Colorimetric Nanoparticle-Embedded Hydrogels for a Biosensing Platform
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
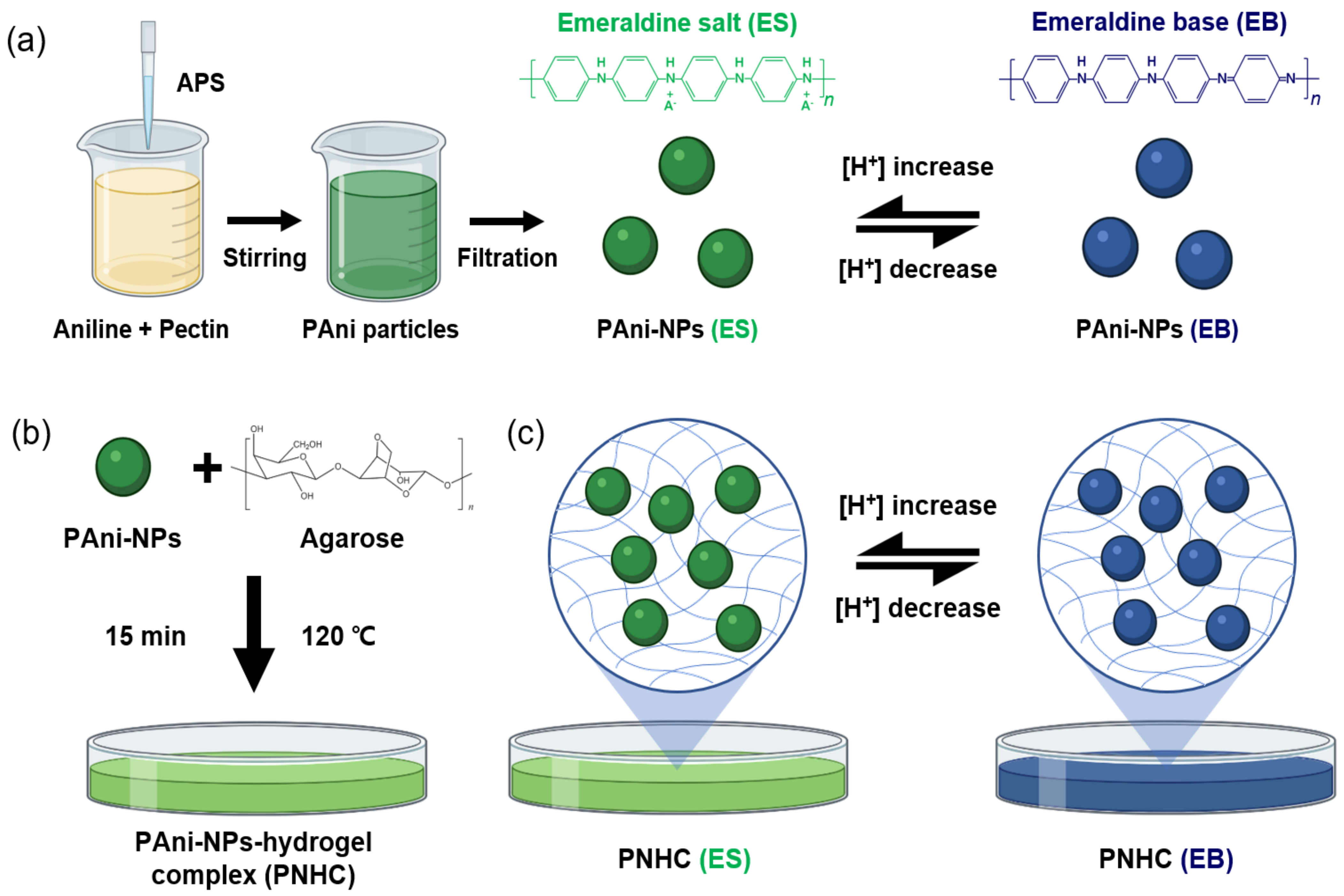
2.2. Synthesis of PAni-NPs
2.3. Fabrication of PNHC
2.4. Characterization
2.5. Cell Culture and Sample Extraction
2.6. Analysis of Cell Viability and Cytotoxicity
3. Results and Discussion
3.1. Fabrication of Biocompatible PNHC
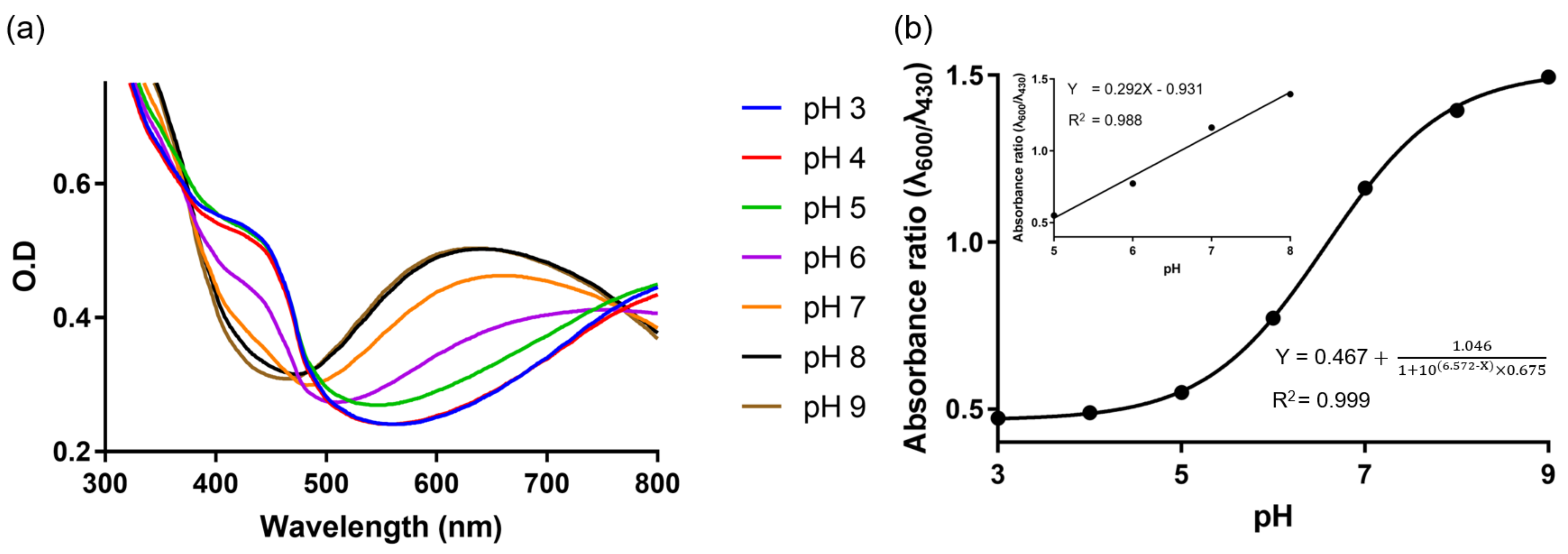
3.2. Optical Properties of PAni-NPs
3.3. Physical Properties of PNHC
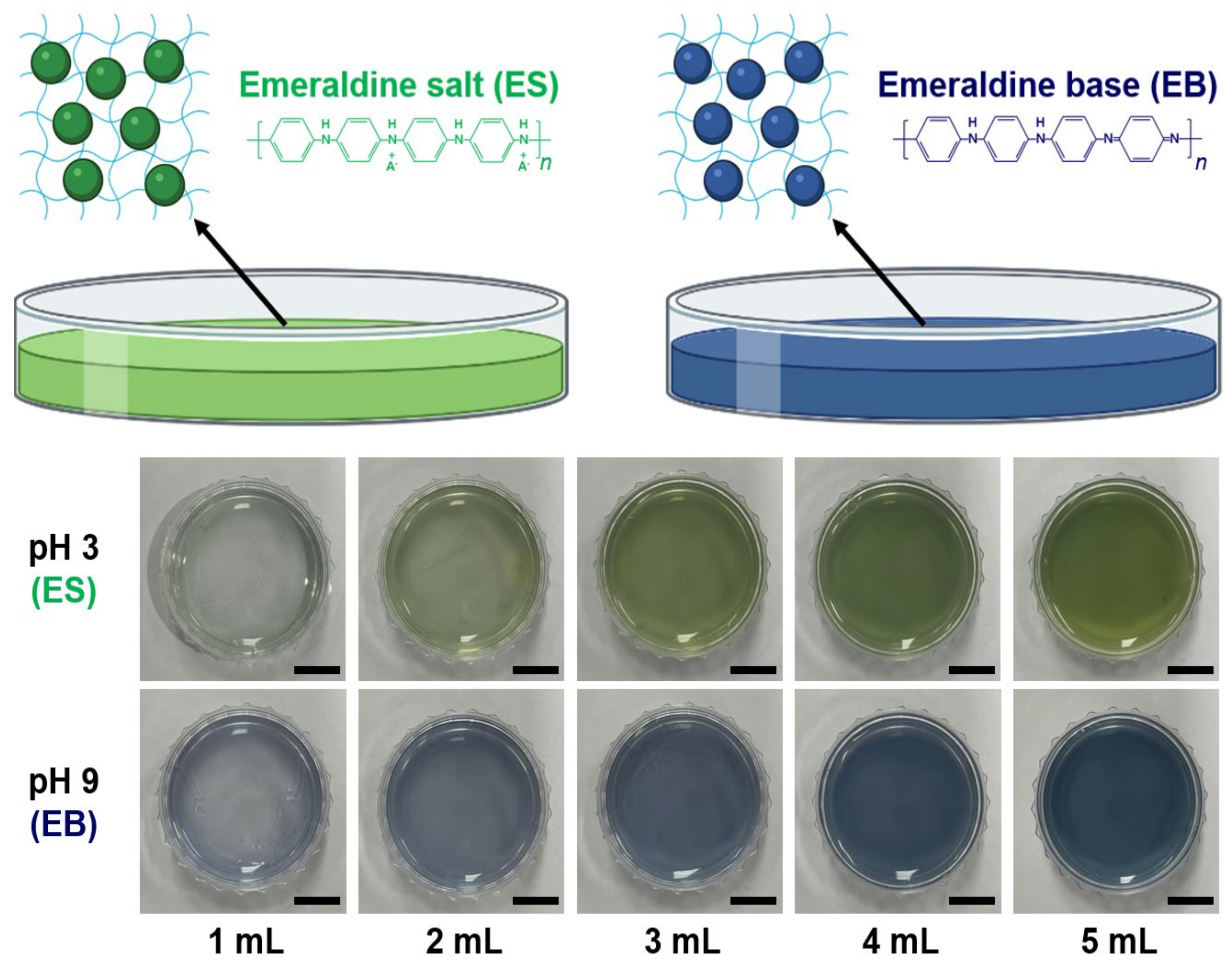
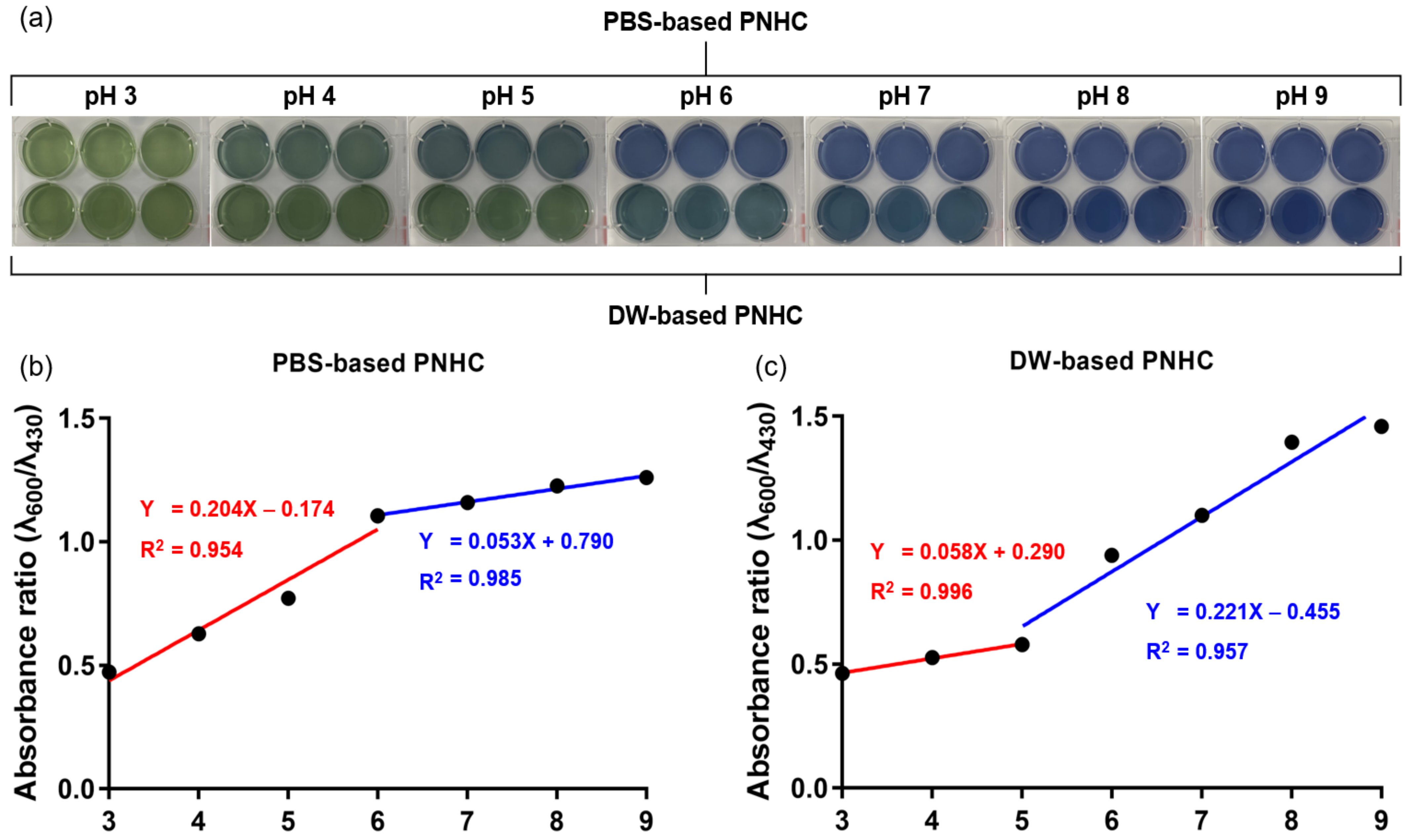
3.4. Effect of Solvent Type on Intrinsic Color of PNHC
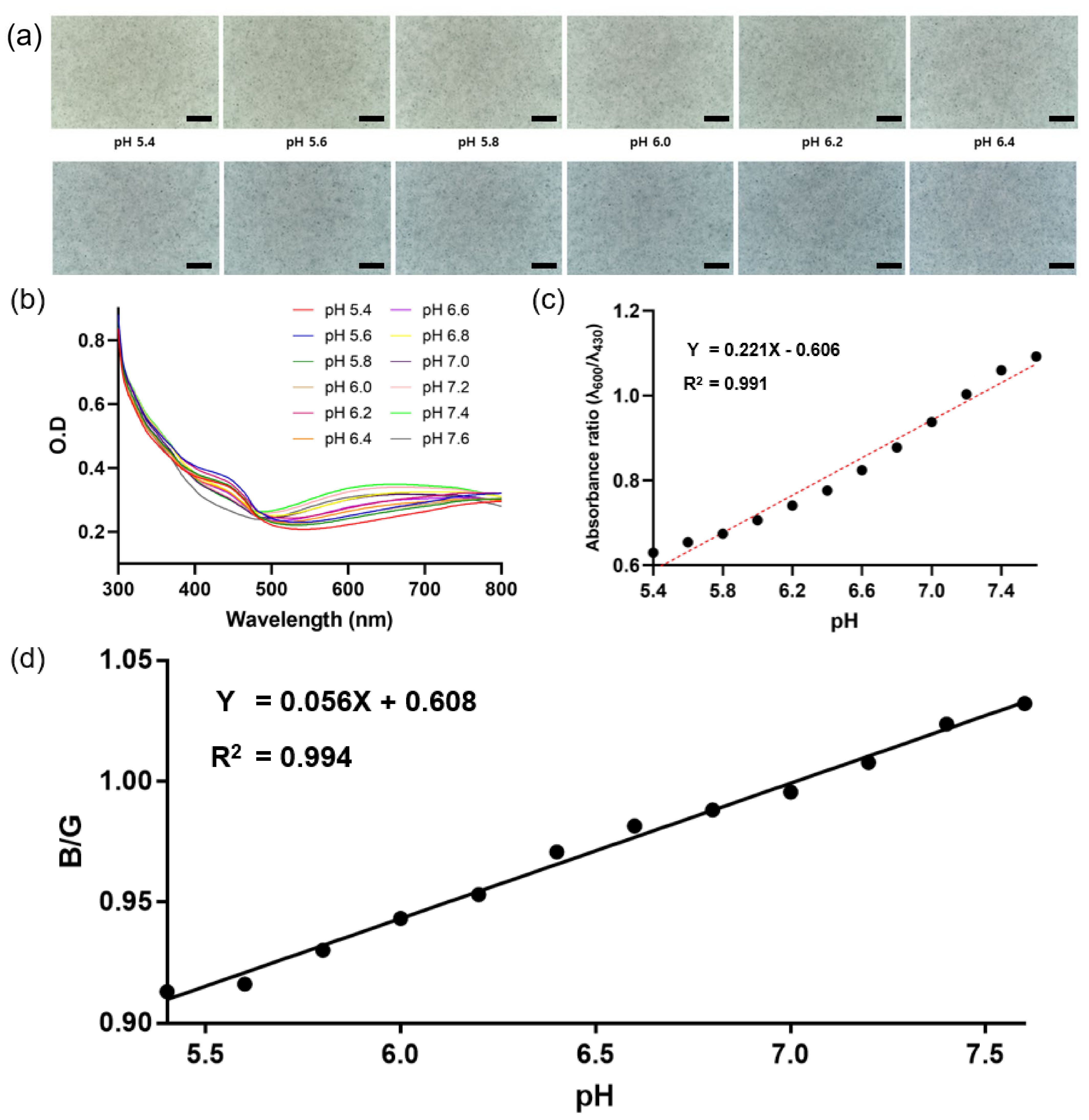
3.5. Colorimetric Responses of PNHC to pH
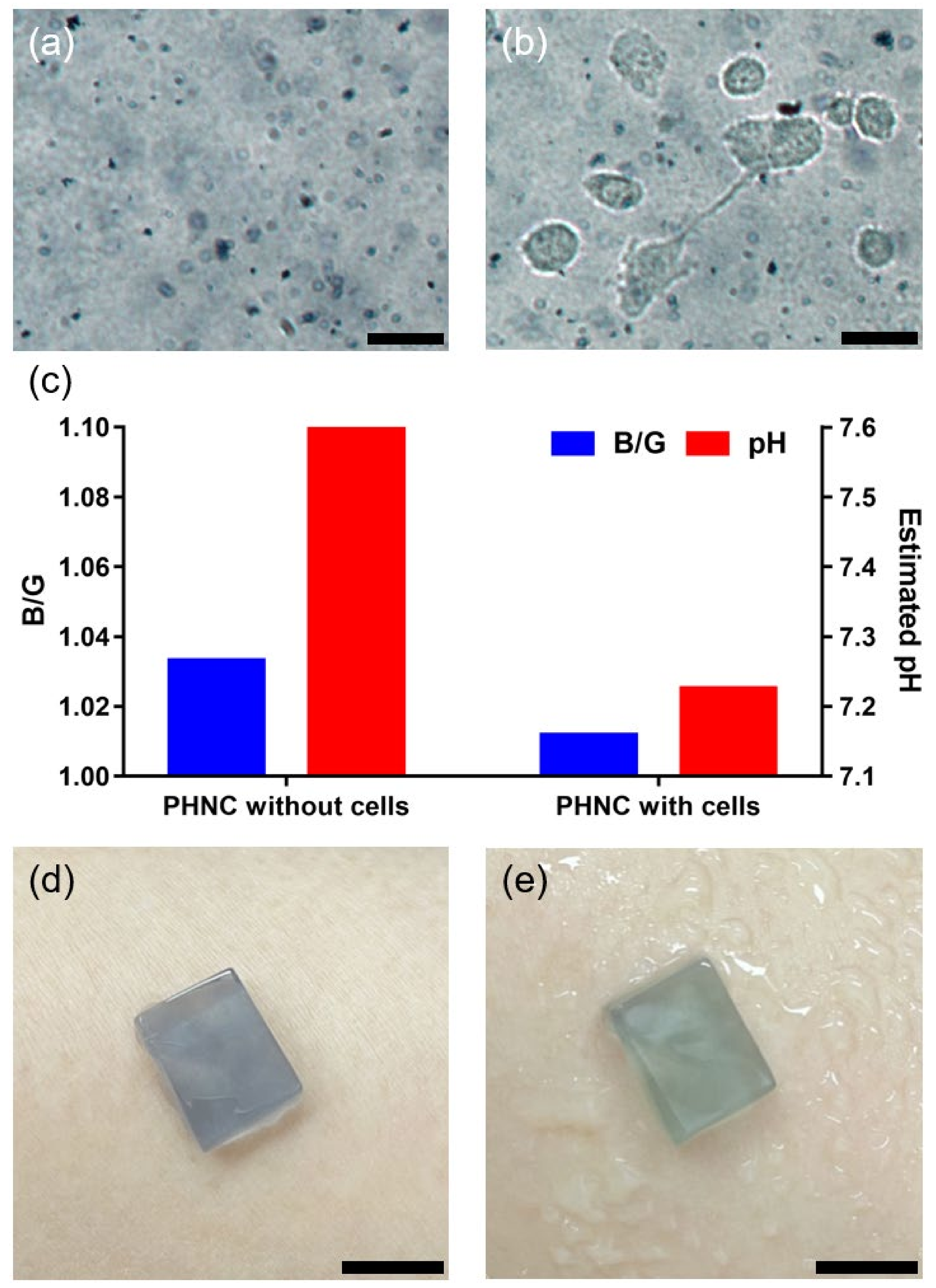
3.6. Application of PNHC as Biosensing Platforms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Elst, L.V.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2010, 110, 2574. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Tiwari, R.N.; Kim, K.S. Zero-dimensional, one-dimensional, two-dimensional and three-dimensional nanostructured materials for advanced electrochemical energy devices. Prog. Mater. Sci. 2012, 57, 724–803. [Google Scholar] [CrossRef]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, W.E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release 2001, 70, 1–20. [Google Scholar] [CrossRef]
- Giner-Casares, J.J.; Henriksen-Lacey, M.; Coronado-Puchau, M.; Liz-Marzán, L.M. Inorganic nanoparticles for biomedicine: Where materials scientists meet medical research. Mater. Today 2016, 19, 19–28. [Google Scholar] [CrossRef]
- Schröfel, A.; Kratošová, G.; Šafařík, I.; Šafaříková, M.; Raška, I.; Shor, L.M. Applications of biosynthesized metallic nanoparticles—A review. Acta Biomater. 2014, 10, 4023–4042. [Google Scholar] [CrossRef]
- Goenka, S.; Sant, V.; Sant, S. Graphene-based nanomaterials for drug delivery and tissue engineering. J. Control. Release 2014, 173, 75–88. [Google Scholar] [CrossRef]
- Söntjens, S.H.M.; Nettles, D.L.; Carnahan, M.A.; Setton, L.A.; Grinstaff, M.W. Biodendrimer-Based Hydrogel Scaffolds for Cartilage Tissue Repair. Biomacromolecules 2006, 7, 310–316. [Google Scholar] [CrossRef]
- Merino, S.; Martin, C.; Kostarelos, K.; Prato, M.; Vázquez, E. Nanocomposite Hydrogels: 3D Polymer–Nanoparticle Synergies for On-Demand Drug Delivery. ACS Nano 2015, 9, 4686–4697. [Google Scholar] [CrossRef] [Green Version]
- Patel, K.D.; Singh, R.K.; Kim, H.-W. Carbon-based nanomaterials as an emerging platform for theranostics. Mater. Horiz. 2019, 6, 434–469. [Google Scholar] [CrossRef]
- Pek, Y.S.; Wan, A.C.A.; Shekaran, A.; Zhuo, L.; Ying, J.Y. A thixotropic nanocomposite gel for three-dimensional cell culture. Nat. Nanotechnol. 2008, 3, 671–675. [Google Scholar] [CrossRef]
- Rose, S.; Prevoteau, A.; Elzière, P.; Hourdet, D.; Marcellan, A.; Leibler, L. Nanoparticle solutions as adhesives for gels and biological tissues. Nature 2014, 505, 382–385. [Google Scholar] [CrossRef]
- Wang, Q.; Mynar, J.L.; Yoshida, M.; Lee, E.; Lee, E.; Lee, M.; Okuro, K.; Kinbara, K.; Aida, T. High-water-content mouldable hydrogels by mixing clay and a dendritic molecular binder. Nature 2010, 463, 339–343. [Google Scholar] [CrossRef]
- Fu, J. Marc in het Panhuis. Hydrogel properties and applications. J. Mater. Chem. B 2019, 7, 1523–1525. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Lee, D.S. Injectable Biodegradable Hydrogels. Macromol. Biosci. 2010, 10, 563–579. [Google Scholar] [CrossRef]
- Yang, X.; Bakaic, E.; Hoare, T.; Cranston, E.D. Injectable Polysaccharide Hydrogels Reinforced with Cellulose Nanocrystals: Morphology, Rheology, Degradation, and Cytotoxicity. Biomacromolecules 2013, 14, 4447–4455. [Google Scholar] [CrossRef]
- Jung, H.G.; Lee, D.; Lee, S.W.; Kim, I.; Kim, Y.; Jang, J.W.; Lee, J.H.; Yoon, D.S. Nanoindentation for Monitoring the Time-Variant Mechanical Strength of Drug-Loaded Collagen Hydrogel Regulated by Hydroxyapatite Nanoparticles. ACS Omega 2021, 6, 9269–9278. [Google Scholar] [CrossRef]
- Arno, M.C.; Inam, M.; Weems, A.C.; Li, Z.; Binch, A.L.A.; Platt, C.I.; Richardson, S.M.; Hoyland, J.A.; Dove, A.P.; O’Reilly, R.K. Exploiting the role of nanoparticle shape in enhancing hydrogel adhesive and mechanical properties. Nat. Commun. 2020, 11, 1420. [Google Scholar] [CrossRef] [Green Version]
- Thakur, B.; Amarnath, C.A.; Mangoli, S.H.; Sawant, S.N. Polyaniline nanoparticle based colorimetric sensor for monitoring bacterial growth. Sens. Actuators B Chem. 2015, 207, 262–268. [Google Scholar] [CrossRef]
- Huang, W.S.; MacDiarmid, A.G. Optical properties of polyaniline. Polymer 1993, 34, 1833–1845. [Google Scholar] [CrossRef]
- Hong, Y.; Kim, H.S.; Lee, T.; Lee, G.; Kwon, O. Polyaniline Nanoskein: Synthetic Method, Characterization, and Redox Sensing. Nanoscale Res. Lett. 2020, 15, 215. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Kim, I.; Cheong, D.Y.; Roh, S.; Jung, H.G.; Lee, S.W.; Kim, H.S.; Yoon, D.S.; Hong, Y.; Lee, G. Selective colorimetric urine glucose detection by paper sensor functionalized with polyaniline nanoparticles and cell membrane. Anal. Chim. Acta 2021, 1158, 338387. [Google Scholar] [CrossRef]
- Amarnath, C.A.; Venkatesan, N.; Doble, M.; Sawant, S.N. Water dispersible Ag@polyaniline-pectin as supercapacitor electrode for physiological environment. J. Mater. Chem. B 2014, 2, 5012–5019. [Google Scholar] [CrossRef]
- Thakur, B.; Amarnath, C.A.; Sawant, S.N. Pectin coated polyaniline nanoparticles for an amperometric glucose biosensor. RSC Adv. 2014, 4, 40917–40923. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.-O.; Kim, H.S.; Kwon, O.; Rho, H.-W.; Huh, Y.-M.; Hong, Y. Active colorimetric lipid-coated polyaniline nanoparticles for redox state sensing in cancer cells. J. Mater. Chem. B 2021, 9, 3131–3135. [Google Scholar] [CrossRef]
- Bhadra, S.; Khastgir, D.; Singha, N.K.; Lee, J.H. Progress in preparation, processing and applications of polyaniline. Prog. Polym. Sci. 2009, 34, 783–810. [Google Scholar] [CrossRef]
- Ma, S.; Yan, C.; Cai, M.; Yang, J.; Wang, X.; Zhou, X.; Zhou, F.; Liu, W. Continuous Surface Polymerization via Fe(II)-Mediated Redox Reaction for Thick Hydrogel Coatings on Versatile Substrates. Adv. Mater. 2018, 30, 1803371. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, X.; Tang, B.; Xu, W.; Li, J.; Hu, J.; Gu, Z. Quantum-Dot-Tagged Bioresponsive Hydrogel Suspension Array for Multiplex Label-Free DNA Detection. Adv. Funct. Mater. 2010, 20, 976–982. [Google Scholar] [CrossRef]
- Wang, L.; Xu, T.; He, X.; Zhang, X. Flexible, self-healable, adhesive and wearable hydrogel patch for colorimetric sweat detection. J. Mater. Chem. C 2021, 9, 14938–14945. [Google Scholar] [CrossRef]
- Tavakoli, J.; Tang, Y. Hydrogel Based Sensors for Biomedical Applications: An Updated Review. Polymers 2017, 9, 364. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.; Wufuer, M.; Kim, I.; Choi, T.H.; Kim, B.J.; Jung, H.G.; Jeon, B.; Lee, G.; Jeon, O.H.; Chang, H.; et al. Sequential dual-drug delivery of BMP-2 and alendronate from hydroxyapatite-collagen scaffolds for enhanced bone regeneration. Sci. Rep. 2021, 11, 746. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.T.; Ahn, H.T.; Kim, M.I. Reagent-Free Colorimetric Assay for Galactose Using Agarose Gel Entrapping Nanoceria and Galactose Oxidase. Nanomaterials 2020, 10, 895. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wang, Y.; Tao, G.; Cai, R.; Wang, P.; Liu, L.; Ai, L.; Zuo, H.; Zhao, P.; Umar, A.; et al. Fabrication of Sericin/Agrose Gel Loaded Lysozyme and Its Potential in Wound Dressing Application. Nanomaterials 2018, 8, 235. [Google Scholar] [CrossRef] [Green Version]
- Mrówczyński, R.; Jędrzak, A.; Szutkowski, K.; Grześkowiak, B.F.; Coy, E.; Markiewicz, R.; Jesionowski, T.; Jurga, S. Cyclodextrin-Based Magnetic Nanoparticles for Cancer Therapy. Nanomaterials 2018, 8, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, N.A.; Heng, L.Y.; Salam, F.; Zaid, M.H.Z.; Hanifah, S.A. A Colorimetric pH Sensor Based on Clitoria sp and Brassica sp for Monitoring of Food Spoilage Using Chromametry. Sensors 2019, 19, 4813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, N.; Yuan, H.; Zhou, H.; Zaho, Y.; Zhang, S. Colorimetric detection of water content in organic solvents via a smartphone with fluorescent Ag nanoclusters. Anal. Methods 2021, 13, 2722–2727. [Google Scholar] [CrossRef]
- Liu, L.; Li, X.; Nagao, M.; Elias, A.L.; Narain, R.; Chung, H. A pH-Indicating Colorimetric Tough Hydrogel Patch towards Applications in a Substrate for Smart Wound Dressings. Polymers 2017, 9, 558. [Google Scholar] [CrossRef] [Green Version]
- Ku, M.; Kim, H.-J.; Yau, S.Y.; Yoon, N.; Kim, N.H.; Yook, J.I.; Suh, J.-S.; Kim, D.E.; Yang, J. Microsphere-Based Nanoindentation for the Monitoring of Cellular Cortical Stiffness Regulated by MT1-MMP. Small 2018, 14, 1803000. [Google Scholar] [CrossRef]
- Brahimi-Horn, M.C.; Chiche, J.; Pouysségur, J. Hypoxia signalling controls metabolic demand. Curr. Opin. Cell Biol. 2007, 19, 223–229. [Google Scholar] [CrossRef]
- Khaparde, S.S.; Roychoudhury, P.K.; Gomes, J.; Mukhopadhyay, A. External modulation of HT-1080 human fibrosarcoma cells improves urokinase production. Biotechnol. Prog. 2008, 24, 1325–1332. [Google Scholar] [CrossRef]
- Han, Q.; Wang, A.; Song, W.; Zhang, M.; Wang, S.; Ren, P.; Hao, L.; Yin, J.; Bai, S. Fabrication of Conductive, Adhesive, and Stretchable Agarose-Based Hydrogels for a Wearable Biosensor. ACS Appl. Bio. Mater. 2021, 4, 6148–6156. [Google Scholar] [CrossRef]
- Lei, K.; Wang, K.; Sun, Y.; Zheng, Z.; Wang, X. Rapid-Fabricated and Recoverable Dual-Network Hydrogel with Inherently Anti-Bacterial Abilities for Potential Adhesive Dressings. Adv. Funct. Mater. 2021, 31, 2008010. [Google Scholar] [CrossRef]
- Lin, P.-H.; Sheu, S.-C.; Chen, C.-W.; Huang, S.-C.; Li, B.-R. Wearable hydrogel patch with noninvasive, electrochemical glucose sensor for natural sweat detection. Talanta 2022, 241, 123187. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, T.; Kim, C.; Kim, J.; Seong, J.B.; Lee, Y.; Roh, S.; Cheong, D.Y.; Lee, W.; Park, J.; Hong, Y.; et al. Colorimetric Nanoparticle-Embedded Hydrogels for a Biosensing Platform. Nanomaterials 2022, 12, 1150. https://doi.org/10.3390/nano12071150
Lee T, Kim C, Kim J, Seong JB, Lee Y, Roh S, Cheong DY, Lee W, Park J, Hong Y, et al. Colorimetric Nanoparticle-Embedded Hydrogels for a Biosensing Platform. Nanomaterials. 2022; 12(7):1150. https://doi.org/10.3390/nano12071150
Chicago/Turabian StyleLee, Taeha, Changheon Kim, Jiyeon Kim, Jung Bae Seong, Youngjeon Lee, Seokbeom Roh, Da Yeon Cheong, Wonseok Lee, Jinsung Park, Yoochan Hong, and et al. 2022. "Colorimetric Nanoparticle-Embedded Hydrogels for a Biosensing Platform" Nanomaterials 12, no. 7: 1150. https://doi.org/10.3390/nano12071150
APA StyleLee, T., Kim, C., Kim, J., Seong, J. B., Lee, Y., Roh, S., Cheong, D. Y., Lee, W., Park, J., Hong, Y., & Lee, G. (2022). Colorimetric Nanoparticle-Embedded Hydrogels for a Biosensing Platform. Nanomaterials, 12(7), 1150. https://doi.org/10.3390/nano12071150







