Sustainable Antibacterial Chitin Nanofiber/ZnO Nanohybrid Materials: Ex Situ and In Situ Synthesis, Characterization and Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
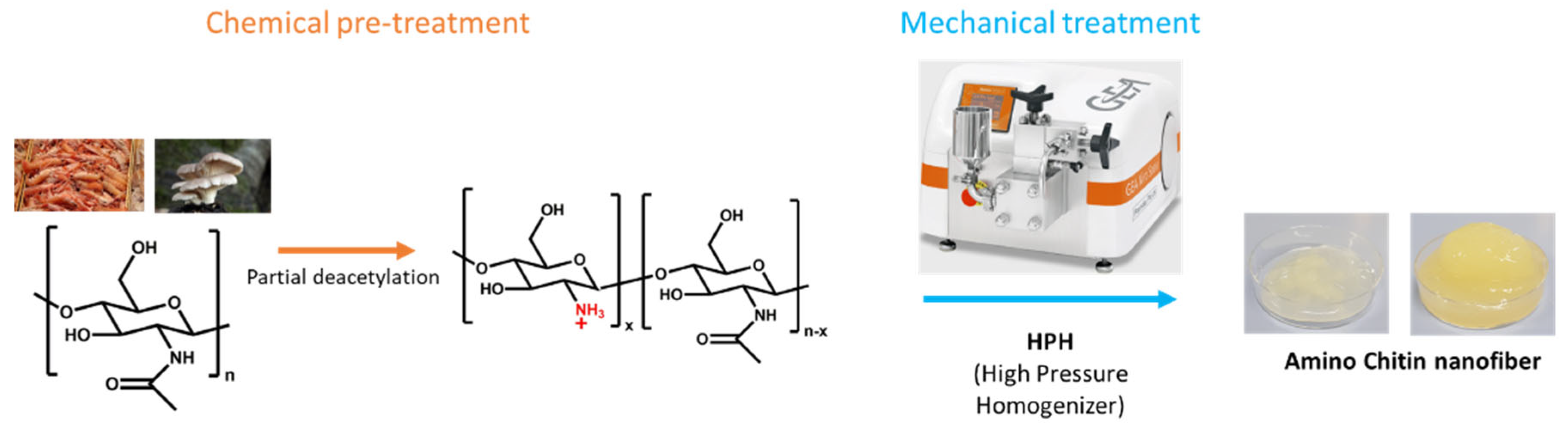
2.2. ChNFs Preparation
2.2.1. Chitin Deacetylation
2.2.2. Chitin Defibrillation
2.3. ChNFs Characterization
2.4. ZnO NPs Preparation and Characterization
2.4.1. ZnO NPs Preparation
2.4.2. ZnO NPs Characterization
2.5. ChNFs/Ex Situ and In Situ ZnO Nanohybrid Preparation and Characterization
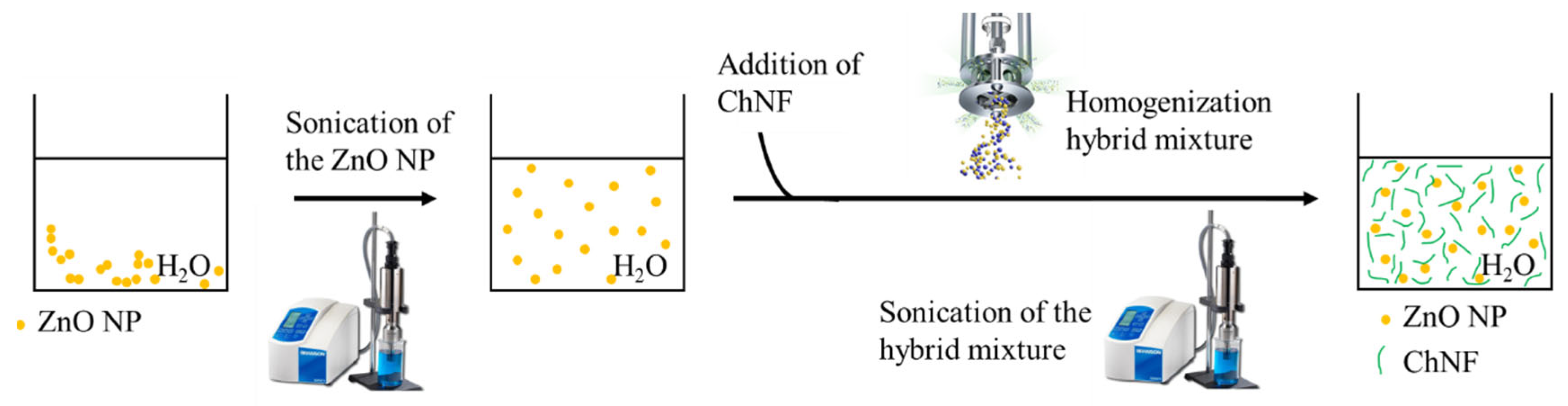
2.5.1. ChNFs/Ex Situ ZnO Nanohybrid Preparation
2.5.2. ChNFs/In Situ ZnO Nanohybrid Preparation
2.5.3. ChNFs/Ex Situ and In Situ ZnO Nanohybrid Physicochemical Characterization
2.5.4. ChNFs/Ex Situ and In Situ ZnO Nanohybrid Antibacterial Evaluation
2.5.5. In Vitro Cytotoxicity Studies
3. Results and Discussion
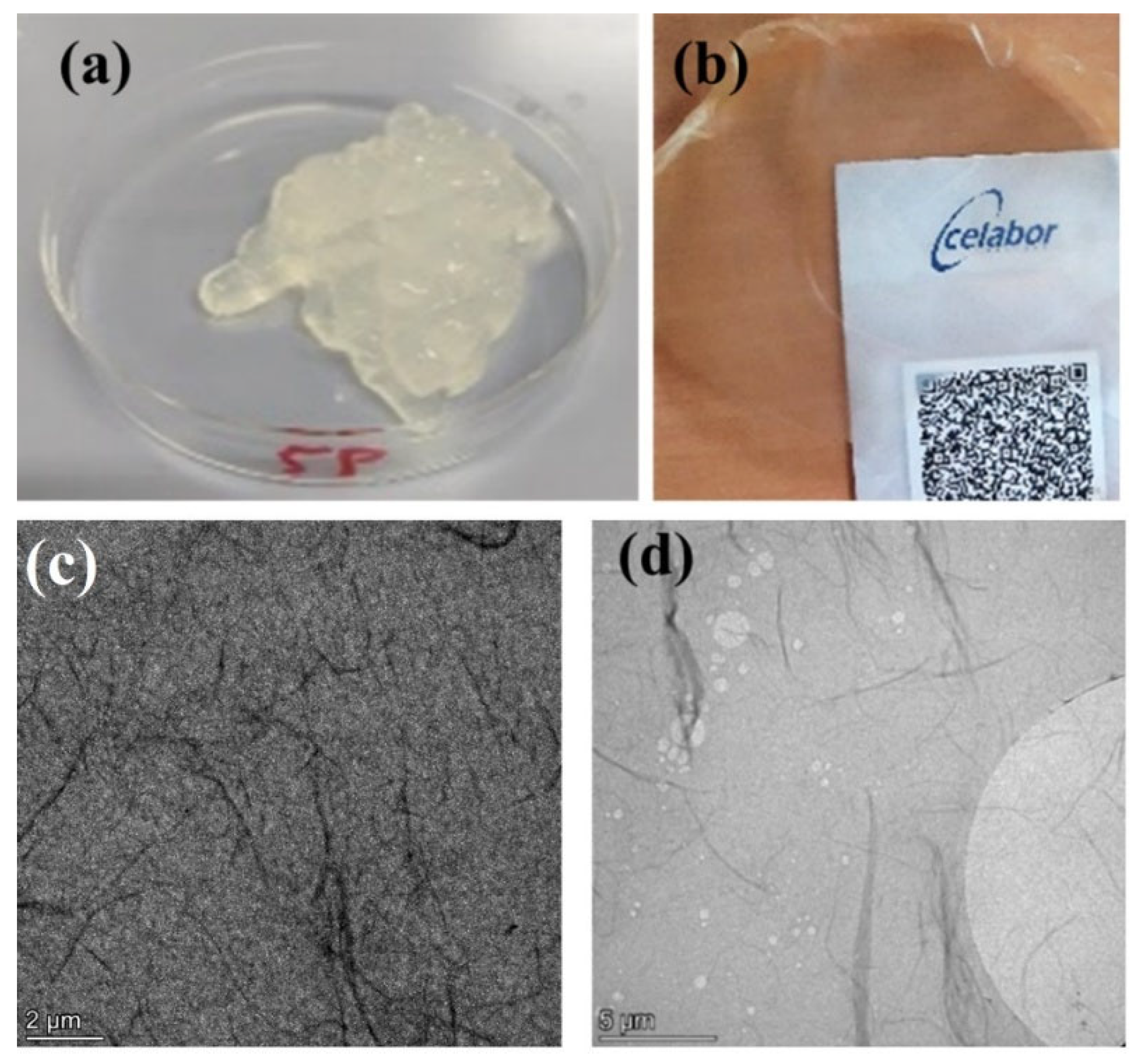
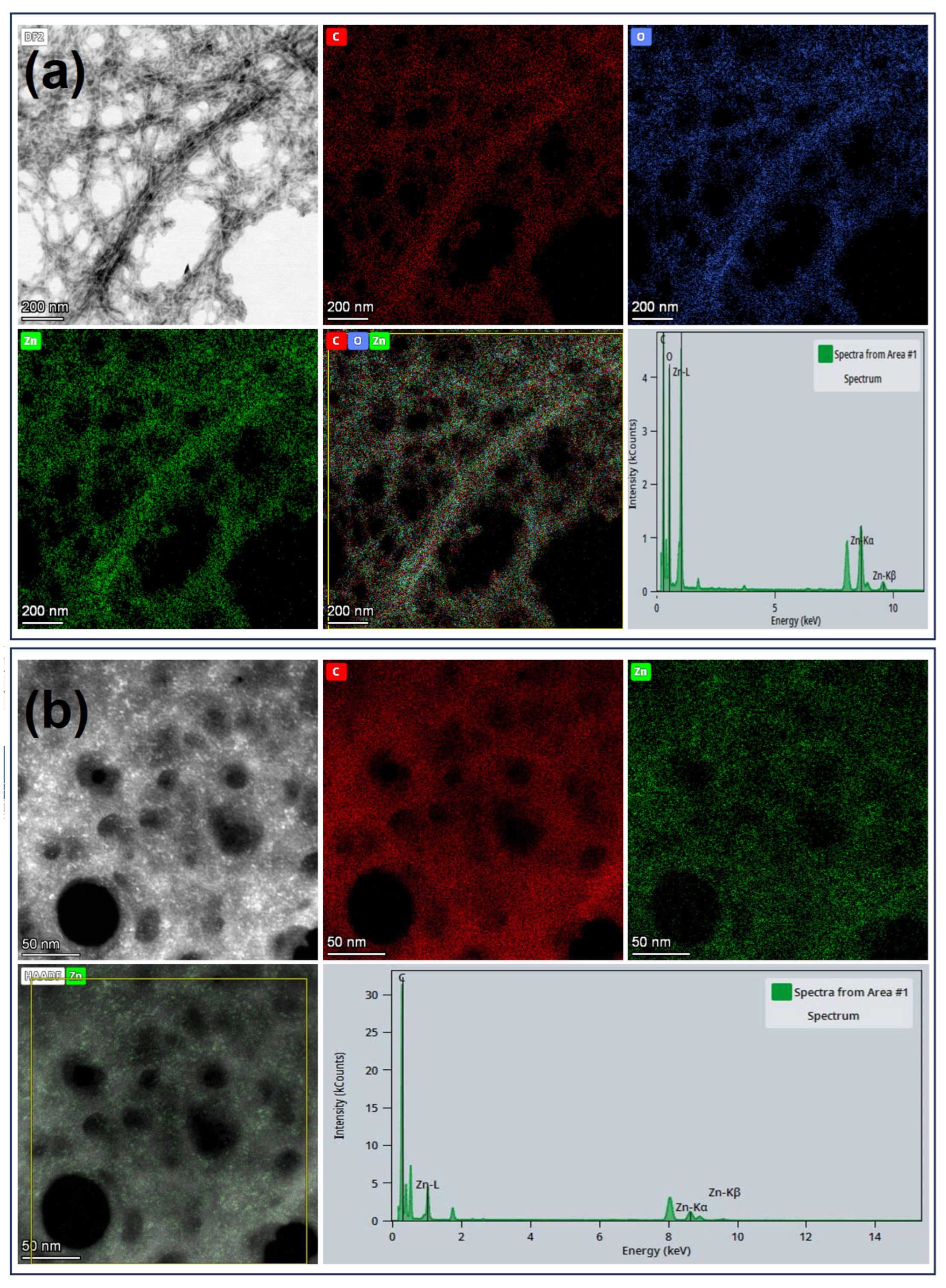
3.1. ChNFs
3.2. Synthesis and Physicochemical Characterization of ChNFs/ZnO Hybrid Nanomaterials
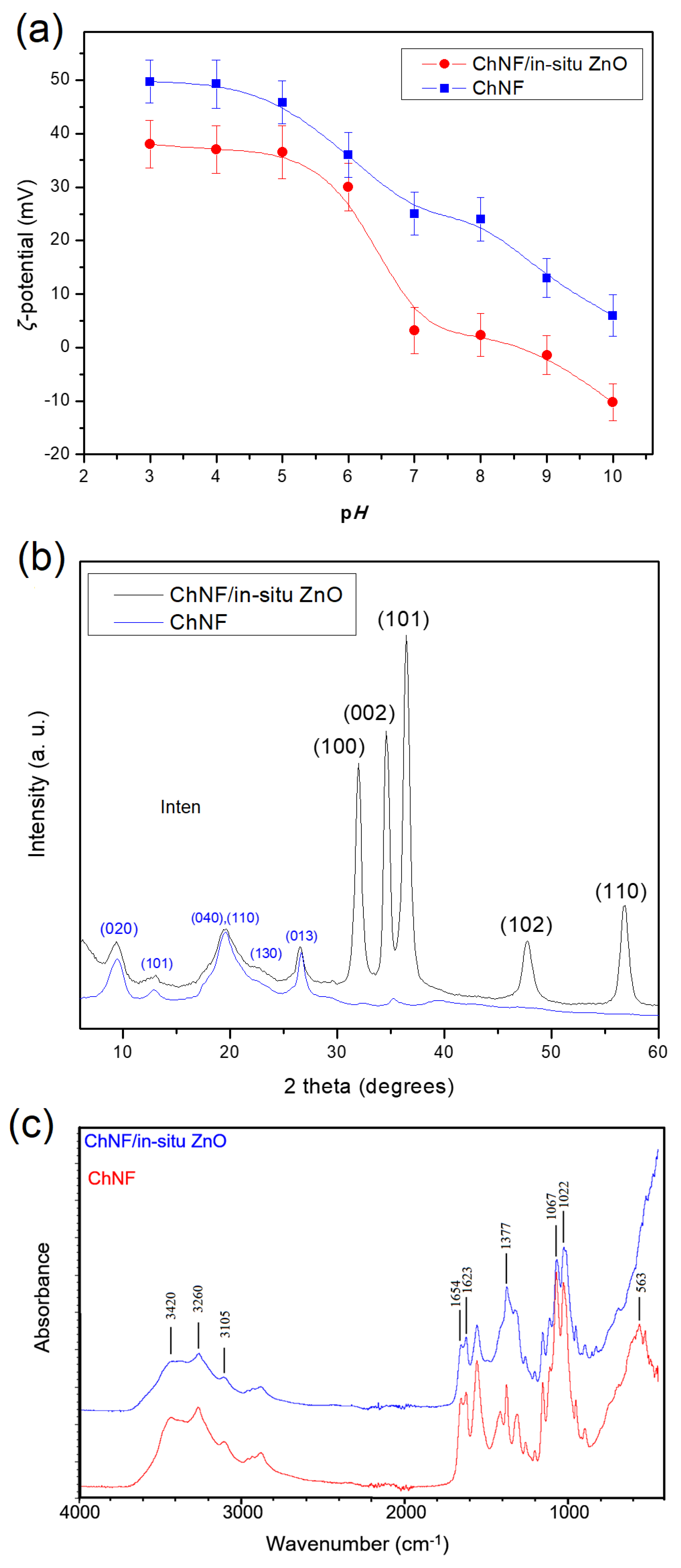
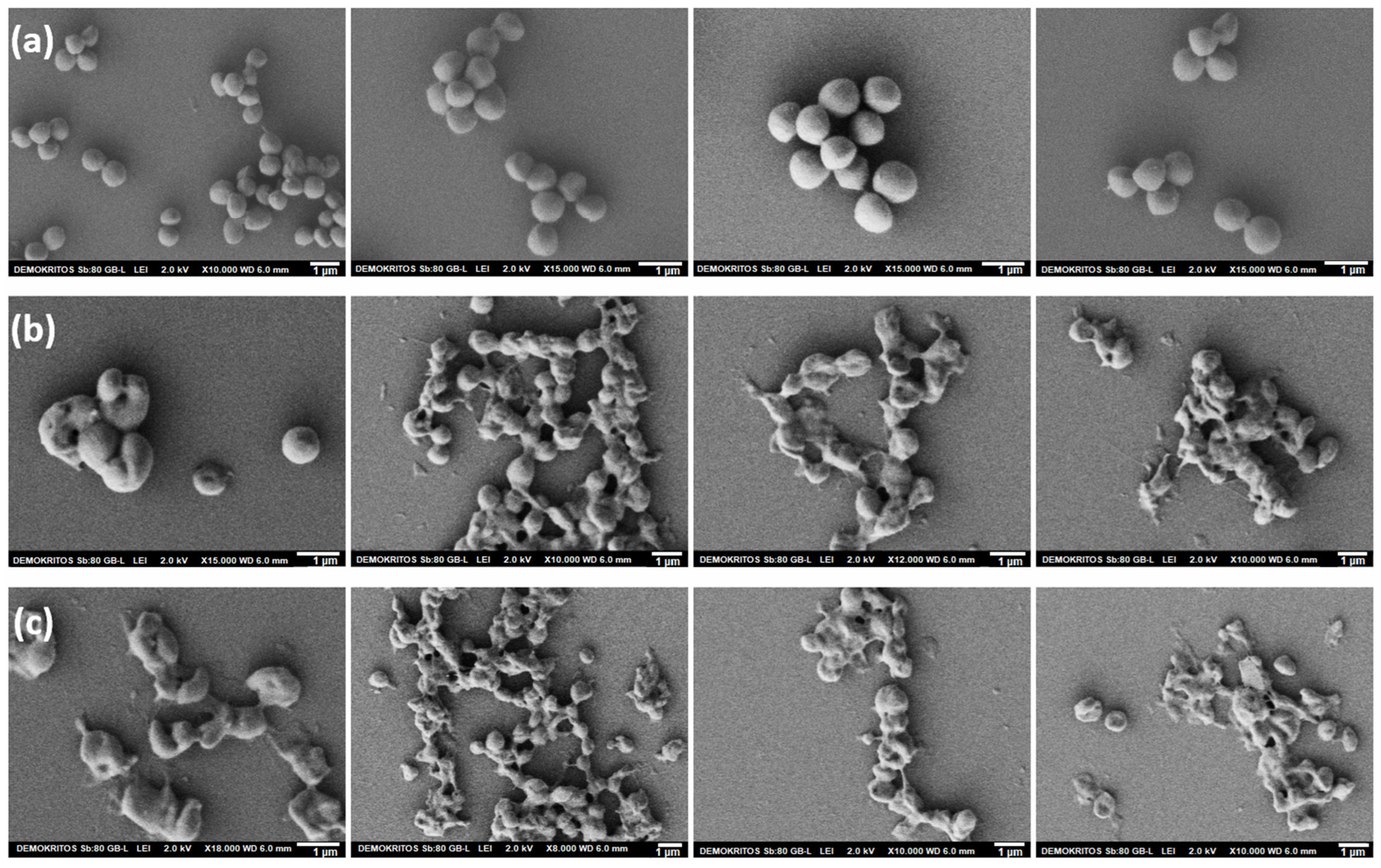
3.2.1. ChNFs/In Situ ZnO Nanohybrid
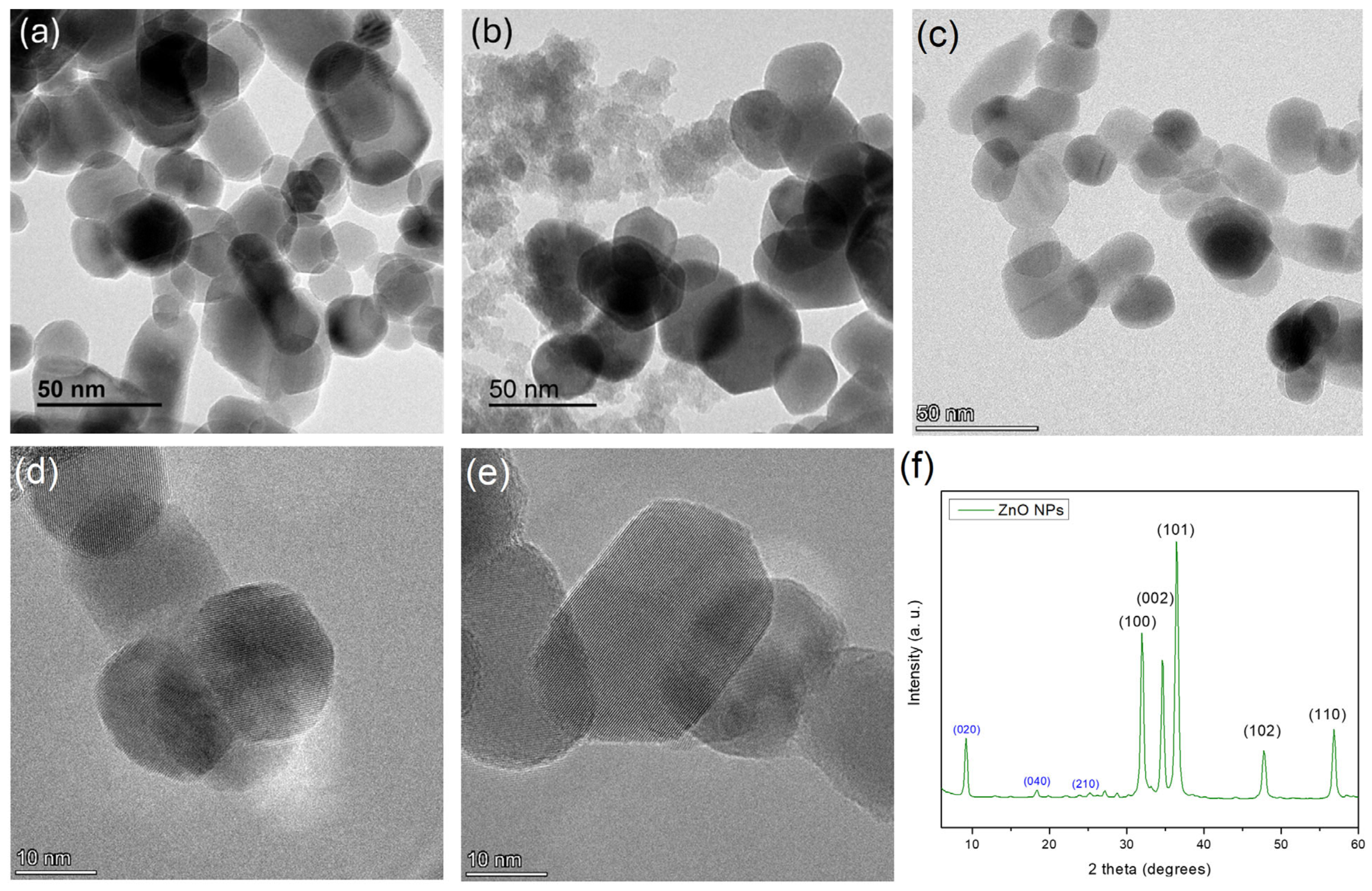
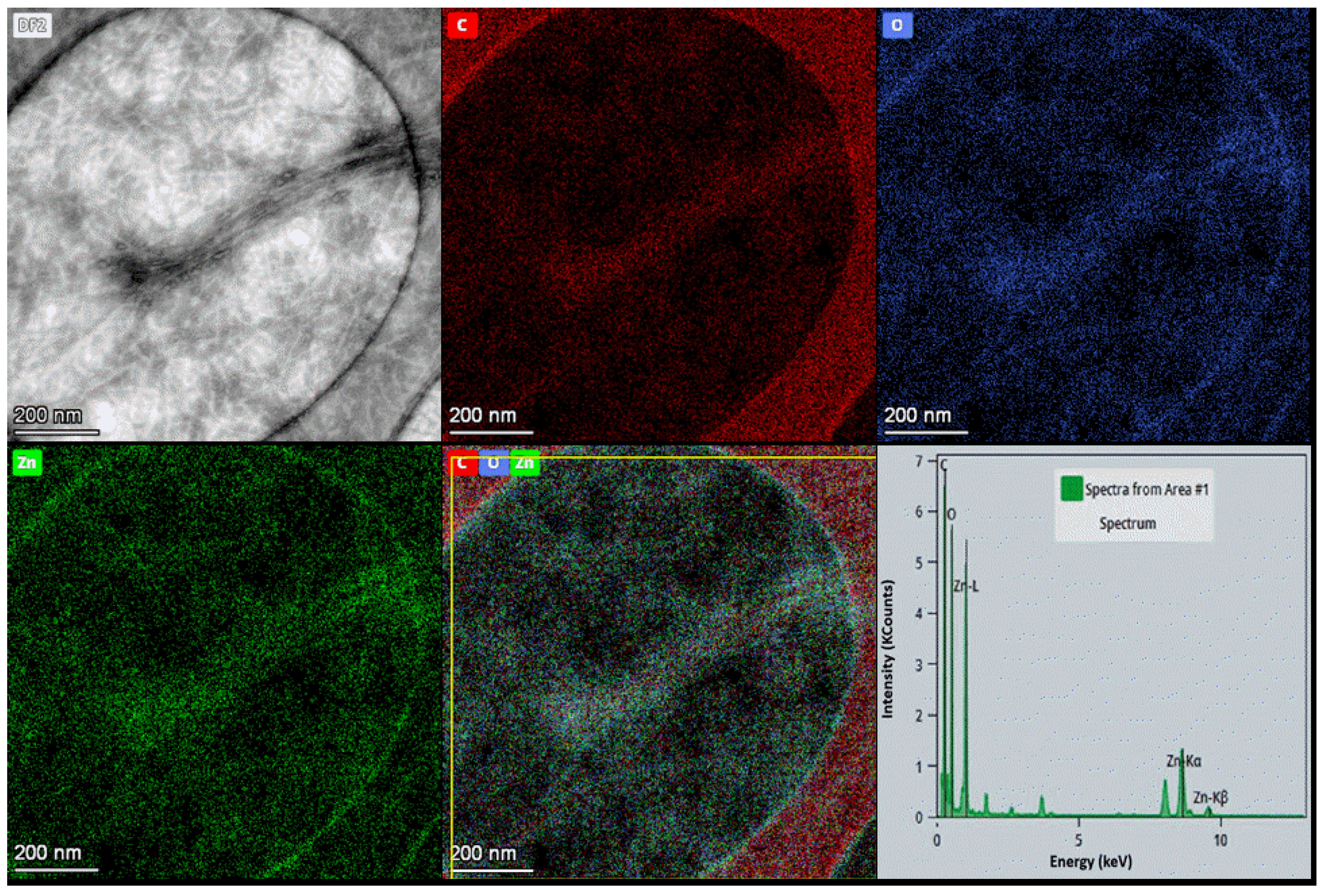
3.2.2. ChNFs/Ex Situ ZnO Nanohybrid
ZnO NPs
ChNFs/Ex Situ ZnO Preparation and Characterization
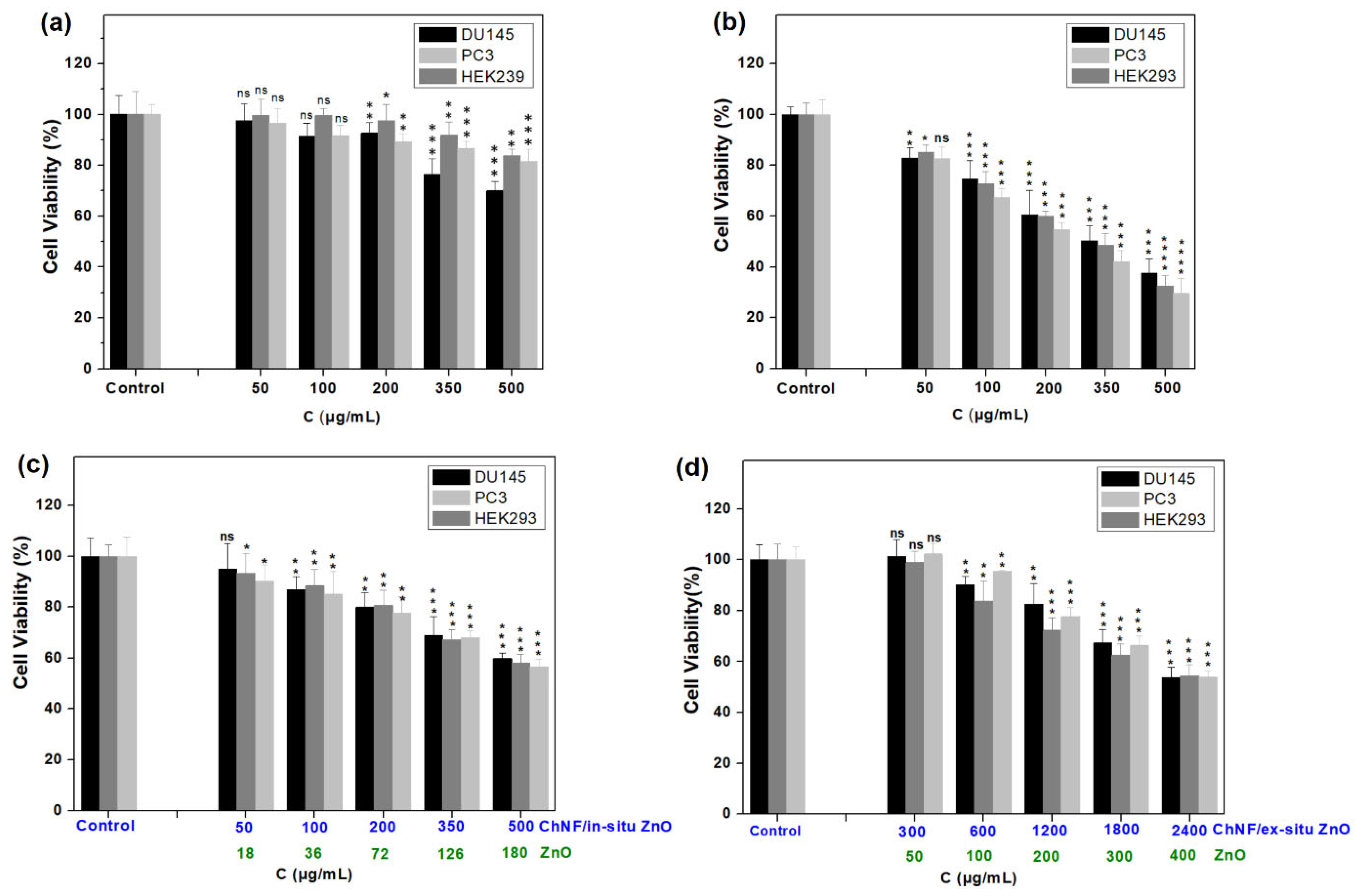
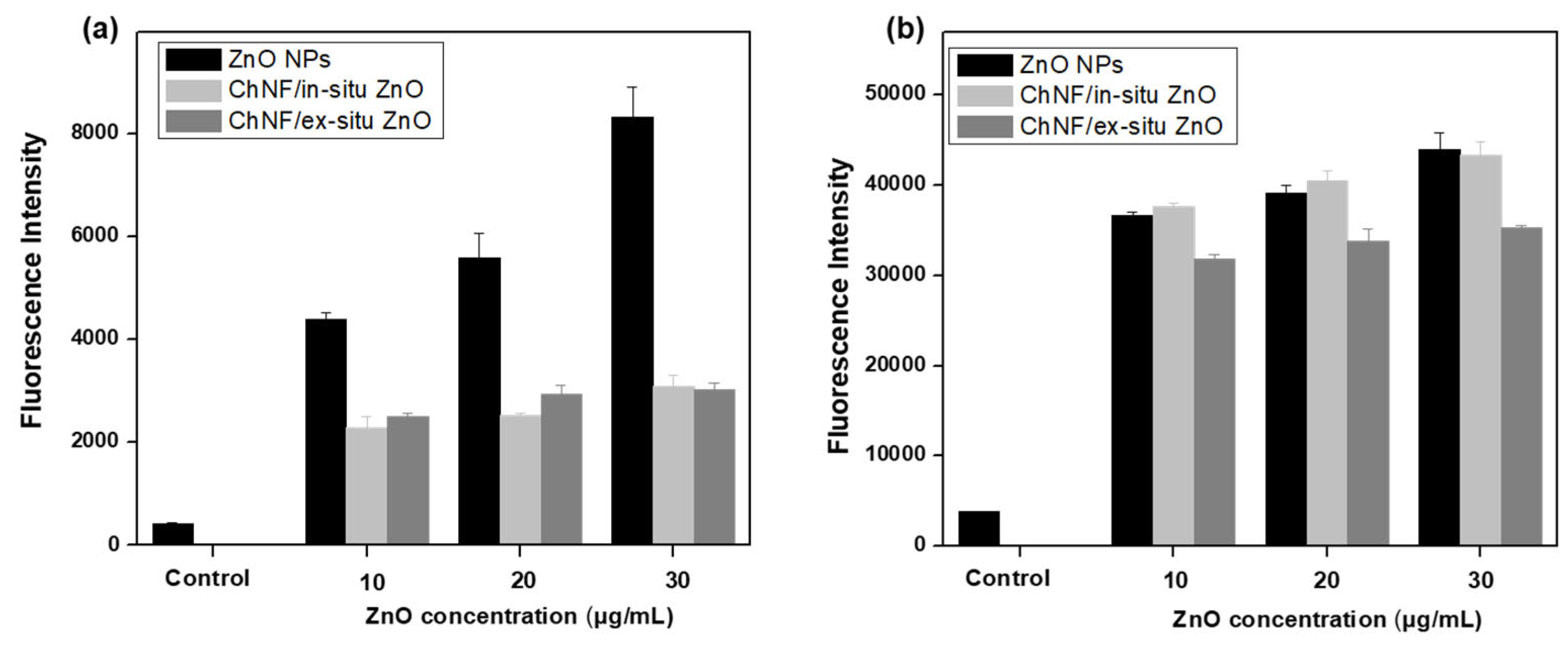
3.3. Cytocompatibility Studies of ChNFs/ZnO Nanohybrids
3.4. Antibacterial Activity of ChNFs/ZnO Nanohybrids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BET specific surface area | Brunauer–Emmett–Teller specific surface area |
| ChNFs | Chitin nanofibers |
| CLSI | Clinical Laboratory Standards Institute |
| DDA | Degree of deacetylation |
| DLS | Dynamic Light Scattering |
| E. coli | Escherichia coli |
| EDTA | Ethylenediaminetetraacetic acid |
| EDX | Energy Dispersive X-ray Spectroscopy |
| FTIR | Fourier Transformed Infrared Spectroscopy |
| FSP | Flame Spray Pyrolysis |
| HPH | High-Pressure Homogenizer |
| H2DCFDA | 2′,7′-dichlorodihydrofluorescein diacetate |
| MBC | Minimum bactericidal concentrations |
| MIC | Minimum inhibitory concentrations |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide |
| NPs | Nanoparticles |
| NFs | Nanofibers |
| ROS | Reactive Oxygen Species |
| SEM | Scanning Electron Microscopy |
| TEM | Transmission Electron Microscopy |
| S. aureus | Staphylococcus Aureus |
| UV-vis | Ultraviolet–visible spectroscopy |
| XRD | X-Ray Diffraction |
References
- Okeke, I.N.; de Kraker, M.E.A.; Van Boeckel, T.P.; Kumar, C.K.; Schmitt, H.; Gales, A.C.; Bertagnolio, S.; Sharland, M.; Laxminarayan, R. The Scope of the Antimicrobial Resistance Challenge. Lancet 2024, 403, 2426–2438. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Li, Y.; Xiao, S.; Yang, X.; Lin, C.; Norris, S.L.; Wei, D.; Hu, Z.; Ji, S. Logistic Growth of a Surface Contamination Network and Its Role in Disease Spread. Sci. Rep. 2017, 7, 14826. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Health Statistics 2024: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Ifuku, S.; Shervani, Z.; Saimoto, H. Chitin Nanofibers, Preparations and Applications. In Advances in Nanofibers; Maguire, R., Ed.; IntechOpen: Rijeka, Croatia, 2013. [Google Scholar]
- Rinaudo, M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Adina, M.; Georghița, M.; Vasile, O. Antibacterial Properties of Chitin and Chitosans. New Front. Chem. 2017, 26, 39–54. [Google Scholar]
- Kucharska, M.; Sikora, M.; Brzoza-Malczewska, K.; Owczarek, M. Antimicrobial Properties of Chitin and Chitosan. In Chitin and Chitosan; Springer: Berlin/Heidelberg, Germany, 2019; pp. 169–187. ISBN 9781119450467. [Google Scholar]
- Khattak, S.; Wahid, F.; Liu, L.-P.; Jia, S.-R.; Chu, L.-Q.; Xie, Y.-Y.; Li, Z.-X.; Zhong, C. Applications of Cellulose and Chitin/Chitosan Derivatives and Composites as Antibacterial Materials: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 1989–2006. [Google Scholar] [CrossRef]
- Mallakpour, S.; Sirous, F.; Hussain, C.M. A Journey to the World of Fascinating ZnO Nanocomposites Made of Chitosan, Starch, Cellulose, and Other Biopolymers: Progress in Recent Achievements in Eco-Friendly Food Packaging, Biomedical, and Water Remediation Technologies. Int. J. Biol. Macromol. 2021, 170, 701–716. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Petrisor, G.; Oprea, O.-C.; Trușcǎ, R.-D.; Ficai, A.; Andronescu, E.; Hudita, A.; Holban, A.M. Antimicrobial Hydroxyethyl-Cellulose-Based Composite Films with Zinc Oxide and Mesoporous Silica Loaded with Cinnamon Essential Oil. Pharmaceutics 2024, 16, 1225. [Google Scholar] [CrossRef]
- Raha, S.; Ahmaruzzaman, M. ZnO Nanostructured Materials and Their Potential Applications: Progress, Challenges and Perspectives. Nanoscale. Adv. 2022, 4, 1868–1925. [Google Scholar] [CrossRef]
- Sharma, V.; Shukla, R.K.; Saxena, N.; Parmar, D.; Das, M.; Dhawan, A. DNA Damaging Potential of Zinc Oxide Nanoparticles in Human Epidermal Cells. Toxicol. Lett. 2009, 185, 211–218. [Google Scholar] [CrossRef]
- Franklin, N.M.; Rogers, N.J.; Apte, S.C.; Batley, G.E.; Gadd, G.E.; Casey, P.S. Comparative Toxicity of Nanoparticulate ZnO, Bulk ZnO, and ZnCl2 to a Freshwater Microalga (Pseudokirchneriella subcapitata): The Importance of Particle Solubility. Environ. Sci. Technol. 2007, 41, 8484–8490. [Google Scholar] [CrossRef] [PubMed]
- Heinlaan, M.; Ivask, A.; Blinova, I.; Dubourguier, H.-C.; Kahru, A. Toxicity of Nanosized and Bulk ZnO, CuO and TiO2 to Bacteria Vibrio fischeri and Crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 2008, 71, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Esparza-González, S.C.; Sánchez-Valdés, S.; Ramírez-Barrón, S.N.; Loera-Arias, M.J.; Bernal, J.; Meléndez-Ortiz, H.I.; Betancourt-Galindo, R. Effects of Different Surface Modifying Agents on the Cytotoxic and Antimicrobial Properties of ZnO Nanoparticles. Toxicol. Vitr. 2016, 37, 134–141. [Google Scholar] [CrossRef]
- Halbus, A.F.; Horozov, T.S.; Paunov, V.N. Surface-Modified Zinc Oxide Nanoparticles for Antialgal and Antiyeast Applications. ACS Appl. Nano. Mater. 2020, 3, 440–451. [Google Scholar] [CrossRef]
- Luo, M.; Shen, C.; Feltis, B.N.; Martin, L.L.; Hughes, A.E.; Wright, P.F.A.; Turney, T.W. Reducing ZnO Nanoparticle Cytotoxicity by Surface Modification. Nanoscale 2014, 6, 5791–5798. [Google Scholar] [CrossRef]
- Mohammad, F.; Bwatanglang, I.B.; Al-Lohedan, H.A.; Shaik, J.P.; Al-Tilasi, H.H.; Soleiman, A.A. Influence of Surface Coating towards the Controlled Toxicity of ZnO Nanoparticles In Vitro. Coatings 2023, 13, 172. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, X.; Xiong, Z.; Huang, Q.; Yang, X.; Yan, H.; Ma, J.; Feng, Q.; Shen, Z. Novel Micro/Nanostructured TiO2/ZnO Coating with Antibacterial Capacity and Cytocompatibility. Ceram. Int. 2018, 44, 9711–9719. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Nair, S.V.; Tamura, H. Novel Chitin and Chitosan Nanofibers in Biomedical Applications. Biotechnol. Adv. 2010, 28, 142–150. [Google Scholar] [CrossRef]
- Tanpichai, S.; Pumpuang, L.; Srimarut, Y.; Woraprayote, W.; Malila, Y. Development of Chitin Nanofiber Coatings for Prolonging Shelf Life and Inhibiting Bacterial Growth on Fresh Cucumbers. Sci. Rep. 2023, 13, 13195. [Google Scholar] [CrossRef]
- Xu, J.; Liu, L.; Yu, J.; Zou, Y.; Wang, Z.; Fan, Y. DDA (Degree of Deacetylation) and PH-Dependent Antibacterial Properties of Chitin Nanofibers against Escherichia coli. Cellulose 2019, 26, 2279–2290. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, Y.; Jin, Y.; Oh, D.-H.; Fu, X. Chitin Nanofibers Prepared by Enzymatic Hydrolysis: Characterization and Application for Pickering Emulsions. Int. J. Biol. Macromol. 2024, 254, 127662. [Google Scholar] [CrossRef] [PubMed]
- Aouadi, A.; Saoud, D.H.; Laouini, S.E.; Rebiai, A.; Achouri, A.; Mohammed, H.A.; Bouafia, A.; Abdullah, J.A.A.; Alharthi, F. Synergistic Chitin-Zinc Nanocomposites from Shrimp Shell Waste: Characterization, Antioxidant, and Antibacterial Properties. Biomass Convers. Biorefin. 2025, 15, 545–561. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Chen, J.; Kalaiselvi, V.; Tungare, K.; Bhori, M.; González-Sánchez, Z.I.; Durán-Lara, E.F. Marine Polysaccharide Laminarin Embedded ZnO Nanoparticles and Their Based Chitosan Capped ZnO Nanocomposites: Synthesis, Characterization and In Vitro and In Vivo Toxicity Assessment. Environ. Res. 2022, 213, 113655. [Google Scholar] [CrossRef] [PubMed]
- Younes, N.; Pintus, G.; Al-Asmakh, M.; Rasool, K.; Younes, S.; Calzolari, S.; Mahmoud, K.A.; Nasrallah, G.K. “Safe” Chitosan/Zinc Oxide Nanocomposite Has Minimal Organ-Specific Toxicity in Early Stages of Zebrafish Development. ACS Biomater. Sci. Eng. 2020, 6, 38–47. [Google Scholar] [CrossRef]
- Wijesena, R.N.; Tissera, N.; Perera, R.; de Silva, K.M.N. Side Selective Surface Modification of Chitin Nanofibers on Anionically Modified Cotton Fabrics. Carbohydr. Polym. 2014, 109, 56–63. [Google Scholar] [CrossRef]
- Manabe, K.; Tanaka, C.; Moriyama, Y.; Tenjimbayashi, M.; Nakamura, C.; Tokura, Y.; Matsubayashi, T.; Kyung, K.-H.; Shiratori, S. Chitin Nanofibers Extracted from Crab Shells in Broadband Visible Antireflection Coatings with Controlling Layer-by-Layer Deposition and the Application for Durable Antifog Surfaces. ACS Appl. Mater. Interfaces 2016, 8, 31951–31958. [Google Scholar] [CrossRef]
- Dimitriou, C.; Psathas, P.; Solakidou, M.; Deligiannakis, Y. Advanced Flame Spray Pyrolysis (FSP) Technologies for Engineering Multifunctional Nanostructures and Nanodevices. Nanomaterials 2023, 13, 3006. [Google Scholar] [CrossRef]
- Teoh, W.Y.; Amal, R.; Mädler, L. Flame Spray Pyrolysis: An Enabling Technology for Nanoparticles Design and Fabrication. Nanoscale 2010, 2, 1324–1347. [Google Scholar] [CrossRef]
- Kasaai, M.R. A Review of Several Reported Procedures to Determine the Degree of N-Acetylation for Chitin and Chitosan Using Infrared Spectroscopy. Carbohydr. Polym. 2008, 71, 497–508. [Google Scholar] [CrossRef]
- CLSI M26; Methods for Determining Bactericidal Activity of Antimicrobial Agents. Clinical and Laboratory Standards Institute (CLSI): Malvern, PA, USA, 1999; Approved Guideline, CLSI Document M26-A.
- CLSI M07; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Clinical and Laboratory Standards Institute (CLSI): Malvern, PA, USA, 2012; Approved Standard, 9th Ed., CLSI Document M07-A9.
- Kuczyńska-Wiśnik, D.; Matuszewska, E.; Furmanek-Blaszk, B.; Leszczyńska, D.; Grudowska, A.; Szczepaniak, P.; Laskowska, E. Antibiotics Promoting Oxidative Stress Inhibit Formation of Escherichia coli Biofilm via Indole Signalling. Res. Microbiol. 2010, 161, 847–853. [Google Scholar] [CrossRef]
- Janardan, S.; Suman, P.; Ragul, G.; Anjaneyulu, U.; Shivendu, R.; Dasgupta, N.; Ramalingam, C.; Swamiappan, S.; Vijayakrishna, K.; Sivaramakrishna, A. Assessment on the Antibacterial Activity of Nanosized Silica Derived from Hypercoordinated Silicon(iv) Precursors. RSC Adv. 2016, 6, 66394–66406. [Google Scholar] [CrossRef]
- Lyra, K.-M.; Tournis, I.; Subrati, M.; Spyrou, K.; Papavasiliou, A.; Athanasekou, C.; Papageorgiou, S.; Sakellis, E.; Karakassides, M.A.; Sideratou, Z. Carbon Nanodisks Decorated with Guanidinylated Hyperbranched Polyethyleneimine Derivatives as Efficient Antibacterial Agents. Nanomaterials 2024, 14, 677. [Google Scholar] [CrossRef]
- Panagiotaki, K.N.; Lyra, K.; Papavasiliou, A.; Stamatakis, K.; Sideratou, Z. Synthesis of N-Sulfopropylated Hyperbranched Polyethyleneimine with Enhanced Biocompatibility and Antimicrobial Activity. Chempluschem 2025, 90. [Google Scholar] [CrossRef] [PubMed]
- Boonmahitthisud, A.; Thongdonson, K.; Tanpichai, S. Preparation of Chitin Nanofibers from Shrimp Shell Waste by Partial Deacetylation and Mechanical Treatment. J. Nat. Fibers 2023, 20, 2229515. [Google Scholar] [CrossRef]
- Chang, S.-H.; Lin, H.-T.V.; Wu, G.-J.; Tsai, G.J. PH Effects on Solubility, Zeta Potential, and Correlation between Antibacterial Activity and Molecular Weight of Chitosan. Carbohydr. Polym. 2015, 134, 74–81. [Google Scholar] [CrossRef]
- Tsai, W.-C.; Wang, S.-T.; Chang, K.-L.B.; Tsai, M.-L. Enhancing Saltiness Perception Using Chitin Nanomaterials. Polymers 2019, 11, 719. [Google Scholar] [CrossRef]
- Desmaisons, J.; Boutonnet, E.; Rueff, M.; Dufresne, A.; Bras, J. A New Quality Index for Benchmarking of Different Cellulose Nanofibrils. Carbohydr. Polym. 2017, 174, 318–329. [Google Scholar] [CrossRef]
- Ifuku, S.; Ikuta, A.; Egusa, M.; Kaminaka, H.; Izawa, H.; Morimoto, M.; Saimoto, H. Preparation of High-Strength Transparent Chitosan Film Reinforced with Surface-Deacetylated Chitin Nanofibers. Carbohydr. Polym. 2013, 98, 1198–1202. [Google Scholar] [CrossRef]
- Machida, J.; Suenaga, S.; Osada, M. Effect of the Degree of Acetylation on the Physicochemical Properties of α-Chitin Nanofibers. Int. J. Biol. Macromol. 2020, 155, 350–357. [Google Scholar] [CrossRef]
- Suenaga, S.; Osada, M. Systematic Dynamic Viscoelasticity Measurements for Chitin Nanofibers Prepared with Various Concentrations, Disintegration Times, Acidities, and Crystalline Structures. Int. J. Biol. Macromol. 2018, 115, 431–437. [Google Scholar] [CrossRef]
- Sigoli, F.A.; Davolos, M.R.; Jafelicci, M. Morphological Evolution of Zinc Oxide Originating from Zinc Hydroxide Carbonate. J. Alloys. Compd. 1997, 262–263, 292–295. [Google Scholar] [CrossRef]
- Vergés, M.A.; Mifsud, A.; Serna, C.J. Formation of Rod-like Zinc Oxide Microcrystals in Homogeneous Solutions. J. Chem. Soc. Faraday Trans. 1990, 86, 959–963. [Google Scholar] [CrossRef]
- Andrés-Vergés, M.; Serna, C.J. Morphological Characterization of ZnO Powders by X-Ray and IR Spectroscopy. J. Mater. Sci. Lett. 1988, 7, 970–972. [Google Scholar] [CrossRef]
- Cárdenas, G.; Cabrera, G.; Taboada, E.; Miranda, S.P. Chitin Characterization by SEM, FTIR, XRD, and 13C Cross Polarization/Mass Angle Spinning NMR. J. Appl. Polym. Sci. 2004, 93, 1876–1885. [Google Scholar] [CrossRef]
- Ivanova, T.; Harizanova, A.; Koutzarova, T.; Vertruyen, B. Study of ZnO Sol–Gel Films: Effect of Annealing. Mater. Lett. 2010, 64, 1147–1149. [Google Scholar] [CrossRef]
- Yang, Z.; Ye, Z.; Xu, Z.; Zhao, B. Effect of the Morphology on the Optical Properties of ZnO Nanostructures. Phys. E Low Dimens. Syst. Nanostruct. 2009, 42, 116–119. [Google Scholar] [CrossRef]
- Yuvaraj, D.; Rao, K.N. Optical and Electrical Properties of ZnO Films Deposited by Activated Reactive Evaporation. Vacuum 2008, 82, 1274–1279. [Google Scholar] [CrossRef]
- Ghasemzadeh, M.A.; Safaei-Ghomi, J. Synthesis and Characterization of ZnO Nanoparticles: Application to One-Pot Synthesis of Benzo[b][1,5]Diazepines. Cogent. Chem. 2015, 1, 1095060. [Google Scholar] [CrossRef]
- Widiyandari, H.; Ketut Umiati, N.A.; Dwi Herdianti, R. Synthesis and Photocatalytic Property of Zinc Oxide (ZnO) Fine Particle Using Flame Spray Pyrolysis Method. J. Phys. Conf. Ser. 2018, 1025, 012004. [Google Scholar] [CrossRef]
- Raoufi, D. Synthesis and microstructural properties of ZnO nanoparticles prepared by precipitation method. Renew. Energy 2013, 50, 932–937. [Google Scholar] [CrossRef]
- Yogamalar, R.; Srinivasan, R.; Vinu, A.; Ariga, K.; Bose, A.C. X-ray peak broadening analysis in ZnO nanoparticles. Solid State Commun. 2009, 149, 1919–1923. [Google Scholar] [CrossRef]
- Ashwini, J.; Aswathy, T.R.; Rahul, A.B.; Thara, G.M.; Nair, A.S. Synthesis and Characterization of Zinc Oxide Nanoparticles Using Acacia Caesia Bark Extract and Its Photocatalytic and Antimicrobial Activities. Catalysts 2021, 11, 1507. [Google Scholar] [CrossRef]
- Suzuki, S.; Kukkadapu, G.; Kiuchi, S.; Wagnon, S.W.; Kinoshita, K.; Takeda, Y.; Sakaida, S.; Konno, M.; Tanaka, K.; Oguma, M.; et al. Formation of PAHs, Phenol, Benzofuran, and Dibenzofuran in a Flow Reactor from the Oxidation of Ethylene, Toluene, and n-Decane. Combust. Flame 2022, 241, 112136. [Google Scholar] [CrossRef]
- Shi, X.; Wang, Q.; Violi, A. Chemical Pathways for the Formation of Benzofuran and Dibenzofuran in Combustion. Combust. Flame 2020, 212, 216–233. [Google Scholar] [CrossRef]
- Collin, G.; Höke, H. Benzofurans. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Cardoso, D.; Narcy, A.; Durosoy, S.; Chevalier, Y. The PH Dependence of Dissolution Kinetics of Zinc Oxide. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129653. [Google Scholar] [CrossRef]
- Liao, C.; Jin, Y.; Li, Y.; Tjong, S.C. Interactions of Zinc Oxide Nanostructures with Mammalian Cells: Cytotoxicity and Photocatalytic Toxicity. Int. J. Mol. Sci. 2020, 21, 6305. [Google Scholar] [CrossRef]
- Kocer, N.N.; Uslu, G.; Cuci, Y. The Adsorption of Zn(II) Ions onto Chitin: Determination of Equilibrium, Kinetic and Thermodynamic Parameters. Adsorpt. Sci. Technol. 2008, 26, 333–344. [Google Scholar] [CrossRef]
- Jaafarzadeh, N.; Mengelizadeh, N.; Takdastan, A.; Farsani, M.; Niknam, N.; Aalipour, M.; Hadei, M.; Bahrami, P. Biosorption of Heavy Metals from Aqueous Solutions onto Chitin. Int. J. Environ. Health Eng. 2015, 4, 7. [Google Scholar] [CrossRef]
- Jaafarzadeh, N.; Mengelizadeh, N.; Takdastan, A.; Heidari-Farsani, M.; Niknam, N. Adsorption of Zn (II) from Aqueous Solution by Using Chitin Extraction from Crustaceous Shell. J. Adv. Environ. Health Res. 2014, 2, 110–119. [Google Scholar] [CrossRef]
- No, H.K.; Young Park, N.; Ho Lee, S.; Meyers, S.P. Antibacterial Activity of Chitosans and Chitosan Oligomers with Different Molecular Weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A Mini Review of Antibacterial Properties of ZnO Nanoparticles. Front. Phys. 2021, 9, 641481. [Google Scholar] [CrossRef]
- Abebe, B.; Zereffa, E.A.; Tadesse, A.; Murthy, H.C.A. A Review on Enhancing the Antibacterial Activity of ZnO: Mechanisms and Microscopic Investigation. Nanoscale Res. Lett. 2020, 15, 190. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nanomicro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; ur Rahman, A.; Tajuddin; Husen, A. Properties of Zinc Oxide Nanoparticles and Their Activity Against Microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef]
- Applerot, G.; Lipovsky, A.; Dror, R.; Perkas, N.; Nitzan, Y.; Lubart, R.; Gedanken, A. Enhanced Antibacterial Activity of Nanocrystalline ZnO Due to Increased ROS-Mediated Cell Injury. Adv. Funct. Mater. 2009, 19, 842–852. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, L.; Wen, D.; Ding, Y. Role of Physical and Chemical Interactions in the Antibacterial Behavior of ZnO Nanoparticles against E. coli. Mater. Sci. Eng. C 2016, 69, 1361–1366. [Google Scholar] [CrossRef]
- Wu, T.; Huang, L.; Sun, J.; Sun, J.; Yan, Q.; Duan, B.; Zhang, L.; Shi, B. Multifunctional Chitin-Based Barrier Membrane with Antibacterial and Osteogenic Activities for the Treatment of Periodontal Disease. Carbohydr. Polym. 2021, 269, 118276. [Google Scholar] [CrossRef]
- Feng, M.; Lu, X.; Jiang, K.; Zhang, J.; Xin, J.; Shi, C.; Wang, K.; Zhang, S. One-Step Preparation of an Antibacterial Chitin/Zn Composite from Shrimp Shells Using Urea-Zn(OAc)2 2H2O Aqueous Solution. Green Chem. 2018, 20, 2212–2217. [Google Scholar] [CrossRef]
- Song, Z.; Wu, Y.; Wang, H.; Han, H. Synergistic Antibacterial Effects of Curcumin Modified Silver Nanoparticles through ROS-Mediated Pathways. Mater. Sci. Eng. C 2019, 99, 255–263. [Google Scholar] [CrossRef]
- Peter, A.; Jose, J.; Bhat, S.G. A Modified Fluorescent Probe Protocol for Evaluating the Reactive Oxygen Species Generation by Metal and Metal Oxide Nanoparticles in Gram-Positive and Gram-Negative Organisms. Results Eng. 2024, 24, 102925. [Google Scholar] [CrossRef]
- Happy Agarwal; Soumya Menon; Venkat Kumar, S.; Rajeshkumar, S. Mechanistic Study on Antibacterial Action of Zinc Oxide Nanoparticles Synthesized Using Green Route. Chem. Biol. Interact. 2018, 286, 60–70. [Google Scholar] [CrossRef]
- Mendes, C.R.; Dilarri, G.; Forsan, C.F.; de Moraes Ruy Sapata, V.; Lopes, P.R.M.; de Moraes, P.B.; Montagnolli, R.N.; Ferreira, H.; Bidoia, E.D. Antibacterial Action and Target Mechanisms of Zinc Oxide Nanoparticles against Bacterial Pathogens. Sci. Rep. 2022, 12, 2658. [Google Scholar] [CrossRef] [PubMed]
- Lallo da Silva, B.; Abuçafy, M.P.; Berbel Manaia, E.; Oshiro Junior, J.A.; Chiari-Andréo, B.G.; Pietro, R.C.L.R.; Chiavacci, L.A. Relationship Between Structure And Antimicrobial Activity Of Zinc Oxide Nanoparticles: An Overview. Int. J. Nanomed. 2019, 14, 9395–9410. [Google Scholar] [CrossRef]
- Patra, J.K.; Baek, K.-H. Antibacterial Activity and Synergistic Antibacterial Potential of Biosynthesized Silver Nanoparticles against Foodborne Pathogenic Bacteria along with Its Anticandidal and Antioxidant Effects. Front. Microbiol. 2017, 8, 167. [Google Scholar] [CrossRef]
- Amro, N.A.; Kotra, L.P.; Wadu-Mesthrige, K.; Bulychev, A.; Mobashery, S.; Liu, G. High-Resolution Atomic Force Microscopy Studies of the Escherichia coli Outer Membrane: Structural Basis for Permeability. Langmuir 2000, 16, 2789–2796. [Google Scholar] [CrossRef]

| E. coli | S. aureus | ||||
|---|---|---|---|---|---|
| MIC (μg/mL) | MBC (μg/mL) | MIC (μg/mL) | MBC (μg/mL) | ||
| ZnO NPs | 10 | 50 | 20 | 50 | |
| ChNFs | >500 | >500 | 100 | 300 | |
| ChNFs/in situ ZnO | Total ZnO | 300 108 | 350 126 | 200 72 | 300 108 |
| ChNFs/ex situ ZnO | Total ZnO | 1200 200 | 1200 200 | 240 40 | 300 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piffet, C.; Thomassin, J.-M.; Stierlin, E.; Tchoumtchoua, J.; Fernández, C.; Mateo, M.; Hernández, L.; Lyra, K.M.; Papavasiliou, A.; Sakellis, E.; et al. Sustainable Antibacterial Chitin Nanofiber/ZnO Nanohybrid Materials: Ex Situ and In Situ Synthesis, Characterization and Evaluation. Nanomaterials 2025, 15, 809. https://doi.org/10.3390/nano15110809
Piffet C, Thomassin J-M, Stierlin E, Tchoumtchoua J, Fernández C, Mateo M, Hernández L, Lyra KM, Papavasiliou A, Sakellis E, et al. Sustainable Antibacterial Chitin Nanofiber/ZnO Nanohybrid Materials: Ex Situ and In Situ Synthesis, Characterization and Evaluation. Nanomaterials. 2025; 15(11):809. https://doi.org/10.3390/nano15110809
Chicago/Turabian StylePiffet, Caroline, Jean-Michel Thomassin, Emilie Stierlin, Job Tchoumtchoua, Claudio Fernández, Marta Mateo, Leyre Hernández, Kyriaki Marina Lyra, Aggeliki Papavasiliou, Elias Sakellis, and et al. 2025. "Sustainable Antibacterial Chitin Nanofiber/ZnO Nanohybrid Materials: Ex Situ and In Situ Synthesis, Characterization and Evaluation" Nanomaterials 15, no. 11: 809. https://doi.org/10.3390/nano15110809
APA StylePiffet, C., Thomassin, J.-M., Stierlin, E., Tchoumtchoua, J., Fernández, C., Mateo, M., Hernández, L., Lyra, K. M., Papavasiliou, A., Sakellis, E., Katsaros, F. K., Sideratou, Z., & Tsiourvas, D. (2025). Sustainable Antibacterial Chitin Nanofiber/ZnO Nanohybrid Materials: Ex Situ and In Situ Synthesis, Characterization and Evaluation. Nanomaterials, 15(11), 809. https://doi.org/10.3390/nano15110809










