Development of a Multi-Enzymatic Biocatalytic System through Immobilization on High Quality Few-Layer bio-Graphene
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of bio-Graphene
2.3. Characterization of bio-Graphene
2.4. Preparation of the Tri-Enzymatic Nanobiocatalyst
2.5. Enzyme Assays
2.6. Determination of Michaelis–Menten Kinetic Parameters
2.7. Thermal Stability Studies
2.8. Reusability Studies
3. Results and Discussion
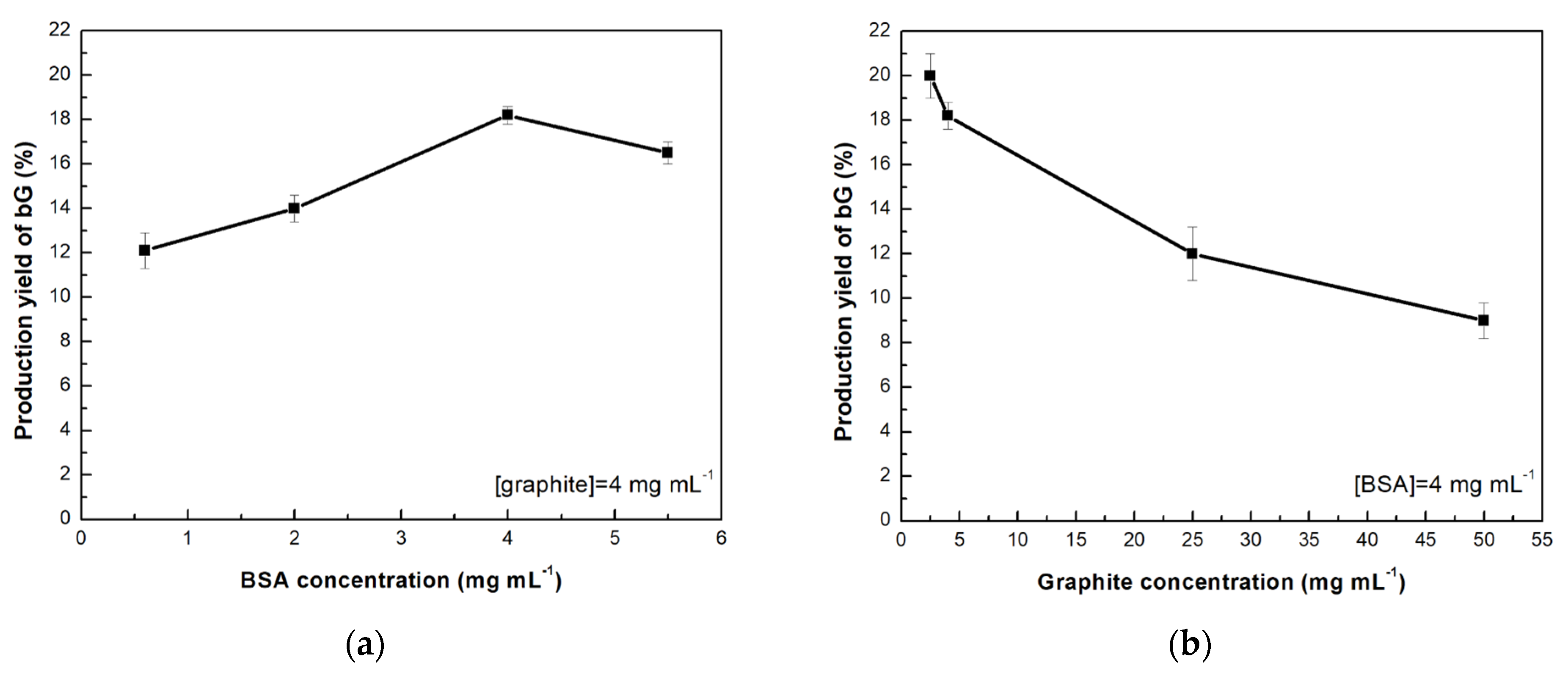
3.1. Effect of the Graphite and Bovine Serum Albumin Concentration on the Production of bio-Graphene
3.2. Microscopic and Spectroscopic Characterization of bio-Graphene
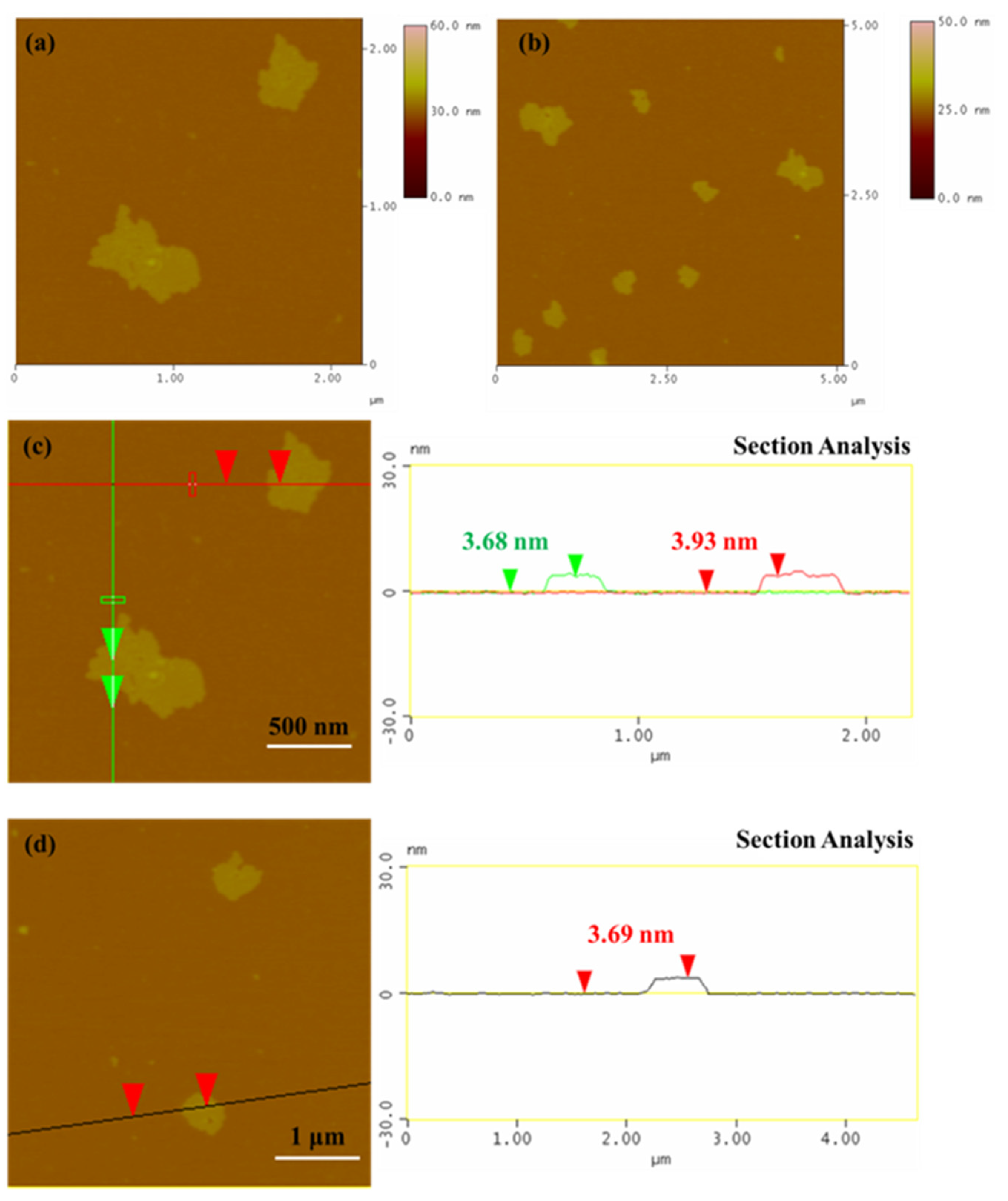
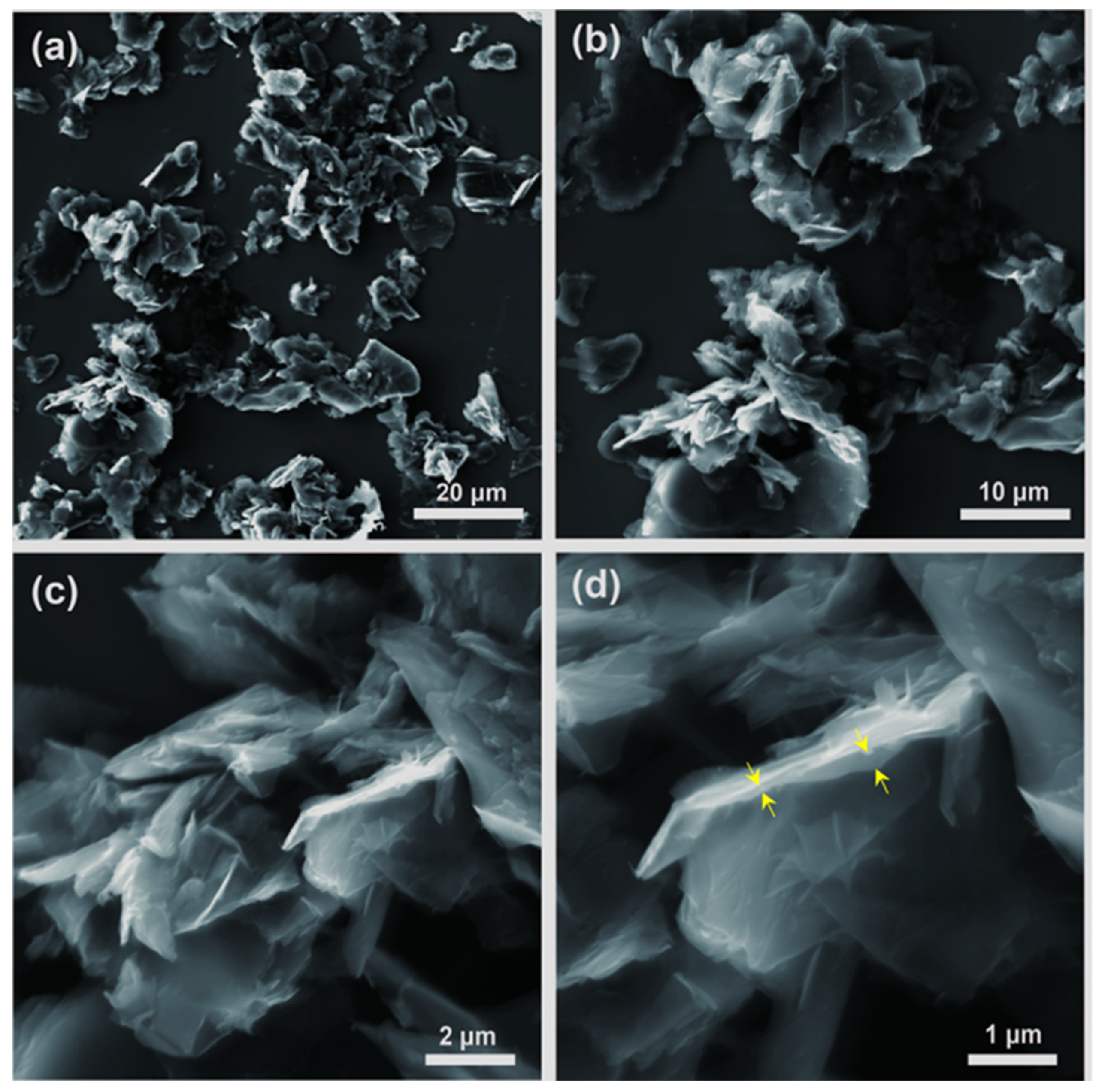
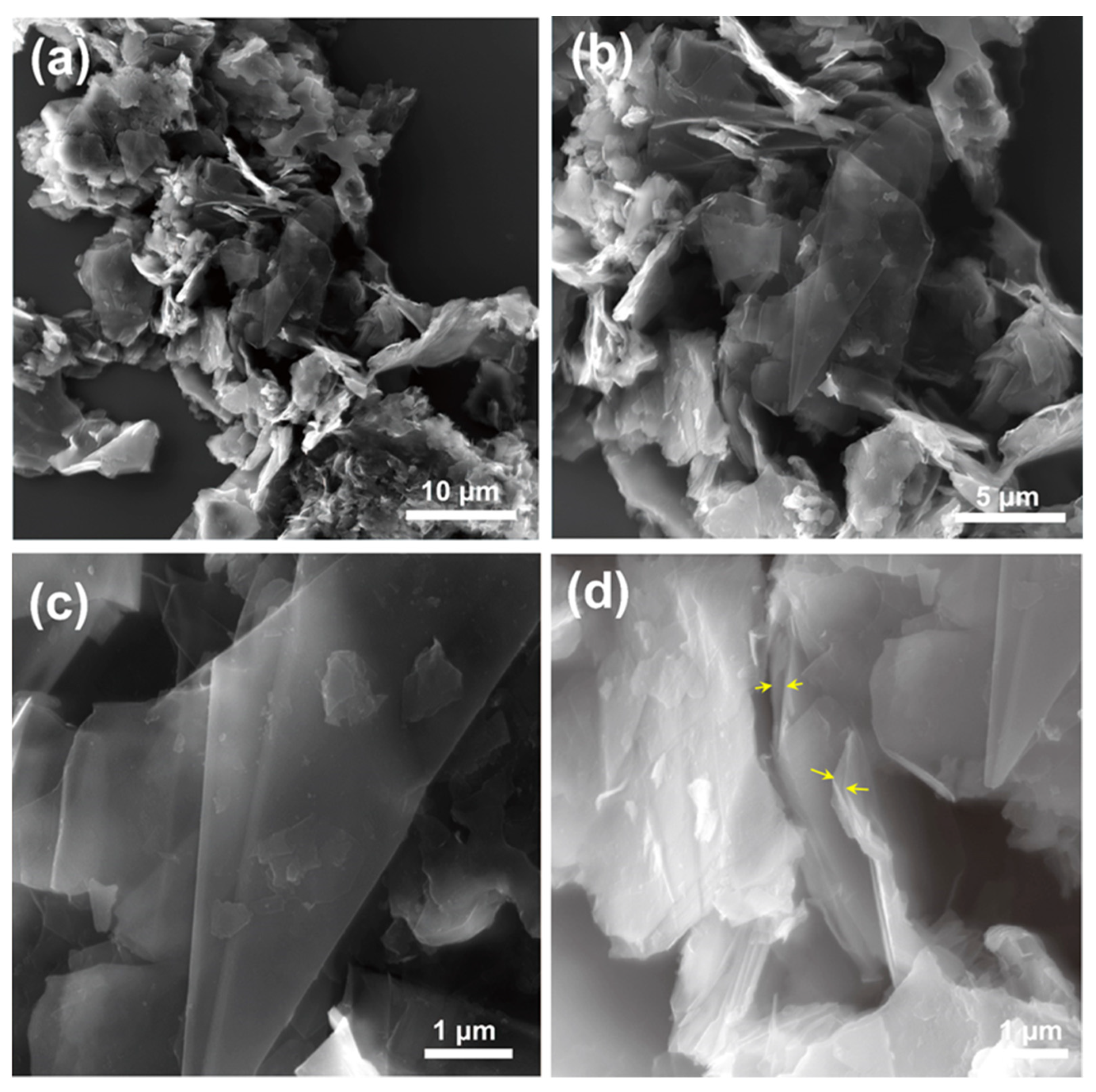
3.2.1. Microscopic Characterization
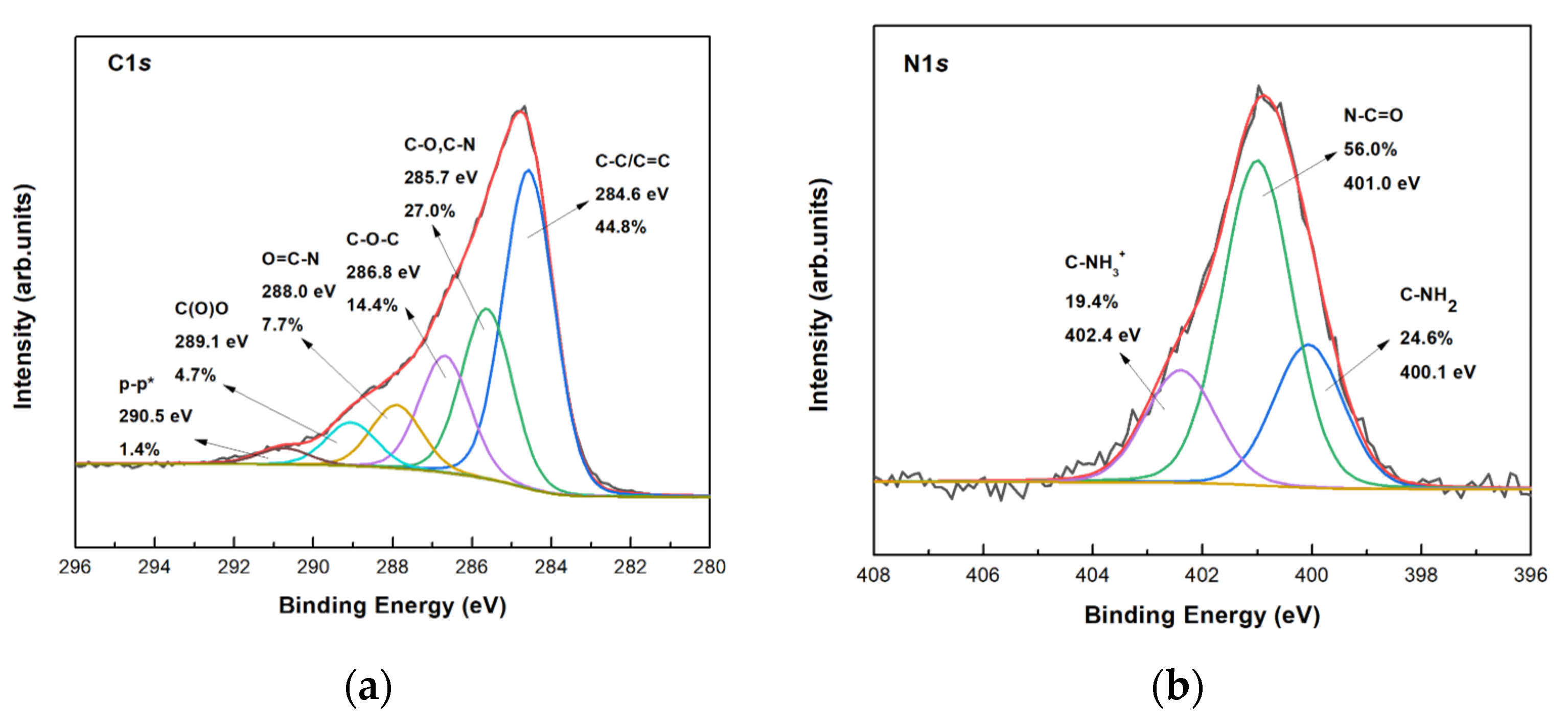
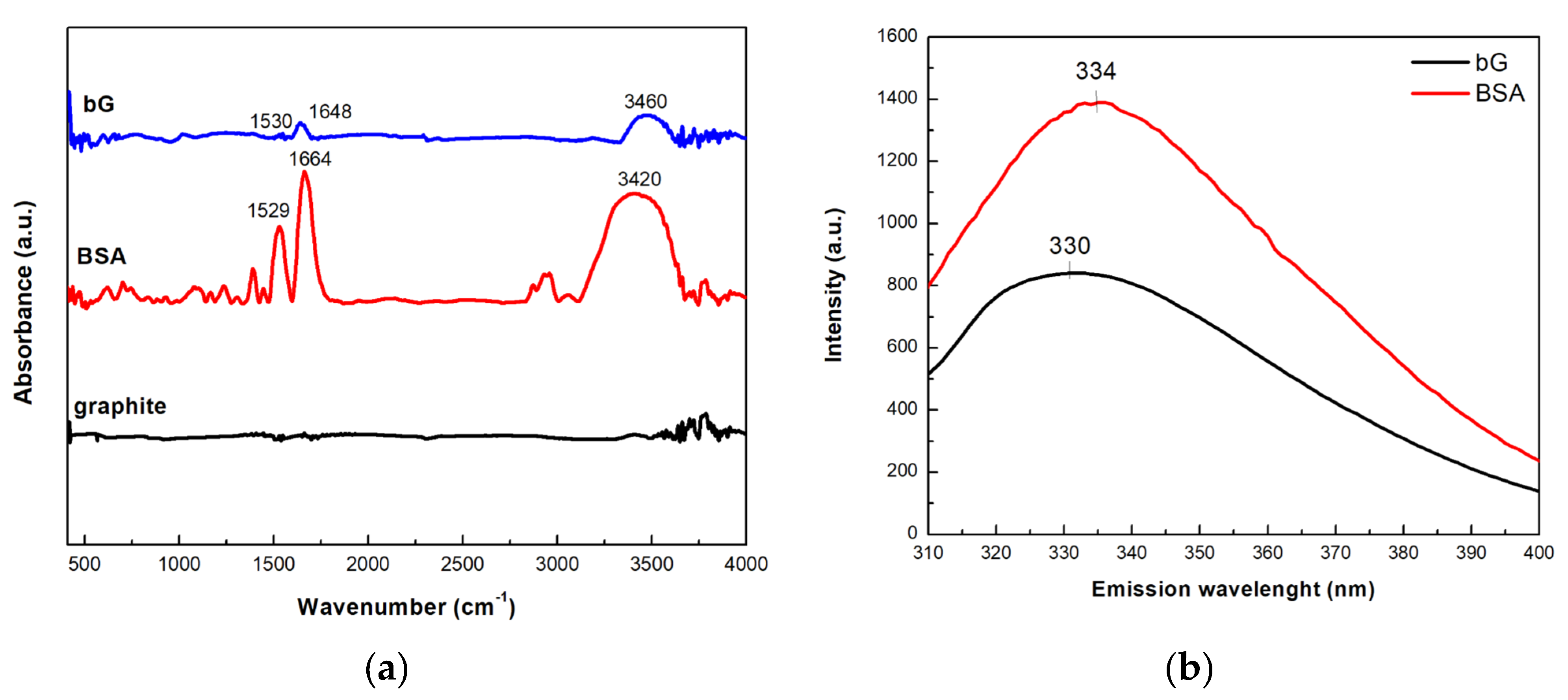
3.2.2. Spectroscopic Characterization
3.3. Bio-Graphene as Support for a Tri-Enzymatic Co-Immobilized System
3.3.1. Preparation of the Tri-Enzymatic Biocatalyst
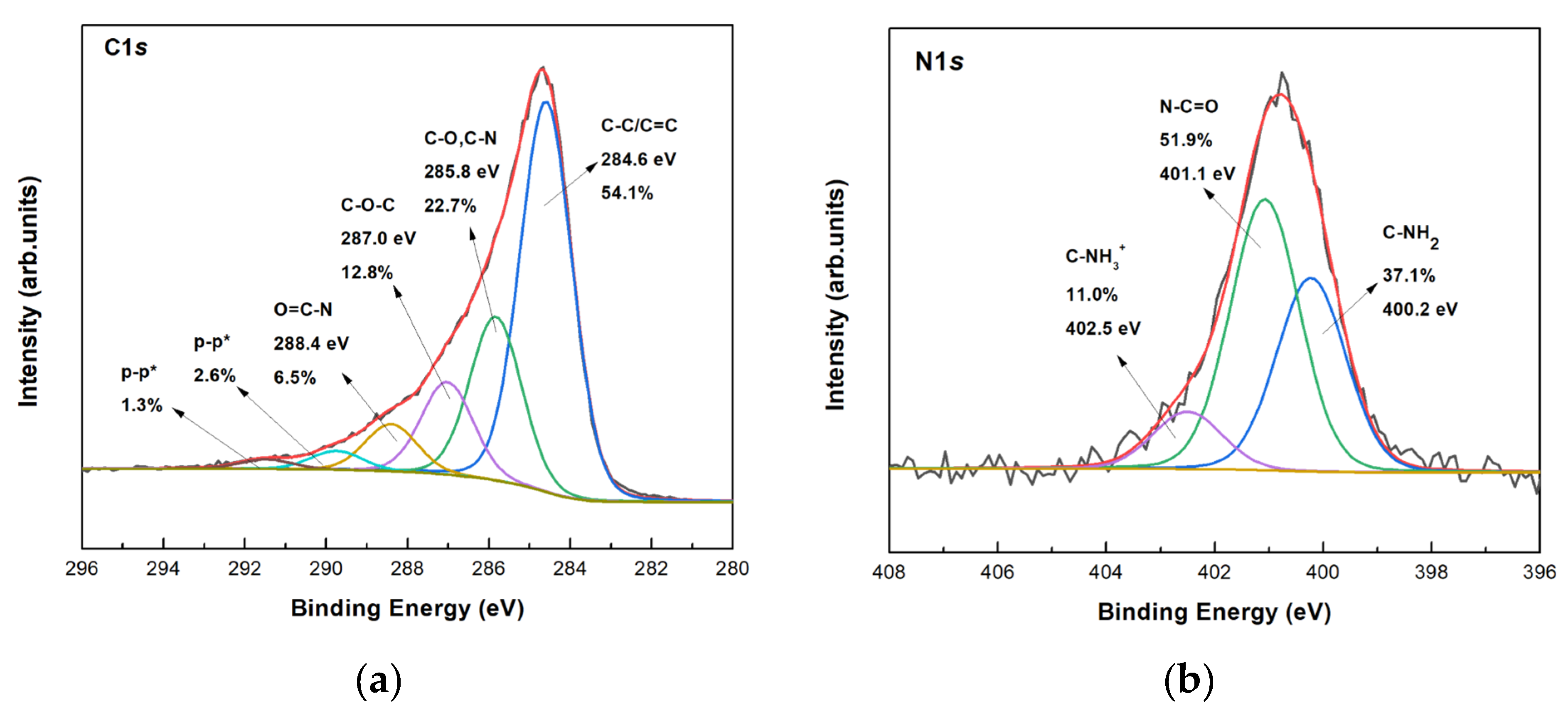
3.3.2. Characterization of the Co-Immobilized Enzymes on bio-Graphene
3.3.3. Kinetic Studies of the Co-Immobilized Enzymes on bio-Graphene
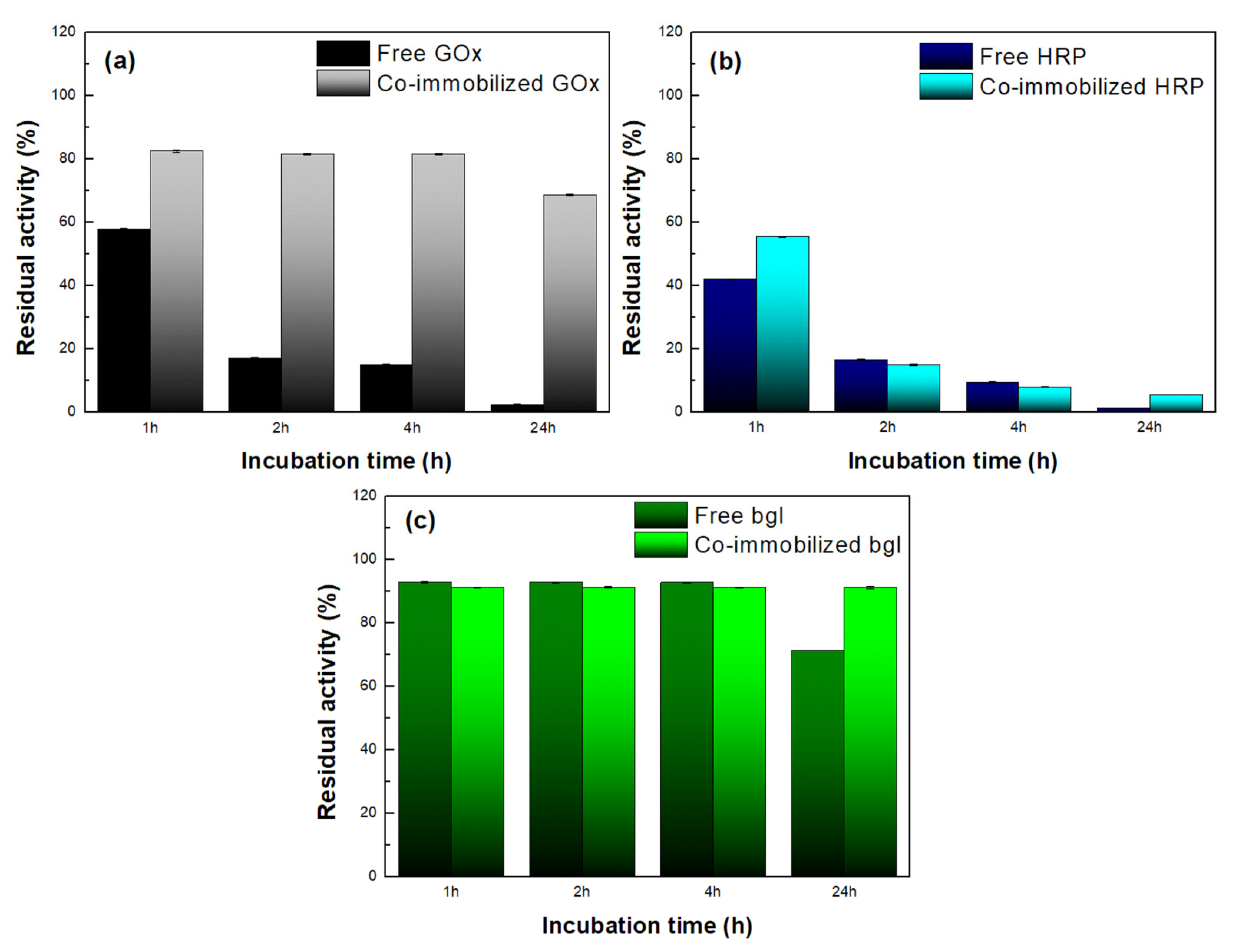
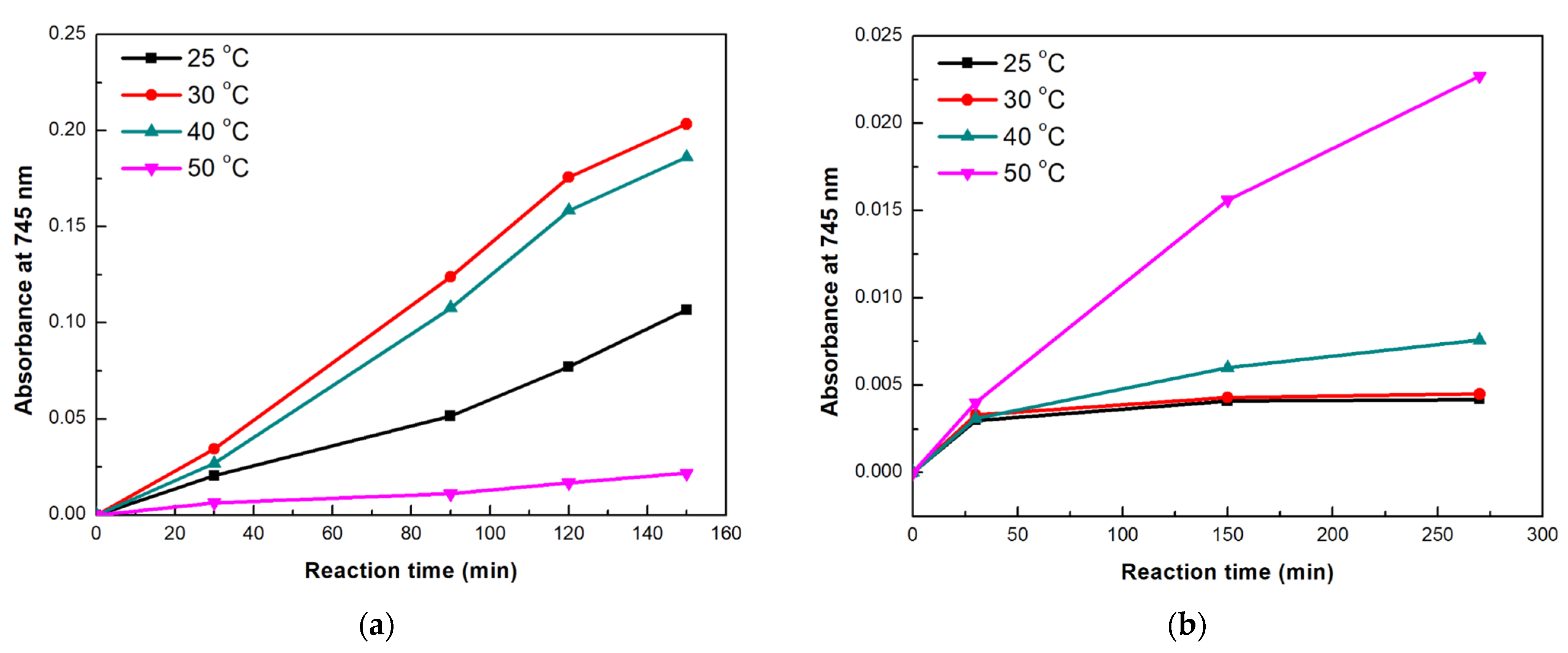
3.3.4. Thermal Stability of the Co-Immobilized Enzymes on bio-Graphene
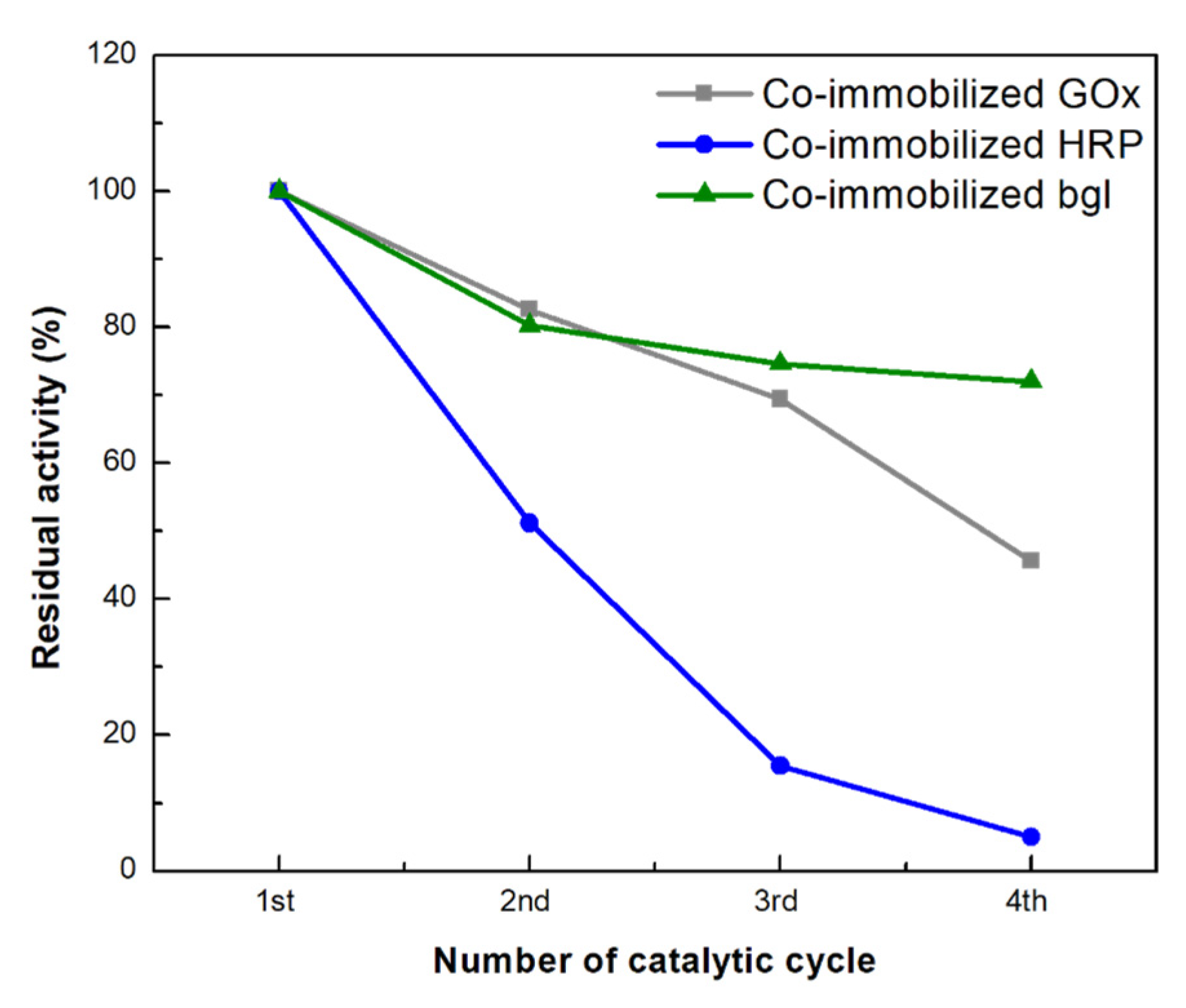
3.3.5. Reusability of Co-Immobilized Enzymes on bio-Graphene
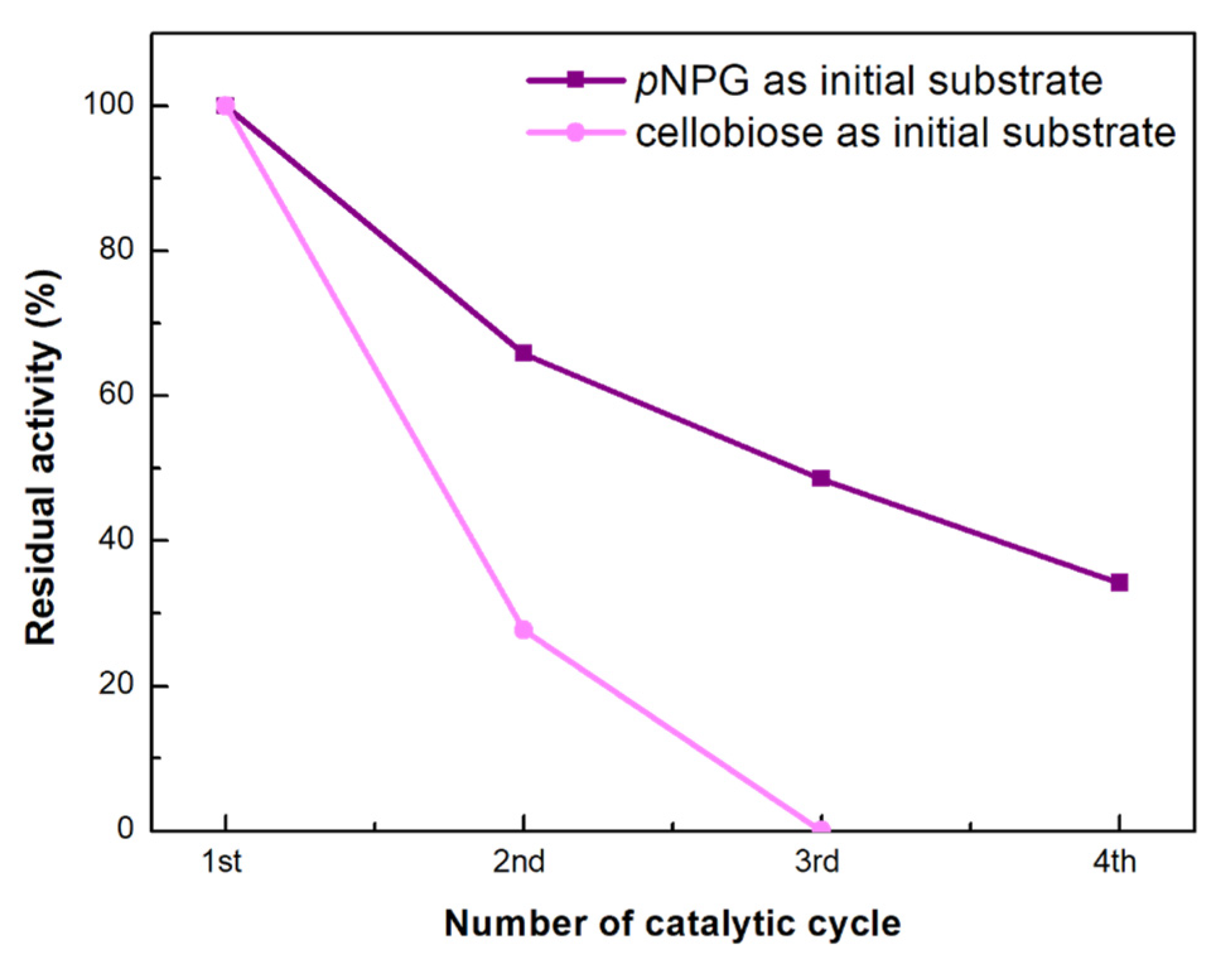
3.3.6. Application of the Immobilized Tri-Enzymatic Biocatalyst on bio-Graphene in Cascade Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Punith Kumar, M.K.; Nidhi, M.; Srivastava, C. Electrochemical exfoliation of graphite to produce graphene using tetrasodium pyrophosphate. RSC Adv. 2015, 5, 24846–24852. [Google Scholar] [CrossRef]
- Bai, Y.; Xu, T.; Zhang, X. Graphene-based biosensors for detection of biomarkers. Micromachines 2020, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Sheshmani, S.; Amini, R. Preparation and characterization of some graphene based nanocomposite materials. Carbohydr. Polym. 2013, 95, 348–359. [Google Scholar] [CrossRef]
- Park, S.H.; Won, D.H.; Choi, H.J.; Hwang, W.P.; Jang, S.I.; Kim, J.H.; Jeong, S.H.; Kim, J.U.; Lee, J.K.; Kim, M.R. Dye-sensitized solar cells based on electrospun polymer blends as electrolytes. Sol. Energy Mater. Sol. Cells 2011, 95, 296–300. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, R.; Chen, W. Graphene-supported nanoelectrocatalysts for fuel cells: Synthesis, properties, and applications. Chem. Rev. 2014, 114, 5117–5160. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Jiang, D.; Schedin, F.; Booth, T.J.; Khotkevich, V.V.; Morozov, S.V.; Geim, A.K. Two-dimensional atomic crystals. Proc. Natl. Acad. Sci. USA 2005, 102, 10451–10453. [Google Scholar] [CrossRef]
- Muñoz, R.; Gómez-Aleixandre, C. Review of CVD synthesis of graphene. Chem. Vap. Depos. 2013, 19, 297–322. [Google Scholar] [CrossRef]
- Amiri, A.; Naraghi, M.; Ahmadi, G.; Soleymaniha, M.; Shanbedi, M. A review on liquid-phase exfoliation for scalable production of pure graphene, wrinkled, crumpled and functionalized graphene and challenges. FlatChem 2018, 8, 40–71. [Google Scholar] [CrossRef]
- Cooil, S.P.; Song, F.; Williams, G.T.; Roberts, O.R.; Langstaff, D.P.; Jørgensen, B.; Høydalsvik, K.; Breiby, D.W.; Wahlström, E.; Evans, D.A.; et al. Iron-mediated growth of epitaxial graphene on SiC and diamond. Carbon N. Y. 2012, 50, 5099–5105. [Google Scholar] [CrossRef]
- Cao, M.; Wang, N.; Wang, L.; Zhang, Y.; Chen, Y.; Xie, Z.; Li, Z.; Pambou, E.; Li, R.; Chen, C.; et al. Direct exfoliation of graphite into graphene in aqueous solutions of amphiphilic peptides. J. Mater. Chem. B 2016, 4, 152–161. [Google Scholar] [CrossRef]
- Laaksonen, P.; Kainlauri, M.; Laaksonen, T.; Shchepetov, A.; Jiang, H.; Ahopelto, J.; Linder, M.B. Interfacial engineering by proteins: Exfoliation and functionalization of graphene by hydrophobins. Angew. Chem. Int. Ed. 2010, 49, 4946–4949. [Google Scholar] [CrossRef] [PubMed]
- Paredes, J.I.; Villar-Rodil, S. Biomolecule-assisted exfoliation and dispersion of graphene and other two-dimensional materials: A review of recent progress and applications. Nanoscale 2016, 8, 15389–15413. [Google Scholar] [CrossRef] [PubMed]
- Ahadian, S.; Estili, M.; Surya, V.J.; Ramón-Azcón, J.; Liang, X.; Shiku, H.; Ramalingam, M.; Matsue, T.; Sakka, Y.; Bae, H.; et al. Facile and green production of aqueous graphene dispersions for biomedical applications. Nanoscale 2015, 7, 6436–6443. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.V.; Pattammattel, A. Chapter 11—BioGraphene Direct Exfoliation of Graphite in a Kitchen Blender for Enzymology Applications. In Methods Enzymol, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 571, pp. 225–244. [Google Scholar] [CrossRef]
- Limbacher, M.R.; Puglia, M.K.; Riccardi, C.M.; Kumar, C.V. Chapter 1—Interlocking Enzymes in Graphene-Coated Cellulose Paper for Increased Enzymatic Efficiency. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 609, pp. 1–22. [Google Scholar] [CrossRef]
- Gustafsson, H.; Küchler, A.; Holmberg, K.; Walde, P. Co-immobilization of enzymes with the help of a dendronized polymer and mesoporous silica nanoparticles. J. Mater. Chem. B 2015, 3, 6174–6184. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.T.; Lee, S. Multienzymatic Cascade Reactions via Enzyme Complex by Immobilization. ACS Catal. 2019, 9, 4402–4425. [Google Scholar] [CrossRef]
- Giannakopoulou, A.; Gkantzou, E.; Polydera, A.; Stamatis, H. Multienzymatic Nanoassemblies: Recent Progress and Applications. Trends Biotechnol. 2020, 38, 202–216. [Google Scholar] [CrossRef]
- Losada, N.; Berenguer-Murcia, A.; Cazorla-Amorós, D.; Palomo, J.M. Green exfoliation of graphite flakes to efficiently synthesize Biographene. ChemRxiv 2019, 1–23. [Google Scholar] [CrossRef]
- Chalmpes, N.; Moschovas, D.; Tantis, I.; Bourlinos, A.B.; Bakandritsos, A.; Fotiadou, R.; Patila, M.; Stamatis, H.; Avgeropoulos, A.; Karakassides, M.A.; et al. Carbon nanostructures derived through hypergolic reaction of conductive polymers with fuming nitric acid at ambient conditions. Molecules 2021, 26, 1595. [Google Scholar] [CrossRef]
- Mendoza, S.M.; Arfaoui, I.; Zanarini, S.; Paolucci, F.; Rudolf, P. Improvements in the characterization of the crystalline structure of acid-terminated alkanethiol self-assembled monolayers on Au(111). Langmuir 2007, 23, 582–588. [Google Scholar] [CrossRef]
- Bowers, G.N.; McComb, R.B.; Christensen, R.G.; Schaffer, R. High-purity 4-nitrophenol: Purification, characterization, and specifications for use as a spectrophotometric reference material. Clin. Chem. 1980, 26, 724–729. [Google Scholar] [CrossRef]
- Scott, S.L.; Chen, W.J.; Bakac, A.; Espenson, J.H. Spectroscopic parameters, electrode potentials, acid ionization constants, and electron exchange rates of the 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonate) radicals and ions. J. Phys. Chem. 1993, 97, 6710–6714. [Google Scholar] [CrossRef]
- Pattammattel, A.; Kumar, C.V. Kitchen Chemistry 101: Multigram Production of High Quality Biographene in a Blender with Edible Proteins. Adv. Funct. Mater. 2015, 25, 7088–7098. [Google Scholar] [CrossRef]
- Jia, X.; Campos-Delgado, J.; Terrones, M.; Meunier, V.; Dresselhaus, M.S. Graphene edges: A review of their fabrication and characterization. Nanoscale 2011, 3, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Karthik, P.S.; Hada, M.; Nishikawa, T.; Hayashi, Y. Simple technique of exfoliation and dispersion of multilayer graphene from natural graphite by ozone-assisted sonication. Nanomaterials 2017, 7, 125. [Google Scholar] [CrossRef] [PubMed]
- Eby, D.M.; Artyushkova, K.; Paravastu, A.K.; Johnson, G.R. Probing the molecular structure of antimicrobial peptide-mediated silica condensation using X-ray photoelectron spectroscopy. J. Mater. Chem. 2012, 22, 9875–9883. [Google Scholar] [CrossRef]
- Patila, M.; Pavlidis, I.V.; Kouloumpis, A.; Dimos, K.; Spyrou, K.; Katapodis, P.; Gournis, D.; Stamatis, H. Graphene oxide derivatives with variable alkyl chain length and terminal functional groups as supports for stabilization of cytochrome c. Int. J. Biol. Macromol. 2016, 84, 227–235. [Google Scholar] [CrossRef]
- Patila, M.; Athanasiou, P.E.; Kortessis, L.; Potsi, G.; Kouloumpis, A.; Gournis, D.; Stamatis, H. Immobilization of Laccase on Hybrid Super-Structured Nanomaterials for the Decolorization of Phenolic Dyes. Processes 2022, 10, 233. [Google Scholar] [CrossRef]
- Mallamace, F.; Corsaro, C.; Mallamace, D.; Vasi, S.; Vasi, C.; Dugo, G. The role of water in protein’s behavior: The two dynamical crossovers studied by NMR and FTIR techniques. Comput. Struct. Biotechnol. J. 2015, 13, 33–37. [Google Scholar] [CrossRef]
- Zhuang, Y.; Yu, F.; Ma, J.; Chen, J. Graphene as a template and structural scaffold for the synthesis of a 3D porous bio-adsorbent to remove antibiotics from water. RSC Adv. 2015, 5, 27964–27969. [Google Scholar] [CrossRef]
- Pavlidis, I.V.; Patila, M.; Polydera, A.C.; Gournis, D.; Stamatis, H. Immobilization of enzymes and other biomolecules on graphene. In Functionalization of Graphene; Georgakilas, V., Ed.; Wiley-VCH Verlag & GmbH & Co. KGaA: Weinheim, Germany, 2014; pp. 139–172. ISBN 9783527672790. [Google Scholar]
- Gkantzou, E.; Chatzikonstantinou, A.V.; Fotiadou, R.; Giannakopoulou, A.; Patila, M.; Stamatis, H. Trends in the development of innovative nanobiocatalysts and their application in biocatalytic transformations. Biotechnol. Adv. 2021, 51, 107738. [Google Scholar] [CrossRef]
- Leskovac, V.; Trivi, S.; Peri, D. Glucose oxidase from Aspergillus niger: The mechanism of action with molecular oxygen, quinones, and one-electron acceptors. Int. J. Biochem. Cell Biol. 2005, 37, 731–750. [Google Scholar] [CrossRef] [PubMed]
- Gabelsberger, J.; Liebl, W.; Schleifer, K.H. Purification and properties of recombinant β-glucosidase of the hyperthermophilic bacterium Thermotoga maritima. Appl. Microbiol. Biotechnol. 1993, 40, 44–52. [Google Scholar] [CrossRef]
- Cans, A.-S.; Dean, S.L.; Reyes, F.E.; Keating, C.D. Synthesis and Characterization of Enzyme-Au Bioconjugates: HRP and Fluorescein-labeled HRP. Nanobiotechnology 2007, 3, 12–22. [Google Scholar] [CrossRef]
- Ciaurriz, P.; Bravo, E.; Hamad-Schifferli, K. Effect of architecture on the activity of glucose oxidase/horseradish peroxidase/carbon nanoparticle conjugates. J. Colloid Interface Sci. 2014, 414, 73–81. [Google Scholar] [CrossRef]
- Giannakopoulou, A.; Patila, M.; Spyrou, K.; Chalmpes, N.; Zarafeta, D.; Skretas, G.; Gournis, D.; Stamatis, H. Development of a four-enzyme magnetic nanobiocatalyst for multi-step cascade reactions. Catalysts 2019, 9, 995. [Google Scholar] [CrossRef]
- Sojitra, U.V.; Nadar, S.S.; Rathod, V.K. A magnetic tri-enzyme nanobiocatalyst for fruit juice clarification. Food Chem. 2016, 213, 296–305. [Google Scholar] [CrossRef]
- Abdulaal, W.H.; Almulaiky, Y.Q.; El-Shishtawy, R.M. Encapsulation of HRP enzyme onto a magnetic fe3o4 Np–PMMA film via casting with sustainable biocatalytic activity. Catalysts 2020, 10, 181. [Google Scholar] [CrossRef]
- Alnadari, F.; Xue, Y.; Zhou, L.; Hamed, Y.S.; Taha, M.; Foda, M.F. Immobilization of β-glucosidase from Thermatoga maritima on chitin-functionalized magnetic nanoparticle via a novel thermostable chitin-binding domain. Sci. Rep. 2020, 10, 1663. [Google Scholar] [CrossRef]
- Zhang, H.; Hua, S.F.; Zhang, L. Co-immobilization of cellulase and glucose oxidase on graphene oxide by covalent bonds: A biocatalytic system for one-pot conversion of gluconic acid from carboxymethyl cellulose. J. Chem. Technol. Biotechnol. 2020, 95, 1116–1125. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Carballares, D.; Morellon-Sterlling, R.; Berenguer-Murcia, Á.; Alcántara, A.R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Enzyme co-immobilization: Always the biocatalyst designers’ choice…or not? Biotechnol. Adv. 2020, 2020, 107584. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, D.; Wu, C.; Yao, K.; Li, Z.; Shi, N.; Wen, F.; Gates, I.D. Co-immobilization of cellulase and lysozyme on amino-functionalized magnetic nanoparticles: An activity-tunable biocatalyst for extraction of lipids from microalgae. Bioresour. Technol. 2018, 263, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Patila, M.; Kouloumpis, A.; Gournis, D.; Rudolf, P.; Stamatis, H. Laccase-functionalized graphene oxide assemblies as efficient nanobiocatalysts for oxidation reactions. Sensors 2016, 16, 287. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, T.; Perriman, A.W.; Sharma, K.P.; Mann, S. Multi-enzyme cascade reactions using protein-polymer surfactant self-standing films. Chem. Commun. 2017, 53, 2094–2097. [Google Scholar] [CrossRef] [PubMed]
- Baynton, K.J.; Bewtra, J.K.; Biswas, N.; Taylor, K.E. Inactivation of horseradish peroxidase by phenol and hydrogen peroxide: A kinetic investigation. Biochim. Biophys. Acta (BBA)/Protein Struct. Mol. 1994, 1206, 272–278. [Google Scholar] [CrossRef]
- Alvarez-Gonzalez, C.; Santos, V.E.; Ladero, M.; Bolivar, J.M. Immobilization-stabilization of β-glucosidase for implementation of intensified hydrolysis of cellobiose in continuous flow reactors. Catalysts 2022, 12, 80. [Google Scholar] [CrossRef]


| Enzyme | Immobilization Yield (%) | Activity Recovery (%) |
|---|---|---|
| Co-immobilized GOx | 80.5 ± 2.3 | 66.5 ± 3.7 |
| Co-immobilized HRP | 51.0 ± 3.2 | 50.0 ± 2.3 |
| Co-immobilized bgl | 98.5 ± 5.6 | 90.0 ± 2.8 |
| Biocatalyst | Apparent Vmax (μΜ min−1) | Apparent KM (mM) |
|---|---|---|
| Free GOx | 13.81 ± 0.48 | 2.79 ± 0.26 |
| Co-immobilized GOx | 3.33 ± 0.21 | 12.57 ± 2.31 |
| Free HRP | 2.93 ± 0.06 | 3.90 ± 0.32 |
| Co-immobilized HRP | 0.23 ± 0.05 | 9.90 ± 2.00 |
| Free bgl | 7.27 ± 0.50 | 0.22 ± 0.08 |
| Co-immobilized bgl | 2.88 ± 0.20 | 0.50 ± 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alatzoglou, C.; Patila, M.; Giannakopoulou, A.; Spyrou, K.; Yan, F.; Li, W.; Chalmpes, N.; Polydera, A.C.; Rudolf, P.; Gournis, D.; et al. Development of a Multi-Enzymatic Biocatalytic System through Immobilization on High Quality Few-Layer bio-Graphene. Nanomaterials 2023, 13, 127. https://doi.org/10.3390/nano13010127
Alatzoglou C, Patila M, Giannakopoulou A, Spyrou K, Yan F, Li W, Chalmpes N, Polydera AC, Rudolf P, Gournis D, et al. Development of a Multi-Enzymatic Biocatalytic System through Immobilization on High Quality Few-Layer bio-Graphene. Nanomaterials. 2023; 13(1):127. https://doi.org/10.3390/nano13010127
Chicago/Turabian StyleAlatzoglou, Christina, Michaela Patila, Archontoula Giannakopoulou, Konstantinos Spyrou, Feng Yan, Wenjian Li, Nikolaos Chalmpes, Angeliki C. Polydera, Petra Rudolf, Dimitrios Gournis, and et al. 2023. "Development of a Multi-Enzymatic Biocatalytic System through Immobilization on High Quality Few-Layer bio-Graphene" Nanomaterials 13, no. 1: 127. https://doi.org/10.3390/nano13010127
APA StyleAlatzoglou, C., Patila, M., Giannakopoulou, A., Spyrou, K., Yan, F., Li, W., Chalmpes, N., Polydera, A. C., Rudolf, P., Gournis, D., & Stamatis, H. (2023). Development of a Multi-Enzymatic Biocatalytic System through Immobilization on High Quality Few-Layer bio-Graphene. Nanomaterials, 13(1), 127. https://doi.org/10.3390/nano13010127








_Stamatis.png)

