Fabrication of Mn3O4-CeO2-rGO as Nanocatalyst for Electro-Oxidation of Methanol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Synthesis of Mn3O4-CeO2 and Mn3O4-CeO2-rGO Nanocatalysts
2.3. Electrochemical Studies
3. Results and Discussion
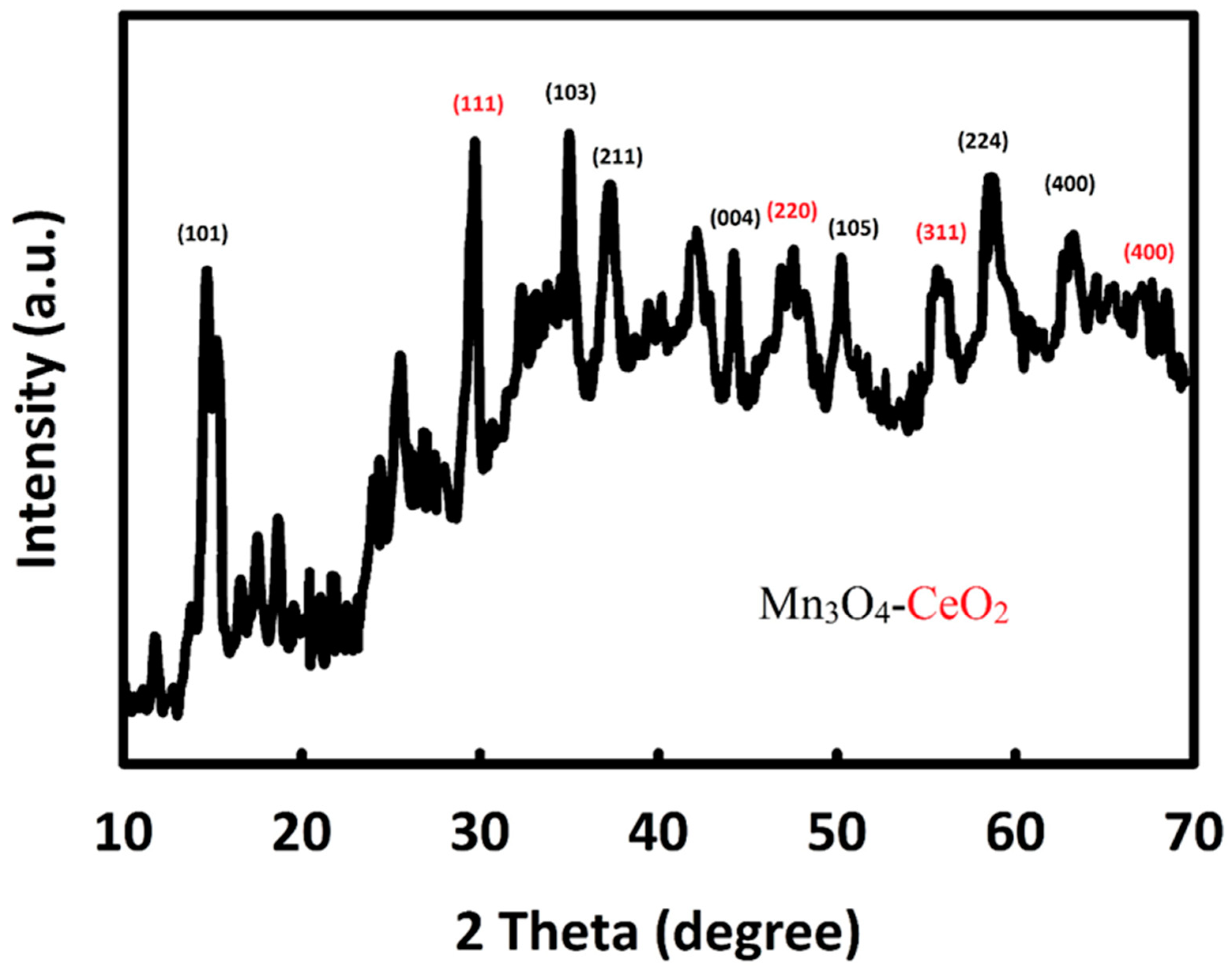
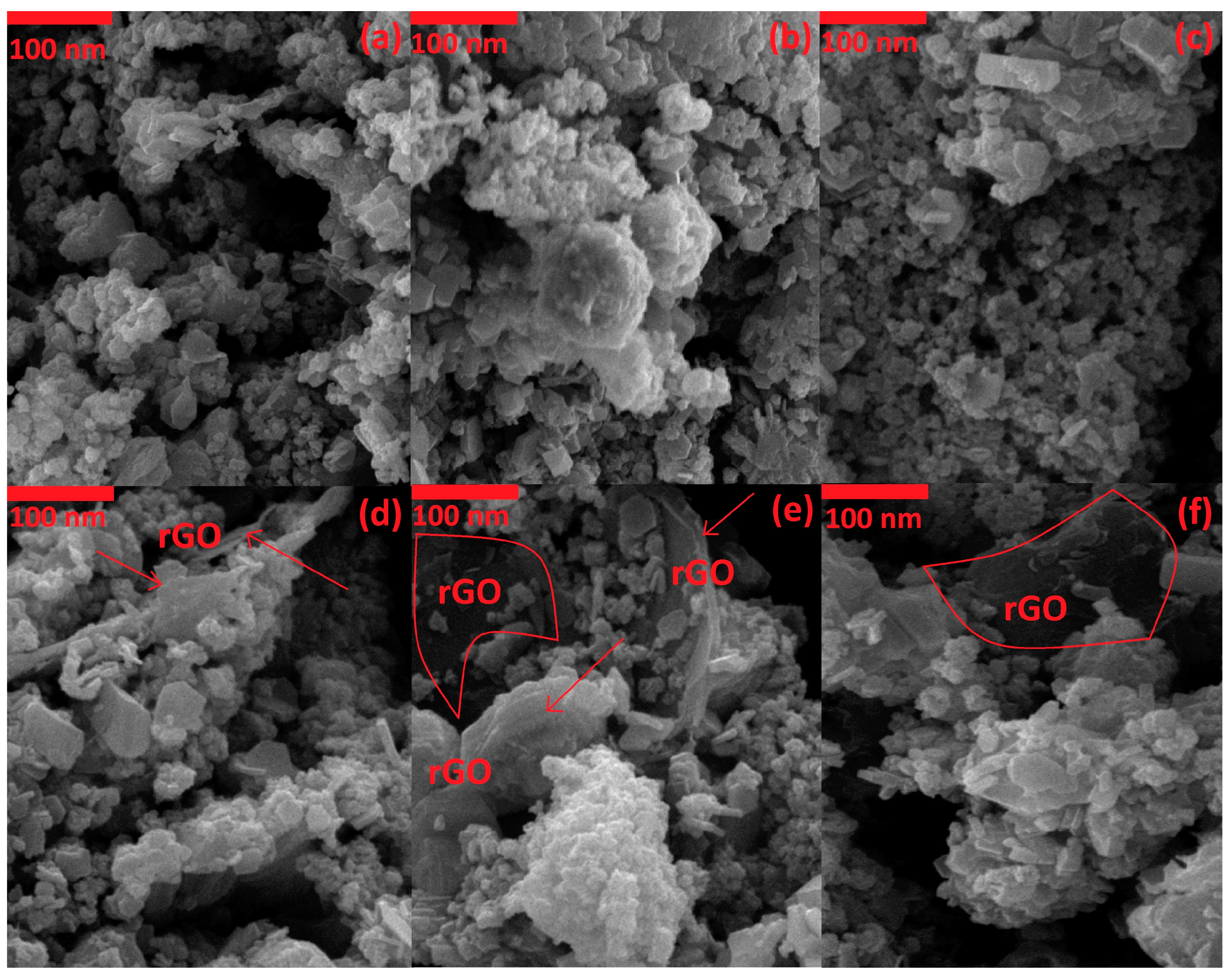
3.1. Characterization
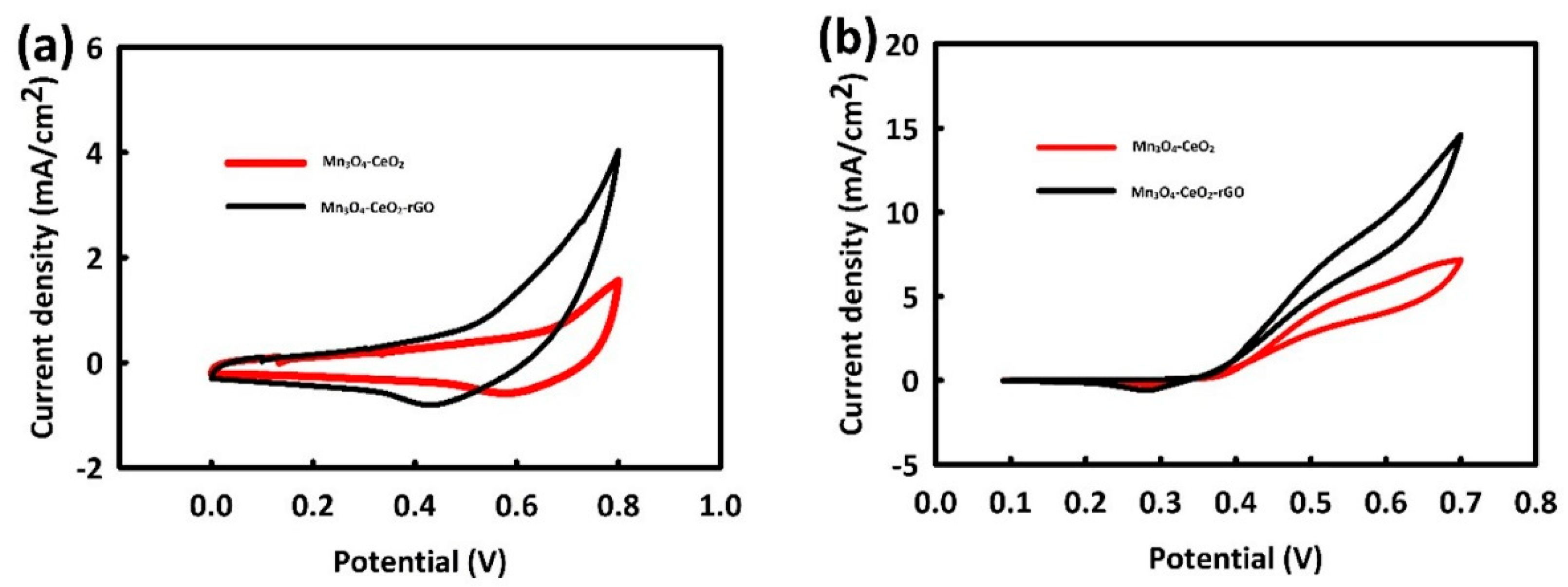
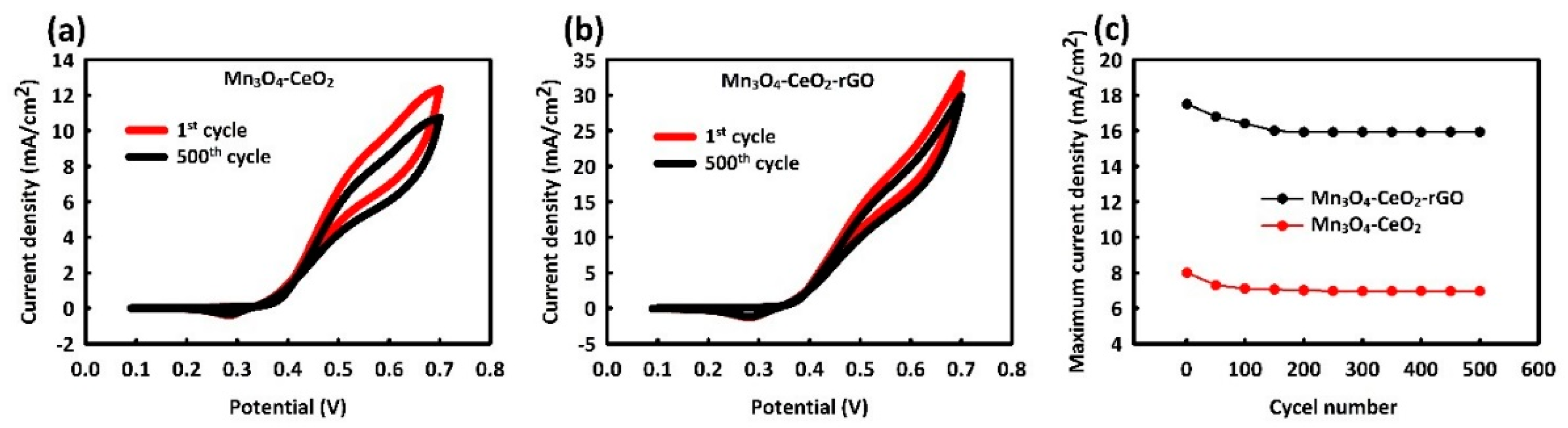
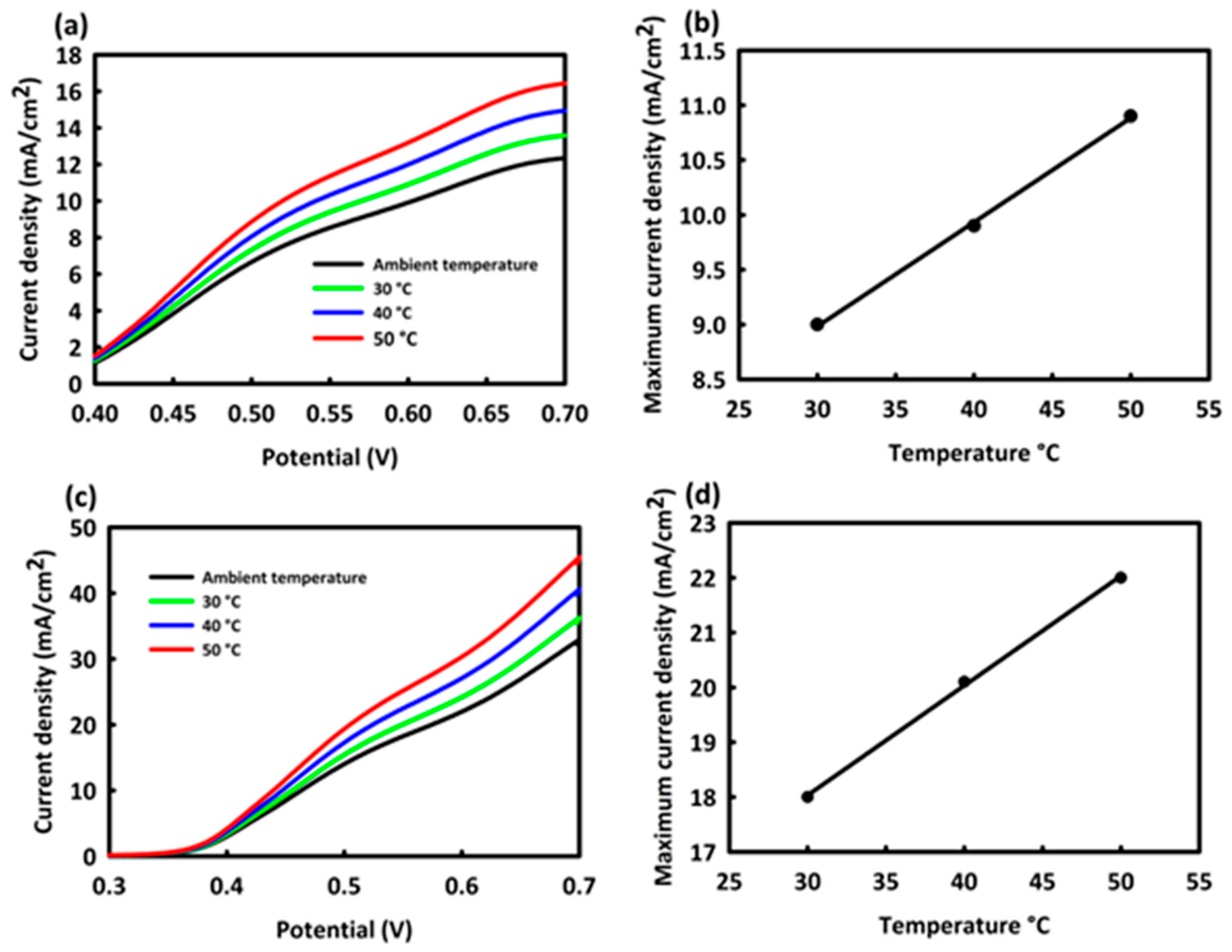
3.2. Electro-Catalytic Investigations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Peter, S.C. Reduction of CO2 to chemicals and fuels: A solution to global warming and energy crisis. ACS Energy Lett. 2018, 3, 1557–1561. [Google Scholar] [CrossRef]
- Salarizadeh, P.; Askari, M.B. MoS2–ReS2/rGO: A novel ternary hybrid nanostructure as a pseudocapacitive energy storage material. J. Alloys Compd. 2021, 874, 159886. [Google Scholar] [CrossRef]
- Martins, F.; Felgueiras, C.; Smitkova, M.; Caetano, N. Analysis of fossil fuel energy consumption and environmental impacts in European countries. Energies 2019, 12, 964. [Google Scholar] [CrossRef] [Green Version]
- Rasoulinezhad, E.; Taghizadeh-Hesary, F.; Taghizadeh-Hesary, F. How is mortality affected by fossil fuel consumption, CO2 emissions and economic factors in CIS region? Energies 2020, 13, 2255. [Google Scholar] [CrossRef]
- Withey, P.; Johnston, C.; Guo, J. Quantifying the global warming potential of carbon dioxide emissions from bioenergy with carbon capture and storage. Renew. Sustain. Energy Rev. 2019, 115, 109408. [Google Scholar] [CrossRef]
- Usman, M.; Jahanger, A.; Makhdum, M.S.A.; Balsalobre-Lorente, D.; Bashir, A. How do financial development, energy consumption, natural resources, and globalization affect Arctic countries’ economic growth and environmental quality? An advanced panel data simulation. Energy 2022, 241, 122515. [Google Scholar] [CrossRef]
- Burke, P.J.; Widnyana, J.; Anjum, Z.; Aisbett, E.; Resosudarmo, B.; Baldwin, K.G. Overcoming barriers to solar and wind energy adoption in two Asian giants: India and Indonesia. Energy Policy 2019, 132, 1216–1228. [Google Scholar] [CrossRef]
- Sharma, P.; Minakshi Sundaram, M.; Watcharatharapong, T.; Jungthawan, S.; Ahuja, R. Tuning the Nanoparticle Interfacial Properties and Stability of the Core–Shell Structure in Zn-Doped NiMoO4@ AWO4. ACS Appl. Mater. Interfaces 2021, 13, 56116–56130. [Google Scholar] [CrossRef]
- Askari, M.B.; Rozati, S.M.; Salarizadeh, P.; Saeidfirozeh, H.; Di Bartolomeo, A. A remarkable three-component RuO2-MnCo2O4/rGO nanocatalyst towards methanol electrooxidation. Int. J. Hydrogen Energy 2021, 46, 36792–36800. [Google Scholar] [CrossRef]
- Minakshi, M.; Singh, P.; Sharma, N.; Blackford, M.; Ionescu, M. Lithium extraction—Insertion from/into LiCoPO4 in aqueous batteries. Ind. Eng. Chem. Res. 2011, 50, 1899–1905. [Google Scholar] [CrossRef]
- Liu, H.; Liu, X.; Wang, S.; Liu, H.K.; Li, L. Transition metal based battery-type electrodes in hybrid supercapacitors: A review. Energy Storage Mater. 2020, 28, 122–145. [Google Scholar] [CrossRef]
- Akdemir, M.; Imanova, G.; Karakaş, D.E.; Kıvrak, H.D.; Kaya, M. High Efficiency Biomass-Based Metal-Free Catalyst as a Promising Supercapacitor Electrode for Energy Storage. 2021. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3908407 (accessed on 20 August 2021).
- Iqbal, S.; Khatoon, H.; Pandit, A.H.; Ahmad, S. Recent development of carbon based materials for energy storage devices. Mater. Sci. Energy Technol. 2019, 2, 417–428. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Gomes, V.G. High efficiency supercapacitor derived from biomass based carbon dots and reduced graphene oxide composite. J. Electroanal. Chem. 2019, 832, 87–96. [Google Scholar]
- Askari, M.B.; Salarizadeh, P.; Beheshti-Marnani, A. A hierarchical hybrid of ZnCo2O4 and rGO as a significant electrocatalyst for methanol oxidation reaction: Synthesis, characterization, and electrocatalytic performance. Int. J. Energy Res. 2020, 44, 8892–8903. [Google Scholar] [CrossRef]
- Chen, S.; Huang, D.; Liu, D.; Sun, H.; Yan, W.; Wang, J.; Fan, W. Hollow and porous NiCo2O4 nanospheres for enhanced methanol oxidation reaction and oxygen reduction reaction by oxygen vacancies engineering. Appl. Catal. B Environ. 2021, 291, 120065. [Google Scholar] [CrossRef]
- Huang, H.; Wei, Y.; Yang, Y.; Yan, M.; He, H.; Jiang, Q.; Zhu, J. Controllable synthesis of grain boundary-enriched Pt nanoworms decorated on graphitic carbon nanosheets for ultrahigh methanol oxidation catalytic activity. J. Energy Chem. 2021, 57, 601–609. [Google Scholar] [CrossRef]
- Jin, D.; Li, Z.; Wang, Z. Hierarchical NiCo2O4 and NiCo2S4 nanomaterials as electrocatalysts for methanol oxidation reaction. Int. J. Hydrogen Energy 2021, 46, 32069–32080. [Google Scholar] [CrossRef]
- Narayanan, N.; Bernaurdshaw, N. Reduced graphene oxide supported NiCo2O4 nano-rods: An efficient, stable and cost-effective electrocatalyst for methanol oxidation reaction. ChemCatChem 2020, 12, 771–780. [Google Scholar] [CrossRef]
- Pattanayak, P.; Pramanik, N.; Kumar, P.; Kundu, P.P. Fabrication of cost-effective non-noble metal supported on conducting polymer composite such as copper/polypyrrole graphene oxide (Cu2O/PPy–GO) as an anode catalyst for methanol oxidation in DMFC. Int. J. Hydrogen Energy 2018, 43, 11505–11519. [Google Scholar] [CrossRef]
- Sharma, P.; Minakshi Sundaram, M.; Watcharatharapong, T.; Laird, D.; Euchner, H.; Ahuja, R. Zn metal atom doping on the surface plane of one-dimesional NiMoO4 nanorods with improved redox chemistry. ACS Appl. Mater. Interfaces 2020, 12, 44815–44829. [Google Scholar] [CrossRef]
- Askari, M.B.; Beheshti-Marnani, A.; Seifi, M.; Rozati, S.M.; Salarizadeh, P. Fe3O4@ MoS2/RGO as an effective nano-electrocatalyst toward electrochemical hydrogen evolution reaction and methanol oxidation in two settings for fuel cell application. J. Colloid Interface Sci. 2019, 537, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Hameed, R.M.A.; Amin, R.S.; El-Khatib, K.M.; Fetohi, A.E. Preparation and characterization of Pt–CeO2/C and Pt–TiO2/C electrocatalysts with improved electrocatalytic activity for methanol oxidation. Appl. Surf. Sci. 2016, 367, 382–390. [Google Scholar] [CrossRef]
- Rezaee, S.; Shahrokhian, S. Facile synthesis of petal-like NiCo/NiO-CoO/nanoporous carbon composite based on mixed-metallic MOFs and their application for electrocatalytic oxidation of methanol. Appl. Catal. B Environ. 2019, 244, 802–813. [Google Scholar] [CrossRef]
- Nagajyothi, P.; Ramaraghavulu, R.; Munirathnam, K.; Yoo, K.; Shim, J. One-pot hydrothermal synthesis: Enhanced MOR and OER performance using low-cost Mn3O4 electrocatalyst. Int. J. Hydrogen Energy 2021, 46, 13946–13951. [Google Scholar] [CrossRef]
- Das, S.K.; Kamila, S.; Satpati, B.; Kandasamy, M.; Chakraborty, B.; Basu, S.; Jena, B.K. Hollow Mn3O4 nanospheres on graphene matrix for oxygen reduction reaction and supercapacitance applications: Experimental and theoretical insight. J. Power Sources 2020, 471, 228465. [Google Scholar] [CrossRef]
- Li, T.; Hu, Y.; Liu, K.; Yin, J.; Li, Y.; Fu, G.; Tang, Y. Hollow yolk-shell nanoboxes assembled by Fe-doped Mn3O4 nanosheets for high-efficiency electrocatalytic oxygen reduction in Zn-Air battery. Chem. Eng. J. 2022, 427, 131992. [Google Scholar] [CrossRef]
- Wang, W.; Chen, J.Q.; Tao, Y.R.; Zhu, S.N.; Zhang, Y.X.; Wu, X.C. Flowerlike Ag-supported Ce-doped Mn3O4 nanosheet heterostructure for a highly efficient oxygen reduction reaction: Roles of metal oxides in Ag surface states. ACS Catal. 2019, 9, 3498–3510. [Google Scholar] [CrossRef]
- Cao, L.; Cai, J.; Deng, W.; Tan, Y.; Xie, Q. NiCoO2@ CeO2 nanoboxes for ultrasensitive electrochemical immunosensing based on the oxygen evolution reaction in a neutral medium: Application for interleukin-6 detection. Anal. Chem. 2020, 92, 16267–16273. [Google Scholar] [CrossRef]
- Xing, H.; Long, G.; Zheng, J.; Zhao, H.; Zong, Y.; Li, X.; Zheng, X. Interface engineering boosts electrochemical performance by fabricating CeO2@ CoP Schottky conjunction for hybrid supercapacitors. Electrochim. Acta 2020, 337, 135817. [Google Scholar] [CrossRef]
- Qiao, Z.; Xia, C.; Cai, Y.; Afzal, M.; Wang, H.; Qiao, J.; Zhu, B. Electrochemical and electrical properties of doped CeO2-ZnO composite for low-temperature solid oxide fuel cell applications. J. Power Sources 2018, 392, 33–40. [Google Scholar] [CrossRef]
- Li, T.; Yin, J.; Sun, D.; Zhang, M.; Pang, H.; Xu, L.; Xue, J. Manipulation of Mott−Schottky Ni/CeO2 Heterojunctions into N-Doped Carbon Nanofibers for High-Efficiency Electrochemical Water Splitting. Small 2022, 2106592. [Google Scholar] [CrossRef] [PubMed]
- Vinothkannan, M.; Hariprasad, R.; Ramakrishnan, S.; Kim, A.R.; Yoo, D.J. Potential bifunctional filler (CeO2–ACNTs) for nafion matrix toward extended electrochemical power density and durability in proton-exchange membrane fuel cells operating at reduced relative humidity. ACS Sustain. Chem. Eng. 2019, 7, 12847–12857. [Google Scholar] [CrossRef]
- Tao, L.; Shi, Y.; Huang, Y.C.; Chen, R.; Zhang, Y.; Huo, J.; Wang, S. Interface engineering of Pt and CeO2 nanorods with unique interaction for methanol oxidation. Nano Energy 2018, 53, 604–612. [Google Scholar] [CrossRef]
- Tan, Q.; Shu, C.; Abbott, J.; Zhao, Q.; Liu, L.; Qu, T.; Wu, G. Highly dispersed Pd-CeO2 nanoparticles supported on N-doped core–shell structured mesoporous carbon for methanol oxidation in alkaline media. ACS Catal. 2019, 9, 6362–6371. [Google Scholar] [CrossRef]
- Van Dao, D.; Adilbish, G.; Le, T.D.; Nguyen, T.T.; Lee, I.H.; Yu, Y.T. Au@ CeO2 nanoparticles supported Pt/C electrocatalyst to improve the removal of CO in methanol oxidation reaction. J. Catal. 2019, 377, 589–599. [Google Scholar]
- Salarizadeh, P.; Askari, M.B.; Mohammadi, M.; Hooshyari, K. Electrocatalytic performance of CeO2-decorated rGO as an anode electrocatalyst for the methanol oxidation reaction. J. Phys. Chem. Solids 2020, 142, 109442. [Google Scholar] [CrossRef]
- Li, W.; Song, Z.; Deng, X.; Fu, X.Z.; Luo, J.L. Decoration of NiO hollow spheres composed of stacked nanosheets with CeO2 nanoparticles: Enhancement effect of CeO2 for electrocatalytic methanol oxidation. Electrochim. Acta 2020, 337, 135684. [Google Scholar] [CrossRef]
- Askari, M.B.; Salarizadeh, P. Superior catalytic performance of NiCo2O4 nanorods loaded rGO towards methanol electro-oxidation and hydrogen evolution reaction. J. Mol. Liq. 2019, 291, 111306. [Google Scholar] [CrossRef]
- Salarizadeh, P.; Askari, M.B.; Di Bartolomeo, A. MoS2/Ni3S2/Reduced Graphene Oxide Nanostructure as an Electrocatalyst for Alcohol Fuel Cells. ACS Appl. Nano Mater. 2022, 5, 3361–3373. [Google Scholar] [CrossRef]
- Askari, M.B.; Salarizadeh, P.; Beheshti-Marnani, A.; Di Bartolomeo, A. NiO-Co3O4-rGO as an Efficient Electrode Material for Supercapacitors and Direct Alcoholic Fuel Cells. Adv. Mater. Interfaces 2021, 8, 2100149. [Google Scholar] [CrossRef]
- Askari, M.B.; Salarizadeh, P.; Di Bartolomeo, A.; Beitollahi, H.; Tajik, S. Hierarchical nanostructures of MgCo2O4 on reduced graphene oxide as a high-performance catalyst for methanol electro-oxidation. Ceram. Int. 2021, 47, 16079–16085. [Google Scholar] [CrossRef]
- Emadi, H.; Salavati-Niasari, M.; Sobhani, A. Synthesis of some transition metal (M: 25Mn, 27Co, 28Ni, 29Cu, 30Zn, 47Ag, 48Cd) sulfide nanostructures by hydrothermal method. Adv. Colloid Interface Sci. 2017, 246, 52–74. [Google Scholar] [CrossRef] [PubMed]
- Majid, F.; Shahin, A.; Ata, S.; Bibi, I.; Malik, A.; Ali, A.; Nazir, A. The effect of temperature on the structural, dielectric and magnetic properties of cobalt ferrites synthesized via hydrothermal method. Z. Für Phys. Chem. 2021, 235, 1279–1296. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, T.; Ma, R.; Du, J.; Xie, C. Synthesis of flower-like CeO2/BiOCl heterostructures with enhanced ultraviolet light photocatalytic activity. Micro Nano Lett. 2018, 13, 1394–1398. [Google Scholar] [CrossRef]
- Lan, D.; Qin, M.; Yang, R.; Wu, H.; Jia, Z.; Kou, K.; Zhang, F. Synthesis, characterization and microwave transparent properties of Mn3O4 microspheres. J. Mater. Sci. Mater. Electron. 2019, 30, 8771–8776. [Google Scholar] [CrossRef]
- Chu, D.; Li, F.; Song, X.; Ma, H.; Tan, L.; Pang, H.; Xiao, B. A novel dual-tasking hollow cube NiFe2O4-NiCo-LDH@ rGO hierarchical material for high preformance supercapacitor and glucose sensor. J. Colloid Interface Sci. 2020, 568, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Askari, M.B.; Salarizadeh, P.; Di Bartolomeo, A. NiCo2O4-rGO/Pt as a robust nanocatalyst for sorbitol electrooxidation. Int. J. Energy Res. 2022, 46, 6745–6754. [Google Scholar] [CrossRef]
- Fajín, J.L.; Cordeiro, M.N.D. Insights into the Mechanism of Methanol Steam Reforming for Hydrogen Production over Ni–Cu-Based Catalysts. ACS Catal. 2021, 12, 512–526. [Google Scholar] [CrossRef]
- Askari, M.B.; Salarizadeh, P.; Seifi, M.; Di Bartolomeo, A. ZnFe2O4 nanorods on reduced graphene oxide as advanced supercapacitor electrodes. J. Alloys Compd. 2021, 860, 158497. [Google Scholar] [CrossRef]
- Askari, M.B.; Salarizadeh, P.; Di Bartolomeo, A.; Şen, F. Enhanced electrochemical performance of MnNi2O4/rGO nanocomposite as pseudocapacitor electrode material and methanol electro-oxidation catalyst. Nanotechnology 2021, 32, 325707. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Askari, M.B.; Rozati, S.M.; Di Bartolomeo, A. Fabrication of Mn3O4-CeO2-rGO as Nanocatalyst for Electro-Oxidation of Methanol. Nanomaterials 2022, 12, 1187. https://doi.org/10.3390/nano12071187
Askari MB, Rozati SM, Di Bartolomeo A. Fabrication of Mn3O4-CeO2-rGO as Nanocatalyst for Electro-Oxidation of Methanol. Nanomaterials. 2022; 12(7):1187. https://doi.org/10.3390/nano12071187
Chicago/Turabian StyleAskari, Mohammad Bagher, Seyed Mohammad Rozati, and Antonio Di Bartolomeo. 2022. "Fabrication of Mn3O4-CeO2-rGO as Nanocatalyst for Electro-Oxidation of Methanol" Nanomaterials 12, no. 7: 1187. https://doi.org/10.3390/nano12071187
APA StyleAskari, M. B., Rozati, S. M., & Di Bartolomeo, A. (2022). Fabrication of Mn3O4-CeO2-rGO as Nanocatalyst for Electro-Oxidation of Methanol. Nanomaterials, 12(7), 1187. https://doi.org/10.3390/nano12071187






