Ultra-Fine Ruthenium Oxide Quantum Dots/Reduced Graphene Oxide Composite as Electrodes for High-Performance Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of GO, RuO2/Reduced Graphene Oxide Nanosheets Nanocomposites
2.3. Instrumentation
2.4. Electrochemical Measurements
3. Results and Discussion
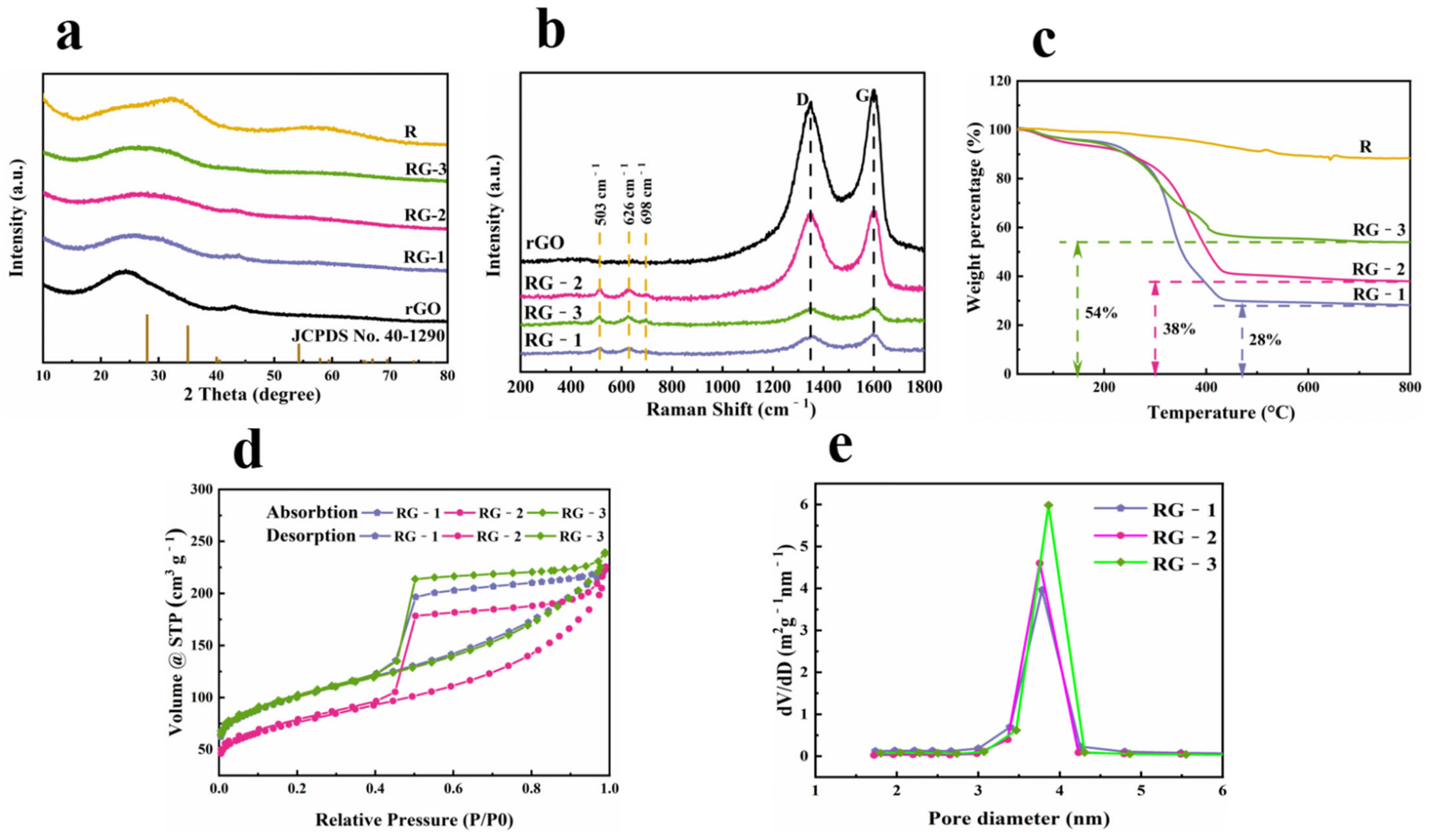
3.1. Morphology Analysis
3.2. Structure Analysis
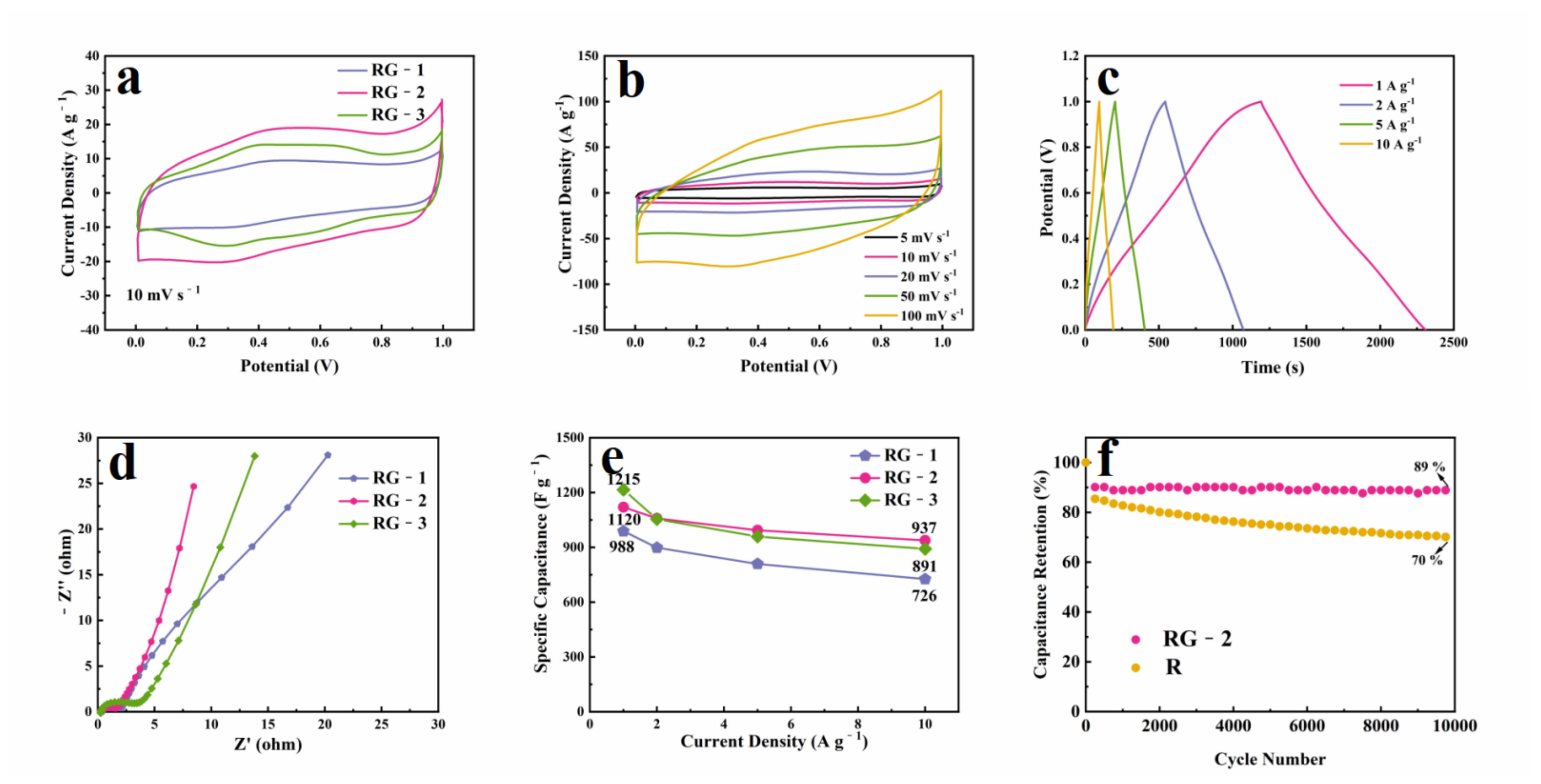
3.3. Electrochemical Performance of Supercapacitor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, P.Y.; Zhao, J.J.; Dong, Z.P.; Liu, Z.L.; Wang, Y.Q. Interwoving polyaniline and a metal-organic framework grown in situ for enhanced supercapacitor behavior. J. Alloys Compd. 2021, 854, 157181. [Google Scholar] [CrossRef]
- Beka, L.G.; Bu, X.; Li, X.; Wang, X.; Han, C.; Liu, W. A 2D metal-organic framework/reduced graphene oxide heterostructure for supercapacitor application. RSC Adv. 2019, 9, 36123–36135. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Gao, S.; Mi, H.; Lei, C.; Ji, C.; Xie, Z.; Yu, C.; Qiu, J. High-energy quasi-solid-state supercapacitors enabled by carbon nanofoam from biowaste and high-voltage inorganic gel electrolyte. Carbon 2019, 149, 273–280. [Google Scholar] [CrossRef]
- Xu, G.; Zhao, J.; Yuan, J.; Zhao, Y.; Yin, H.; Wang, F.; Tang, J.; Zhang, J. A facile one-pot microwave assisted hydrothermal synthesis of hierarchical cobalt oxide/reduced graphene oxide composite electrode for high-performance supercapacitors. J. Alloys Compd. 2022, 897, 163163. [Google Scholar] [CrossRef]
- Mei, B.A.; Munteshari, O.; Lau, J.; Dunn, B.; Pilon, L. Physical interpretations of Nyquist plots for EDLC electrodes and devices. J. Phys. Chem. C 2018, 122, 194–206. [Google Scholar] [CrossRef]
- Portet, C.; Yushin, G.; Gogotsi, Y. Effect of carbon particle size on electrochemical performance of EDLC. J. Electrochem. Soc. 2008, 155, A531. [Google Scholar] [CrossRef]
- Lei, C.; Amini, N.; Markoulidis, F.; Wilson, P.; Tennison, S.; Lekakou, C. Activated carbon from phenolic resin with controlled mesoporosity for an electric double-layer capacitor (EDLC). J. Mater. Chem. A 2013, 1, 6037–6042. [Google Scholar] [CrossRef]
- Naskar, P.; Maiti, A.; Chakraborty, P.; Kundu, D.; Biswas, B.; Banerjee, A. Chemical supercapacitors: A review focusing on metallic compounds and conducting polymers. J. Mater. Chem. A 2021, 9, 1970–2017. [Google Scholar] [CrossRef]
- Zhao, J.; Burke, A.F. Review on supercapacitors: Technologies and performance evaluation. J. Energy Chem. 2021, 59, 276–291. [Google Scholar] [CrossRef]
- Gao, R.; Tang, J.; Yu, X.; Tang, S.; Ozawa, K.; Sasaki, T.; Qin, L.C. In situ synthesis of MOF-derived carbon shells for silicon anode with improved lithium-ion storage. Nano Energy 2020, 70, 104444. [Google Scholar] [CrossRef]
- Gao, R.; Tang, J.; Zhang, K.; Ozawa, K.; Qin, L.C. A sandwich-like silicon–carbon composite prepared by surface-polymerization for rapid lithium-ion storage. Nano Energy 2020, 78, 105341. [Google Scholar] [CrossRef]
- Cheng, F.; Yang, X.; Zhang, S.; Lu, W. Boosting the supercapacitor performances of activated carbon with carbon nanomaterials. J. Power Sources 2020, 450, 227678. [Google Scholar] [CrossRef]
- Bairi, P.; Maji, S.; Hill, J.P.; Kim, J.H.; Ariga, K.; Shrestha, L.K. Mesoporous carbon cubes derived from fullerene crystals as a high rate performance electrode material for supercapacitors. J. Mater. Chem. A 2019, 7, 12654–12660. [Google Scholar] [CrossRef]
- Huang, L.; Santiago, D.; Loyselle, P.; Dai, L. Graphene-Based Nanomaterials for Flexible and Wearable Supercapacitors. Small 2018, 14, 1800879. [Google Scholar] [CrossRef]
- Yassine, M.; Fabris, D. Performance of Commercially Available Supercapacitors. Energies 2017, 10, 1340. [Google Scholar] [CrossRef] [Green Version]
- Xia, H.; Hong, C.; Li, B.; Zhao, B.; Lin, Z.; Zheng, M.; Savilov, S.V.; Aldoshin, S.M. Facile synthesis of hematite quantum—dot/functionalized graphene—sheet composites as advanced anode materials for asymmetric supercapacitors. Adv. Funct. Mater. 2015, 25, 627–635. [Google Scholar] [CrossRef]
- Jabeen, N.; Hussain, A.; Xia, Q.; Sun, S.; Zhu, J.; Xia, H. High-performance 2.6 V aqueous asymmetric supercapacitors based on in situ formed Na0. 5MnO2 nanosheet assembled nanowall arrays. Adv. Mater. 2017, 29, 1700804. [Google Scholar] [CrossRef]
- Xia, H.; Meng, Y.S.; Yuan, G.; Cui, C.; Lu, L. A symmetric RuO2/RuO2 supercapacitor operating at 1.6 V by using a neutral aqueous electrolyte. Electrochem. Solid-State Lett. 2012, 15, A60. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Huang, X.; Dou, S.; Wu, J.; Wang, S. One-pot synthesis of Fe2O3 nanoparticles on nitrogen-doped graphene as advanced supercapacitor electrode materials. J. Phys. Chem. C 2014, 118, 17231–17239. [Google Scholar] [CrossRef]
- Zhang, Q.Z.; Zhang, D.; Miao, Z.C.; Zhang, X.L.; Chou, S.L. Research progress in MnO2-carbon based supercapacitor electrode materials. Small 2018, 14, 1702883. [Google Scholar] [CrossRef]
- Xiang, C.; Li, M.; Zhi, M.; Manivannan, A.; Wu, N. A reduced graphene oxide/Co3O4 composite for supercapacitor electrode. J. Power Sources 2013, 226, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Wang, R.; Cao, R. Physical and electrochemical characterization of hydrous ruthenium oxide/ordered mesoporous carbon composites as supercapacitor. Microporous Mesoporous Mater. 2008, 111, 32–38. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, Y.; Huang, X.; Chen, N.; Qu, L. Three-dimensional graphitic carbon nitride functionalizeds graphene-based high-performance supercapacitors. J. Mater.Chem. A 2015, 3, 6761–6766. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, L.; Wu, H.B.; Lou, X.W. Formation of NixCo3−xS4 Hollow Nanoprisms with Enhanced Pseudocapacitive Properties. Angew. Chem. Int. Ed. 2014, 53, 3711–3714. [Google Scholar] [CrossRef]
- Wu, Z.S.; Ren, W.; Wang, D.W.; Li, F.; Liu, B.; Cheng, H.M. High-Energy MnO2 Nanowire/Graphene and Graphene Asymmetric Electrochemical Capacitors. ACS Nano 2010, 4, 5835–5842. [Google Scholar] [CrossRef]
- Liu, M.; Tjiu, W.W.; Pan, J.; Zhang, C.; Gao, W.; Liu, T. One-step synthesis of graphene nanoribbon–MnO2 hybrids and their all-solid-state asymmetric supercapacitors. Nanoscale 2014, 6, 4233–4242. [Google Scholar] [CrossRef]
- Gujar, T.; Shinde, V.; Lokhande, C.; Kim, W.Y.; Jung, K.D.; Joo, O.S. Spray deposited amorphous RuO2 for an effective use in electrochemical supercapacitor. Electrochem. Commun. 2007, 9, 504–510. [Google Scholar] [CrossRef]
- Wu, Z.S.; Wang, D.W.; Ren, W.; Zhao, J.; Zhou, G.; Li, F.; Cheng, H.M. Anchoring hydrous RuO2 on graphene sheets for high-performance electrochemical capacitors. Adv. Funct. Mater. 2010, 20, 3595–3602. [Google Scholar] [CrossRef]
- Ghasemi, S.; Ahmadi, F. Effect of surfactant on the electrochemical performance of graphene/iron oxide electrode for supercapacitor. J. Power Sources 2015, 289, 129–137. [Google Scholar] [CrossRef]
- Guo, D.; Luo, Y.; Yu, X.; Li, Q.; Wang, T. High performance NiMoO4 nanowires supported on carbon cloth as advanced electrodes for symmetric supercapacitors. Nano Energy 2014, 8, 174–182. [Google Scholar] [CrossRef]
- Wu, H.B.; Xia, B.Y.; Yu, L.; Yu, X.Y.; Lou, X.W.D. Porous molybdenum carbide nano-octahedrons synthesized via confined carburization in metal-organic frameworks for efficient hydrogen production. Nat. Commun. 2015, 6, 6512. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.S.; Chien, P.Y.; Lin, J.Y.; Chou, S.W.; Wu, W.K.; Li, P.H.; Wu, K.Y.; Lin, T.W. Hierarchically Structured Ni3S2/Carbon Nanotube Composites as High Performance Cathode Materials for Asymmetric Supercapacitors. ACS Appl. Mater. Interfaces 2013, 5, 12168–12174. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Tang, J.; Lin, Y.; Li, J.; Qin, F.; Yuan, J.; Qin, L.C. Carbon nanotube spaced graphene aerogels with enhanced capacitance in aqueous and ionic liquid electrolytes. J. Power Sources 2015, 278, 751–759. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Chen, T.; Chu, H.; Niu, L.; Sun, Z.; Pan, L.; Sun, C.Q. Fe2O3-reduced graphene oxide composites synthesized via microwave-assisted method for sodium ion batteries. Electrochim. Acta 2015, 166, 12–16. [Google Scholar] [CrossRef]
- Saraf, M.; Natarajan, K.; Mobin, S.M. Microwave assisted fabrication of a nanostructured reduced graphene oxide (rGO)/Fe2O3 composite as a promising next generation energy storage material. RSC Adv. 2017, 7, 309–317. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.J.; Wang, Y.X.; Chou, S.L.; Li, H.J.; Liu, H.K.; Wang, J.Z. Rapid synthesis of α-Fe2O3/rGO nanocomposites by microwave autoclave as superior anodes for sodium-ion batteries. J. Power Sources 2015, 280, 107–113. [Google Scholar] [CrossRef]
- Hu, C.C.; Yang, Y.L.; Lee, T.C. Microwave-Assisted Hydrothermal Synthesis of RuO2 ⋅ xH2O–TiO2 Nanocomposites for High Power Supercapacitors. Electrochem. Solid-State Lett. 2010, 13, A173. [Google Scholar] [CrossRef]
- Thanh, N.T.; Maclean, N.; Mahiddine, S. Mechanisms of nucleation and growth of nanoparticles in solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar] [CrossRef]
- Yin, H.; Zhao, Y.; Hua, Q.; Zhang, J.; Zhang, Y.; Xu, X.; Long, Y.; Tang, J.; Wang, F. Controlled synthesis of hollow α-Fe2O3 microspheres assembled with ionic liquid for enhanced visible-light photocatalytic activity. Front. Chem. 2019, 7, 58. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Zhang, D.; Ma, Y. One-pot hydrothermal synthesis of ruthenium oxide nanodots on reduced graphene oxide sheets for supercapacitors. J. Alloys Compd. 2012, 511, 251–256. [Google Scholar] [CrossRef]
- Petrović, Ž.; Ristić, M.; Marciuš, M.; Sepiol, B.; Peterlik, H.; Ivanda, M.; Musić, S. Formation of RuO2 nanoparticles by thermal decomposition of Ru (NO)(NO3)3. Ceram. Int. 2015, 41, 7811–7815. [Google Scholar] [CrossRef]
- Yan, J.; Liu, J.; Fan, Z.; Wei, T.; Zhang, L. High-performance supercapacitor electrodes based on highly corrugated graphene sheets. Carbon 2012, 50, 2179–2188. [Google Scholar] [CrossRef]
- Zhang, L.S.; Jiang, L.Y.; Yan, H.J.; Wang, W.D.; Wang, W.; Song, W.G.; Guo, Y.G.; Wan, L.J. Mono dispersed SnO2 nanoparticles on both sides of single layer graphene sheets as anode materials in Li-ion batteries. J. Mater. Chem. 2010, 20, 5462–5467. [Google Scholar] [CrossRef]
- Lei, Z.; Shi, F.; Lu, L. Incorporation of MnO2-Coated Carbon Nanotubes between Graphene Sheets as Supercapacitor Electrode. ACS Appl. Mater. Interfaces 2012, 4, 1058–1064. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Zhang, J.; Yin, H.; Zhao, Y.; Xu, G.; Yuan, J.; Mo, X.; Tang, J.; Wang, F. Ultra-Fine Ruthenium Oxide Quantum Dots/Reduced Graphene Oxide Composite as Electrodes for High-Performance Supercapacitors. Nanomaterials 2022, 12, 1210. https://doi.org/10.3390/nano12071210
Zhao J, Zhang J, Yin H, Zhao Y, Xu G, Yuan J, Mo X, Tang J, Wang F. Ultra-Fine Ruthenium Oxide Quantum Dots/Reduced Graphene Oxide Composite as Electrodes for High-Performance Supercapacitors. Nanomaterials. 2022; 12(7):1210. https://doi.org/10.3390/nano12071210
Chicago/Turabian StyleZhao, Jie, Jianmin Zhang, Hang Yin, Yuling Zhao, Guangxu Xu, Jinshi Yuan, Xiaoyao Mo, Jie Tang, and Fengyun Wang. 2022. "Ultra-Fine Ruthenium Oxide Quantum Dots/Reduced Graphene Oxide Composite as Electrodes for High-Performance Supercapacitors" Nanomaterials 12, no. 7: 1210. https://doi.org/10.3390/nano12071210
APA StyleZhao, J., Zhang, J., Yin, H., Zhao, Y., Xu, G., Yuan, J., Mo, X., Tang, J., & Wang, F. (2022). Ultra-Fine Ruthenium Oxide Quantum Dots/Reduced Graphene Oxide Composite as Electrodes for High-Performance Supercapacitors. Nanomaterials, 12(7), 1210. https://doi.org/10.3390/nano12071210





