Abstract
Due to the significant rise in atmospheric carbon dioxide (CO2) concentration and its detrimental environmental effects, the electrochemical CO2 conversion to valuable liquid products has received great interest. In this work, the copper-melamine complex was used to synthesize copper-based electrocatalysts comprising copper nanoparticles decorating thin layers of nitrogen-doped carbon nanosheets (Cu/NC). The as-prepared electrocatalysts were characterized by XRD, SEM, EDX, and TEM and investigated in the electrochemical CO2 reduction reaction (ECO2RR) to useful liquid products. The electrochemical CO2 reduction reaction was carried out in two compartments of an electrochemical H-Cell, using 0.5 M potassium bicarbonate (KHCO3) as an electrolyte; nuclear magnetic resonance (1H NMR) was used to analyze and quantify the liquid products. The electrode prepared at 700 °C (Cu/NC-700) exhibited the best dispersion for the copper nanoparticles on the carbon nanosheets (compared to Cu/NC-600 & Cu/NC-800), highest current density, highest electrochemical surface area, highest electrical conductivity, and excellent stability and faradic efficiency (FE) towards overall liquid products of 56.9% for formate and acetate at the potential of −0.8V vs. Reversible Hydrogen Electrode (RHE).
1. Introduction
Recently, intensive fossil usage (such as coal, petroleum, and natural gas) is considered globally as the major energy source and has led to a dramatic increase in CO2 emission [1,2]. The present CO2 level is greater than 414 ppm [3]. Therefore, great efforts for the capture, sequestration, and utilization of CO2 should be devoted. Several techniques including biochemical, thermal, and electrochemical methods, have been extensively studied for their potential to convert CO2 into valuable chemicals [4,5]. The electrochemical CO2 reduction (ECO2RR) draws substantial attention due to its several advantages. In ECO2RR, the conversion process is controlled by the applied potential in the process [6]. The process also operates with electricity at ambient conditions, resulting in zero carbon emission. However, the ECO2RR required relatively high energy due to the stability of the CO2 molecule in an aqueous electrolyte. In order to lower the energy barrier and improve the performance and selectivity, an effective and long-lasting electrocatalyst is needed [7,8].
In the previous few years, several transition metals catalysts have been investigated, such as (Cu, Co, Zn, Sn, Ni, Bi, etc.) [9,10,11,12,13,14], bi-metallic (Cu-Zn, Cu-Ag, Cu-Sn, etc.) [15,16,17,18], oxides (CuOx, CuO-ZnO, etc.) [19,20], metal-organic frameworks [21,22,23,24], and zeolites [25,26]. Carbon-based electrocatalysts showed additional benefits beyond those already described, including low cost and availability, high electrical conductivity, and a large surface area that allows for the even distribution of active sites and the efficient adsorption of reactants [27,28,29]. Additionally, when nitrogen is doped in the carbon, the electrical conductivity is improved, and CO2 molecules are drawn to the catalyst’s surface more readily [30,31,32,33,34,35]. Han and co-authors claimed in their reports the synergy between the active sites of the Cu NPs and the N terminals in the supports facilitate the coupling of the CO (produced in the N-sites) and secondary (C), which lead to the formation of higher carbon alcohols products [36]. Recently, Bhunia et al. [31] reported the production of several liquid products with FE of 54 % at a potential of 1.0 VRHE using Cu NPs supported on N-doped graphene. The selective ethanol production was also reported by Wang et al. [30], using N-doped carbon nanospikes decorated by the Cu NPs; this catalyst exhibited FE of 63% at a potential of 1.2 VRHE. Zhou et al. synthesized Cu@Cu2O coated with N-doped carbon derived from Cu-BTC MOF. The reported electrocatalysts showed 45% FE toward methanol production at −0.7 V potential [37].
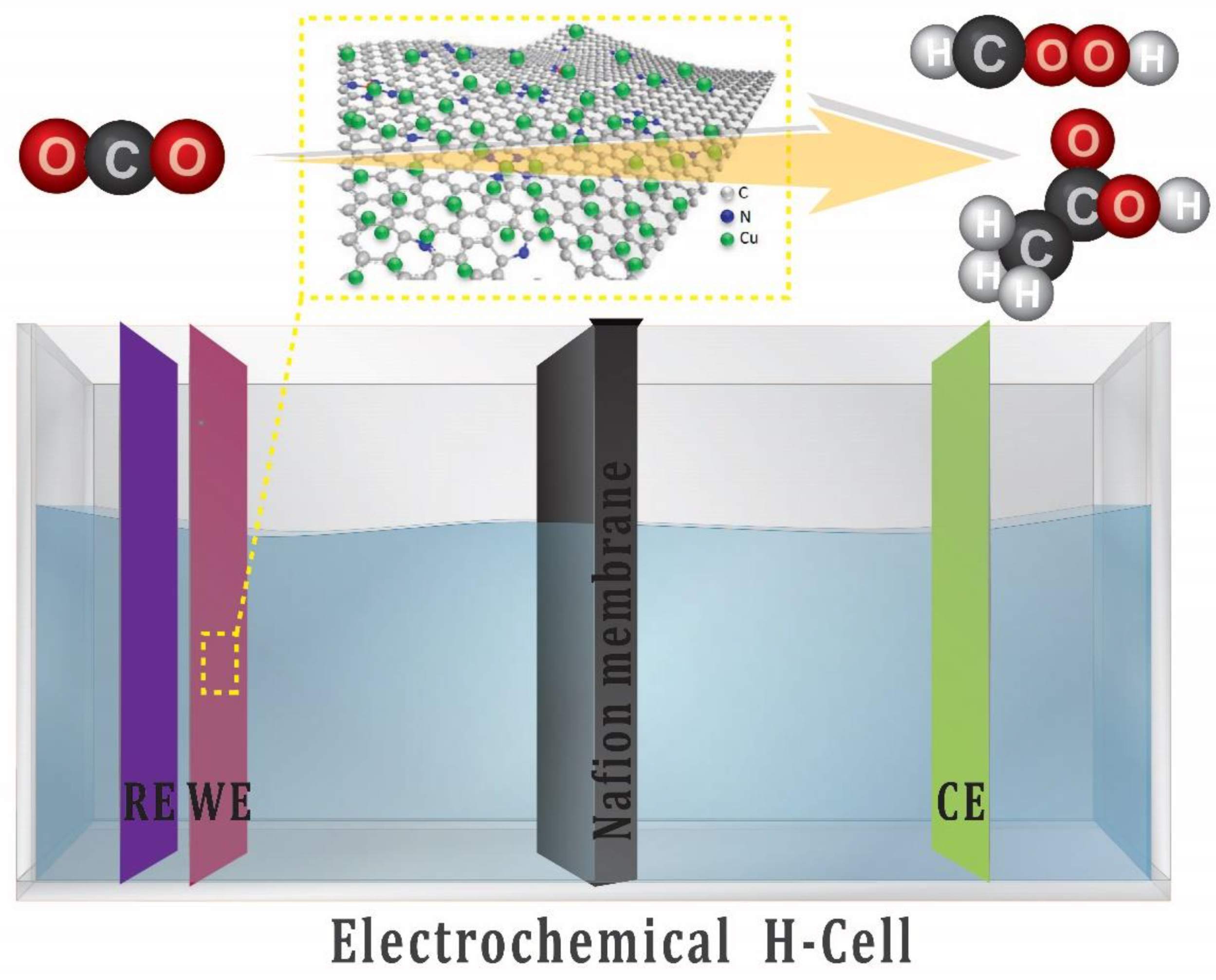
This work involved the fabrication of copper nanoparticle-decorated nitrogen-doped carbon nanosheets. The copper precursor for this electrocatalyst was complexed with a cheap organic linker (melamine), and then the resulting complex was pyrolyzed at various temperatures to produce the final electrocatalyst. After pyrolysis, small and evenly scattered Cu-NPs are formed due to the complexation of copper with melamine, which aids in the homogenous dispersion of copper atoms. Yuan and co-workers [38] reported the use of melamine crosslinked with 1-hydroxyethylidene-1,1-diphosphonic acid and some transition metal to form core-shell transition metal phosphides in N-doped carbon for water electrolysis and zinc air battery applications. However, in this study, the condition is optimized and melamine is directly crosslinked with the metal (Cu). The as-prepared Cu NPs/NC is used for the ECO2RR in H-Cell for the production of liquid products, as shown in Figure 1.
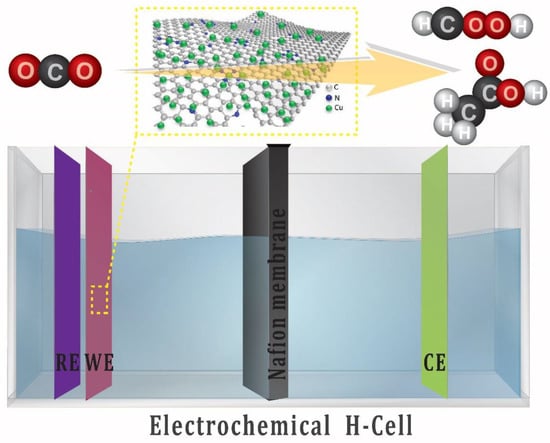
Figure 1.
Schematic presentation of the three-electrode setup (H-Cell) for electrochemical CO2 reduction.
2. Experimental
2.1. Materials
Copper chloride dihydrate (CuCl2.2H2O) (99.95%) and melamine (99.0%) were purchased from Sigma Aldrich, US. Methanol (CH3OH) (99.8%) and diethyl ether ((CH3CH2)2O) (99.9%) were procured from Sharlu (Sharjah, United Arab Emirates). Nitrogen gas (N2) was supplied by Abdullah Hashem Industrial Gas Co., Ltd., Dammam, Saudi Arabia.
2.2. Preparation of Copper Melamine Complex
A total of 170 mg copper chloride dihydrate was dissolved in 20 mL of N2-purged methanol, then melamine (250 mg) was added to the solution. The mixture was heated to 100 °C for 14 h. After that, the solution was kept to cool at room temperature. The green powder was collected, washed three times with diethyl ether, and dried under vacuum at 50 °C.
2.3. Preparation of Copper Nanoparticles Decorated on Thin Carbon Nanosheets
The as-prepared copper melamine complex was placed in crucible and heated under N2 atmosphere at different temperatures (600, 700 and 800 °C) with 5 °C/min heating rate for 2 h to obtain Cu-NP/NC.
2.4. Preparation of Electrocatalyst
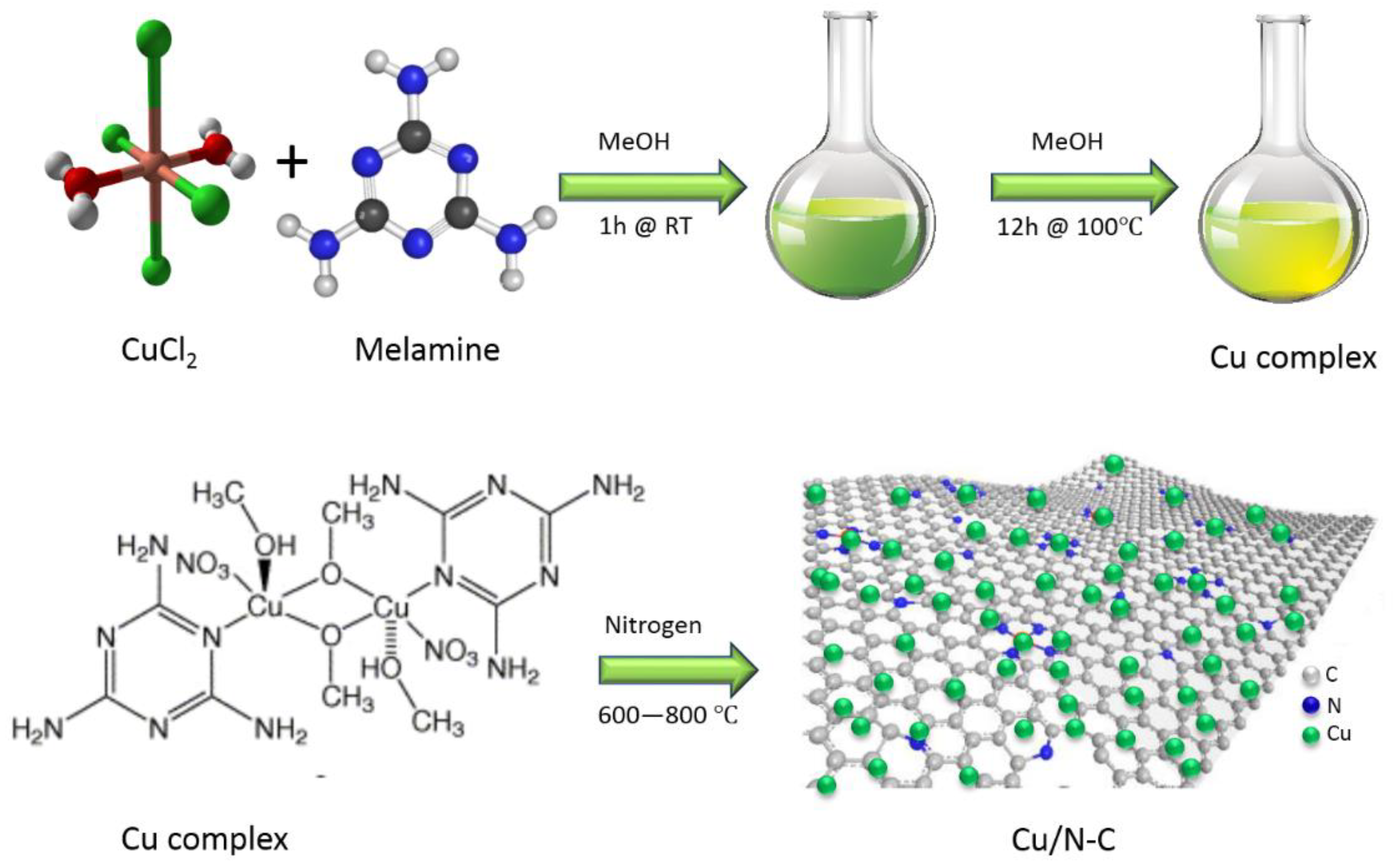
10 mg of the Cu-NP/NC catalyst was dispersed in 1 mL mixture of 750 µL isopropanol, 200 µL DI water and 50 µL Nafion (5%). The mixture was sonicated for 20 min. Then 100 µL of the suspension was drop casted onto 1 cm2 conductive carbon paper and dried at room temperature. This preparation method is schematically presented in Figure 2.
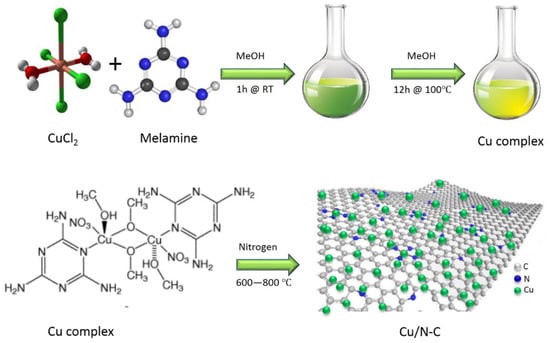
Figure 2.
The synthesis schematic of copper-decorated nitrogen-doped carbon nanosheets.
2.5. Characterization
Morphological and detailed microstructural attributes of the materials were discerned by transmission and high-resolution transmission electron microscopy techniques (TEM/HR-TEM, Tecnai TF20) and field emission scanning electron microscopy (FESEM, Tescan Lyra-3). Other techniques employed for the characterization of the samples were X-ray diffraction (XRD, Rigaku MiniFlex) and 1H NMR spectroscopy (LAMBDA 500 spectrophotometer). Potentiostat (Gammray 620) was used for electrochemical analysis.
2.6. The Electrochemical Studies
The ECO2RR performance is investigated with an H-cell system consisting of a sliver silver chloride electrode (Ag/AgCl) as a reference electrode. A platinum mesh was used as a counter electrode. The as-prepared Cu-NP/NC film on conductive carbon paper was used as working electrode. A potentiostat (Gammray 620) is connected to the electrodes in the H-Cell. The ECO2RR performance was evaluated by carrying out linear sweep voltammetry (LSV) techniques and calculated the overpotential at different current densities (current normalized to the geometric surface area of the electrode). The cyclic voltammetry (CV) and LSV experiments were performed in 0.5 M potassium bicarbonate (KHCO3). All the electrochemical measurements were normalized to the RHE by using the following formula:
where [17].
The potential was swept from 0.0 to −1.4 V vs. RHE. The electrochemical impedance spectroscopy (EIS) was performed by varying the frequency from 105 to 0.1 Hz under identical electrolyte and electrodes to the LSV.
The reduction products were evaluated by running the potentiostatic measurements at different potentials (−0.5 to −1.2) for 2 h, the liquid products were collected from the cell and quantified with 1H NMR.
3. Results and Discussion
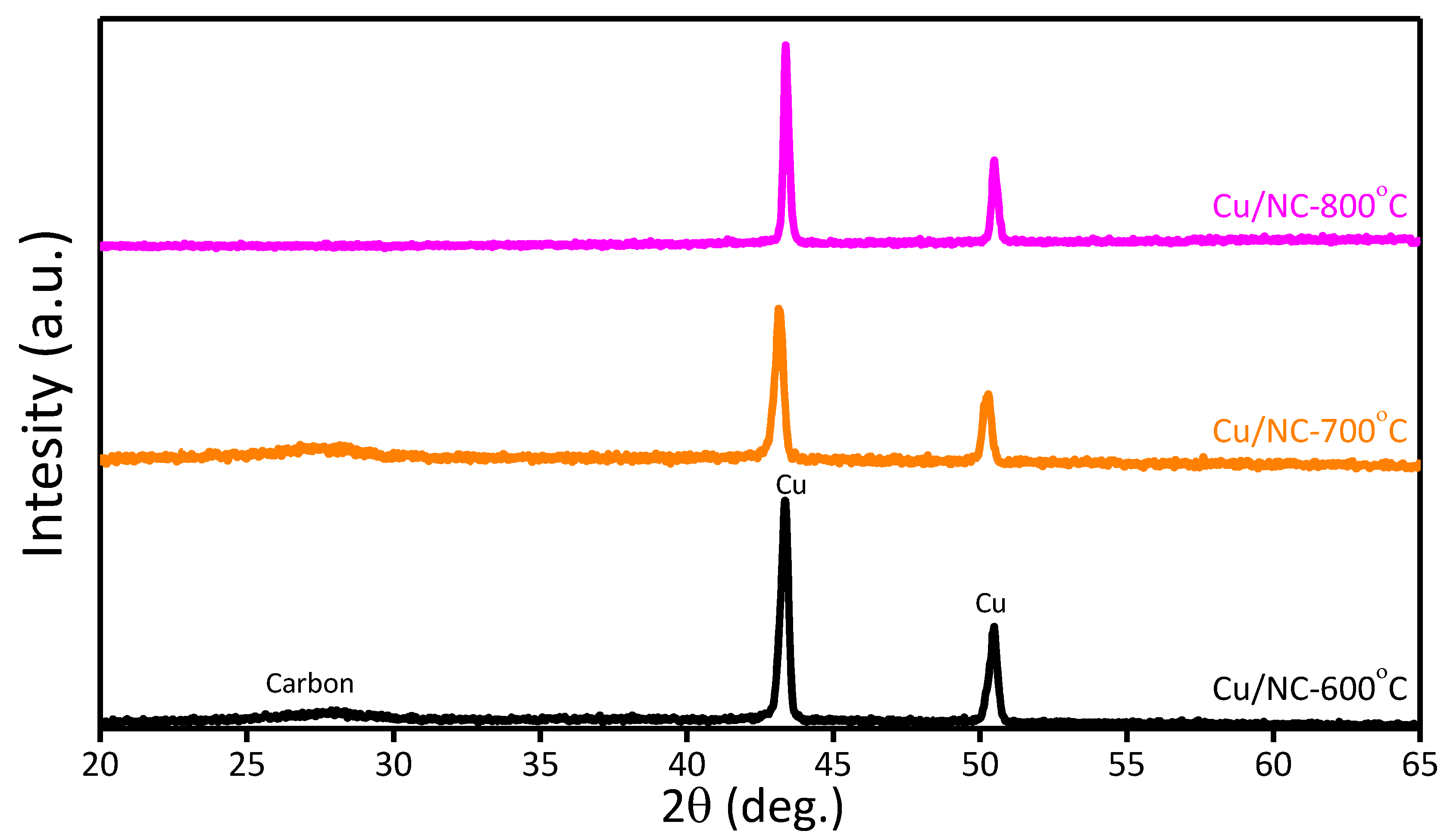
The phase structure of Cu-NP/NC was investigated with powder XRD as shown in Figure 3. For the three catalysts Cu-NP/NC-600, Cu-NP/NC-700, and Cu-NP/NC-800, reflections at 43.4° and 50.3° were recorded ascribed for the planes (111) and (200), respectively (JCPDS number 01-085-1326) [39]. Additionally, the reflection peak at 26.2° (002) corresponded to NC (JCPDS# 03-065-6212) [40], which indicates the successful formation of metallic copper of nitrogen-doped carbon.
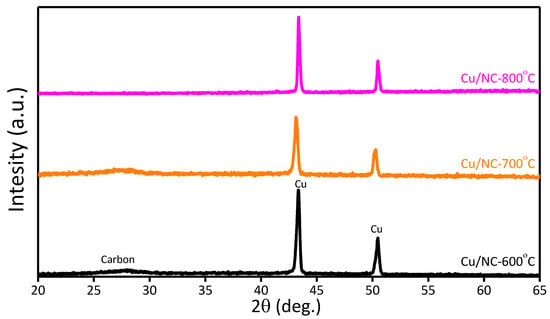
Figure 3.
XRD of Cu-NP/NC.
Further information about the composites’ chemical composition was explored using the EDS (Figure S1), which confirms the existence of the elements (Cu, C and N). Therefore, from the XRD and the EDS, the formation of metallic copper on nitrogen-doped carbon was confirmed.
The microstructure and morphology of the Cu-NP/NC were inspected with the SEM and the TEM. Figure 4a shows the SEM image of Cu-NP/NC-600, which reveals sheet-like morphology and the showed a thin sheet in the case of Cu-NP/NC-700 (Figure 4b). However, upon increasing the temperature to 800 °C the copper particles start to grow and agglomerate, as it is observed in Figure 4c for the catalyst Cu-NP/NC-800. The TEM (Figure 4d) confirms the formation of small and uniform dispersed copper nanoparticles (<20 nm) onto the thin sheet of carbon. The copper nanoparticles were smaller than 10 nm in size (Figure 4e), and in Figure 4f the high-resolution TEM (HRTEM) was carried out for the highlighted particles and the interplanar distance was estimated to be 0.2 nm, corresponding to the phase (111) for the metallic copper.

Figure 4.
SEM of (a) Cu-NP/NC-600, (b) Cu-NP/NC-700, (c) Cu-NP/NC-700, (d,e) TEM of Cu-NP/NC-700 and (f) HRTEM of Cu-NP/NC-700.
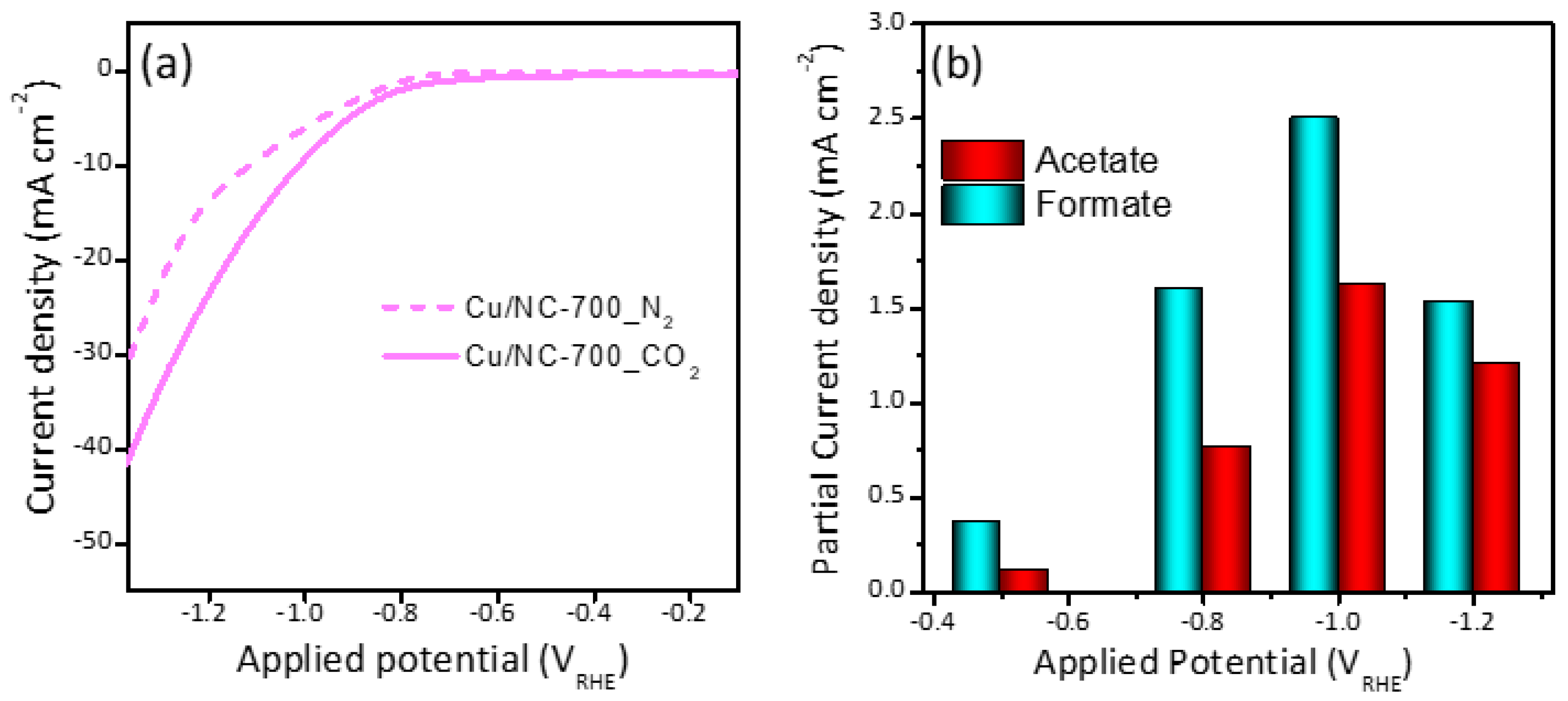
The LSV was recorded for the three electrocatalysts in CO2 saturated 0.5 M KHCO3 and compared with N2 saturated in the same electrolyte as shown in Figure 5a. The polarization curves were demonstrated in Figure S2, which shows that the current density (CD) was increasing with increasing potential. It can be noted that there is significant enhancement upon the saturation of the electrolyte with CO2 (solid lines) compared to N2 (dashed lines). The observed CD at a potential of 1.0 VRHE was −10, −6.2, and −3.7 mA cm−2 for the electrocatalysts Cu-NP/NC-700, Cu-NP/NC-600 and Cu-NP/NC-800, respectively. This activity order could be explained as follows: for the sample prepared at 600 °C less graphitic carbon and nitrogen were formed compared to the catalyst Cu-NP/NC-700, which significantly influences the electrode’s conductivity. However, the electrode Cu-NP/NC-800 preparation of the sample at a higher temperature led to higher degree of agglomeration as observed in the SEM (Figure 2c), which led to a drop in the surface area and accordingly decrease in the electrochemical performance. The partial current densities were shown in Figure 5b, which is the current required to generate formate and acetate. It can be observed that the partial current density is increasing with an increase the potential until −1.0 V. Moving to more cathodic potential (−1.2 V), the partial current densities of acetate and formate decreased.
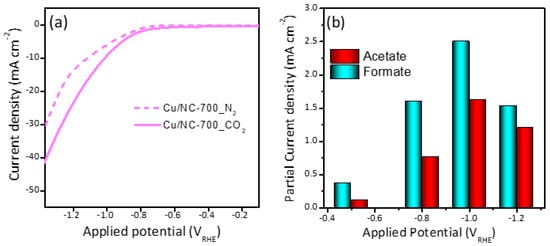
Figure 5.
(a) LSV curves of Cu-NP/NC-700 electrocatalysts in N2 and CO2 saturated 0.5 M KHCO3 electrolyte. (b) Partial current densities of Cu-NP/NC-700.
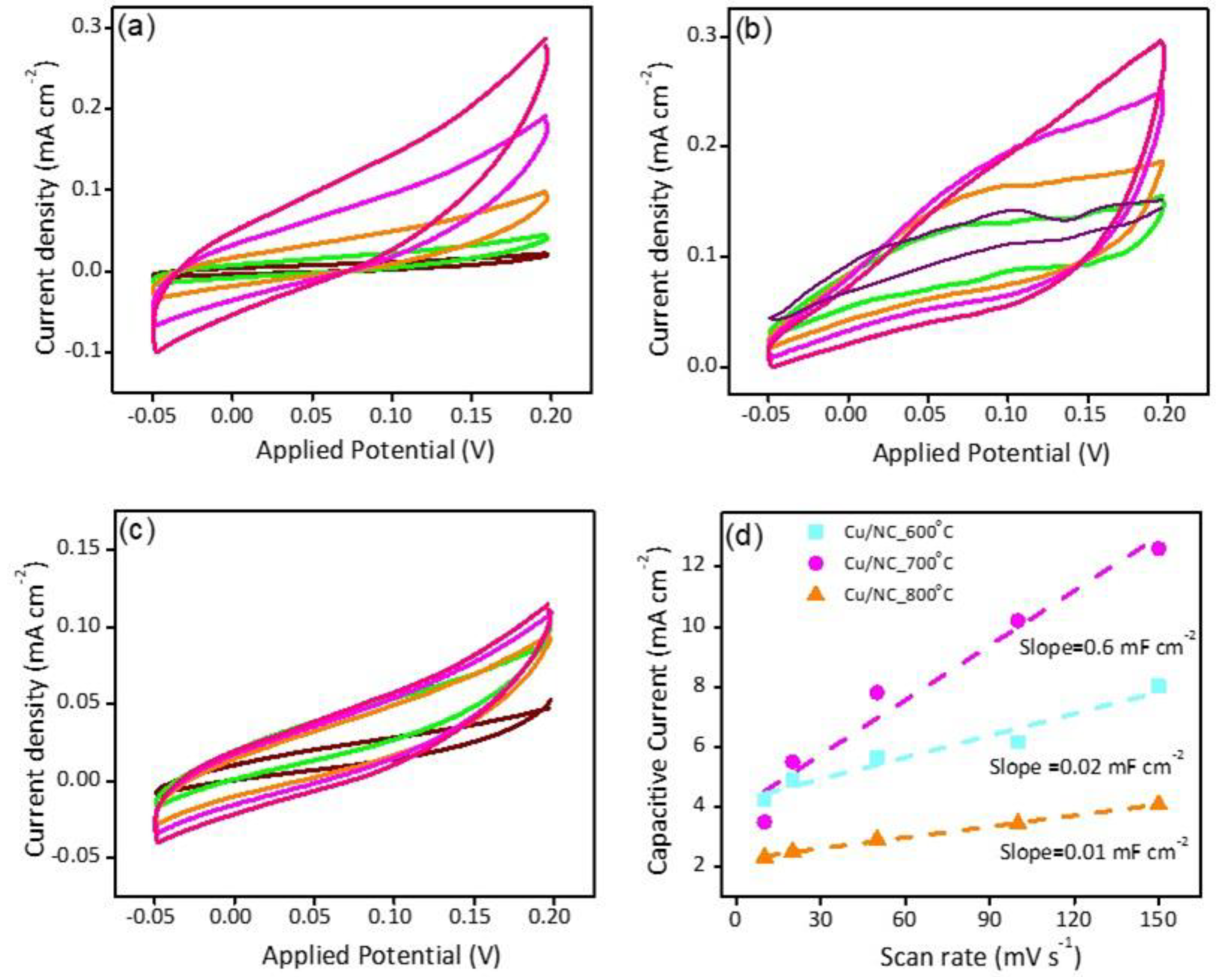
Electrochemical surface area (ECSA) was estimated by calculating the double layer capacitance (Cdl) [28,41]. Figure 6a–c shows the recorded CVs in the capacitive region for the electrodes Cu-NP/NC-600, Cu-NP/NC-700, and Cu-NP/NC-800, respectively. Figure 6d shows the respective slopes calculated from the previous figures which represent the Cdl. The electrode Cu-NP/NC-800 exhibited the lower Cdl (0.1 mF cm−2) due to the agglomeration of the Cu particles, followed by Cu-NP/NC-600 (0.2 mF cm−2) and finally, the electrocatalyst Cu-NP/NC-700, which possessed the highest ECSA
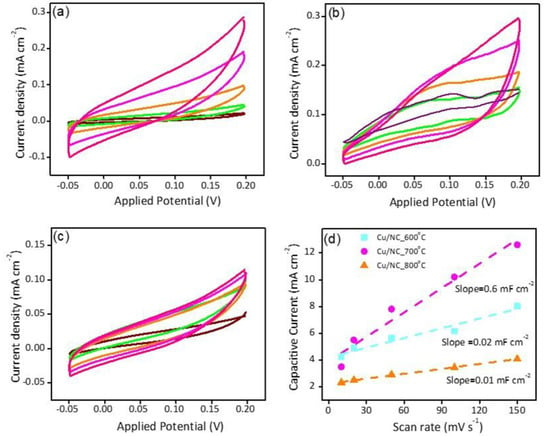
Figure 6.
CVs of (a) Cu-NP/NC-600, (b) Cu-NP/NC-700, (c) Cu-NP/NC-800 electrocatalysts in CO2 saturated 0.5 M KHCO3 electrolyte, and (d) is the respected Cdl slopes of the electrodes. The scan rate (a to c) purple: 50 mVs−1, Green: 100 mVs−1, Orange: 150 mVs−1, Magenta: 200 mVs−1, Pink: 250 mVs−1.
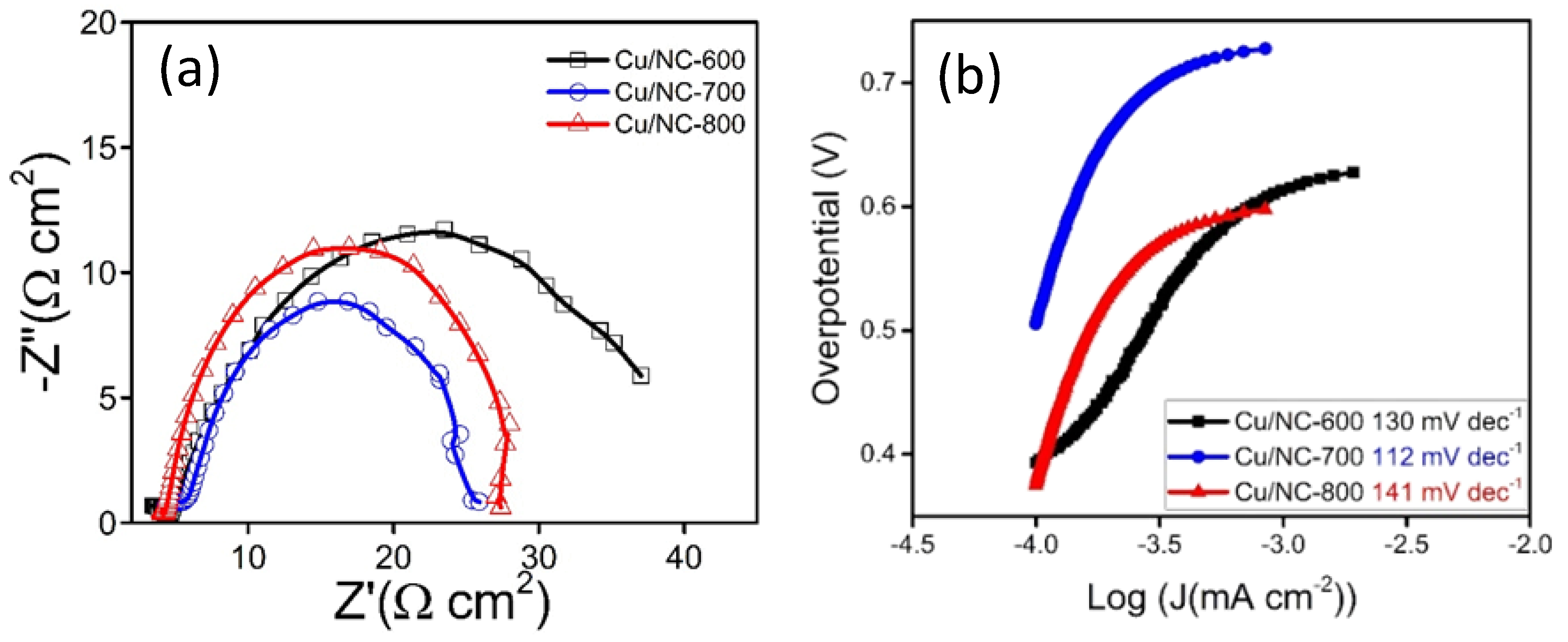
With the highest Cdl (0.6 mF cm−2). Moreover, the electrode conductivity is considered as a critical factor in the electrochemical performance; hence, the conductivity of the catalysts was investigated with the electrochemical impedance spectroscopy (EIS). EIS is a very important tool used to understand the electrode conductivity and the charge transfer resistance (Rct). The Nyquist plot is obtained from the EIS experiment; the smaller semicircle represents the higher conductivity. Figure 7a reveals the Nyquist plot for the three electrodes at applied potential of −1.0 vs. RHE. The Rct values were 31.5, 26.0, and 27.5 Ω cm2 for the electrodes Cu-NP/NC-600, Cu-NP/NC-700, and Cu-NP/NC-800, respectively. As expected, the sample prepared at 700 °C with less degree of agglomeration with graphitic carbon and nitrogen exhibited the highest conductivity (lower Rct).
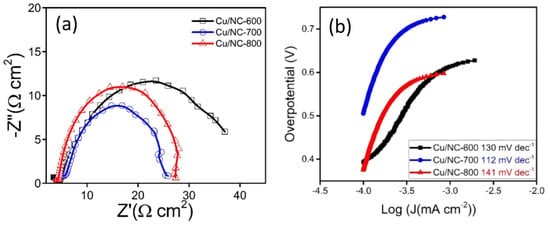
Figure 7.
(a) Nyquist plots of Cu-NP/NC electrodes in CO2 saturated 0.5 M KHCO3 electrolyte. (b) Tafel slopes for the Cu/NC electrodes.
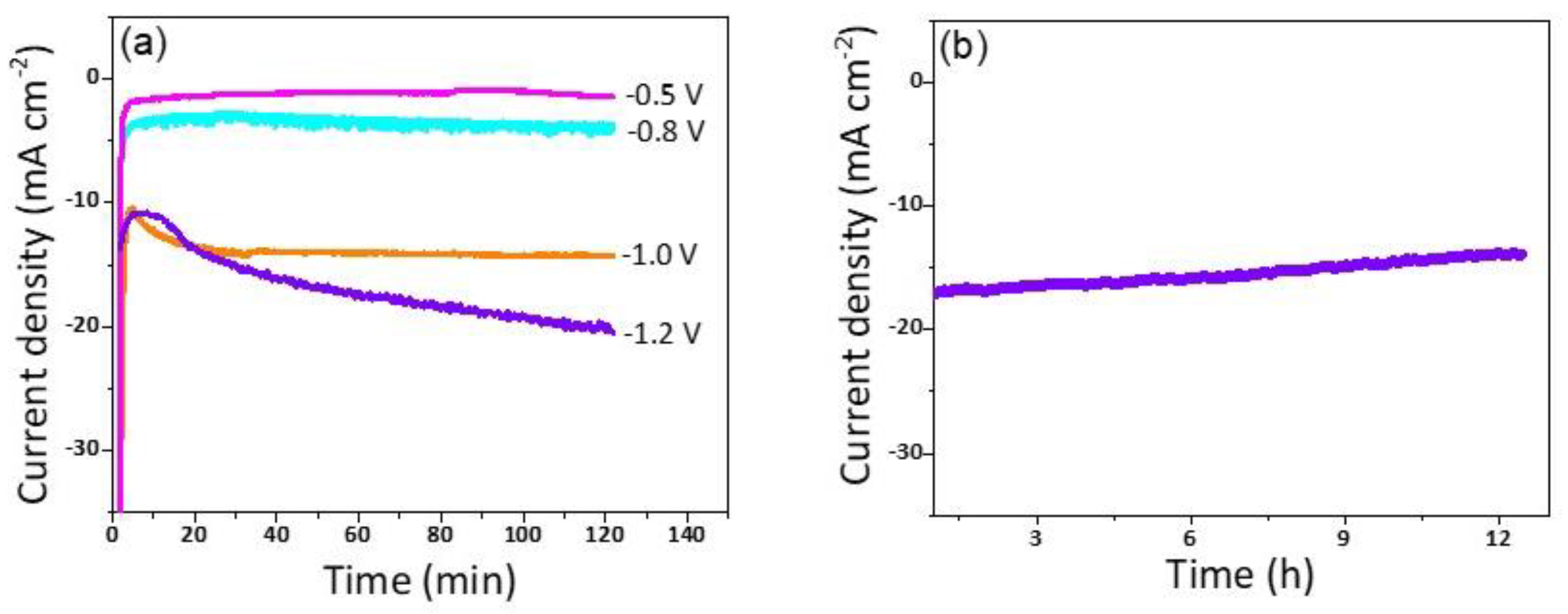
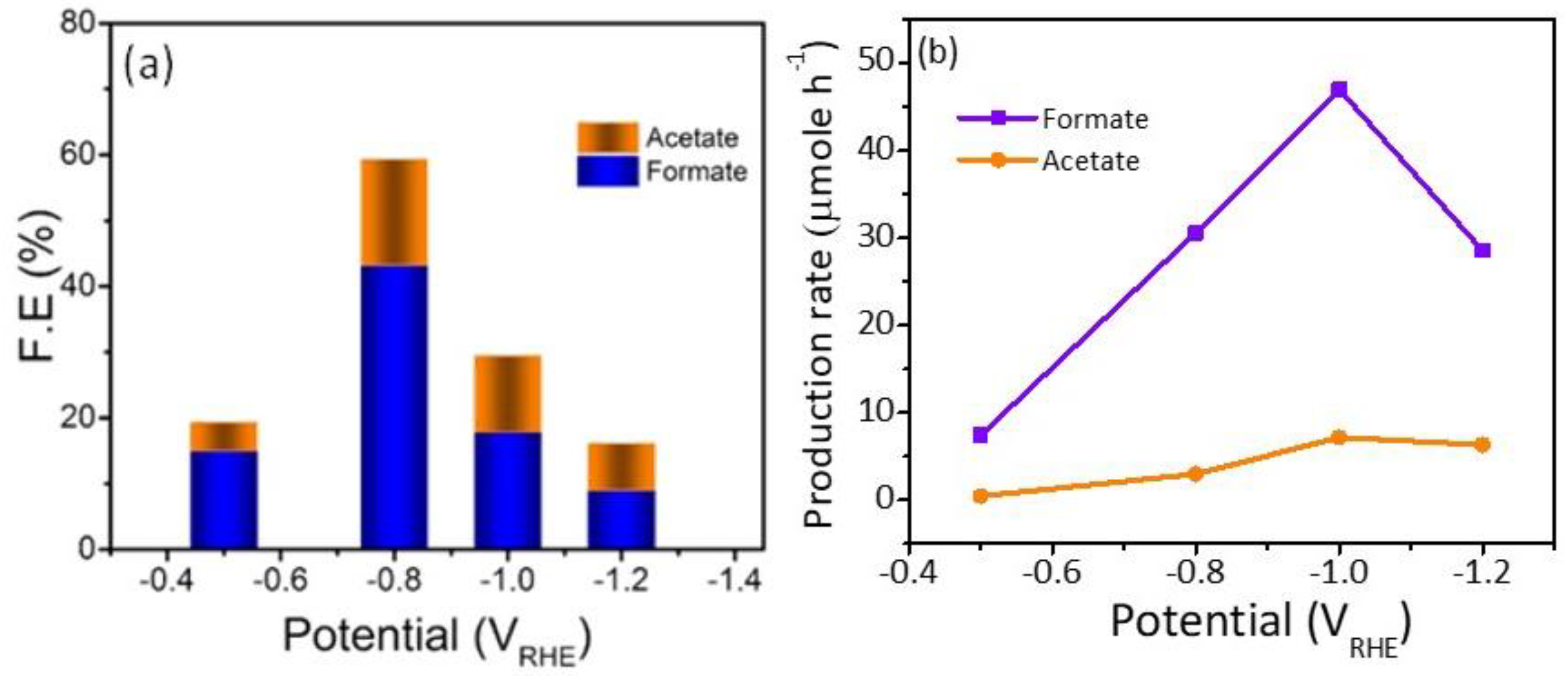
To obtain an idea about the kinetics and the mechanism of the reduction reaction Tafel slopes were investigated and compared for the three Cu/NC electrodes. Tafel slopes were estimated for the three electrodes using Tafel plots (Figure 7b). From the figure, the estimated values were 130, 112, and 141 mV dec−1 for the electrodes Cu/NC-600, Cu/NC-700, and Cu/NC-800, respectively. The Tafel equation suggests that the smaller slope value is translated into faster reaction kinetics [42]. The Cu/NC-700 exhibited the lowest Tafel value with an excellent agreement with values reported in the literature for Cu-based electrocatalysts. This small value suggests facilitated activation of the adsorbed CO2 on the surface of the catalyst (by the stabilization of the CO2 •—). Additionally, it has been reported that the N atom doped in the carbon is considered as an excellent active site for CO production due to its weak adsorption energy, which led to the desorption of CO [36]. Moreover, the stability of the Cu/NC-700 was investigated using chronoamperometry by applying constant potential for a period of time and recording the produced current density. As it is observed in Figure 8b, the electrode Cu/NC-700 exhibited excellent stability at −15 mA cm−2 for 12 h in CO2 saturated in 0.5 M KHCO3 with no significant drop in the current. Chronoamperometry was carried out at different potentials (−0.5, −0.8, −1.0 and −1.2 V) for 2 h (Figure 8a), then after the chrono, the solution was evaluated using 1H NMR (Figure S3). As in Figure 9, two conversion products were observed: formic and acetic acid. The highest FE of 59.1% was a conversion rate of 31.0 and 3.2 µmol h−1 for the formic acid and acetic acid, respectively. The higher cathodic current observed at higher potential is predominant by the hydrogen evolution reaction (HER), obtained from the water reduction [4].
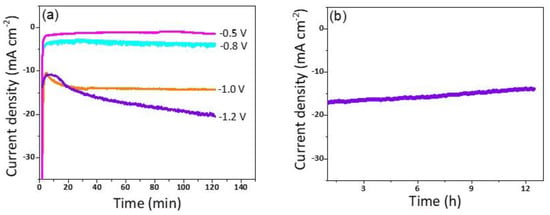
Figure 8.
(a) The chronoamperometry of Cu/NC-700 at potentials of −0.5, −0.8, −1.0, and −1.2 V vs RHE for 2 h. (b) The long-term stability of Cu/NC-700 at potentials of −1.0 V vs RHE for 12 h.
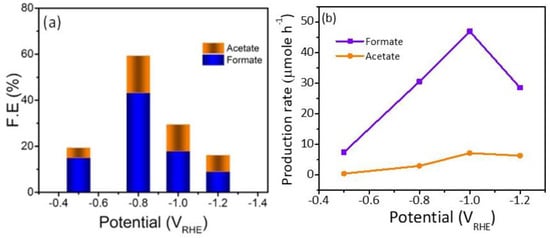
Figure 9.
(a) FEs. (b) Production rate of the liquid products by Cu/NC-700 at the function of potential.
The electrochemical performance and conversion efficiency were compared with recent similar reports in Table 1, which compares other Cu-based, carbon based, and Cu-carbon composites for the electroreduction of CO2 into useful liquid products. The Cu/NC-700 can produce formic acid with a FE of 40.9% at low potential of −0.8 V vs. RHE. Acetic acid can also be significantly detected with a FE of 16% is in the range of the reported literature.

Table 1.
Comparison of the catalytic performances of Cu/NC-700 and the similar electrocatalysts reported in literature for the reduction of CO2 to liquid products.
The proposed mechanism for CO2 reduction using this catalyst is as follows: firstly adsorption and reduction of CO2 (to the catalyst surface) to form CO2 radical (CO2•—). The formed radical is got protonated by the electrolyte to form (HCOO—)ads, which desorb to generate formate. In the case of acetate, prior to the protonation step of the first (CO2•—) radical, a second (CO2•—) radical is combined with first one to form (—OOC—COO—); similarly, this intermediate is protonated and forms acetate. Since the yield of formate is higher than acetate, this means the rate of (CO2•—) protonation is faster than the rate of (—OOC—COO—) formation [43,44].
4. Conclusions
In this work, N-doped carbon nanosheets supported copper nanoparticles (Cu/NC) were prepared via pyrolysis of copper melamine complex at different temperatures and were investigated for the electrochemical CO2 reduction reaction in 0.5 M KHCO3 solution. The Cu/NC-700 exhibited the highest current density and selectivity for the conversion of CO2 with faradic efficiencies of 43.2% for formic acid and 16.1% for acetic acid, with a conversion rate of 34.0 and 3.2 µmol h−1, respectively at a reduction potential of −0.8 V vs. RHE and a current density of −4.9 mA cm−2. Moreover, the optimized electrocatalyst shows long term stability without significant loss in current density for 12 h. The Cu/NC-700 electrode exhibited a higher ECSA than Cu/NC-600 and Cu/NC-800. The EIS measurements showed better electrical conductivity of the electrode Cu/NC-700 compared to the other two electrocatalysts.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/nano13010047/s1: Figure S1: EDS of Cu/NC-700; Figure S2: LSV curves of Cu-NP/NC electrocatalysts in N2 and CO2 saturated 0.5 M KHCO3 electrolyte.; Figure S3: (a) Comparative NMR spectra for the blank electrolyte sample with the internal standard & D2O and (b) the sample after the chrono for 2 h at −0.8 VRHE with the internal standard & D2O.
Author Contributions
Conceptualization, M.H.S. and M.U.; methodology, M.H.S.; software, M.U.; validation, M.H.S., M.U. and Z.H.Y.; formal analysis, M.H.S., M.U. and Z.H.Y.; writing—original draft preparation, M.H.S., M.U. and Z.H.Y.; writing—review and editing, M.H.S., M.U. and Z.H.Y.; visualization, M.H.S., M.U. and Z.H.Y.; supervision, M.U.; project administration, Z.H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deanship of Research Oversight and Coordination (DROC) at King Fahd University of Petroleum and Minerals (KFUPM).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The author would like to acknowledge the support provided by the Deanship of Research Oversight and Coordination (DROC) at King Fahd University of Petroleum and Minerals (KFUPM) and the Saudi Aramco Chair Programme (ORCP2390).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shahsavari, A.; Akbari, M. Potential of solar energy in developing countries for reducing energy-related emissions. Renew. Sustain. Energy Rev. 2018, 90, 275–291. [Google Scholar] [CrossRef]
- Li, W.; Yu, X.; Hu, N.; Huang, F.; Wang, J.; Peng, Q. Study on the relationship between fossil energy consumption and carbon emission in Sichuan Province. Energy Rep. 2022, 8, 53–62. [Google Scholar] [CrossRef]
- Garba, M.D.; Usman, M.; Khan, S.; Shehzad, F.; Galadima, A.; Ehsan, M.F.; Ghanem, A.S.; Humayun, M. CO2 towards fuels: A review of catalytic conversion of carbon dioxide to hydrocarbons. J. Environ. Chem. Eng. 2021, 9, 104756. [Google Scholar] [CrossRef]
- Gabrielli, P.; Gazzani, M.; Mazzotti, M. The Role of Carbon Capture and Utilization, Carbon Capture and Storage, and Biomass to Enable a Net-Zero-CO2 Emissions Chemical Industry. Ind. Eng. Chem. Res. 2020, 59, 7033–7045. [Google Scholar] [CrossRef]
- Saravanan, A.; Senthil kumar, P.; Vo, D.-V.N.; Jeevanantham, S.; Bhuvaneswari, V.; Anantha Narayanan, V.; Yaashikaa, P.R.; Swetha, S.; Reshma, B. A comprehensive review on different approaches for CO2 utilization and conversion pathways. Chem. Eng. Sci. 2021, 236, 116515. [Google Scholar] [CrossRef]
- Tufa, R.A.; Chanda, D.; Ma, M.; Aili, D.; Demissie, T.B.; Vaes, J.; Li, Q.; Liu, S.; Pant, D. Towards highly efficient electrochemical CO2 reduction: Cell designs, membranes and electrocatalysts. Appl. Energy 2020, 277, 115557. [Google Scholar] [CrossRef]
- Ma, J.; Sun, N.; Zhang, X.; Zhao, N.; Xiao, F.; Wei, W.; Sun, Y. A short review of catalysis for CO2 conversion. Catal. Today 2009, 148, 221–231. [Google Scholar] [CrossRef]
- Etim, U.J.; Zhang, C.; Zhong, Z. Impacts of the Catalyst Structures on CO2 Activation on Catalyst Surfaces. Nanomaterials 2021, 11, 3265. [Google Scholar] [CrossRef]
- Dongare, S.; Singh, N.; Bhunia, H. Electrocatalytic reduction of CO2 to useful chemicals on copper nanoparticles. Appl. Surf. Sci. 2021, 537, 148020. [Google Scholar] [CrossRef]
- Won, D.H.; Shin, H.; Koh, J.; Chung, J.; Lee, H.S.; Kim, H.; Woo, S.I. Highly Efficient, Selective, and Stable CO2 Electroreduction on a Hexagonal Zn Catalyst. Angew. Chem. Int. Ed. 2016, 55, 9297–9300. [Google Scholar] [CrossRef]
- Zhang, S.; Kang, P.; Meyer, T.J. Nanostructured Tin Catalysts for Selective Electrochemical Reduction of Carbon Dioxide to Formate. J. Am. Chem. Soc. 2014, 136, 1734–1737. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Jia, Y.; Ji, Y.; Kuang, P.; Zhu, B.; Liu, X.; Yu, J. Curved Surface Boosts Electrochemical CO2 Reduction to Formate via Bismuth Nanotubes in a Wide Potential Window. ACS Catal. 2020, 10, 358–364. [Google Scholar] [CrossRef]
- Tsiotsias, A.I.; Charisiou, N.D.; Yentekakis, I.V.; Goula, M.A. Bimetallic Ni-Based Catalysts for CO2 Methanation: A Review. Nanomaterials 2021, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Humayun, M.; Garba, M.D.; Ullah, L.; Zeb, Z.; Helal, A.; Suliman, M.H.; Alfaifi, B.Y.; Iqbal, N.; Abdinejad, M.; et al. Electrochemical Reduction of CO2: A Review of Cobalt Based Catalysts for Carbon Dioxide Conversion to Fuels. Nanomaterials 2021, 11, 2029. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Zhang, X.; Cheng, J.; Chen, R.; Wu, L.; Shi, J.; Luo, J. Bimetallic Cu–Zn Catalysts for Electrochemical CO2 Reduction: Phase-Separated versus Core–Shell Distribution. ACS Catal. 2022, 12, 2741–2748. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Z.; Zhao, Z.; Huang, H.; Anjum, D.H.; Wang, D.; He, J.-h.; Huang, K.-W. Tunable Selectivity for Electrochemical CO2 Reduction by Bimetallic Cu–Sn Catalysts: Elucidating the Roles of Cu and Sn. ACS Catal. 2021, 11, 11103–11108. [Google Scholar] [CrossRef]
- Wang, Y.; Niu, C.; Zhu, Y. Copper–Silver Bimetallic Nanowire Arrays for Electrochemical Reduction of Carbon Dioxide. Nanomaterials 2019, 9, 173. [Google Scholar] [CrossRef]
- Razzaq, R.; Zhu, H.; Jiang, L.; Muhammad, U.; Li, C.; Zhang, S. Catalytic Methanation of CO and CO2 in Coke Oven Gas over Ni–Co/ZrO2–CeO2. Ind. Eng. Chem. Res. 2013, 52, 2247–2256. [Google Scholar] [CrossRef]
- Möller, T.; Scholten, F.; Thanh, T.N.; Sinev, I.; Timoshenko, J.; Wang, X.; Jovanov, Z.; Gliech, M.; Roldan Cuenya, B.; Varela, A.S.; et al. Electrocatalytic CO2 Reduction on CuOx Nanocubes: Tracking the Evolution of Chemical State, Geometric Structure, and Catalytic Selectivity using Operando Spectroscopy. Angew. Chem. Int. Ed. 2020, 59, 17974–17983. [Google Scholar] [CrossRef]
- Li, Z.; Yadav, R.M.; Sun, L.; Zhang, T.; Zhang, J.; Ajayan, P.M.; Wu, J. CuO/ZnO/C electrocatalysts for CO2-to-C2+ products conversion with high yield: On the effect of geometric structure and composition. Appl. Catal. A-Gen. 2020, 606, 117829. [Google Scholar] [CrossRef]
- Helal, A.; Al-ahmari, F.S.; Usman, M.; Yamani, Z.H. Chalcopyrite UiO-67 Metal-Organic Framework Composite for CO2 Fixation as Cyclic Carbonates. J. Environ. Chem. Eng. 2022, 108061. [Google Scholar] [CrossRef]
- Helal, A.; Fettouhi, M.; Arafat, M.E.; Khan, M.Y.; Sanhoob, M.A. Nickel based metal-organic framework as catalyst for chemical fixation of CO2 in oxazolidinone synthesis. J. CO2 Util. 2021, 50, 101603. [Google Scholar] [CrossRef]
- Usman, M.; Iqbal, N.; Noor, T.; Zaman, N.; Asghar, A.; Abdelnaby, M.M.; Galadima, A.; Helal, A. Advanced strategies in Metal-Organic Frameworks for CO2 Capture and Separation. Chem. Rec. 2021. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Helal, A.; Abdelnaby, M.M.; Alloush, A.M.; Zeama, M.; Yamani, Z.H. Trends and Prospects in UiO-66 Metal-Organic Framework for CO2 Capture, Separation, and Conversion. Chem. Rec. 2021, 21, 1771–1791. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Ghanem, A.S.; Ali Shah, S.N.; Garba, M.D.; Khan, M.Y.; Khan, S.; Humayun, M.; Khan, A.L. A review on SAPO-34 zeolite materials for CO2 capture and conversion. Chem. Rec. 2022. [Google Scholar] [CrossRef]
- Zhou, W.; Cheng, K.; Kang, J.; Zhou, C.; Subramanian, V.; Zhang, Q.; Wang, Y. New horizon in C1 chemistry: Breaking the selectivity limitation in transformation of syngas and hydrogenation of CO2 into hydrocarbon chemicals and fuels. Chem. Soc. Rev. 2019, 48, 3193–3228. [Google Scholar] [CrossRef]
- Suliman, M.H.; Adam, A.; Siddiqui, M.N.; Yamani, Z.H.; Qamar, M. Facile synthesis of ultrathin interconnected carbon nanosheets as a robust support for small and uniformly-dispersed iron phosphide for the hydrogen evolution reaction. Carbon 2019, 144, 764–771. [Google Scholar] [CrossRef]
- Suliman, M.H.; Adam, A.; Siddiqui, M.N.; Yamani, Z.H.; Qamar, M. The impact of microstructural features of carbon supports on the electrocatalytic hydrogen evolution reaction. Catal. Sci. Technol. 2019, 9, 1497–1503. [Google Scholar] [CrossRef]
- Suliman, M.H.; Baroud, T.N.; Siddiqui, M.N.; Qamar, M.; Giannelis, E.P. Confined growth and dispersion of FeP nanoparticles in highly mesoporous carbons as efficient electrocatalysts for the hydrogen evolution reaction. Int. J. Hydrogen Energy 2021, 46, 8507–8518. [Google Scholar] [CrossRef]
- Yuan, J.; Yang, M.-P.; Zhi, W.-Y.; Wang, H.; Wang, H.; Lu, J.-X. Efficient electrochemical reduction of CO2 to ethanol on Cu nanoparticles decorated on N-doped graphene oxide catalysts. J. CO2 Util. 2019, 33, 452–460. [Google Scholar] [CrossRef]
- Dongare, S.; Singh, N.; Bhunia, H. Nitrogen-doped graphene supported copper nanoparticles for electrochemical reduction of CO2. J. CO2 Util. 2021, 44, 101382. [Google Scholar] [CrossRef]
- Usman, M.; Zeb, Z.; Ullah, H.; Suliman, M.H.; Humayun, M.; Ullah, L.; Shah, S.N.A.; Ahmed, U.; Saeed, M. A review of metal-organic frameworks/graphitic carbon nitride composites for solar-driven green H2 production, CO2 reduction, and water purification. J. Environ. Chem. Eng. 2022, 10, 107548. [Google Scholar] [CrossRef]
- Usman, M.; Humayun, M.; Shah, S.S.; Ullah, H.; Tahir, A.A.; Khan, A.; Ullah, H. Bismuth-Graphene Nanohybrids: Synthesis, Reaction Mechanisms, and Photocatalytic Applications—A Review. Energies 2021, 14, 2281. [Google Scholar] [CrossRef]
- Adio, S.O.; Ganiyu, S.A.; Usman, M.; Abdulazeez, I.; Alhooshani, K. Facile and efficient nitrogen modified porous carbon derived from sugarcane bagasse for CO2 capture: Experimental and DFT investigation of nitrogen atoms on carbon frameworks. Chem. Eng. J. 2020, 382, 122964. [Google Scholar] [CrossRef]
- Lu, Q.; Eid, K.; Li, W. Heteroatom-Doped Porous Carbon-Based Nanostructures for Electrochemical CO2 Reduction. Nanomaterials 2022, 12, 2379. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Noh, Y.; Kim, Y.; Park, S.; Yoon, W.; Jang, D.; Choi, S.M.; Kim, W.B. Selective electrochemical CO2 conversion to multicarbon alcohols on highly efficient N-doped porous carbon-supported Cu catalysts. Green Chem. 2020, 22, 71–84. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, J.; Yang, X.; Xu, Y.; Sun, W.; Zhou, J. MOF-derived Cu@Cu2O heterogeneous electrocatalyst with moderate intermediates adsorption for highly selective reduction of CO2 to methanol. Chem. Eng. J. 2022, 431, 134171. [Google Scholar] [CrossRef]
- Yin, J.; Jin, J.; Lin, H.; Yin, Z.; Li, J.; Lu, M.; Guo, L.; Xi, P.; Tang, Y.; Yan, C.-H. Optimized Metal Chalcogenides for Boosting Water Splitting. Adv. Sci. 2020, 7, 1903070. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Davar, F.; Mir, N. Synthesis and characterization of metallic copper nanoparticles via thermal decomposition. Polyhedron 2008, 27, 3514–3518. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Huang, M.; Ma, H.-L.; Zhang, Z.-Q.; Gao, J.-M.; Zhu, Y.-L.; Han, X.-J.; Guo, X.-Y. Preparation of a Carbon-Based Solid Acid Catalyst by Sulfonating Activated Carbon in a Chemical Reduction Process. Molecules 2010, 15, 7188–7196. [Google Scholar] [CrossRef]
- Suliman, M.H.; Adam, A.; Li, L.; Tian, Z.; Siddiqui, M.N.; Yamani, Z.H.; Qamar, M. FeP/MoS2 Enriched with Dense Catalytic Sites and High Electrical Conductivity for the Hydrogen Evolution Reaction. ACS Sustain. Chem. Eng. 2019, 7, 17671–17681. [Google Scholar] [CrossRef]
- Merino-Garcia, I.; Albo, J.; Solla-Gullón, J.; Montiel, V.; Irabien, A. Cu oxide/ZnO-based surfaces for a selective ethylene production from gas-phase CO2 electroconversion. J. CO2 Util. 2019, 31, 135–142. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Quan, X.; Yu, H. Efficient Electrochemical Reduction of Carbon Dioxide to Acetate on Nitrogen-Doped Nanodiamond. J. Am. Chem. Soc. 2015, 137, 11631–11636. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, K.P.; Cave, E.R.; Abram, D.N.; Jaramillo, T.F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 2012, 5, 7050–7059. [Google Scholar] [CrossRef]
- Geioushy, R.A.; Khaled, M.M.; Hakeem, A.S.; Alhooshani, K.; Basheer, C. High efficiency graphene/Cu2O electrode for the electrochemical reduction of carbon dioxide to ethanol. J. Electroanal. Chem. 2017, 785, 138–143. [Google Scholar] [CrossRef]
- Zhao, K.; Liu, Y.; Quan, X.; Chen, S.; Yu, H. CO2 Electroreduction at Low Overpotential on Oxide-Derived Cu/Carbons Fabricated from Metal Organic Framework. ACS Appl. Mater. Interfaces 2017, 9, 5302–5311. [Google Scholar] [CrossRef]
- Sreekanth, N.; Nazrulla, M.A.; Vineesh, T.V.; Sailaja, K.; Phani, K.L. Metal-free boron-doped graphene for selective electroreduction of carbon dioxide to formic acid/formate. Chem. Commun. 2015, 51, 16061–16064. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Hou, X.; Ma, C.; Tan, T. Nitrogen-doped graphenes as efficient electrocatalysts for the selective reduction of carbon dioxide to formate in aqueous solution. Green Chem. 2016, 18, 3250–3256. [Google Scholar] [CrossRef]
- Song, Y.; Peng, R.; Hensley, D.K.; Bonnesen, P.V.; Liang, L.; Wu, Z.; Meyer, H.M., III; Chi, M.; Ma, C.; Sumpter, B.G.; et al. High-Selectivity Electrochemical Conversion of CO2 to Ethanol using a Copper Nanoparticle/N-Doped Graphene Electrode. ChemistrySelect 2016, 1, 6055–6061. [Google Scholar] [CrossRef]
- Geioushy, R.A.; Khaled, M.M.; Alhooshani, K.; Hakeem, A.S.; Rinaldi, A. Graphene/ZnO/Cu2O electrocatalyst for selective conversion of CO2 into n-propanol. Electrochim. Acta 2017, 245, 456–462. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).