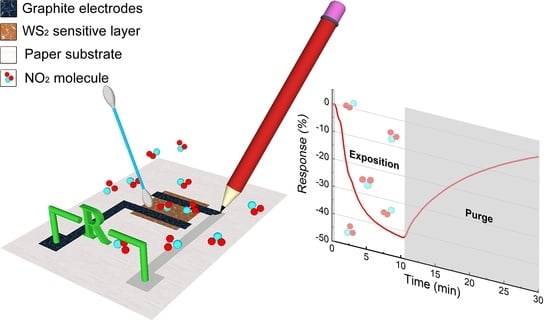
Eco-Friendly Disposable WS2 Paper Sensor for Sub-ppm NO2 Detection at Room Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
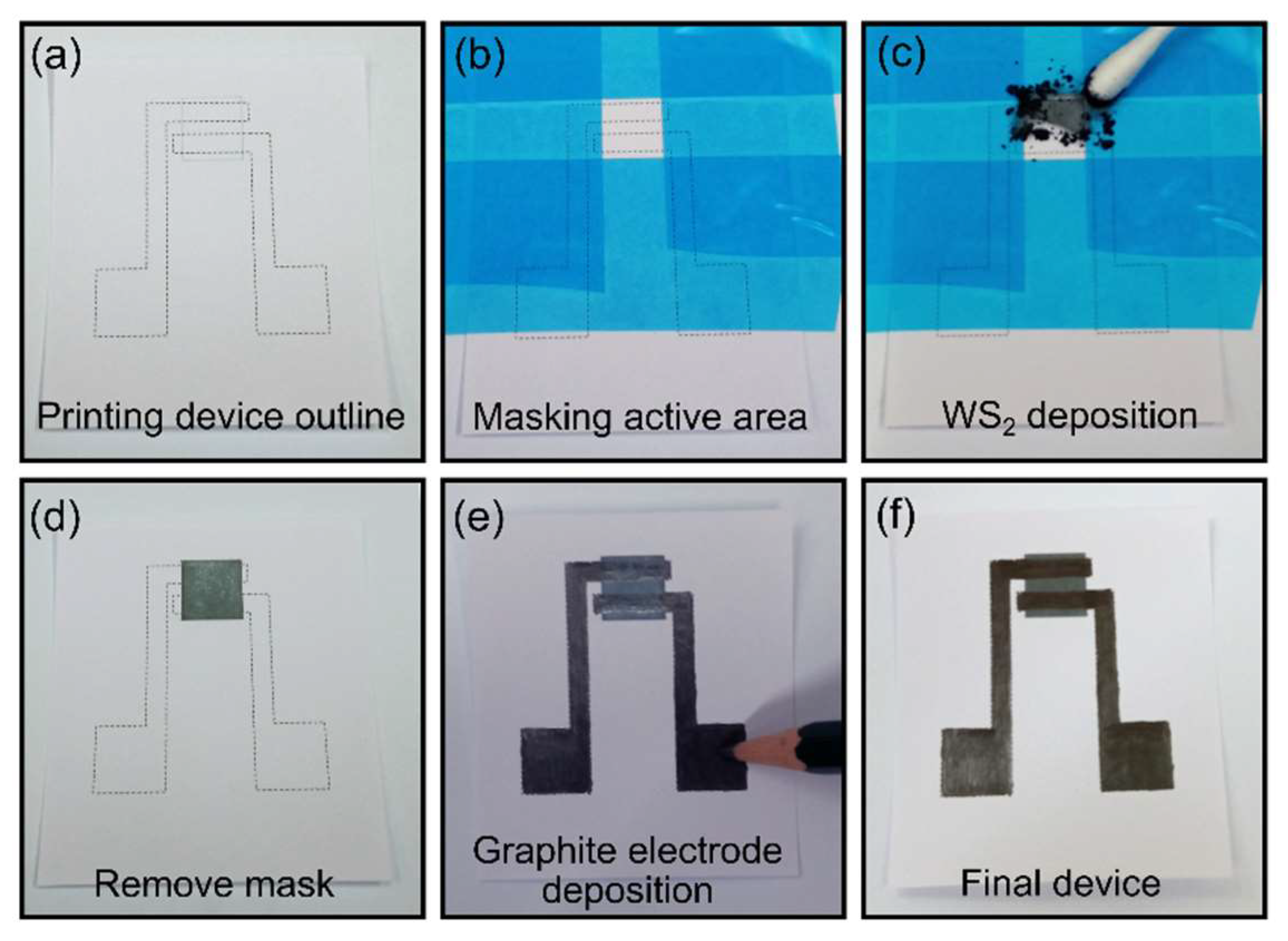
2.2. Sensor Fabrication
2.3. Material Characterization
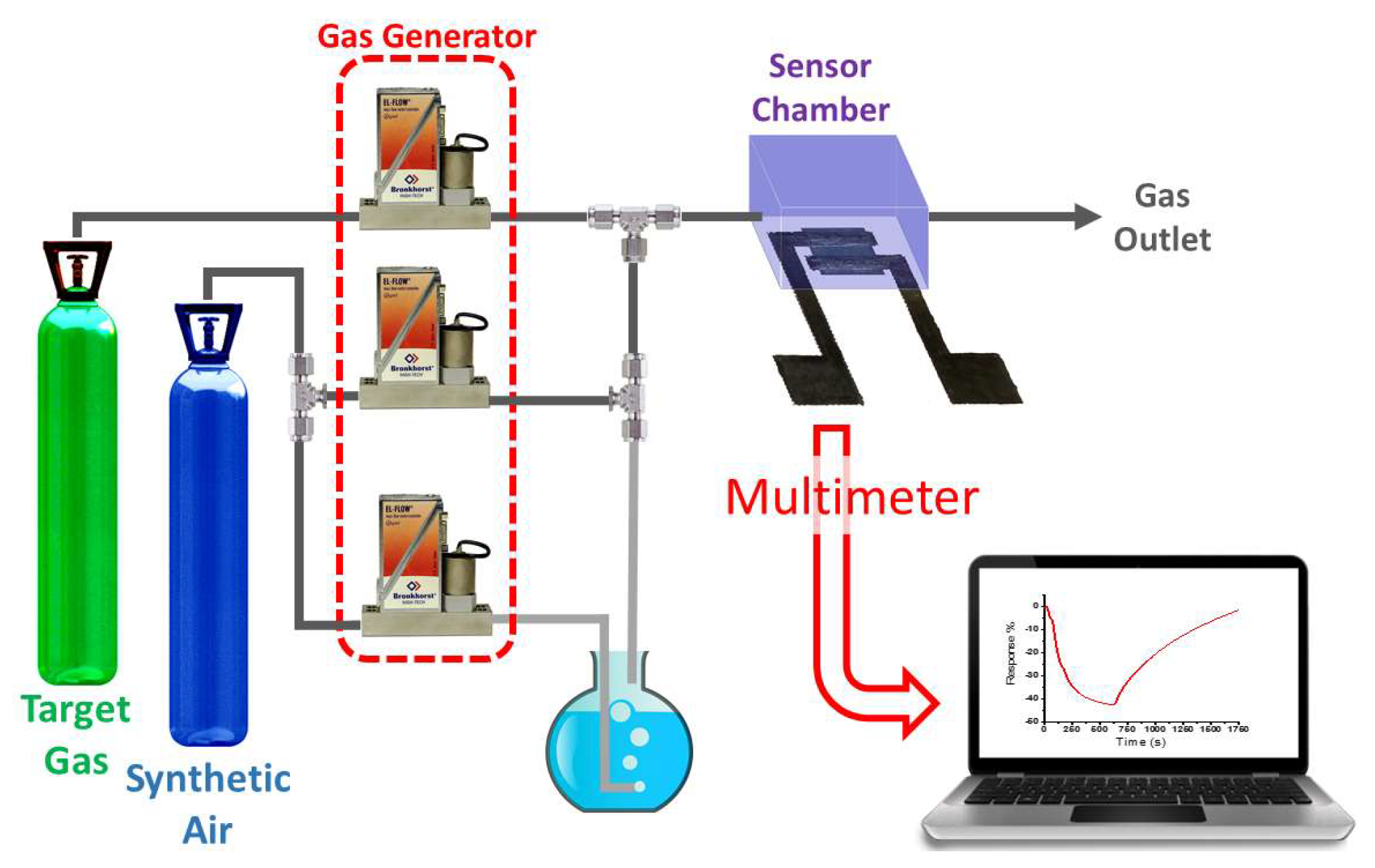
2.4. Experimental Setup
3. Results
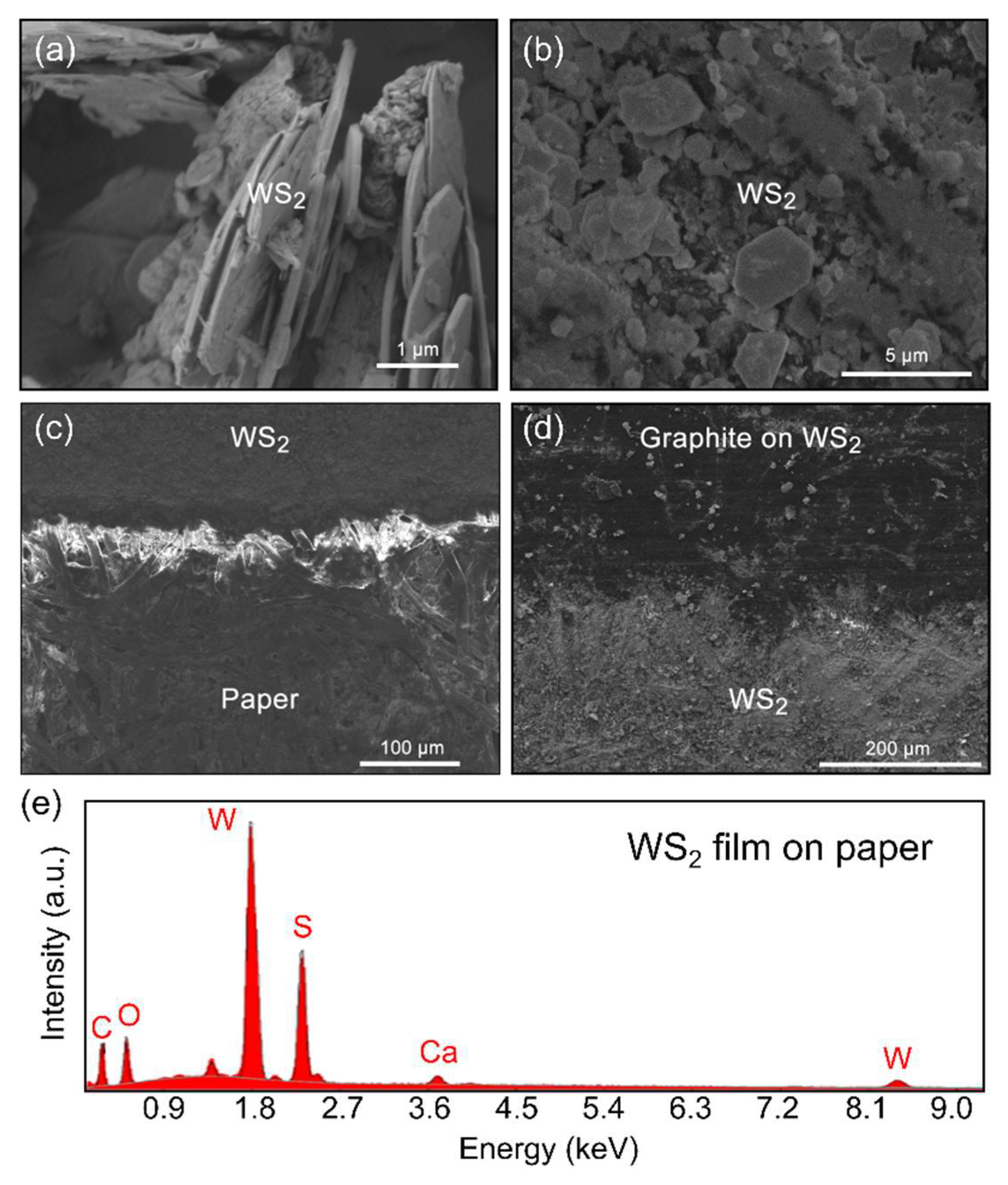
3.1. Structural and Morphological Characterization
3.2. Electrical Characterization
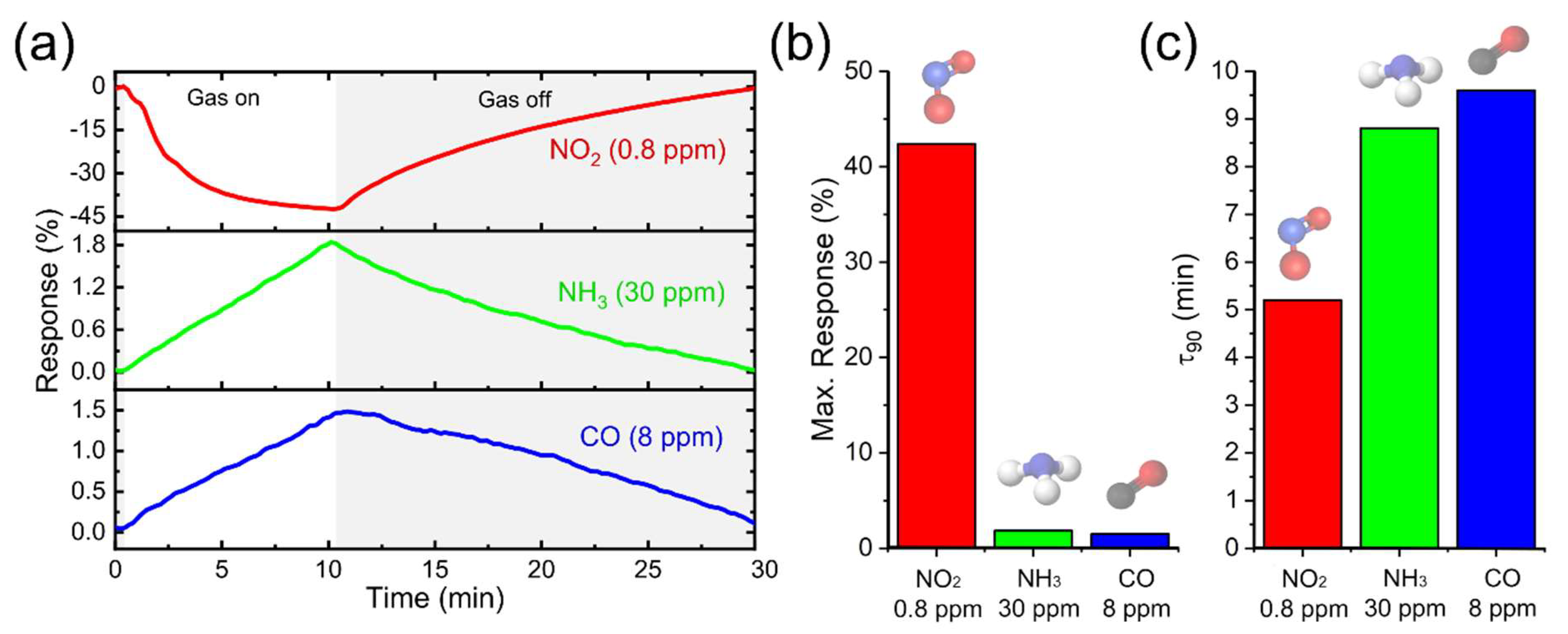
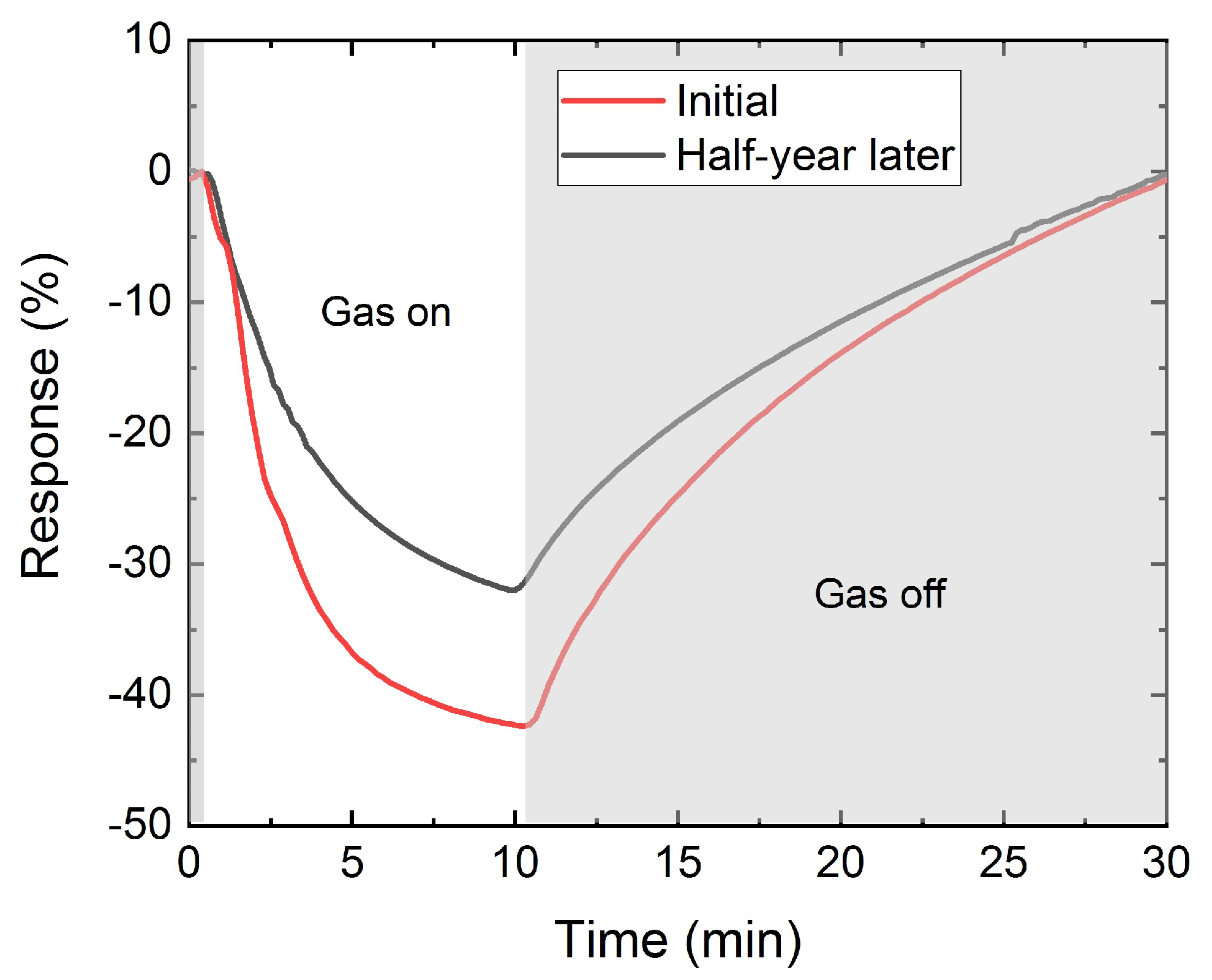
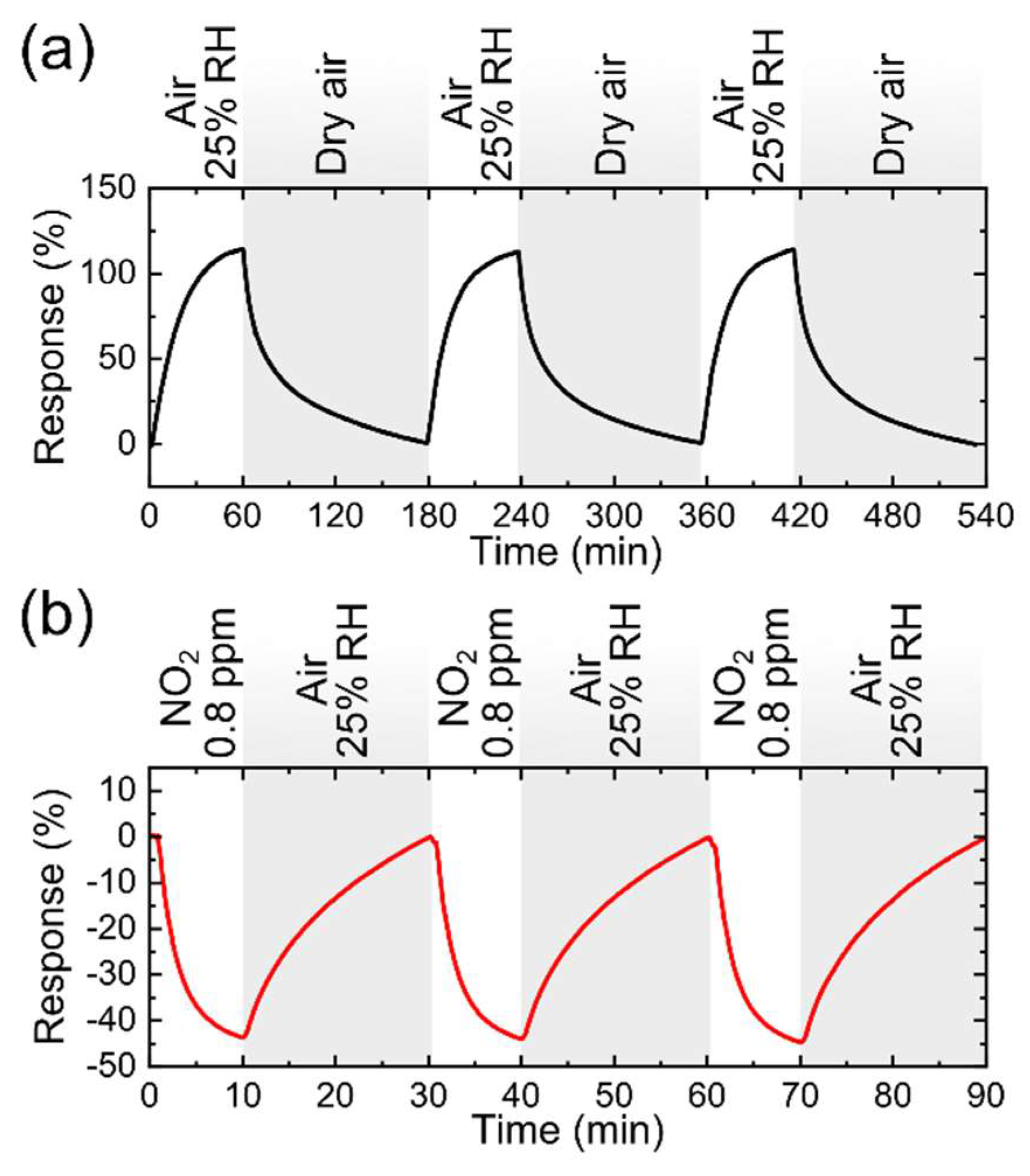
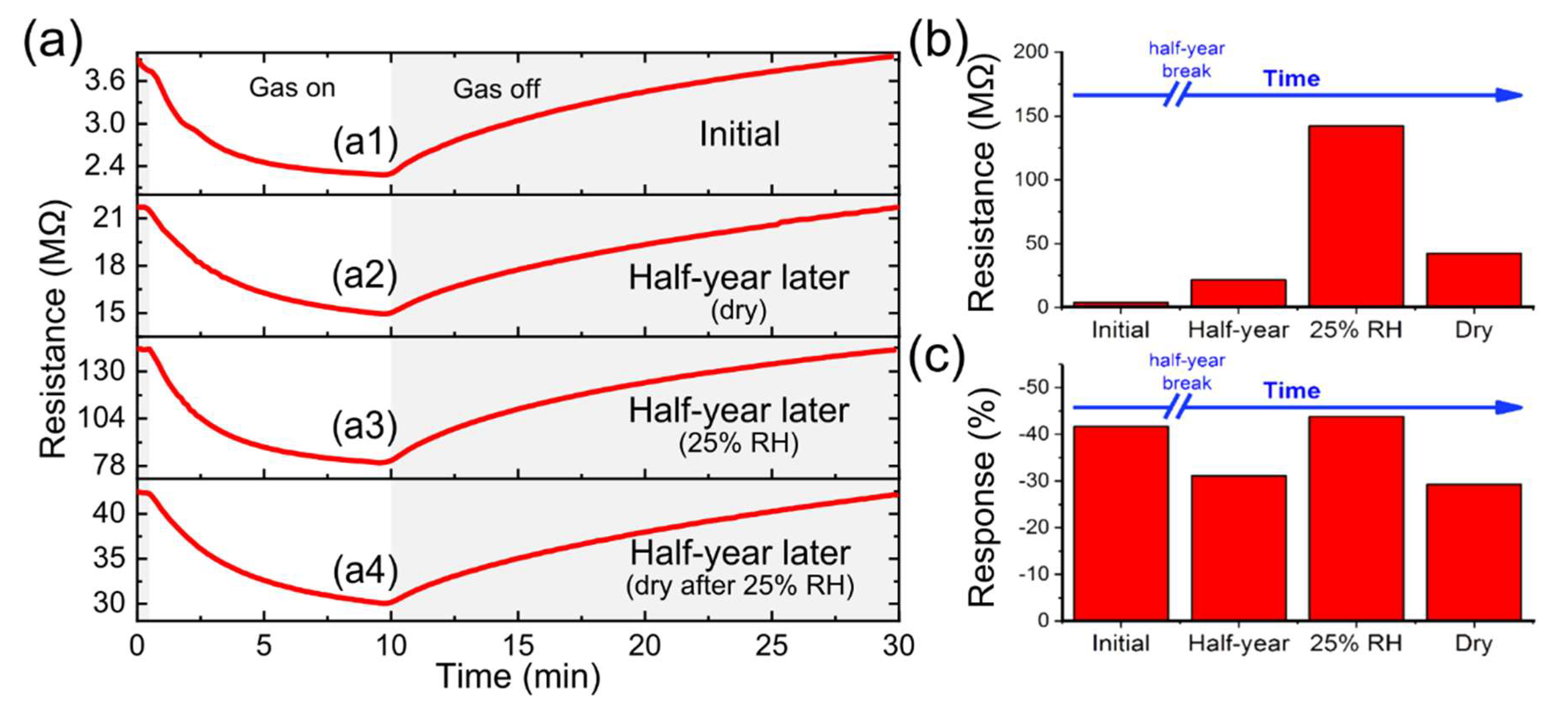
3.3. Gas Sensor Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, X.; Han, L.; Zhang, X.; Zhou, W.; Li, W.; Qian, Y. A review of current air quality indexes and improvements under the multi-contaminant air pollution exposure. J. Environ. Manag. 2021, 279, 111681. [Google Scholar] [CrossRef] [PubMed]
- Lambey, V.; Prasad, A.D. A Review on Air Quality Measurement Using an Unmanned Aerial Vehicle. Water. Air. Soil Pollut. 2021, 232, 1–32. [Google Scholar] [CrossRef]
- Touma, D.; Stevenson, S.; Lehner, F.; Coats, S. Human-driven greenhouse gas and aerosol emissions cause distinct regional impacts on extreme fire weather. Nat. Commun. 2021, 12, 1–8. [Google Scholar] [CrossRef]
- Buszewski, B.; Kesy, M.; Ligor, T.; Amann, A. Human exhaled air analytics: Biomarkers of diseases. Biomed. Chromatogr. 2007, 21, 553–566. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Jeerapan, I.; Krishnan, S.; Wang, J. Wearable Chemical Sensors: Emerging Systems for On-Body Analytical Chemistry. Anal. Chem. 2019, 92, 378–396. [Google Scholar] [CrossRef]
- Murray, L.T. Lightning NOX and Impacts on Air Quality. Curr. Pollut. Rep. 2016, 2, 115–133. [Google Scholar] [CrossRef] [Green Version]
- Dutta, R.; Hines, E.L.; Gardner, J.W.; Udrea, D.D.; Boilot, P. Non-destructive egg freshness determination: An electronic nose based approach. Meas. Sci. Technol. 2003, 14, 190–198. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Recommendation from Scientific Expert Group on Occupational Exposure Limits for Nitrogen Monoxide; SCOEL/SUM/53; European Commission: Brussels, Belgium, 2003. [Google Scholar]
- Azad, N.; Rojanasakul, Y.; Vallyathan, V. Inflammation and lung cancer: Roles of reactive oxygen/nitrogen species. J. Toxicol. Environ. Health Part B Crit. Rev. 2008, 11, 1–15. [Google Scholar] [CrossRef]
- Chen, Y.; Li, M.; Yan, W.; Zhuang, X.; Ng, K.W.; Cheng, X. Sensitive and Low-Power Metal Oxide Gas Sensors with a Low-Cost Microelectromechanical Heater. ACS Omega 2021, 6, 1216–1222. [Google Scholar] [CrossRef]
- Ji, H.; Zeng, W.; Li, Y. Gas sensing mechanisms of metal oxide semiconductors: A focus review. Nanoscale 2019, 11, 22664–22684. [Google Scholar] [CrossRef]
- Wang, J.; Shen, H.; Xia, Y.; Komarneni, S. Light-activated room-temperature gas sensors based on metal oxide nanostructures: A review on recent advances. Ceram. Int. 2021, 47, 7353–7368. [Google Scholar] [CrossRef]
- Matatagui, D.; Sainz-Vidal, A.; Gràcia, I.; Figueras, E.; Cané, C.; Saniger, J.M. Chemoresistive gas sensor based on ZIF-8/ZIF-67 nanocrystals. Sens. Actuators B Chem. 2018, 274, 601–608. [Google Scholar] [CrossRef]
- Santos, J.P.; Fernández, M.J.; Fontecha, J.L.; Matatagui, D.; Sayago, I.; Horrillo, M.C.; Gracia, I. Nanocrystalline tin oxide nanofibers deposited by a novel focused electrospinning method. Application to the detection of TATP precursors. Sensors 2014, 14, 24231–24243. [Google Scholar] [CrossRef] [PubMed]
- Horrillo, M.C.; Martí, J.; Matatagui, D.; Santos, J.P.; Sayago, I.; Gutiérrez, J.; Martin-Fernandez, I.; Ivanov, P.; Grcia, I.; Cané, C. Single-walled carbon nanotube microsensors for nerve agent simulant detection. Sens. Actuators B Chem. 2011, 157, 253–259. [Google Scholar] [CrossRef]
- Teja Vijjapu, M.; Surya, S.G.; Yuvaraja, S.; Zhang, X.; Alshareef, H.N.; Salama, K.N. Fully Integrated Indium Gallium Zinc Oxide NO2 Gas Detector. ACS Sens. 2020, 5, 984–993. [Google Scholar] [CrossRef]
- Goutham, S.; Sadasivuni, K.K.; Kumar, D.S.; Rao, K.V. Flexible ultra-sensitive and resistive NO2 gas sensor based on nanostructured Zn(X)Fe(1−x)2O. RSC Adv. 2018, 8, 3243–3249. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.; Sharma, A.; Myung, J.-H. Selective ppb-level NO2 gas sensor based on SnO2-boron nitride nanotubes. Sens. Actuators B Chem. 2021, 331, 129464. [Google Scholar] [CrossRef]
- Casals, O.; Markiewicz, N.; Fabrega, C.; Gràcia, I.; Cane, C.; Wasisto, H.S.; Waag, A.; Prades, J.D. A Parts per Billion (ppb) Sensor for NO2 with Microwatt (μW) Power Requirements Based on Micro Light Plates. ACS Sens. 2019, 4, 822–826. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal Oxide Gas Sensors: Sensitivity and Influencing Factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [Green Version]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Tai, H.; Duan, Z.; Wang, Y.; Wang, S.; Jiang, Y. Paper-Based Sensors for Gas, Humidity, and Strain Detections: A Review. ACS Appl. Mater. Interfaces 2020, 12, 31037–31053. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, M.; Kang, F. Graphene derivatives: Graphane, fluorographene, graphene oxide, graphyne and graphdiyne. J. Mater. Chem. A 2014, 2, 13193–13206. [Google Scholar] [CrossRef]
- Lee, X.J.; Hiew, B.Y.Z.; Lai, K.C.; Lee, L.Y.; Gan, S.; Thangalazhy-Gopakumar, S.; Rigby, S. Review on graphene and its derivatives: Synthesis methods and potential industrial implementation. J. Taiwan Inst. Chem. Eng. 2019, 98, 163–180. [Google Scholar] [CrossRef]
- Hong Choi, J.; Lee, J.; Byeon, M.; Eun Hong, T.; Park, H.; Young Lee, C. Graphene-Based Gas Sensors with High Sensitivity and Minimal Sensor-to-Sensor Variation. ACS Appl. Nano Mater. 2020, 3, 2257–2265. [Google Scholar] [CrossRef]
- Matatagui, D.; López-Sánchez, J.; Peña, A.; Serrano, A.; del Campo, A.; de la Fuente, O.R.; Carmona, N.; Navarro, E.; Marín, P.; del Carmen Horrillo, M. Ultrasensitive NO2 gas sensor with insignificant NH3-interference based on a few-layered mesoporous graphene. Sens. Actuators B Chem. 2021, 335, 129657. [Google Scholar] [CrossRef]
- Sayago, I.; Matatagui, D.; Fernández, M.J.; Fontecha, J.L.; Jurewicz, I.; Garriga, R.; Muñoz, E. Graphene oxide as sensitive layer in Love-wave surface acoustic wave sensors for the detection of chemical warfare agent simulants. Talanta 2016, 148, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Ren, B.; Xu, K.; Jeerapan, I.; Chen, H.; Li, Z.; Ou, J.Z. Recent progress in intrinsic and stimulated room-temperature gas sensors enabled by low-dimensional materials. J. Mater. Chem. C 2021, 9, 3026–3051. [Google Scholar] [CrossRef]
- Reddeppa, M.; Park, B.-G.; Murali, G.; Choi, S.H.; Chinh, N.D.; Kim, D.; Yang, W.; Kim, M.-D. NOX gas sensors based on layer-transferred n-MoS2p-GaN heterojunction at room temperature: Study of UV light illuminations and humidity. Sens. Actuators B Chem. 2020, 308, 127700. [Google Scholar] [CrossRef]
- Eom, T.H.; Cho, S.H.; Suh, J.M.; Kim, T.; Lee, T.H.; Jun, S.E.; Yang, J.W.; Lee, J.; Hong, S.-H.; Jang, H.W. Substantially improved room temperature NO2 sensing in 2-dimensional SnS2 nanoflowers enabled by visible light illumination. J. Mater. Chem. A 2021, 9, 11168–11178. [Google Scholar] [CrossRef]
- Reddeppa, M.; KimPhung, N.T.; Murali, G.; Pasupuleti, K.S.; Park, B.-G.; In, I.; Kim, M.-D. Interaction activated interfacial charge transfer in 2D g-C3N4/GaN nanorods heterostructure for self-powered UV photodetector and room temperature NO2 gas sensor at ppb level. Sens. Actuators B Chem. 2021, 329, 127700. [Google Scholar] [CrossRef]
- Suh, J.M.; Kwon, K.C.; Lee, T.H.; Kim, C.; Lee, C.W.; Song, Y.G.; Choi, M.-J.; Choi, S.; Cho, S.H.; Kim, S.; et al. Edge-exposed WS2 on 1D nanostructures for highly selective NO2 sensor at room temperature. Sens. Actuators B Chem. 2021, 333, 129566. [Google Scholar] [CrossRef]
- Lien, D.-H.; Kao, Z.-K.; Huang, T.-H.; Liao, Y.-C.; Lee, S.-C.; He, J.-H. All-Printed Paper Memory. ACS Nano 2014, 8, 7613–7619. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-P.; Lai, K.-Y.; Lin, C.-A.; Li, C.-T.; Ho, K.-C.; Wu, C.-I.; Lau, S.-P.; He, J.-H. A paper-based electrode using a graphene dot/PEDOT:PSS composite for flexible solar cells. Nano Energy 2017, 36, 260–267. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.; Zhu, H.; Yuan, Y.; Ha, D.; Zhu, S.; Preston, C.; Chen, Q.; Li, Y.; Han, X.; Lee, S.; et al. Novel Nanostructured Paper with Ultrahigh Transparency and Ultrahigh Haze for Solar Cells. Nano Lett. 2014, 14, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Lakafosis, V.; Rida, A.; Vyas, R.; Yang, L.; Nikolaou, S.; Tentzeris, M.M. Progress towards the first wireless sensor networks consisting of inkjet-printed, paper-based RFID-enabled sensor tags. Proc. IEEE 2010, 98, 1601–1609. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Xiao, X.; Ding, T.; Zhong, J.; Zhang, X.; Shen, Y.; Hu, B.; Huang, Y.; Zhou, J.; Wang, Z.L. Paper-based supercapacitors for self-powered nanosystems. Angew. Chem. Int. Ed. 2012, 51, 4934–4938. [Google Scholar] [CrossRef]
- Nutting, D.; Felix, J.F.; Tillotson, E.; Shin, D.-W.; De Sanctis, A.; Chang, H.; Cole, N.; Russo, S.; Woodgate, A.; Leontis, I.; et al. Heterostructures formed through abraded van der Waals materials. Nat. Commun. 2020, 11, 3047. [Google Scholar] [CrossRef]
- Mazaheri, A.; Lee, M.; van der Zant, H.S.J.; Frisenda, R.; Castellanos-Gomez, A. MoS2-on-paper optoelectronics: Drawing photodetectors with van der Waals semiconductors beyond graphite. Nanoscale 2020, 12, 19068–19074. [Google Scholar] [CrossRef]
- Lee, M.; Mazaheri, A.; van der Zant, H.S.J.; Frisenda, R.; Castellanos-Gomez, A. Drawing WS2 thermal sensors on paper substrates. Nanoscale 2020, 12, 22091–22096. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, Q.; Munuera, C.; Lee, M.; Flores, E.; Rodrigues, J.E.F.; Ares, J.R.; Sanchez, C.; Gainza, J.; van der Zant, H.S.J.; et al. Integrating van der Waals materials on paper substrates for electrical and optical applications. Appl. Mater. Today 2021, 23, 101012. [Google Scholar] [CrossRef]
- Eder, F.; Klauk, H.; Halik, M.; Zschieschang, U.; Schmid, G.; Dehm, C. Organic electronics on paper. Appl. Phys. Lett. 2004, 84, 2673–2675. [Google Scholar] [CrossRef]
- Russo, A.; Ahn, B.Y.; Adams, J.J.; Duoss, E.B.; Bernhard, J.T.; Lewis, J.A. Pen-on-paper flexible electronics. Adv. Mater. 2011, 23, 3426–3430. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhao, G.; Zhu, L.; Fei, Q.; Zhang, Z.; Chen, Z.; An, F.; Chen, Y.; Ling, Y.; Guo, P.; et al. Pencil–paper on-skin electronics. Proc. Natl. Acad. Sci. USA 2020, 117, 18292–18301. [Google Scholar] [CrossRef] [PubMed]
- Tobjörk, D.; Österbacka, R. Paper electronics. Adv. Mater. 2011, 23, 1935–1961. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, L.; Lin, Y.; Chen, L.; Zeng, Z.; Shen, L.; Chen, Q.; Shi, W. Pencil-trace on printed silver interdigitated electrodes for paper-based NO2 gas sensors. Appl. Phys. Lett. 2015, 106, 143101. [Google Scholar] [CrossRef]
- Rasia, L.A.; Andrades, C.E.; Heck, T.G.; Rasia, J. Approach Pencil-on-Paper to Flexible Piezoresistive Respiration Sensor; Springer: Cham, Germany, 2021; Volume 1431, ISBN 9783030867010. [Google Scholar]
- Kurra, N.; Kulkarni, G.U. Pencil-on-paper: Electronic devices. Lab. Chip 2013, 13, 2866–2873. [Google Scholar] [CrossRef]
- Mahadeva, S.K.; Walus, K.; Stoeber, B. Paper as a platform for sensing applications and other devices: A review. ACS Appl. Mater. Interfaces 2015, 7, 8345–8362. [Google Scholar] [CrossRef]
- Rekha, P.; Panda, S.K.; Suresh, M.S. Pencil drawn films as Ammonia gas sensors. In Proceedings of the 2017 International Conference on Smart Grids, Power and Advanced Control Engineering, ICSPACE 2017, Bangalore, India, 17–19 August 2017; pp. 366–371. [Google Scholar]
- Šetka, M.; Bahos, F.A.; Matatagui, D.; Potoček, M.; Kral, Z.; Drbohlavová, J.; Gràcia, I.; Vallejos, S. Love wave sensors based on gold nanoparticle-modified polypyrrole and their properties to ammonia and ethylene. Sens. Actuators B Chem. 2020, 304, 127337. [Google Scholar] [CrossRef]
- Narayana, A.; Bhat, S.A.; Fathima, A.; Lokesh, S.V.; Surya, S.G.; Yelamaggad, C.V. Green and low-cost synthesis of zinc oxide nanoparticles and their application in transistor-based carbon monoxide sensing. RSC Adv. 2020, 10, 13532–13542. [Google Scholar] [CrossRef] [Green Version]
- Reddeppa, M.; Chandrakalavathi, T.; Park, B.-G.; Murali, G.; Siranjeevi, R.; Nagaraju, G.; Su Yu, J.; Jayalakshmi, R.; Kim, S.-G.; Kim, M.-D. UV-light enhanced CO gas sensors based on InGaN nanorods decorated with p-Phenylenediamine-graphene oxide composite. Sens. Actuators B Chem. 2020, 307, 127649. [Google Scholar] [CrossRef]
- Sousa, M.C.; Buchanan, J.W. Observational models of graphite pencil materials. In Computer Graphics Forum; Blackwell: Oxford, UK; Boston, MA, USA, 2000; Volume 19, pp. 27–49. [Google Scholar]
- Ryter, S.W.; Choi, A.M.K. Carbon monoxide in exhaled breath testing and therapeutics. J. Breath Res. 2013, 7, 017111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, F.M.; Vaittinen, O.; Metsälä, M.; Lehto, M.; Forsblom, C.; Groop, P.-H.; Halonen, L. Ammonia in breath and emitted from skin. J. Breath Res. 2013, 7, 017109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azizi, A.; Dogan, M.; Long, H.; Cain, J.D.; Lee, K.; Eskandari, R.; Varieschi, A.; Glazer, E.C.; Cohen, M.L.; Zettl, A. High-Performance Atomically-Thin Room-Temperature NO2 Sensor. Nano Lett. 2020, 20, 6120–6127. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matatagui, D.; Cruz, C.; Carrascoso, F.; Al-Enizi, A.M.; Nafady, A.; Castellanos-Gomez, A.; Horrillo, M.d.C. Eco-Friendly Disposable WS2 Paper Sensor for Sub-ppm NO2 Detection at Room Temperature. Nanomaterials 2022, 12, 1213. https://doi.org/10.3390/nano12071213
Matatagui D, Cruz C, Carrascoso F, Al-Enizi AM, Nafady A, Castellanos-Gomez A, Horrillo MdC. Eco-Friendly Disposable WS2 Paper Sensor for Sub-ppm NO2 Detection at Room Temperature. Nanomaterials. 2022; 12(7):1213. https://doi.org/10.3390/nano12071213
Chicago/Turabian StyleMatatagui, Daniel, Carlos Cruz, Felix Carrascoso, Abdullah M. Al-Enizi, Ayman Nafady, Andres Castellanos-Gomez, and María del Carmen Horrillo. 2022. "Eco-Friendly Disposable WS2 Paper Sensor for Sub-ppm NO2 Detection at Room Temperature" Nanomaterials 12, no. 7: 1213. https://doi.org/10.3390/nano12071213
APA StyleMatatagui, D., Cruz, C., Carrascoso, F., Al-Enizi, A. M., Nafady, A., Castellanos-Gomez, A., & Horrillo, M. d. C. (2022). Eco-Friendly Disposable WS2 Paper Sensor for Sub-ppm NO2 Detection at Room Temperature. Nanomaterials, 12(7), 1213. https://doi.org/10.3390/nano12071213