Chemical Vapor Deposition-Fabricated Manganese-Doped and Potassium-Doped Hexagonal Tungsten Trioxide Nanowires with Enhanced Gas Sensing and Photocatalytic Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Tungsten Oxide Nanowires
2.2. Synthesis of Manganese-Doped and Potassium-Doped Tungsten Oxide Nanowires
2.3. Electrical Measurements
2.4. Preparation of Gas-Sensing Micro-Devices
2.5. Photodegradation Experiments
2.6. Gas Sensing Experiments
3. Results
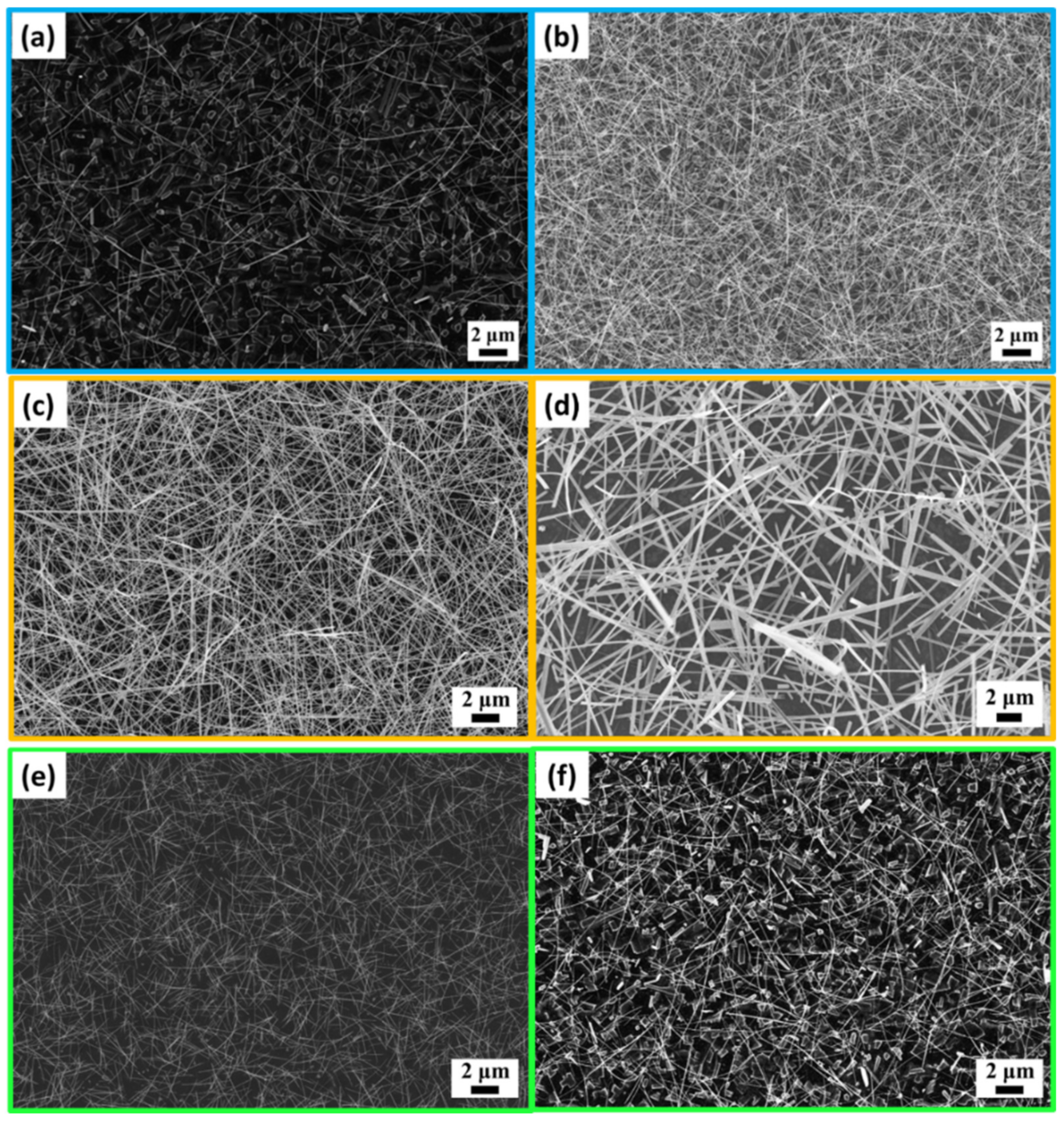
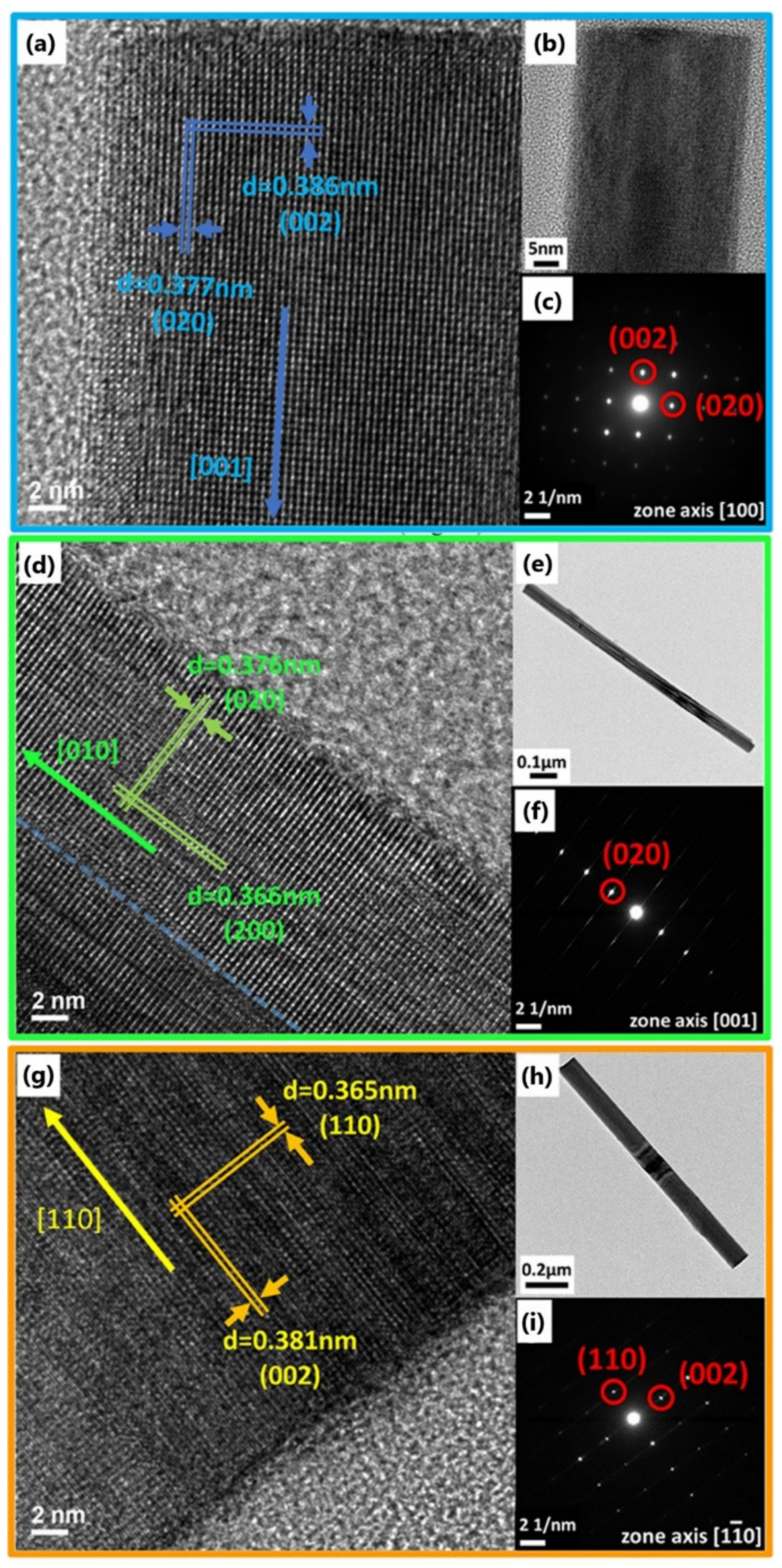
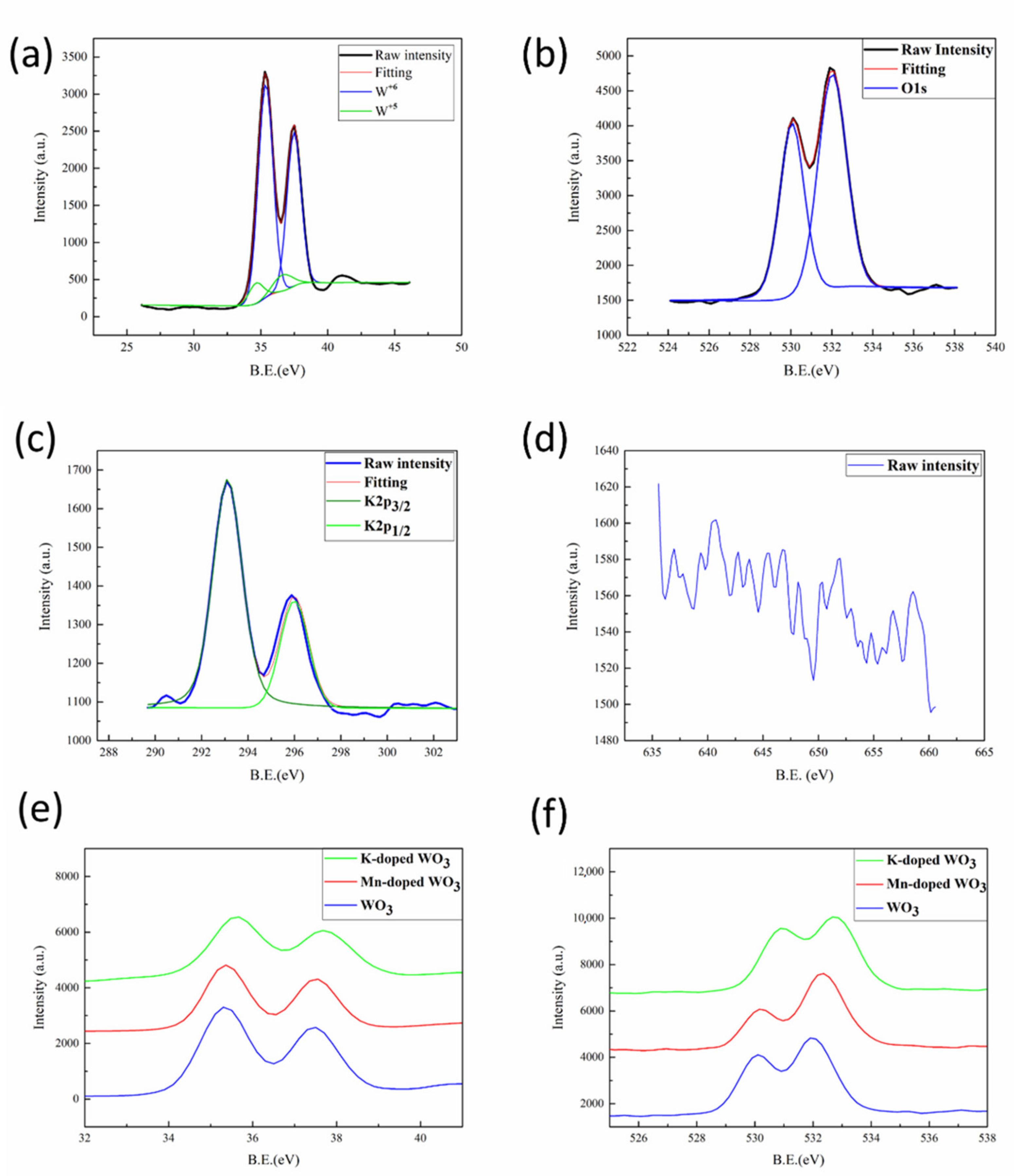
3.1. Synthesis and Characterization of WO3, Mn-Doped WO3, and K-Doped WO3 Nanowires
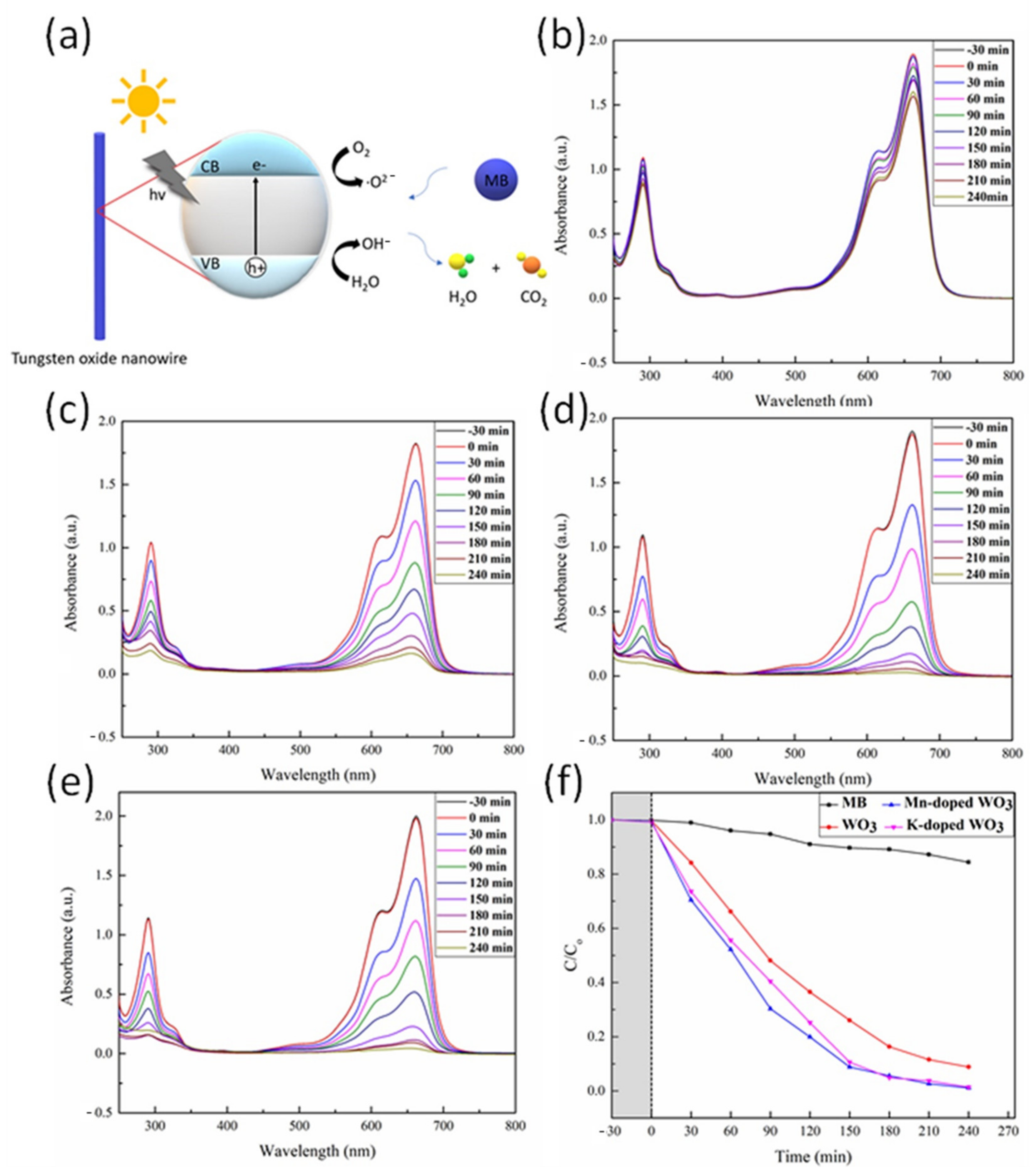
3.2. Photodegradation of Methlene Blue
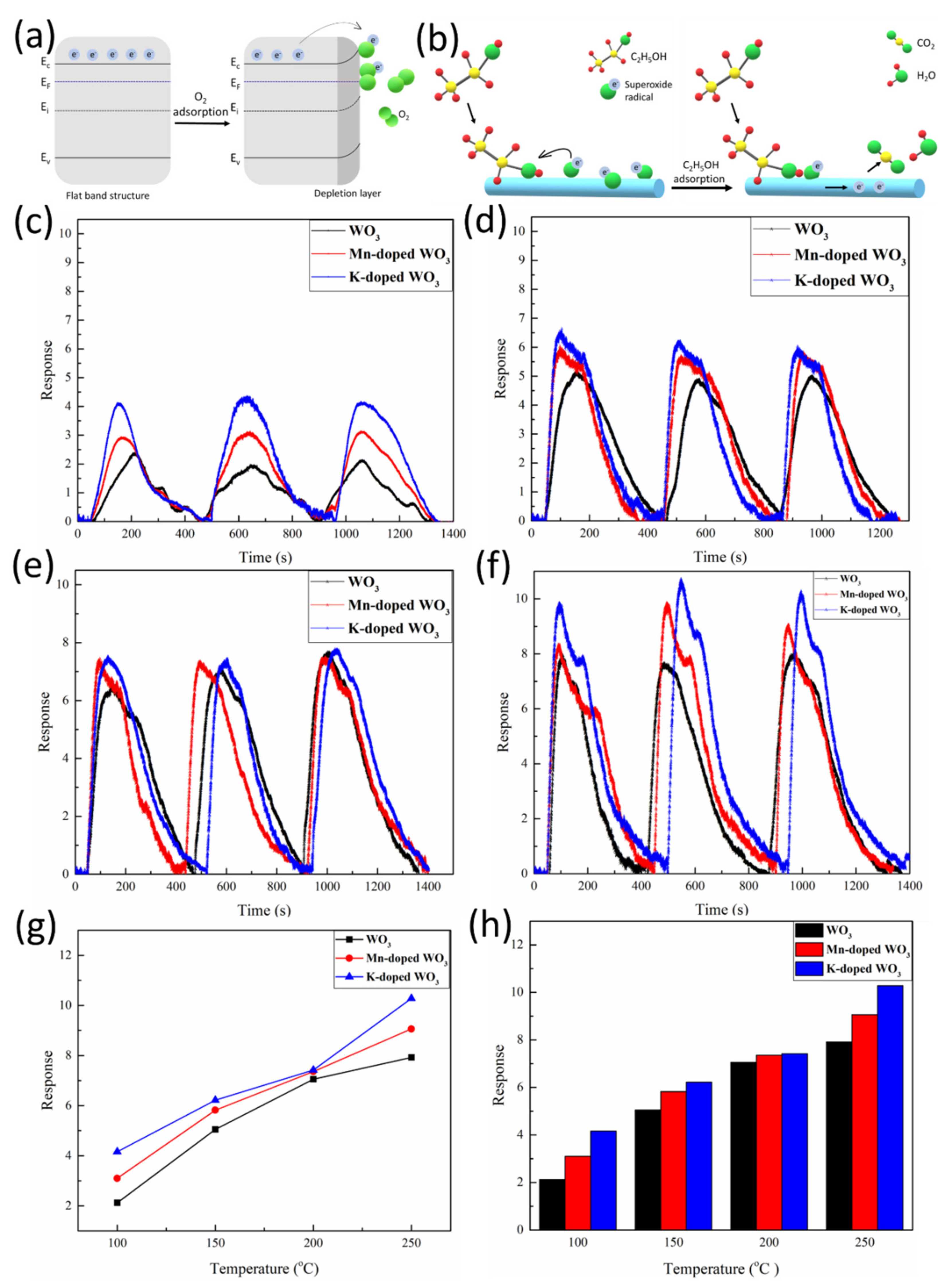
3.3. Gas Sensing Mechanism
3.4. Gas Sensing Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mohammad, A.; Khan, M.E.; Yoon, T.; Cho, M.H. Na,O-co-doped-graphitic-carbon nitride (Na,O-g-C3N4) for nonenzymatic electrochemical sensing of hydrogen peroxide. Appl. Surf. Sci. 2020, 525, 146353. [Google Scholar] [CrossRef]
- Pal, B.; Vijayan, B.L.; Krishnan, S.G.; Harilal, M.; Basirun, W.J.; Lowe, A.; Yusoff, M.M.; Jose, R. Hydrothermal syntheses of tungsten doped TiO2 and TiO2/WO3. J. Alloys Compd. 2018, 740, 703–710. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.; Zhang, Q. Rechargeable lithium battery based on a single hexagonal tungsten trioxide nanowire. Nano Energy 2012, 1, 172–175. [Google Scholar] [CrossRef]
- Zhang, W.H.; Yue, L.; Zhang, F.; Zhang, Q.F.; Gui, X.C.; Guan, R.F.; Hou, G.H.; Xu, N. One-step in situ synthesis of ultrathin tungsten oxide@carbon nanowire webs as an anode material for high performance. J. Mater. Chem. A 2015, 3, 6102–6109. [Google Scholar] [CrossRef]
- Huang, K.; Pan, Q.T.; Yang, F.; Ni, S.B.; He, D.Y. Synthesis and field-emission properties of the tungsten oxide nanowire arrays. Physica E 2007, 39, 219–222. [Google Scholar] [CrossRef]
- Chakrapani, V.; Thangala, J.; Sunkara, M.K. WO3 and W2N nanowire arrays for photoelectrochemical hydrogen production. Int. J. Hydrogen Energy 2009, 34, 9050–9059. [Google Scholar] [CrossRef]
- Li, Y.; Chopra, N. Structural evolution of cobalt oxide–tungsten oxide nanowire heterostructures for photocatalysis. J. Catal. 2015, 329, 514–521. [Google Scholar] [CrossRef]
- Zhao, B.S.; Qiang, X.Y.; Qin, Y.; Hu, M. Tungsten oxide nanowire gas sensor preparation and P-type NO2 sensing properties at room temperature. Acta Phys. Sin. 2018, 67, 8. [Google Scholar]
- Tomic, M.; Setka, M.; Chmela, O.; Gracia, I.; Figueras, E.; Cane, C.; Vallejos, S. Cerium Oxide-Tungsten Oxide Core-Shell Nanowire-Based Microsensors Sensitive to Acetone. Biosensors 2018, 8, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.L.; Liu, F.; Xu, N.S.; Chen, J.; Deng, S.Z. A study of control growth of three-dimensional nanowire networks of tungsten oxides: From aligned nanowires through hybrid nanostructures to 3D networks. J. Cryst. Growth 2010, 312, 520–526. [Google Scholar] [CrossRef]
- Zhou, D.; Shi, F.; Xie, D.; Wang, D.H.; Xia, X.H.; Wang, X.L.; Gu, C.D.; Tu, J.P. Bi-functional Mo-doped WO3 nanowire array electrochromism-plus electrochemical energy storage. J. Colloid Interface Sci. 2016, 465, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.L.; Tabata, I.; Hirogaki, K.; Hisada, K.; Wang, T.; Wang, S.; Hori, T. Preparation of platinum-loaded cubic tungsten oxide: A highly efficient visible light-driven photocatalyst. Mater. Lett. 2011, 65, 1252–1256. [Google Scholar] [CrossRef]
- Zheng, H.D.; Ou, J.Z.; Strano, M.S.; Kaner, R.B.; Mitchell, A.; Kalantar-Zadeh, K. Nanostructured Tungsten Oxide—Properties, Synthesis, and Applications. Adv. Funct. Mater. 2011, 21, 2175–2196. [Google Scholar] [CrossRef]
- Wang, Y.L.; Wang, X.L.; Li, Y.H.; Fang, L.J.; Zhao, J.J.; Du, X.L.; Chen, A.P.; Yang, H.G. Controllable Synthesis of Hexagonal WO3 Nanoplates for Efficient Visible-Light-Driven Photocatalytic Oxygen Production. Chem. Asian J. 2017, 12, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.Y.; Gong, L.; Liu, X.; Tao, Y.T.; Zhang, W.H.; Chen, S.H.; Meng, H.; Chen, J. XPS studies on surface reduction of tungsten oxide nanowire film by Ar+ bombardment. J. Electron. Spectros. Relat. Phenomena 2012, 185, 112–118. [Google Scholar] [CrossRef]
- Khan, M.E.; Khan, M.M.; Cho, M.H. Fabrication of WO3 nanorods on graphene nanosheets for improved visible light-induced photocapacitive and photocatalytic performance. RSC Adv. 2016, 6, 20824–20833. [Google Scholar] [CrossRef]
- Szilágyi, I.M.; Madarász, J.; Pokol, G.; Király, P.; Tárkányi, G.; Saukko, S.; Mizsei, J.; Tóth, A.L.; Szabó, A.; Josepovits, K.V. Stability and Controlled Composition of Hexagonal WO3. Chem. Mater. 2008, 20, 4116–4125. [Google Scholar] [CrossRef]
- Zhu, M.S.; Meng, W.J.; Huang, Y.; Huang, Y.; Zhi, C.Y. Proton-Insertion-Enhanced Pseudocapacitance Based on the Assembly Structure of Tungsten Oxide. ACS Appl. Mater. Interfaces 2014, 6, 18901–18910. [Google Scholar] [CrossRef] [PubMed]
- Nwanya, A.C.; Jafta, C.J.; Ejikeme, P.M.; Ugwuoke, P.E.; Reddy, M.V.; Osuji, R.U.; Ozoemena, K.I.; Ezema, F.I. Electrochromic and electrochemical capacitive properties of tungsten oxide and its polyaniline nanocomposite films obtained by chemical bath deposition method. Electrochim. Acta 2014, 128, 218–225. [Google Scholar] [CrossRef] [Green Version]
- Su, J.Z.; Feng, X.J.; Sloppy, J.D.; Guo, L.J.; Grimes, C.A. Vertically Aligned WO3 Nanowire Arrays Grown Directly on Transparent Conducting Oxide Coated Glass: Synthesis and Photoelectrochemical Properties. Nano Lett. 2011, 11, 203–208. [Google Scholar] [CrossRef]
- Kunyapat, T.; Xu, F.; Neate, N.; Wang, N.N.; De Sanctis, A.; Russo, S.; Zhang, S.W.; Xia, Y.D.; Zhu, Y.Q. Ce-Doped bundled ultrafine diameter tungsten oxide nanowires with enhanced electrochromic performance. Nanoscale 2018, 10, 4718–4726. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Yang, S.M.; Lu, K.C. Synthesis of High-Density Indium Oxide Nanowires with Low Electrical Resistivity. Nanomaterials 2020, 10, 2100. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.M.; Yen, H.K.; Lu, K.C. Synthesis and Characterization of Indium Tin oxide Nanowires with Surface Modification with Silver Nanoparticles by Electrochemical Method. Nanomaterials 2022, 12, 897. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Yang, S.M.; Liao, T.T.; Lu, K.C. Synthesis of morphology-improved single-crystalline iron silicide nanowires with enhanced physical characteristics. CrystEngComm 2021, 23, 3270–3275. [Google Scholar] [CrossRef]
- Gu, W.; Choi, H.; Kim, K.K. Universal approach to accurate resistivity measurement for a single nanowire: Theory and application. Appl. Phys. 2006, 89, 253102. [Google Scholar] [CrossRef]
- Huang, M.H.; Naresh, G.; Chen, H.S. Facet-Dependent Electrical, Photocatalytic, and Optical Properties of Semiconductor Crystals and Their Implications for Applications. ACS Appl. Mater. Interfaces 2018, 10, 4–15. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Zhang, P.X.; Peng, F.; Bowen, C.; Huo, J.; Deng, L.B. Tailoring the geometric and electronic structure of tungsten oxide with manganese or vanadium doping toward highly efficient electrochemical and photoelectrochemical water splitting. J. Mater. Chem. A 2019, 7, 6161–6172. [Google Scholar] [CrossRef]
- Kalanur, S.S.; Yoo, I.H.; Seo, H. Fundamental investigation of Ti doped WO3 photoanode and their influence on photoelectrochemical water splitting activity. Electrochim. Acta 2017, 254, 348–357. [Google Scholar] [CrossRef]
- Dalavi, D.S.; Devan, R.S.; Patil, R.A.; Patil, R.S.; Ma, Y.R.; Sadale, S.B.; Kim, I.; Kim, J.H.; Patil, P.S. Efficient electrochromic performance of nanoparticulate WO3 thin films. J. Mater. Chem. C 2013, 1, 3722–3728. [Google Scholar] [CrossRef]
- Saasa, V.; Malwela, T.; Lemmer, Y.; Beukes, M.; Mwakikunga, B. The hierarchical nanostructured Co-doped WO3/carbon and their improved acetone sensing perfomance. Mater. Sci. Semicond. Process 2020, 117, 9. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Hou, T.T.; Xu, Q.; Wang, Q.Y.; Bai, Y.; Yang, S.K.; Rao, D.W.; Wu, L.H.; Pan, H.B.; Chen, J.F.; et al. Dual-Metal Sites Boosting Polarization of Nitrogen Molecules for Efficient Nitrogen Photofixation. Adv. Sci. 2021, 8, 2100302. [Google Scholar] [CrossRef]
- Ding, J.; Zhanga, L.; Liua, Q.; Dai, W.L.; Guan, G. Synergistic effects of electronic structure of WO3 nanorods with the dominant {001} exposed facets combined with silver size-dependent on the visible-light photocatalytic activity. Appl. Catal. B 2017, 203, 335–342. [Google Scholar] [CrossRef]
- Liang, Y.C.; Chang, C.W. Preparation of Orthorhombic WO3 Thin Films and Their Crystal Quality-Dependent Dye Photodegradation Ability. Coatings 2019, 9, 90. [Google Scholar] [CrossRef] [Green Version]
- Chiang, Y.J.; Pan, F.M. PdO Nanoflake Thin Films for CO Gas Sensing at Low Temperatures. J. Phys. Chem. C 2013, 117, 15593–15601. [Google Scholar] [CrossRef]
- Singh, O.; Kohli, N.; Singh, R.C. Precursor controlled morphology of zinc oxide and its sensing behaviour. Sens. Actuators B—Chem. 2013, 178, 149–154. [Google Scholar] [CrossRef]
- Ahsan, M.; Ahmad, M.Z.; Tesfamichael, T.; Bell, J.; Wlodarski, W.; Motta, N. Low temperature response of nanostructured tungsten oxide thin films toward hydrogen and ethanol. Sens. Actuators B—Chem. 2012, 173, 789–796. [Google Scholar] [CrossRef] [Green Version]
| WO3 NW | Mn-Doped WO3 NW | K-Doped WO3 NW | |
|---|---|---|---|
| Resistivity | 8.27 × 10−6 Ω·m | 1.81 × 10−5 Ω·m | 1.93 × 10−5 Ω·m |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.-R.; Fu, H.-W.; Yang, S.-M.; Lu, K.-C. Chemical Vapor Deposition-Fabricated Manganese-Doped and Potassium-Doped Hexagonal Tungsten Trioxide Nanowires with Enhanced Gas Sensing and Photocatalytic Properties. Nanomaterials 2022, 12, 1208. https://doi.org/10.3390/nano12071208
Chen P-R, Fu H-W, Yang S-M, Lu K-C. Chemical Vapor Deposition-Fabricated Manganese-Doped and Potassium-Doped Hexagonal Tungsten Trioxide Nanowires with Enhanced Gas Sensing and Photocatalytic Properties. Nanomaterials. 2022; 12(7):1208. https://doi.org/10.3390/nano12071208
Chicago/Turabian StyleChen, Pin-Ru, Hsuan-Wei Fu, Shu-Meng Yang, and Kuo-Chang Lu. 2022. "Chemical Vapor Deposition-Fabricated Manganese-Doped and Potassium-Doped Hexagonal Tungsten Trioxide Nanowires with Enhanced Gas Sensing and Photocatalytic Properties" Nanomaterials 12, no. 7: 1208. https://doi.org/10.3390/nano12071208
APA StyleChen, P.-R., Fu, H.-W., Yang, S.-M., & Lu, K.-C. (2022). Chemical Vapor Deposition-Fabricated Manganese-Doped and Potassium-Doped Hexagonal Tungsten Trioxide Nanowires with Enhanced Gas Sensing and Photocatalytic Properties. Nanomaterials, 12(7), 1208. https://doi.org/10.3390/nano12071208






