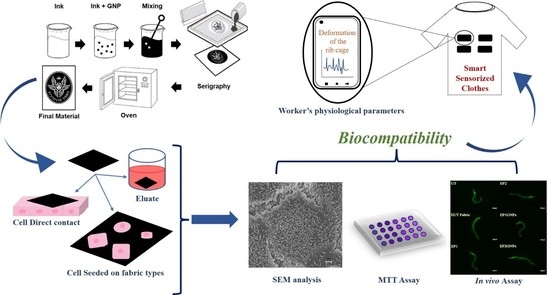
In Vitro and In Vivo Biocompatibility Studies on Engineered Fabric with Graphene Nanoplatelets
Abstract
1. Introduction
2. Materials and Methods
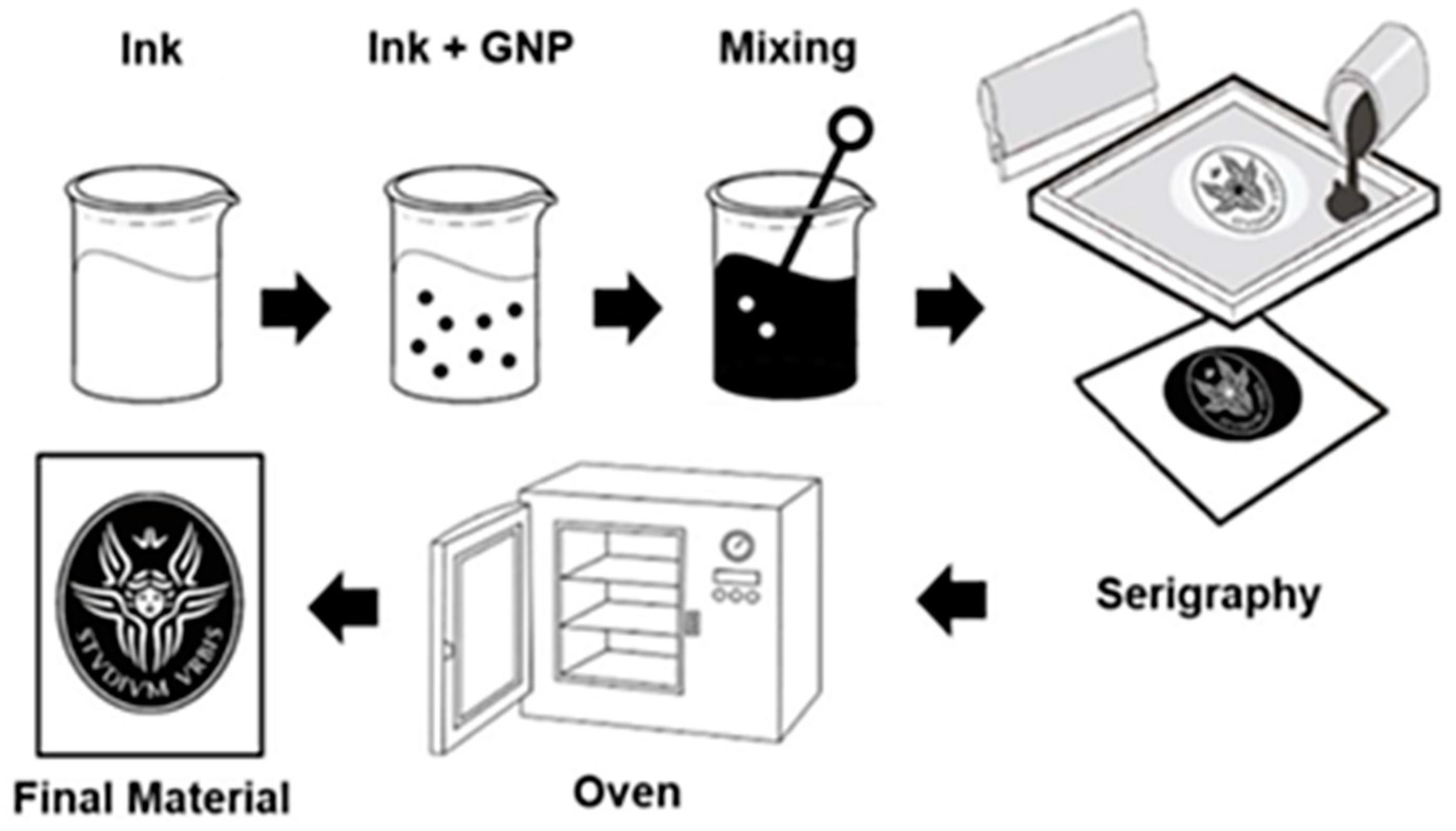
2.1. Smart Fabric
2.2. Fabric Types Used
2.3. Eluates
2.4. In Vitro Assays
2.4.1. HaCaT Cultures and Treatments
2.4.2. Cell Viability Assay
2.4.3. Sample Preparation for FESEM and Analysis
2.4.4. Immunofluorescence Microscopy Analysis
2.5. In Vivo Assays
2.5.1. Nematode Strains and Maintenance
2.5.2. Sample Preparation for C. elegans Experiments
2.5.3. Survival Assay
2.5.4. Brood Size Assays
2.5.5. C. elegans Body Lengthiness
2.5.6. Pharyngeal Pumping Analysis
2.5.7. Body Bend Analysis
2.5.8. Fluorescence Microscopy Analysis for the Oxidative Stress Evaluation
2.6. Statistical Analysis
3. Results
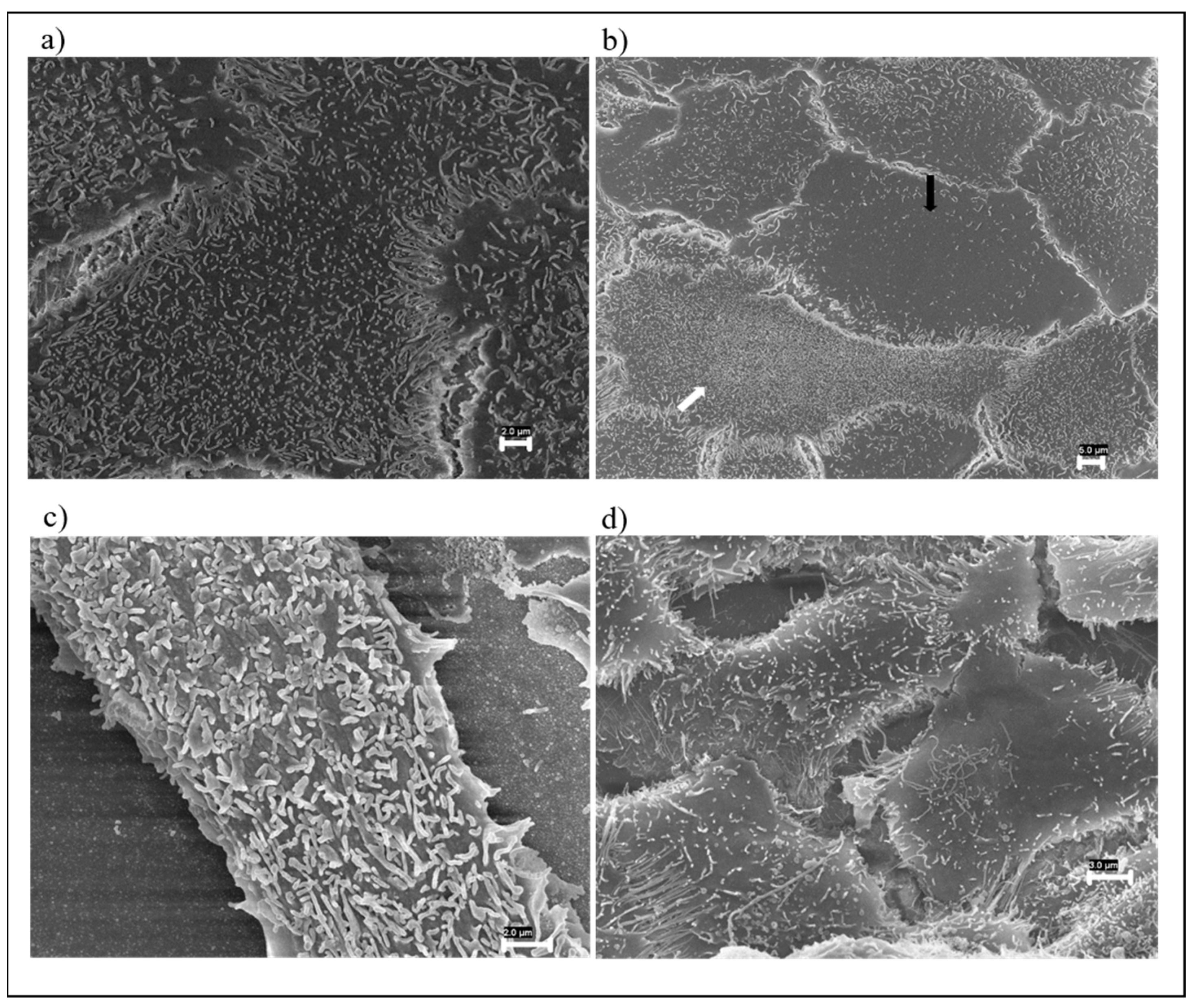
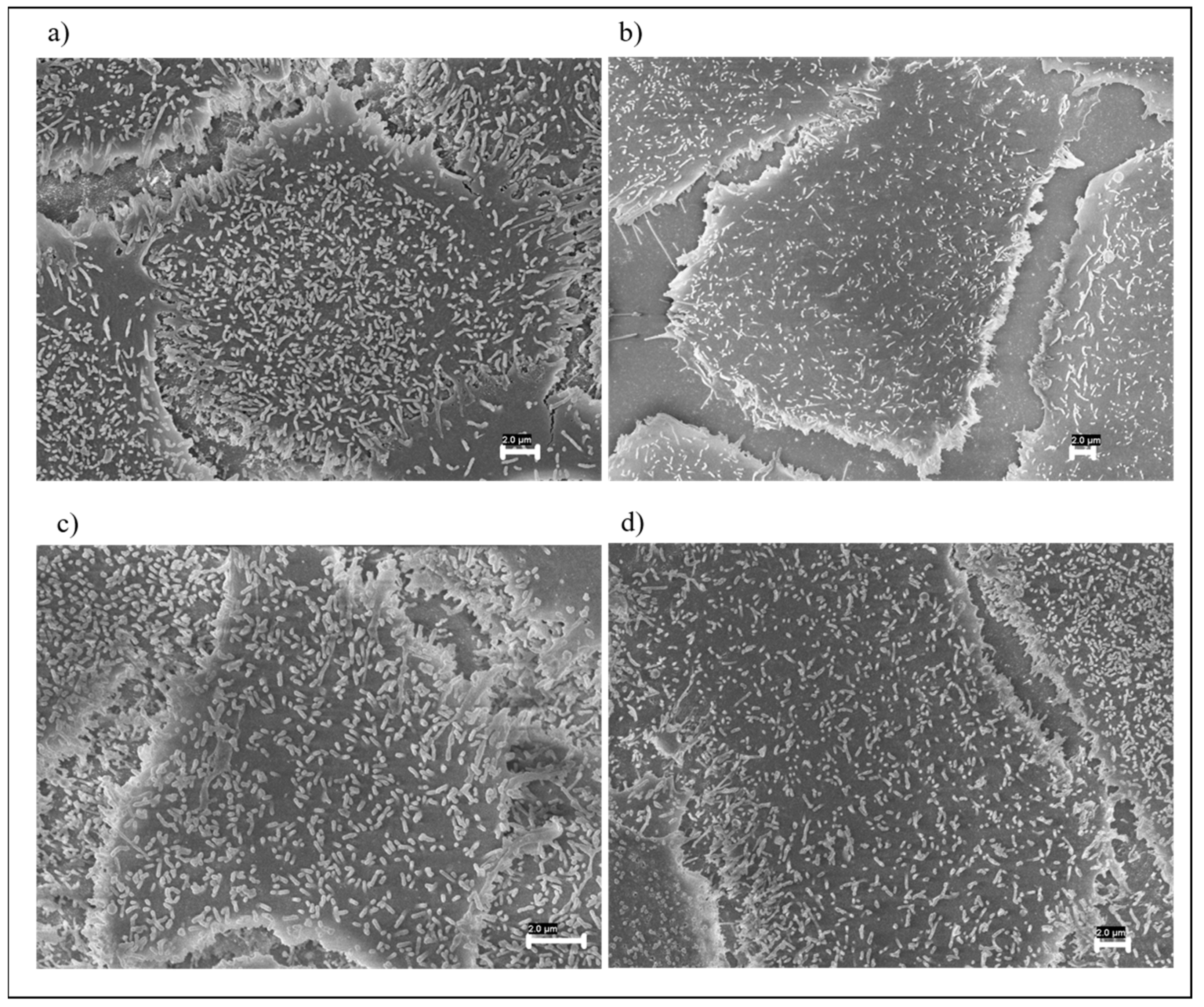
3.1. FESEM Morphological Analysis of Fabrics
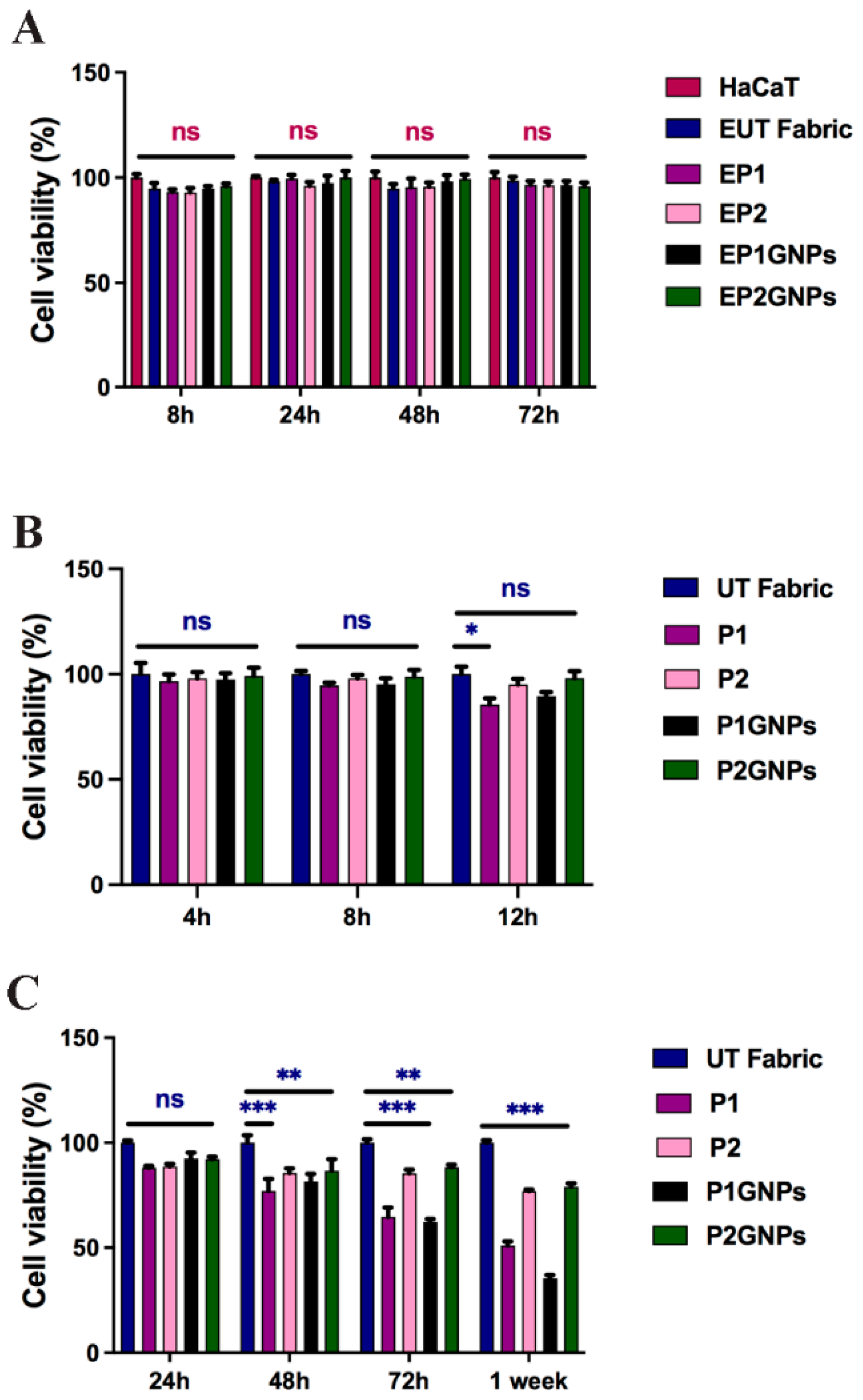
3.2. Cell Viability
3.3. Morphological Analysis
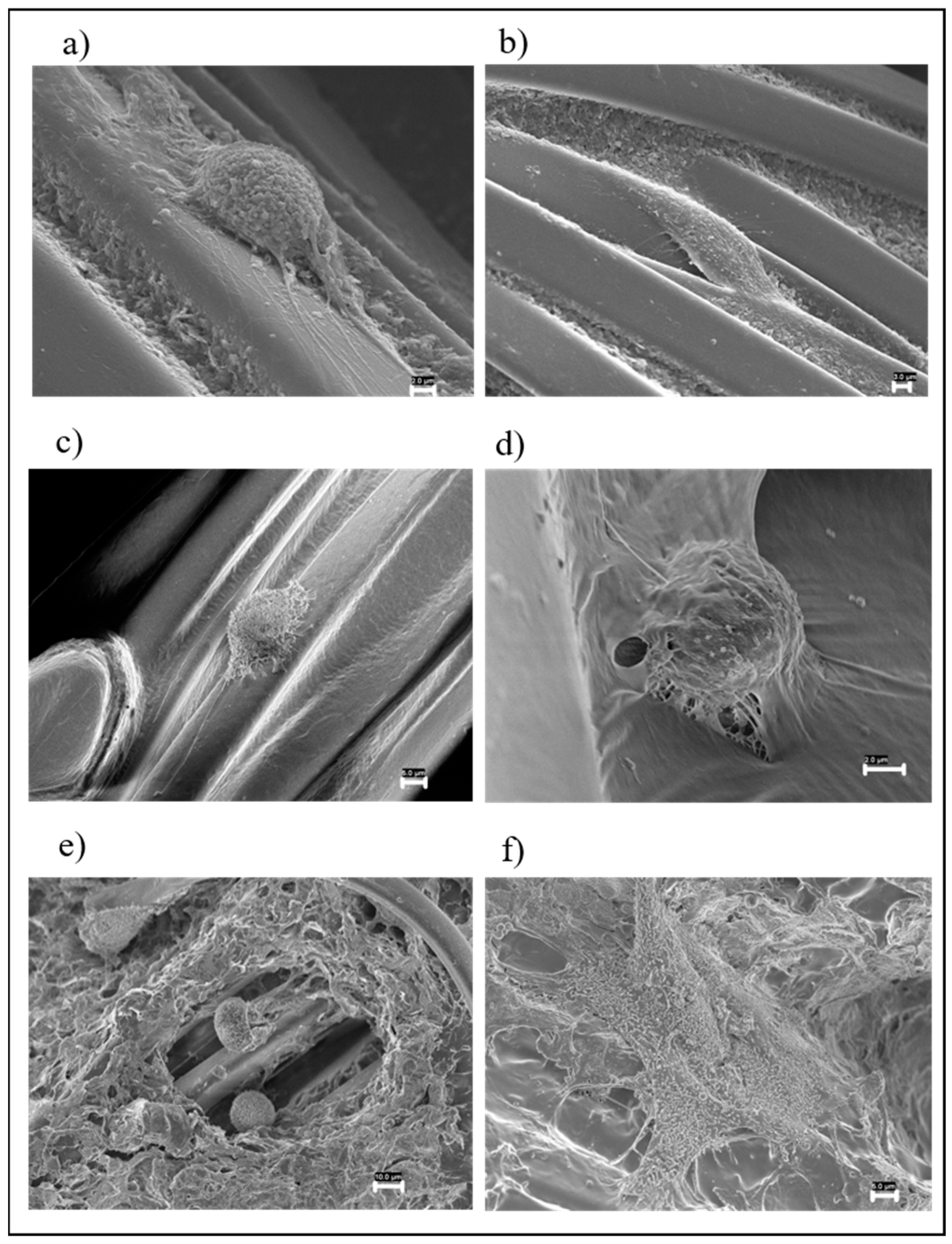
3.3.1. FESEM
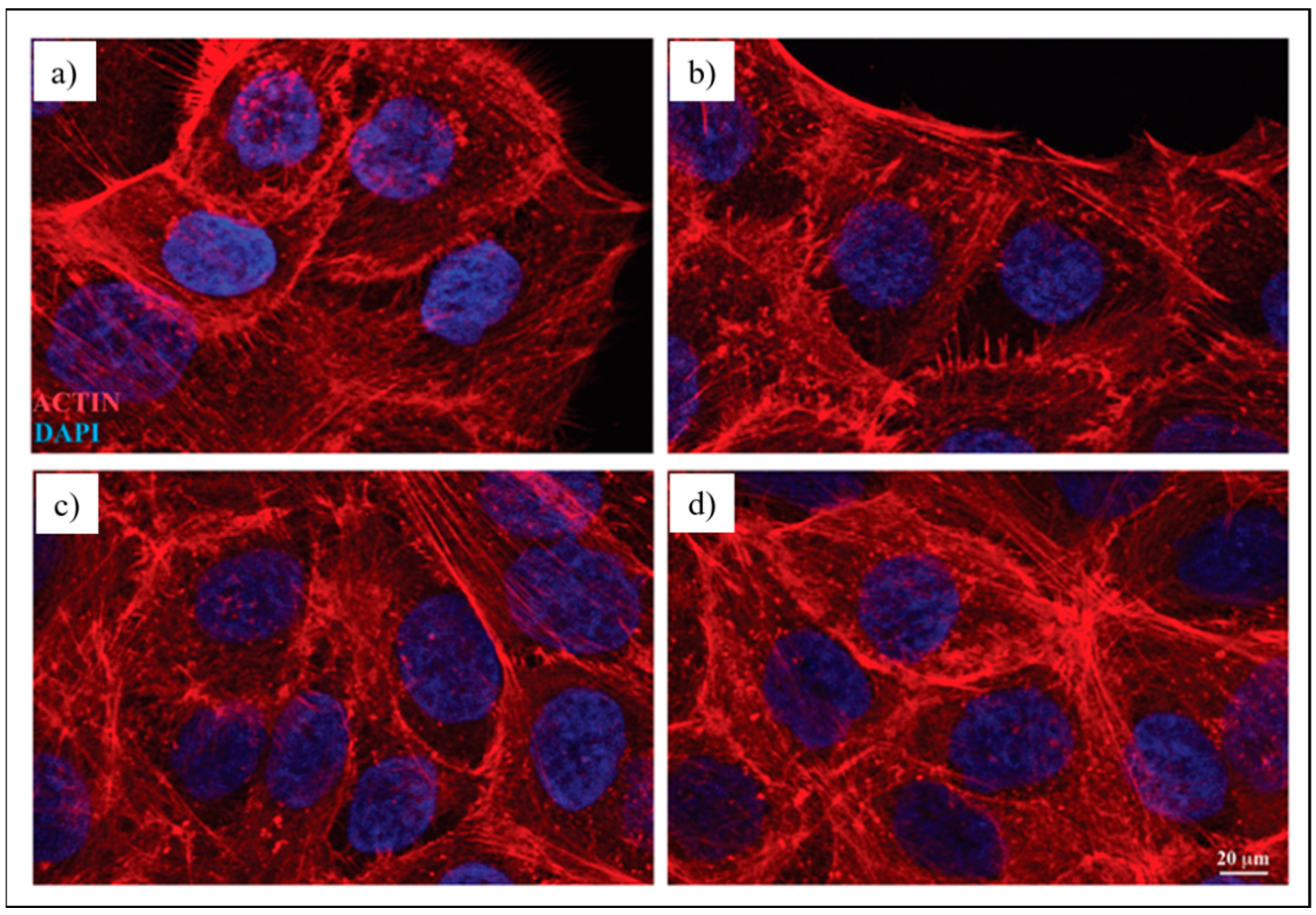
3.3.2. Immunofluorescence Analysis
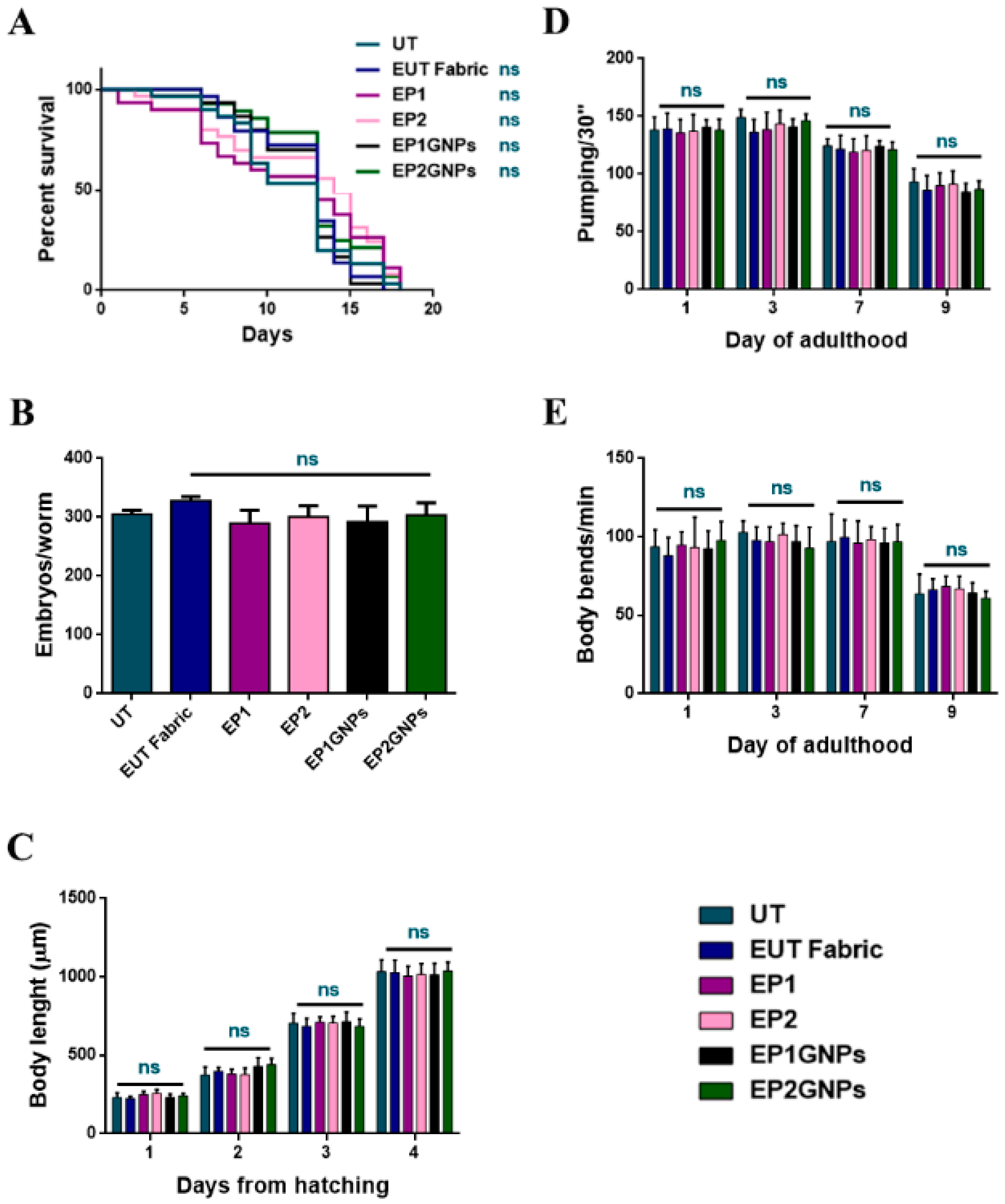
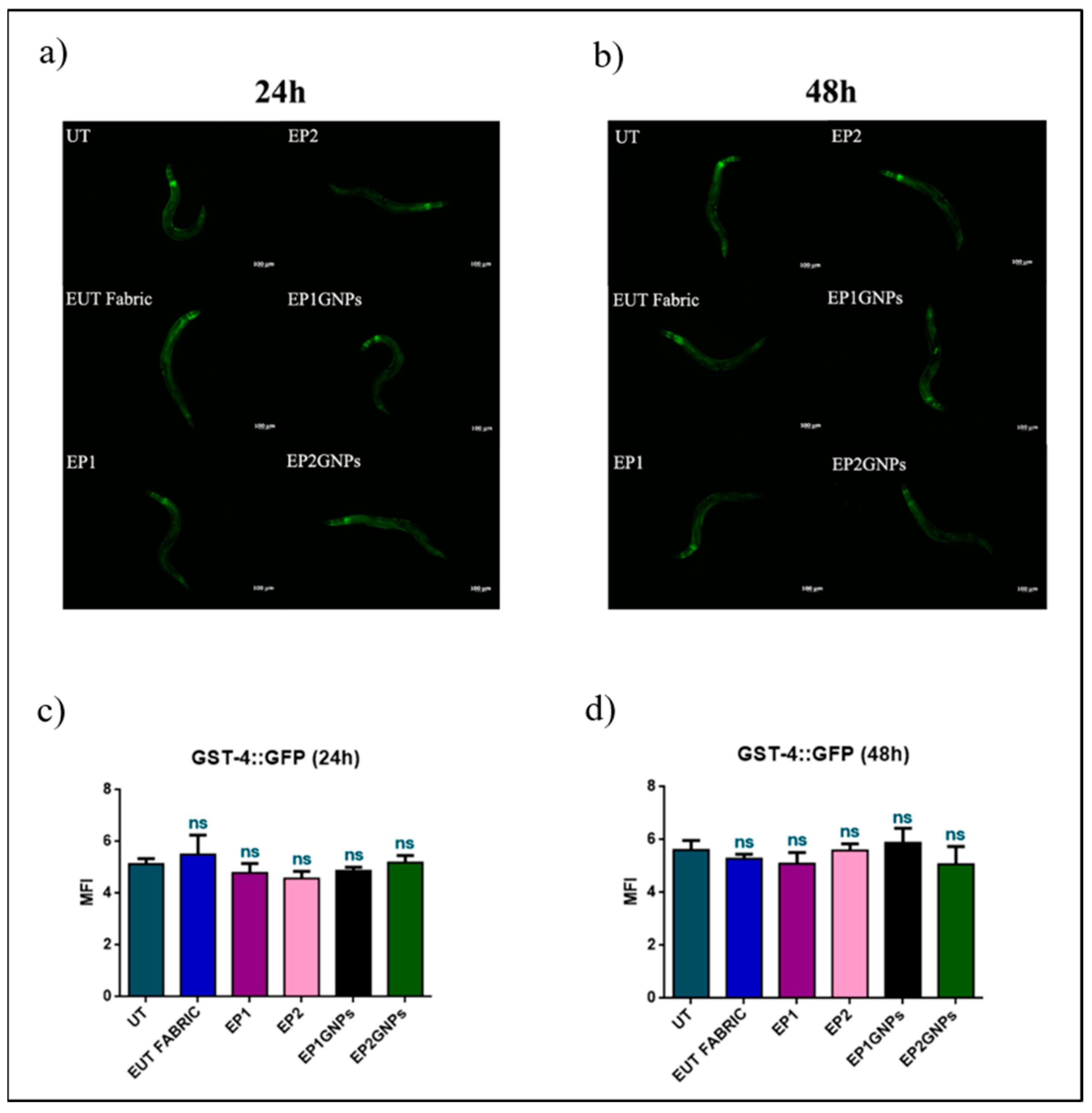
3.4. In Vivo Results
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yetisen, A.K.; Qu, H.; Manbachi, A.; Butt, H.; Dokmeci, M.R.; Hinestroza, J.P.; Skorobogatiy, M.; Khademhosseini, A.; Yun, S.H. Nanotechnology in Textiles. ACS Nano 2016, 10, 3042–3068. [Google Scholar] [CrossRef] [PubMed]
- Ehrman, A.; Nguyen, T.A.; Tri, P.N. Nanosensors and Nanodevices for Smart Multifunctional Textiles, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Axisa, F.; Schmitt, P.; Gehin, C.; Delhomme, G.; McAdams, E.; Dittmar, A. Flexible Technologies and Smart Clothing for Citizen Medicine, Home Healthcare, and Disease Prevention. IEEE Trans. Inf. Technol. Biomed. 2005, 9, 325–336. [Google Scholar] [CrossRef]
- Castano, L.M.; Flatau, A.B. Smart Fabric Sensors and E-textile Technologies: A Review. Smart Mater. Struct. 2014, 23, 053001. [Google Scholar] [CrossRef]
- Ju, N.; Lee, K.-H. Consumer Resistance to Innovation: Smart Clothing. Fash. Text. 2020, 7, 21. [Google Scholar] [CrossRef]
- Sayem, A.S.M.; Teay, S.H.; Shahariar, H.; Fink, P.L.; Albarbar, A. Review on Smart Electro-Clothing Systems (SeCSs). Sensors 2020, 20, 587. [Google Scholar] [CrossRef]
- Zhang, L.; Baima, M.; Andrew, T.L. Transforming Commercial Textiles and Threads into Sewable and Weavable Electric Heaters. ACS Appl. Mater. Interfaces 2017, 9, 32299–32307. [Google Scholar] [CrossRef] [PubMed]
- Ilanchezhiyan, P.; Zakirov, A.S.; Kumar, G.M.; Yuldashev, S.U.; Cho, H.D.; Kang, T.W.; Mamadalimov, A.T. Highly Efficient CNT Functionalized Cotton Fabrics for Flexible/Wearable Heating Applications. RSC Adv. 2015, 5, 10697–10702. [Google Scholar] [CrossRef]
- Yao, S.; Zhu, Y. Wearable Multifunctional Sensors Using Printed Stretchable Conductors Made of Silver Nanowires. Nanoscale 2014, 6, 2345–2352. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Lee, H.B.; Yeon, S.M.; Park, J.; Lee, N.K. Flexible and Stretchable Piezoelectric Sensor with Thickness-Tunable Configuration of Electrospun Nanofiber Mat and Elastomeric Substrates. ACS Appl. Mater. Interfaces 2016, 8, 24773–24781. [Google Scholar] [CrossRef]
- Ai, Y.; Lou, Z.; Chen, S.; Chen, D.; Wang, Z.M.; Jiang, K.; Shen, G. All rGO-on-PVDF-Nanofibers Based Self-Powered Electronic Skins. Nano Energy 2017, 35, 121–127. [Google Scholar] [CrossRef]
- Limpiteeprakan, P.; Babel, S.; Lohwacharin, J.; Takizawa, S. Release of Silver Nanoparticles from Fabrics during the Course of Sequential Washing. Environ. Sci. Pollut. Res. 2016, 23, 22810–22818. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.; Ramos, D. Health and Safety Concerns of Textiles with Nanomaterials. IOP Conf. Ser. Mater. Sci. Eng. 2017, 254, 102002. [Google Scholar] [CrossRef]
- Kulthong, K.; Srisung, S.; Boonpavanitchakul, K.; Kangwansupamonkon, W.; Maniratanachote, R. Determination of Silver Nanoparticle Release from Antibacterial Fabrics into Aartificial Sweat. Part. Fibre Toxicol. 2010, 7, 8. [Google Scholar] [CrossRef]
- OECD. Work Plan for the Test Guidelines Programme. Organisation for Economic Co-operation and Development (OECD): Paris, France, 2018. Available online: http://www.oecd.org/env/ehs/testing/ENV_JM_WRPR_2019__TGP-work-plan.pdf (accessed on 1 January 2020).
- Bengalli, R.; Colantuoni, A.; Perelshtein, I.; Gedanken, A.; Collini, M.; Mantecca, P.; Fiandra, L. In Vitro Skin Toxicity of CuO and ZnO Nanoparticles: Application in the Safety Assessment of Antimicrobial Coated Textiles. NanoImpact 2021, 21, 100282. [Google Scholar] [CrossRef]
- Bengalli, R.; Fiandra, L.; Vineis, C.; Sanchez-Ramirez, D.O.; Azoia, N.G.; Varesano, A.; Mantecca, P. Safety Assessment of Polypyrrole Nanoparticles and Spray-Coated Textiles. Nanomaterials 2021, 11, 1991. [Google Scholar] [CrossRef]
- Shvedova, A.; Castranova, V.; Kisin, E.R.; Schwegler-Berry, D.; Murray, A.R.; Gandelsman, V.Z.; Maynard, A.; Baron, P. Exposure to Carbon Nanotube Material: Assessment of Nanotube Cytotoxicity using Human Keratinocyte Cells. J. Toxicol. Environ. Health Part A 2003, 66, 1909–1926. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.-H.; Lin, Y.-S.; Macosko, C.W.; Haynes, C.L. Cytotoxicity of Graphene Oxide and Graphene in Human Erythrocytes and Skin Fibroblasts. ACS Appl. Mater. Interfaces 2011, 3, 2607–2615. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, H.; Bussche, A.V.D.; Creighton, M.; Hurt, R.H.; Kane, A.B.; Gao, H. Graphene Microsheets Enter Cells through Spontaneous Membrane Penetration at Edge Asperities and Corner Sites. Proc. Natl. Acad. Sci. USA 2013, 110, 12295–12300. [Google Scholar] [CrossRef]
- Pelin, M.; Fusco, L.; León, V.; Martín, C.; Criado, A.; Sosa, S.; Vázquez, E.; Tubaro, A.; Prato, M. Differential Cytotoxic Effects of Graphene and Graphene Oxide on Skin Keratinocytes. Sci. Rep. 2017, 7, 40572. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Duan, L.; Chen, W. Carbon Nanomaterials: Their Environmental Behavior and Effects on the Transport and Fate of Pollutants in Environment. Yingyong Shengtai Xuebao 2009, 20, 205–212. [Google Scholar] [PubMed]
- Giese, B.; Klaessig, F.; Park, B.; Kaegi, R.; Steinfeldt, M.; Wigger, H.; Von Gleich, A.; Gottschalk, F. Risks, Release and Concentrations of Engineered Nanomaterial in the Environment. Sci. Rep. 2018, 8, 1565. [Google Scholar] [CrossRef] [PubMed]
- Bundschuh, M.; Filser, J.; Lüderwald, S.; McKee, M.S.; Metreveli, G.; Schaumann, G.E.; Schulz, R.; Wagner, S. Nanoparticles in the Environment: Where Do We Come From, Where Do We Go To? Environ. Sci. Eur. 2018, 30, 6. [Google Scholar] [CrossRef]
- Mishra, S.; Singh, H.B.; Yang, X. Creating a Global Database “Nanomaterials in the Soil Environment”: Future Need for the Terrestrial Ecosystem. Energy Ecol. Environ. 2019, 4, 271–285. [Google Scholar] [CrossRef]
- Qi, L.-F.; Xu, Z.-R.; Li, Y.; Jiang, X.; Han, X.-Y. In Vitro Effects of Chitosan Nanoparticles on Proliferation of Human Gastric Carcinoma Cell Line MGC803 Cells. World J. Gastroenterol. 2005, 11, 5136–5141. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, D.; Fanizza, C.; Ursini, C.L.; Casciardi, S.; Paba, E.; Ciervo, A.; Fresegna, A.M.; Maiello, R.; Marcelloni, A.M.; Buresti, G.; et al. Multi-Walled Carbon Nanotubes Induce Cytotoxicity and Genotoxicity in Human Lung Epithelial Cells. J. Appl. Toxicol. 2012, 32, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Baiguera, S.; Casciardi, S.; Incoronato, F.; Cavallo, D.; Ursini, C.L.; Ciervo, A.; Maiello, R.; Fresegna, A.M.; Marcelloni, A.M.; Lega, D.; et al. Cell Surface Modifications Induced by Ttitanium Dioxide Nanoparticles in A549 Cells. In Microscopy, Advances in Scientific Research and Education; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2014; Volume 1, pp. 24–31. [Google Scholar]
- Fanizza, C.; Casciardi, S.; Incoronato, F.; Cavallo, D.; Ursini, C.; Ciervo, A.; Maiello, R.; Fresegna, A.; Marcelloni, A.; Lega, D.; et al. Human Epithelial Cells Exposed to Functionalized Multiwalled Carbon Nanotubes: Interactions and Cell Surface Modifications. J. Microsc. 2015, 259, 173–184. [Google Scholar] [CrossRef]
- Wang, D. Evaluation of In Vitro Methods for Human Hazard Assessment Applied in the OECD Testing Programme for the Safety of Manufactured Nanomaterials Series on the Safety of Manufactured Nanomaterials. Available online: https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV/JM/MONO(2018)4&doclanguage=en (accessed on 12 February 2022).
- Li, Y.; Zhong, L.; Zhang, L.; Shen, X.; Kong, L.; Wu, T. Research Advances on the Adverse Effects of Nanomaterials in a Model Organism, Caenorhabditis elegans. Environ. Toxicol. Chem. 2021, 40, 2406–2424. [Google Scholar] [CrossRef]
- Wu, T.; Xu, H.; Liang, X.; Tang, M. Caenorhabditis elegans as a Complete Model Organism for Biosafety Assessments of Nanoparticles. Chemosphere 2019, 221, 708–726. [Google Scholar] [CrossRef]
- Sarto, M.S.; D’Aloia, A.G.; Tamburrano, A.; De Bellis, G. Synthesis, Modeling, and Experimental Characterization of Graphite Nanoplatelet-Based Composites for EMC Applications. IEEE Trans. Electromagn. Compat. 2012, 54, 17–27. [Google Scholar] [CrossRef]
- Marra, F.; Minutillo, S.; Tamburrano, A.; Sarto, M.S. Production and Characterization of Graphene Nanoplatelet-Based Ink for Smart Textile Strain Sensors via Screen Printing Technique. Mater. Des. 2021, 198, 109306. [Google Scholar] [CrossRef]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal Keratinization in a Spontaneously Immortalized Aneuploid Human Keratinocyte Cell Line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef]
- Nica, I.C.; Stan, M.S.; Dinischiotu, A.; Popa, M.; Chifiriuc, M.C.; Lazar, V.; Pircalabioru, G.G.; Bezirtzoglou, E.; Iordache, O.G.; Varzaru, E.; et al. Innovative Self-Cleaning and Biocompatible Polyester Textiles Nano-Decorated with Fe–N-Doped Titanium Dioxide. Nanomaterials 2016, 6, 214. [Google Scholar] [CrossRef]
- Stan, M.S.; Nica, I.C.; Dinischiotu, A.; Varzaru, E.; Iordache, O.G.; Dumitrescu, I.; Popa, M.; Chifiriuc, M.C.; Pircalabioru, G.G.; Lazar, V.; et al. Photocatalytic, Antimicrobial and Biocompatibility Features of Cotton Knit Coated with Fe-N-Doped Titanium Dioxide Nanoparticles. Materials 2016, 9, 789. [Google Scholar] [CrossRef]
- Bossù, M.; Mancini, P.; Bruni, E.; Uccelletti, D.; Preziosi, A.; Rulli, M.; Relucenti, M.; Donfrancesco, O.; Iaculli, F.; Di Giorgio, G.; et al. Biocompatibility and Antibiofilm Properties of Calcium Silicate-Based Cements: An In Vitro Evaluation and Report of Two Clinical Cases. Biology 2021, 10, 470. [Google Scholar] [CrossRef]
- Robards, A.W.; Wilson, A.J.; Stewart, M. Procedures in Electron Microscopy. Trends Cell Biol. 1993, 4, 187–188. [Google Scholar] [CrossRef]
- Fischer, E.R.; Hansen, B.T.; Nair, V.; Hoyt, F.H.; Dorward, D.W. Scanning Electron Microscopy. Curr. Protoc. Microbiol. 2012, 25, 2B.2.1–2B.2.47. [Google Scholar] [CrossRef]
- Stiernagle, T. Maintenance of C. elegans. WormBook 2006, 2, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Uccelletti, D.; Pascoli, A.; Farina, F.; Alberti, A.; Mancini, P.; Hirschberg, C.B.; Palleschi, C. APY-1, a Novel Caenorhabditis elegans Apyrase Involved in Unfolded Protein Response Signalling and Stress Responses. Mol. Biol. Cell 2008, 19, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Nawa, M.; Matsuoka, M. The Method of the Body Bending Assay Using Caenorhabditis elegans. Bio-Protocol 2012, 2, e253. [Google Scholar] [CrossRef]
- Raizen, D.; Song, B.-M.; Trojanowski, N.; You, Y.-J. Methods for Measuring Pharyngeal Behaviors. WormBook Online Rev. C. Elegans Biol. 2012, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Van Der Hoeven, R.; McCallum, K.C.; Cruz, M.R.; Garsin, D.A. Ce-Duox1/BLI-3 Generated Reactive Oxygen Species Trigger Protective SKN-1 Activity via p38 MAPK Signaling During Infection in C. elegans. PLoS Pathog. 2011, 7, e1002453. [Google Scholar] [CrossRef]
- Melo, J.A.; Ruvkun, G. Inactivation of Conserved C. elegans Genes Engages Pathogen- and Xenobiotic-Associated Defenses. Cell 2012, 149, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Fusco, L.; Pelin, M.; Mukherjee, S.; Keshavan, S.; Sosa, S.; Martín, C.; González, V.; Vázquez, E.; Prato, M.; Fadeel, B.; et al. Keratinocytes Are Capable of Selectively Sensing Low Amounts of Graphene-Based Materials: Implications for Cutaneous Applications. Carbon 2020, 159, 598–610. [Google Scholar] [CrossRef]
- Salesa, B.; Tuñón-Molina, A.; Cano-Vicent, A.; Assis, M.; Andrés, J.; Serrano-Aroca, Á. Graphene Nanoplatelets: In Vivo and In Vitro Toxicity, Cell Proliferative Activity, and Cell Gene Expression. Appl. Sci. 2022, 12, 720. [Google Scholar] [CrossRef]
- Wiegand, C.; Hipler, U.-C. Evaluation of Biocompatibility and Cytotoxicity Using Keratinocyte and Fibroblast Cultures. Ski. Pharmacol. Physiol. 2009, 22, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.L.; Vale, A.C.; Sousa, M.P.; Barbosa, A.M.; Torrado, E.; Mano, J.F.; Alves, N.M. Antibacterial Bioadhesive Layer-By-Layer Coatings for Orthopedic Applications. J. Mater. Chem. B 2016, 4, 5385–5393. [Google Scholar] [CrossRef]
- Slepička, P.; Trostová, S.; Kasálková, N.S.; Kolská, Z.; Malinský, P.; Macková, A.; Bačáková, L.; Švorčík, V. Nanostructuring of polymethylpentene by plasma and heat treatment for improved biocompatibility. Polym. Degrad. Stab. 2012, 97, 1075–1082. [Google Scholar] [CrossRef]
- Lammel, T.; Boisseaux, P.; Fernández-Cruz, M.-L.; Navas, J.M. Internalization and Cytotoxicity of Graphene Oxide and Carboxyl Graphene Nanoplatelets in the Human Hepatocellular Carcinoma Cell Line Hep G2. Part. Fibre Toxicol. 2013, 10, 27. [Google Scholar] [CrossRef]
- Slepička, P.; Peterková, L.; Rimpelová, S.; Pinkner, A.; Slepičková Kasálkova, N.; Kolská, Z.; Ruml, T.; Švorčík, V. Plasma Activation of Perfluoroethylenepropylene for Cytocompatibility Enhancement. Polym. Degrad. Stab. 2016, 130, 277–287. [Google Scholar] [CrossRef]
- Lange, K. Fundamental Role of Microvilli in the Main Functions of Differentiated Cells: Outline of an Universal Regulating and Signaling System at the Cell Periphery. J. Cell. Physiol. 2011, 226, 896–927. [Google Scholar] [CrossRef]
- Koeneman, B.A.; Zhang, Y.; Westerhoff, P.; Chen, Y.; Crittenden, J.C.; Capco, D.G. Toxicity and Cellular Responses of Intestinal Cells Exposed to Titanium Dioxide. Cell Biol. Toxicol. 2010, 26, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Mak, K.Y.; Shi, J.; Koon, H.K.; Leung, C.H.; Wong, C.M.; Leung, C.W.; Mak, C.S.K.; Chan, N.M.M.; Zhong, W.; et al. Comparative In Vitro Cytotoxicity Study on Uncoated Magnetic Nanoparticles: Effects on Cell Viability, Cell Morphology, and Cellular Uptake. J. Nanosci. Nanotechnol. 2012, 12, 9010–9017. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tenuzzo, B.; Chionna, A.; Panzarini, E.; Lanubile, R.; Tarantino, P.; di Jeso, B.; Dwikat, M.; Dini, L. Biological Effects of 6 mT Static Magnetic Fields: A Comparative Study in Different Cell Types. Bioelectromagnetics 2006, 27, 560–577. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Zhu, X.; Huang, Z.; Peng, M.; Luo, H. Incorporation of Dual Nanoplatelets to a Natural Polymer for Foldable, Robust, Bioactive, and Biocompatible Nacre-like Nanocomposites. Compos. Part B Eng. 2021, 214, 108747. [Google Scholar] [CrossRef]
- Gonzalez-Moragas, L.; Roig, A.; Laromaine, A. C. elegans as a Tool for in Vivo Nanoparticle Assessment. Adv. Colloid Interface Sci. 2015, 219, 10–26. [Google Scholar] [CrossRef]
- Seabra, A.B.; Paula, A.J.; de Lima, R.; Alves, O.L.; Durán, N. Nanotoxicity of Graphene and Graphene Oxide. Chem. Res. Toxicol. 2014, 27, 159–168. [Google Scholar] [CrossRef]
- Ou, L.; Song, B.; Liang, H.; Liu, J.; Feng, X.; Deng, B.; Sun, T.; Shao, L. Toxicity of Graphene-Family Nanoparticles: A General Review of the Origins and Mechanisms. Part. Fibre Toxicol. 2016, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Sivaselvam, S.; Mohankumar, A.; Thiruppathi, G.; Sundararaj, P.; Viswanathan, C.; Ponpandian, N. Engineering the Surface of Graphene Oxide with Bovine Serum Albumin for Improved Biocompatibility in Caenorhabditis elegans. Nanoscale Adv. 2020, 2, 5219–5230. [Google Scholar] [CrossRef]
- Jin, L.; Dou, T.-T.; Chen, J.-Y.; Duan, M.-X.; Zhen, Q.; Wu, H.-Z.; Zhao, Y.-L. Sublethal Toxicity of Graphene Oxide in Caenorhabditis elegans under Multi-Generational Exposure. Ecotoxicol. Environ. Saf. 2022, 229, 113064. [Google Scholar] [CrossRef]
- Li, P.; Xu, T.; Wu, S.; Lei, L.; He, D. Chronic Exposure to Graphene-Based Nanomaterials Induces Behavioral Deficits and Neural Damage in Caenorhabditis elegans. J. Appl. Toxicol. 2017, 37, 1140–1150. [Google Scholar] [CrossRef]
- Manjunatha, B.; Seo, E.; Park, S.H.; Kundapur, R.R.; Lee, S.J. Pristine Graphene and Graphene Oxide Induce Multi-Organ Defects in Zebrafish (Danio rerio) Larvae/Juvenile: An in Vivo Study. Environ. Sci. Pollut. Res. 2021, 28, 34664–34675. [Google Scholar] [CrossRef] [PubMed]
- Demir, E. Mechanisms and Biological Impacts of Graphene and Multi-Walled Carbon Nanotubes on Drosophila Melanogaster: Oxidative Stress, Genotoxic Damage, Phenotypic Variations, Locomotor Behavior, Parasitoid Resistance, and Cellular Immune Response. J. Appl. Toxicol. 2021, 42, 450–474. [Google Scholar] [CrossRef] [PubMed]
- Zanni, E.; De Bellis, G.; Bracciale, M.P.; Broggi, A.; Santarelli, M.L.; Sarto, M.S.; Palleschi, C.; Uccelletti, D. Graphite Nanoplatelets and Caenorhabditis elegans: Insights from an in Vivo Model. Nano Lett. 2012, 12, 2740–2744. [Google Scholar] [CrossRef] [PubMed]
- Wang, D. Confirmation of Nanomaterials with Low-Toxicity or Non-toxicity Property. In Nanotoxicology in Caenorhabditis elegans; Springer: Singapore, 2018; pp. 205–226. [Google Scholar] [CrossRef]
- Zanni, E.; Bruni, E.; Chandraiahgari, C.R.; De Bellis, G.; Santangelo, M.G.; Leone, M.; Bregnocchi, A.; Mancini, P.; Sarto, M.S.; Uccelletti, D. Evaluation of the Antibacterial Power and Biocompatibility of Zinc Oxide Nanorods Decorated Graphene Nanoplatelets: New Perspectives for Antibiodeteriorative Approaches. J. Nanobiotechnol. 2017, 15, 57. [Google Scholar] [CrossRef]
- Fakhrullina, G.; Khakimova, E.; Akhatova, F.; Lazzara, G.; Parisi, F.; Fakhrullin, R.F. Selective Antimicrobial Effects of Curcumin@Halloysite Nanoformulation: A Caenorhabditis elegans Study. ACS Appl. Mater. Interfaces 2019, 11, 23050–23064. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fanizza, C.; Stefanelli, M.; Risuglia, A.; Bruni, E.; Ietto, F.; Incoronato, F.; Marra, F.; Preziosi, A.; Mancini, P.; Sarto, M.S.; et al. In Vitro and In Vivo Biocompatibility Studies on Engineered Fabric with Graphene Nanoplatelets. Nanomaterials 2022, 12, 1405. https://doi.org/10.3390/nano12091405
Fanizza C, Stefanelli M, Risuglia A, Bruni E, Ietto F, Incoronato F, Marra F, Preziosi A, Mancini P, Sarto MS, et al. In Vitro and In Vivo Biocompatibility Studies on Engineered Fabric with Graphene Nanoplatelets. Nanomaterials. 2022; 12(9):1405. https://doi.org/10.3390/nano12091405
Chicago/Turabian StyleFanizza, Carla, Mara Stefanelli, Anna Risuglia, Erika Bruni, Federica Ietto, Federica Incoronato, Fabrizio Marra, Adele Preziosi, Patrizia Mancini, Maria Sabrina Sarto, and et al. 2022. "In Vitro and In Vivo Biocompatibility Studies on Engineered Fabric with Graphene Nanoplatelets" Nanomaterials 12, no. 9: 1405. https://doi.org/10.3390/nano12091405
APA StyleFanizza, C., Stefanelli, M., Risuglia, A., Bruni, E., Ietto, F., Incoronato, F., Marra, F., Preziosi, A., Mancini, P., Sarto, M. S., & Uccelletti, D. (2022). In Vitro and In Vivo Biocompatibility Studies on Engineered Fabric with Graphene Nanoplatelets. Nanomaterials, 12(9), 1405. https://doi.org/10.3390/nano12091405