Effect of Steam to Carbon Dioxide Ratio on the Performance of a Solid Oxide Cell for H2O/CO2 Co-Electrolysis
Abstract
1. Introduction
2. Materials and Methods
- 31% H2O-62% CO2-4% H2 in He (S/C= 0.5)
- 31% H2O-31% CO2-19% H2 in He (S/C= 1)
- 62% H2O-31% CO2-4% H2 in He (S/C= 2)
- 31% H2O-62% CO2 in He (S/C= 0.5)
- 31% H2O-31% CO2 in He (S/C= 1)
- 62% H2O-31% CO2 in He (S/C= 2)
3. Results and Discussion
3.1. Electrochemical Characterization
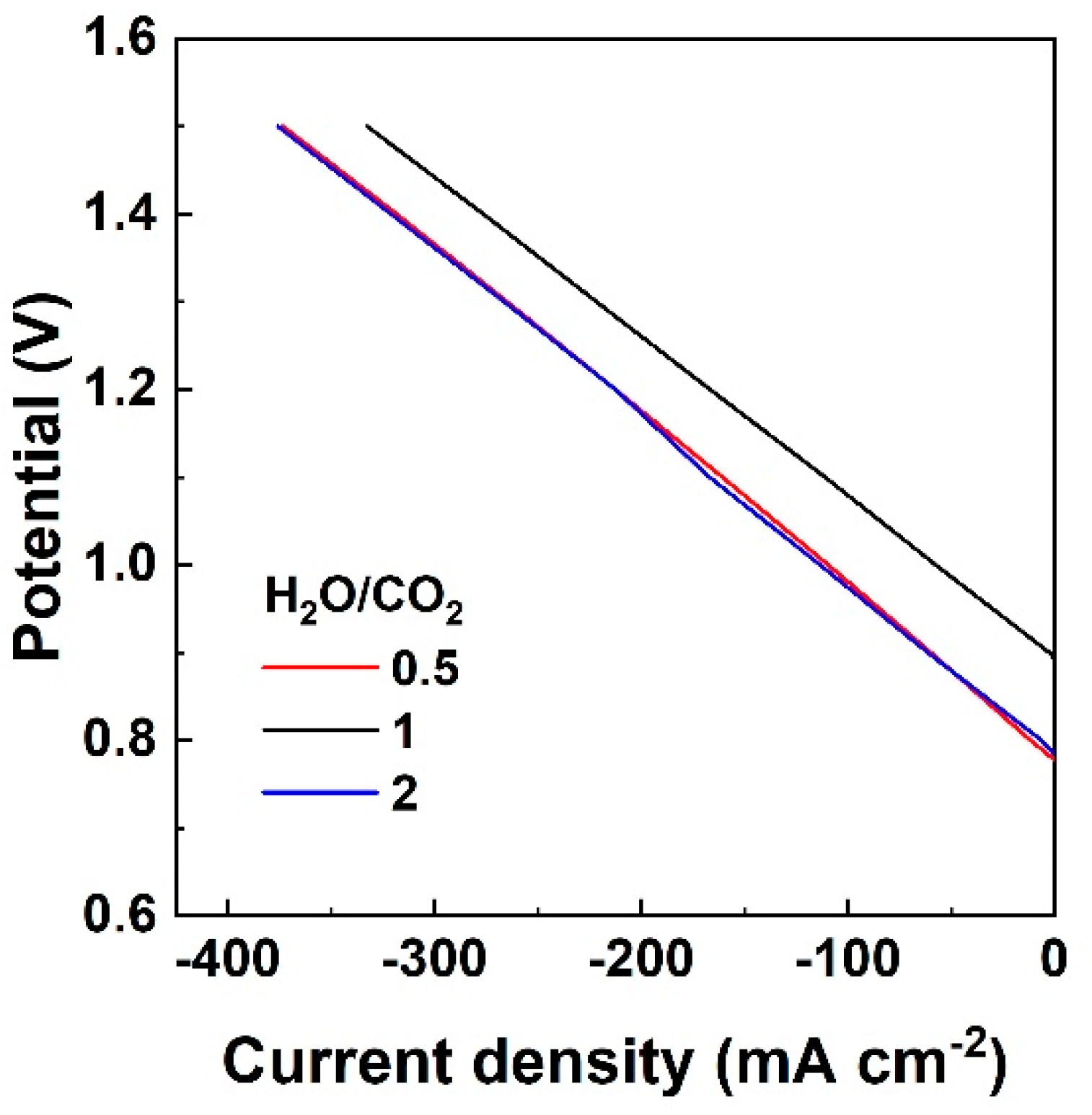
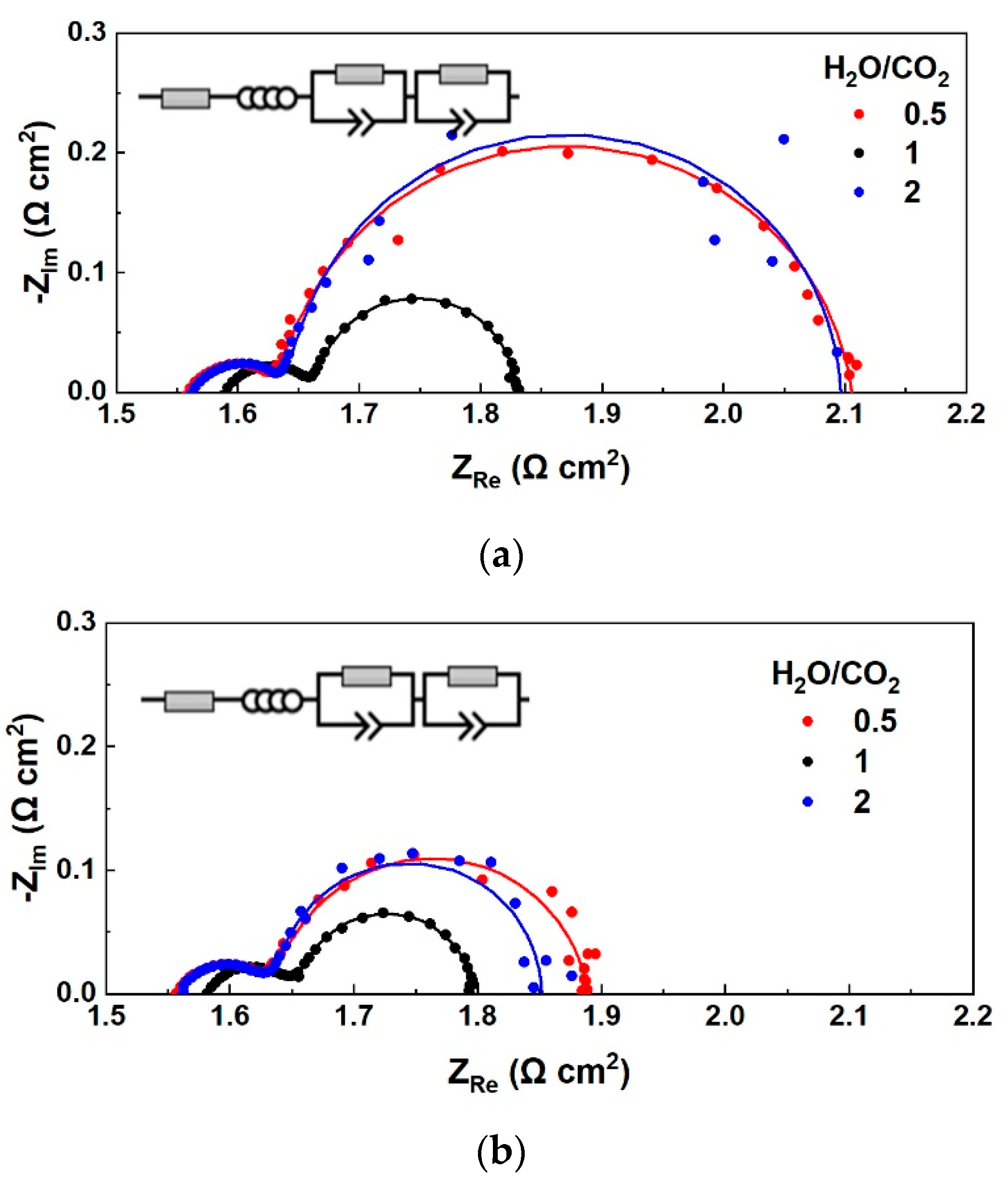
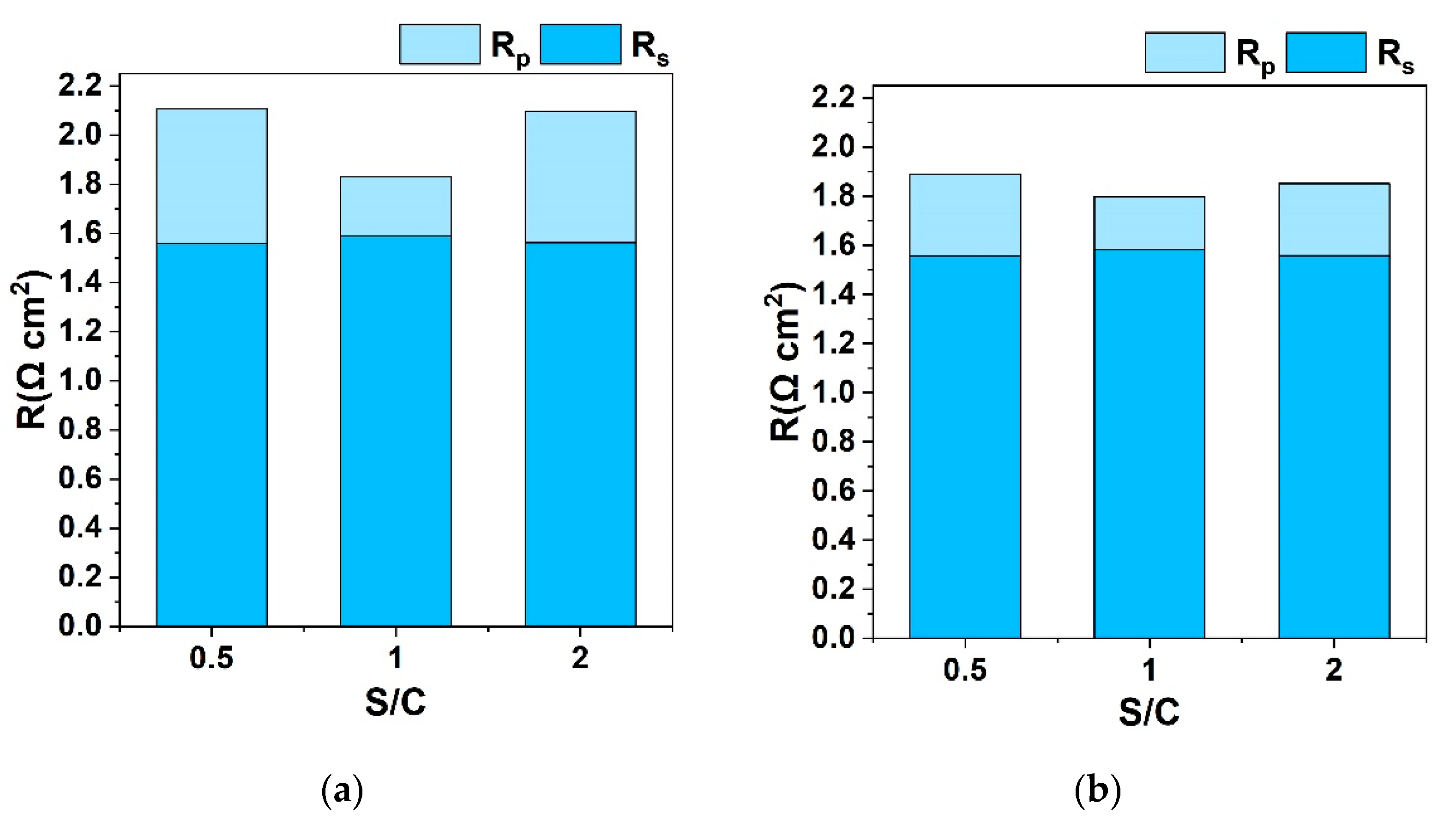
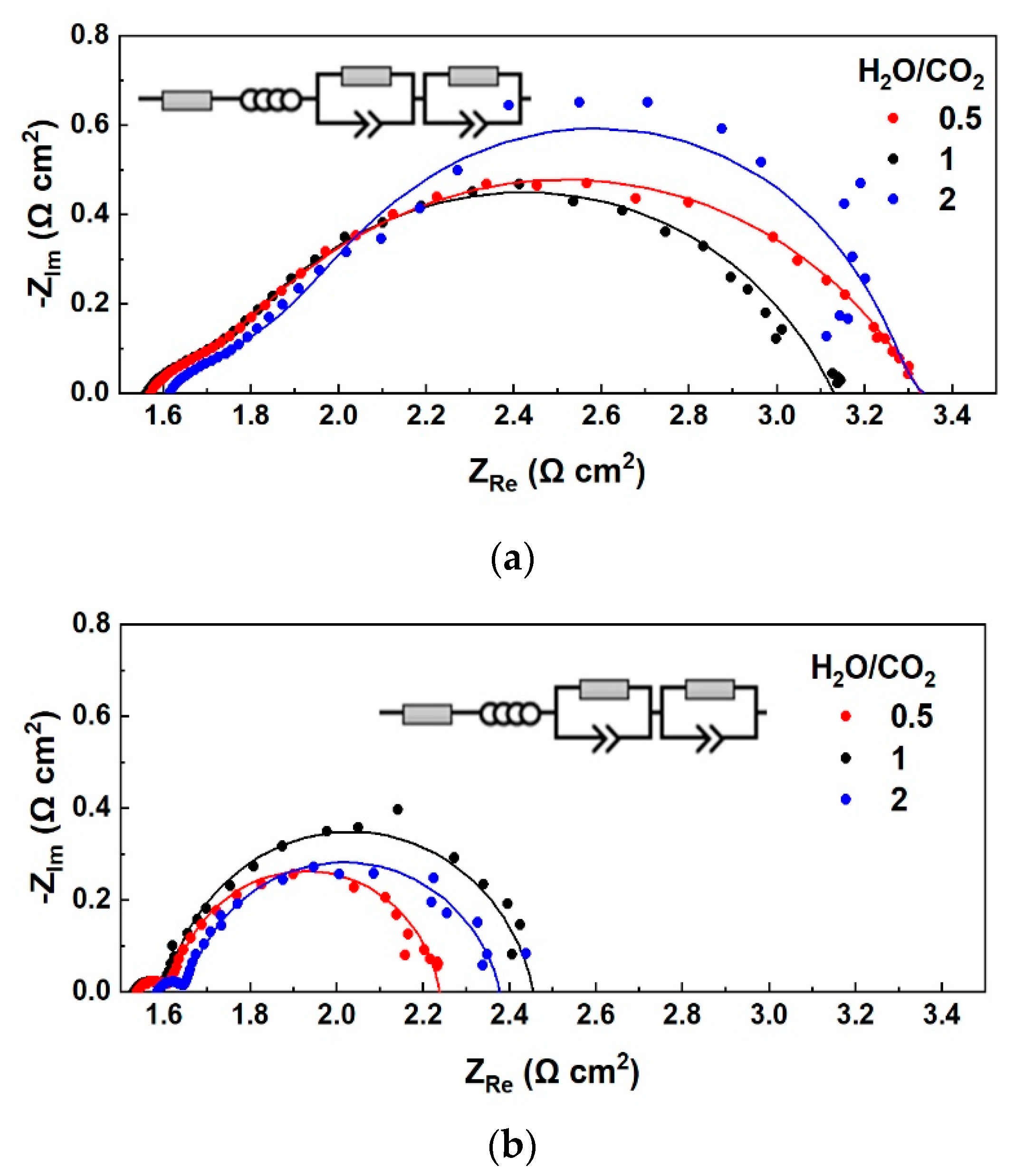
3.1.1. Effect of S/C Ratio with H2 Co-Feed in the Fuel Electrode LSCF
3.1.2. Effect of S/C Ratio without the Co-Feed of a Reducing Agent on the Fuel Electrode LSCF
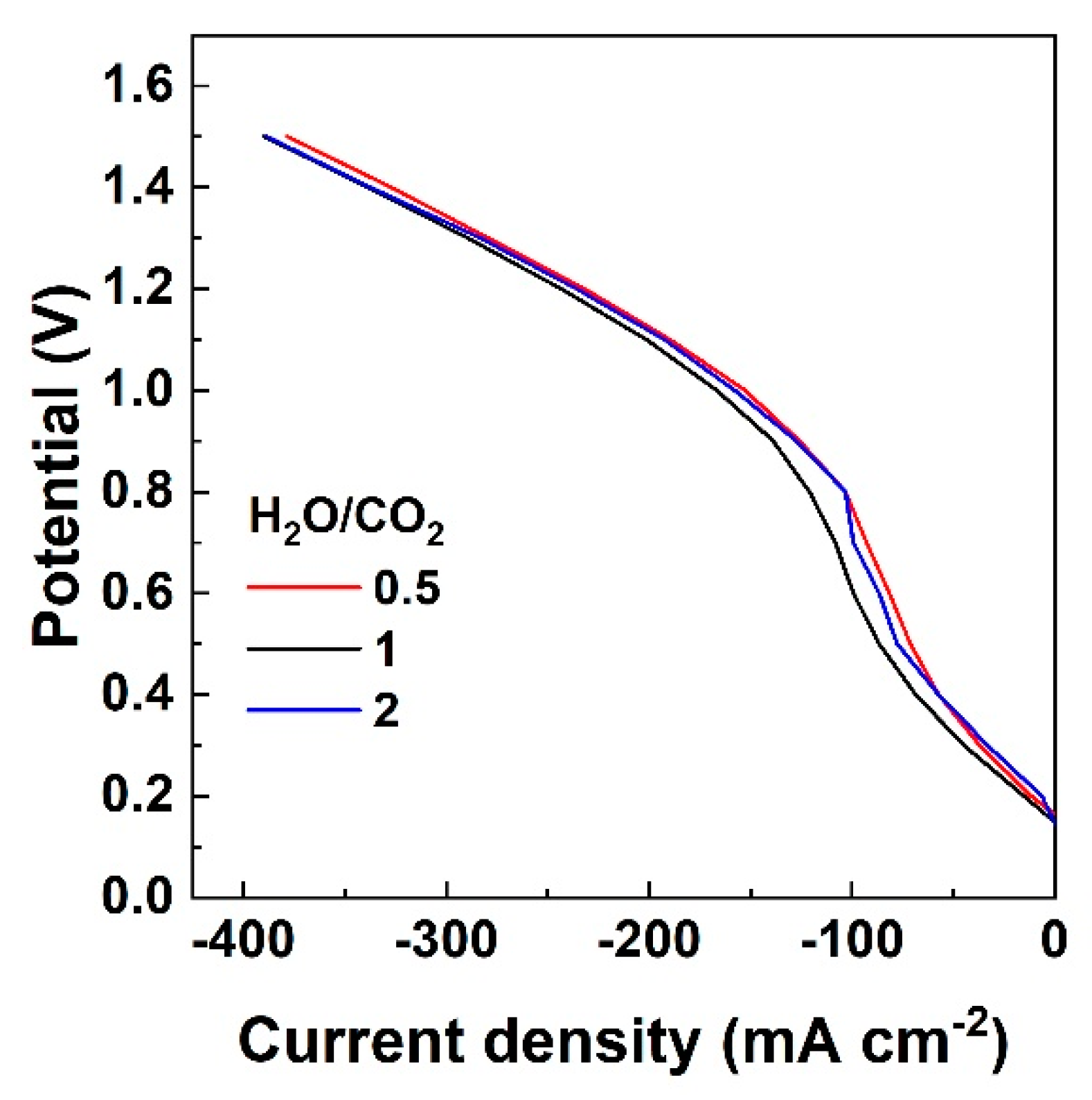
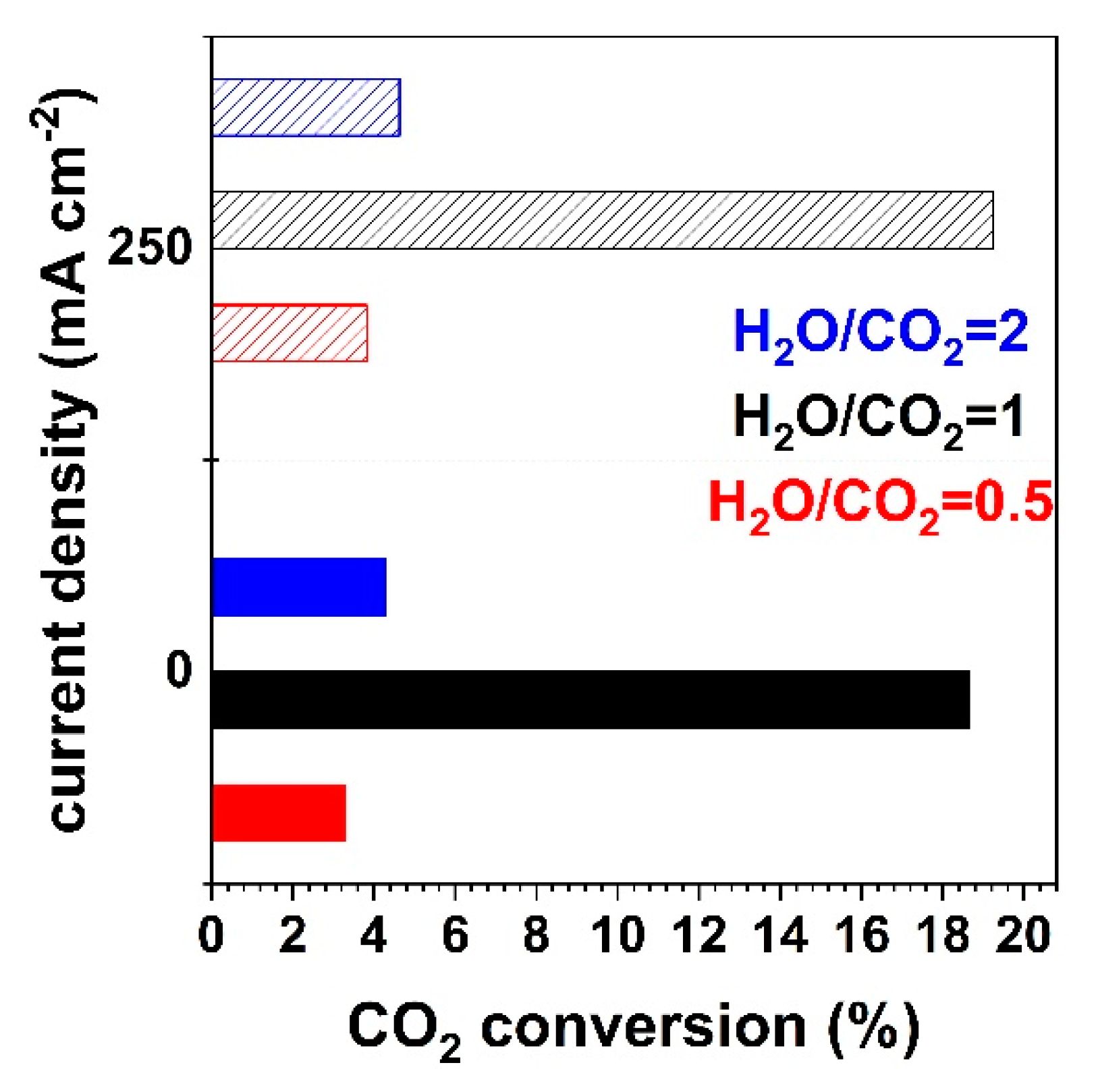
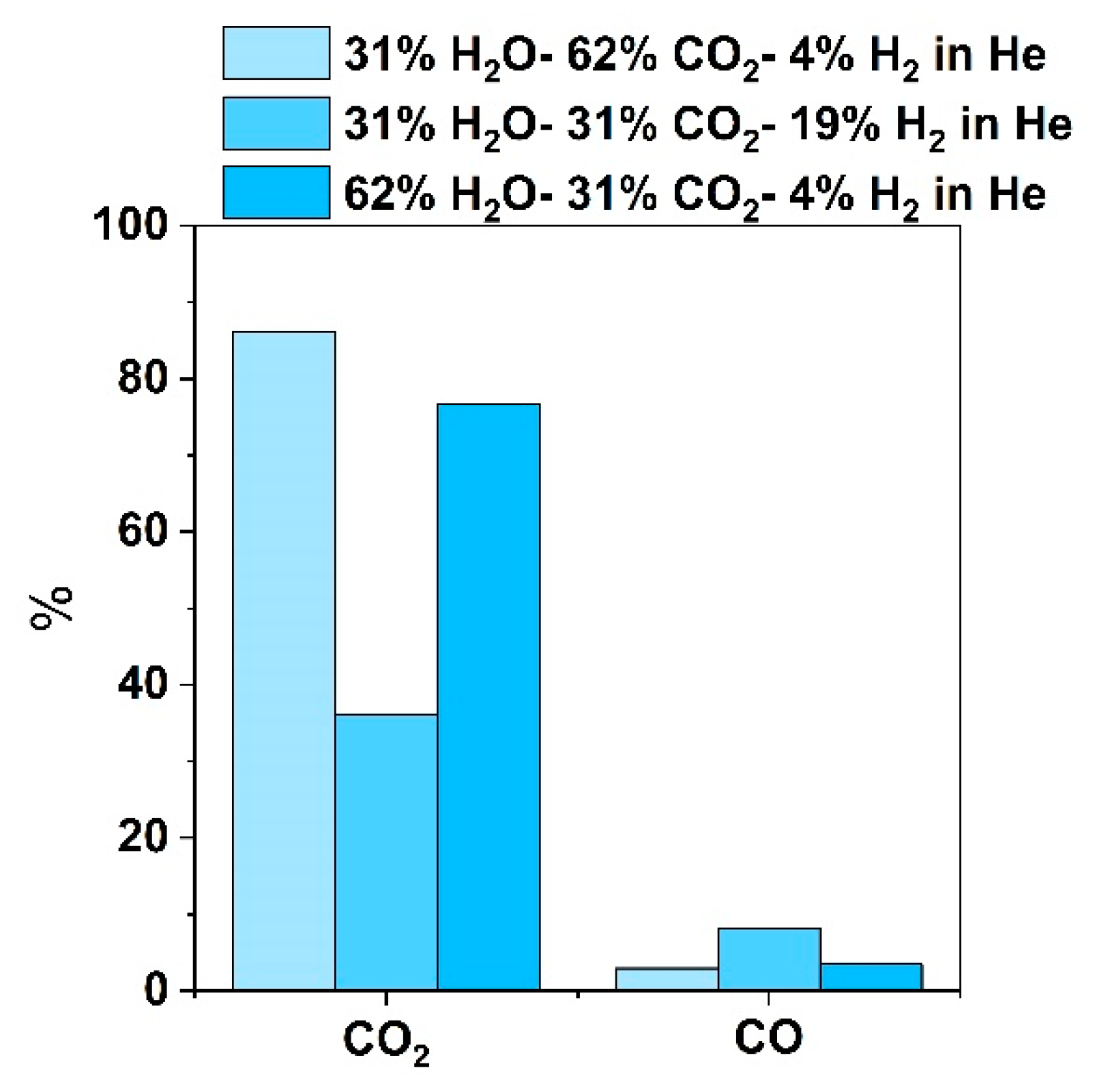
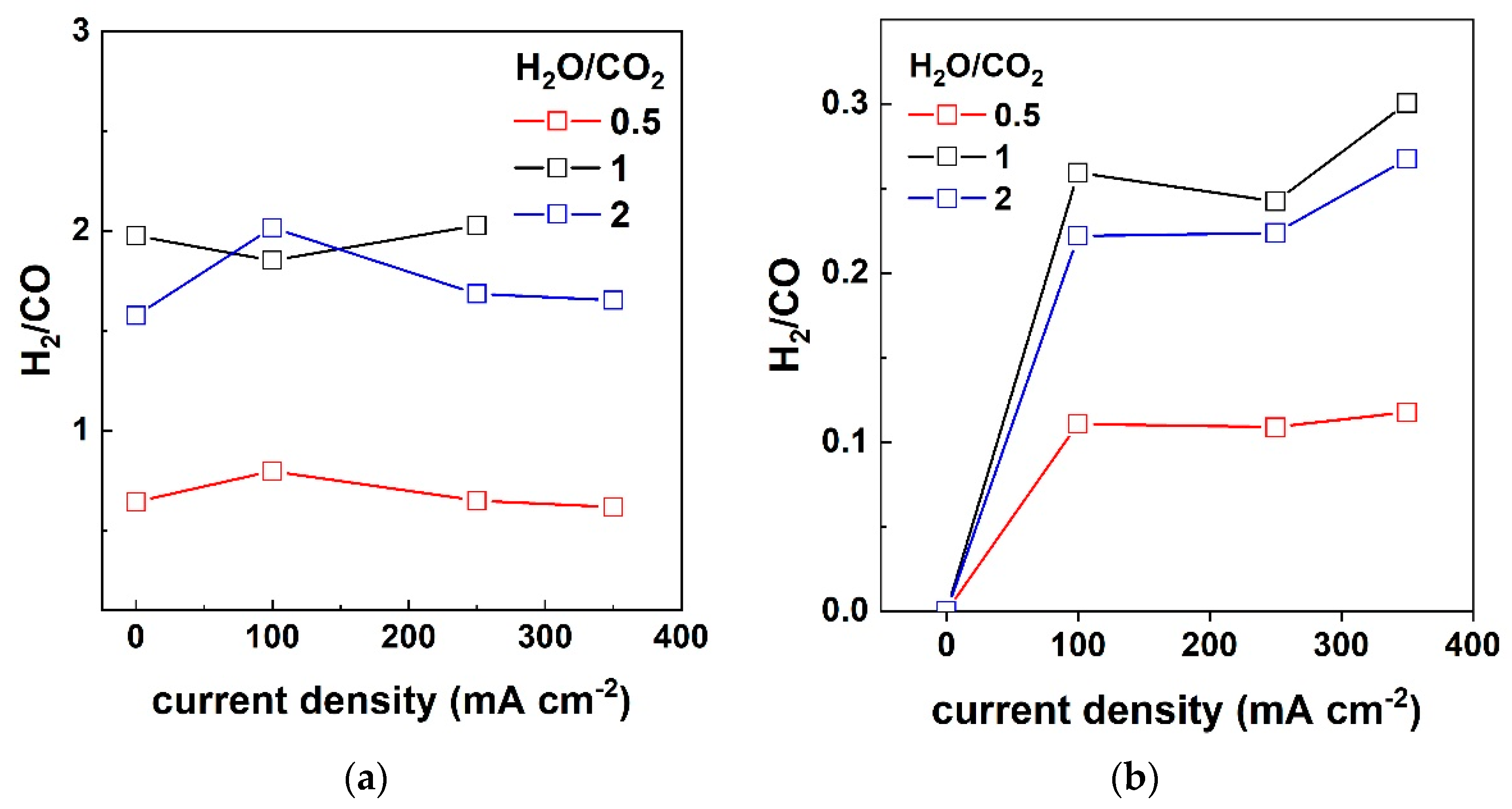
3.2. Outlet Gas Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Becker, W.L.; Braun, R.J.; Penev, M.; Melaina, M. Production of Fischer–Tropsch Liquid Fuels from High Temperature Solid Oxide Co-Electrolysis Units. Energy 2012, 47, 99–115. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Y.; Wang, G.; Bao, X. Co-Electrolysis of CO2 and H2O in High-Temperature Solid Oxide Electrolysis Cells: Recent Advance in Cathodes. J. Energy Chem. 2017, 26, 839–853. [Google Scholar] [CrossRef]
- Herranz, J.; Pătru, A.; Fabbri, E.; Schmidt, T.J. Co-Electrolysis of CO2 and H2O: From Electrode Reactions to Cell-Level Development. Curr. Opin. Electrochem. 2020, 23, 89–95. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, F.; Xia, C. A Review on Cathode Processes and Materials for Electro-Reduction of Carbon Dioxide in Solid Oxide Electrolysis Cells. J. Power Sources 2021, 493, 229713. [Google Scholar] [CrossRef]
- Redissi, Y.; Bouallou, C. Valorization of Carbon Dioxide by Co-Electrolysis of CO2/H2O at High Temperature for Syngas Production. Energy Procedia 2013, 37, 6667–6678. [Google Scholar] [CrossRef]
- Aicart, J.; Petitjean, M.; Laurencin, J.; Tallobre, L.; Dessemond, L. Accurate Predictions of H2O and CO2 Co-Electrolysis Outlet Compositions in Operation. Int. J. Hydrog. Energy 2015, 40, 3134–3148. [Google Scholar] [CrossRef]
- Torrell, M.; García-Rodríguez, S.; Morata, A.; Penelas, G.; Tarancón, A. Co-Electrolysis of Steam and CO2 in Full-Ceramic Symmetrical SOECs: A Strategy for Avoiding the Use of Hydrogen as a Safe Gas. Faraday Discuss 2015, 182, 241–255. [Google Scholar] [CrossRef]
- Laurencin, J.; Kane, D.; Delette, G.; Deseure, J.; Lefebvre-Joud, F. Modelling of Solid Oxide Steam Electrolyser: Impact of the Operating Conditions on Hydrogen Production. J. Power Sources 2011, 196, 2080–2093. [Google Scholar] [CrossRef]
- Foit, S.R.; Vinke, I.C.; de Haart, L.G.J.; Eichel, R.-A. New Energy Systems Power-to-Syngas:An Enabling Technology for the Transition of the Energy System? Angew. Chem. Int. Ed. 2017, 56, 5402–5411. [Google Scholar] [CrossRef]
- Dittrich, L.; Nohl, M.; Jaekel, E.E.; Foit, S.; (Bert) de Haart, L.G.J.; Eichel, R.-A. High-Temperature Co-Electrolysis: A Versatile Method to Sustainably Produce Tailored Syngas Compositions. J. Electrochem. Soc. 2019, 166, F971–F975. [Google Scholar] [CrossRef]
- Ebbesen, S.; Knibbe, R.; Mogensen, M. Co-Electrolysis of Steam and Carbon Dioxide in Solid Oxide Cells. J. Electrochem. Soc. 2012, 159, F482–F489. [Google Scholar] [CrossRef]
- Sapountzi, F.M.; Gracia, J.M.; Weststrate, C.J.; Fredriksson, H.O.A.; Niemantsverdriet, J.W. Electrocatalysts for the Generation of Hydrogen, Oxygen and Synthesis Gas. Prog. Energy Combust. Sci. 2017, 58, 1–35. [Google Scholar] [CrossRef]
- Cui, C.; Wang, Y.; Tong, Y.; Wang, S.; Chen, C.; Zhan, Z. Syngas Production through CH4-Assisted Co-Electrolysis of H2O and CO2 in La0.8Sr0.2Cr0.5Fe0.5O3-δ-Zr0.84Y0.16O2-δ Electrode-Supported Solid Oxide Electrolysis Cells. Int. J. Hydrog. Energy 2021, 46, 20305–20312. [Google Scholar] [CrossRef]
- Graves, C.; Ebbesen, S.D.; Mogensen, M. Co-Electrolysis of CO2 and H2O in Solid Oxide Cells: Performance and Durability. Solid State Ion 2011, 192, 398–403. [Google Scholar] [CrossRef]
- Li, W.; Shi, Y.; Luo, Y.; Cai, N. Elementary Reaction Modeling of Solid Oxide Electrolysis Cells: Main Zones for Heterogeneous Chemical/Electrochemical Reactions. J. Power Sources 2015, 273, 1–13. [Google Scholar] [CrossRef]
- Breeze, P. The Solid Oxide Fuel Cell. Fuel Cells 2017, 63–73. [Google Scholar] [CrossRef]
- Zhan, Z.; Kobsiriphat, W.; Wilson, J.R.; Pillai, M.; Kim, I.; Barnett, S.A. Syngas Production By Coelectrolysis of CO2/H2O: The Basis for a Renewable Energy Cycle. Energy Fuels 2009, 23, 3089–3096. [Google Scholar] [CrossRef]
- Deka, D.J.; Gunduz, S.; Fitzgerald, T.; Miller, J.T.; Co, A.C.; Ozkan, U.S. Production of Syngas with Controllable H2/CO Ratio by High Temperature Co-Electrolysis of CO2 and H2O over Ni and Co- Doped Lanthanum Strontium Ferrite Perovskite Cathodes. Appl. Catal. B 2019, 248, 487–503. [Google Scholar] [CrossRef]
- Bian, L.; Duan, C.; Wang, L.; Chen, Z.; Hou, Y.; Peng, J.; Song, X.; An, S.; O’Hayre, R. An All-Oxide Electrolysis Cells for Syngas Production with Tunable H2/CO Yield via Co-Electrolysis of H2O and CO2. J. Power Sources 2021, 482, 228887. [Google Scholar] [CrossRef]
- Papazisi, K.; Balomenou, S.; Tsiplakides, D. Synthesis and Characterization of La0.75Sr0.25Cr0.9M0.1O3 Perovskites as Anodes for CO-Fuelled Solid Oxide Fuel Cells. J. Appl. Electrochem. 2010, 40, 1875–1881. [Google Scholar] [CrossRef]
- Papazisi, K.-M.; Tsiplakides, D.; Balomenou, S. High Temperature Co-Electrolysis of CO2 and Water on Doped Lanthanum Chromites. ECS Trans. 2017, 78, 3197–3204. [Google Scholar] [CrossRef]
- Knibbe, R.; Hjelm, J.; Menon, M.; Pryds, N.; Jen Wang, H.; Neufeld, K. Cathode-Electrolyte Interfaces with CGO Barrier Layers in SOFC. J. Am. Ceram. Soc. 2010, 93, 2877–2883. [Google Scholar] [CrossRef]
- Szymczewska, D.; Karczewski, J.; Chrzan, A.; Jasinski, P. CGO as a Barrier Layer between LSCF Electrodes and YSZ Electrolyte Fabricated by Spray Pyrolysis for Solid Oxide Fuel Cells. Solid State Ion 2017, 302, 113–117. [Google Scholar] [CrossRef]
- Railsback, J.; Choi, S.H.; Barnett, S.A. Effectiveness of Dense Gd-Doped Ceria Barrier Layers for (La,Sr)(Co,Fe)O3 Cathodes on Yttria-Stabilized Zirconia Electrolytes. Solid State Ion 2019, 335, 74–81. [Google Scholar] [CrossRef]
- Liang, J.; Han, M. Different Performance and Mechanisms of CO2 Electrolysis with CO and H2 as Protective Gases in Solid Oxide Electrolysis Cell. Int. J. Hydrog. Energy 2022, 47, 18606–18618. [Google Scholar] [CrossRef]
- Ioannidou, E.; Chavani, M.; Neophytides, S.G.; Niakolas, D.K. Effect of the PH2O/PCO2 and PH2 on the Intrinsic Electro-Catalytic Interactions and the CO Production Pathway on Ni/GDC during Solid Oxide H2O/CO2 Co-Electrolysis. J. Catal. 2021, 404, 174–186. [Google Scholar] [CrossRef]
- Leonide, A.; Apel, Y.; Ivers-Tiffee, E. SOFC Modeling and Parameter Identification by Means of Impedance Spectroscopy. ECS Trans. 2009, 19, 81–109. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, Y.; Yu, B.; Wang, J.; Chen, J. Electrochemical Characterization and Mechanism Analysis of High Temperature Co-Electrolysis of CO2 and H2O in a Solid Oxide Electrolysis Cell. Int. J. Hydrog. Energy 2017, 42, 29911–29920. [Google Scholar] [CrossRef]
- Laycock, C.J.; Panagi, K.; Reed, J.P.; Guwy, A.J. The Importance of Fuel Variability on the Performance of Solid Oxide Cells Operating on H2/CO2 Mixtures from Biohydrogen Processes. Int. J. Hydrog. Energy 2018, 43, 8972–8982. [Google Scholar] [CrossRef]
- Setz, L.F.G.; Santacruz, I.; León-Reina, L.; de la Torre, A.G.; Aranda, M.A.G.; Mello-Castanho, S.R.H.; Moreno, R.; Colomer, M.T. Strontium and Cobalt Doped-Lanthanum Chromite: Characterisation of Synthesised Powders and Sintered Materials. Ceram Int. 2015, 41, 1177–1187. [Google Scholar] [CrossRef]
- Bernadet, L.; Moncasi, C.; Torrell, M.; Tarancón, A. High-Performing Electrolyte-Supported Symmetrical Solid Oxide Electrolysis Cells Operating under Steam Electrolysis and Co-Electrolysis Modes. Int. J. Hydrog. Energy 2020, 45, 14208–14217. [Google Scholar] [CrossRef]
- Ruan, C.; Xie, K. A Redox-Stable Chromate Cathode Decorated with in Situ Grown Nickel Nanocatalyst for Efficient Carbon Dioxide Electrolysis. Catal. Sci. Technol. 2015, 5, 1929–1940. [Google Scholar] [CrossRef]
- lo Faro, M.; Trocino, S.; Zignani, S.C.; Antonucci, V.; Aricò, A.S. Production of Syngas by Solid Oxide Electrolysis: A Case Study. Int. J. Hydrog. Energy 2017, 42, 27859–27865. [Google Scholar] [CrossRef]
- lo Faro, M.; Campagna Zignani, S.; Antonucci, V.; Aricò, A.S. The Effect of Ni-Modified LSFCO Promoting Layer on the Gas Produced through Co-Electrolysis of CO2 and H2O at Intermediate Temperatures. Catalysts 2021, 11, 56. [Google Scholar] [CrossRef]

| Inlet Feed H2O/CO2 Ratio | Equilibrium Gas Composition 1,2 | OCVtheo/CO2-CO (mV) | OCVtheo/H2O-H2 (mV) | OCVexp (mV) |
|---|---|---|---|---|
| 0.5 | 33.0%H2O- 59.4% CO2- 1.6% H2- 2.2% CO | 779 | 778 | 773 |
| 1 | 37.2%H2O- 24.2% CO2- 12.8% H2- 6.5% CO | 885 | 884 | 893 |
| 2 | 62.6%H2O- 29.8% CO2- 2.8% H2- 1.0% CO | 783 | 782 | 782 |
| Inlet Feed Composition 1 | OCVexp (mV) | OCVGC-CO2/CO (mV) | OCVGC-H2O/H2 (mV) |
|---|---|---|---|
| 31% H2O-62% CO2-4% H2 (S/C = 0.5) | 773 | 765 | 784 |
| 31% H2O-31% CO2-19% H2 (S/C = 1) | 893 | 862 | 894 |
| 62% H2O-31% CO2-4% H2 (S/C = 2) | 782 | 779 | 775 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bimpiri, N.; Konstantinidou, A.; Tsiplakides, D.; Balomenou, S.; Papazisi, K.M. Effect of Steam to Carbon Dioxide Ratio on the Performance of a Solid Oxide Cell for H2O/CO2 Co-Electrolysis. Nanomaterials 2023, 13, 299. https://doi.org/10.3390/nano13020299
Bimpiri N, Konstantinidou A, Tsiplakides D, Balomenou S, Papazisi KM. Effect of Steam to Carbon Dioxide Ratio on the Performance of a Solid Oxide Cell for H2O/CO2 Co-Electrolysis. Nanomaterials. 2023; 13(2):299. https://doi.org/10.3390/nano13020299
Chicago/Turabian StyleBimpiri, Naouma, Argyro Konstantinidou, Dimitrios Tsiplakides, Stella Balomenou, and Kalliopi Maria Papazisi. 2023. "Effect of Steam to Carbon Dioxide Ratio on the Performance of a Solid Oxide Cell for H2O/CO2 Co-Electrolysis" Nanomaterials 13, no. 2: 299. https://doi.org/10.3390/nano13020299
APA StyleBimpiri, N., Konstantinidou, A., Tsiplakides, D., Balomenou, S., & Papazisi, K. M. (2023). Effect of Steam to Carbon Dioxide Ratio on the Performance of a Solid Oxide Cell for H2O/CO2 Co-Electrolysis. Nanomaterials, 13(2), 299. https://doi.org/10.3390/nano13020299






