn-Doped MAPbBr3 NCs
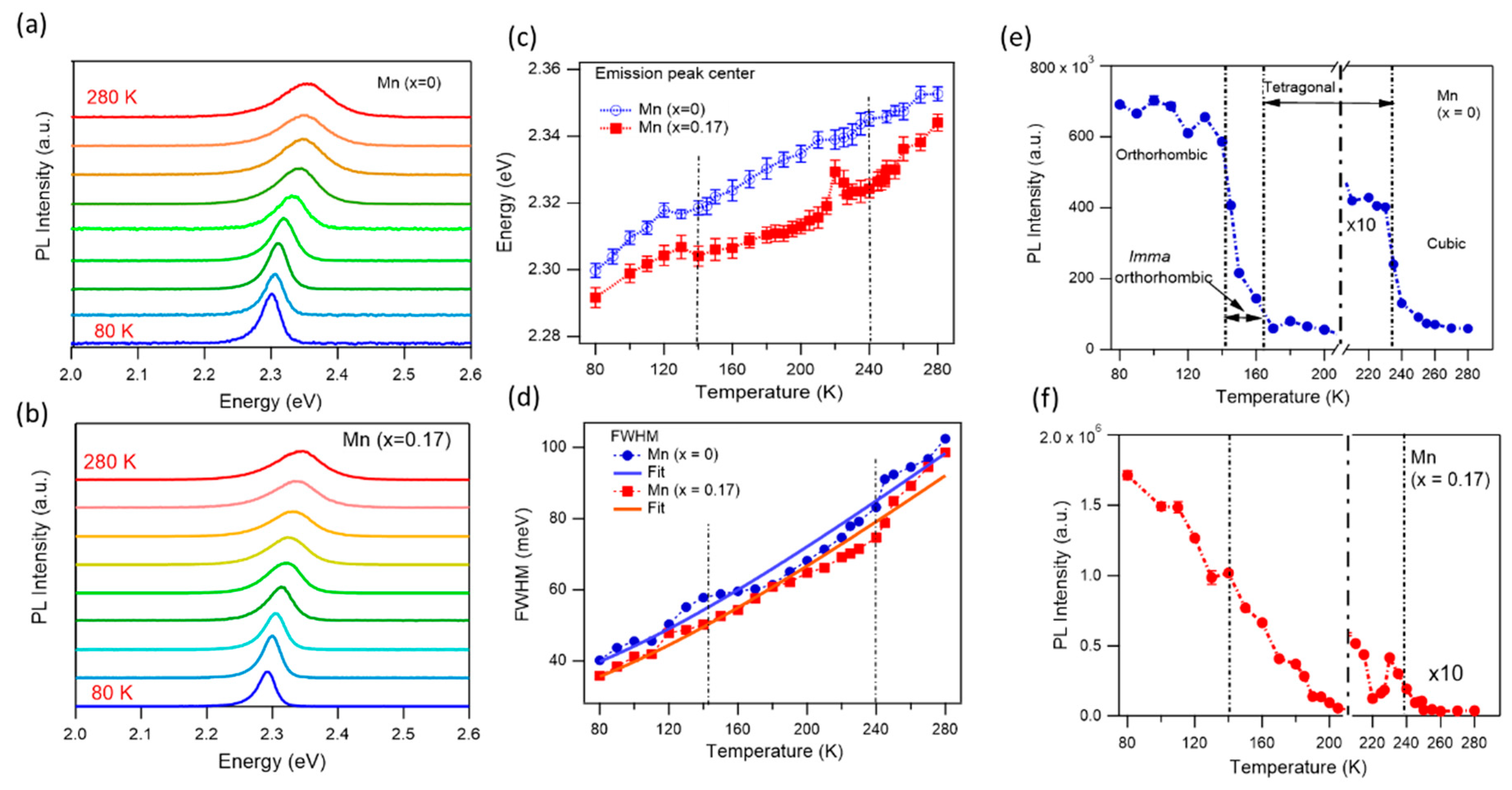
3.4. Optical Properties at Low Temperature and Crystal Phase Transitions
3.5. Superlattice Formation of xMn-MAPbBr3 NCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hassan, Y.; Song, Y.; Pensack, R.D.; Abdelrahman, A.I.; Kobayashi, Y.; Winnik, M.A.; Scholes, G.D. Structure-Tuned Lead. Halide Perovskite Nanocrystals. Adv. Mater. 2016, 28, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Malgras, V.; Tominaka, S.; Ryan, J.W.; Henzie, J.; Takei, T.; Ohara, K.; Yamauchi, Y. Observation of Quantum Confinement in Monodisperse Methylammonium Lead Halide Perovskite Nanocrystals Embedded in Mesoporous Silica. J. Am. Chem. Soc. 2016, 138, 13874–13881. [Google Scholar] [CrossRef] [PubMed]
- Droseros, N.; Longo, G.; Brauer, J.C.; Sessolo, M.; Bolink, H.J.; Banerji, N. Origin of the Enhanced Photoluminescence Quantum Yield in MAPbBr3 Perovskite with Reduced Crystal Size. ACS Energy Lett. 2018, 3, 1458–1466. [Google Scholar] [CrossRef]
- Zhu, C.; Boehme, S.C.; Feld, L.G.; Moskalenko, A.; Dirin, D.N.; Mahrt, R.F.; Stöferle, T.; Bodnarchuk, M.I.; Efros, A.L.; Sercel, P.C.; et al. Single-Photon Superradiance in Individual Caesium Lead Halide Quantum Dots. Nature 2024, 626, 535–541. [Google Scholar] [CrossRef]
- Chiba, T.; Kido, J. Lead Halide Perovskite Quantum Dots for Light-Emitting Devices. J. Mater. Chem. C 2018, 6, 11868–11877. [Google Scholar] [CrossRef]
- Li, Y.-F.; Chou, S.-Y.; Huang, P.; Xiao, C.; Liu, X.; Xie, Y.; Zhao, F.; Huang, Y.; Feng, J.; Zhong, H.; et al. Stretchable Organometal-Halide-Perovskite Quantum-Dot Light-Emitting Diodes. Adv. Mater. 2019, 31, e1807516. [Google Scholar] [CrossRef]
- Zou, T.; Liu, X.; Qiu, R.; Wang, Y.; Huang, S.; Liu, C.; Dai, Q.; Zhou, H. Enhanced UV-C Detection of Perovskite Photodetector Arrays via Inorganic CsPbBr3 Quantum Dot Down-Conversion Layer. Adv. Opt. Mater. 2019, 7, e1801812. [Google Scholar] [CrossRef]
- Jia, D.; Chen, J.; Zhuang, R.; Hua, Y.; Zhang, X. Inhibiting Lattice Distortion of CsPbI 3 Perovskite Quantum Dots for Solar Cells with Efficiency over 16.6%. Energy Environ. Sci. 2022, 15, 4201–4212. [Google Scholar] [CrossRef]
- Jia, D.; Chen, J.; Qiu, J.; Ma, H.; Yu, M.; Liu, J.; Zhang, X. Tailoring Solvent-Mediated Ligand Exchange for CsPbI3 Perovskite Quantum Dot Solar Cells with Efficiency Exceeding 16.5%. Joule 2022, 6, 1632–1653. [Google Scholar] [CrossRef]
- Jia, D.; Chen, J.; Zhuang, R.; Hua, Y.; Zhang, X.; Jia, D.; Chen, J.; Zhang, X.; Zhuang, R.; Hua, Y. Antisolvent-Assisted In Situ Cation Exchange of Perovskite Quantum Dots for Efficient Solar Cells. Adv. Mater. 2023, 35, e2212160. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Zou, C.; Mao, C.; Corp, K.L.; Yao, Y.-C.; Lee, Y.-J.; Schlenker, C.W.; Jen, A.K.Y.; Lin, L.Y. CsPbBr3 Perovskite Quantum Dot Vertical Cavity Lasers with Low Threshold and High Stability. ACS Photonics 2017, 4, 2281–2289. [Google Scholar] [CrossRef]
- Chen, L.-J.; Dai, J.-H.; Lin, J.-D.; Mo, T.-S.; Lin, H.-P.; Yeh, H.-C.; Chuang, Y.-C.; Jiang, S.-A.; Lee, C.-R. Wavelength-Tunable and Highly Stable Perovskite-Quantum-Dot-Doped Lasers with Liquid Crystal Lasing Cavities. ACS Appl. Mater. Interfaces 2018, 10, 33307–33315. [Google Scholar] [CrossRef]
- Utzat, H.; Sun, W.; Kaplan, A.E.K.; Krieg, F.; Ginterseder, M.; Spokoyny, B.; Klein, N.D.; Shulenberger, K.E.; Perkinson, C.F.; Kovalenko, M.V.; et al. Coherent Single-Photon Emission from Colloidal Lead Halide Perovskite Quantum Dots. Science 2019, 363, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Marczak, M.; Feld, L.; Boehme, S.C.; Bernasconi, C.; Moskalenko, A.; Cherniukh, I.; Dirin, D.; Bodnarchuk, M.I.; Kovalenko, M.V.; et al. Room-Temperature, Highly Pure Single-Photon Sources from All-Inorganic Lead Halide Perovskite Quantum Dots. Nano Lett. 2022, 22, 3751–3760. [Google Scholar] [CrossRef] [PubMed]
- Kirakosyan, A.; Yun, S.; Yoon, S.-G.; Choi, J. Surface Engineering for Improved Stability of CH3NH3PbBr3 Perovskite Nanocrystals. Nanoscale 2018, 10, 1885–1891. [Google Scholar] [CrossRef]
- Mosconi, E.; Umari, P.; De Angelis, F. Electronic and Optical Properties of MAPbX3 Perovskites (X = I, Br, Cl): A Unified DFT and GW Theoretical Analysis. Phys. Chem. Chem. Phys. 2016, 18, 27158–27164. [Google Scholar] [CrossRef]
- Dai, S.-W.; Hsu, B.-W.; Chen, C.-Y.; Lee, C.-A.; Liu, H.-Y.; Wang, H.-F.; Huang, Y.-C.; Wu, T.-L.; Manikandan, A.; Ho, R.-M.; et al. Perovskite Quantum Dots with Near Unity Solution and Neat-Film Photoluminescent Quantum Yield by Novel Spray Synthesis. Adv. Mater. 2018, 30, 1705532. [Google Scholar] [CrossRef]
- Xu, K.; Vickers, E.T.; Rao, L.; Lindley, S.A.; Allen, A.C.; Luo, B.; Li, X.; Zhang, J.Z. Synergistic Surface Passivation of CH3NH3PbBr3 Perovskite Quantum Dots with Phosphonic Acid and (3-Aminopropyl) Triethoxysilane. Chem.—A Eur. J. 2019, 25, 5014–5021. [Google Scholar] [CrossRef]
- Ahmed, G.H.; Yin, J.; Bakr, O.M.; Mohammed, O.F. Successes and Challenges of Core/Shell Lead. Halide Perovskite Nanocrystals. ACS Energy Lett. 2021, 6, 1340–1357. [Google Scholar] [CrossRef]
- Lu, Y.; Alam, F.; Shamsi, J.; Abdi-Jalebi, M. Doping Up the Light: A Review of A/B-Site Doping in Metal Halide Perovskite Nanocrystals for Next-Generation LEDs. J. Phys. Chem. C 2024, 128, 10084–10107. [Google Scholar] [CrossRef]
- Ricciarelli, D.; Meggiolaro, D.; Belanzoni, P.; Alothman, A.A.; Mosconi, E.; De Angelis, F. Energy vs. Charge Transfer in Manganese-Doped Lead Halide Perovskites. ACS Energy Lett. 2021, 6, 1869–1878. [Google Scholar] [CrossRef]
- Vargas, B.; Reyes-Castillo, D.T.; Coutino-Gonzalez, E.; Sánchez-Aké, C.; Ramos, C.; Falcony, C.; Solis-Ibarra, D. Enhanced Luminescence and Mechanistic Studies on Layered Double-Perovskite Phosphors: Cs4Cd1-XMnxBi2Cl12. Chem. Mater. 2020, 32, 9307–9315. [Google Scholar] [CrossRef]
- Wei, Z.; Liao, W.Q.; Tang, Y.Y.; Li, P.F.; Shi, P.P.; Cai, H.; Xiong, R.G. Discovery of an Antiperovskite Ferroelectric in [(CH3)3NH]3(MnBr3)(MnBr4). J. Am. Chem. Soc. 2018, 140, 8110–8113. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, X.; Yang, C.; Li, J.; Wang, L. Fe2+ Doped in CsPbCl3 Perovskite Nanocrystals: Impact on the Luminescence and Magnetic Properties. RSC Adv. 2019, 9, 33017–33022. [Google Scholar] [CrossRef] [PubMed]
- Ferro, S.M.; Wobben, M.; Ehrler, B. Rare-Earth Quantum Cutting in Metal Halide Perovskites—A Review. Mater. Horiz. 2021, 8, 1072–1083. [Google Scholar] [CrossRef]
- Zeng, M.; Artizzu, F.; Liu, J.; Singh, S.; Locardi, F.; Mara, D.; Hens, Z.; Van Deun, R. Boosting the Er3+1.5 Μm Luminescence in CsPbCl3Perovskite Nanocrystals for Photonic Devices Operating at Telecommunication Wavelengths. ACS Appl. Nano Mater. 2020, 3, 4699–4707. [Google Scholar] [CrossRef]
- Wang, W.; Song, S.; Lv, P.; Li, J.; Cao, B.; Liu, Z. Thermally Stable Rare-Earth Eu3+-Doped CsPbCl3 Perovskite Quantum Dots with Controllable Blue Emission. J. Lumin. 2023, 260, 119894. [Google Scholar] [CrossRef]
- Milstein, T.J.; Kroupa, D.M.; Gamelin, D.R. Picosecond Quantum Cutting Generates Photoluminescence Quantum Yields Over 100% in Ytterbium-Doped CsPbCl3 Nanocrystals. Nano Lett. 2018, 18, 3792–3799. [Google Scholar] [CrossRef]
- Kroupa, D.M.; Roh, J.Y.; Milstein, T.J.; Creutz, S.E.; Gamelin, D.R. Quantum-Cutting Ytterbium-Doped CsPb(Cl1–XBrx)3 Perovskite Thin Films with Photoluminescence Quantum Yields over 190%. ACS Energy Lett. 2018, 3, 2390–2395. [Google Scholar] [CrossRef]
- Kim, B.-J.; Fabbri, E.; Abbott, D.F.; Cheng, X.; Clark, A.H.; Nachtegaal, M.; Borlaf, M.; Castelli, I.E.; Graule, T.; Schmidt, T.J. Functional Role of Fe-Doping in Co-Based Perovskite Oxide Catalysts for Oxygen Evolution Reaction. J. Am. Chem. Soc. 2019, 141, 5231–5240. [Google Scholar] [CrossRef]
- Pan, G.; Bai, X.; Yang, D.; Chen, X.; Jing, P.; Qu, S.; Zhang, L.; Zhou, D.; Zhu, J.; Xu, W.; et al. Doping Lanthanide into Perovskite Nanocrystals: Highly Improved and Expanded Optical Properties. Nano Lett. 2017, 17, 8005–8011. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Fan, J.; Chen, B.; Qin, X.; Wang, J.; Wang, F.; Deng, R.; Liu, X. Rare-Earth Doping in Nanostructured Inorganic Materials. Chem. Rev. 2022, 122, 5519–5603. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Vickers, E.T.; Luo, B.; Allen, A.C.; Chen, E.; Roseman, G.; Wang, Q.; Kliger, D.S.; Millhauser, G.L.; Yang, W.; et al. First Synthesis of Mn-Doped Cesium Lead. Bromide Perovskite Magic. Sized Clusters at Room Temperature. J. Phys. Chem. Lett. 2020, 11, 1162–1169. [Google Scholar] [CrossRef]
- Lin, C.Q.; Liu, M.L.; Yang, Z.; Wang, H.; Pan, C.Y. Mn2+ Doped CsPbBr3 Perovskite Quantum Dots with High Quantum Yield and Stability for Flexible Array Displays. J. Solid. State Chem. 2023, 327, 124295. [Google Scholar] [CrossRef]
- Parobek, D.; Roman, B.J.; Dong, Y.; Jin, H.; Lee, E.; Sheldon, M.; Son, D.H. Exciton-to-Dopant Energy Transfer in Mn-Doped Cesium Lead. Halide Perovskite Nanocrystals. Nano Lett. 2016, 16, 7376–7380. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, Y.; Xu, Z.; Li, L.; Zhang, D.; Zheng, W.; Liang, D.; Zheng, B.; Liu, H.; Sun, X.; et al. Magnetic Doping Induced Strong Circularly Polarized Light Emission and Detection in 2D Layered Halide Perovskite. Adv. Opt. Mater. 2022, 10, 2200183. [Google Scholar] [CrossRef]
- Guria, A.K.; Dutta, S.K.; Adhikari, S.D.; Pradhan, N. Doping Mn2+ in Lead Halide Perovskite Nanocrystals: Successes and Challenges. ACS Energy Lett. 2017, 2, 1014–1021. [Google Scholar] [CrossRef]
- Gao, D.; Qiao, B.; Xu, Z.; Song, D.; Song, P.; Liang, Z.; Shen, Z.; Cao, J.; Zhang, J.; Zhao, S. Postsynthetic, Reversible Cation Exchange between Pb2+ and Mn2+ in Cesium Lead Chloride Perovskite Nanocrystals. J. Phys. Chem. C 2017, 121, 20387–20395. [Google Scholar] [CrossRef]
- Travis, W.; Glover, E.N.K.; Bronstein, H.; Scanlon, D.O.; Palgrave, R.G. On the Application of the Tolerance Factor. to Inorganic and Hybrid. Halide Perovskites: A Revised System. Chem. Sci. 2016, 7, 4548–4556. [Google Scholar] [CrossRef]
- Manser, J.S.; Christians, J.A.; Kamat, P.V. Intriguing Optoelectronic Properties of Metal. Halide Perovskites. Chem. Rev. 2016, 116, 12956–13008. [Google Scholar] [CrossRef]
- Liu, W.; Lin, Q.; Li, H.; Wu, K.; Robel, I.; Pietryga, J.M.; Klimov, V.I. Mn2+-Doped Lead Halide Perovskite Nanocrystals with Dual-Color Emission Controlled by Halide Content. J. Am. Chem. Soc. 2016, 138, 14954–14961. [Google Scholar] [CrossRef]
- Chen, D.; Zhou, S.; Fang, G.; Chen, X.; Zhong, J. Fast Room-Temperature Cation Exchange Synthesis of Mn-Doped CsPbCl3 Nanocrystals Driven by Dynamic Halogen Exchange. ACS Appl. Mater. Interfaces 2018, 10, 39872–39878. [Google Scholar] [CrossRef]
- Zhou, S.; Zhu, Y.; Zhong, J.; Tian, F.; Huang, H.; Chen, J.; Chen, D. Chlorine-Additive-Promoted Incorporation of Mn2+ Dopants into CsPbCl3 Perovskite Nanocrystals. Nanoscale 2019, 11, 12465–12470. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Wang, C.; Xu, S.; Zong, S.; Lu, J.; Wang, Z.; Lu, C.; Cui, Y. Postsynthetic Doping of MnCl2 Molecules into Preformed CsPbBr3 Perovskite Nanocrystals via a Halide Exchange-Driven Cation Exchange. Adv. Mater. 2017, 29, 1700095. [Google Scholar] [CrossRef] [PubMed]
- Boehme, S.C.; Bodnarchuk, M.I.; Burian, M.; Bertolotti, F.; Cherniukh, I.; Bernasconi, C.; Zhu, C.; Erni, R.; Amenitsch, H.; Naumenko, D.; et al. Strongly Confined CsPbBr3 Quantum Dots as Quantum Emitters and Building Blocks for Rhombic Superlattices. ACS Nano 2023, 17, 2089–2100. [Google Scholar] [CrossRef] [PubMed]
- Bartel, C.J.; Sutton, C.; Goldsmith, B.R.; Ouyang, R.; Musgrave, C.B.; Ghiringhelli, L.M.; Scheffler, M. New Tolerance Factor to Predict the Stability of Perovskite Oxides and Halides. Sci. Adv. 2019, 5, eaav0693. [Google Scholar] [CrossRef]
- Shi, D.; Adinolfi, V.; Comin, R.; Yuan, M.; Alarousu, E.; Buin, A.; Chen, Y.; Hoogland, S.; Rothenberger, A.; Katsiev, K.; et al. Low Trap-State Density and Long Carrier Diffusion in Organolead Trihalide Perovskite Single Crystals. Science 2015, 347, 519–522. [Google Scholar] [CrossRef]
- Saidaminov, M.I.; Abdelhady, A.L.; Murali, B.; Alarousu, E.; Burlakov, V.M.; Peng, W.; Dursun, I.; Wang, L.; He, Y.; MacUlan, G.; et al. High-Quality Bulk Hybrid Perovskite Single Crystals within Minutes by Inverse Temperature Crystallization. Nat. Commun. 2015, 6, 7586. [Google Scholar] [CrossRef]
- Biswas, A.; Bakthavatsalam, R.; Kundu, J. Efficient Exciton to Dopant Energy Transfer in Mn2+-Doped (C4H9NH3)2PbBr4 Two-Dimensional (2D) Layered Perovskites. Chem. Mater. 2017, 29, 7816–7825. [Google Scholar] [CrossRef]
- Zhou, G.; Jiang, X.; Molokeev, M.; Lin, Z.; Zhao, J.; Wang, J.; Xia, Z. Optically Modulated Ultra-Broad-Band Warm White Emission in Mn2+-Doped (C6H18N2O2)PbBr4 Hybrid Metal Halide Phosphor. Chem. Mater. 2019, 31, 5788–5795. [Google Scholar] [CrossRef]
- Dutta, A.; Behera, R.K.; Deb, S.; Baitalik, S.; Pradhan, N. Doping Mn(II) in All-Inorganic Ruddlesden–Popper Phase of Tetragonal Cs2PbCl2I2 Perovskite Nanoplatelets. J. Phys. Chem. Lett. 2019, 10, 1954–1959. [Google Scholar] [CrossRef] [PubMed]
- Kirberger, M.; Yang, J.J. Structural Differences Between Pb2+- and Ca2+-Binding Sites in Proteins: Implications with Respect to Toxicity. J. Inorg. Biochem. 2008, 102, 1901. [Google Scholar] [CrossRef]
- Dutta, S.; Yang, L.; Liu, S.Y.; Liu, C.M.; Liaw, L.J.; Som, S.; Mohapatra, A.; Sankar, R.; Lin, W.C.; Chao, Y.C. Impact of Co2+ on the Magneto-Optical Response of MAPbBr3: An Inspective Study of Doping and Quantum Confinement Effect. Mater. Today Phys. 2022, 27, 100843. [Google Scholar] [CrossRef]
- Moj, K.; Owsiński, R.; Robak, G.; Gupta, M.K.; Scholz, S.; Mehta, H. Measurement of Precision and Quality Characteristics of Lattice Structures in Metal-Based Additive Manufacturing Using Computer Tomography Analysis. Measurement 2024, 231, 114582. [Google Scholar] [CrossRef]
- Jiang, M.C.; Pan, C.Y. Research on the Stability of Luminescence of CsPbBr3 and Mn:CsPbBr3 PQDs in Polar Solution. RSC Adv. 2022, 12, 15420–15426. [Google Scholar] [CrossRef]
- Lin, C.C.; Xu, K.Y.; Wang, D.; Meijerink, A. Luminescent Manganese-Doped CsPbCl3 Perovskite Quantum Dots. Sci. Rep. 2017, 7, 45906. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Zhu, Y.; Zhan, Y.; Matras-Postolek, K.; Yang, P. Ion Exchange Derived CsPbBr3:Mn Nanocrystals with Stable and Bright Luminescence towards White Light-Emitting Diodes. Mater. Res. Bull. 2022, 153, 111915. [Google Scholar] [CrossRef]
- Chen, B.; Jiang, C.; Wang, Y.; Du, L. Investigation for Structural and Electronic Properties of Mn-Doped Perovskite at Different Doping Concentrations. Comput. Mater. Sci. 2025, 252, 113782. [Google Scholar] [CrossRef]
- Du, L.; An, J.; Katayama, T.; Duan, M.; Shi, X.P.; Wang, Y.; Furube, A. Photogenerated Carrier Dynamics of Mn2+ Doped CsPbBr3 Assembled with TiO2 Systems: Effect of Mn Doping Content. J. Chem. Phys. 2024, 160, 164713. [Google Scholar] [CrossRef]
- Trinh, C.T.; Minh, D.N.; Ahn, K.J.; Kang, Y.; Lee, K.G. Verification of Type-A and Type-B-HC Blinking Mechanisms of Organic–Inorganic Formamidinium Lead Halide Perovskite Quantum Dots by FLID Measurements. Sci. Rep. 2020, 10, 2172. [Google Scholar] [CrossRef]
- Hu, F.; Yin, C.; Zhang, H.; Sun, C.; Yu, W.W.; Zhang, C.; Wang, X.; Zhang, Y.; Xiao, M. Slow Auger Recombination of Charged Excitons in Nonblinking Perovskite Nanocrystals without Spectral Diffusion. Nano Lett. 2016, 16, 6425–6430. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Zhang, J.B.; Shen, H.R.; Su, Z.; Cui, H.; Lan, T.; Wang, J.Q.; Chen, Y.H.; Liu, L.; Cao, K.; et al. Improving the Stability of α-CsPbI3Nanocrystals in Extreme Conditions Facilitated by Mn2+Doping. ACS Omega 2021, 6, 13831–13838. [Google Scholar] [CrossRef]
- Futscher, M.H.; Gangishetty, M.K.; Congreve, D.N.; Ehrler, B. Manganese Doping Stabilizes Perovskite Light-Emitting Diodes by Reducing Ion Migration. ACS Appl. Electron. Mater. 2020, 2, 1522–1528. [Google Scholar] [CrossRef]
- Phan, T.T.T.; Nguyen, T.K.; Mac, T.K.; Trinh, M.T. Understanding and Eliminating Transient Photoluminescence Quenching in Lead. Halide Perovskite Quantum Dots for Photonic Applications. ACS Appl. Nano Mater. 2025. [Google Scholar] [CrossRef]
- Lee, S.M.; Moon, C.J.; Lim, H.; Lee, Y.; Choi, M.Y.; Bang, J. Temperature-Dependent Photoluminescence of Cesium Lead Halide Perovskite Quantum Dots: Splitting of the Photoluminescence Peaks of CsPbBr3 and CsPb(Br/I)3 Quantum Dots at Low Temperature. J. Phys. Chem. C 2017, 121, 26054–26062. [Google Scholar] [CrossRef]
- Woo, H.C.; Choi, J.W.; Shin, J.; Chin, S.H.; Ann, M.H.; Lee, C.L. Temperature-Dependent Photoluminescence of CH3NH3PbBr3 Perovskite Quantum Dots and Bulk Counterparts. J. Phys. Chem. Lett. 2018, 9, 4066–4074. [Google Scholar] [CrossRef]
- Varshni, Y.P. Temperature Dependence of the Energy Gap in Semiconductors. Physica 1967, 34, 149–154. [Google Scholar] [CrossRef]
- Meloni, S.; Palermo, G.; Ashari-Astani, N.; Grätzel, M.; Grätzel, G.; Rothlisberger, U. Valence and Conduction Band Tuning in Halide Perovskites for Solar Cell Applications †. J. Mater. Chem. A 2016, 4, 15997–16002. [Google Scholar] [CrossRef]
- Wu, K.; Bera, A.; Ma, C.; Du, Y.; Yang, Y.; Li, L.; Wu, T. Temperature-Dependent Excitonic Photoluminescence of Hybrid Organometal Halide Perovskite Films. Phys. Chem. Chem. Phys. 2014, 16, 22476–22481. [Google Scholar] [CrossRef]
- Wright, A.D.; Verdi, C.; Milot, R.L.; Eperon, G.E.; Pérez-Osorio, M.A.; Snaith, H.J.; Giustino, F.; Johnston, M.B.; Herz, L.M. Electron–Phonon Coupling in Hybrid Lead Halide Perovskites. Nat. Commun. 2016, 7, 11755. [Google Scholar] [CrossRef]
- Wolf, C.; Kim, J.S.; Lee, T.W. Structural and Thermal Disorder of Solution-Processed CH3NH3PbBr3 Hybrid Perovskite Thin Films. ACS Appl. Mater. Interfaces 2017, 9, 10344–10348. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Ming, W.; Du, M.-H.; Keum, J.K.; Puretzky, A.A.; Rouleau, C.M.; Huang, J.; Geohegan, D.B.; Wang, X.; Xiao, K.; et al. Real-Time Observation of Order-Disorder Transformation of Organic Cations Induced Phase Transition and Anomalous Photoluminescence in Hybrid Perovskites. Adv. Mater. 2018, 30, 1705801. [Google Scholar] [CrossRef] [PubMed]
- López, C.A.; Martínez-Huerta, M.V.; Alvarez-Galván, M.C.; Kayser, P.; Gant, P.; Castellanos-Gomez, A.; Fernández-Díaz, M.T.; Fauth, F.; Alonso, J.A. Elucidating the Methylammonium (MA) Conformation in MAPbBr3 Perovskite with Application in Solar Cells. Inorg. Chem. 2017, 56, 14214–14219. [Google Scholar] [CrossRef] [PubMed]
- Abia, C.; López, C.A.; Cañadillas-Delgado, L.; Fernández-Diaz, M.T.; Alonso, J.A. Crystal Structure Thermal Evolution and Novel Orthorhombic Phase of Methylammonium Lead Bromide, CH3NH3PbBr3. Sci. Rep. 2022, 12, 18647. [Google Scholar] [CrossRef]
- Rainò, G.; Becker, M.A.; Bodnarchuk, M.I.; Mahrt, R.F.; Kovalenko, M.V.; Stöferle, T. Superfluorescence from Lead Halide Perovskite Quantum Dot Superlattices. Nature 2018, 563, 671–675. [Google Scholar] [CrossRef]





| MAPbBr3 | Mn-Doped MAPbBr3 (x = 0.17) | |||
|---|---|---|---|---|
| Time constants | t1 (ns) | t2 (ns) | t1 (ns) | t2 (ns) |
| Drop-cast film | 1 | 4.4 | 1.46 | 5.9 |
| Superlattice | 0.9 | 3.4 | 0.6 | 2.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phan, T.T.T.; Nguyen, T.T.K.; Mac, T.K.; Trinh, M.T. Enhancing Stability and Emissions in Metal Halide Perovskite Nanocrystals Through Mn2⁺ Doping. Nanomaterials 2025, 15, 847. https://doi.org/10.3390/nano15110847
Phan TTT, Nguyen TTK, Mac TK, Trinh MT. Enhancing Stability and Emissions in Metal Halide Perovskite Nanocrystals Through Mn2⁺ Doping. Nanomaterials. 2025; 15(11):847. https://doi.org/10.3390/nano15110847
Chicago/Turabian StylePhan, Thi Thu Trinh, Thi Thuy Kieu Nguyen, Trung Kien Mac, and Minh Tuan Trinh. 2025. "Enhancing Stability and Emissions in Metal Halide Perovskite Nanocrystals Through Mn2⁺ Doping" Nanomaterials 15, no. 11: 847. https://doi.org/10.3390/nano15110847
APA StylePhan, T. T. T., Nguyen, T. T. K., Mac, T. K., & Trinh, M. T. (2025). Enhancing Stability and Emissions in Metal Halide Perovskite Nanocrystals Through Mn2⁺ Doping. Nanomaterials, 15(11), 847. https://doi.org/10.3390/nano15110847







