Solvent Etching Process for Graphitic Carbon Nitride Photocatalysts Containing Platinum Cocatalyst: Effects of Water Hydrolysis on Photocatalytic Properties and Hydrogen Evolution Behaviors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of CNx
2.2. Synthesis of Pt/CNx
2.3. Characterization
2.4. H2 Evolution Test
3. Results and Discussion
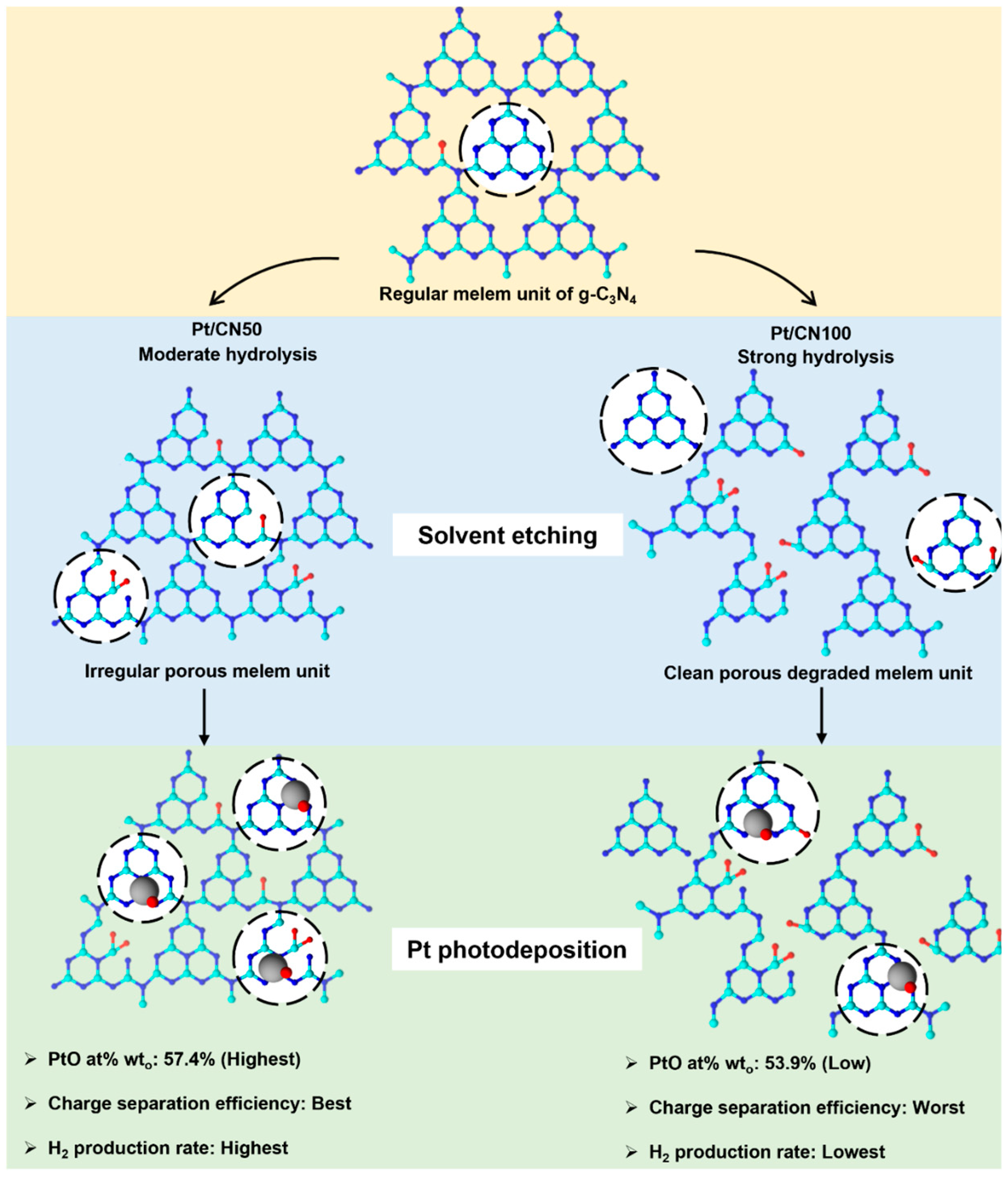
3.1. Physicochemical and Structural Properties
3.2. Optical Properties
3.3. Hydrogen Evolution Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Banos, R.; Manzano-Agugliaro, F.; Montoya, F.; Gil, C.; Alcayde, A.; Gómez, J. Optimization methods applied to renewable and sustainable energy: A review. Renew. Sustain. Energy Rev. 2011, 15, 1753–1766. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.; Leung, D.Y.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Currao, A. Photoelectrochemical water splitting. CHIMIA Int. J. Chem. 2007, 61, 815–819. [Google Scholar] [CrossRef]
- Elton, L.; Jackson, D.F. X-ray diffraction and the Bragg law. Am. J. Phys. 1966, 34, 1036–1038. [Google Scholar] [CrossRef]
- Luan, P.; Xie, M.; Liu, D.; Fu, X.; Jing, L. Effective charge separation in the rutile TiO2 nanorod-coupled α-Fe2O3 with exceptionally high visible activities. Sci. Rep. 2014, 4, 6180. [Google Scholar] [CrossRef]
- An, L.; Han, X.; Li, Y.; Wang, H.; Hou, C.; Zhang, Q. One step synthesis of self-doped F–Ta2O5 nanoshuttles photocatalyst and enhanced photocatalytic hydrogen evolution. Int. J. Hydrog. Energy 2021, 46, 3996–4006. [Google Scholar] [CrossRef]
- Pérez, A.; Orfila, M.; Linares, M.; Sanz, R.; Marugán, J.; Molina, R.; Botas, J.A. Hydrogen production by thermochemical water splitting with La0.8Al0.2MeO3-δ (Me = Fe, Co, Ni and Cu) perovskites prepared under controlled pH. Catal. Today 2021, 390–391, 22–33. [Google Scholar] [CrossRef]
- Chen, Z.G.; Xia, K.X.; She, X.J.; Mo, Z.; Zhao, S.W.; Yi, J.J.; Xu, Y.G.; Chen, H.X.; Xu, H.; Li, H.M. 1D metallic MoO2-C as co-catalyst on 2D g-C3N4 semiconductor to promote photocatlaytic hydrogen production. Appl. Surf. Sci. 2018, 447, 732–739. [Google Scholar] [CrossRef]
- Huang, H.W.; Liu, C.Y.; Ou, H.L.; Ma, T.Y.; Zhang, Y.H. Self-sacrifice transformation for fabrication of type-I and type-II heterojunctions in hierarchical BixOyIz/g-C3N4 for efficient visible-light photocatalysis. Appl. Surf. Sci. 2019, 470, 1101–1110. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.; Tomer, V.K. State-of-the-art review of morphological advancements in graphitic carbon nitride (g-CN) for sustainable hydrogen production. Renew. Sustain. Energy Rev. 2021, 135, 110235. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, Y.; Dong, F. Graphitic carbon nitride based nanocomposites: A review. Nanoscale 2015, 7, 15–37. [Google Scholar] [CrossRef]
- Ratshiedana, R.; Kuvarega, A.T.; Mishra, A.K. Titanium dioxide and graphitic carbon nitride–based nanocomposites and nanofibres for the degradation of organic pollutants in water: A review. Environ. Sci. Pollut. Res. 2021, 28, 10357–10374. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Mehta, A.; Basu, S.; Shetti, N.P.; Reddy, K.R.; Aminabhavi, T.M. Graphitic carbon nitride (g–C3N4)–based metal-free photocatalysts for water splitting: A review. Carbon 2019, 149, 693–721. [Google Scholar] [CrossRef]
- Tian, J.; Zhou, Z.; Zhang, S.; Li, Z.; Shi, L.; Li, Q.; Wang, J. Synergistic modulation of metal-free photocatalysts by the composition ratio change and heteroatom doping for overall water splitting. J. Mater. Chem. A 2021, 9, 11753–11761. [Google Scholar] [CrossRef]
- Mei, F.; Zhang, J.; Liang, C.; Dai, K. Fabrication of novel CoO/porous graphitic carbon nitride S-scheme heterojunction for efficient CO2 photoreduction. Mater. Lett. 2021, 282, 128722. [Google Scholar] [CrossRef]
- Zuo, W.; Liang, L.; Ye, F.; Zhao, S. Construction of visible light driven silver sulfide/graphitic carbon nitride pn heterojunction for improving photocatalytic disinfection. Chemosphere 2021, 283, 131167. [Google Scholar] [CrossRef]
- Liu, J.; Yan, X.-T.; Qin, X.-S.; Wu, S.-J.; Zhao, H.; Yu, W.-B.; Chen, L.-H.; Li, Y.; Su, B.-L. Light-assisted preparation of heterostructured g-C3N4/ZnO nanorods arrays for enhanced photocatalytic hydrogen performance. Catal. Today 2020, 355, 932–936. [Google Scholar] [CrossRef]
- Kim, J.S.; Oh, J.W.; Woo, S.I. Improvement of the photocatalytic hydrogen production rate of g-C3N4 following the elimination of defects on the surface. Catal. Today 2017, 293, 8–14. [Google Scholar] [CrossRef]
- Xu, L.; Zeng, J.; Li, Q.; Xia, L.; Luo, X.; Ma, Z.; Peng, B.; Xiong, S.; Li, Z.; Wang, L.-L. Defect-engineered 2D/2D hBN/g-C3N4 Z-scheme heterojunctions with full visible-light absorption: Efficient metal-free photocatalysts for hydrogen evolution. Appl. Surf. Sci. 2021, 547, 149207. [Google Scholar] [CrossRef]
- Meng, J.; Zhang, X.; Liu, Y.; Ren, M.; Guo, Y.; Yang, X.; Yang, Y. Engineering of graphitic carbon nitride with simultaneous potassium doping sites and nitrogen defects for notably enhanced photocatalytic oxidation performance. Sci. Total Environ. 2021, 796, 148946. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ng, S.F.; Putri, L.K.; Tan, L.L.; Mohamed, A.R.; Ong, W.J. Point-Defect Engineering: Leveraging Imperfections in Graphitic Carbon Nitride (g-C3N4) Photocatalysts toward Artificial Photosynthesis. Small 2021, 17, 2006851. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wei, C.; Zhuzhang, H.; Zhou, J.; Pan, Z.; Lin, W.; Yu, Z.; Zhang, G.; Wang, X. Fully Condensed Poly (Triazine Imide) Crystals: Extended π-Conjugation and Structural Defects for Overall Water Splitting. Angew. Chem. Int. Ed. 2022, 61, e202113389. [Google Scholar]
- Li, F.; Yue, X.; Zhang, D.; Fan, J.; Xiang, Q. Targeted regulation of exciton dissociation in graphitic carbon nitride by vacancy modification for efficient photocatalytic CO2 reduction. Appl. Catal. B Environ. 2021, 292, 120179. [Google Scholar] [CrossRef]
- Hong, Z.; Shen, B.; Chen, Y.; Lin, B.; Gao, B. Enhancement of photocatalytic H2 evolution over nitrogen-deficient graphitic carbon nitride. J. Mater. Chem. A 2013, 1, 11754–11761. [Google Scholar] [CrossRef]
- Sano, T.; Tsutsui, S.; Koike, K.; Hirakawa, T.; Teramoto, Y.; Negishi, N.; Takeuchi, K. Activation of graphitic carbon nitride (g-C3N4) by alkaline hydrothermal treatment for photocatalytic NO oxidation in gas phase. J. Mater. Chem. A 2013, 1, 6489–6496. [Google Scholar] [CrossRef]
- Dao, D.Q.; Anh Nguyen, T.K.; Kang, S.G.; Shin, E.W. Engineering Oxidation States of a Platinum Cocatalyst over Chemically Oxidized Graphitic Carbon Nitride Photocatalysts for Photocatalytic Hydrogen Evolution. ACS Sustain. Chem. Eng. 2021, 9, 14537–14549. [Google Scholar] [CrossRef]
- Xu, H.; Yan, J.; She, X.; Xu, L.; Xia, J.; Xu, Y.; Song, Y.; Huang, L.; Li, H. Graphene-analogue carbon nitride: Novel exfoliation synthesis and its application in photocatalysis and photoelectrochemical selective detection of trace amount of Cu2+. Nanoscale 2014, 6, 1406–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, H.; Ou, M.; Zhong, Q.; Zhang, S.; Yu, L. Efficient visible-light photocatalytic oxidation of gaseous NO with graphitic carbon nitride (g-C3N4) activated by the alkaline hydrothermal treatment and mechanism analysis. J. Hazard. Mater. 2015, 300, 598–606. [Google Scholar] [CrossRef]
- Nguyen, P.A.; Nguyen, T.K.A.; Dao, D.Q.; Shin, E.W. Ethanol Solvothermal Treatment on Graphitic Carbon Nitride Materials for Enhancing Photocatalytic Hydrogen Evolution Performance. Nanomaterials 2022, 12, 179. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, F.; Wang, X.; Yu, H. In situ one-step hydrothermal synthesis of oxygen-containing groups-modified g-C3N4 for the improved photocatalytic H2-evolution performance. Appl. Surf. Sci. 2018, 427, 645–653. [Google Scholar] [CrossRef]
- Ming, L.; Yue, H.; Xu, L.; Chen, F. Hydrothermal synthesis of oxidized g-C3N4 and its regulation of photocatalytic activity. J. Mater. Chem. A 2014, 2, 19145–19149. [Google Scholar] [CrossRef]
- Nguyen, T.K.A.; Pham, T.-T.; Gendensuren, B.; Oh, E.-S.; Shin, E.W. Defect engineering of water-dispersible g-C3N4 photocatalysts by chemical oxidative etching of bulk g-C3N4 prepared in different calcination atmospheres. J. Mater. Sci. Technol. 2022, 103, 232–243. [Google Scholar] [CrossRef]
- Nguyen, T.K.A.; Pham, T.-T.; Nguyen-Phu, H.; Shin, E.W. The effect of graphitic carbon nitride precursors on the photocatalytic dye degradation of water-dispersible graphitic carbon nitride photocatalysts. Appl. Surf. Sci. 2021, 537, 148027. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Ma, T.; Zhang, Y.; Huang, H. 2D Graphitic Carbon Nitride for Energy Conversion and Storage. Adv. Funct. Mater. 2021, 31, 2102540. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, Z.; Long, J.; Liang, J.; Lin, H.; Lin, H.; Wang, X. Vacuum heat-treatment of carbon nitride for enhancing photocatalytic hydrogen evolution. J. Mater. Chem. A 2014, 2, 17797–17807. [Google Scholar] [CrossRef]
- Madhusudan, P.; Shi, R.; Xiang, S.; Jin, M.; Chandrashekar, B.N.; Wang, J.; Wang, W.; Peng, O.; Amini, A.; Cheng, C. Construction of highly efficient Z-scheme ZnxCd1−xS/Au@g-C3N4 ternary heterojunction composite for visible-light-driven photocatalytic reduction of CO2 to solar fuel. Appl. Catal. B Environ. 2021, 282, 119600. [Google Scholar] [CrossRef]
- Obregón, S.; Colón, G. Improved H2 production of Pt-TiO2/g-C3N4-MnOx composites by an efficient handling of photogenerated charge pairs. Appl. Catal. B Environ. 2014, 144, 775–782. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, Z.; Dong, F.; Zhang, Y. Solvent-assisted synthesis of porous g-C3N4 with efficient visible-light photocatalytic performance for NO removal. Chin. J. Catal. 2017, 38, 372–378. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, J.; Yu, H.; Yu, J. A facile hydrothermal synthesis of carbon dots modified g-C3N4 for enhanced photocatalytic H2-evolution performance. Dalton Trans. 2017, 46, 6417–6424. [Google Scholar] [CrossRef]
- Du, C.; Yan, B.; Lin, Z.; Yang, G. Cross-linked bond accelerated interfacial charge transfer in monolayer zinc indium sulfide (ZnIn2S4)/reduced graphene oxide (RGO) heterostructure for photocatalytic hydrogen production with mechanistic insight. Catal. Sci. Technol. 2019, 9, 4066–4076. [Google Scholar] [CrossRef]
- Neena, D.; Humayun, M.; Zuo, W.; Liu, C.; Gao, W.; Fu, D.J. Hierarchical hetero-architectures of in-situ g-C3N4-coupled Fe-doped ZnO micro-flowers with enhanced visible-light photocatalytic activities. Appl. Surf. Sci. 2020, 506, 145017. [Google Scholar] [CrossRef]
- Li, H.; Ning, F.; Chen, X.; Shi, A. Effect of carbon and nitrogen double vacancies on the improved photocatalytic hydrogen evolution over porous carbon nitride nanosheets. Catal. Sci. Technol. 2021, 11, 3270–3278. [Google Scholar] [CrossRef]
- Jin, Z.; Chen, J.; Huang, S.; Wu, J.; Zhang, Q.; Zhang, W.; Zeng, Y.-J.; Ruan, S.; Ohno, T. A facile approach to fabricating carbonaceous material/g-C3N4 composites with superior photocatalytic activity. Catal. Today 2018, 315, 149–154. [Google Scholar] [CrossRef]
- Wang, S.; Li, D.; Sun, C.; Yang, S.; Guan, Y.; He, H. Synthesis and characterization of g-C3N4/Ag3VO4 composites with significantly enhanced visible-light photocatalytic activity for triphenylmethane dye degradation. Appl. Catal. B Environ. 2014, 144, 885–892. [Google Scholar] [CrossRef]
- Jiang, J.; Yu, J.; Cao, S. Au/PtO nanoparticle-modified g-C3N4 for plasmon-enhanced photocatalytic hydrogen evolution under visible light. J. Colloid Interface Sci. 2016, 461, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Niu, P.; Wang, L.; Lu, G.Q.M.; Cheng, H.-M. Achieving maximum photo-oxidation reactivity of Cs0.68Ti1.83O4−xNx photocatalysts through valence band fine-tuning. Catal. Sci. Technol. 2011, 1, 222–225. [Google Scholar] [CrossRef]
- Zhu, J.; Zäch, M. Nanostructured materials for photocatalytic hydrogen production. Curr. Opin. Colloid Interface Sci. 2009, 14, 260–269. [Google Scholar] [CrossRef]
- Ma, H.; Li, Y.; Li, S.; Liu, N. Novel PO codoped g-C3N4 with large specific surface area: Hydrothermal synthesis assisted by dissolution–precipitation process and their visible light activity under anoxic conditions. Appl. Surf. Sci. 2015, 357, 131–138. [Google Scholar] [CrossRef]
- Xu, Q.; Zhu, B.; Cheng, B.; Yu, J.; Zhou, M.; Ho, W. Photocatalytic H2 evolution on graphdiyne/g-C3N4 hybrid nanocomposites. Appl. Catal. B Environ. 2019, 255, 117770. [Google Scholar] [CrossRef]
- Sun, N.; Zhu, Y.; Li, M.; Zhang, J.; Qin, J.; Li, Y.; Wang, C. Thermal coupled photocatalysis over Pt/g-C3N4 for selectively reducing CO2 to CH4 via cooperation of the electronic metal–support interaction effect and the oxidation state of Pt. Appl. Catal. B Environ. 2021, 298, 120565. [Google Scholar] [CrossRef]
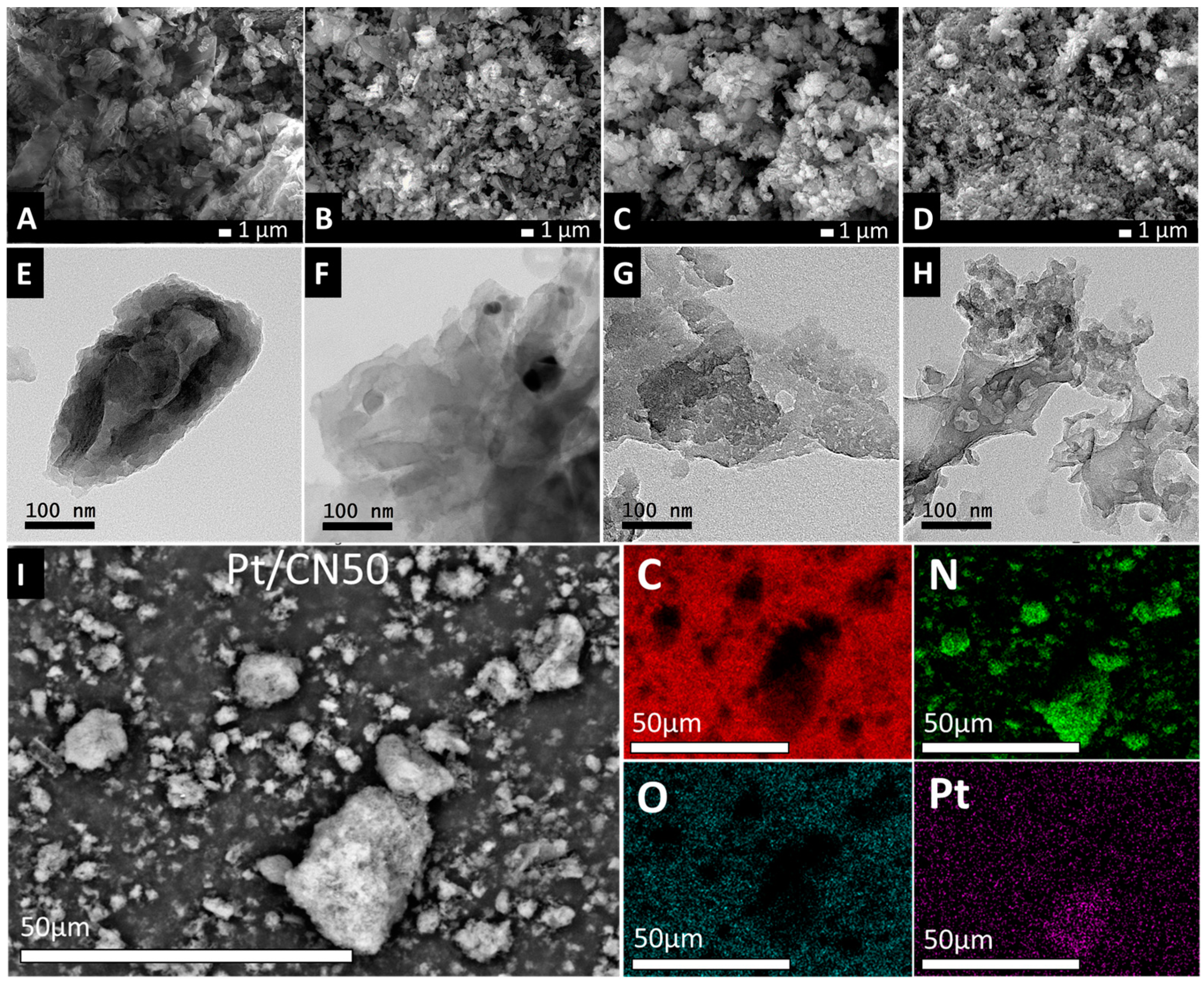
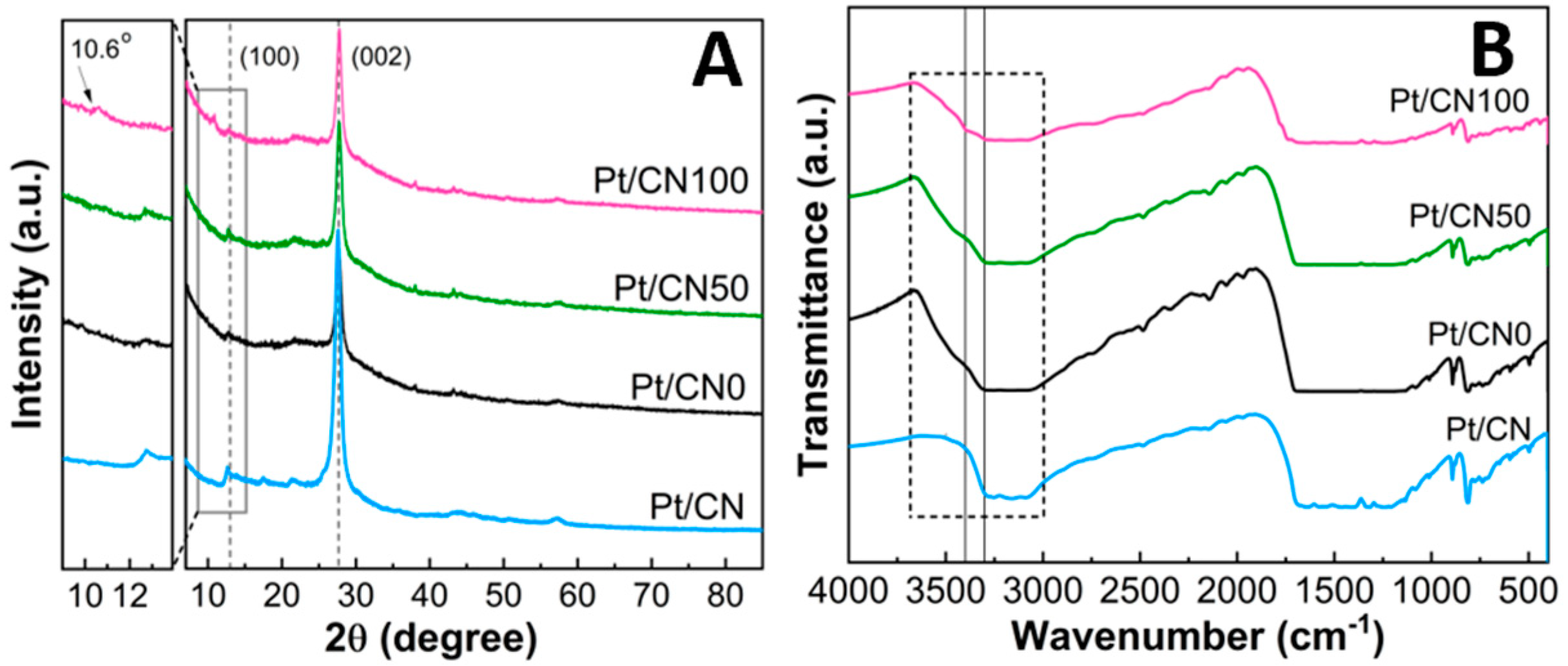

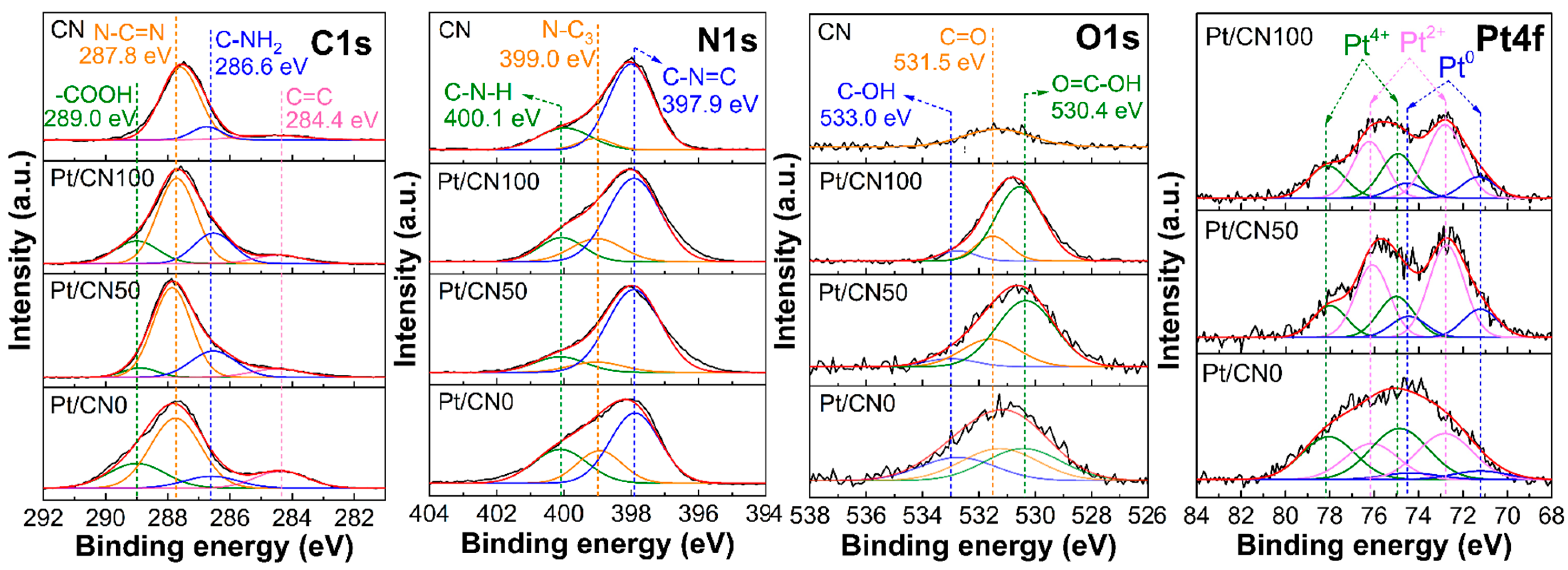
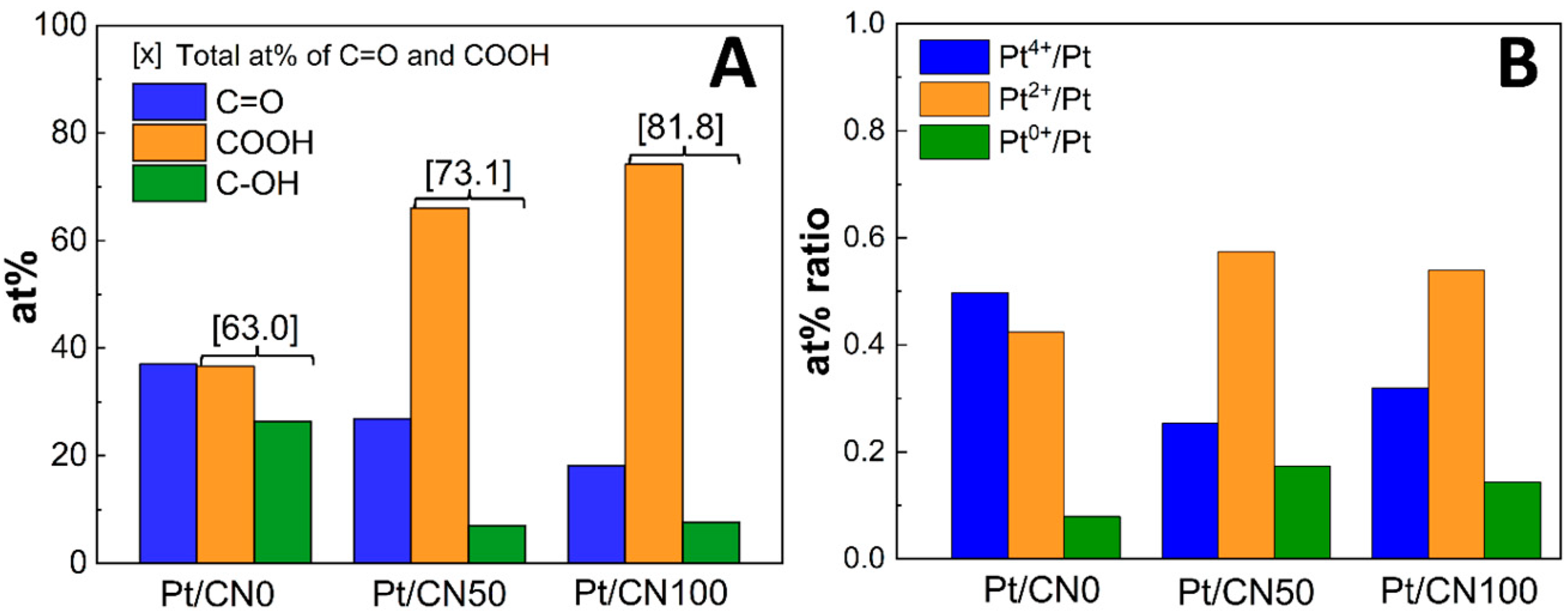
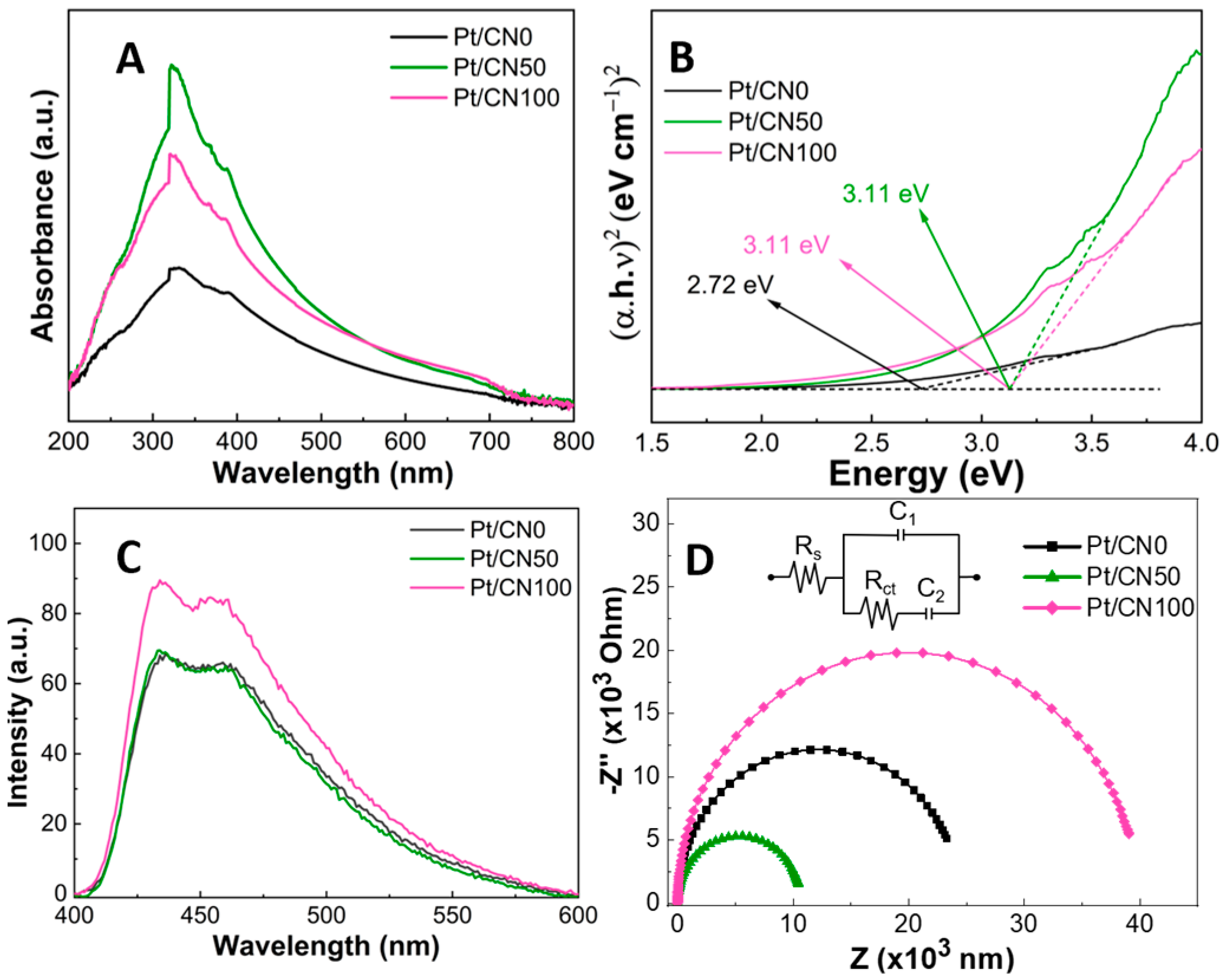

| Sample | Pt (%) a | SBET (m2/g) b | V (cm3/g) b | L (nm) b | Band Gap (eV) c |
|---|---|---|---|---|---|
| Pt/CN | 3.24 | 36.3 | 0.254 | 27.8 | 2.58 |
| Pt/CN0 | 2.29 | 56.3 | 0.483 | 3.83 | 2.72 |
| Pt/CN50 | 1.54 | 70.5 | 0.435 | 3.82 | 3.11 |
| Pt/CN100 | 3.03 | 51.6 | 0.424 | 24 | 3.11 |
| Element Analysis | ||||
|---|---|---|---|---|
| Samples | Atom (wt%) | Atomic Ratio | ||
| C | N | O | O/N | |
| Pt/CN | 35.7 | 59.1 | 0.46 | 0.008 |
| Pt/CN0 | 33.9 | 63.6 | 0.61 | 0.008 |
| Pt/CN50 | 33.5 | 62.2 | 2.05 | 0.029 |
| Pt/CN100 | 31.6 | 60.6 | 4.71 | 0.068 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoang, T.V.A.; Nguyen, T.K.A.; Dao, D.Q.; Nguyen, P.A.; Jeong, D.H.; Shin, E.W. Solvent Etching Process for Graphitic Carbon Nitride Photocatalysts Containing Platinum Cocatalyst: Effects of Water Hydrolysis on Photocatalytic Properties and Hydrogen Evolution Behaviors. Nanomaterials 2022, 12, 1188. https://doi.org/10.3390/nano12071188
Hoang TVA, Nguyen TKA, Dao DQ, Nguyen PA, Jeong DH, Shin EW. Solvent Etching Process for Graphitic Carbon Nitride Photocatalysts Containing Platinum Cocatalyst: Effects of Water Hydrolysis on Photocatalytic Properties and Hydrogen Evolution Behaviors. Nanomaterials. 2022; 12(7):1188. https://doi.org/10.3390/nano12071188
Chicago/Turabian StyleHoang, Thi Van Anh, Thi Kim Anh Nguyen, Duc Quang Dao, Phuong Anh Nguyen, Dong Hwi Jeong, and Eun Woo Shin. 2022. "Solvent Etching Process for Graphitic Carbon Nitride Photocatalysts Containing Platinum Cocatalyst: Effects of Water Hydrolysis on Photocatalytic Properties and Hydrogen Evolution Behaviors" Nanomaterials 12, no. 7: 1188. https://doi.org/10.3390/nano12071188
APA StyleHoang, T. V. A., Nguyen, T. K. A., Dao, D. Q., Nguyen, P. A., Jeong, D. H., & Shin, E. W. (2022). Solvent Etching Process for Graphitic Carbon Nitride Photocatalysts Containing Platinum Cocatalyst: Effects of Water Hydrolysis on Photocatalytic Properties and Hydrogen Evolution Behaviors. Nanomaterials, 12(7), 1188. https://doi.org/10.3390/nano12071188






