Nickel—Alumina Catalysts for the Transformation of Vegetable Oils into Green Diesel: The Role of Preparation Method, Activation Temperature, and Reaction Conditions
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Effect of Preparation Method and Activation Temperature on the Porosity and Structure of the Catalysts Studied
3.2. Effect of Preparation Method and Activation Temperature on the Catalytic Efficiency of the Catalysts Studied
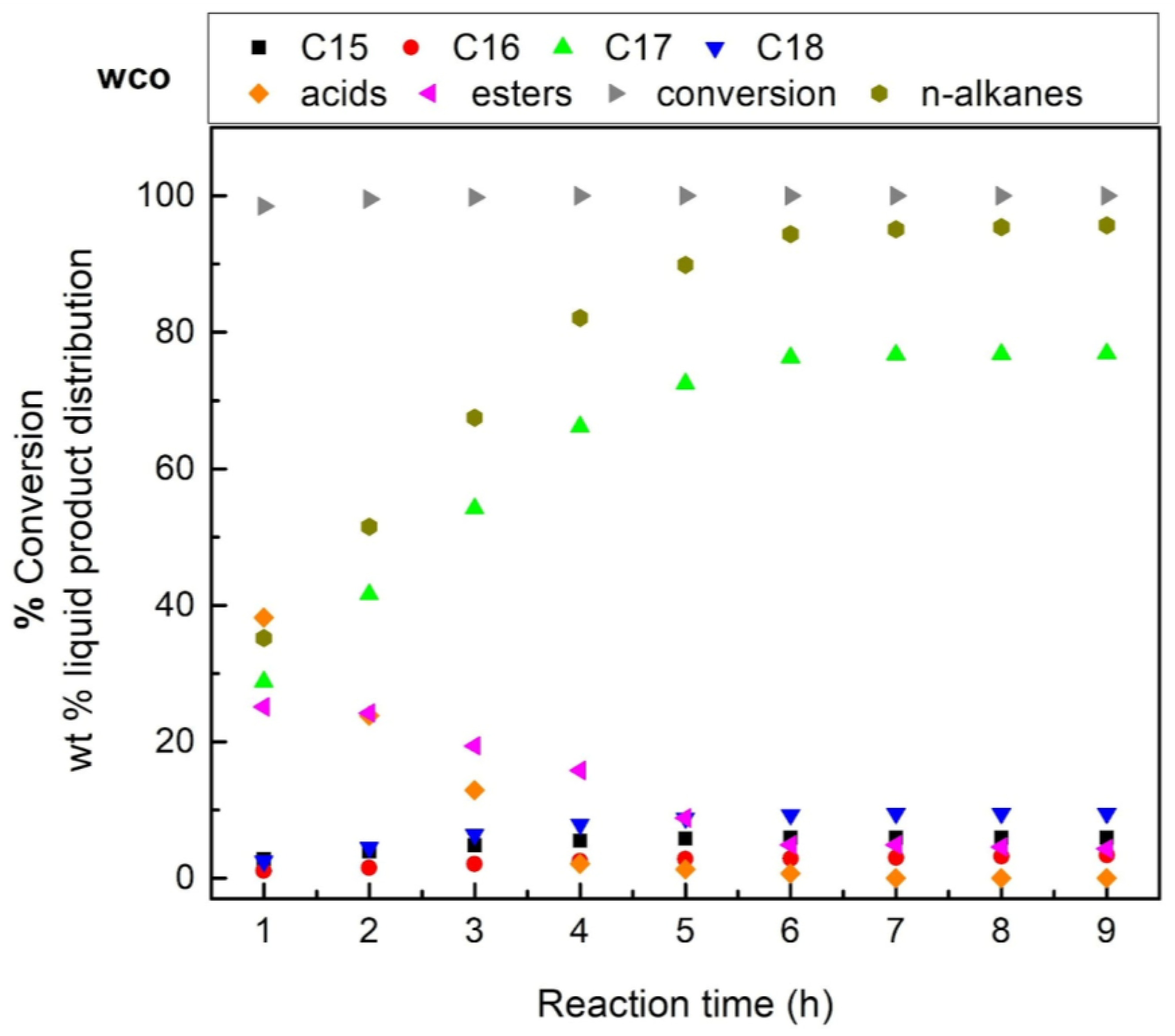
3.3. Effect of the Reaction Temperature on the Performance of Most Efficient Catalyst Studied
3.4. Influence of Reaction Temperature and Feedstock on Spent Catalyst Characteristics
3.5. Comparison of Catalyst Efficiency for Green Diesel Production with Relevant Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lycourghiotis, A.; Kordulis, C.; Lycourghiotis, S. Beyond Fossil Fuels: The Return Journey to Renewable Energy; Crete University Press: Herakleion, Greece, 2017. [Google Scholar]
- Kubickova, I.; Kubicka, D. Utilization of Triglycerides and Related Feedstocks for Production of Clean Hydrocarbon Fuels and Petrochemicals: A Review. Waste Biomass Valorization 2010, 1, 293–308. [Google Scholar] [CrossRef]
- Zhao, C.; Brückb, T.; Lercher, J.A. Catalytic deoxygenation of microalgae oil to green hydrocarbons. Green Chem. 2013, 15, 1720–1739. [Google Scholar] [CrossRef]
- de Sousa, F.P.; Cardoso, C.C.; Pasa, V.M. Producing hydrocarbons for green diesel and jet fuel formulation from palm kernel fat over Pd/C. Fuel Process. Technol. 2016, 143, 35–42. [Google Scholar] [CrossRef]
- Coumans, A.E.; Hensen, E.J.M. A model compound (methyl oleate, oleic acid, triolein) study of triglycerides hydrodeoxygenation over alumina-supported NiMo sulfide. Appl. Catal. B Environ. 2017, 201, 290–301. [Google Scholar] [CrossRef]
- Arora, P.; Ojagh, H.; Woo, J.; Grennfelt, E.L.; Olsson, L.; Creaser, D. Investigating the effect of Fe as a poison for catalytic HDO over sulfided NiMo alumina catalysts. Appl. Catal. B Environ. 2018, 227, 240–251. [Google Scholar] [CrossRef]
- Kordulis, C.; Bourikas, K.; Gousi, M.; Kordouli, E.; Lycourghiotis, A. Development of nickel based catalysts for the transformation of natural triglycerides and related compounds into green diesel: A critical review. Appl. Catal. B Environ. 2016, 181, 156–196. [Google Scholar] [CrossRef]
- Phimsen, S.; Kiatkittipong, W.; Yamada, H.; Tagawa, T.; Kiatkittipong, K.; Laosiripojana, N.; Assabumrungrat, S. Nickel sulfide, nickel phosphide and nickel carbide catalysts for bio-hydrotreated fuel production. Energy Convers. Manag. 2017, 151, 324–333. [Google Scholar] [CrossRef]
- Deliy, I.V.; Shamanaev, I.V.; Gerasimov, E.Y.; Pakharukova, V.P.; Yakovlev, I.V.; Lapina, O.B.; Aleksandrov, P.V.; Bukhtiyarova, G.A. HDO of Methyl Palmitate over Silica-Supported Ni Phosphides: Insight into Ni/P Effect. Catalysts 2017, 7, 298. [Google Scholar] [CrossRef]
- Taromi, A.A.; Kaliaguine, S. Hydrodeoxygenation of triglycerides over reduced mesostructured Ni/γ-alumina catalysts prepared via one-pot sol-gel route for green diesel production. Appl. Catal. A Gen. 2018, 558, 140–149. [Google Scholar] [CrossRef]
- Alvarez-Galvan, M.C.; Campos-Martin, J.M.; Fierro, J.L.G. Transition metal phosphides for the catalytic hydrodeoxygenation of waste oils into green diesel. Catalysts 2019, 9, 93. [Google Scholar] [CrossRef]
- Papanikolaou, G.; Lanzafame, P.; Giorgianni, G.; Abate, S.; Perathoner, S.; Centi, G. Highly selective bifunctional Ni zeo-type catalysts for hydroprocessing of methyl palmitate to green diesel. Catal. Today 2020, 345, 14–21. [Google Scholar] [CrossRef]
- de Oliveira Camargo, M.; Castagnari Willimann Pimenta, J.L.; de Oliveira Camargo, M.; Arroyo, P.A. Green diesel production by solvent-free deoxygenation of oleic acid over nickel phosphide bifunctional catalysts: Effect of the support. Fuel 2020, 281, 118719. [Google Scholar] [CrossRef]
- Shi, Y.; Li, M.; Yu, Y.; Zhang, B. Recent advances in nanostructured transition metal phosphides: Synthesis and energy-related applications. Energy Environ. Sci. 2020, 13, 4564–4582. [Google Scholar] [CrossRef]
- Du, X.; Liang, W.; Hao, X.; Zhou, K.; Yang, H.; Lei, X.; Li, D.; Hu, C. The effect of support on nickel phosphide catalysts for one-pot conversion of jatropha oil into high grade hydrocarbons. Catal. Today 2021, 367, 83–94. [Google Scholar] [CrossRef]
- Zhou, K.; Du, X.; Zhou, L.; Yang, H.; Lei, X.; Zeng, Y.; Li, D.; Hu, C. The Deoxygenation of Jatropha Oil to High Quality Fuel via the Synergistic Catalytic Effect of Ni, W2C and WC Species. Catalysts 2021, 11, 469. [Google Scholar] [CrossRef]
- García-Pérez, D.; Alvarez-Galvan, M.C.; Capel-Sanchez, M.C.; Blanco-Brieva, G.; Morales-de la Rosa, S.J.; Campos-Martin, M.; Fierro, J.L.G. Influence of bimetallic characteristics on the performance of MoCoP and MoFeP catalysts for methyl laurate hydrodeoxygenation. Catal. Today 2021, 367, 43–50. [Google Scholar] [CrossRef]
- Crisostomo, C.A.B.; Almeida, T.S.S.; Soares, R.R. Towards triglycerides-based biorefineries: Hydrolysis-reforming-hydrogenation in one-pot over Ni/γ-Al2O3 based catalysts. Catal. Today 2021, 367, 124–136. [Google Scholar] [CrossRef]
- Hongloi, N.; Prapainainar, P.; Prapainainar, C. Review of green diesel production from fatty acid deoxygenation over Ni-based catalysts. Mol. Catal. 2022, 523, 111696. [Google Scholar] [CrossRef]
- Gosselink, R.W.; Hollak, S.A.W.; Chang, S.-W.; van Haveren, J.; de Jong, K.P.; Bitter, J.H.; van Es, D.S. Reaction Pathways for the Deoxygenation of Vegetable Oils and Related Model Compounds. ChemSusChem 2013, 6, 1576–1594. [Google Scholar] [CrossRef]
- Gousi, M.; Andriopoulou, C.; Bourikas, K.; Ladas, S.; Sotiriou, M.; Kordulis, C.; Lycourghiotis, A. Green diesel production over nickel-alumina co-precipitated catalysts. Appl. Catal. A Gen. 2017, 536, 45–56. [Google Scholar] [CrossRef]
- Nikolopoulos, I.; Kogkos, G.; Kordouli, E.; Bourikas, K.; Kordulis, C.; Lycourghiotis, A. Waste cooking oil transformation into third generation green diesel catalyzed by nickel—Alumina catalysts. Mol. Catal. 2020, 482, 110697. [Google Scholar] [CrossRef]
- Zafeiropoulos, G.; Nikolopoulos, N.; Kordouli, E.; Sygellou, L.; Bourikas, K.; Kordulis, C.; Lycourghiotis, A. Developing Nickel–Zirconia Co-Precipitated Catalysts for Production of Green Diesel. Catalysts 2019, 9, 210. [Google Scholar] [CrossRef]
- Gousi, M.; Kordouli, E.; Bourikas, K.; Symianakis, E.; Ladas, S.; Kordulis, C.; Lycourghiotis, A. Green Diesel Production over Nickel-Alumina Nanostructured Catalysts Promoted by Copper. Energies 2020, 13, 3707. [Google Scholar] [CrossRef]
- Gousi, M.; Kordouli, E.; Bourikas, K.; Simianakis, E.; Ladas, S.; Panagiotou, G.D.; Kordulis, C.; Lycourghiotis, A. Green diesel production over nickel-alumina nanostructured catalysts promoted by zinc. Catal. Today 2020, 355, 903–909. [Google Scholar] [CrossRef]
- Kordouli, E.; Sigellou, L.; Kordulis, C.; Bourikas, K.; Lycourghiotis, A. Probing the synergistic ratio of the NiMo/γ-Al2O3 reduced catalysts for the transformation of natural triglycerides into green diesel. Appl. Catal. B Environ. 2017, 209, 12–22. [Google Scholar] [CrossRef]
- Kordouli, E.; Pawelec, B.; Bourikas, K.; Kordulis, C.; Fierro, J.L.G.; Lycourghiotis, A. Mo promoted Ni-Al2O3 co-precipitated catalysts for green diesel production. Appl. Catal. B Environ. 2018, 229, 139–154. [Google Scholar] [CrossRef]
- Papadopoulos, C.; Kordouli, E.; Sygellou, L.; Bourikas, K.; Kordulis, C.; Lycourghiotis, A. W promoted Ni-Al2O3 co-precipitated catalysts for green diesel production. Fuel Proc. Technol. 2021, 217, 106820. [Google Scholar] [CrossRef]
- Faroldi, B.; Paviotti, M.A.; Camino-Manjarrés, M.; González-Carrazán, S.; López-Olmos, C.; Rodríguez-Ramos, I. Hydrogen Production by Formic Acid Decomposition over Ca Promoted Ni/SiO2 Catalysts: Effect of the Calcium Content. Nanomaterials 2019, 9, 1516. [Google Scholar] [CrossRef]
- Zafeiropoulos, J.; Petropoulos, G.; Kordouli, E.; Kordulis, C.; Lycourghiotis, A.; Bourikas, K. Development of nickel catalysts supported on silica for green diesel production. Catal. Today, 2022; in press. [Google Scholar] [CrossRef]
- Weisz, P.B.; Haag, W.O.; Rodewald, P.G. Catalytic Production of High-Grade Fuel (Gasoline) from Biomass Compounds by Shape-Selective Catalysis. Science 1979, 206, 57–58. [Google Scholar] [CrossRef]
- Knothe, G. Biodiesel and renewable diesel: A comparison. Prog. Energy Combust. Sci. 2010, 36, 364–373. [Google Scholar] [CrossRef]
- Glisic, S.B.; Pajnik, J.M.; Orlovic, A.M. Process and techno-economic analysis of green diesel production from waste vegetable oil and the comparison with ester type biodiesel production. Appl. Energy 2016, 170, 176–185. Available online: https://ideas.repec.org/a/eee/appene/v170y2016icp176-185.html#:~:text=DOI%3A%2010.1016/j.apenergy.2016.02.102 (accessed on 1 January 2023). [CrossRef]
- Zhao, X.; Wei, L.; Cheng, S.; Julson, J.; Anderson, G.; Muthukumarappan, K.; Qiu, C. Development of hydrocarbon biofuel from sunflower seed and sunflower meat oils over ZSM-5. J. Renew. Sustain. Energy 2016, 8, 013109. [Google Scholar] [CrossRef]
- Lycourghiotis, S.; Kordouli, E.; Sygellou, L.; Bourikas, K.; Kordulis, C. Nickel catalysts supported on palygorskite for transformation of waste cooking oils into green diesel. Appl. Catal. B Environ. 2019, 259, 118059. [Google Scholar] [CrossRef]
- Zeng, Y.; Ma, H.; Zhang, H.; Ying, W.; Fang, D. Highly efficient NiAl2O4-free Ni/γ-Al2O3 catalysts prepared by solution combustion method for CO methanation. Fuel 2014, 137, 155–163. [Google Scholar] [CrossRef]
- Córdova-Pérez, G.E.; Cortez-Elizalde, J.; Silahua-Pavón, A.A.; Cervantes-Uribe, A.; Arévalo-Pérez, J.C.; Cordero-Garcia, A.; de los Monteros, A.E.E.; Espinosa-González, C.G.; Godavarthi, S.; Ortiz-Chi, F.; et al. γ-Valerolactone Production from Levulinic Acid Hydrogenation Using Ni Supported Nanoparticles: Influence of Tungsten Loading and pH of Synthesis. Nanomaterials 2022, 12, 2017. [Google Scholar] [CrossRef]
- Al-Ali, L.I.; Elmutasim, O.; Al Ali, K.; Singh, N.; Polychronopoulou, K. Transition Metal Phosphides (TMP) as a Versatile Class of Catalysts for the Hydrodeoxygenation Reaction (HDO) of Oil-Derived Compounds. Nanomaterials 2022, 12, 1435. [Google Scholar] [CrossRef]
- Lin, D.; Mao, Z.; Feng, X.; Zhou, X.; Yan, H.; Zhu, H.; Liu, Y.; Chen, X.; Tuo, Y.; Peng, C.; et al. Kinetic insights into deoxygenation of vegetable oils to produce second-generation biodiesel. Fuel 2023, 333, 126416. [Google Scholar] [CrossRef]
- Kaluža, L.; Kubička, D. The comparison of Co, Ni, Mo, CoMo and NiMo sulfided catalysts in rapeseed oil hydrodeoxygenation. Reac. Kinet. Mech. Cat. 2017, 122, 333–341. [Google Scholar] [CrossRef]
- Kaluža, L.; Soukup, K.; Koštejn, M.; Čejka, J.; Karban, J.; Palcheva, R.; Laube, M.; Gulková, D. On Stability of High-Surface-Area Al2O3, TiO2, SiO2-Al2O3, and Activated Carbon Supports during Preparation of NiMo Sulfide Catalysts for Parallel Deoxygenation of Octanoic Acid and Hydrodesulfurization of 1-Benzothiophene. Catalysts 2022, 12, 1559. [Google Scholar] [CrossRef]
- Anand, M.; Sinha, A.K. Temperature-dependent reaction pathways for the anomalous hydrocracking of triglycerides in the presence of sulfided Co–Mo-catalyst. Bioresour. Technol. 2012, 126, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Anand, M.; Farooqui, S.A.; Kumar, R.; Joshi, R.; Kumar, R.; Sibi, M.G.; Singh, H.; Sinha, A.K. Kinetics, thermodynamics and mechanisms for hydroprocessing of renewable oils. Appl. Catal. A Gen. 2016, 516, 144–152. [Google Scholar] [CrossRef]
- Smirnov, A.A.; Alekseeva, M.V.; Bulavchenko, O.A.; Yakovlev, V.A. Studying the Effect of the Process Temperature on the Degree of Bio-Oil Hydrotreatment at Low Hydrogen Contents over NiCu–SiO2 Catalyst with a High Metal Loading. Catal. Ind. 2019, 11, 65–73. [Google Scholar] [CrossRef]
- Kaviani, M.; Rezaei, M.; Alavi, M.S.; Akbari, E. High coke resistance Ni-SiO2@SiO2 core-shell catalyst for biogas dry reforming: Effects of Ni loading and calcination temperature. Fuel 2022, 330, 125609. [Google Scholar] [CrossRef]
- Nugrahaningtyas, K.D.; Lukitawati, R.; Mukhsin, S.A.; Fadlulloh, Z.; Sabiilagusti, A.I.; Budiman, A.W.; Kurniawati, M.F. Conversion of waste cooking oil into green diesel using Ni/MOR and Cu/MOR catalysts. J. Phys. Conf. Ser. 2022, 2190, 012037. [Google Scholar] [CrossRef]
- Jeon, K.-W.; Park, H.-R.; Lee, Y.-L.; Kim, J.-E.; Jang, W.-J.; Shim, J.-O.; Roh, H.-S. Deoxygenation of non-edible fatty acid for green diesel production: Effect of metal loading amount over Ni/MgO–Al2O3 on the catalytic performance and reaction pathway. Fuel 2022, 311, 122488. [Google Scholar] [CrossRef]
- Prihadiyono, F.I.; Lestari, W.W.; Putra, R.; Aqna, A.N.L.; Cahyani, I.S.; Kadja, G.T.M. Heterogeneous Catalyst based on Nickel Modified into Indonesian Natural Zeolite in Green Diesel Production from Crude Palm Oil. Int. J. Technol. 2022, 13, 931–943. [Google Scholar] [CrossRef]
- Sowe, M.S.; Lestari, A.R.; Novitasari, E.; Masruri, M.; Ulfa, S.M. The Production of Green Diesel Rich Pentadecane (C15) from Catalytic Hydrodeoxygenation of Waste Cooking Oil using Ni/Al2O3-ZrO2 and Ni/SiO2-ZrO2. Bull. Chem. React. Eng. Catal. 2022, 17, 135–145. [Google Scholar] [CrossRef]
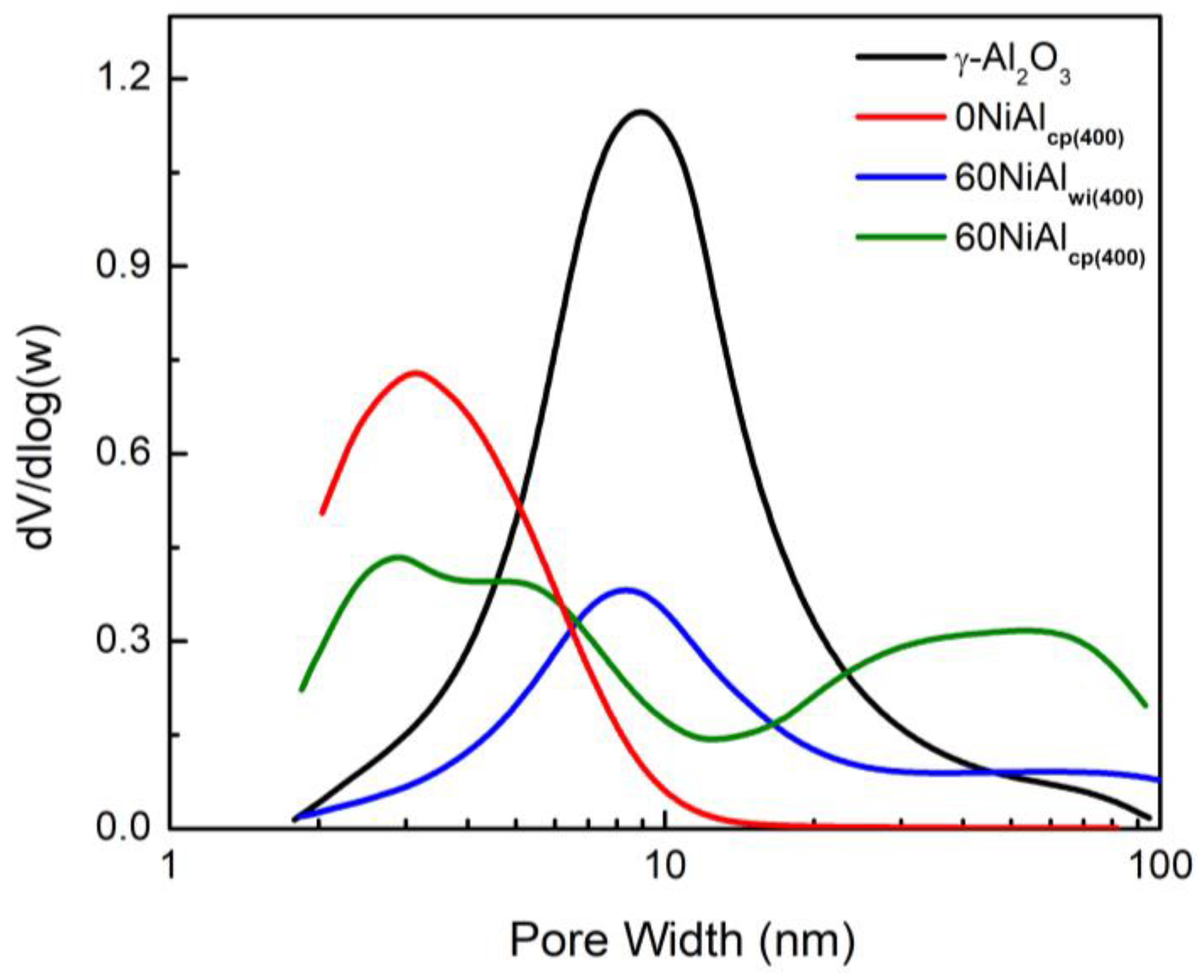
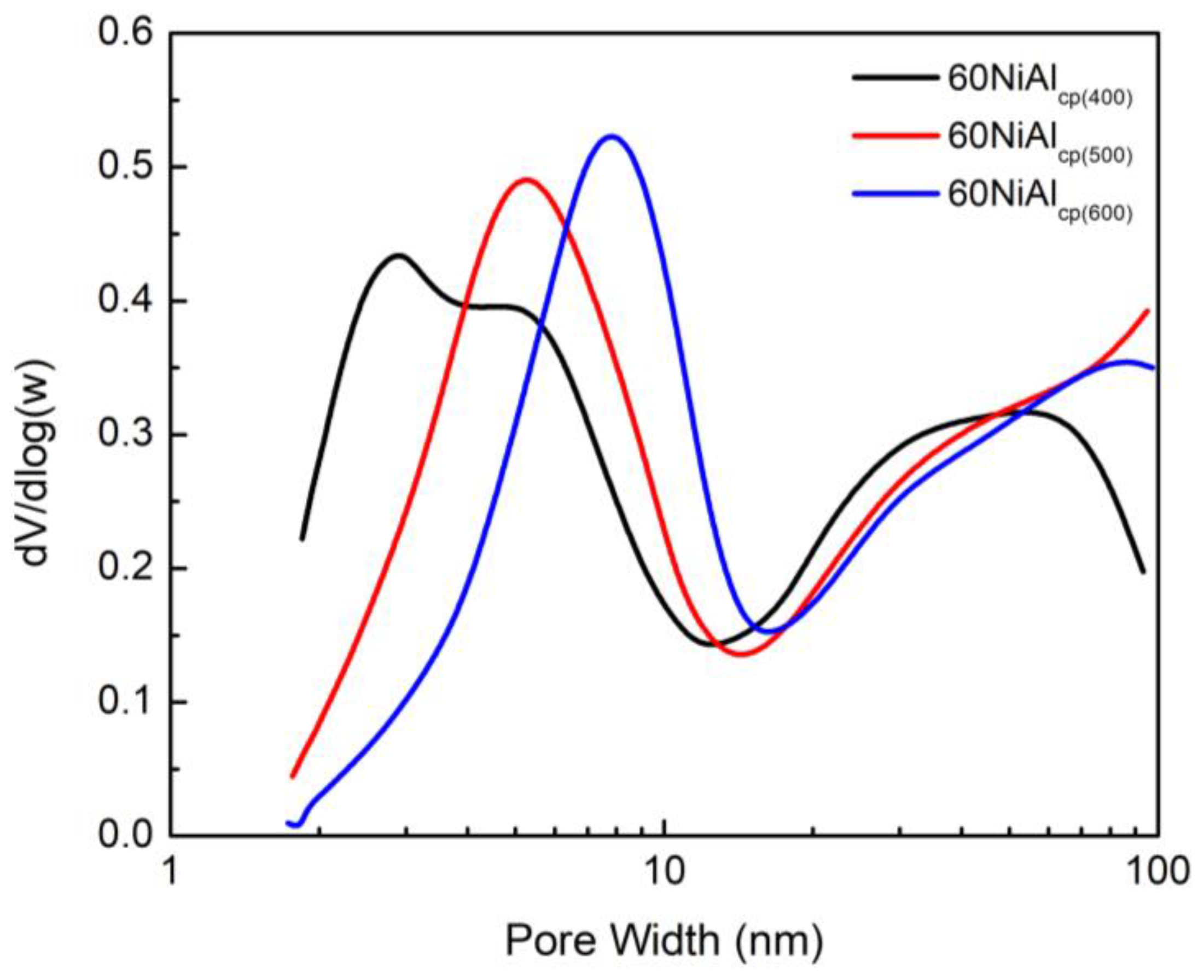
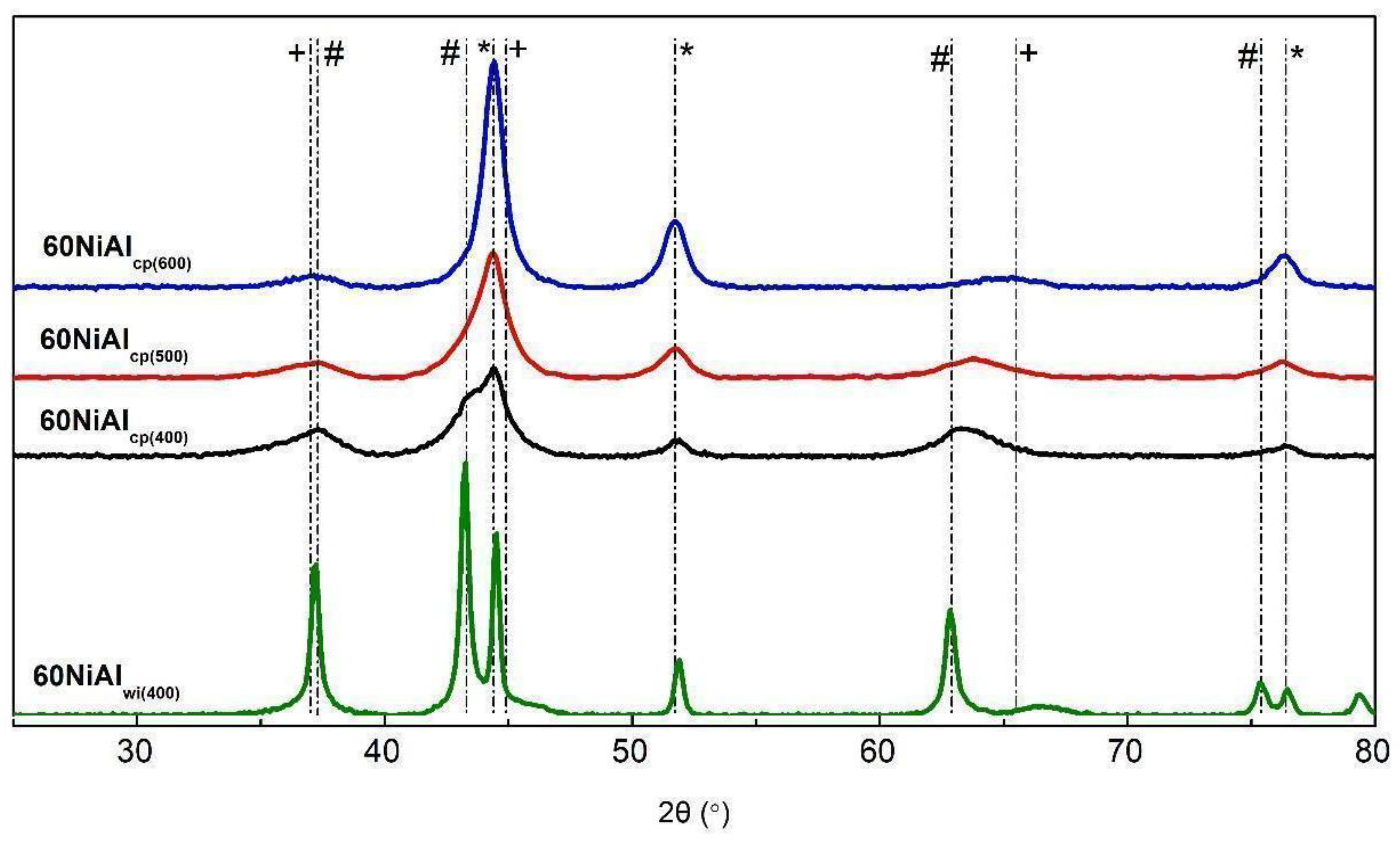

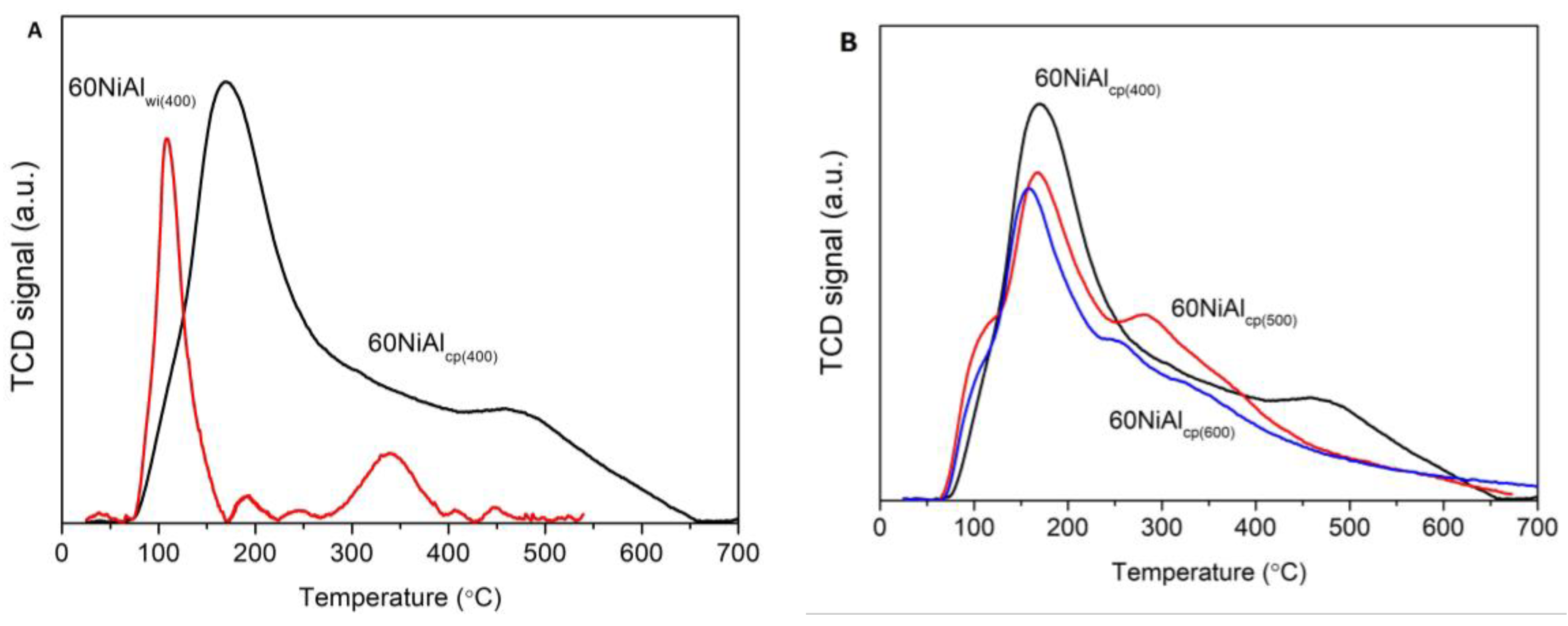
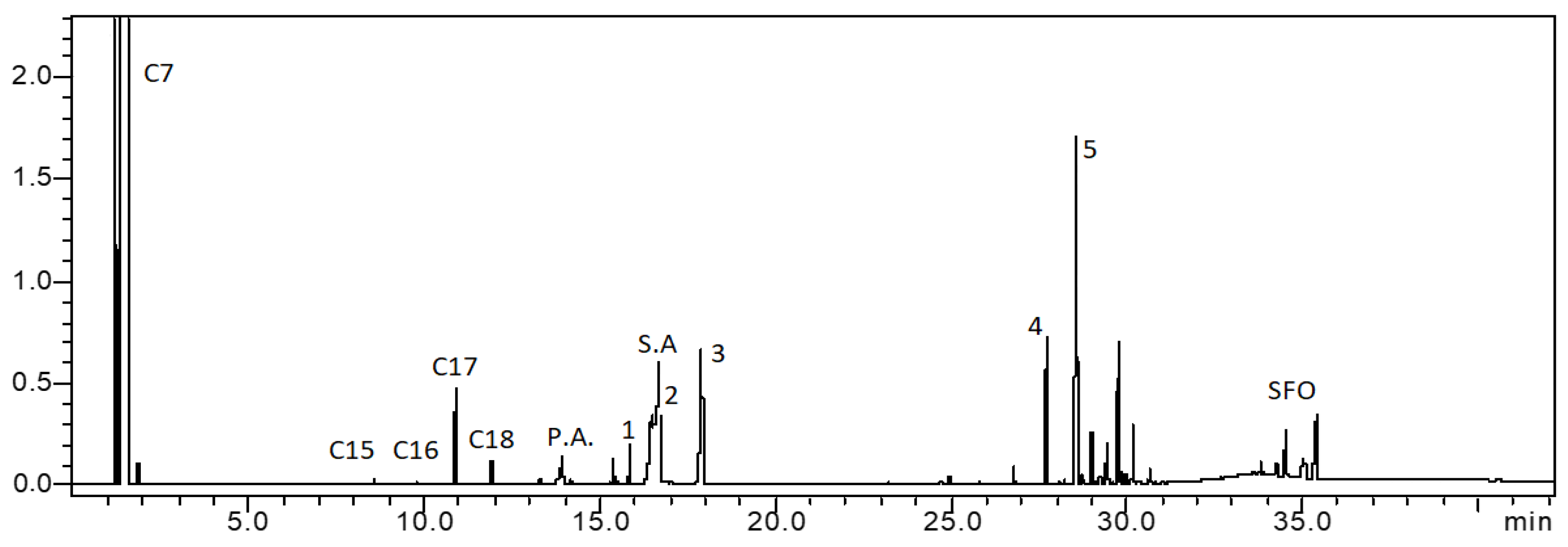

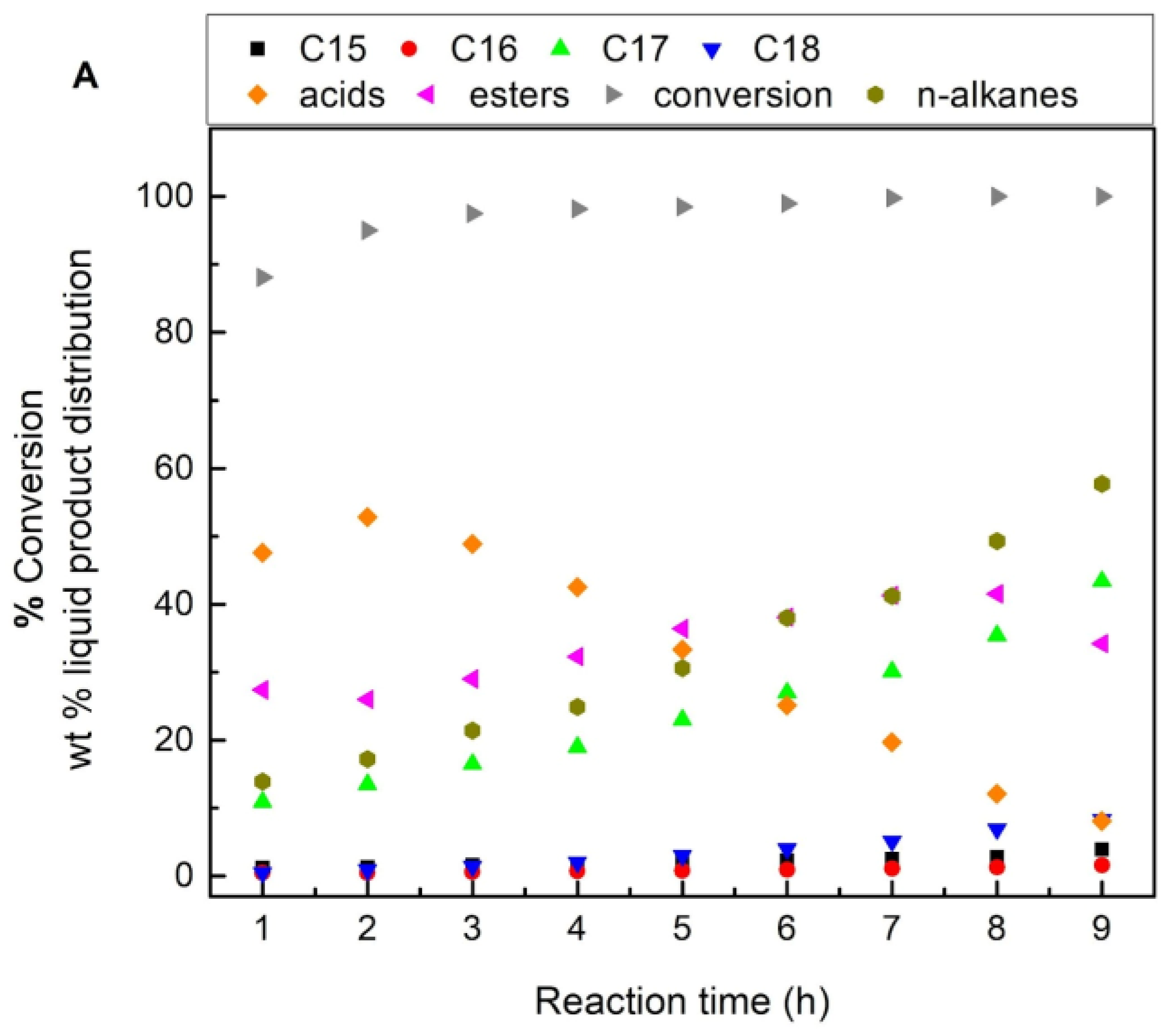
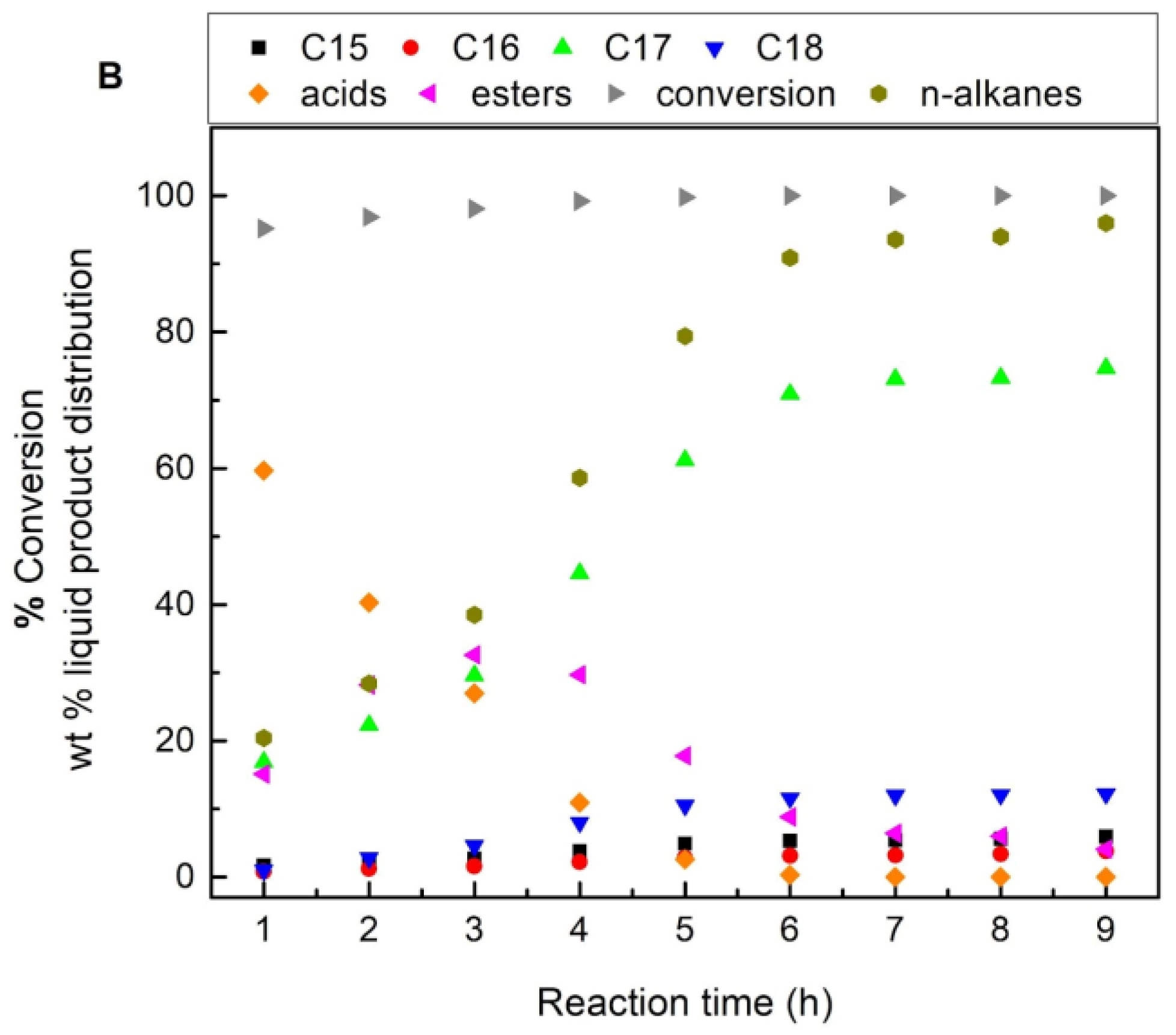
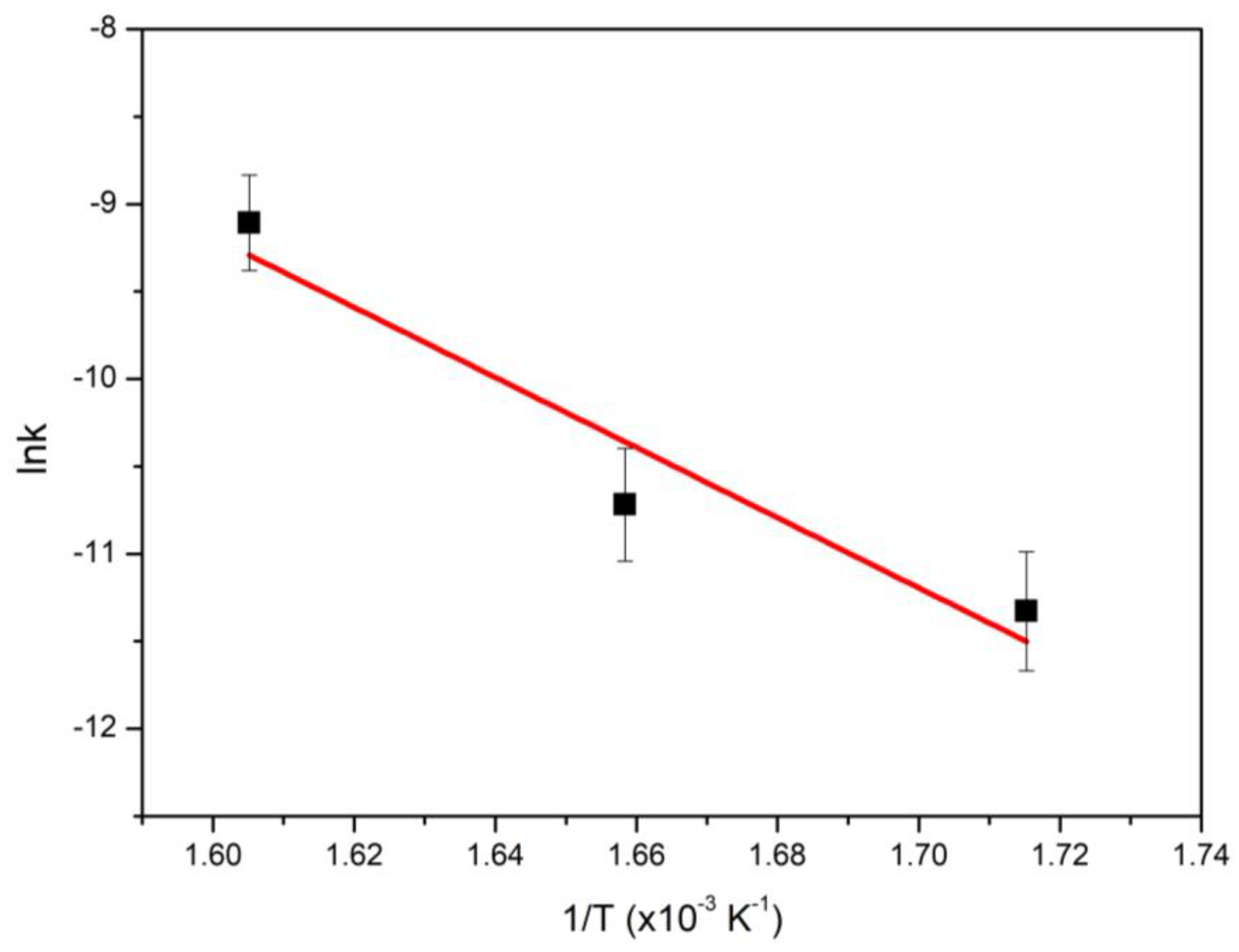
| Supports/Catalysts | SBET m2·g−1 | 1 SPVBJH cm3·g−1 | 2 MPDBJH nm | 3 MCDNi nm | 4 SNi m2·g−1 | 5 COchem μmol/gcat | 6 Acidity a.u. |
|---|---|---|---|---|---|---|---|
| γ-Al2O3 | 260 | 0.66 | 6.6 | - | - | - | - |
| 0NiAlcp(400) | 344 | 0.34 | 3.2 | - | - | - | - |
| 60NiAlw.i(400) | 111 | 0.29 | 7.6 | 20.2 | 20.0 | 7 | 14 |
| 60NiAlcp(400) | 272 | 0.60 | 6.6 | <4.0 | >60 | 15 7 | 63 |
| 60NiAcp(500) | 193 | 0.59 | 8.7 | 7.6 | 53.2 | 10.5 | 57 |
| 60NiAlcp(600) | 143 | 0.54 | 10.9 | 8.2 | 49.3 | 9 | 54 |
| Catalyst | X (%) | Liquid Phase Composition (wt. %) | R * | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Acids | Esters | Alkanes | n-C17 | n-C18 | n-C15 | n-C16 | |||
| 60NiAlwi(400) | 78.8 | 9.7 | 51.0 | 18.2 | 14.3 | 2.7 | 0.9 | 0.3 | 5.1 |
| 60NiAlcp(400) | 100 | 11.5 | 45.9 | 42.6 | 35.0 | 4.7 | 1.4 | 1.5 | 7.5 |
| 60NiAlcp(500) | 95.1 | 10.2 | 61.3 | 23.6 | 19.8 | 2.0 | 1.4 | 0.4 | 8.8 |
| 60NiAlcp(600) | 93.2 | 12.0 | 60.6 | 20.7 | 17.1 | 1.9 | 1.2 | 0.4 | 8.0 |
| Reaction Temperature °C | SBET m2g−1 | SPVBJH cm3g−1 | MPDBJH nm | MCDNi nm | Carbon Content % |
|---|---|---|---|---|---|
| - * | 272 | 0.60 | 6.6 | <4 | -- |
| 310 | 27 | 0.19 | 20.4 | 10.2 | 19.71 |
| 330 | 73 | 0.18 | 16.1 | 14.0 | 7.40 |
| 350 | 110 | 0.52 | 13.5 | 20.9 | 5.01 |
| 350 ** | 52 | 0.3 | 15.6 | 19.2 | 9.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolopoulos, I.; Kogkos, G.; Tsavatopoulou, V.D.; Kordouli, E.; Bourikas, K.; Kordulis, C.; Lycourghiotis, A. Nickel—Alumina Catalysts for the Transformation of Vegetable Oils into Green Diesel: The Role of Preparation Method, Activation Temperature, and Reaction Conditions. Nanomaterials 2023, 13, 616. https://doi.org/10.3390/nano13030616
Nikolopoulos I, Kogkos G, Tsavatopoulou VD, Kordouli E, Bourikas K, Kordulis C, Lycourghiotis A. Nickel—Alumina Catalysts for the Transformation of Vegetable Oils into Green Diesel: The Role of Preparation Method, Activation Temperature, and Reaction Conditions. Nanomaterials. 2023; 13(3):616. https://doi.org/10.3390/nano13030616
Chicago/Turabian StyleNikolopoulos, Ioannis, George Kogkos, Vasiliki D. Tsavatopoulou, Eleana Kordouli, Kyriakos Bourikas, Christos Kordulis, and Alexis Lycourghiotis. 2023. "Nickel—Alumina Catalysts for the Transformation of Vegetable Oils into Green Diesel: The Role of Preparation Method, Activation Temperature, and Reaction Conditions" Nanomaterials 13, no. 3: 616. https://doi.org/10.3390/nano13030616
APA StyleNikolopoulos, I., Kogkos, G., Tsavatopoulou, V. D., Kordouli, E., Bourikas, K., Kordulis, C., & Lycourghiotis, A. (2023). Nickel—Alumina Catalysts for the Transformation of Vegetable Oils into Green Diesel: The Role of Preparation Method, Activation Temperature, and Reaction Conditions. Nanomaterials, 13(3), 616. https://doi.org/10.3390/nano13030616









