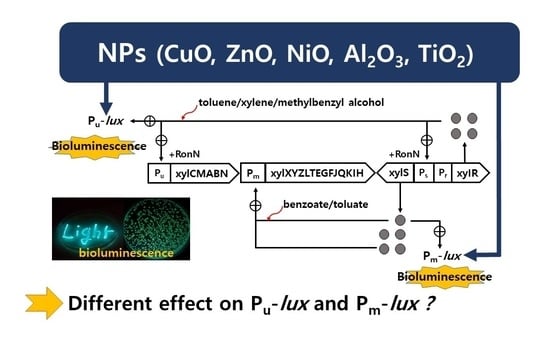
Exposure of Metal Oxide Nanoparticles on the Bioluminescence Process of Pu- and Pm-lux Recombinant P. putida mt-2 Strains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characteristics of Recombinant Strains
2.2. Culture Conditions and Bioluminescence Activity
2.3. Effects of NPs on Bioluminescence Activity and Metal Analysis
3. Results and Discussion
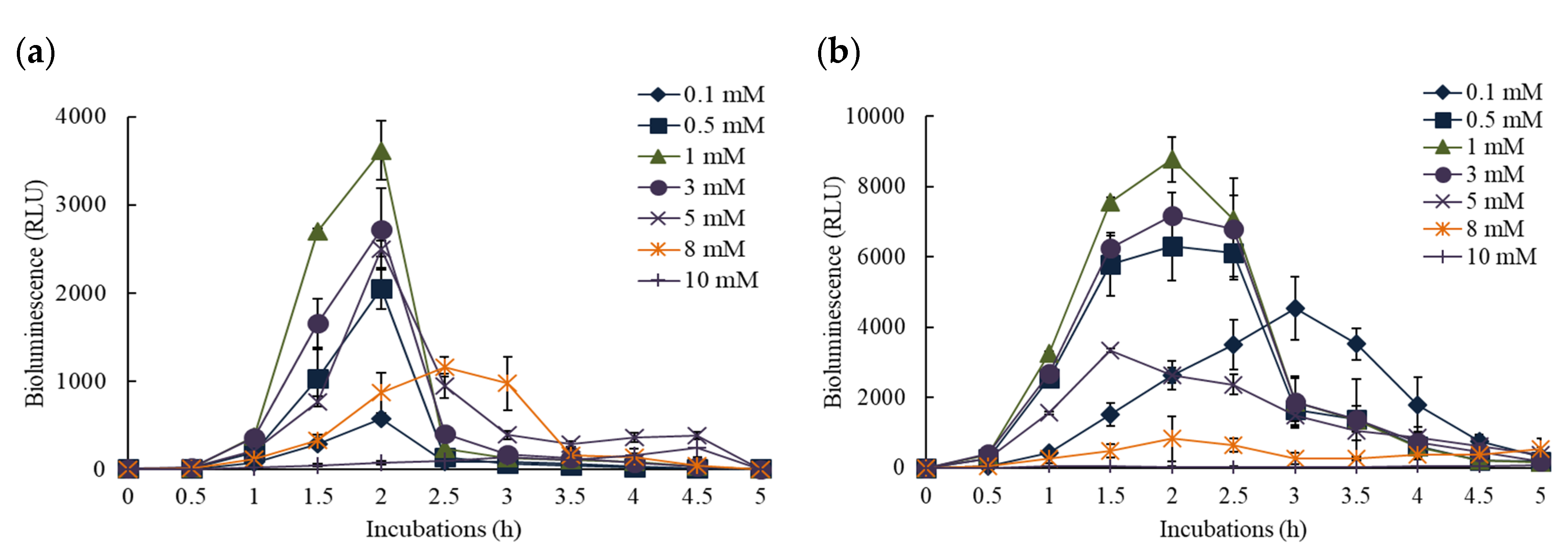
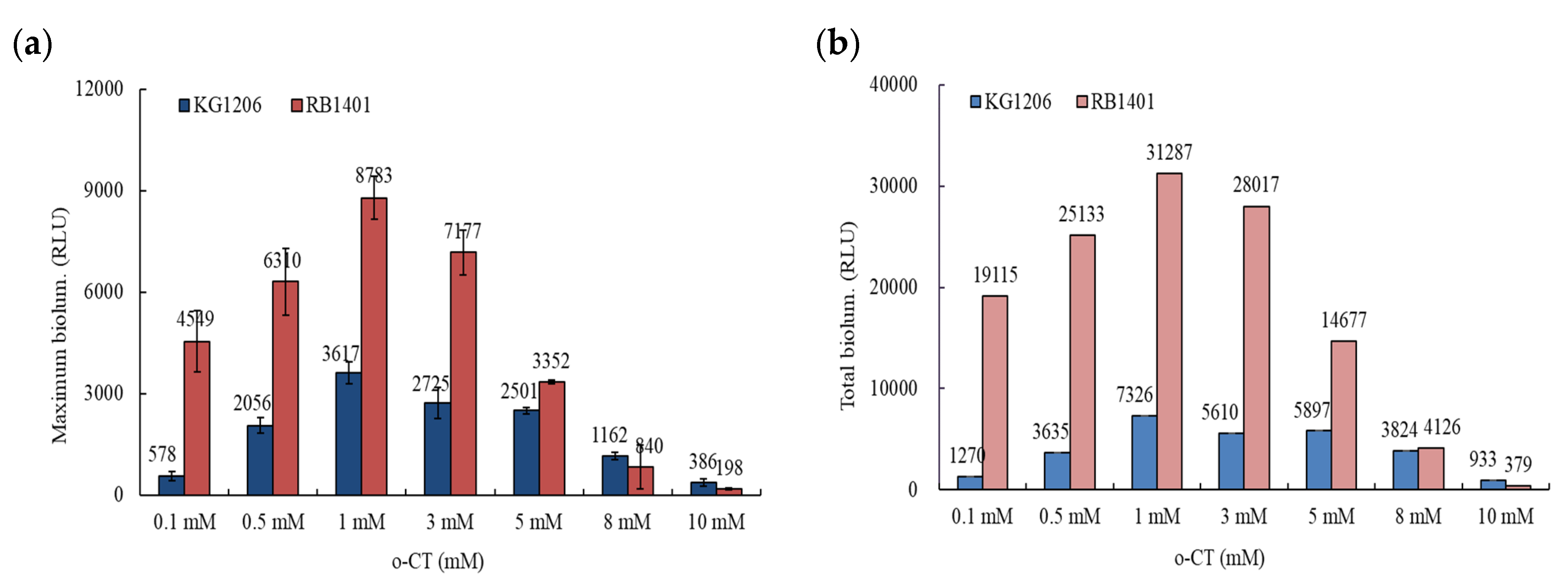
3.1. Bioluminescence Activity of Pm- and Pu-lux Gene Fusion to Inducer, o-CT
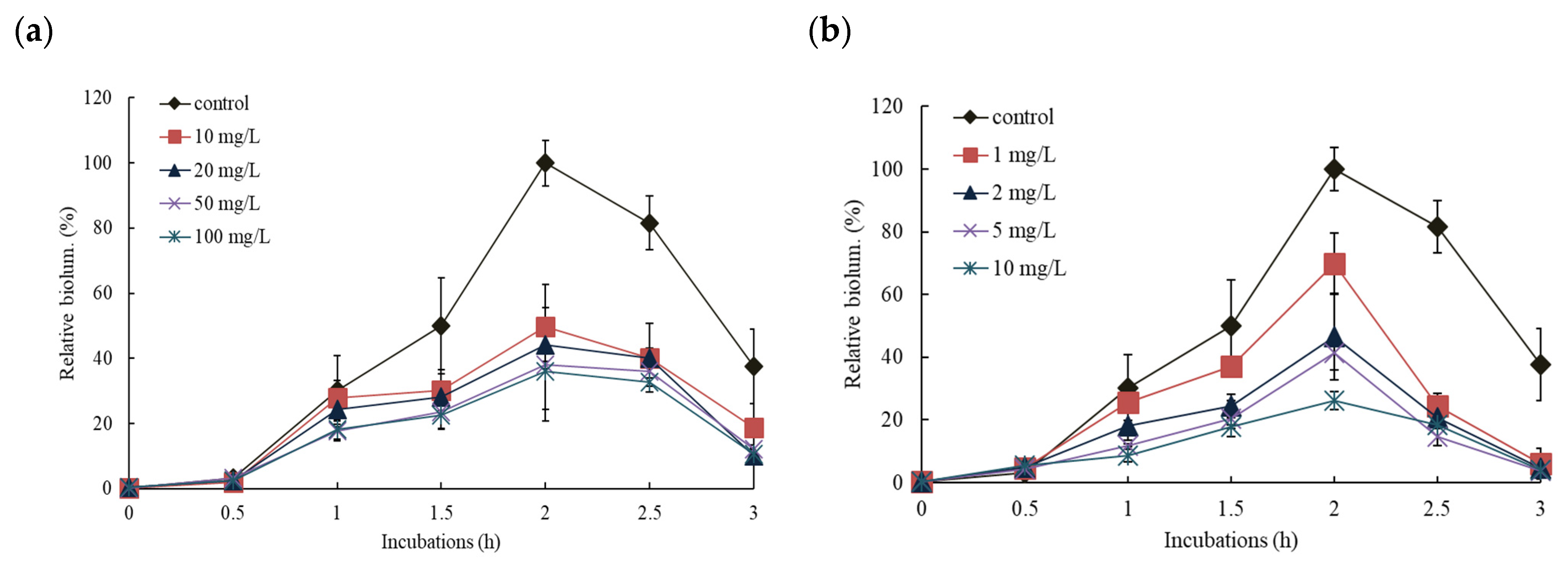
3.2. Effects of NPs on the Response of Pm-lux Gene Fusion Strain, KG1206
3.3. Effects of NPs on the Response of Pu-lux Gene Fusion Strain, RB1401
3.4. Comparisons of the Effects of NPs on the Response of Pu- and Pm-lux Gene Fusion Strains
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Onwurah, I.N.E.; Ogugua, V.N.; Onyike, N.B.; Ochonogor, A.E.; Otitoju, O.F. Crude oil spills in the environment, effects and some innovative clean-up biotechnologies. Int. J. Environ. Res. 2007, 1, 307–320. [Google Scholar]
- Varjani, S.J.; Rana, D.P.; Jain, A.K.; Bateja, S.; Upasani, V.N. Synergistic ex-situ biodegradation of crude oil by halotolerant bacterial consortium of indigenous strains isolated from on shore sites of Gujarat, India. Int. Biodeterior. Biodegrad. 2015, 103, 116–124. [Google Scholar] [CrossRef]
- Ukpaka, C.P.; Lezorghia, S.B.; Nwosu, H. Crude oil degradation in loamy soil using Neem root extracts: An experimental study. Chem. Int. 2020, 6, 160–167. [Google Scholar]
- Mirjani, M.; Soleimani, M.; Salari, V. Toxicity assessment of total petroleum hydrocarbon in aquatic environments using the bioluminescent bacterium Aliivibrio fischeri. Ecotoxicol. Environ. Saf. 2021, 207, 111554. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Gu, M.B. Toxicity biomonitoring of degradation byproducts using freeze-dried recombinant bioluminescence bacteria. Anal. Chim. Acta 2003, 481, 229–238. [Google Scholar] [CrossRef]
- Abbas, M.; Adil, M.; Ehtisham-ul-Haque, S.; Munir, B.; Yameen, M.; Ghaffar, A.; Shar, G.; Tahir, M.A.; Iqbal, M. Vibrio fischeri bioluminescence inhibition assay for ecotoxicity assessment: A review. Sci. Total Environ. 2018, 26, 1295–1309. [Google Scholar] [CrossRef]
- Iqbal, M.; Abbas, M.; Nisar, J.; Nazir, A.; Qamar, A. Bioassays based on higher plants as excellent dosimeters for ecotoxicity monitoring: A review. Chem. Int. 2019, 5, 1–80. [Google Scholar]
- Saadati, M.; Soleimani, M.; Sadeghsaba, M.; Hemami, M.R. Bioaccumulation of heavy metals (Hg, Cd and Ni) by sentinel crab (Macrophthalmus depressus) from sediments of Mousa Bay, Persian Gulf. Ecotoxicol. Environ. Saf. 2020, 191, 109986–109992. [Google Scholar] [CrossRef]
- Kong, I.C. Effects of binary mixtures of inducers (toluene analogs) and of metals on bioluminescence induction of a recombinant bioreporter strain. Sensors 2014, 14, 18993–19006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burlage, R.S. Emerging Technologies: Bioreporters, Biosensors, and Microprobes. In Manual of Environmental Microbiology, 2nd ed.; Hurst, C.J., Ed.; ASM: Washington, DC, USA, 2002; pp. 147–157. [Google Scholar]
- Ko, K.-S.; Kong, I.C. Application of the freeze-dried bioluminescence bioreporter Pseudomonas putida mt-2 KG1206 to the biomonitoring of groundwater samples from monitoring wells near gasoline leakage sites. Appl. Microbiol. Biotechnol. 2017, 101, 1709–1716. [Google Scholar] [CrossRef]
- Auffan, M.; Rose, J.; Bottero, J.Y.; Lowry, G.V.; Jolivet, J.P.; Wiesner, M.R. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat. Nanotechnol. 2009, 4, 634–641. [Google Scholar] [CrossRef]
- Lowry, G.V.; Gregory, K.B.; Apte, S.C.; Lead, J.R. Transformations of nanomaterials in the environment. Environ. Sci. Technol. 2012, 46, 6893–6899. [Google Scholar] [CrossRef]
- Thuesombat, P.; Hannongbua, S.; Akasit, S.; Chadchawan, S. Effect of silver nanoparticles on rice (Oryza sativa L. cv. KDML 105) seed germination and seedling growth. Ecotoxicol. Environ. Saf. 2014, 104, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Lines, M.G. Nanomaterials for practical funtional uses. J. Alloys Comp. 2008, 449, 242–245. [Google Scholar] [CrossRef]
- Nowack, B.; Bucheli, T.D. Occurrence, behavior and effects of nanoparticles in the environment. Environ. Pollut. 2007, 150, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Hänsch, M.; Emmerling, C. Effects of silver nanoparticles on the microbiota and enzyme activity in soil. J. Plant Nut. Soil Sci. 2010, 173, 554–558. [Google Scholar] [CrossRef]
- Wang, A.; Zhang, L.; Zhao, J.; Xing, B. Environmental processes and toxicity of metallic nanoparticles in aquatic systems as affected by natural organic matter. Environ. Sci. Nano 2016, 3, 240–255. [Google Scholar] [CrossRef]
- Misra, S.K.; Dybowska, A.; Berhanu, D.; Luoma, S.N.; Valsami, J.E. The complexity of nanoparticle dissolution and its importance in nanotoxicological studies. Sci. Total Environ. 2012, 438, 225–232. [Google Scholar] [CrossRef]
- Burlage, R.S.; Palumbo, A.V.; Heitzer, A.; Sayler, G.S. Bioluminescent reporter bacteria detect contaminants in soil samples. Appl. Biochem. Biotech. 1993, 45/46, 731–740. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Kong, I.C. An optimization of a bioassay for toluene analogs using bioluminescence reporter strain KG1206. Soil Sed. Contam. 2006, 15, 231–239. [Google Scholar] [CrossRef]
- Holtel, A.; Marques, S.; Mohler, I.; Jakubzik, U.; Timmis, K.N. Carbon source-dependent inhibition of xyl operon expression of the Pseudomonas putida TOL plasmid. J. Bacteriol. 1994, 176, 1773–1776. [Google Scholar] [CrossRef] [Green Version]
- Inouye, S.; Nakazawa, A.; Nakazawa, T. Overproduction of the XylS gene product and activation of the xylDLEGF operon on the TOL plasmid. J. Bacteriol. 1987, 169, 3587–3592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva-Rocha, R.; de Jong, H.; Tamames, J.; de Lorenzo, V. The logic layout of the TOL network of Pseudomonas putida pWW0 plasmid stems from a metabolic amplifier motif (MAM) that optimizes biodegradation of m-xylene. BMC Syst. Biol. 2011, 5, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, K.-S.; Kong, I.C. Toxic effects of nanoparticles on bioluminescence activity, seed germination, and gene mutation. Appl. Microbiol. Biotechnol. 2014, 98, 3295–3303. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.-S.; Ha, J.H.; Kong, I.C. Effects of monotypic and binary mixtures of metal oxide nanoparticles on microbial growth in sandy soil collected from artificial recharge sites. Int. J. Mol. Sci. 2015, 16, 27967–27977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, I.C.; Ko, K.S.; Koh, D.C. Comparisons of the effect of different metal oxide nanoparticles on the root and shoot growth under shaking and non-shaking incubation, different plants, and binary mixture conditions. Nanomaterials 2021, 11, 1653. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef]
- Hadjispyrou, S.; Kungolos, A.; Anagnostopoulos, A. Toxicity, bioaccumulation and interactive effects of organotin, cadmium and chromium on Artemia franciscana. Ecotox. Environ. Saf. 2001, 49, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Franklin, N.M.; Rogers, N.J.; Apte, S.C.; Batley, G.E.; Gadd, G.E.; Casey, P.S. Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): The importance of particle solubility. Environ. Sci. Technol. 2004, 41, 8484–8490. [Google Scholar] [CrossRef]
- Horvat, T.; Vidakovic, C.Ž.; Orešcanin, V.; Tkalec, M.; Pevalek, K.B. Toxicity assessment of heavy metal mixtures by Lemna minor L. Sci. Total Environ. 2007, 384, 229–238. [Google Scholar] [CrossRef]
- Heinlaan, M.; Ivask, A.; Blinova, I.; Dubourguier, H.C.; Kahru, A. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 2008, 71, 1308–1316. [Google Scholar] [CrossRef]
- Petersen, E.J.; Pinto, R.A.; Landrum, P.F.; Weber, W.J. Influence of carbon nanotubes in pyrene bioaccumulation from contaminated soils by earthworms. Environ. Sci. Technol. 2009, 43, 4181–4187. [Google Scholar] [CrossRef] [PubMed]
- Maccoramack, T.J.; Clark, R.J.; Dang, M.K.M.; Ma, G.; Kelly, J.A.; Veinot, J.G.C.; Goss, G.G. Inhibition of enzyme activity by nanomaterials: Potentials: Potential mechanisms and implications for nanotoxicity testing. Nanotoxicology 2012, 6, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, K.J.; Cho, J.W.; Jeon, S.L.; Kang, S.H. A study on comparison and analysis of chlorophyll sensor with aceton extraction for chlorophyll measurement in the Nakdong River. J. Kor. Soc. Wat. Wastewat. 2015, 29, 325–335. [Google Scholar] [CrossRef]
- Liu, Y.; Baas, J.; Peijnenburg, W.G.M.; Vijver, M.G. Evaluating the combined toxicity of Cu and ZnO nanoparticles: Utility of the concept of additivity and a nested experimental design. Environ. Sci. Technol. 2016, 50, 5328–5337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhidimea, I.D.; Saubadea, F.; Bensona, P.S.; Butlera, J.A.; Olivierb, S.; Kellyb, P.; Verrana, J.; Whiteheada, K.A. The antimicrobial effect of metal substrates on food pathogens. Food Bioprod. Process. 2019, 113, 68–76. [Google Scholar] [CrossRef]
- Brayner, R.; Ferrari-Iliou, R.; Brivois, N.; Djediat, S.; Benedetti, M.F.; Fiévet, F. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 2006, 6, 866–870. [Google Scholar] [CrossRef]
- Choi, O.; Hu, Z. Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ. Sci. Technol. 2008, 42, 4583–4588. [Google Scholar] [CrossRef]
- Priester, J.H.; Stoimenov, P.K.; Mielke, R.E.; Webb, S.M.; Ehrhardt, C.; Zhang, J.P.; Stucky, G.D.; Holden, P.A. Effects of soluble cadmium salts versus CdSe quantum dots on the growth of planktonic Pseudomonas aeruginosa. Environ. Sci. Technol. 2009, 43, 2589–2594. [Google Scholar] [CrossRef]
- Gou, N.; Onnis-Hayden, A.; Gu, A.Z. Mechanistic toxicity assessment of nanomaterials by whole-cell-array stress genes expression analysis. Environ. Sci. Technol. 2010, 44, 5964–5970. [Google Scholar] [CrossRef]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylibacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohen, R.; Nyska, A. Oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 2002, 6, 620–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D. Investigation into the antibacterial behavior of suspensions of ZnO nanoparticles (ZnO nanofluids). J. Nanopart. Res. 2007, 9, 479–489. [Google Scholar] [CrossRef]
- Dhas, S.P.; Shiny, P.J.; Khan, S.; Mukherjee, A.; Chandrasekaran, N. Toxic behavior of silver and zinc oxide nanoparticles on environmental microorganisms. J. Basic Microbiol. 2014, 54, 916–927. [Google Scholar] [CrossRef]
- Peyrot, C.; Wilkinson, K.J.; Desrosiers, M.; Sauvé, S. Effects of silver nanoaprticles on soil enzyme activities with and without added organic matter. Environ. Toxicol. Chem. 2014, 33, 115–125. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, L.; Duan, Z.; Qi, R.; Li, Y.; Lang, Y. Comparative toxicity of several metal oxide nanoparticle aqueous suspensions to Zebrafish (Danio rerio) early developmental stage. J. Environ. Sci. Health A 2008, 43, 278–284. [Google Scholar] [CrossRef]
- Lee, W.M.; An, Y.J.; Yoon, H.K.; Kweon, H.-S. Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiates) and wheat (Triticum aestivum): Plant agar test for water-insoluble nanoparticles. Environ. Toxicol. Chem. 2008, 27, 103–112. [Google Scholar] [CrossRef]
- Papis, E.; Rossi, F.; Raspanti, M.; Dalle-Donne, I.; Colombo, G.; Milzani, A.; Bernardini, G.; Gornati, R. Engineered cobalt oxide nanoparticles readily enter cells. Toxicol. Lett. 2009, 189, 253–259. [Google Scholar] [CrossRef]
- Hu, C.W.; Li, M.; Cui, Y.B.; Chen, J.; Yang, L.Y. Toxicological effects of TiO2 and ZnO nanoparticles in soil on earthworm Eisenia fetida. Soil Biol. Biochem. 2010, 42, 586–591. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Dash, S.K.; Tripathy, S.; Das, B.; Mandal, D.; Pramanik, P.; Roy, S. Toxicity of cobalt oxide nanoparticles to normal cells; an in vitro and in vivo study. Chemico-Biolog. Interact. 2015, 226, 58–71. [Google Scholar] [CrossRef]
- Lu, B.; Smith, T.; Schmid, J.J. Nanoparticle-lipid bilayer interactions studies with lipid bilayer arrays. Nanoscale 2015, 7, 7858–7866. [Google Scholar] [CrossRef] [PubMed]
- Bossi, E.; Zanella, D.; Gornati, R.; Nernardini, G. Cobalt oxide nanoparticles can enter inside the cells by crossing plasma membranes. Sci. Rep. 2016, 6, 22254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoroddu, M.A.; Medici, S.; Ledda, A.; Nurchi, V.M.; Lachowicz, J.I.; Peana, M. Toxicity of nanoparticles. Curr. Med. Chem. 2014, 21, 3837–3853. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Harper, B.J.; Harper, S.L. Differential dissolution and toxicity of surface functionalized silver nanoparticles in small-scale microcosms: Impacts of community complexity. Environ. Sci. Nano 2017, 4, 359–372. [Google Scholar] [CrossRef]
- Al-Bairuty, G.A.; Boyle, D.; Henry, T.B.; Handy, R.D. Sublethal effects of copper sulphate compared to copper nanoparticles in rainbow trout (Oncorhynchus mykiss) at low pH: Physiology and metal accumulation. Aquat. Toxicol. 2016, 174, 188–198. [Google Scholar] [CrossRef]
- Razmara, P.; Lari, E.; Mohaddes, E.; Zhang, Y.G.; Goss, G.; Pyle, G.G. The effect of copper nanoparticles on olfaction in rainbow trout (Oncorhynchus mykiss). Environ. Sci. Nano 2019, 6, 2094–2104. [Google Scholar] [CrossRef]
- Vandenbrouck, T.; Soetaert, A.; van der Ven, K.; Blust, R.; de Coem, W. Nickel and binary metal mixture responses in Daphnia magna: Molecular fingerprints and (sub)organismal effects. Aquat. Toxicol. 2009, 92, 18–29. [Google Scholar] [CrossRef] [PubMed]



| Strains | EC50 (mg/L) | ||||
|---|---|---|---|---|---|
| ZnO NP | CuO NP | NiO NP | Al2O3 NP | TiO2 NP | |
| KG1206 | 0.25 a (0.15–0.34) b | 26.8 (22.16–32.47) | 0.47 (0.38–0.59) | 0.68 (0.52–0.89) | 1.57 (0.97–2.52) |
| RB1401 | 0.42 (0.11–1.54) | 46.4 (14.35–80.12) | 2.92 (1.76–4.84) | 1.58 (1.12–2.22) | 3.60 (2.12–6.12) |
| Methods | EC50 (mg/L) | Reference | ||||
|---|---|---|---|---|---|---|
| ZnO | CuO | NiO | TiO2 | |||
| Bioluminescence activity | 1.05 a | 54 | 198 | >1000 | [26] | |
| Seed germination | Lactuca | 11 | 0.46 | 17 | >1000 | |
| Raphanus | 46 | 26 | 114 | >1000 | ||
| ATP contents | 11 | 55 | 87 | 530 | [27] | |
| Lactuca root/shoot growth | 0.50/>2 | 0.25/>1 | 0.57/>5 | ND | [28] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, I.C.; Ko, K.-S.; Lee, S.; Koh, D.-C.; Burlage, R. Exposure of Metal Oxide Nanoparticles on the Bioluminescence Process of Pu- and Pm-lux Recombinant P. putida mt-2 Strains. Nanomaterials 2021, 11, 2822. https://doi.org/10.3390/nano11112822
Kong IC, Ko K-S, Lee S, Koh D-C, Burlage R. Exposure of Metal Oxide Nanoparticles on the Bioluminescence Process of Pu- and Pm-lux Recombinant P. putida mt-2 Strains. Nanomaterials. 2021; 11(11):2822. https://doi.org/10.3390/nano11112822
Chicago/Turabian StyleKong, In Chul, Kyung-Seok Ko, Sohyeon Lee, Dong-Chan Koh, and Robert Burlage. 2021. "Exposure of Metal Oxide Nanoparticles on the Bioluminescence Process of Pu- and Pm-lux Recombinant P. putida mt-2 Strains" Nanomaterials 11, no. 11: 2822. https://doi.org/10.3390/nano11112822
APA StyleKong, I. C., Ko, K.-S., Lee, S., Koh, D.-C., & Burlage, R. (2021). Exposure of Metal Oxide Nanoparticles on the Bioluminescence Process of Pu- and Pm-lux Recombinant P. putida mt-2 Strains. Nanomaterials, 11(11), 2822. https://doi.org/10.3390/nano11112822