Nanoengineering of NiO/MnO2/GO Ternary Composite for Use in High-Energy Storage Asymmetric Supercapacitor and Oxygen Evolution Reaction (OER)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of GO
2.3. Synthesis of NiO
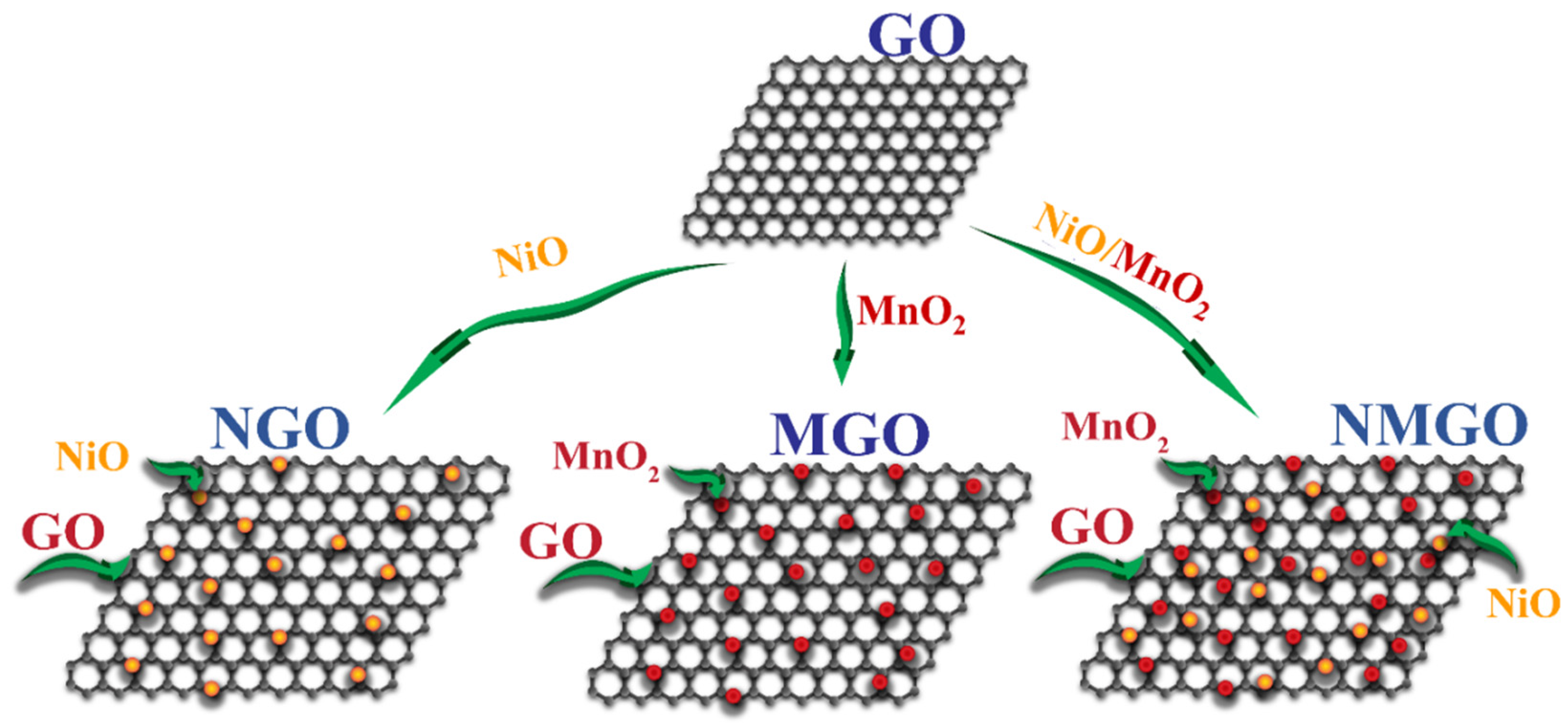
2.4. Formation of NiO/MnO2/GO Composite
2.5. Characterizations
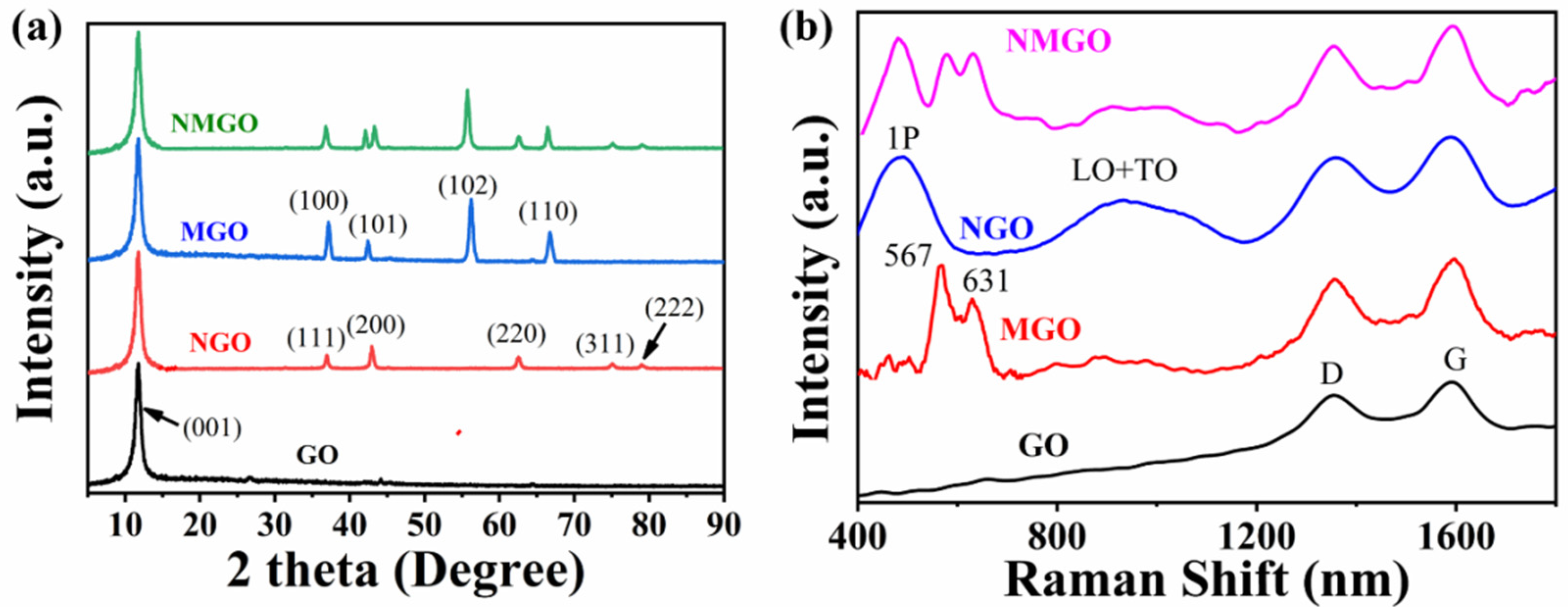
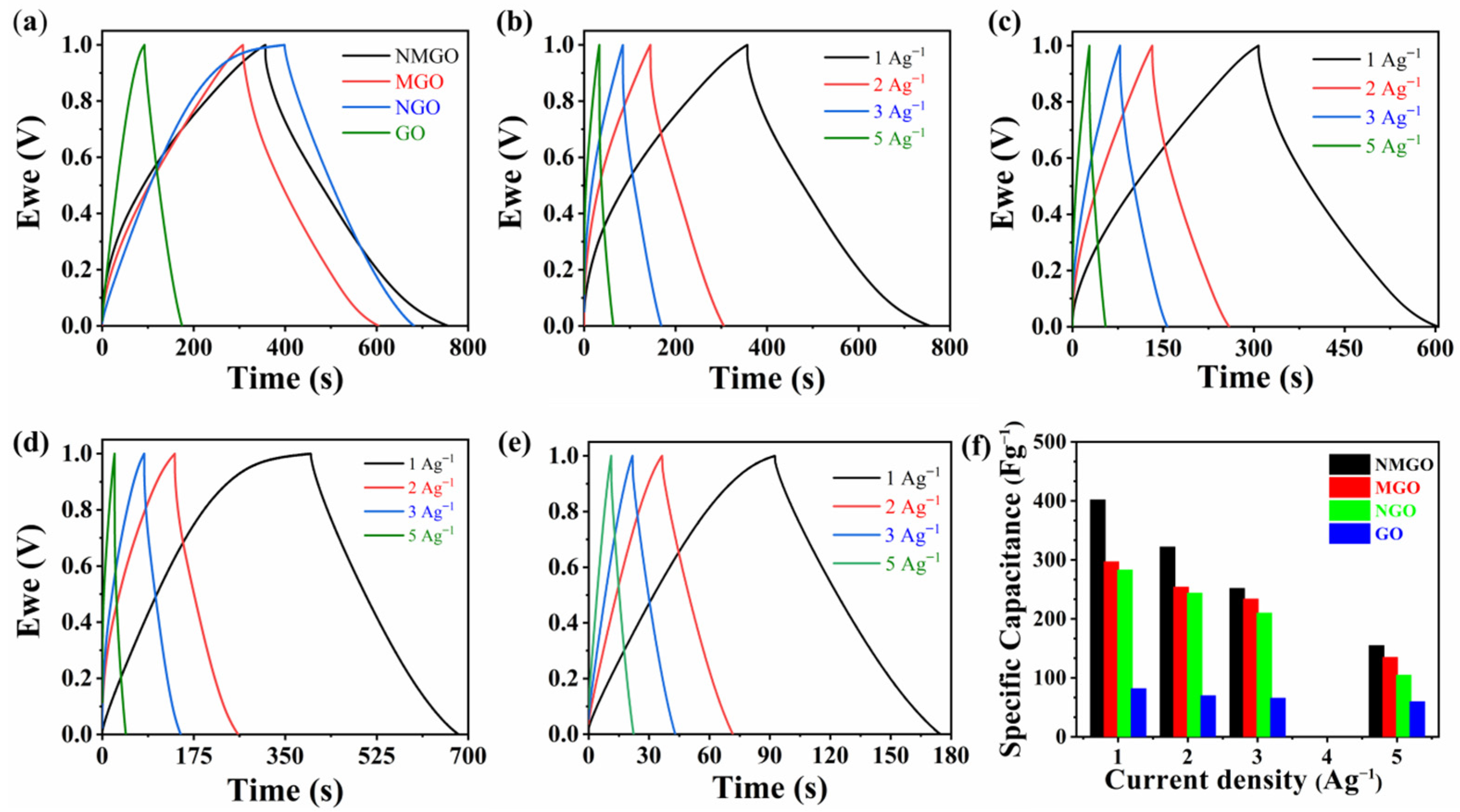
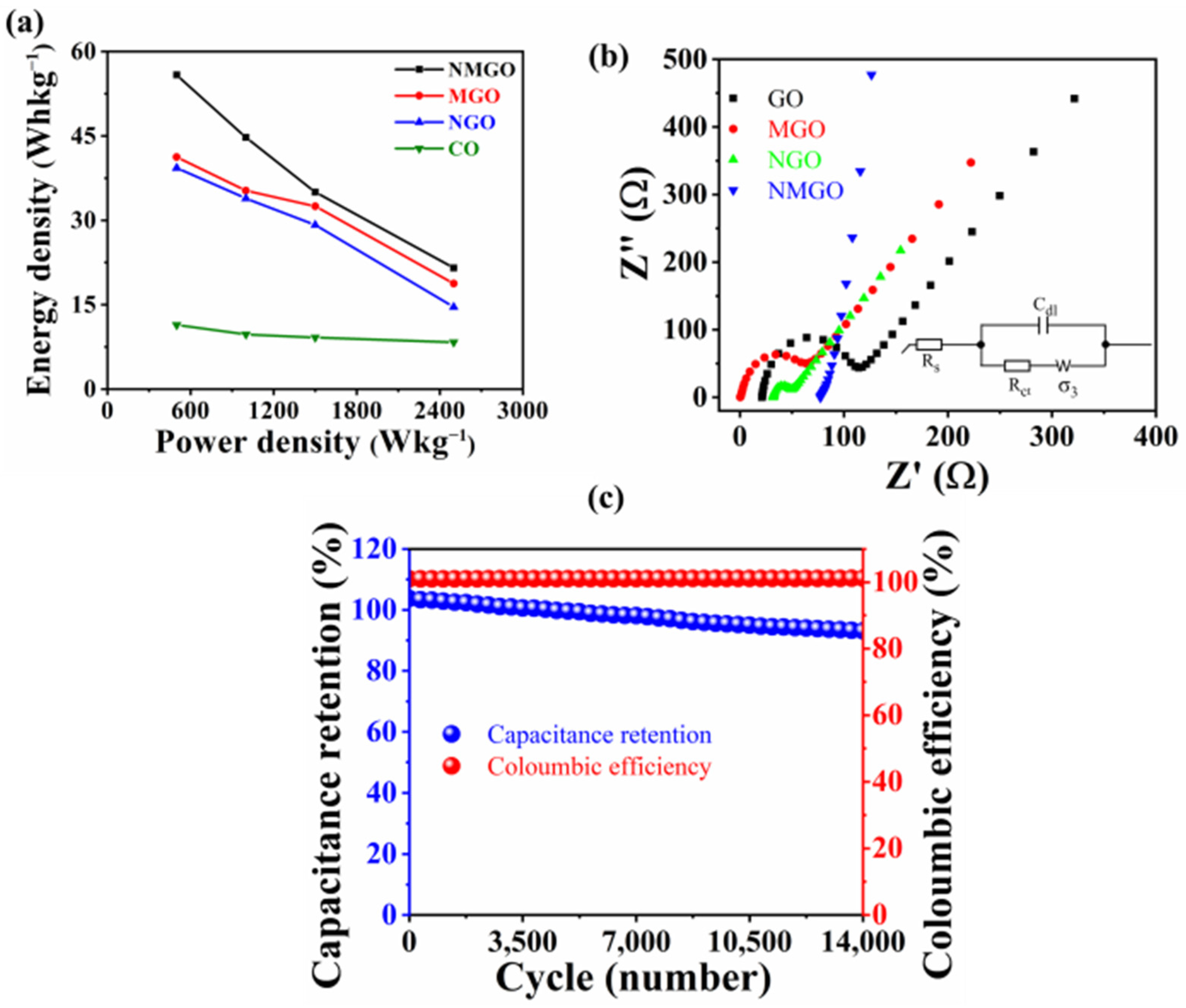
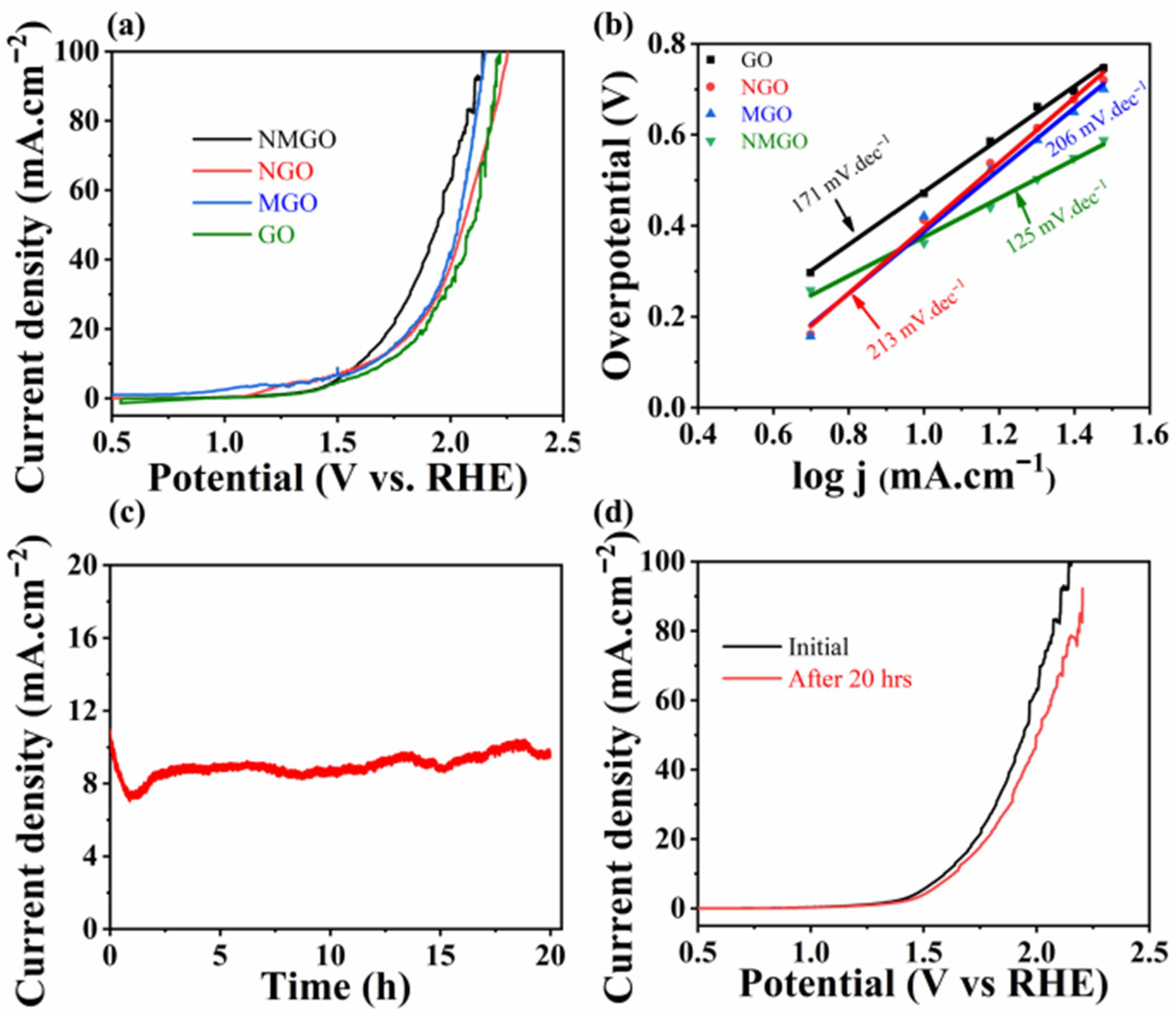
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Abraham, S.; Prasankumar, T.; Vinoth Kumar, K.; Zh Karazhanov, S.; Jose, S. Novel Lead Dioxide Intercalated Polypyrrole/Graphene Oxide Ternary Composite for High Throughput Supercapacitors. Mater. Lett. 2020, 273, 127943. [Google Scholar] [CrossRef]
- Hong, J.; Mengesha, T.T.; Hong, S.-W.; Kim, H.-K.; Hwang, Y.-H. A Comparative Study of the Effects of Different Methods for Preparing Rgo/Metal-Oxide Nanocomposite Electrodes on Supercapacitor Performance. J. Kor. Phys. Soc. 2020, 76, 264–272. [Google Scholar] [CrossRef]
- Miniach, E.; Śliwak, A.; Moyseowicz, A.; Fernández-Garcia, L.; González, Z.; Granda, M.; Menendez, R.; Gryglewicz, G. MnO2/Thermally Reduced Graphene Oxide Composites for High-Voltage Asymmetric Supercapacitors. Electrochim. Acta 2017, 240, 53–62. [Google Scholar] [CrossRef]
- Tamilselvi, R.; Ramesh, M.; Lekshmi, G.S.; Bazaka, O.; Levchenko, I.; Bazaka, K.; Mandhakini, M. Graphene Oxide – Based Supercapacitors from Agricultural Wastes: A Step to Mass Production of Highly Efficient Electrodes for Electrical Transportation Systems. Renew. Energy 2020, 151, 731–739. [Google Scholar] [CrossRef]
- Xia, H.; Wang, Y.; Lin, J.; Lu, L. Hydrothermal Synthesis of MnO2/Cnt Nanocomposite with a Cnt Core/Porous MnO2 Sheath Hierarchy Architecture for Supercapacitors. Nanoscale Res. Lett. 2012, 7, 33. [Google Scholar] [CrossRef]
- Miah, M.; Mondal, T.K.; Ghosh, A.; Saha, S.K. Study of Highly Porous Zno Nanospheres Embedded Reduced Graphene Oxide for High Performance Supercapacitor Application. Electrochim. Acta 2020, 354. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, X.; Li, F.; Huang, M. MnO2@ Nio Nanosheets@ Nanowires Hierarchical Structures with Enhanced Supercapacitive Properties. J. Mater. Sci. 2020, 55, 2482–2491. [Google Scholar] [CrossRef]
- Ahsan, M.T.; Usman, M.; Ali, Z.; Javed, S.; Ali, R.; Farooq, M.U.; Akram, M.A.; Mahmood, A. 3d Hierarchically Mesoporous Zinc-Nickel-Cobalt Ternary Oxide (Zn0.6ni0.8co1.6o4) Nanowires for High-Performance Asymmetric Supercapacitors. Front. Chem. 2020, 8, 487. [Google Scholar] [CrossRef]
- Wang, M.; Yang, J.; Liu, S.; Li, M.; Hu, C.; Qiu, J. Nitrogen-Doped Hierarchically Porous Carbon Nanosheets Derived from Polymer/Graphene Oxide Hydrogels for High-Performance Supercapacitors. J. Colloid Interface Sci. 2020, 560, 69–76. [Google Scholar] [CrossRef]
- Chen, I.W.P.; Chou, Y.-C.; Wang, P.-Y. Integration of Ultrathin Mos2/Pani/Cnt Composite Paper in Producing All-Solid-State Flexible Supercapacitors with Exceptional Volumetric Energy Density. J. Phys. Chem. C 2019, 123, 17864–17872. [Google Scholar] [CrossRef]
- Roy, A.; Ray, A.; Saha, S.; Ghosh, M.; Das, T.; Satpati, B.; Nandi, M.; Das, S. Nio-Cnt Composite for High Performance Supercapacitor Electrode and Oxygen Evolution Reaction. Electrochim. Acta 2018, 283, 327–337. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, Y.; Xie, X.; Du, W.; Kang, L.; Wang, Y.; Sun, X.; Li, Z.; Wang, B. One-Step Synthesis of the Reduced Graphene Oxide@Nio Composites for Supercapacitor Electrodes by Electrode-Assisted Plasma Electrolysis. Mater. Des. 2020, 196, 109111. [Google Scholar] [CrossRef]
- Usman, M.; Adnan, M.; Ahsan, M.T.; Javed, S.; Butt, M.S.; Akram, M.A. In Situ Synthesis of a Polyaniline/ Fe-Ni Codoped Co3o4 Composite for the Electrode Material of Supercapacitors with Improved Cyclic Stability. ACS Omega 2021, 6, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, A.; Muhammad, U.; Muhammad Aftab, A.; Sofia, J.; Saqib, A.; Iftikhar, A.; Mohammad, I. Study of Magnetic and Dielectric Properties of Znfe2o4/Cocr2o4 Nanocomposites Produced Using Sol-Gel and Hydrothermal Processes. J. Alloys Comp. 2021, 865, 158953. [Google Scholar]
- Zhou, X.; Meng, T.; Yi, F.; Shu, D.; Han, D.; Zhu, Z.; Gao, A.; Liu, C.; Li, X.; Yang, K.; et al. Supramolecular-Induced Confining Methylene Blue in Three-Dimensional Reduced Graphene Oxide for High-Performance Supercapacitors. J. Power Sources 2020, 475. [Google Scholar] [CrossRef]
- Cheng, H.; Duong, H.M.; Jewell, D. Three Dimensional Manganese Oxide on Carbon Nanotube Hydrogels for Asymmetric Supercapacitors. RSC Adv. 2016, 6, 36954–36960. [Google Scholar] [CrossRef]
- Hwang, S.-G.; Hong, J.-E.; Kim, G.-O.; Jeong, H.M.; Ryu, K.-S. Graphene Anchored with Nio-MnO2 Nanocomposites for Use as an Electrode Material in Supercapacitors. ECS Sol. State Lett. 2012, 2, M8. [Google Scholar] [CrossRef]
- Seredych, M.; Bandosz, T.J. Evaluation of Go/MnO2 Composites as Supercapacitors in Neutral Electrolytes: Role of Graphite Oxide Oxidation Level. J. Mater. Chem. 2012, 22. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, Y.; Song, C.; Qiu, H.; Xue, H. Study on Preparation and Performance of Flexible All-Solid-State Supercapacitor Based on Nitrogen-Doped Rgo/Cnt/MnO2 Composite Fibers. J. Alloys Comp. 2020, 859, 157816. [Google Scholar] [CrossRef]
- Zhao, Z.; Shen, T.; Liu, Z.; Zhong, Q.; Qin, Y. Facile Fabrication of Binder-Free Reduced Graphene Oxide/MnO2/Ni Foam Hybrid Electrode for High-Performance Supercapacitors. J. Alloys Comp. 2020, 812. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, Y.; Li, F.; Zhang, L.; Wen, Z.; Liu, Q. Facile Synthesis of Hierarchical Co3o4@MnO2 Core–Shell Arrays on Ni Foam for Asymmetric Supercapacitors. J. Power Sources 2014, 252, 98–106. [Google Scholar] [CrossRef]
- Qiu, Z.; He, D.; Wang, Y.; Zhao, X.; Zhao, W.; Wu, H. High Performance Asymmetric Supercapacitors with Ultrahigh Energy Density Based on Hierarchical Carbon Nanotubes@Nio Core–Shell Nanosheets and Defect-Introduced Graphene Sheets with Hole Structure. RSC Adv. 2017, 7, 7843–7856. [Google Scholar] [CrossRef]
- Kate, R.S.; Khalate, S.A.; Deokate, R.J. Overview of Nanostructured Metal Oxides and Pure Nickel Oxide (Nio) Electrodes for Supercapacitors: A Review. J. Alloys Comp. 2018, 734, 89–111. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Y.-C.; Pan, L.; Shen, G.-Q.; Mahmood, N.; Ma, Y.-H.; Shi, Y.; Jia, W.; Wang, L.; Zhang, X.; et al. Engineering Cobalt Defects in Cobalt Oxide for Highly Efficient Electrocatalytic Oxygen Evolution. ACS Catal. 2018, 8, 3803–3811. [Google Scholar] [CrossRef]
- Aslam, S.; Sagar, R.U.R.; Kumar, H.; Zhang, G.; Nosheen, F.; Namvari, M.; Mahmood, N.; Zhang, M.; Qiu, Y. Mixed-Dimensional Heterostructures of Hydrophobic/Hydrophilic Graphene Foam for Tunable Hydrogen Evolution Reaction. Chemosphere 2020, 245, 125607. [Google Scholar] [CrossRef]
- Tahir, M.; Cao, C.; Mahmood, N.; Butt, F.K.; Mahmood, A.; Idrees, F.; Hussain, S.; Tanveer, M.; Ali, Z.; Aslam, I. Multifunctional G-C(3)N(4) Nanofibers: A Template-Free Fabrication and Enhanced Optical, Electrochemical, and Photocatalyst Properties. ACS Appl. Mater. Interfaces 2014, 6, 1258–1265. [Google Scholar] [CrossRef]
- Babar, N.U.A.; Joya, K.S.; Ehsan, M.A.; Khan, M.; Sharif, M. Noble-Metal-Free Colloidal-Copper Based Low Overpotential Water Oxidation Electrocatalyst. ChemCatChem 2019, 11, 6022–6030. [Google Scholar] [CrossRef]
- Narwade, S.S.; Mali, S.M.; Digraskar, R.V.; Sapner, V.S.; Sathe, B.R. Ni/Nio@Rgo as an Efficient Bifunctional Electrocatalyst for Enhanced Overall Water Splitting Reactions. Int. J. Hydrogen Energy 2019, 44, 27001–27009. [Google Scholar] [CrossRef]
- Usman, M.; Adnan, M.; Ali, S.; Javed, S.; Akram, M.A. Preparation and Characterization of Pani@Nio Visible Light Photocatalyst for Wastewater Treatment. Chem. Sel. 2020, 5, 12618–12623. [Google Scholar] [CrossRef]
- Zhang, S.; Pan, N. Supercapacitors Performance Evaluation. Adv. Energy Mater. 2015, 5. [Google Scholar]
- Abid, A.G.; Manzoor, S.; Usman, M.; Munawar, T.; Nisa, M.U.; Iqbal, F.; Ashiq, M.N.; Najam-ul-Haq, M.; Shah, A.; Imran, M. Scalable Synthesis of Sm2o3/Fe2o3 Hierarchical Oxygen Vacancy-Based Gyroid-Inspired Morphology: With Enhanced Electrocatalytic Activity for Oxygen Evolution Performance. Energy Fuels 2021, 35, 17820–17832. [Google Scholar] [CrossRef]
- Sadaqat, M.; Manzoor, S.; Aman, S.; Gouadria, S.; Usman, M.; Joya, K.S.; Najam-Ul-Haq, M.; Hassan, H.M.A.; Ashiq, M.N.; Taha, T. Mn-Based Hierarchical Polyhedral 2d/3d Nanostructures Mnx2 (X= S, Se, Te) Derived from Mn-Based Metal–Organic Frameworks as High-Performance Electrocatalysts for the Oxygen Evolution Reaction. Energy Fuels 2022, 36, 10327–10338. [Google Scholar] [CrossRef]
- Wang, H.; Fu, Q.; Pan, C. Green Mass Synthesis of Graphene Oxide and Its MnO2 Composite for High Performance Supercapacitor. Electrochim. Acta 2019, 312, 11–21. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, S.-Y.; Sinh, L.H.; Yeo, C.S.; Son, Y.R.; Cho, K.R.; Song, Y.; Ju, S.; Shin, M.K.; Park, S.-J.; et al. Maximizing Volumetric Energy Density of All-Graphene-Oxide-Supercapacitors and Their Potential Applications for Energy Harvest. J. Power Sources 2017, 346, 113–119. [Google Scholar] [CrossRef]
- Xiong, S.; Zhang, X.; Chu, J.; Wang, X.; Zhang, R.; Gong, M.; Wu, B. Hydrothermal Synthesis of Porous Sugarcane Bagasse Carbon/MnO2 Nanocomposite for Supercapacitor Application. J. Elect. Mater. 2018, 47, 6575–6582. [Google Scholar] [CrossRef]
- Han, X.; Cheng, F.; Chen, C.; Hu, Y.; Chen, J. Uniform MnO2 Nanostructures Supported on Hierarchically Porous Carbon as Efficient Electrocatalysts for Rechargeable Li-O2 Batteries. Nano Res. 2014, 8, 156–164. [Google Scholar] [CrossRef]
- Freitas, R.M.; Perilli, T.A.G.; Ladeira, A.C.Q. Oxidative Precipitation of Manganese from Acid Mine Drainage by Potassium Permanganate. J. Chem. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Liu, C.; Li, C.; Ahmed, K.; Mutlu, Z.; Ozkan, C.S.; Ozkan, M. Template Free and Binderless Nio Nanowire Foam for Li-Ion Battery Anodes with Long Cycle Life and Ultrahigh Rate Capability. Sci. Rep. 2016, 6, 29183. [Google Scholar] [CrossRef]
- Mironova-Ulmane, N.; Kuzmin, A.; Sildos, I.; Puust, L.; Grabis, J. Magnon and Phonon Excitations in Nanosized Nio. Latv. J. Phys. Tech. Sci 2019, 56, 61–72. [Google Scholar] [CrossRef]
- Thema, F.T.; Manikandan, E.; Gurib-Fakim, A.; Maaza, M. Single Phase Bunsenite Nio Nanoparticles Green Synthesis by Agathosma Betulina Natural Extract. J. Alloys Comp. 2016, 657, 655–661. [Google Scholar] [CrossRef]
- Tian, Y.; Gong, L.; Qi, X.; Yang, Y.; Zhao, X. Effect of Substrate Temperature on the Optical and Electrical Properties of Nitrogen-Doped Nio Thin Films. Coatings 2019, 9, 634. [Google Scholar] [CrossRef]
- Ramadan, A.; Anas, M.; Ebrahim, S.; Soliman, M.; Abou-Aly, A. Effect of Co-Doped Graphene Quantum Dots to Polyaniline Ratio on Performance of Supercapacitor. J. Mater. Sci. Mater. Electr. 2020, 31, 7247–7259. [Google Scholar] [CrossRef]
- Ramadan, A.; Anas, M.; Ebrahim, S.; Soliman, M.; Abou-Aly, A.I. Polyaniline/Fullerene Derivative Nanocomposite for Highly Efficient Supercapacitor Electrode. Int. J. Hydrogen Energy 2020, 45, 16254–16265. [Google Scholar] [CrossRef]
- Usman, M.; Ahsan, M.T.; Javed, S.; Ali, Z.; Zhan, Y.; Ahmed, I.; Butt, S.; Islam, M.; Mahmood, A.; Akram, M.A. Facile Synthesis of Iron Nickel Cobalt Ternary Oxide (Fnco) Mesoporous Nanowires as Electrode Material for Supercapacitor Application. J. Mater. 2022, 8, 221–228. [Google Scholar] [CrossRef]
- Zhang, K.; Park, M.; Zhou, L.; Lee, G.-H.; Li, W.; Kang, Y.-M.; Chen, J. Urchin-Like Cose2 as a High-Performance Anode Material for Sodium-Ion Batteries. Adv. Funct. Mater. 2016, 26, 6278–6735. [Google Scholar] [CrossRef]
- Ali, Z.; Tang, T.; Huang, X.; Wang, Y.; Asif, M.; Hou, Y. Cobalt Selenide Decorated Carbon Spheres for Excellent Cycling Performance of Sodium Ion Batteries. Energy Storage Mater. 2018, 13, 19–28. [Google Scholar] [CrossRef]
- Lai, Y.-H.; Gupta, S.; Hsiao, C.-H.; Lee, C.-Y.; Tai, N.-H. Multilayered Nickel Oxide/Carbon Nanotube Composite Paper Electrodes for Asymmetric Supercapacitors. Electrochim. Acta 2020, 354. [Google Scholar] [CrossRef]
- Fan, L.-Q.; Liu, G.-J.; Wu, J.-H.; Liu, L.; Lin, J.-M.; Wei, Y.-L. Asymmetric Supercapacitor Based on Graphene Oxide/Polypyrrole Composite and Activated Carbon Electrodes. Electrochim. Acta 2014, 137, 26–33. [Google Scholar] [CrossRef]
- Liu, Y.; He, D.; Wu, H.; Duan, J.; Zhang, Y. Hydrothermal Self-Assembly of Manganese Dioxide/Manganese Carbonate/Reduced Graphene Oxide Aerogel for Asymmetric Supercapacitors. Electrochim. Acta 2015, 164, 154–162. [Google Scholar] [CrossRef]
- Wang, G.; Xu, H.; Lu, L.; Zhao, H. One-Step Synthesis of Mesoporous MnO2/Carbon Sphere Composites for Asymmetric Electrochemical Capacitors. J. Mater. Chem. A 2015, 3, 1127–1132. [Google Scholar] [CrossRef]
- Chai, Y.; Li, Z.; Wang, J.; Mo, Z.; Yang, S. Construction of hierarchical holey graphene/MnO2 composites as potential electrode materials for supercapacitors. J. Alloys Compd. 2019, 775, 1206–1212. [Google Scholar] [CrossRef]
- Qiu, S.; Li, R.; Huang, Z.; Huang, Z.; Tsui, C.P.; He, C.; Han, X.; Yang, Y. Scalable sonochemical synthesis of petal-like MnO2/graphene hierarchical composites for high-performance supercapacitors. Compos. Part B Eng. 2019, 161, 37–43. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Li, M.; Yang, C.; Zhang, L.; Wang, C.; Lu, H. Strong interface coupling and few-crystalline MnO2/Reduced graphene oxide composites for supercapacitors with high cycle stability. Electrochim. Acta 2018, 292, 115–124. [Google Scholar] [CrossRef]
- Hao, H.; Wang, J.; Lv, Q.; Jiao, Y.; Li, J.; Li, W.; Akpinar, I.; Shen, W.; He, G. Interfacial engineering of reduced graphene oxide for high-performance supercapacitor materials. J. Electroanal. Chem. 2020, 878, 114679. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arshad, N.; Usman, M.; Adnan, M.; Ahsan, M.T.; Rehman, M.R.; Javed, S.; Ali, Z.; Akram, M.A.; Demopoulos, G.P.; Mahmood, A. Nanoengineering of NiO/MnO2/GO Ternary Composite for Use in High-Energy Storage Asymmetric Supercapacitor and Oxygen Evolution Reaction (OER). Nanomaterials 2023, 13, 99. https://doi.org/10.3390/nano13010099
Arshad N, Usman M, Adnan M, Ahsan MT, Rehman MR, Javed S, Ali Z, Akram MA, Demopoulos GP, Mahmood A. Nanoengineering of NiO/MnO2/GO Ternary Composite for Use in High-Energy Storage Asymmetric Supercapacitor and Oxygen Evolution Reaction (OER). Nanomaterials. 2023; 13(1):99. https://doi.org/10.3390/nano13010099
Chicago/Turabian StyleArshad, Natasha, Muhammad Usman, Muhammad Adnan, Muhammad Tayyab Ahsan, Mah Rukh Rehman, Sofia Javed, Zeeshan Ali, Muhammad Aftab Akram, George P. Demopoulos, and Asif Mahmood. 2023. "Nanoengineering of NiO/MnO2/GO Ternary Composite for Use in High-Energy Storage Asymmetric Supercapacitor and Oxygen Evolution Reaction (OER)" Nanomaterials 13, no. 1: 99. https://doi.org/10.3390/nano13010099
APA StyleArshad, N., Usman, M., Adnan, M., Ahsan, M. T., Rehman, M. R., Javed, S., Ali, Z., Akram, M. A., Demopoulos, G. P., & Mahmood, A. (2023). Nanoengineering of NiO/MnO2/GO Ternary Composite for Use in High-Energy Storage Asymmetric Supercapacitor and Oxygen Evolution Reaction (OER). Nanomaterials, 13(1), 99. https://doi.org/10.3390/nano13010099





