Waste Citrus reticulata Assisted Preparation of Cobalt Oxide Nanoparticles for Supercapacitors
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Method
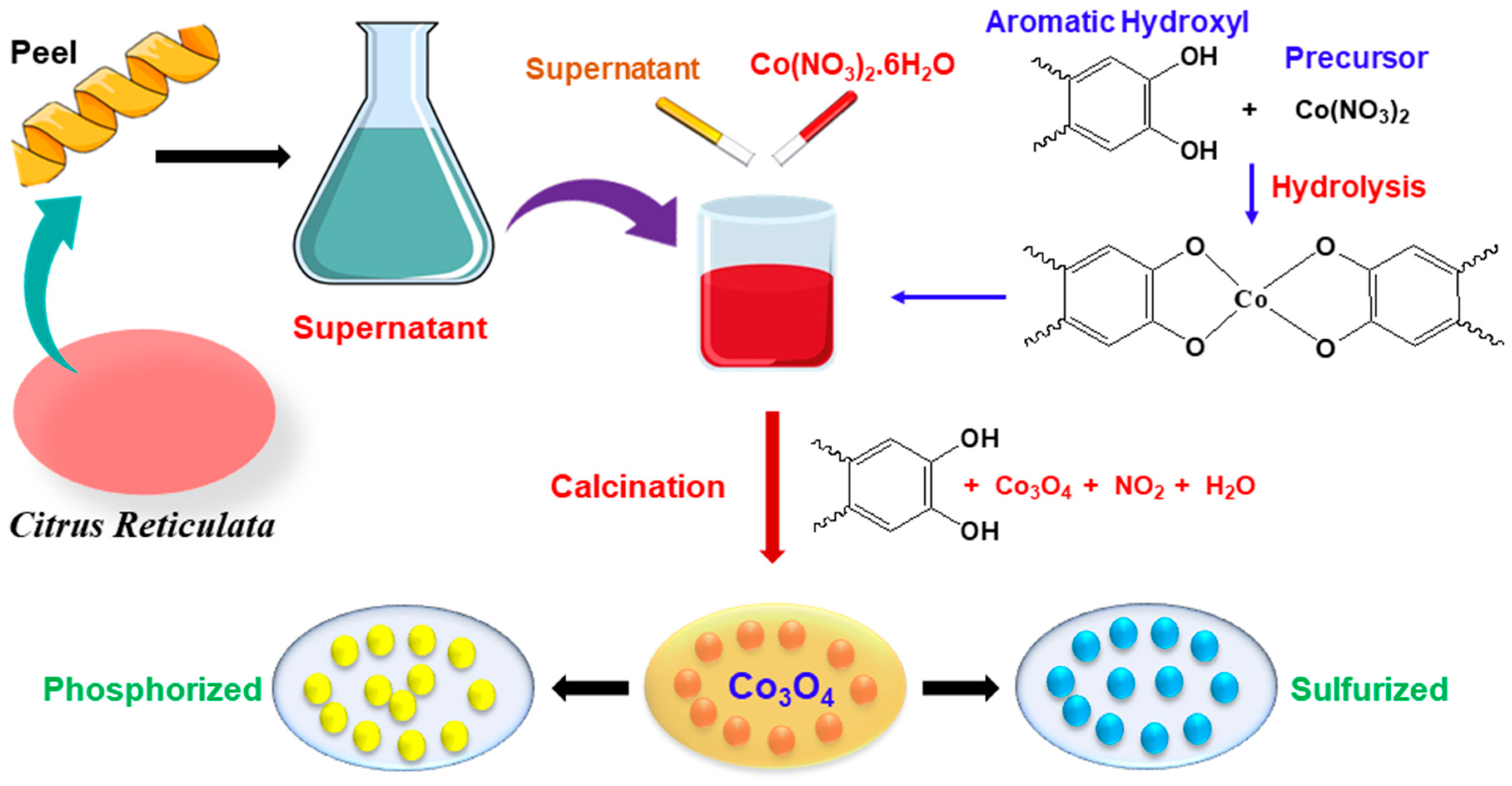
2.2. Preparation of Peel Extracts
2.3. Synthesis of CoO/Co3O4 Nanoparticles
2.4. Synthesis of Cobalt Oxide Using NaOH
2.5. Sulfurization
2.6. Phosphorization
3. Results and Discussion
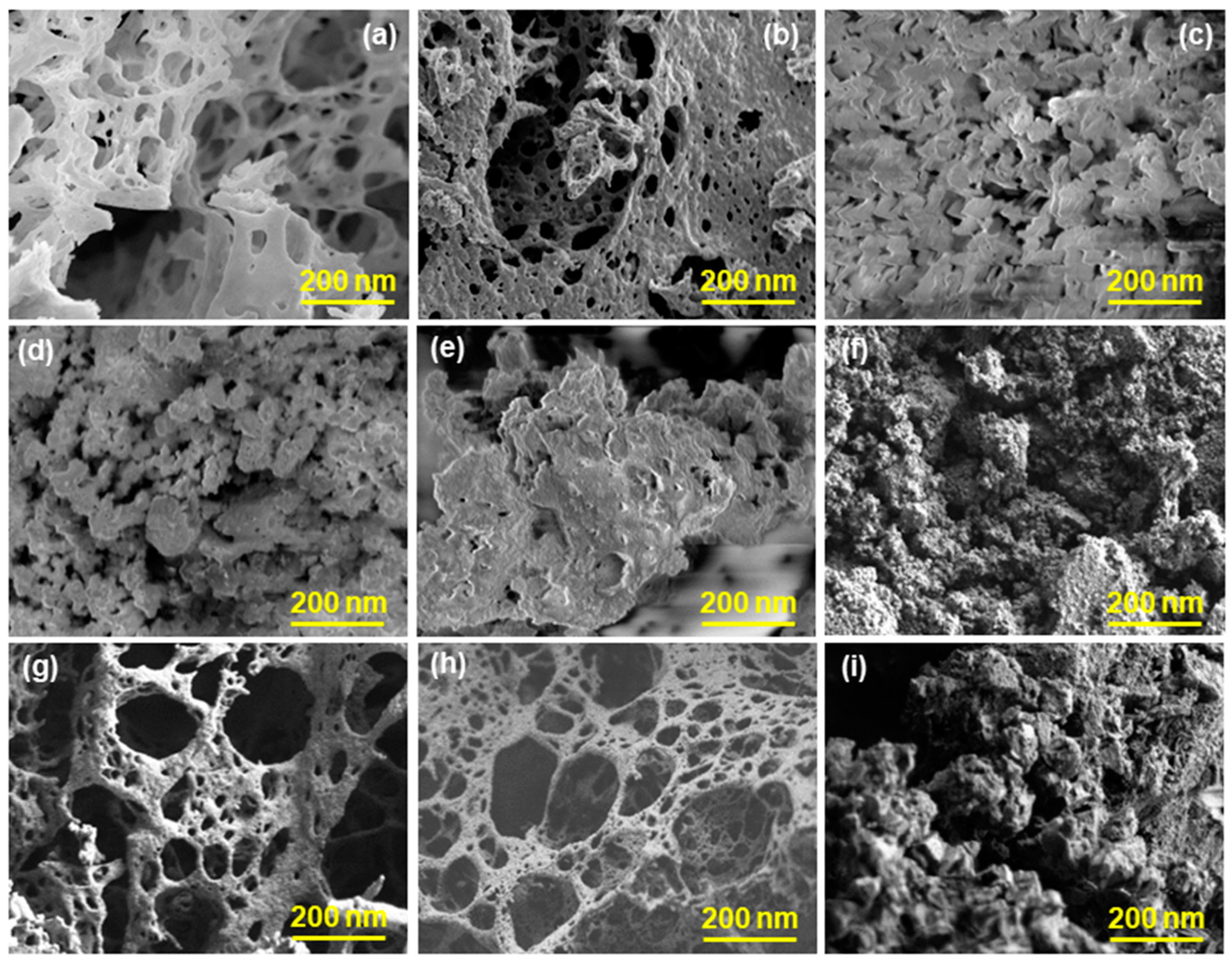
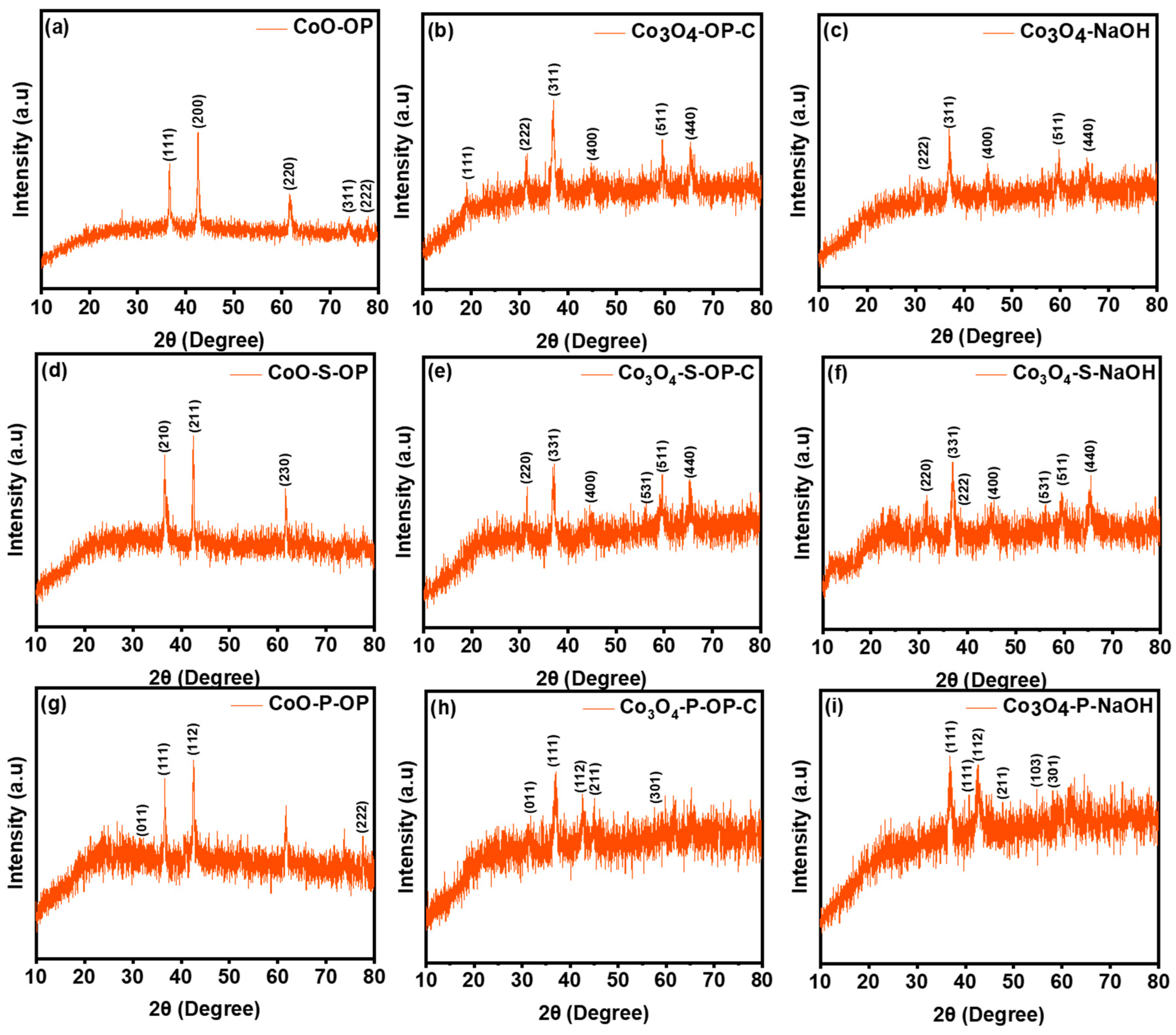
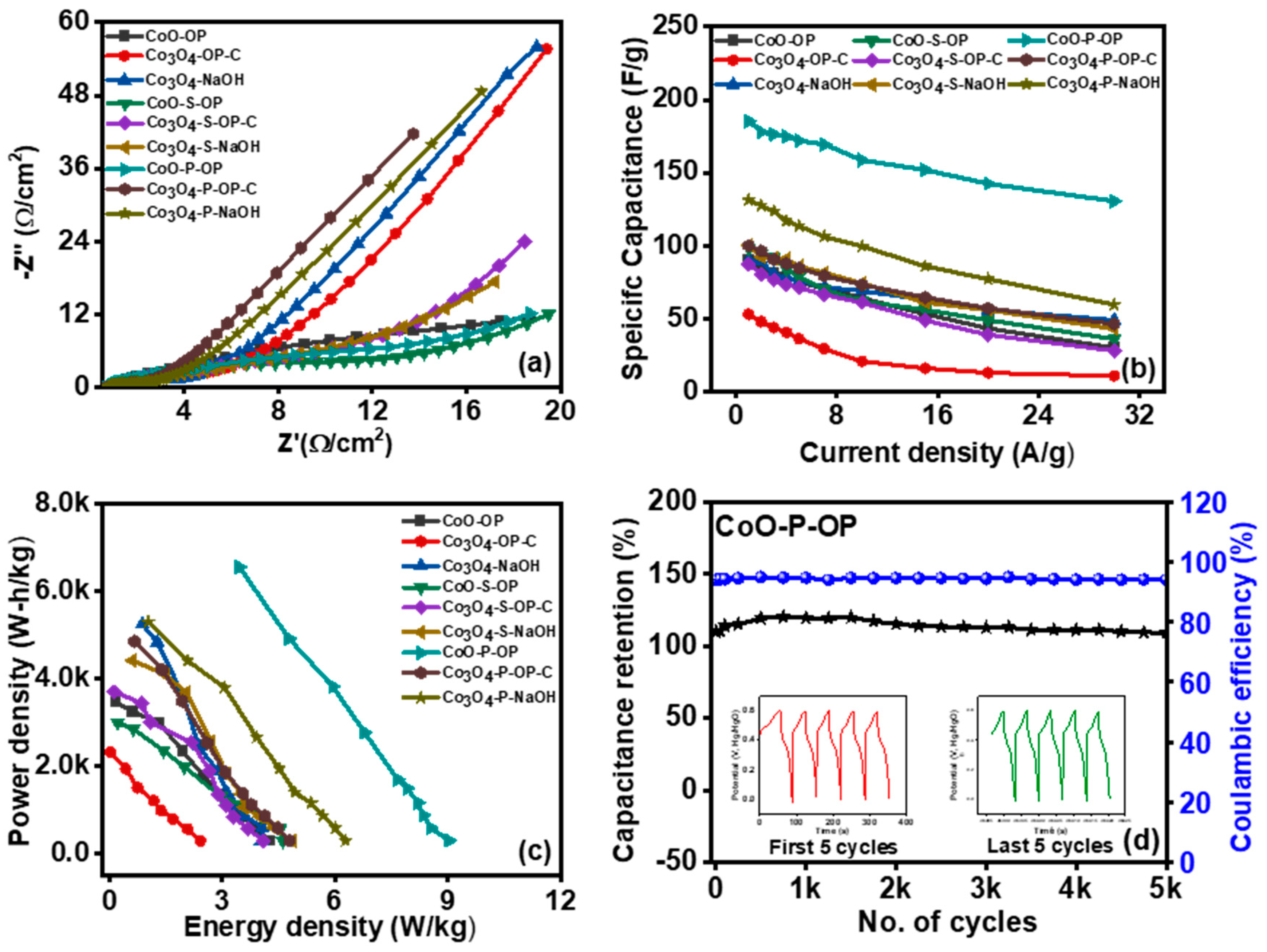
3.1. Physicochemical Characterization
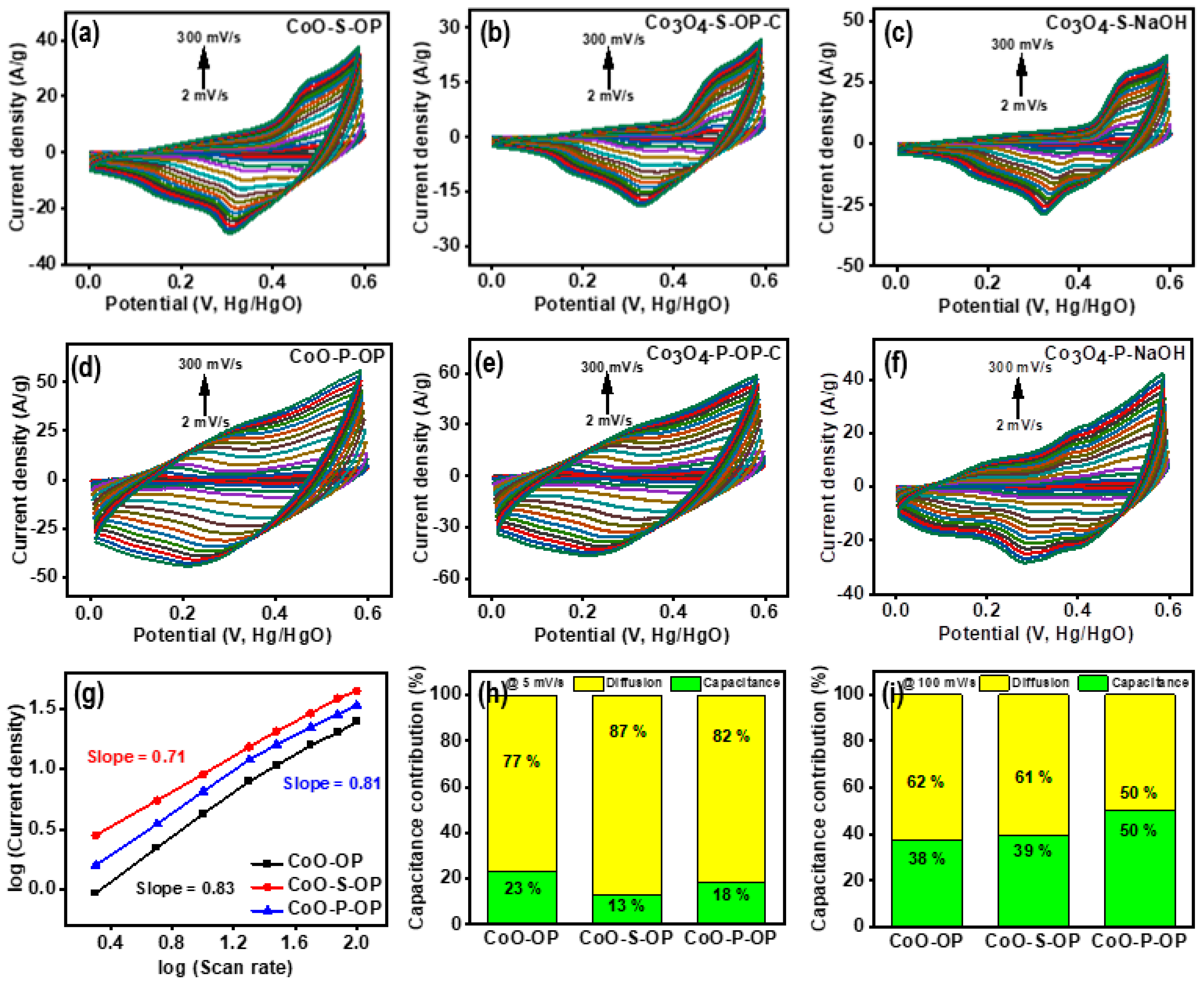
3.2. Electrochemical Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bhardwaj, S.; Martins de Souza, F.; Gupta, R.K. 4-High-performance lithium–sulfur batteries: Role of nanotechnology and nanoengineering. In Lithium-Sulfur Batteries; Gupta, R.K., Nguyen, T.A., Song, H., Yasin, G.B.T.-L.-S.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 57–73. [Google Scholar]
- Van Overmeere, Q.; Kerman, K.; Ramanathan, S. Energy Storage in Ultrathin Solid Oxide Fuel Cells. Nano Lett. 2012, 12, 3756–3760. [Google Scholar] [CrossRef] [PubMed]
- Poonam; Sharma, K.; Arora, A.; Tripathi, S.K. Review of supercapacitors: Materials and devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar] [CrossRef]
- Choi, J.; Ingsel, T.; Neupane, D.; Mishra, S.R.; Kumar, A.; Gupta, R.K. Metal-organic framework-derived cobalt oxide and sulfide having nanoflowers architecture for efficient energy conversion and storage. J. Energy Storage 2022, 50, 104145. [Google Scholar] [CrossRef]
- Itoi, H.; Suzuki, R.; Miyaji, M.; Matsuura, M.; Takagi, K.; Goto, Y.; Kameoka, S.; Nagai, Y.; Usami, T.; Adachi, Y.; et al. Synthesis of Polynorbornadiene within the Pores of Activated Carbons: Effects on EDLC and Hydrogen Adsorption Performances. Langmuir 2022, 38, 12024–12034. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Yang, Y.; Li, K.; Ma, Z.; Zhou, Y.; Xue, D. CoCl2 Designed as Excellent Pseudocapacitor Electrode Materials. ACS Sustain. Chem. Eng. 2014, 2, 440–444. [Google Scholar] [CrossRef]
- Xiang, C.; Liu, Y.; Yin, Y.; Huang, P.; Zou, Y.; Fehse, M.; She, Z.; Xu, F.; Banerjee, D.; Hermida Merino, D.; et al. Facile Green Route to Ni/Co Oxide Nanoparticle Embedded 3D Graphitic Carbon Nanosheets for High Performance Hybrid Supercapacitor Devices. ACS Appl. Energy Mater. 2019, 2, 3389–3399. [Google Scholar] [CrossRef]
- Dedova, T.; Acik, I.O.; Krunks, M.; Mikli, V.; Volobujeva, O.; Mere, A. Effect of substrate morphology on the nucleation and growth of ZnO nanorods prepared by spray pyrolysis. Thin Solid Films 2012, 520, 4650–4653. [Google Scholar] [CrossRef]
- Ayom, G.E.; Khan, M.D.; Choi, J.; Gupta, R.K.; van Zyl, W.E.; Revaprasadu, N. Synergistically enhanced performance of transition-metal doped Ni2P for supercapacitance and overall water splitting. Dalt. Trans. 2021, 50, 11821–11833. [Google Scholar] [CrossRef]
- Jensen, K.F. Chemical Vapor Deposition. In Microelectronics Processing; American Chemical Society: Washington, DC, USA, 1989; Volume 221, pp. 199–263. ISBN 9780841214750. [Google Scholar]
- Baskoutas, S.; Giabouranis, P.; Yannopoulos, S.N.; Dracopoulos, V.; Toth, L.; Chrissanthopoulos, A.; Bouropoulos, N. Preparation of ZnO nanoparticles by thermal decomposition of zinc alginate. Thin Solid Films 2007, 515, 8461–8464. [Google Scholar] [CrossRef]
- Lei, Y.; Li, J.; Wang, Y.; Gu, L.; Chang, Y.; Yuan, H.; Xiao, D. Rapid Microwave-Assisted Green Synthesis of 3D Hierarchical Flower-Shaped NiCo2O4 Microsphere for High-Performance Supercapacitor. ACS Appl. Mater. Interfaces 2014, 6, 1773–1780. [Google Scholar] [CrossRef]
- Chaudhary, J.P.; Gupta, R.; Mahto, A.; Vadodariya, N.; Dharmalingm, K.; Sanna Kotrappanavar, N.; Meena, R. Self-Doped Interwoven Carbon Network Derived from Ulva fasciata for All-Solid Supercapacitor Devices: Solvent-Free Approach to a Scalable Synthetic Route. ACS Sustain. Chem. Eng. 2019, 7, 174–186. [Google Scholar] [CrossRef]
- Veerakumar, P.; Sangili, A.; Manavalan, S.; Thanasekaran, P.; Lin, K.-C. Research Progress on Porous Carbon Supported Metal/Metal Oxide Nanomaterials for Supercapacitor Electrode Applications. Ind. Eng. Chem. Res. 2020, 59, 6347–6374. [Google Scholar] [CrossRef]
- Yao, W.R.; Wang, H.Y.; Wang, S.T.; Sun, S.L.; Zhou, J.; Luan, Y.Y. Assessment of the Antibacterial Activity and the Antidiarrheal Function of Flavonoids from Bayberry Fruit. J. Agric. Food Chem. 2011, 59, 5312–5317. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.-G.; Luo, X.-D. Meliaceous Limonoids: Chemistry and Biological Activities. Chem. Rev. 2011, 111, 7437–7522. [Google Scholar] [CrossRef]
- Chen, B.H.; Tang, Y.C. Processing and Stability of Carotenoid Powder from Carrot Pulp Waste. J. Agric. Food Chem. 1998, 46, 2312–2318. [Google Scholar] [CrossRef]
- Han, C.; Andersen, J.; Pillai, S.C.; Fagan, R.; Falaras, P.; Byrne, J.A.; Dunlop, P.S.M.; Choi, H.; Jiang, W.; O’Shea, K.; et al. Chapter green nanotechnology: Development of nanomaterials for environmental and energy applications. ACS Symp. Ser. 2013, 1124, 201–229. [Google Scholar] [CrossRef]
- Jiang, K.; Xiong, P.; Ji, J.; Zhu, J.; Ma, R.; Sasaki, T.; Geng, F. Two-Dimensional Molecular Sheets of Transition Metal Oxides toward Wearable Energy Storage. Acc. Chem. Res. 2020, 53, 2443–2455. [Google Scholar] [CrossRef]
- Chang, S.; Pu, J.; Wang, J.; Du, H.; Zhou, Q.; Liu, Z.; Zhu, C.; Li, J.; Zhang, H. Electrochemical Fabrication of Monolithic Electrodes with Core/Shell Sandwiched Transition Metal Oxide/Oxyhydroxide for High-Performance Energy Storage. ACS Appl. Mater. Interfaces 2016, 8, 25888–25895. [Google Scholar] [CrossRef]
- Tedstone, A.A.; Lewis, D.J.; O’Brien, P. Synthesis, Properties, and Applications of Transition Metal-Doped Layered Transition Metal Dichalcogenides. Chem. Mater. 2016, 28, 1965–1974. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, H.; Han, Z.; Bo, Z.; Yan, J.; Cen, K.; Ostrikov, K.K. MXene-Based Electrodes for Supercapacitor Energy Storage. Energy Fuels 2022, 36, 2390–2406. [Google Scholar] [CrossRef]
- Salunkhe, R.R.; Kaneti, Y.V.; Yamauchi, Y. Metal–Organic Framework-Derived Nanoporous Metal Oxides toward Supercapacitor Applications: Progress and Prospects. ACS Nano 2017, 11, 5293–5308. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, K.; Tiwari, A.P.; Dahal, B.; Ojha, G.P.; Mukhiya, T.; Lee, M.; Kim, T.; Chae, S.H.; Muthurasu, A.; Kim, H.Y. A ZIF-8-Derived Nanoporous Carbon Nanocomposite Wrapped with Co3O4-Polyaniline as an Efficient Electrode Material for an Asymmetric Supercapacitor; Elsevier B.V: Amsterdam, The Netherlands, 2020; Volume 856, ISBN 8263270235. [Google Scholar]
- Bo, Z.; Yi, K.; Yang, H.; Guo, X.; Huang, Z.; Zheng, Z.; Yan, J.; Cen, K.; Ostrikov, K. (Ken) More from Less but Precise: Industry-relevant Pseudocapacitance by Atomically-precise Mass-loading MnO2 within Multifunctional MXene Aerogel. J. Power Sources 2021, 492, 229639. [Google Scholar] [CrossRef]
- Liu, X.Y.; Gao, Y.Q.; Yang, G.W. A flexible, transparent and super-long-life supercapacitor based on ultrafine Co3O4 nanocrystal electrodes. Nanoscale 2016, 8, 4227–4235. [Google Scholar] [CrossRef] [PubMed]
- Madhu, R.; Veeramani, V.; Chen, S.-M.; Manikandan, A.; Lo, A.-Y.; Chueh, Y.-L. Honeycomb-like Porous Carbon–Cobalt Oxide Nanocomposite for High-Performance Enzymeless Glucose Sensor and Supercapacitor Applications. ACS Appl. Mater. Interfaces 2015, 7, 15812–15820. [Google Scholar] [CrossRef] [PubMed]
- Kandalkar, S.G.; Dhawale, D.S.; Kim, C.-K.; Lokhande, C.D. Chemical synthesis of cobalt oxide thin film electrode for supercapacitor application. Synth. Met. 2010, 160, 1299–1302. [Google Scholar] [CrossRef]
- Zapata, B.; Balmaseda, J.; Fregoso-Israel, E.; Torres-García, E. Thermo-kinetics study of orange peel in air. J. Therm. Anal. Calorim. 2009, 98, 309–315. [Google Scholar] [CrossRef]
- Doan Thi, T.U.; Nguyen, T.T.; Thi, Y.D.; Ta Thi, K.H.; Phan, B.T.; Pham, K.N. Green synthesis of ZnO nanoparticles using orange fruit peel extract for antibacterial activities. RSC Adv. 2020, 10, 23899–23907. [Google Scholar] [CrossRef]
- Manteghi, F.; Kazemi, S.H.; Peyvandipour, M.; Asghari, A. Preparation and application of cobalt oxide nanostructures as electrode materials for electrochemical supercapacitors. RSC Adv. 2015, 5, 76458–76463. [Google Scholar] [CrossRef]
- Ji, Y.; Liu, X.; Liu, W.; Wang, Y.; Zhang, H.; Yang, M.; Wang, X.; Zhao, X.; Feng, S. A facile template-free approach for the solid-phase synthesis of CoS2 nanocrystals and their enhanced storage energy in supercapacitors. RSC Adv. 2014, 4, 50220–50225. [Google Scholar] [CrossRef]
- Yang, Z.; Bai, X.; Zhu, S.; Qi, C. Synthesis of porous Co3S4 for enhanced voltammetric nonenzymatic determination of glucose. Microchim. Acta 2020, 187, 98. [Google Scholar] [CrossRef]
- Pan, H.; Huang, X.; Lu, Z.; Zhang, Z.; An, B.; Wu, D.; Wang, T.; Chen, X.; Cheng, F. Dual oxidation and sulfurization enabling hybrid Co/Co3O4@CoS in S/N-doped carbon matrix for bifunctional oxygen electrocatalysis and rechargeable Zn-air batteries. Chem. Eng. J. 2021, 419, 129619. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Q.; Bai, T.; Wang, W.; He, F.; Ye, M. Nickel and cobalt sulfide-based nanostructured materials for electrochemical energy storage devices. Chem. Eng. J. 2021, 409, 127237. [Google Scholar] [CrossRef]
- Pramanik, M.; Tominaka, S.; Wang, Z.-L.; Takei, T.; Yamauchi, Y. Mesoporous Semimetallic Conductors: Structural and Electronic Properties of Cobalt Phosphide Systems. Angew. Chem. Int. Ed. 2017, 56, 13508–13512. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Tu, J.; Mai, Y.; Wang, X.; Gu, C.; Zhao, X. Self-supported hydrothermal synthesized hollow Co3O4 nanowire arrays with high supercapacitor capacitance. J. Mater. Chem. 2011, 21, 9319–9325. [Google Scholar] [CrossRef]
- Liu, F.; Su, H.; Jin, L.; Zhang, H.; Chu, X.; Yang, W. Facile synthesis of ultrafine cobalt oxide nanoparticles for high-performance supercapacitors. J. Colloid Interface Sci. 2017, 505, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, R.M.; Chhetri, K.; Saud, S.; Teke, S.; Kim, S.J.; Mok, Y.S. Eco-Friendly Synthesis of Cobalt Molybdenum Hydroxide 3d Nanostructures on Carbon Fabric Coupled with Cherry Flower Waste-Derived Activated Carbon for Quasi-Solid-State Flexible Asymmetric Supercapacitors. ACS Appl. Nano Mater. 2022, 5, 160–175. [Google Scholar] [CrossRef]
- Xing, J.-C.; Zhu, Y.-L.; Zhou, Q.-W.; Zheng, X.-D.; Jiao, Q.-J. Fabrication and shape evolution of CoS2 octahedrons for application in supercapacitors. Electrochim. Acta 2014, 136, 550–556. [Google Scholar] [CrossRef]
- Mao, M.; Mei, L.; Wu, L.; Li, Q.; Zhang, M. Facile synthesis of cobalt sulfide/carbon nanotube shell/core composites for high performance supercapacitors. RSC Adv. 2014, 4, 12050–12056. [Google Scholar] [CrossRef]
- Jin, Y.; Zhao, C.; Jiang, Q.; Ji, C. Hierarchically mesoporous micro/nanostructured CoP nanowire electrodes for enhanced performance supercapacitors. Colloids Surf. A Physicochem. Eng. Asp. 2018, 553, 58–65. [Google Scholar] [CrossRef]
- Zhou, Q.; Gong, Y.; Tao, K. Calcination/phosphorization of dual Ni/Co-MOF into NiCoP/C nanohybrid with enhanced electrochemical property for high energy density asymmetric supercapacitor. Electrochim. Acta 2019, 320, 134582. [Google Scholar] [CrossRef]
- Guragain, D.; Zequine, C.; Bhattarai, R.; Choi, J.; Gupta, R.K.; Shen, X.; Mishra, S.R. Effect of dopant on the morphology and electrochemical performance of Ni1-xCaxCo2O4 (0 = x = 0.8) oxide hierarchical structures. MRS Adv. 2020, 5, 2487–2494. [Google Scholar] [CrossRef]
- Chhetri, K.; Kim, T.; Acharya, D.; Muthurasu, A.; Dahal, B.; Bhattarai, R.M.; Lohani, P.C.; Pathak, I.; Ji, S.; Ko, T.H.; et al. Hollow Carbon Nanofibers with Inside-outside Decoration of Bi-metallic MOF Derived Ni-Fe Phosphides as Electrode Materials for Asymmetric Supercapacitors. Chem. Eng. J. 2022, 450, 138363. [Google Scholar] [CrossRef]
- Choi, J.; Morey, K.; Kumar, A.; Neupane, D.; Mishra, S.R.; Perez, F.; Gupta, R.K. Self-assembled cotton-like copper–molybdenum sulfide and phosphide as a bifunctional electrode for green energy storage and production. Mater. Today Chem. 2022, 24, 100848. [Google Scholar] [CrossRef]
- Liang, H.; Xia, C.; Jiang, Q.; Gandi, A.N.; Schwingenschlögl, U.; Alshareef, H.N. Low temperature synthesis of ternary metal phosphides using plasma for asymmetric supercapacitors. Nano Energy 2017, 35, 331–340. [Google Scholar] [CrossRef]
- Gautam, K.P.; Acharya, D.; Bhatta, I.; Subedi, V.; Das, M.; Neupane, S.; Kunwar, J.; Chhetri, K.; Yadav, A.P. Nickel Oxide-Incorporated Polyaniline Nanocomposites as an Efficient Electrode Material for Supercapacitor Application. Inorganics 2022, 10, 86. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, L.; Lai, G.; Wei, M.; Jiang, X.; Bai, L. Nitrogen-Doped Hierarchically Porous Carbons Derived from Polybenzoxazine for Enhanced Supercapacitor Performance. Nanomaterials 2019, 9, 131. [Google Scholar] [CrossRef]
- Tiwari, A.P.; Chhetri, K.; Kim, H.; Ji, S.; Chae, S.H.; Kim, T.; Kim, H.Y. Self-assembled polypyrrole hierarchical porous networks as the cathode and porous three dimensional carbonaceous networks as the anode materials for asymmetric supercapacitor. J. Energy Storage 2021, 33, 102080. [Google Scholar] [CrossRef]
- Sahoo, S.; Satpati, A.K.; Sahoo, P.K.; Naik, P.D. Incorporation of Carbon Quantum Dots for Improvement of Supercapacitor Performance of Nickel Sulfide. ACS Omega 2018, 3, 17936–17946. [Google Scholar] [CrossRef]
- Lu, X.; Yang, H.; Bo, Z.; Gong, B.; Cao, M.; Chen, X.; Wu, E.; Yan, J.; Cen, K.; Ostrikov, K. Aligned Ti3C2TX Aerogel with High Rate Performance, Power Density and Sub-Zero-Temperature Stability. Energies 2022, 15, 1191. [Google Scholar] [CrossRef]
- Zallouz, S.; Réty, B.; Vidal, L.; Le Meins, J.M.; Matei Ghimbeu, C. Co3O4Nanoparticles Embedded in Mesoporous Carbon for Supercapacitor Applications. ACS Appl. Nano Mater. 2021, 4, 5022–5037. [Google Scholar] [CrossRef]
- Bhattarai, R.M.; Chhetri, K.; Natarajan, S.; Saud, S.; Kim, S.J.; Mok, Y.S. Activated carbon derived from cherry flower biowaste with a self-doped heteroatom and large specific surface area for supercapacitor and sodium-ion battery applications. Chemosphere 2022, 303, 135290. [Google Scholar] [CrossRef] [PubMed]
- Al Jahdaly, B.A.; Abu-Rayyan, A.; Taher, M.M.; Shoueir, K. Phytosynthesis of Co3O4 Nanoparticles as the High Energy Storage Material of an Activated Carbon/Co3O4 Symmetric Supercapacitor Device with Excellent Cyclic Stability Based on a Na2SO4 Aqueous Electrolyte. ACS Omega 2022, 7, 23673–23684. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, I.; Ahmad, K.S.; Zequine, C.; Gupta, R.K.; Thomas, A.; Malik, M.A. Organic template-assisted green synthesis of CoMoO4 nanomaterials for the investigation of energy storage properties. RSC Adv. 2020, 10, 8115–8129. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liu, J.; Chen, T.; Tan, K.S.; Jia, X.; Luo, Z.; Cong, C.; Yang, H.; Li, C.M.; Yu, T. Fabrication of Co3O4-reduced graphene oxide scrolls for high-performance supercapacitor electrodes. Phys. Chem. Chem. Phys. 2011, 13, 14462–14465. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Wang, Y.; Shi, Y.; Wong, J.I.; Yang, H.Y. CoO nanoflowers woven by CNT network for high energy density flexible micro-supercapacitor. Nano Energy 2014, 3, 46–54. [Google Scholar] [CrossRef]
- Kim, B.H.; Yang, K.S. Enhanced electrical capacitance of porous carbon nanofibers derived from polyacrylonitrile and boron trioxide. Electrochim. Acta 2013, 88, 597–603. [Google Scholar] [CrossRef]
- Dai, M.; Song, L.; Labelle, J.T.; Vogt, B.D. Ordered mesoporous carbon composite films containing cobalt oxide and vanadia for electrochemical applications. Chem. Mater. 2011, 23, 2869–2878. [Google Scholar] [CrossRef]
- Guan, Q.; Cheng, J.; Wang, B.; Ni, W.; Gu, G.; Li, X.; Huang, L.; Yang, G.; Nie, F. Needle-like Co3O4 anchored on the graphene with enhanced electrochemical performance for aqueous supercapacitors. ACS Appl. Mater. Interfaces 2014, 6, 7626–7632. [Google Scholar] [CrossRef]
- Lima-Tenório, M.K.; Ferreira, C.S.; Felix Rebelo, Q.H.; Brambilla de Souza, R.F.; Passos, R.R.; Gómes Pineda, E.A.; Pocrifka, L.A. Pseudocapacitance properties of Co3O4 nanoparticles synthesized using a modified sol-gel method. Mater. Res. 2018, 21, 1–7. [Google Scholar] [CrossRef]

| Sample Name | CoO-OP | Co3O4-OP-C | Co3O4-NaOH | CoO-S-OP | Co3O4-S-OP-C | Co3O4-S-NaOH | CoO-P-OP | Co3O4-P-OP-C | Co3O4-P-NaOH |
|---|---|---|---|---|---|---|---|---|---|
| Rs (Ω) | 0.95 | 0.75 | 0.99 | 1.32 | 1.26 | 1.59 | 0.70 | 0.705 | 0.76 |
| Rct (Ω) | 3.85 | 3.25 | 3.71 | 6.19 | 4.24 | 4.73 | 1.52 | 2.81 | 2.42 |
| Sample | Specific Capacitance F/g @ 1A/g | Energy Density Wh/kg | Power Density W/kg | Ref. |
|---|---|---|---|---|
| Ultrafine Co3O4 | 165 | 3 | 31 | [26] |
| C/Co3O4-750 | 44 | 1.66 | 18.75 | [53] |
| CFAC-800 | 133 | - | - | [54] |
| AC/Co3O4-NP | 182 | 25.27 | 585 | [55] |
| CoMoO4 | 128 | 7.3 | 146 | [56] |
| Co3O4/r-GO | 163.8 | - | - | [57] |
| CoO/CNT | 17.4 @ 0.25 A/g | 3.48 mWh/g @ 0.25 A/g | - | [58] |
| N,B, Co doped C | 184 | 18.7 | 400 | [59] |
| Mesoporous C/Co | 113 | - | - | [60] |
| Needle-like cobalt oxide/graphene composite | 60 | - | - | [61] |
| Pongam seed shell derived Carbon and Co3O4 | 94 | - | - | [27] |
| Co3O4 NPs | 120 | - | - | [62] |
| Co3O4 | 118 | 5.8 | 330 | [28] |
| CoO-OP | 90 | 4.2 | 292 | Our work |
| CoO-S-OP | 98 | 4.6 | 291 | Our work |
| CoO-P-OP | 185 | 9 | 296 | Our work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srivastava, R.; Bhardwaj, S.; Kumar, A.; Singhal, R.; Scanley, J.; Broadbridge, C.C.; Gupta, R.K. Waste Citrus reticulata Assisted Preparation of Cobalt Oxide Nanoparticles for Supercapacitors. Nanomaterials 2022, 12, 4119. https://doi.org/10.3390/nano12234119
Srivastava R, Bhardwaj S, Kumar A, Singhal R, Scanley J, Broadbridge CC, Gupta RK. Waste Citrus reticulata Assisted Preparation of Cobalt Oxide Nanoparticles for Supercapacitors. Nanomaterials. 2022; 12(23):4119. https://doi.org/10.3390/nano12234119
Chicago/Turabian StyleSrivastava, Rishabh, Shiva Bhardwaj, Anuj Kumar, Rahul Singhal, Jules Scanley, Christine C. Broadbridge, and Ram K. Gupta. 2022. "Waste Citrus reticulata Assisted Preparation of Cobalt Oxide Nanoparticles for Supercapacitors" Nanomaterials 12, no. 23: 4119. https://doi.org/10.3390/nano12234119
APA StyleSrivastava, R., Bhardwaj, S., Kumar, A., Singhal, R., Scanley, J., Broadbridge, C. C., & Gupta, R. K. (2022). Waste Citrus reticulata Assisted Preparation of Cobalt Oxide Nanoparticles for Supercapacitors. Nanomaterials, 12(23), 4119. https://doi.org/10.3390/nano12234119








