Bioactive Keratin and Fibroin Nanoparticles: An Overview of Their Preparation Strategies
Abstract
1. Introduction
2. Synthesis of Keratin and Silk Fibroin Nanoparticles
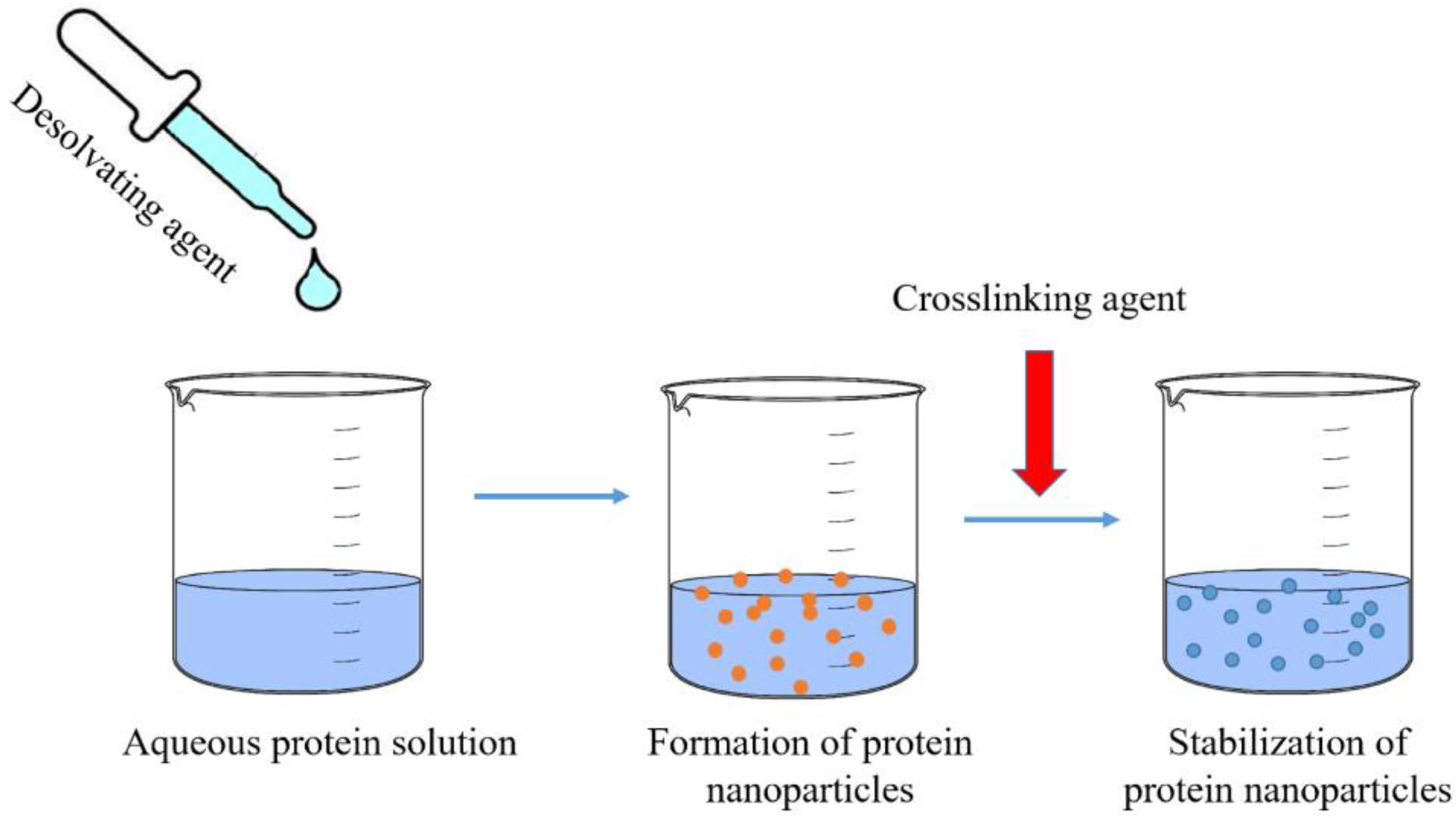
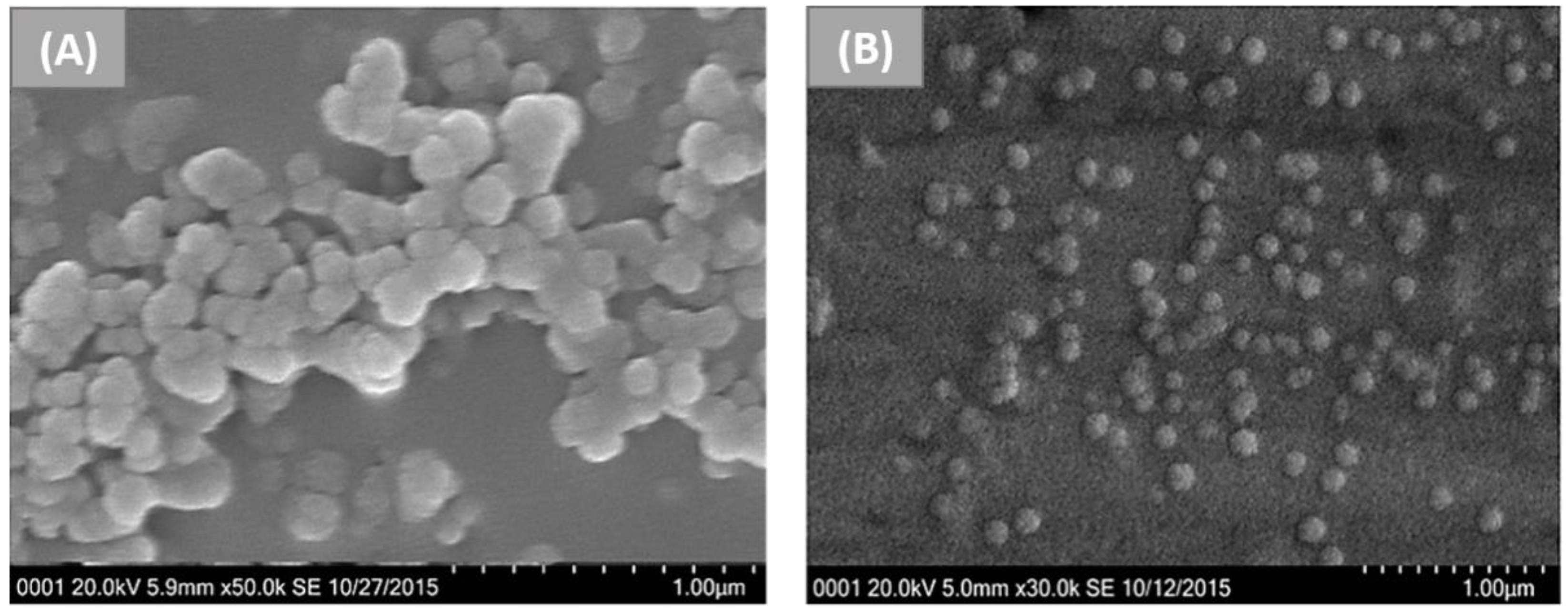
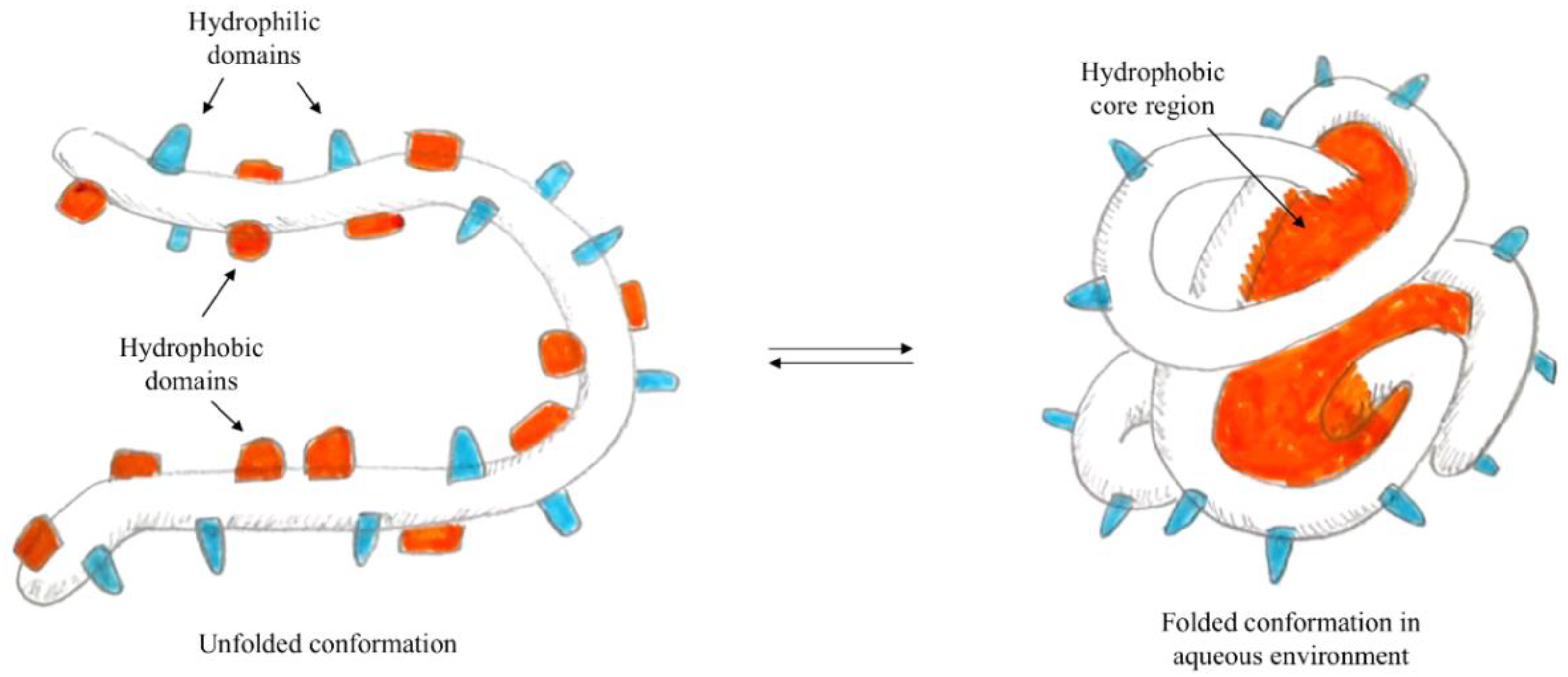
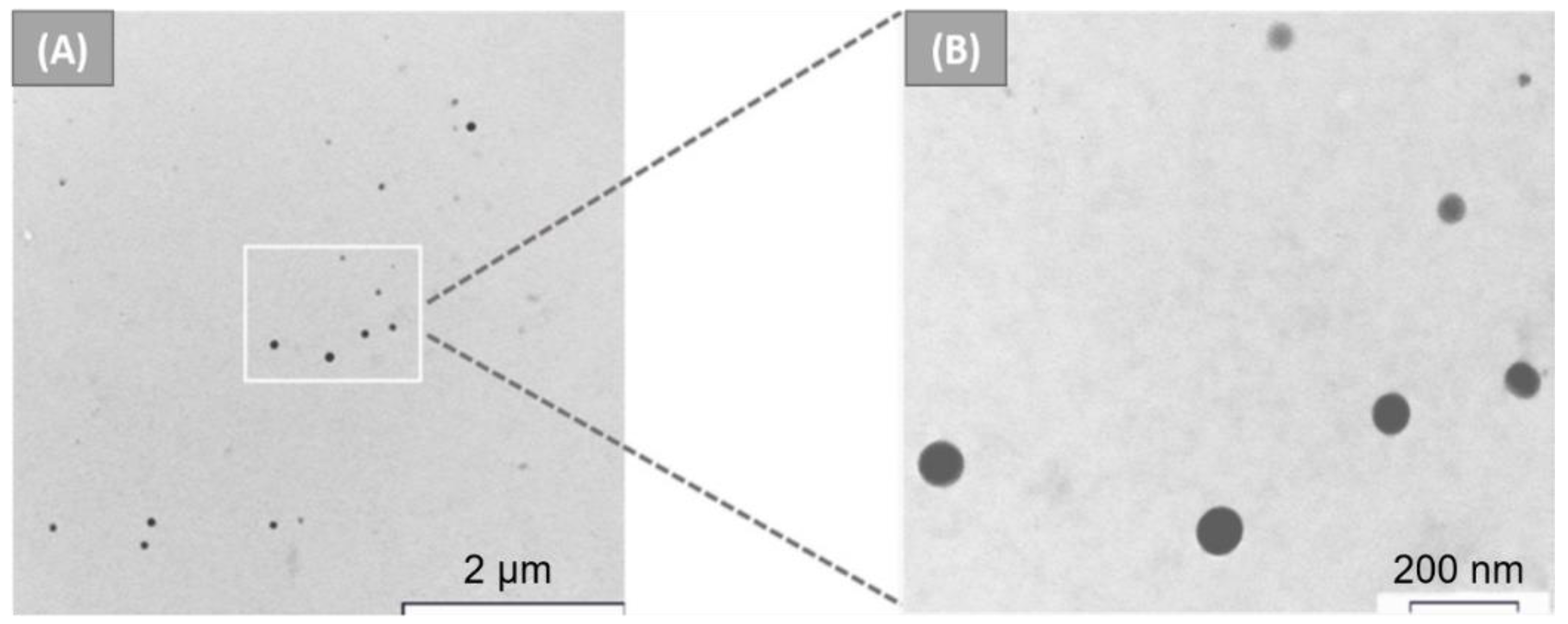
2.1. Desolvation
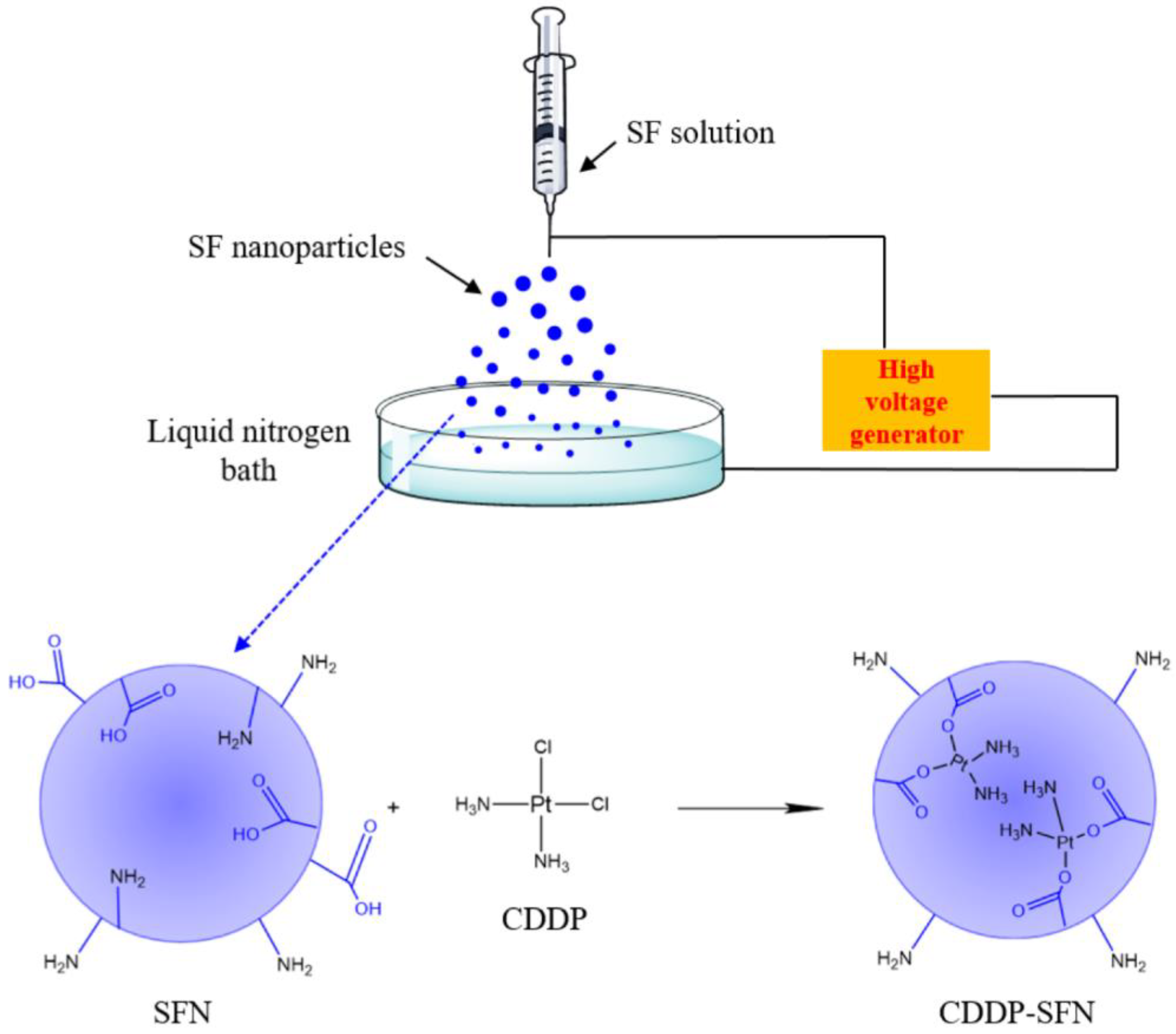
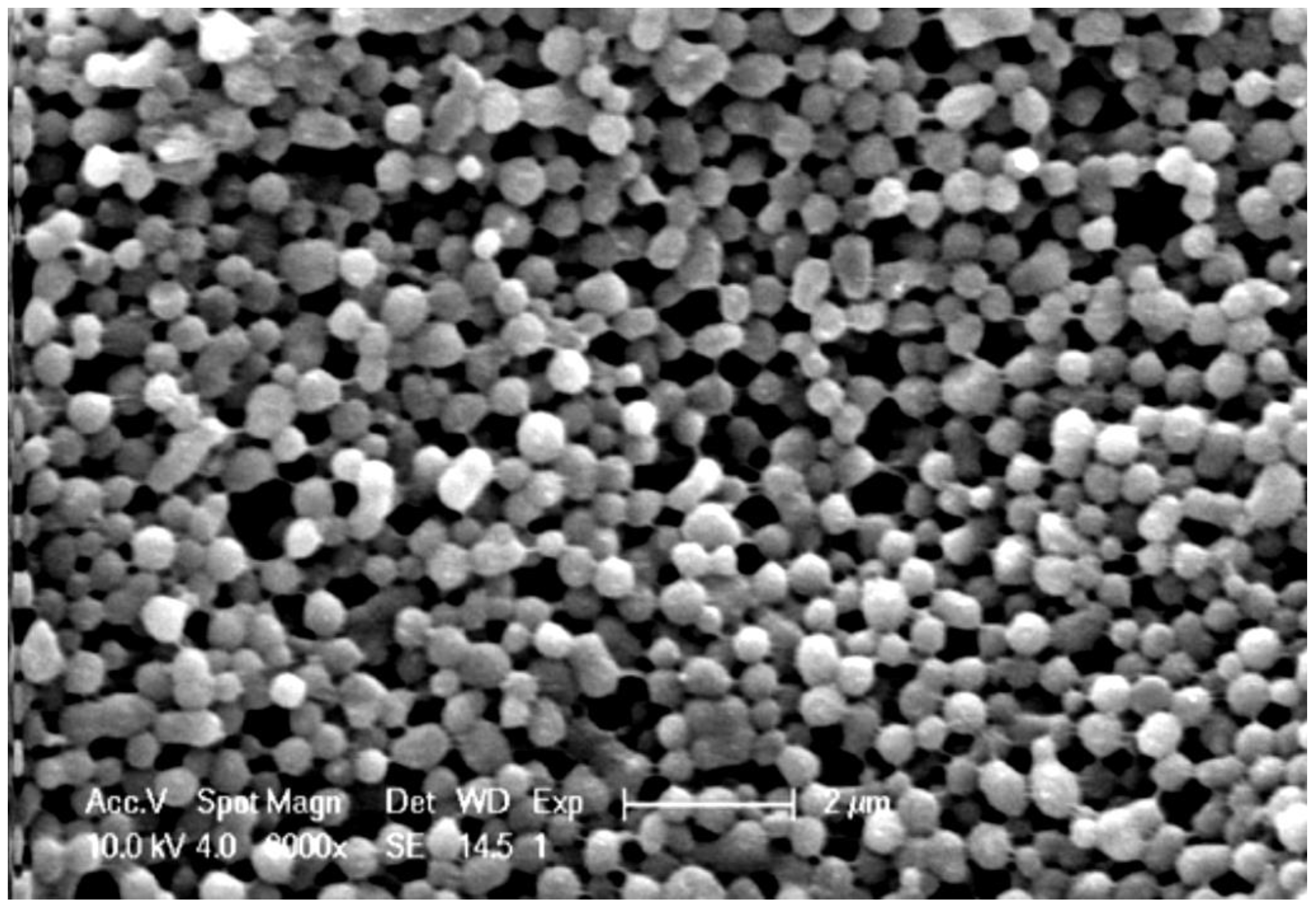
2.2. Electrospraying
- -
- Electrospraying in solution: the electrosprayed polymer solution is dropped into an immiscible solution, which contains a crosslinking agent, in order to obtain stable particles [59];
- -
- -
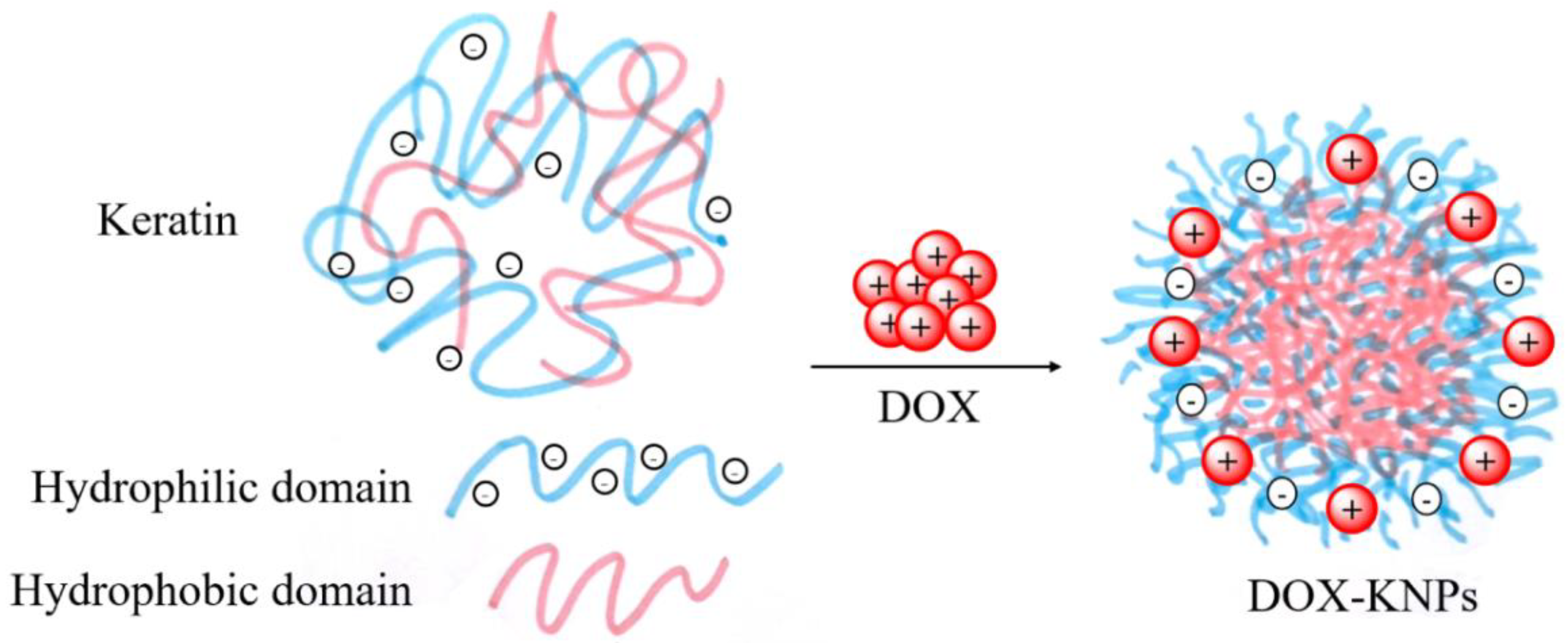
2.3. Self-Assembly and/or Aggregation
- -
- Static: occurs in the absence of external factors, and is determined by the minimisation of energy;
- -
- Dynamic: the process is influenced by external factors.
2.4. Microemulsion
2.5. Salting Out
2.6. Ionic Gelation
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Strebhardt, K.; Ullrich, A. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat. Rev. Cancer 2008, 8, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Crucho, C.I.C.; Barros, M.T. Polymeric nanoparticles: A study on the preparation variables and characterization methods. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 80, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Kemp, J.A.; Shim, M.S.; Heo, C.Y.; Kwon, Y.J. “Combo” nanomedicine: Co-delivery of multi-modal therapeutics for efficient, targeted, and safe cancer therapy. Adv. Drug Deliv. Rev. 2016, 98, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.H.; Harford, J.B.; Eaton, M.A.W.; Boisseau, P.M.; Dube, A.; Hayeshi, R.; Swai, H.; Lee, D.S. Nanomedicine: Past, present and future—A global perspective. Biochem. Biophys. Res. Commun. 2015, 468, 511–517. [Google Scholar] [CrossRef]
- Masood, F. Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater. Sci. Eng. C 2016, 60, 569–578. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.T.; Saelim, N.; Tiyaboonchai, W. Alpha mangostin loaded crosslinked silk fibroin-based nanoparticles for cancer chemotherapy. Colloids Surf. B Biointerfaces 2019, 181, 705–713. [Google Scholar] [CrossRef]
- Xu, H.; Shi, Z.; Reddy, N.; Yang, Y. Intrinsically Water-Stable Keratin Nanoparticles and Their in Vivo Biodistribution for Targeted Delivery. J. Agric. Food Chem. 2014, 62, 9145–9150. [Google Scholar] [CrossRef]
- Jahanshahi, M.; Babaei, Z. Protein nanoparticle: A unique system as drug delivery vehicles. Afr. J. Biotechnol. 2008, 7, 4926–4934. [Google Scholar] [CrossRef]
- Zhang, Y.; Chan, H.F.; Leong, K.W. Advanced materials and processing for drug delivery: The past and the future. Adv. Drug Deliv. Rev. 2013, 65, 104–120. [Google Scholar] [CrossRef]
- Pham, D.T.; Tiyaboonchai, W. Fibroin nanoparticles: A promising drug delivery system. Drug Deliv. 2020, 27, 431–448. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Long-circulating and target-specific nanoparticles: Theory to practice. Pharmacol. Rev. 2001, 53, 283–318. [Google Scholar] [PubMed]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Albumin-based nanoparticles as potential controlled release drug delivery systems. J. Control. Release 2012, 157, 168–182. [Google Scholar] [CrossRef] [PubMed]
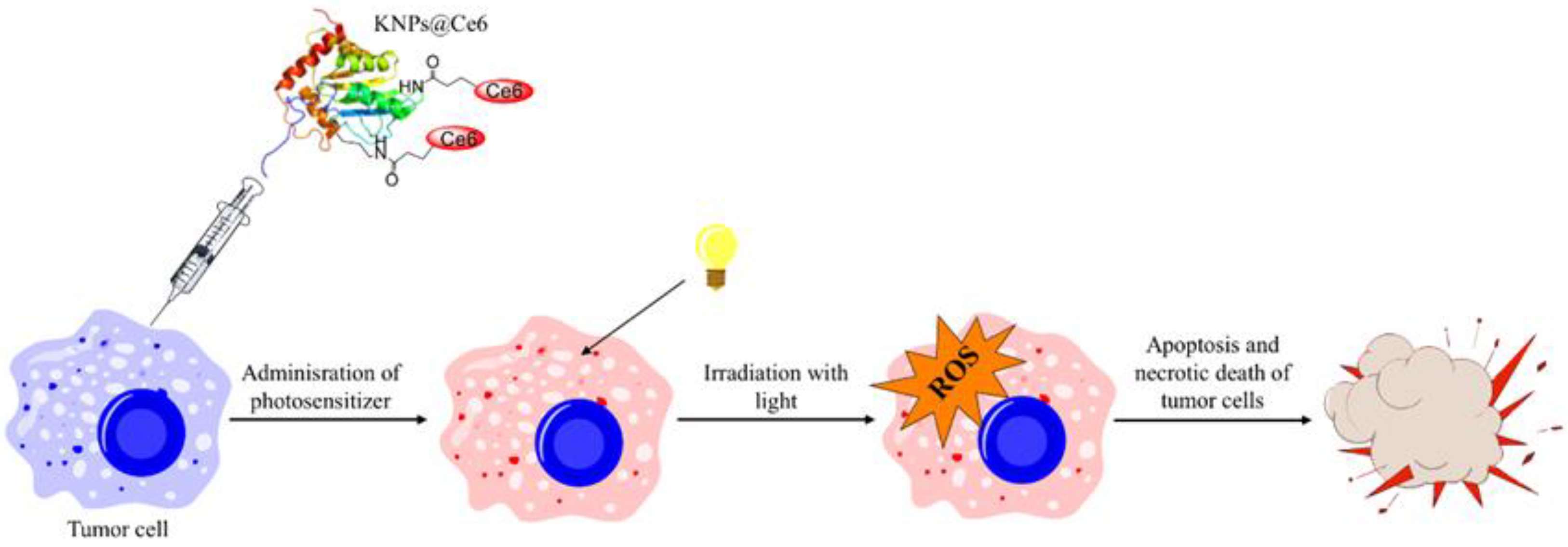
- Aluigi, A.; Sotgiu, G.; Ferroni, C.; Duchi, S.; Lucarelli, E.; Martini, C.; Posati, T.; Guerrini, A.; Ballestri, M.; Corticelli, F.; et al. Chlorin e6 keratin nanoparticles for photodynamic anticancer therapy. RSC Adv. 2016, 6, 33910–33918. [Google Scholar] [CrossRef]
- Lohcharoenkal, W.; Wang, L.; Chen, Y.C.; Rojanasakul, Y. Protein Nanoparticles as Drug Delivery Carriers for Cancer Therapy. Biomed Res. Int. 2014, 2014, 180549. [Google Scholar] [CrossRef] [PubMed]
- Halwani, A.A. Development of Pharmaceutical Nanomedicines: From the Bench to the Market. Pharmaceutics 2022, 14, 106. [Google Scholar] [CrossRef]
- Chilakamarry, C.R.; Mahmood, S.; Saffe, S.N.B.M.; Arifin, M.A.B.; Gupta, A.; Sikkandar, M.Y.; Begum, S.S.; Narasaiah, B. Extraction and application of keratin from natural resources: A review. 3 Biotech 2021, 11, 220. [Google Scholar] [CrossRef]
- Zhi, X.; Wang, Y.; Li, P.; Yuan, J.; Shen, J. Preparation of keratin/chlorhexidine complex nanoparticles for long-term and dual stimuli-responsive release. RSC Adv. 2015, 5, 82334–82341. [Google Scholar] [CrossRef]
- Xu, H.; Cai, S.; Xu, L.; Yang, Y. Water-Stable Three-Dimensional Ultrafine Fibrous Scaffolds from Keratin for Cartilage Tissue Engineering. Langmuir 2014, 30, 8461–8470. [Google Scholar] [CrossRef]
- Numata, K.; Kaplan, D.L. Silk-based delivery systems of bioactive molecules. Adv. Drug Deliv. Rev. 2010, 62, 1497–1508. [Google Scholar] [CrossRef]
- Sun, W.; Gregory, D.A.; Tomeh, M.A.; Zhao, X. Silk Fibroin as a Functional Biomaterial for Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 1499. [Google Scholar] [CrossRef] [PubMed]
- Lujerdean, C.; Baci, G.-M.; Cucu, A.-A.; Dezmirean, D.S. The Contribution of Silk Fibroin in Biomedical Engineering. Insects 2022, 13, 286. [Google Scholar] [CrossRef]
- Jastrzebska, K.; Kucharczyk, K.; Florczak, A.; Dondajewska, E.; Mackiewicz, A.; Dams-Kozlowska, H. Silk as an innovative biomaterial for cancer therapy. Rep. Pract. Oncol. Radiother. 2015, 20, 87–98. [Google Scholar] [CrossRef]
- Jastrzebska, K.; Florczak, A.; Kucharczyk, K.; Lin, Y.; Wang, Q.; Mackiewicz, A.; Kaplan, D.L.; Dams-Kozlowska, H. Delivery of chemotherapeutics using spheres made of bioengineered spider silks derived from MaSp1 and MaSp2 proteins. Nanomedicine 2018, 13, 439–454. [Google Scholar] [CrossRef]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef]
- Preda, R.C.; Leisk, G.; Omenetto, F.; Kaplan, D.L. Bioengineered silk proteins to control cell and tissue functions. Methods Mol. Biol. 2013, 996, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Posati, T.; Benfenati, V.; Sagnella, A.; Pistone, A.; Nocchetti, M.; Donnadio, A.; Ruani, G.; Zamboni, R.; Muccini, M. Innovative multifunctional silk fibroin and hydrotalcite nanocomposites: A synergic effect of the components. Biomacromolecules 2014, 15, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Posati, T.; Aluigi, A.; Donnadio, A.; Sotgiu, G.; Mosconi, M.; Muccini, M.; Ruani, G.; Zamboni, R.; Seri, M. Keratin Film as Natural and Eco-Friendly Support for Organic Optoelectronic Devices. Adv. Sustain. Syst. 2019, 3, 1900080. [Google Scholar] [CrossRef]
- Giannelli, M.; Barbalinardo, M.; Riminucci, A.; Belvedere, K.; Boccalon, E.; Sotgiu, G.; Corticelli, F.; Ruani, G.; Zamboni, R.; Aluigi, A.; et al. Magnetic keratin/hydrotalcites sponges as potential scaffolds for tissue regeneration. Appl. Clay Sci. 2021, 207, 106090. [Google Scholar] [CrossRef]
- Giuri, D.; Barbalinardo, M.; Sotgiu, G.; Zamboni, R.; Nocchetti, M.; Donnadio, A.; Corticelli, F.; Valle, F.; Gennari, C.G.M.; Selmin, F.; et al. Nano-hybrid electrospun non-woven mats made of wool keratin and hydrotalcites as potential bio-active wound dressings. Nanoscale 2019, 11, 6422–6430. [Google Scholar] [CrossRef]
- Cavallaro, G.; Milioto, S.; Konnova, S.; Fakhrullina, G.; Akhatova, F.; Lazzara, G.; Fakhrullin, R.; Lvov, Y. Halloysite/Keratin Nanocomposite for Human Hair Photoprotection Coating. ACS Appl. Mater. Interfaces 2020, 12, 24348–24362. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Chen, X.; Zhai, D.; Gao, F.; Guo, T.; Li, W.; Hao, S.; Ji, J.; Wang, B. Development of keratin nanoparticles for controlled gastric mucoadhesion and drug release. J. Nanobiotechnol. 2018, 16, 24. [Google Scholar] [CrossRef] [PubMed]
- DeFrates, K.; Moore, R.; Borgesi, J.; Lin, G.; Mulderig, T.; Beachley, V.; Hu, X. Protein-Based Fiber Materials in Medicine: A Review. Nanomaterials 2018, 8, 457. [Google Scholar] [CrossRef]
- Zhong, T.; Jiang, Z.; Wang, P.; Bie, S.; Zhang, F.; Zuo, B. Silk fibroin/copolymer composite hydrogels for the controlled and sustained release of hydrophobic/hydrophilic drugs. Int. J. Pharm. 2015, 494, 264–270. [Google Scholar] [CrossRef]
- Wongpinyochit, T.; Uhlmann, P.; Urquhart, A.J.; Seib, F.P. PEGylated Silk Nanoparticles for Anticancer Drug Delivery. Biomacromolecules 2015, 16, 3712–3722. [Google Scholar] [CrossRef] [PubMed]
- Nasir, A.; Kausar, A.; Younus, A. A Review on Preparation, Properties and Applications of Polymeric Nanoparticle-Based Materials. Polym. Plast. Technol. Eng. 2015, 54, 325–341. [Google Scholar] [CrossRef]
- Castro, K.C.D.; Costa, J.M.; Campos, M.G.N. Drug-loaded polymeric nanoparticles: A review. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 1–13. [Google Scholar] [CrossRef]
- Ko, S.; Gunasekaran, S. Preparation of sub-100-nm beta-lactoglobulin (BLG) nanoparticles. J. Microencapsul. 2006, 23, 887–898. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Xie, M.-B. Silk fibroin-based nanoparticles for drug delivery. Int. J. Mol. Sci. 2015, 16, 4880–4903. [Google Scholar] [CrossRef]
- Langer, K.; Balthasar, S.; Vogel, V.; Dinauer, N.; von Briesen, H.; Schubert, D. Optimization of the preparation process for human serum albumin (HSA) nanoparticles. Int. J. Pharm. 2003, 257, 169–180. [Google Scholar] [CrossRef]
- Li, Y.; Zhi, X.; Lin, J.; You, X.; Yuan, J. Preparation and characterization of DOX loaded keratin nanoparticles for pH/GSH dual responsive release. Mater. Sci. Eng. C 2017, 73, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Uzdensky, A.B. The biophysical aspects of photodynamic therapy. Biophysics 2016, 61, 461–469. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Buytaert, E.; Dewaele, M.; Agostinis, P. Molecular effectors of multiple cell death pathways initiated by photodynamic therapy. Biochim. Biophys. Acta 2007, 1776, 86–107. [Google Scholar] [CrossRef]
- Robertson, C.A.; Evans, D.H.; Abrahamse, H. Photodynamic therapy (PDT): A short review on cellular mechanisms and cancer research applications for PDT. J. Photochem. Photobiol. B Biol. 2009, 96, 1–8. [Google Scholar] [CrossRef]
- Ash, C.; Dubec, M.; Donne, K.; Bashford, T. Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers Med. Sci. 2017, 32, 1909–1918. [Google Scholar] [CrossRef]
- Boyle, R.W.; Dolphin, D. Structure and biodistribution relationships of photodynamic sensitizers. Photochem. Photobiol. 1996, 64, 469–485. [Google Scholar] [CrossRef]
- Duchi, S.; Sotgiu, G.; Lucarelli, E.; Ballestri, M.; Dozza, B.; Santi, S.; Guerrini, A.; Dambruoso, P.; Giannini, S.; Donati, D.; et al. Mesenchymal stem cells as delivery vehicle of porphyrin loaded nanoparticles: Effective photoinduced in vitro killing of osteosarcoma. J. Control. Release 2013, 168, 225–237. [Google Scholar] [CrossRef]
- Martella, E.; Dozza, B.; Ferroni, C.; Obeyok, C.O.; Guerrini, A.; Tedesco, D.; Manet, I.; Sotgiu, G.; Columbaro, M.; Ballestri, M.; et al. Two Beats One: Osteosarcoma Therapy with Light-Activated and Chemo-Releasing Keratin Nanoformulation in a Preclinical Mouse Model. Pharmaceutics 2022, 14, 677. [Google Scholar] [CrossRef]
- Duchi, S.; Ramos-Romero, S.; Dozza, B.; Guerra-Rebollo, M.; Cattini, L.; Ballestri, M.; Dambruoso, P.; Guerrini, A.; Sotgiu, G.; Varchi, G.; et al. Development of near-infrared photoactivable phthalocyanine-loaded nanoparticles to kill tumor cells: An improved tool for photodynamic therapy of solid cancers. Nanomedicine 2016, 12, 1885–1897. [Google Scholar] [CrossRef]
- Aluigi, A.; Tonetti, C.; Vineis, C.; Tonin, C.; Casasola, R.; Ferrero, F. Wool keratin nanofibres for copper(II) adsorption. J. Biobased Mater. Bioenergy 2012, 6, 230–236. [Google Scholar] [CrossRef]
- Ferroni, C.; Varchi, G. Keratin-Based Nanoparticles as Drug Delivery Carriers. Appl. Sci. 2021, 11, 9417. [Google Scholar] [CrossRef]
- Kundu, J.; Chung, Y.-I.; Kim, Y.H.; Tae, G.; Kundu, S.C. Silk fibroin nanoparticles for cellular uptake and control release. Int. J. Pharm. 2010, 388, 242–250. [Google Scholar] [CrossRef]
- Jin, H.J.; Park, J.; Karageorgiou, V.; Kim, U.J.; Valluzzi, R.; Cebe, P.; Kaplan, D.L. Water-stable silk films with reduced β-sheet content. Adv. Funct. Mater. 2005, 15, 1241–1247. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Shen, W.-D.; Xiang, R.-L.; Zhuge, L.-J.; Gao, W.-J.; Wang, W.-B. Formation of silk fibroin nanoparticles in water-miscible organic solvent and their characterization. J. Nanoparticle Res. 2007, 9, 885–900. [Google Scholar] [CrossRef]
- Jaworek, A.; Sobczyk, A.T. Electrospraying route to nanotechnology: An overview. J. Electrostat. 2008, 66, 197–219. [Google Scholar] [CrossRef]
- Bock, N.; Dargaville, T.R.; Woodruff, M.A. Electrospraying of polymers with therapeutic molecules: State of the art. Prog. Polym. Sci. 2012, 37, 1510–1551. [Google Scholar] [CrossRef]
- Tapia-Hernández, J.A.; Rodríguez-Félix, F.; Katouzian, I. 9-Nanocapsule formation by electrospraying. In Nanoencapsulation Technologies for the Food and Nutraceutical Industries; Jafari, S.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 320–345. ISBN 978-0-12-809436-5. [Google Scholar]
- Chen, H.; Talaty, N.N.; Takáts, Z.; Cooks, R.G. Desorption Electrospray Ionization Mass Spectrometry for High-Throughput Analysis of Pharmaceutical Samples in the Ambient Environment. Anal. Chem. 2005, 77, 6915–6927. [Google Scholar] [CrossRef]
- Ghayempour, S.; Mortazavi, S.M. Fabrication of micro–nanocapsules by a new electrospraying method using coaxial jets and examination of effective parameters on their production. J. Electrostat. 2013, 71, 717–727. [Google Scholar] [CrossRef]
- Casula, G.; Cosseddu, P.; Busby, Y.; Pireaux, J.-J.; Rosowski, M.; Tkacz Szczesna, B.; Soliwoda, K.; Celichowski, G.; Grobelny, J.; Novák, J.; et al. Air-stable, non-volatile resistive memory based on hybrid organic/inorganic nanocomposites. Org. Electron. 2015, 18, 17–23. [Google Scholar] [CrossRef]
- Zubair, M.; Mustafa, M.; Lee, K.; Yoon, C.; Doh, Y.H.; Choi, K.H. Fabrication of CdSe/ZnS quantum dots thin film by electrohydrodynamics atomization technique for solution based flexible hybrid OLED application. Chem. Eng. J. 2014, 253, 325–331. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, G.; Lee, J.; Lee, K. Morphology controlled bulk-heterojunction layers of fully electro-spray coated organic solar cells. Sol. Energy Mater. Sol. Cells 2012, 105, 272–279. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Gavara, R.; Hernández-Muñoz, P. Encapsulation of curcumin in electrosprayed gelatin microspheres enhances its bioaccessibility and widens its uses in food applications. Innov. Food Sci. Emerg. Technol. 2015, 29, 302–307. [Google Scholar] [CrossRef]
- López-Rubio, A.; Lagaron, J.M. Whey protein capsules obtained through electrospraying for the encapsulation of bioactives. Innov. Food Sci. Emerg. Technol. 2012, 13, 200–206. [Google Scholar] [CrossRef]
- Asadi, M.; Salami, M.; Hajikhani, M.; Emam-Djomeh, Z.; Aghakhani, A.; Ghasemi, A. Electrospray Production of Curcumin-walnut Protein Nanoparticles. Food Biophys. 2021, 16, 15–26. [Google Scholar] [CrossRef]
- Ebrahimgol, F.; Tavanai, H.; Alihosseini, F.; Khayamian, T. Electrosprayed recovered wool keratin nanoparticles. Polym. Adv. Technol. 2014, 25, 1001–1007. [Google Scholar] [CrossRef]
- Schrooyen, P.M.M.; Dijkstra, P.J.; Oberthür, R.C.; Bantjes, A.; Feijen, J. Stabilization of Solutions of Feather Keratins by Sodium Dodecyl Sulfate. J. Colloid Interface Sci. 2001, 240, 30–39. [Google Scholar] [CrossRef]
- Guo, T.; Yang, X.; Deng, J.; Zhu, L.; Wang, B.; Hao, S. Keratin nanoparticles-coating electrospun PVA nanofibers for potential neural tissue applications. J. Mater. Sci. Mater. Med. 2019, 30, 9. [Google Scholar] [CrossRef]
- Hill, P.; Brantley, H.; Van Dyke, M. Some properties of keratin biomaterials: Kerateines. Biomaterials 2010, 31, 585–593. [Google Scholar] [CrossRef]
- Wang, J.; Hao, S.; Luo, T.; Zhou, T.; Yang, X.; Wang, B. Keratose/poly (vinyl alcohol) blended nanofibers: Fabrication and biocompatibility assessment. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 72, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.D.; Mututuvari, T.M. Cellulose, Chitosan and Keratin Composite Materials: Facile and Recyclable Synthesis, Conformation and Properties. ACS Sustain. Chem. Eng. 2016, 4, 1850–1861. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Shibayama, M.; Tanabe, T.; Yamauchi, K. Preparation and Properties of Keratin-Poly(vinyl alcohol) Blend Fiber. J. Appl. Polym. Sci. 2004, 91, 756–762. [Google Scholar] [CrossRef]
- Qu, J.; Liu, Y.; Yu, Y.; Li, J.; Luo, J.; Li, M. Silk fibroin nanoparticles prepared by electrospray as controlled release carriers of cisplatin. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 44, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xia, Z.R.; Lv, L.L.; Tang, Q.; Li, M.Z. Preparation of Silk Fibroin Microspheres. Adv. Mater. Res. 2011, 236–238, 1902–1905. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, F.; Chen, Y.; Yu, T.; Lou, D.; Guo, Y.; Li, P.; Wang, Z.; Ran, H. Drug release from core-shell PVA/silk fibroin nanoparticles fabricated by one-step electrospraying. Sci. Rep. 2017, 7, 11913. [Google Scholar] [CrossRef]
- Ozin, G.A.; Hou, K.; Lotsch, B.V.; Cademartiri, L.; Puzzo, D.P.; Scotognella, F.; Ghadimi, A.; Thomson, J. Nanofabrication by self-assembly. Mater. Today 2009, 12, 12–23. [Google Scholar] [CrossRef]
- Grzybowski, B.A.; Wilmer, C.E.; Kim, J.; Browne, K.P.; Bishop, K.J.M. Self-assembly: From crystals to cells. Soft Matter 2009, 5, 1110–1128. [Google Scholar] [CrossRef]
- Mattia, E.; Otto, S. Supramolecular systems chemistry. Nat. Nanotechnol. 2015, 10, 111–119. [Google Scholar] [CrossRef]
- Mendes, A.C.; Baran, E.T.; Reis, R.L.; Azevedo, H.S. Self-assembly in nature: Using the principles of nature to create complex nanobiomaterials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 582–612. [Google Scholar] [CrossRef]
- Pakdel, M.; Moosavi-Nejad, Z.; Kermanshahi, R.K.; Hosano, H. Self-assembled uniform keratin nanoparticles as building blocks for nanofibrils and nanolayers derived from industrial feather waste. J. Clean. Prod. 2022, 335, 130331. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, J.; Rao, H. Preparation and drug-loading properties of human hair keratin nanoparticles. Int. J. Sci. 2017, 4, 93–96. [Google Scholar]
- Xu, S.; Sang, L.; Zhang, Y.; Wang, X.; Li, X. Biological evaluation of human hair keratin scaffolds for skin wound repair and regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, L.; Han, X.; Dou, J.; Yuan, J.; Shen, J. Keratin-tannic acid complex nanoparticles as pH/GSH dual responsive drug carriers for doxorubicin. J. Biomater. Sci. Polym. Ed. 2021, 32, 1125–1139. [Google Scholar] [CrossRef]
- Natarajan, V.; Krithica, N.; Madhan, B.; Sehgal, P.K. Preparation and properties of tannic acid cross-linked collagen scaffold and its application in wound healing. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 560–567. [Google Scholar] [CrossRef]
- Heijmen, F.H.; du Pont, J.S.; Middelkoop, E.; Kreis, R.W.; Hoekstra, M.J. Cross-linking of dermal sheep collagen with tannic acid. Biomaterials 1997, 18, 749–754. [Google Scholar] [CrossRef]
- Lomova, M.V.; Brichkina, A.I.; Kiryukhin, M.V.; Vasina, E.N.; Pavlov, A.M.; Gorin, D.A.; Sukhorukov, G.B.; Antipina, M.N. Multilayer Capsules of Bovine Serum Albumin and Tannic Acid for Controlled Release by Enzymatic Degradation. ACS Appl. Mater. Interfaces 2015, 7, 11732–11740. [Google Scholar] [CrossRef]
- Jin, X.; Wang, Y.; Yuan, J.; Shen, J. Extraction, characterization, and NO release potential of keratin from human hair. Mater. Lett. 2016, 175, 188–190. [Google Scholar] [CrossRef]
- Foglietta, F.; Spagnoli, G.C.; Muraro, M.G.; Ballestri, M.; Guerrini, A.; Ferroni, C.; Aluigi, A.; Sotgiu, G.; Varchi, G. Anticancer activity of paclitaxel-loaded keratin nanoparticles in two-dimensional and perfused three-dimensional breast cancer models. Int. J. Nanomed. 2018, 13, 4847–4867. [Google Scholar] [CrossRef]
- Aluigi, A.; Varchi, G.; Sotgiu, G.; Guerrini, A.; Ballestri, M. Nanoparticles as delivery vehicles of active ingredients and methods for the production thereof. European Patent Office EP3638214B1, 26 January 2022. [Google Scholar]
- Meng, Z.; Lv, Q.; Lu, J.; Yao, H.; Lv, X.; Jiang, F.; Lu, A.; Zhang, G. Prodrug Strategies for Paclitaxel. Int. J. Mol. Sci. 2016, 17, 796. [Google Scholar] [CrossRef]
- Busi, A.; Aluigi, A.; Guerrini, A.; Boga, C.; Sartor, G.; Calonghi, N.; Sotgiu, G.; Posati, T.; Corticelli, F.; Fiori, J.; et al. Unprecedented Behavior of (9 R)-9-Hydroxystearic Acid-Loaded Keratin Nanoparticles on Cancer Cell Cycle. Mol. Pharm. 2019, 16, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Boanini, E.; Torricelli, P.; Boga, C.; Micheletti, G.; Cassani, M.C.; Fini, M.; Bigi, A. (9R)-9-Hydroxystearate-Functionalized Hydroxyapatite as Antiproliferative and Cytotoxic Agent toward Osteosarcoma Cells. Langmuir 2016, 32, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Goh, J.C.H. Release and cellular acceptance of multiple drugs loaded silk fibroin particles. Int. J. Pharm. 2011, 420, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Min, B.-M.; Lee, G.; Kim, S.H.; Nam, Y.S.; Lee, T.S.; Park, W.H. Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro. Biomaterials 2004, 25, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tian, J.; Wu, A.; Wang, J.; Ge, C.; Sun, Z. Self-assembled silk fibroin nanoparticles loaded with binary drugs in the treatment of breast carcinoma. Int. J. Nanomed. 2016, 11, 4373–4380. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, S.; Li, M. Silk Fibroin Based Porous Materials. Materials 2009, 2, 2276–2295. [Google Scholar] [CrossRef]
- Sager, W.F.C. Controlled formation of nanoparticles from microemulsions. Curr. Opin. Colloid Interface Sci. 1998, 3, 276–283. [Google Scholar] [CrossRef]
- Park, C.; Rhue, M.; Lim, J.; Kim, C. Metal nanoparticles in the template of poly(2-ethyl-2-oxazoline)-block-poly(ε-caprolactone) micelle. Macromol. Res. 2007, 15, 39–43. [Google Scholar] [CrossRef]
- Myung, S.J.; Kim, H.S.; Kim, Y.; Chen, P.; Jin, H.J. Fluorescent silk fibroin nanoparticles prepared using a reverse microemulsion. Macromol. Res. 2008, 16, 604–608. [Google Scholar] [CrossRef]
- Sofia, S.; McCarthy, M.B.; Gronowicz, G.; Kaplan, D.L. Functionalized silk-based biomaterials for bone formation. J. Biomed. Mater. Res. 2001, 54, 139–148. [Google Scholar] [CrossRef]
- Hofmann, S.; Wong Po Foo, C.T.; Rossetti, F.; Textor, M.; Vunjak-Novakovic, G.; Kaplan, D.L.; Merkle, H.P.; Meinel, L. Silk fibroin as an organic polymer for controlled drug delivery. J. Control. Release 2006, 111, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Arruebo, M.; Fernández-Pacheco, R.; Ibarra, M.R.; Santamaría, J. Magnetic nanoparticles for drug delivery. Nano Today 2007, 2, 22–32. [Google Scholar] [CrossRef]
- Mikhaylov, G.; Mikac, U.; Magaeva, A.A.; Itin, V.I.; Naiden, E.P.; Psakhye, I.; Babes, L.; Reinheckel, T.; Peters, C.; Zeiser, R.; et al. Ferri-liposomes as an MRI-visible drug-delivery system for targeting tumours and their microenvironment. Nat. Nanotechnol. 2011, 6, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sun, X.; Cheng, L.; Yin, S.; Yang, G.; Li, Y.; Liu, Z. Multifunctional theranostic red blood cells for magnetic-field-enhanced in vivo combination therapy of cancer. Adv. Mater. 2014, 26, 4794–4802. [Google Scholar] [CrossRef]
- Tian, Y.; Jiang, X.; Chen, X.; Shao, Z.; Yang, W. Doxorubicin-loaded magnetic silk fibroin nanoparticles for targeted therapy of multidrug-resistant cancer. Adv. Mater. 2014, 26, 7393–7398. [Google Scholar] [CrossRef]
- Lammel, A.S.; Hu, X.; Park, S.-H.; Kaplan, D.L.; Scheibel, T.R. Controlling silk fibroin particle features for drug delivery. Biomaterials 2010, 31, 4583–4591. [Google Scholar] [CrossRef]
- Ji, D.; Deng, Y.B.; Zhou, P. Folding process of silk fibroin induced by ferric and ferrous ions. J. Mol. Struct. 2009, 938, 305–310. [Google Scholar] [CrossRef]
- Song, W.; Muthana, M.; Mukherjee, J.; Falconer, R.J.; Biggs, C.A.; Zhao, X. Magnetic-Silk Core–Shell Nanoparticles as Potential Carriers for Targeted Delivery of Curcumin into Human Breast Cancer Cells. ACS Biomater. Sci. Eng. 2017, 3, 1027–1038. [Google Scholar] [CrossRef]
- Huang, X.; Brazel, C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control. Release 2001, 73, 121–136. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef]
- Li, Y.; Lin, J.; Zhi, X.; Li, P.; Jiang, X.; Yuan, J. Triple stimuli-responsive keratin nanoparticles as carriers for drug and potential nitric oxide release. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Yan, W.; Xu, Z.; Ni, H. Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids Surf. B Biointerfaces 2012, 90, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Vaezifar, S.; Razavi, S.; Golozar, M.A.; Karbasi, S.; Morshed, M.; Kamali, M. Effects of Some Parameters on Particle Size Distribution of Chitosan Nanoparticles Prepared by Ionic Gelation Method. J. Clust. Sci. 2013, 24, 891–903. [Google Scholar] [CrossRef]
- Schrooyen, P.M.M.; Dijkstra, P.J.; Oberthür, R.C.; Bantjes, A.; Feijen, J. Partially Carboxymethylated Feather Keratins. 1. Properties in Aqueous Systems. J. Agric. Food Chem. 2000, 48, 4326–4334. [Google Scholar] [CrossRef] [PubMed]
- Hoes, C.J.T.; Grootoonk, J.; Duncan, R.; Hume, I.C.; Bhakoo, M.; Bouma, J.M.W.; Feijen, J. Biological properties of adriamycin bound to biodegradable polymeric carriers. J. Control. Release 1993, 23, 37–53. [Google Scholar] [CrossRef][Green Version]
- Aluigi, A.; Ballestri, M.; Guerrini, A.; Sotgiu, G.; Ferroni, C.; Corticelli, F.; Gariboldi, M.B.; Monti, E.; Varchi, G. Organic solvent-free preparation of keratin nanoparticles as doxorubicin carriers for antitumour activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 476–484. [Google Scholar] [CrossRef]
- Cardamone, J.M.; Nuñez, A.; Garcia, R.A.; Aldema-Ramos, M. Characterizing Wool Keratin. Res. Lett. Mater. Sci. 2009, 2009, 147175. [Google Scholar] [CrossRef]
- Kalyane, D.; Raval, N.; Maheshwari, R.; Tambe, V.; Kalia, K.; Tekade, R.K. Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater. Sci. Eng. C 2019, 98, 1252–1276. [Google Scholar] [CrossRef]



| Methods | Advantages | Disadvantages |
|---|---|---|
| Desolvation | Operational simplicity; Mild conditions; Small particle size | Use of crosslinking agents and organic solvents |
| Electrospray | Ability to process different materials; Low cost; One-step procedure; Controllable particle size | Expensive; Complex equipment |
| Self-assembly/aggregation | Mild conditions; No organic solvents | Low size control |
| Microemulsion | Good control of particle size | Use of organic solvents and surfactant agents; Tedious purification steps |
| Salting out | Mild conditions; Easy to scale up; Low cost | Large particle size; Several purification steps |
| Ionic gelation | Organic-solvent-free; Suitable; No surfactant agents | Large particle size |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannelli, M.; Guerrini, A.; Ballestri, M.; Aluigi, A.; Zamboni, R.; Sotgiu, G.; Posati, T. Bioactive Keratin and Fibroin Nanoparticles: An Overview of Their Preparation Strategies. Nanomaterials 2022, 12, 1406. https://doi.org/10.3390/nano12091406
Giannelli M, Guerrini A, Ballestri M, Aluigi A, Zamboni R, Sotgiu G, Posati T. Bioactive Keratin and Fibroin Nanoparticles: An Overview of Their Preparation Strategies. Nanomaterials. 2022; 12(9):1406. https://doi.org/10.3390/nano12091406
Chicago/Turabian StyleGiannelli, Marta, Andrea Guerrini, Marco Ballestri, Annalisa Aluigi, Roberto Zamboni, Giovanna Sotgiu, and Tamara Posati. 2022. "Bioactive Keratin and Fibroin Nanoparticles: An Overview of Their Preparation Strategies" Nanomaterials 12, no. 9: 1406. https://doi.org/10.3390/nano12091406
APA StyleGiannelli, M., Guerrini, A., Ballestri, M., Aluigi, A., Zamboni, R., Sotgiu, G., & Posati, T. (2022). Bioactive Keratin and Fibroin Nanoparticles: An Overview of Their Preparation Strategies. Nanomaterials, 12(9), 1406. https://doi.org/10.3390/nano12091406






