Cellulose Nanocrystals (CNC)-Based Functional Materials for Supercapacitor Applications
Abstract
:1. Introduction
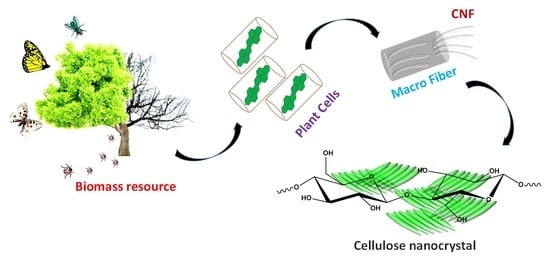
2. Cellulose Nanocrystals (CNCs): Structure and Properties
3. Pretreatment Process
4. Surface Modification of CNCs
5. Oxidation of Cellulose Nanocrystals (CNCs)
6. Acetylation of CNCs
7. Sulfonation of CNC
8. Silylation of CNCs
9. Carbamation
10. CNCs: Key to Developing Sustainable Electrochemical Energy Material
11. Conductive Polymer CNCs
12. Cellulose Nanocrystal-Conductive Hybrid Electrode
13. Carbonized CNC Electrodes
14. Conclusions and Outlook
- i.
- The need to fabricate hierarchical CNCs with an existing millimeter thickness and multi-scale porous microstructure. Hierarchical CNCs would become a great potential candidate for electrochemical systems.
- ii.
- The control and optimization of the surface functionalization on the CNC will be crucial to making novel CNCs for both hydrophobic and hydrophilic matrices.
- iii.
- Biosynthetic alternatives allow for the development of advanced high-performance CNC materials. The incorporation of biological molecules on the CNC surface by bacterial cellulose can make a better impact on their superior properties.
- iv.
- There is still a large research gap in improving the performance of super capacitance, energy and power densities.
- v.
- The construction of metal-free porous and heteroatom-doped CNC using a low cost and sustainable approach from abundant biomass is still needed.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Philippot, M.; Alvarez, G.; Ayerbe, E.; Van Mierlo, J.; Messagie, M. Eco-Efficiency of a Lithium-Ion Battery for Electric Vehicles: Influence of Manufacturing Country and Commodity Prices on GHG Emissions and Costs. Batteries 2019, 5, 23. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, P.; Mishra, P.K.; Mondal, M.K.; Srivastava, N. Non-Biodegradable Polymeric Waste Pyrolysis for Energy Recovery. Heliyon 2019, 5, e02198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dilshad, E.; Waheed, H.; Ali, U.; Amin, A.; Ahmed, I. General Structure and Classification of Bioplastics and Biodegradable Plastics. In Bioplastics Sustainable Development; Kuddus, M., Roohi, Eds.; Springer Nature: Singapore, 2021; pp. 61–82. [Google Scholar] [CrossRef]
- Guo, A.F.; Li, J.F.; Li, F.Y.; Xu, J.; Zhang, C.W.; Chen, S. Compression Behavior of Biodegradable Thermoplastic Plasticizer-Containing Composites. Strength Mater. 2019, 51, 18–25. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chemie Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Bhaladhare, S.; Das, D. Cellulose: A Fascinating Biopolymer for Hydrogel Synthesis. J. Mater. Chem. B 2022, 10, 1923–1945. [Google Scholar] [CrossRef]
- Karimi, K.; Taherzadeh, M.J. A Critical Review of Analytical Methods in Pretreatment of Lignocelluloses: Composition, Imaging, and Crystallinity. Bioresour. Technol. 2016, 200, 1008–1018. [Google Scholar] [CrossRef] [Green Version]
- Mishra, R.K.; Sabu, A.; Tiwari, S.K. Materials Chemistry and the Futurist Eco-Friendly Applications of Nanocellulose: Status and Prospect. J. Saudi Chem. Soc. 2018, 22, 949–978. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef]
- Pui, K.; Shak, Y.; Pang, Y.L.; Keat, S.; Review, M.; Rahman, A.; Long, J.S.; Long, S.; Kajang, D.; Ehsan, M.; et al. Nanocellulose: Recent Advances and Its Prospects in Environmental Remediation. Beilstein J. Nanotechnol. 9232 2018, 9, 2479–2498. [Google Scholar] [CrossRef]
- Dhali, K.; Ghasemlou, M.; Daver, F.; Cass, P.; Adhikari, B. A Review of Nanocellulose as a New Material towards Environmental Sustainability. Sci. Total Environ. 2021, 775, 145871. [Google Scholar] [CrossRef]
- Jaffar, S.S.; Saallah, S.; Misson, M.; Siddiquee, S.; Roslan, J.; Saalah, S.; Lenggoro, W. Recent Development and Environmental Applications of Nanocellulose-Based Membranes. Membranes 2022, 12, 287. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Nanocellulose in Biomedicine: Current Status and Future Prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef] [Green Version]
- Leung, A.C.W.; Hrapovic, S.; Lam, E.; Liu, Y.; Male, K.B.; Mahmoud, K.A.; Luong, J.H.T. Characteristics and Properties of Carboxylated Cellulose Nanocrystals Prepared from a Novel One-step Procedure. Small 2011, 7, 302–305. [Google Scholar] [CrossRef] [Green Version]
- Kargarzadeh, H.; Mariano, M.; Huang, J.; Lin, N.; Ahmad, I.; Dufresne, A.; Thomas, S. Recent Developments on Nanocellulose Reinforced Polymer Nanocomposites: A Review. Polymer 2017, 132, 368–393. [Google Scholar] [CrossRef]
- Salas, C.; Nypelö, T.; Rodriguez-Abreu, C.; Carrillo, C.; Rojas, O.J. Nanocellulose Properties and Applications in Colloids and Interfaces. Curr. Opin. Colloid Interface Sci. 2014, 19, 383–396. [Google Scholar] [CrossRef]
- Mondal, S. Preparation, Properties and Applications of Nanocellulosic Materials. Carbohydr. Polym. 2017, 163, 301–316. [Google Scholar] [CrossRef]
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a Tiny Fiber with Huge Applications. Curr. Opin. Biotechnol. 2016, 39, 76–88. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, S.N. Cellulose Nanocrystals: Synthesis, Functional Properties, and Applications. Nanotechnol. Sci. Appl. 2015, 8, 45. [Google Scholar] [CrossRef] [Green Version]
- Tavakolian, M.; Jafari, S.M.; van de Ven, T.G.M. A Review on Surface-Functionalized Cellulosic Nanostructures as Biocompatible Antibacterial Materials. Nano-Micro Lett. 2020, 12, 73. [Google Scholar] [CrossRef] [Green Version]
- Lam, E.; Male, K.B.; Chong, J.H.; Leung, A.C.W.; Luong, J.H.T. Applications of Functionalized and Nanoparticle-Modified Nanocrystalline Cellulose. Trends Biotech. 2012, 30, 283–290. [Google Scholar] [CrossRef]
- He, X.; Male, K.B.; Nesterenko, P.N.; Brabazon, D.; Paull, B.; Luong, J.H.T. Adsorption and Desorption of Methylene Blue on Porous Carbon Monoliths and Nanocrystalline Cellulose. ACS Appl. Mater. Interf. 2013, 5, 8796–8804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoud, K.A.; Male, K.B.; Hrapovic, S.; Luong, J.H.T. Cellulose Nanocrystal/Gold Nanoparticle Composite as a Matrix for Enzyme Immobilization. ACS Appl. Mater. Interf. 2009, 1, 1383–1386. [Google Scholar] [CrossRef] [PubMed]
- Tafete, G.A.; Abera, M.K.; Thothadri, G. Review on Nanocellulose-Based Materials for Supercapacitors Applications. J. Energy Storage 2022, 48, 103938. [Google Scholar] [CrossRef]
- Afif, A.; Rahman, S.M.; Tasfiah Azad, A.; Zaini, J.; Islam, M.A.; Azad, A.K. Advanced Materials and Technologies for Hybrid Supercapacitors for Energy Storage—A Review. J. Energy Storage 2019, 25, 100852. [Google Scholar] [CrossRef]
- Trahey, L.; Brushett, F.R.; Balsara, N.P.; Ceder, G.; Cheng, L.; Chiang, Y.M.; Hahn, N.T.; Ingram, B.J.; Minteer, S.D.; Moore, J.S.; et al. Energy Storage Emerging: A Perspective from the Joint Center for Energy Storage Research. Proc. Natl. Acad. Sci. USA 2020, 117, 12550–12557. [Google Scholar] [CrossRef] [PubMed]
- Bashir, T.; Irfan, R.M. A Review of the Energy Storage Aspects of Chemical Elements for Lithium-Ion Based Batteries. Energy Mater. 2021, 1, 100019. [Google Scholar] [CrossRef]
- Kebede, A.A.; Kalogiannis, T.; Van Mierlo, J.; Berecibar, M. A Comprehensive Review of Stationary Energy Storage Devices for Large Scale Renewable Energy Sources Grid Integration. Renew. Sustain. Energy Rev. 2022, 159, 112213. [Google Scholar] [CrossRef]
- Sam, D.K.; Sam, E.K.; Durairaj, A.; Lv, X.; Zhou, Z.; Liu, J. Synthesis of Biomass-Based Carbon Aerogels in Energy and Sustainability. Carbohydr. Res. 2020, 491, 107986. [Google Scholar] [CrossRef]
- Durairaj, A.; Sakthivel, T.; Ramanathan, S.; Obadiah, A.; Vasanthkumar, S. Conversion of Laboratory Paper Waste into Useful Activated Carbon: A Potential Supercapacitor Material and a Good Adsorbent for Organic Pollutant and Heavy Metals. Cellulose 2019, 26, 3313–3324. [Google Scholar] [CrossRef]
- Durairaj, A.; Liu, J.; Lv, X.; Vasanthkumar, S.; Sakthivel, T. Facile Synthesis of Waste-Derived Carbon/MoS2 Composite for Energy Storage and Water Purification Applications. Biomass Convers. Biorefinery 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Li, B.; Dai, F.; Xiao, Q.; Yang, L.; Shen, J.; Zhang, C.; Cai, M. Nitrogen-Doped Activated Carbon for a High Energy Hybrid Supercapacitor. Energy Environ. Sci. 2016, 9, 102–106. [Google Scholar] [CrossRef]
- Zhang, M.; Fan, H.; Zhao, N.; Peng, H.; Ren, X.; Wang, W.; Li, H.; Chen, G.; Zhu, Y.; Jiang, X.; et al. 3D Hierarchical CoWO4/Co3O4 Nanowire Arrays for Asymmetric Supercapacitors with High Energy Density. Chem. Eng. J. 2018, 347, 291–300. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, W.H. Development of Electrolytes towards Achieving Safe and High-Performance Energy-Storage Devices: A Review. ChemElectroChem 2015, 2, 22–36. [Google Scholar] [CrossRef]
- Kim, B.K.; Sy, S.; Yu, A.; Zhang, J. Electrochemical Supercapacitors for Energy Storage and Conversion. In Handbook of Clean Energy Systems; John Wiley & Sons, Ltd.: Chichester, UK, 2015; pp. 1–25. [Google Scholar]
- Tang, Z.; Tang, C.H.; Gong, H. A High Energy Density Asymmetric Supercapacitor from Nano-Architectured Ni(OH)2/Carbon Nanotube Electrodes. Adv. Funct. Mater. 2012, 22, 1272–1278. [Google Scholar] [CrossRef]
- Han, S.; Wu, D.; Li, S.; Zhang, F.; Feng, X. Porous Graphene Materials for Advanced Electrochemical Energy Storage and Conversion Devices. Adv. Mater. 2014, 26, 849–864. [Google Scholar] [CrossRef]
- Wesley, R.J.; Durairaj, A.; Ramanathan, S.; Obadiah, A.; Justinabraham, R.; Lv, X.; Vasanthkumar, S. Potato Peels Biochar Composite with Copper Phthalocyanine for Energy Storage Application. Diam. Relat. Mater. 2021, 115, 108360. [Google Scholar] [CrossRef]
- Zhang, Q.Z.; Zhang, D.; Miao, Z.C.; Zhang, X.L.; Chou, S.L. Research Progress in MnO2–Carbon Based Supercapacitor Electrode Materials. Small 2018, 14, 1702883. [Google Scholar] [CrossRef]
- Hussain, S.Z.; Ihrar, M.; Hussain, S.B.; Oh, W.C.; Ullah, K. A Review on Graphene Based Transition Metal Oxide Composites and Its Application towards Supercapacitor Electrodes. SN Appl. Sci. 2020, 2, 764. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhao, X.S. Carbon-Based Materials as Supercapacitor Electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Zhou, Y.; Qi, H.; Yang, J.; Bo, Z.; Huang, F.; Islam, M.S.; Lu, X.; Dai, L.; Amal, R.; Wang, C.H.; et al. Two-Birds-One-Stone: Multifunctional Supercapacitors beyond Traditional Energy Storage. Energy Environ. Sci. 2021, 14, 1854–1896. [Google Scholar] [CrossRef]
- Hou, J.; Shao, Y.; Ellis, M.W.; Moore, R.B.; Yi, B. Graphene-Based Electrochemical Energy Conversion and Storage: Fuel Cells, Supercapacitors and Lithium Ion Batteries. Phys. Chem. Chem. Phys. 2011, 13, 15384–15402. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Z. Supercapacitor and Supercapattery as Emerging Electrochemical Energy Stores. Int. Mater. Rev. 2016, 62, 173–202. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Lee, P.S.; Li, C. 3D Carbon Based Nanostructures for Advanced Supercapacitors. Energy Environ. Sci. 2012, 6, 41–53. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Liang, H.W.; Chen, L.F.; Hu, B.C.; Yu, S.H. Bacterial Cellulose: A Robust Platform for Design of Three Dimensional Carbon-Based Functional Nanomaterials. Acc. Chem. Res. 2016, 49, 96–105. [Google Scholar] [CrossRef]
- Wang, X.; Yao, C.; Wang, F.; Li, Z. Cellulose-Based Nanomaterials for Energy Applications. Small 2017, 13, 1702240. [Google Scholar] [CrossRef]
- Cheng, H.; Lijie, L.; Wang, B.; Feng, X.; Mao, Z.; Vancso, G.J.; Sui, X. Multifaceted Applications of Cellulosic Porous Materials in Environment, Energy, and Health. Prog. Polym. Sci. 2020, 106, 101253. [Google Scholar] [CrossRef]
- Du, X.; Zhang, Z.; Liu, W.; Deng, Y. Nanocellulose-Based Conductive Materials and Their Emerging Applications in Energy Devices—A Review. Nano Energy 2017, 35, 299–320. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Hua, K.; Carlsson, D.O.; Ålander, E.; Lindström, T.; Strømme, M.; Mihranyan, A.; Ferraz, N. Translational Study between Structure and Biological Response of Nanocellulose from Wood and Green Algae. RSC Adv. 2013, 4, 2892–2903. [Google Scholar] [CrossRef]
- Mokhena, T.C.; John, M.J. Cellulose Nanomaterials: New Generation Materials for Solving Global Issues. Cellulose 2019, 27, 1149–1194. [Google Scholar] [CrossRef]
- Farooq, A.; Patoary, M.K.; Zhang, M.; Mussana, H.; Li, M.; Naeem, M.A.; Mushtaq, M.; Farooq, A.; Liu, L. Cellulose from Sources to Nanocellulose and an Overview of Synthesis and Properties of Nanocellulose/Zinc Oxide Nanocomposite Materials. Int. J. Biol. Macromol. 2020, 154, 1050–1073. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chemie Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of Hydrogels and Aerogels Containing Nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Lam, E.; Leung, A.C.W.; Liu, Y.; Majid, E.; Hrapovic, S.; Male, K.B.; Luong, J.H.T. Green Strategy Guided by Raman Spectroscopy for the Synthesis of Ammonium Carboxylated Nanocrystalline Cellulose and the Recovery of Byproducts. ACS Sustain. Chem. Eng. 2013, 1, 278–283. [Google Scholar] [CrossRef]
- Yang, X.; Cranston, E.D. Chemically Cross-Linked Cellulose Nanocrystal Aerogels with Shape Recovery and Superabsorbent Properties. Chem. Mater. 2014, 26, 6016–6025. [Google Scholar] [CrossRef]
- Lam, E.; Hrapovic, S.; Majid, E.; Chong, J.H.; Luong, J.H.T. Catalysis using Gold Nanoparticles Decorated on Nanocrystalline Cellulose. Nanoscale 2012, 4, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tammela, P.; Strømme, M.; Nyholm, L. Cellulose-Based Supercapacitors: Material and Performance Considerations. Adv. Energy Mater. 2017, 7, 1700130. [Google Scholar] [CrossRef]
- Blanco, A.; Monte, M.C.; Campano, C.; Balea, A.; Merayo, N.; Negro, C. Nanocellulose for Industrial Use: Cellulose Nanofibers (CNF), Cellulose Nanocrystals (CNC), and Bacterial Cellulose (BC). Handb. Nanomater. Ind. Appl. 2018, 74–126. [Google Scholar] [CrossRef]
- Shankaran, D.R. Cellulose Nanocrystals for Health Care Applications. Appl. Nanomater. 2018, 415–459. [Google Scholar] [CrossRef]
- Leung, A.C.W.; Lam, E.; Chong, J.H.; Hrapovic, S.; Luong, J.H.T. Reinforced Plastics and Aerogels by Nanocrystalline Cellulose. J. Nanoparticle Res. 2013, 15, 1636. [Google Scholar] [CrossRef]
- Brinchi, L.; Cotana, F.; Fortunati, E.; Kenny, J.M. Production of Nanocrystalline Cellulose from Lignocellulosic Biomass: Technology and Applications. Carbohydr. Polym. 2013, 94, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Julie Chandra, C.S.; George, N.; Narayanankutty, S.K. Isolation and Characterization of Cellulose Nanofibrils from Arecanut Husk Fibre. Carbohydr. Polym. 2016, 142, 158–166. [Google Scholar] [CrossRef]
- Nickerson, R.F.; Harree, J.A. Cellulose Intercrystalline Structure. Ind. Eng. Chem. 1947, 39, 1507–1512. [Google Scholar] [CrossRef]
- Lamaming, J.; Hashim, R.; Leh, C.P.; Sulaiman, O. Properties of Cellulose Nanocrystals from Oil Palm Trunk Isolated by Total Chlorine Free Method. Carbohydr. Polym. 2017, 156, 409–416. [Google Scholar] [CrossRef]
- Babicka, M.; Woźniak, M.; Dwiecki, K.; Borysiak, S.; Ratajczak, I. Preparation of Nanocellulose Using Ionic Liquids: 1-Propyl-3-Methylimidazolium Chloride and 1-Ethyl-3-Methylimidazolium Chloride. Molecules 2020, 25, 1544. [Google Scholar] [CrossRef] [Green Version]
- Surov, O.V.; Voronova, M.I.; Rubleva, N.V.; Kuzmicheva, L.A. A Novel Effective Approach of Nanocrystalline Cellulose Production: Oxidation–Hydrolysis Strategy. Cellulose 2018, 25, 5035–5048. [Google Scholar] [CrossRef]
- Cui, S.; Zhang, S.; Ge, S.; Xiong, L.; Sun, Q. Green Preparation and Characterization of Size-Controlled Nanocrystalline Cellulose via Ultrasonic-Assisted Enzymatic Hydrolysis. Ind. Crops Prod. 2016, 83, 346–352. [Google Scholar] [CrossRef]
- Chowdhury, Z.Z.; Hamid, S.B.A. Preparation and Characterization of Nanocrystalline Cellulose Using Ultrasonication Combined with a Microwave-Assisted Pretreatment Process. BioResources 2016, 11, 3397–3415. [Google Scholar] [CrossRef]
- Rohaizu, R.; Wanrosli, W.D. Sono-Assisted TEMPO Oxidation of Oil Palm Lignocellulosic Biomass for Isolation of Nanocrystalline Cellulose. Ultrason. Sonochem. 2017, 34, 631–639. [Google Scholar] [CrossRef]
- Mendoza, D.J.; Browne, C.; Raghuwanshi, C.S.; Simon, G.P.; Garnier, G. One-shot TEMPO-Periodate Oxidation of Native Cellulose. Carbohydr. Polym. 2019, 226, 115292. [Google Scholar] [CrossRef]
- Leung, C.W.; Luong, J.H.T.; Hrapovic, S.; Lam, E.; Liu, Y.; Male, K.B.; Mahmoud, K.; Rho, D. Cellulose Nanocrystals from Renewable Biomass. U.S. Patent No. 8,900, 706 B2, 2 December 2014. [Google Scholar]
- Seta, F.T.; An, X.; Liu, L.; Zhang, H.; Yang, J.; Zhang, W.; Nie, S.; Yao, S.; Cao, H.; Xu, Q.; et al. Preparation and Characterization of High Yield Cellulose Nanocrystals (CNC) Derived from Ball Mill Pretreatment and Maleic Acid Hydrolysis. Carbohydr. Polym. 2020, 234, 115942. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Wang, P.; Dong, C. Ultrasonic Pretreatment of Cellulose in Ionic Liquid for Efficient Preparation of Cellulose Nanocrystals. Cellulose 2018, 25, 7053–7064. [Google Scholar] [CrossRef]
- Singh, S.; Gaikwad, K.K.; Lee, Y.S. Antimicrobial and Antioxidant Properties of Polyvinyl Alcohol Bio Composite Films Containing Seaweed Extracted Cellulose Nano-Crystal and Basil Leaves Extract. Int. J. Biol. Macromol. 2018, 107, 1879–1887. [Google Scholar] [CrossRef]
- Smirnov, M.A.; Sokolova, M.P.; Tolmachev, D.A.; Vorobiov, V.K.; Kasatkin, I.A.; Smirnov, N.N.; Klaving, A.V.; Bobrova, N.V.; Lukasheva, N.V.; Yakimansky, A.V. Green Method for Preparation of Cellulose Nanocrystals Using Deep Eutectic Solvent. Cellulose 2020, 27, 4305–4317. [Google Scholar] [CrossRef]
- Calvino, C.; Macke, N.; Kato, R.; Rowan, S.J. Development, Processing and Applications of Bio-Sourced Cellulose Nanocrystal Composites. Prog. Polym. Sci. 2020, 103, 101221. [Google Scholar] [CrossRef]
- Trache, D.; Thakur, V.K.; Boukherroub, R. Cellulose Nanocrystals/Graphene Hybrids—A Promising New Class of Materials for Advanced Applications. Nanomaterials 2020, 10, 1523. [Google Scholar] [CrossRef]
- Eyley, S.; Thielemans, W. Surface Modification of Cellulose Nanocrystals. Nanoscale 2014, 6, 7764–7779. [Google Scholar] [CrossRef] [Green Version]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of Cellulose Nanofibrils: A Review of Recent Advances. Ind. Crops Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Araki, J.; Wada, M.; Kuga, S. Steric Stabilization of a Cellulose Microcrystal Suspension by Poly(Ethylene Glycol) Grafting. Langmuir 2000, 17, 21–27. [Google Scholar] [CrossRef]
- Habibi, Y. Key Advances in the Chemical Modification of Nanocelluloses. Chem. Soc. Rev. 2014, 43, 1519–1542. [Google Scholar] [CrossRef]
- Cheng, M.; Qin, Z.; Liu, Y.; Qin, Y.; Li, T.; Chen, L.; Zhu, M. Efficient Extraction of Carboxylated Spherical Cellulose Nanocrystals with Narrow Distribution through Hydrolysis of Lyocell Fibers by Using Ammonium Persulfate as an Oxidant. J. Mater. Chem. A 2013, 2, 251–258. [Google Scholar] [CrossRef]
- Daud, J.B.; Lee, K.-Y. Surface Modification of Nanocellulose. Handb. Nanocellulose Cellul. Nanocomposites 2017, 101–122. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, S.H.; Kim, S.H. Surface Alkylation of Cellulose Nanocrystals to Enhance Their Compatibility with Polylactide. Polymers 2020, 12, 178. [Google Scholar] [CrossRef] [Green Version]
- Çetin, N.S.; Tingaut, P.; Özmen, N.; Henry, N.; Harper, D.; Dadmun, M.; Sèbe, G. Acetylation of Cellulose Nanowhiskers with Vinyl Acetate under Moderate Conditions. Macromol. Biosci. 2009, 9, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Mariano, M.; El Kissi, N.; Dufresne, A. Cellulose Nanomaterials: Size and Surface Influence on the Thermal and Rheological Behavior. Polímeros 2018, 28, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Thakur, V.K.; Thakur, M.K. Processing and Characterization of Natural Cellulose Fibers/Thermoset Polymer Composites. Carbohydr. Polym. 2014, 109, 102–117. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial Application of Cellulose Nano-Composites—A Review. Biotechnol. Rep. 2019, 21, e00316. [Google Scholar] [CrossRef]
- Issa, A.A.; Luyt, A.S. Kinetics of Alkoxysilanes and Organoalkoxysilanes Polymerization: A Review. Polymers 2019, 11, 537. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wang, L.; Dong, S.; Zhang, X.; Wu, Q.; Zhao, L.; Shi, Y. Nanocellulose 3, 5-Dimethylphenylcarbamate Derivative Coated Chiral Stationary Phase: Preparation and Enantioseparation Performance. Chirality 2016, 28, 376–381. [Google Scholar] [CrossRef]
- Guo, J.; Du, W.; Gao, Y.; Cao, Y.; Yin, Y. Cellulose Nanocrystals as Water-in-Oil Pickering Emulsifiers via Intercalative Modification. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 634–642. [Google Scholar] [CrossRef]
- Girouard, N.M.; Xu, S.; Schueneman, G.T.; Shofner, M.L.; Meredith, J.C. Site-Selective Modification of Cellulose Nanocrystals with Isophorone Diisocyanate and Formation of Polyurethane-CNC Composites. ACS Appl. Mater. Interfaces 2016, 8, 1458–1467. [Google Scholar] [CrossRef] [PubMed]
- Abushammala, H. On the Para/Ortho Reactivity of Isocyanate Groups during the Carbamation of Cellulose Nanocrystals Using 2,4-Toluene Diisocyanate. Polymers 2019, 11, 1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Lu, C.; Han, Y.; Zhou, Z.; Yuan, G.; Zhang, X. Cellulose Nanowhisker Modulated 3D Hierarchical Conductive Structure of Carbon Black/Natural Rubber Nanocomposites for Liquid and Strain Sensing Application. Compos. Sci. Technol. 2016, 124, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Zhuo, H.; Hu, Y.; Tong, X.; Zhong, L.; Peng, X.; Sun, R. Sustainable Hierarchical Porous Carbon Aerogel from Cellulose for High-Performance Supercapacitor and CO2 Capture. Ind. Crops Prod. 2016, 87, 229–235. [Google Scholar] [CrossRef]
- Bhanvase, B.A.; Sonawane, S.H. Ultrasound Assisted in Situ Emulsion Polymerization for Polymer Nanocomposite: A Review. Chem. Eng. Process. Process Intensif. 2014, 85, 86–107. [Google Scholar] [CrossRef]
- Liang, H.W.; Guan, Q.F.; Zhu, Z.Z.; Song, L.T.; Yao, H.B.; Lei, X.; Yu, S.H. Highly Conductive and Stretchable Conductors Fabricated from Bacterial Cellulose. NPG Asia Mater. 2012, 4, e19. [Google Scholar] [CrossRef] [Green Version]
- Luong, J.H.T.; Narayan, T.; Solanki, S.; Malhotra, B.D. Recent Advances of Conducting Polymers and Their Composites for ElectrochemicalBiosensing Applications. J. Funct. Biomat. 2021, 11, 71. [Google Scholar] [CrossRef]
- Shi, Y.; Peng, L.; Ding, Y.; Zhao, Y.; Yu, G. Nanostructured Conductive Polymers for Advanced Energy Storage. Chem. Soc. Rev. 2015, 44, 6684–6696. [Google Scholar] [CrossRef] [Green Version]
- Ravit, R.; Abdullah, J.; Ahmad, I.; Sulaiman, Y. Electrochemical Performance of Poly(3, 4-Ethylenedioxythipohene)/Nanocrystalline Cellulose (PEDOT/NCC) Film for Supercapacitor. Carbohydr. Polym. 2019, 203, 128–138. [Google Scholar] [CrossRef]
- Wu, X.; Chabot, V.L.; Kim, B.K.; Yu, A.; Berry, R.M.; Tam, K.C. Cost-Effective and Scalable Chemical Synthesis of Conductive Cellulose Nanocrystals for High-Performance Supercapacitors. Electrochim. Acta 2014, 138, 139–147. [Google Scholar] [CrossRef]
- Liew, S.Y.; Thielemans, W.; Walsh, D.A. Electrochemical Capacitance of Nanocomposite Polypyrrole/Cellulose Films. J. Phys. Chem. C 2010, 114, 17926–17933. [Google Scholar] [CrossRef]
- Liew, S.Y.; Thielemans, W.; Walsh, D.A. Polyaniline- and Poly(Ethylenedioxythiophene)-Cellulose Nanocomposite Electrodes for Supercapacitors. J. Solid State Electrochem. 2014, 18, 3307–3315. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Tang, J.; Duan, Y.; Yu, A.; Berry, R.M.; Tam, K.C. Conductive Cellulose Nanocrystals with High Cycling Stability for Supercapacitor Applications. J. Mater. Chem. A 2014, 2, 19268–19274. [Google Scholar] [CrossRef]
- Forner-Cuenca, A.; Brushett, F.R. Engineering Porous Electrodes for Next-Generation Redox Flow Batteries: Recent Progress and Opportunities. Curr. Opin. Electrochem. 2019, 18, 113–122. [Google Scholar] [CrossRef]
- Valentini, L.; Cardinali, M.; Fortunati, E.; Torre, L.; Kenny, J.M. A Novel Method to Prepare Conductive Nanocrystalline Cellulose/Graphene Oxide Composite Films. Mater. Lett. 2013, 105, 4–7. [Google Scholar] [CrossRef]
- Imai, M.; Akiyama, K.; Tanaka, T.; Sano, E. Highly Strong and Conductive Carbon Nanotube/Cellulose Composite Paper. Compos. Sci. Technol. 2010, 70, 1564–1570. [Google Scholar] [CrossRef] [Green Version]
- Al Haj, Y.; Mousavihashemi, S.; Robertson, D.; Borghei, M.; Pääkkönen, T.; Rojas, O.J.; Kontturi, E.; Kallio, T.; Vapaavuori, J. Biowaste-Derived Electrode and Electrolyte Materials for Flexible Supercapacitors. Chem. Eng. J. 2022, 435, 135058. [Google Scholar] [CrossRef]
- Han, J.; Wang, S.; Zhu, S.; Huang, C.; Yue, Y.; Mei, C.; Xu, X.; Xia, C. Electrospun Core-Shell Nanofibrous Membranes with Nanocellulose-Stabilized Carbon Nanotubes for Use as High-Performance Flexible Supercapacitor Electrodes with Enhanced Water Resistance, Thermal Stability, and Mechanical Toughness. ACS Appl. Mater. Interfaces 2019, 11, 44624–44635. [Google Scholar] [CrossRef]
- Shi, K.; Yang, X.; Cranston, E.D.; Zhitomirsky, I. Efficient Lightweight Supercapacitor with Compression Stability. Adv. Funct. Mater. 2016, 26, 6437–6445. [Google Scholar] [CrossRef]
- Chen, L.M.; Yu, H.Y.; Wang, D.C.; Yang, T.; Yao, J.M.; Tam, K.C. Simple Synthesis of Flower-like Manganese Dioxide Nanostructures on Cellulose Nanocrystals for High-Performance Supercapacitors and Wearable Electrodes. ACS Sustain. Chem. Eng. 2019, 7, 11823–11831. [Google Scholar] [CrossRef]
- Ravit, R.; Azman, N.H.N.; Kulandaivalu, S.; Abdullah, J.; Ahmad, I.; Sulaiman, Y. Cauliflower-like Poly(3,4-Ethylenedioxythipohene)/Nanocrystalline Cellulose/Manganese Oxide Ternary Nanocomposite for Supercapacitor. J. Appl. Polym. Sci. 2020, 137, 49162. [Google Scholar] [CrossRef]
- Johnsen, D.L.; Zhang, Z.; Emamipour, H.; Yan, Z.; Rood, M.J. Effect of Isobutane Adsorption on the Electrical Resistivity of Activated Carbon Fiber Cloth with Select Physical and Chemical Properties. Carbon 2014, 76, 435–445. [Google Scholar] [CrossRef]
- Lakhi, K.S.; Park, D.H.; Al-Bahily, K.; Cha, W.; Viswanathan, B.; Choy, J.H.; Vinu, A. Mesoporous Carbon Nitrides: Synthesis, Functionalization, and Applications. Chem. Soc. Rev. 2017, 46, 72–101. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, Y.; Wang, X.; Ahn, W.; Jiang, G.; Feng, K.; Lui, G.; Chen, Z. Gas Pickering Emulsion Templated Hollow Carbon for High Rate Performance Lithium Sulfur Batteries. Adv. Funct. Mater. 2016, 26, 8408–8417. [Google Scholar] [CrossRef]
- Lu, W.; Wang, T.; He, X.; Sun, K.; Huang, Z.; Tan, G.; Eddings, E.G.; Adidharma, H.; Fan, M. A New Method for Preparing Excellent Electrical Conductivity Carbon Nanofibers from Coal Extraction Residual. Clean. Eng. Technol. 2021, 4, 100109. [Google Scholar] [CrossRef]
- Shopsowitz, K.E.; Hamad, W.Y.; MacLachlan, M.J. Chiral Nematic Mesoporous Carbon Derived From Nanocrystalline Cellulose. Angew. Chem. Int. Ed. 2011, 50, 10991–10995. [Google Scholar] [CrossRef]
- Li, Z.; Ahadi, K.; Jiang, K.; Ahvazi, B.; Li, P.; Anyia, A.O.; Cadien, K.; Thundat, T. Freestanding Hierarchical Porous Carbon Film Derived from Hybrid Nanocellulose for High-Power Supercapacitors. Nano Res. 2017, 10, 1847–1860. [Google Scholar] [CrossRef]
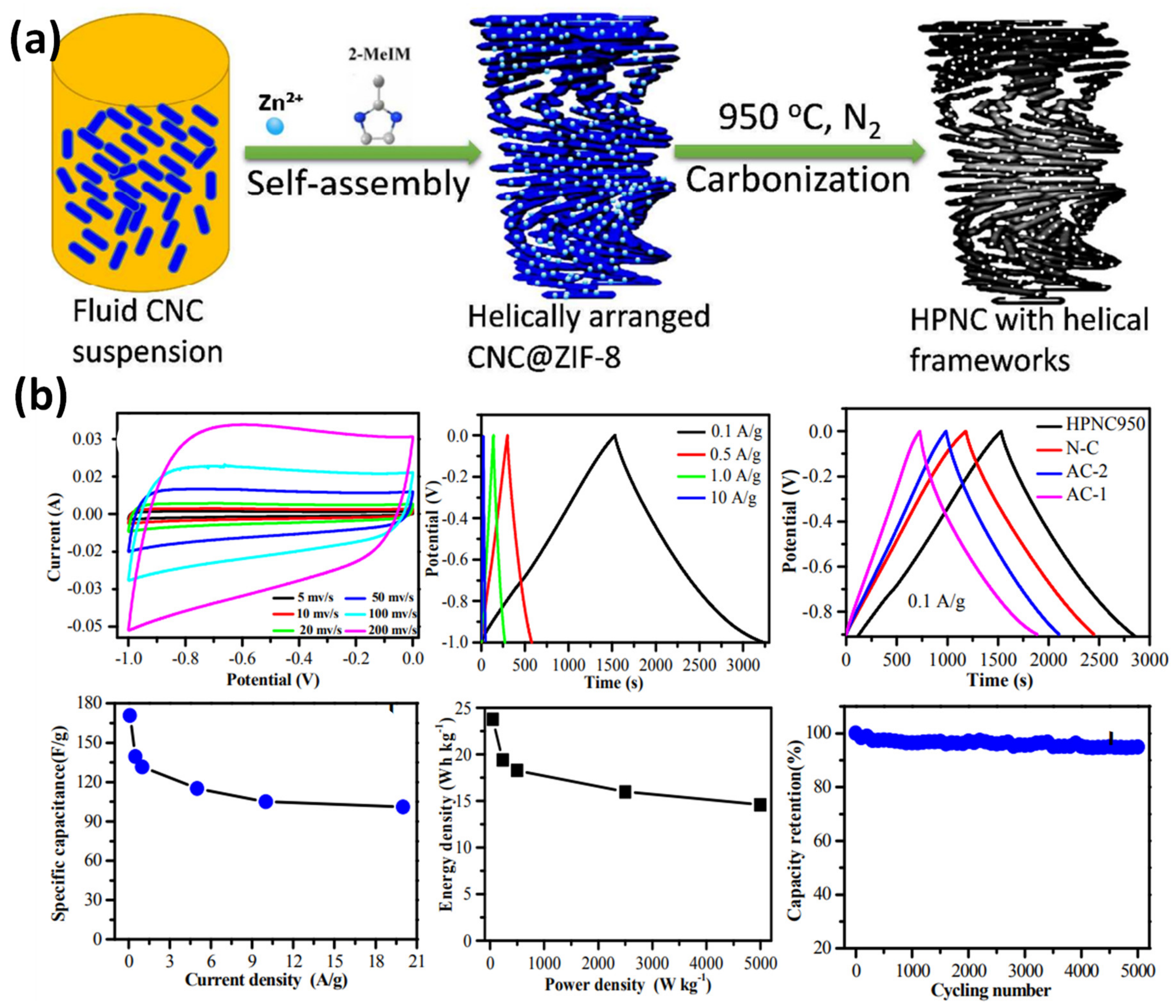
- Wu, X.; Shi, Z.; Tjandra, R.; Cousins, A.J.; Sy, S.; Yu, A.; Berry, R.M.; Tam, K.C. Nitrogen-Enriched Porous Carbon Nanorods Templated by Cellulose Nanocrystals as High Performance Supercapacitor Electrodes. J. Mater. Chem. A 2015, 3, 23768–23777. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, T.; Lin, X.; Chen, H.; Chen, S.; Jiang, Z.; Chen, Y.; Liu, J.; Huang, J.; Liu, M. Self-Templated Synthesis of Hierarchically Porous N-Doped Carbon Derived from Biomass for Supercapacitors. ACS Sustain. Chem. Eng. 2018, 6, 13932–13939. [Google Scholar] [CrossRef]
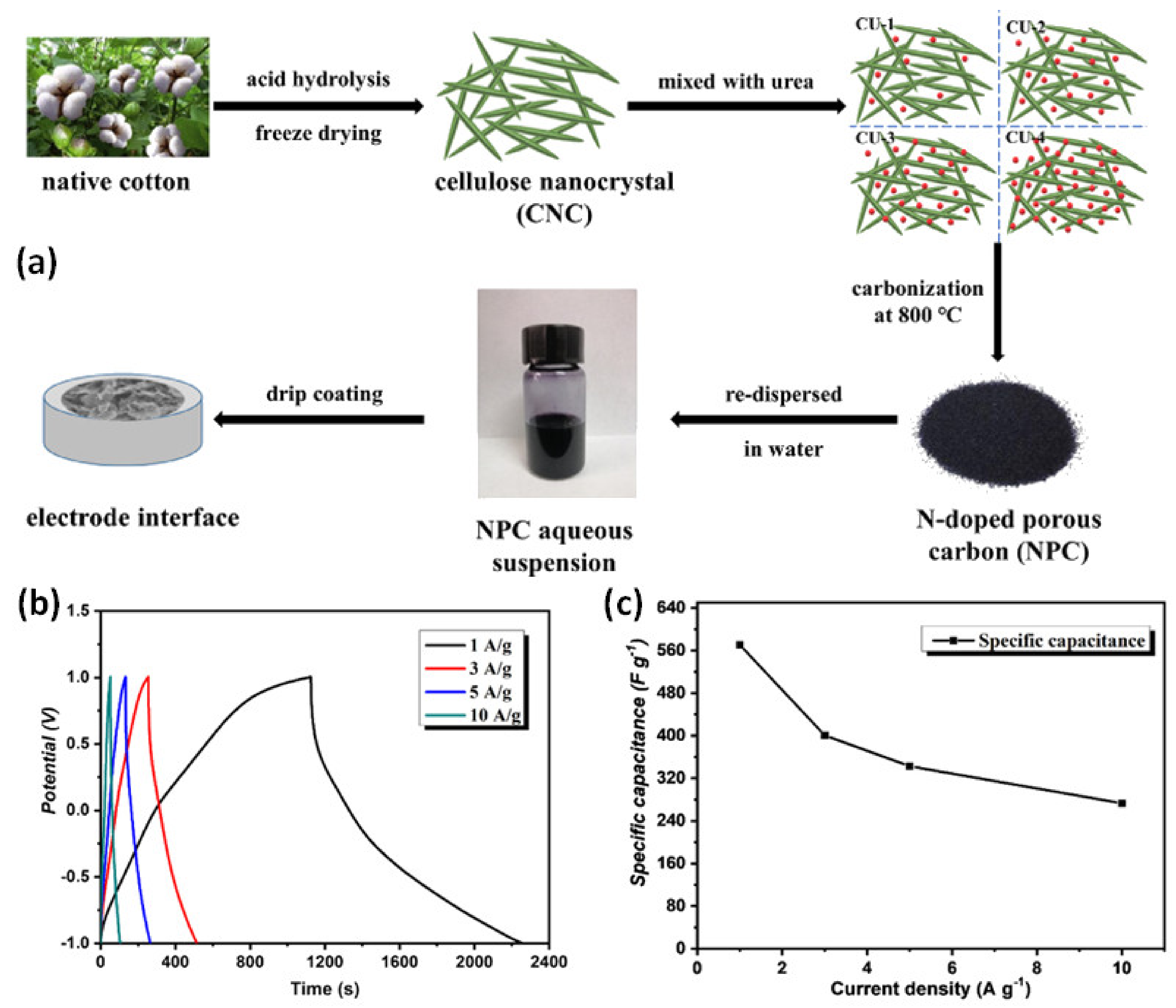
- Wang, S.; Dong, L.; Li, Z.; Lin, N.; Xu, H.; Gao, S. Sustainable Supercapacitors of Nitrogen-Doping Porous Carbon Based on Cellulose Nanocrystals and Urea. Int. J. Biol. Macromol. 2020, 164, 4095–4103. [Google Scholar] [CrossRef]
- Luong, J.H.T.; Lam, E.; Leung, C.W.; Hrapovic, S.; Male, K.B. Chitin Nanocrystals and Process for Preparation Thereof. US Patent No. US10214596B2, 2019. [Google Scholar]
- Mahmoud, K.A.; Mena, J.A.; Male, K.B.; Hrapovic, S.; Kamen, A.; Luong, J.H.T. Effect of Surface Charge on the Cellular Uptake and Cytotoxicity of Fluorescent Labeled Cellulose Nanocrystals. ACS Appl. Mater. Interf. 2010, 2, 2924–2932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Male, K.B.; Leung, A.C.W.; Montes, J.; Kamen, A.; Luong, J.H.T. Probing Inhibitory Effects of Nanocrystalline Cellulose: Inhibition versus Surface Charge. Nanoscale 2012, 4, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Halder, L.; Das, A.K.; Maitra, A.; Bera, A.; Paria, S.; Karan, S.K.; Si, S.K.; Ojha, S.; De, A.; Bhanu Bhusan Khatua, B.B. A Polypyrrole-adorned, Self-supported, Pseudocapacitive Zinc Vanadium Oxide Nanoflower and Nitrogen-doped Reduced Graphene Oxide-based Asymmetric Supercapacitor Device for Power Density Applications. New J. Chem. 2020, 44, 1063–1075. [Google Scholar] [CrossRef]
- Siwa, S.S.; Zhang, Q.; Sun, C.; Thakur, V.T. Graphitic Carbon Nitride Doped Copper–Manganese Alloy as High–Performance Electrode Material in Supercapacitor for Energy Storage. Nanomaterials 2020, 10, 2. [Google Scholar]
- Maitra, A.; Bera, R.; Halder, L.; Bera, A.; Paria, S.; Karan, S.K.; Si, S.K.; De, A.; Ojha, S.; Khatua, B.B. Photovoltaic and triboelectrification empowered light-weight flexible self-charging asymmetric supercapacitor cell for self-powered multifunctional electronics. Renew. Sustain. Energy Rev. 2021, 151, 111595. [Google Scholar] [CrossRef]
- Liu, C.; Yi, C.; Wang, K.; Yang, Y.; Bhatta, R.S.; Tsige, M.; Xiao, S.; Gong, X. Single-Junction Polymer Solar Cells with Over 10% Efficiency by a Novel Two-Dimensional Donor–Acceptor Conjugated Copolymer. ACS Appl. Mater. Interfaces 2015, 7, 4928–4935. [Google Scholar] [CrossRef]
- Liu, S.; Wei, L.; Wang, H. Review on Reliability of Supercapacitors in Energy Storage Applications. Appl. Energy 2020, 278, 115436. [Google Scholar] [CrossRef]

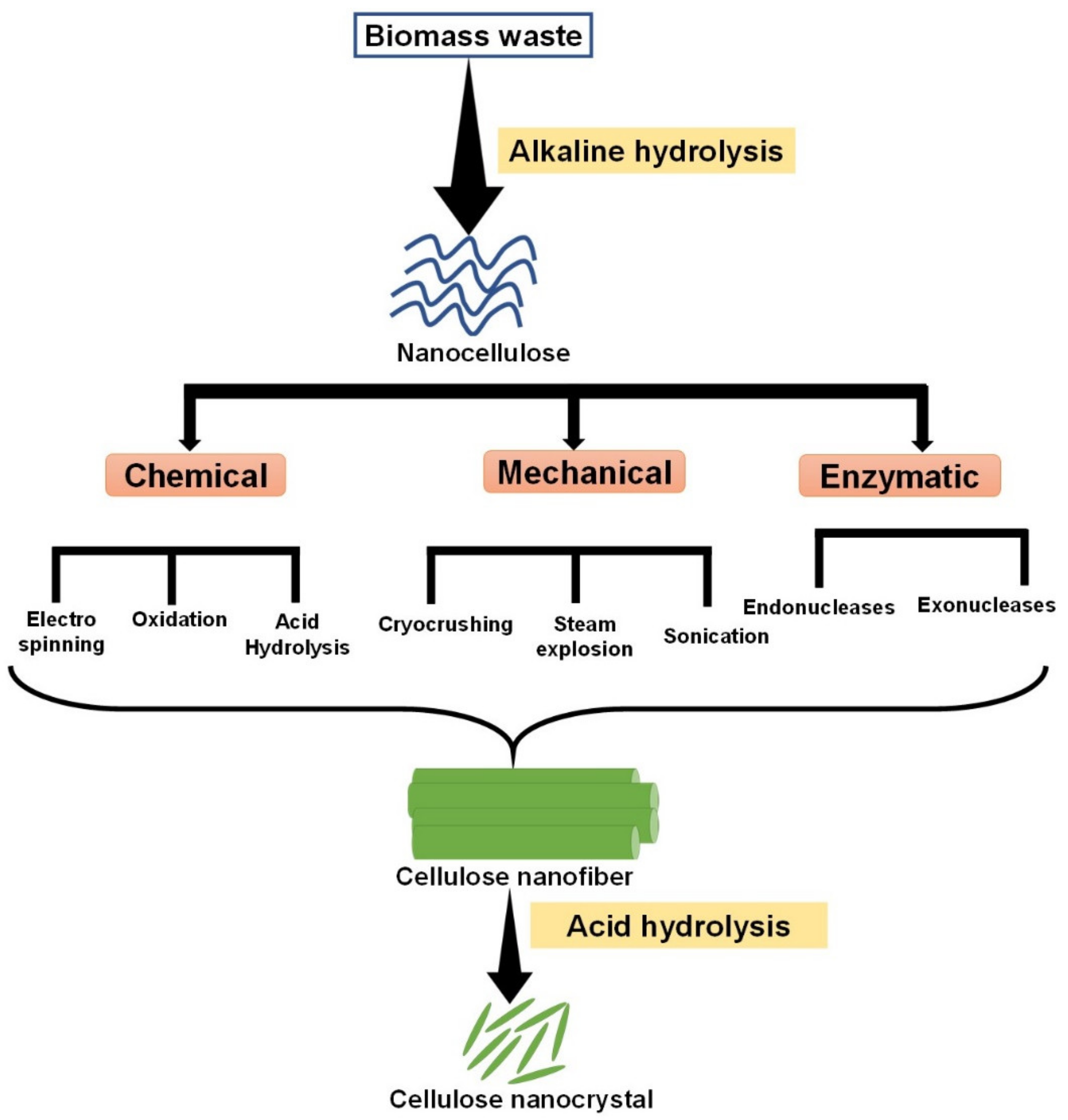
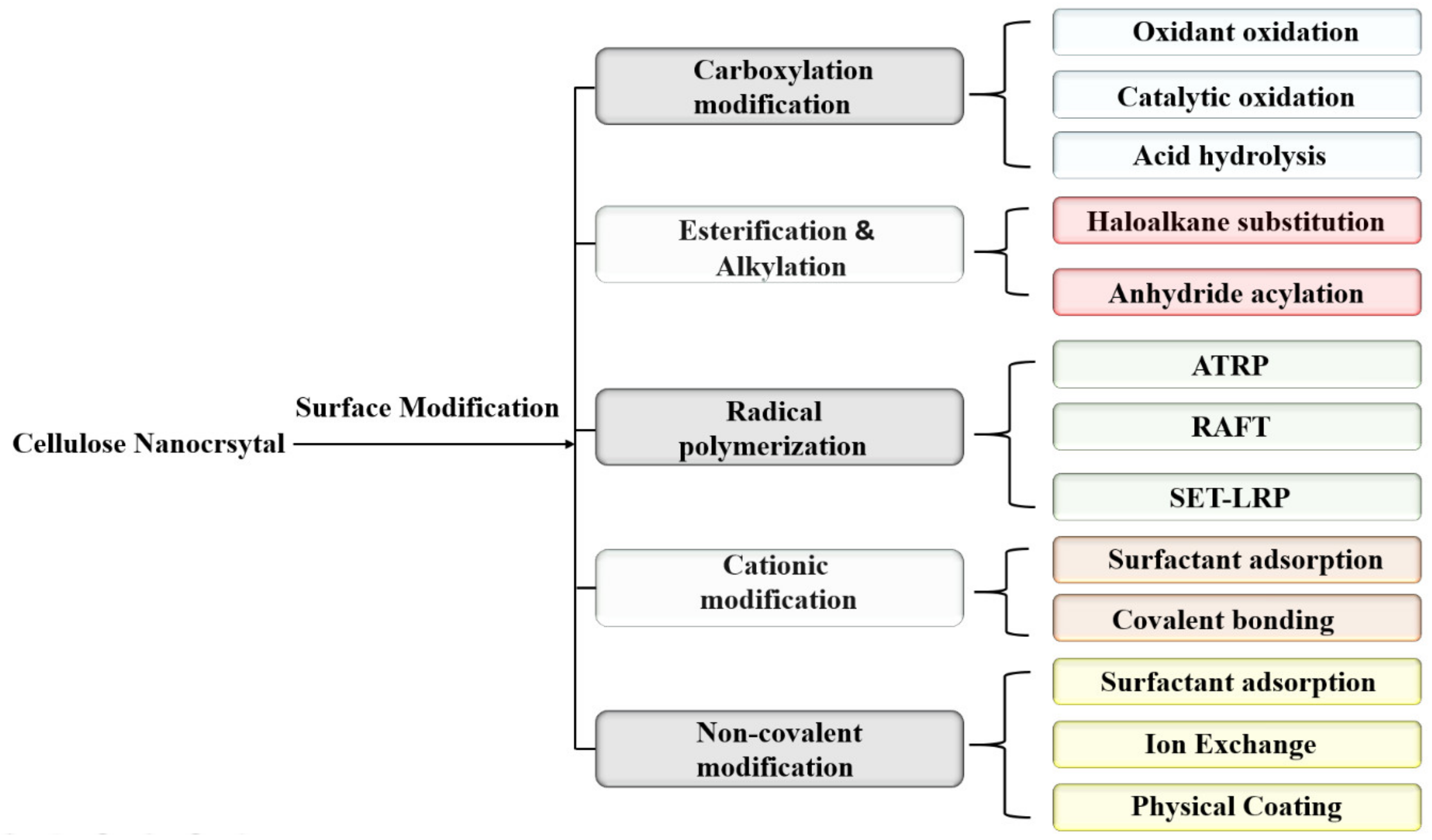
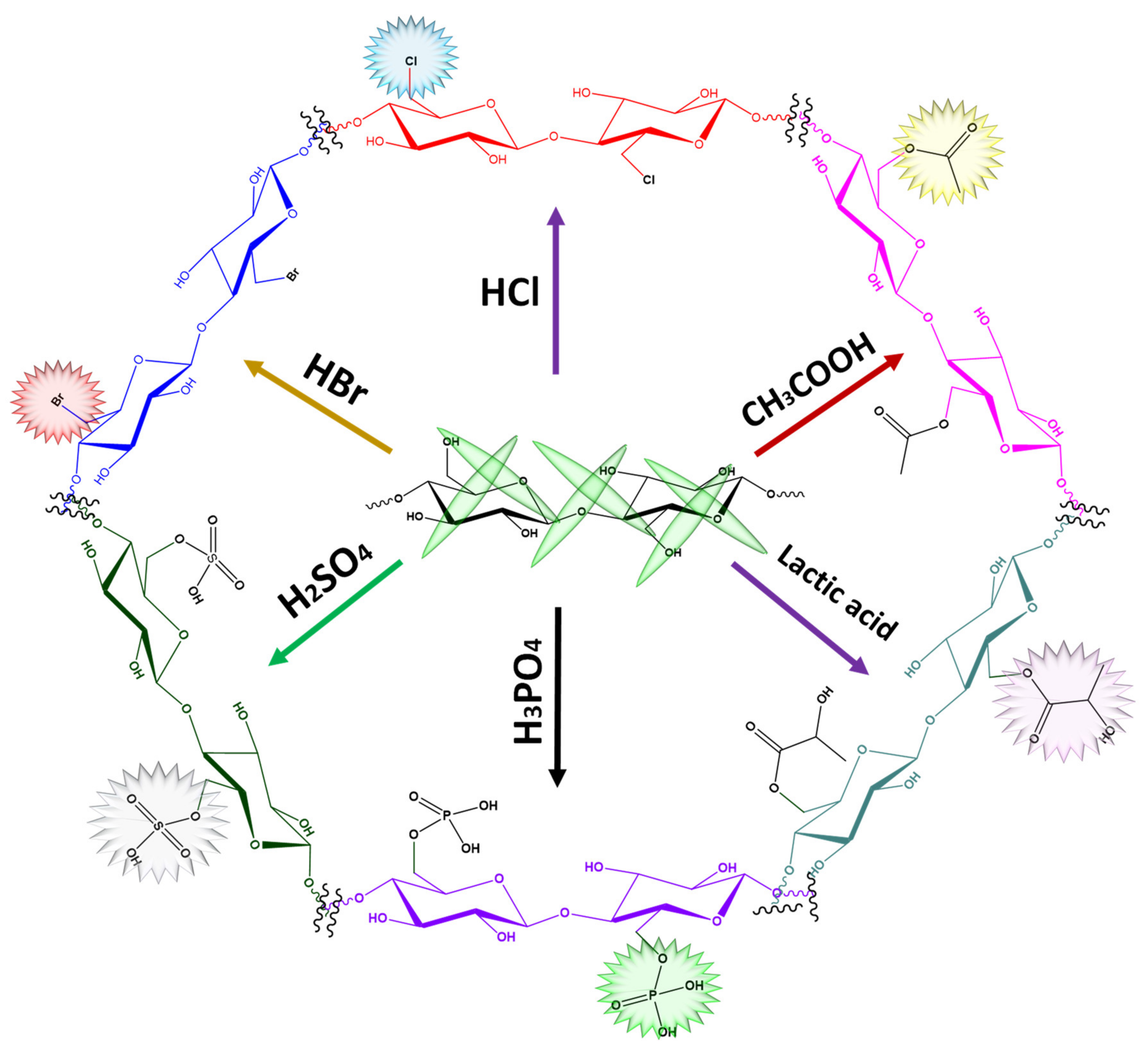


| Methods | Size (nm) | Advantages | Disadvantages |
|---|---|---|---|
| Acid hydrolysis | Diameter: 3–15 Length: 100–300 |
|
|
| Enzymolysis | Diameter: 3–50 Length: 100–1800 |
|
|
| Physical (mechanical, ultrasonic) | Diameter: 3–50 Length: 100–2000 |
|
|
| Physical + green chemistry | Diameter: <20 Length: 100–500 |
|
|
| Composite | Fabrication | Electrolyte | Capacitance (F/g) | Power Density (Wh/kg) | Energy Density (Wkg−1) | Ref |
|---|---|---|---|---|---|---|
| PEDOT/NCC | Electrochemical deposition | 1.0 M KCl | 117.02 | 11.44 | 99.85 | [101] |
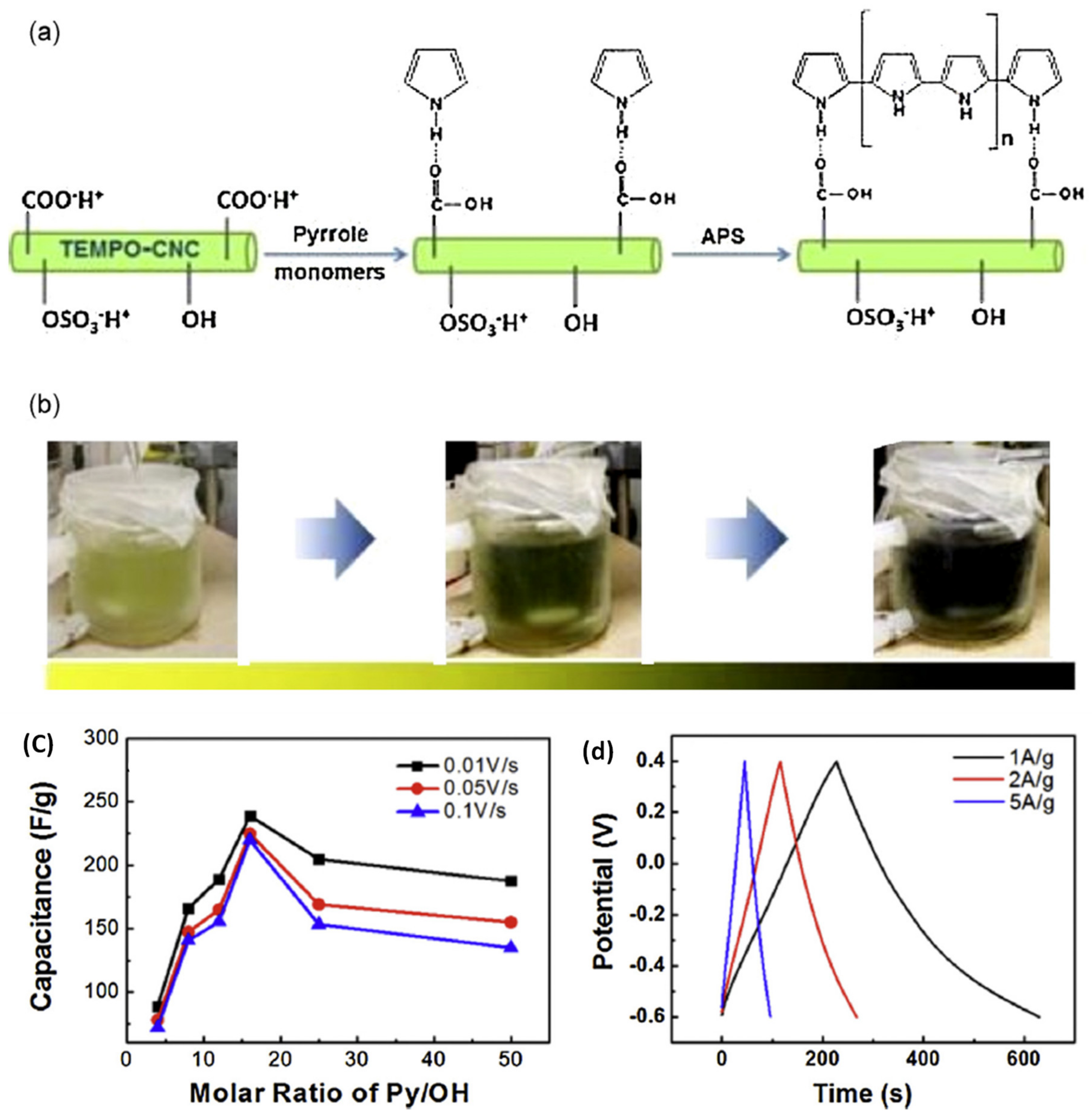
| PPY/CNC | TEMPO/polymerization | 0.5 M KCl | 248 | - | - | [102] |
| PPY/CNC | Electrochemical deposition | 0.1 M KCl | 256 | - | - | [103] |
| PANI/CNC | Electrochemical deposition | 1.0 M HCl | 488 | - | - | [104] |
| PEDOT/CNC | Electrochemical deposition | 1.0 M HCl | 69 | - | - | [104] |
| PPY/PVP/CNC | Polymerization | 0.5 M KCl | 338.6 | - | - | [105] |
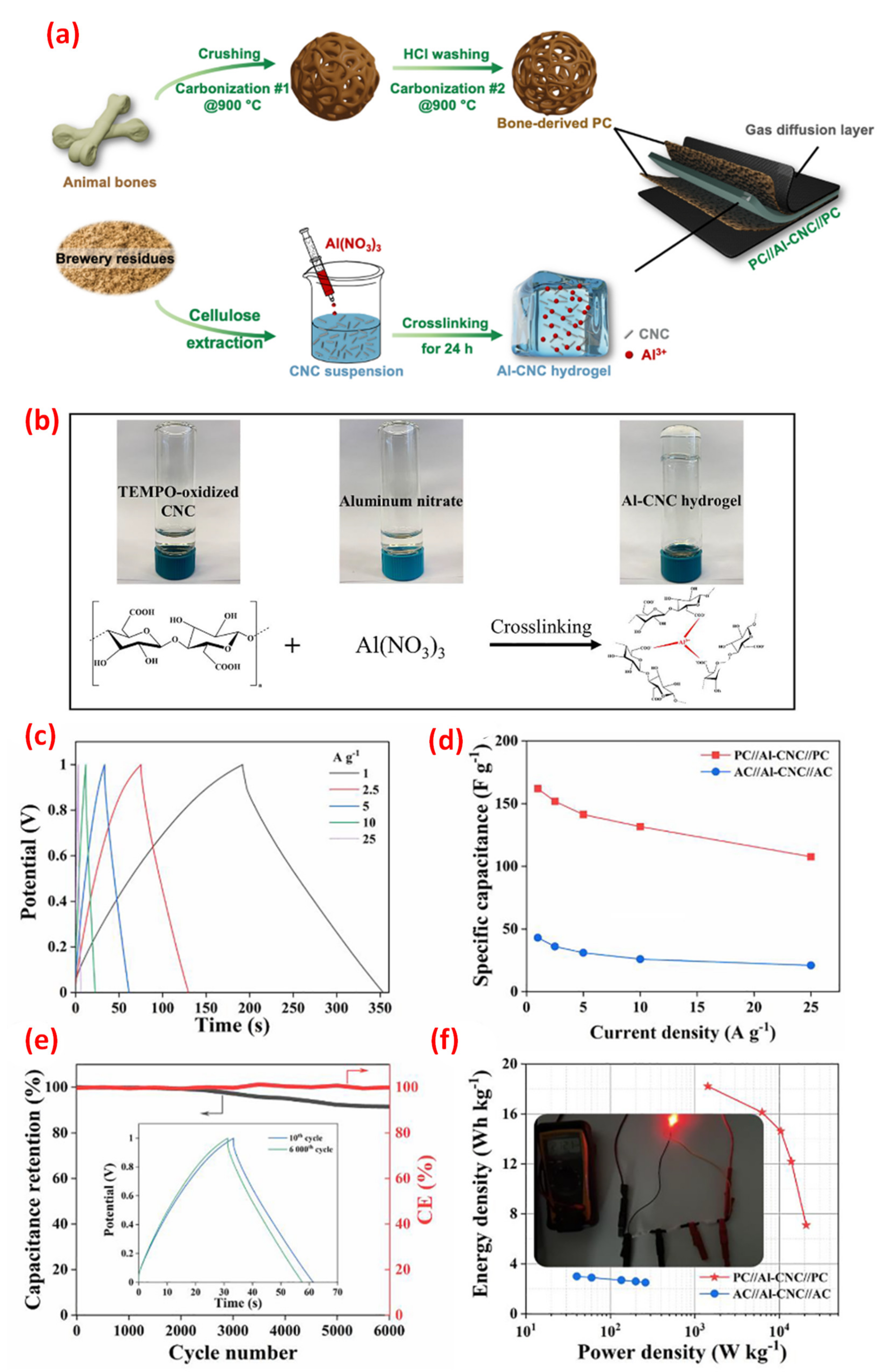
| Biowaste-derived carbon | Carbonization | Al-CNC hydrogel | 804 | 425 | 18.2 | [109] |
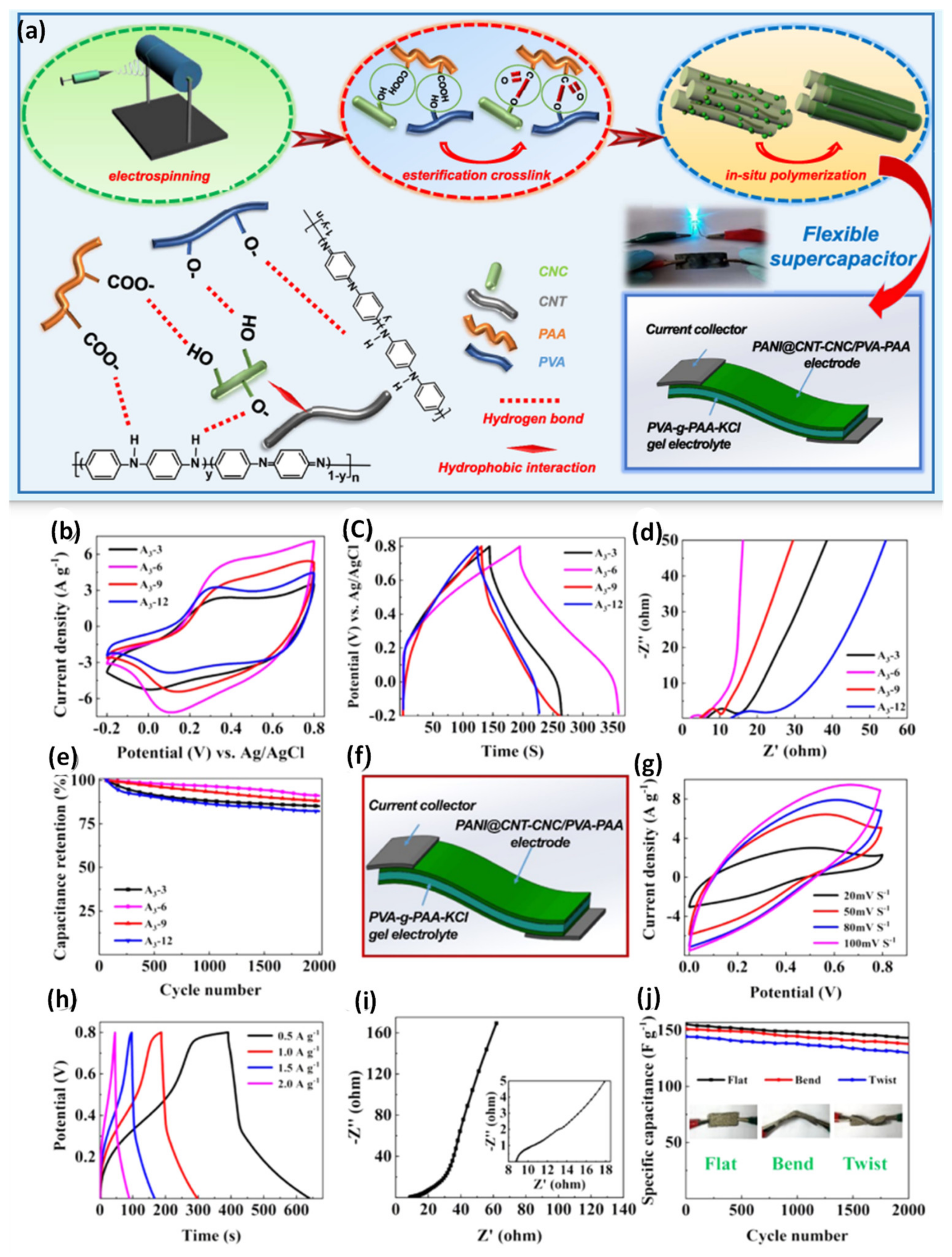
| PANI@ CNT− CNC/PVA−PAA | Electrospinning/ Polymerization | 1 M H2SO4 | 164.6 | 13.8 | 200.3 | [110] |
| CNC-MWCNT-PPY | Polymerization | 0.5 M Na2SO4 | 2.1 F cm−2 | - | - | [111] |
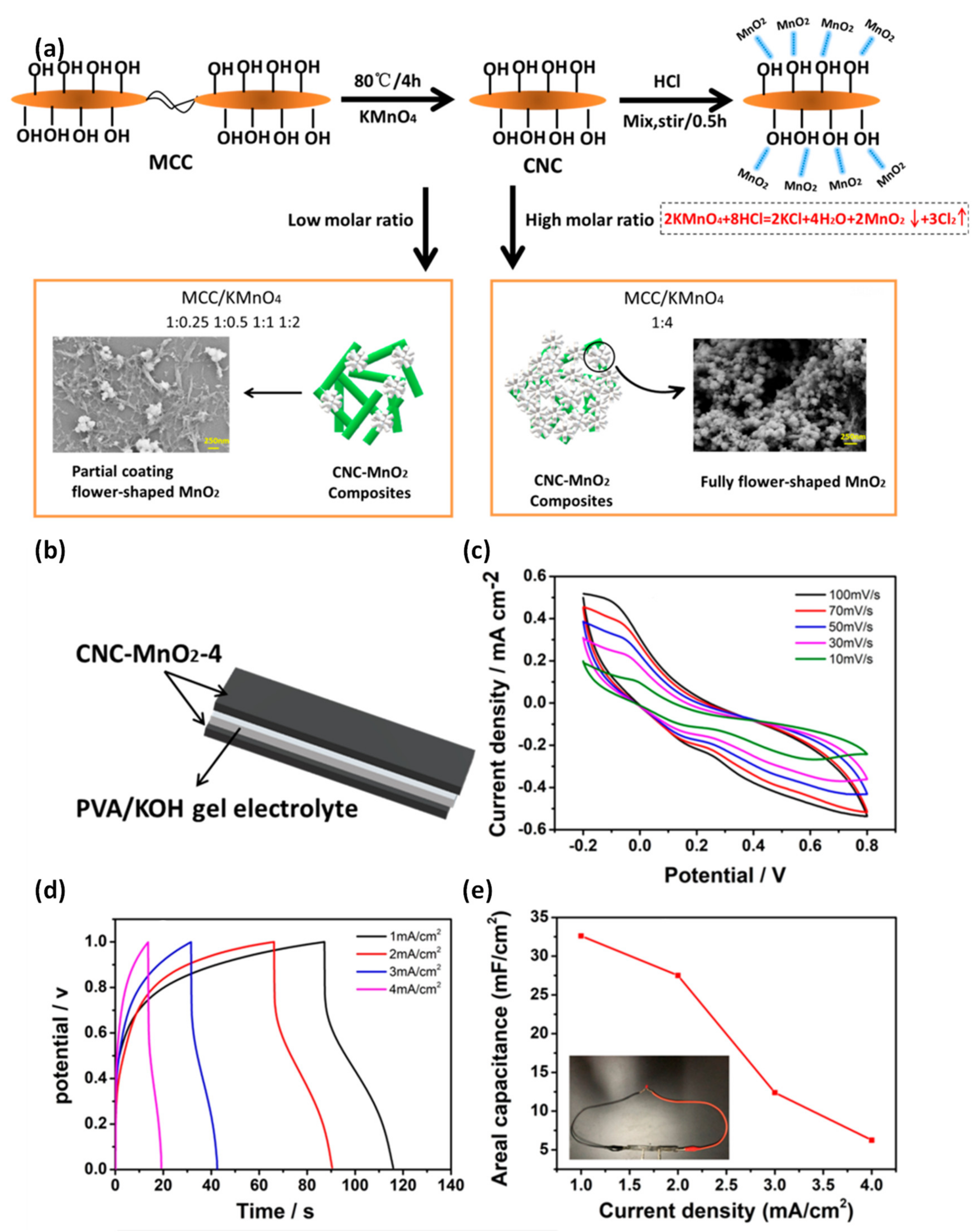
| CNC-MnO2 | Thermal synthesis | PVA/KOH | 306.3 | 42.59 | - | [112] |
| PEDOT/CNC /MnO2 | Electro polymerization | 1 M KCl | 144.69 | 10.3 | 494.9 | [113] |
| Porous carbon from CNC | Silica etching | 1 M H2SO4 | 170 | - | - | [118] |
| CNC/CNF | ALD | 2 M KOH | 152 | - | - | [119] |
| N-CNC | Pyrolysis | 1 M H2SO4 | 352 | 48.8 | 39.85 | [120] |
| N-CNC | Pyrolysis | 6 M KOH | 172 | 23.75 | 70 | [121] |
| N-CNC | pyrolysis | 6 M KOH | 570.6 | 23.75 | 50 | [122] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durairaj, A.; Maruthapandi, M.; Saravanan, A.; Luong, J.H.T.; Gedanken, A. Cellulose Nanocrystals (CNC)-Based Functional Materials for Supercapacitor Applications. Nanomaterials 2022, 12, 1828. https://doi.org/10.3390/nano12111828
Durairaj A, Maruthapandi M, Saravanan A, Luong JHT, Gedanken A. Cellulose Nanocrystals (CNC)-Based Functional Materials for Supercapacitor Applications. Nanomaterials. 2022; 12(11):1828. https://doi.org/10.3390/nano12111828
Chicago/Turabian StyleDurairaj, Arulppan, Moorthy Maruthapandi, Arumugam Saravanan, John H. T. Luong, and Aharon Gedanken. 2022. "Cellulose Nanocrystals (CNC)-Based Functional Materials for Supercapacitor Applications" Nanomaterials 12, no. 11: 1828. https://doi.org/10.3390/nano12111828
APA StyleDurairaj, A., Maruthapandi, M., Saravanan, A., Luong, J. H. T., & Gedanken, A. (2022). Cellulose Nanocrystals (CNC)-Based Functional Materials for Supercapacitor Applications. Nanomaterials, 12(11), 1828. https://doi.org/10.3390/nano12111828