Graphene Oxide Thin Films with Drug Delivery Function
Abstract
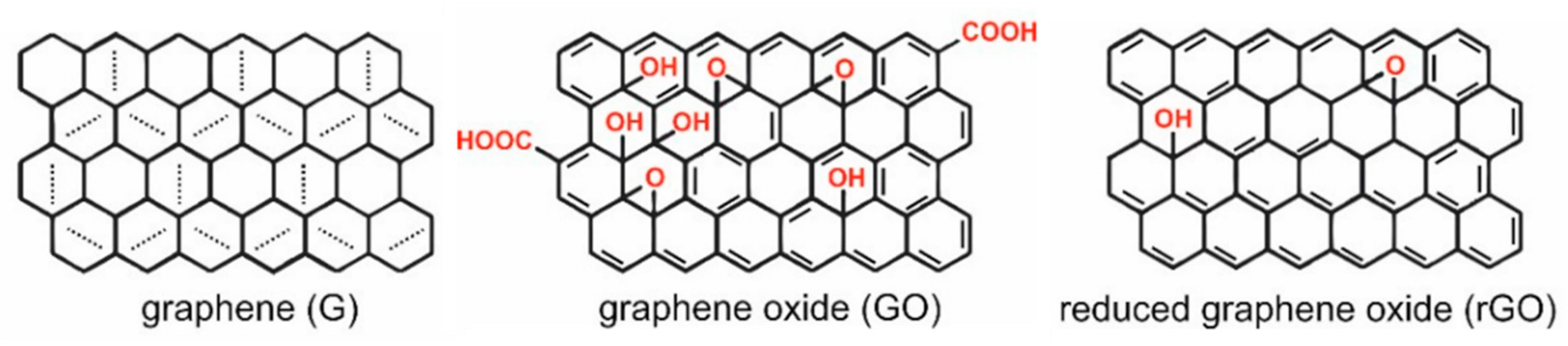
1. Introduction
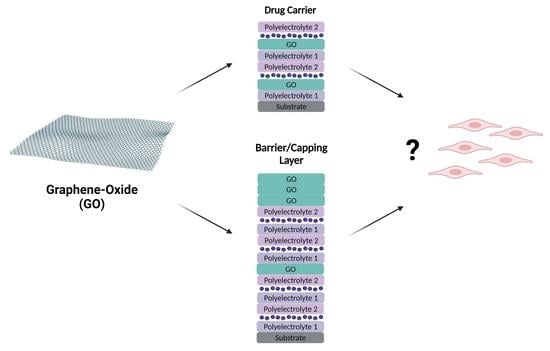
- GO incorporated in multilayered systems in which it takes the role of carrier, helping to transport the therapeutic agents, making sure that they reach the target before being released, and helping to protect them from early degradation.
- GO incorporated in multilayered systems in which it acts as a barrier or capping layer with the purpose of delaying and/or controlling the drug release across time, in a precise and stratified manner, with control of the release sequence.
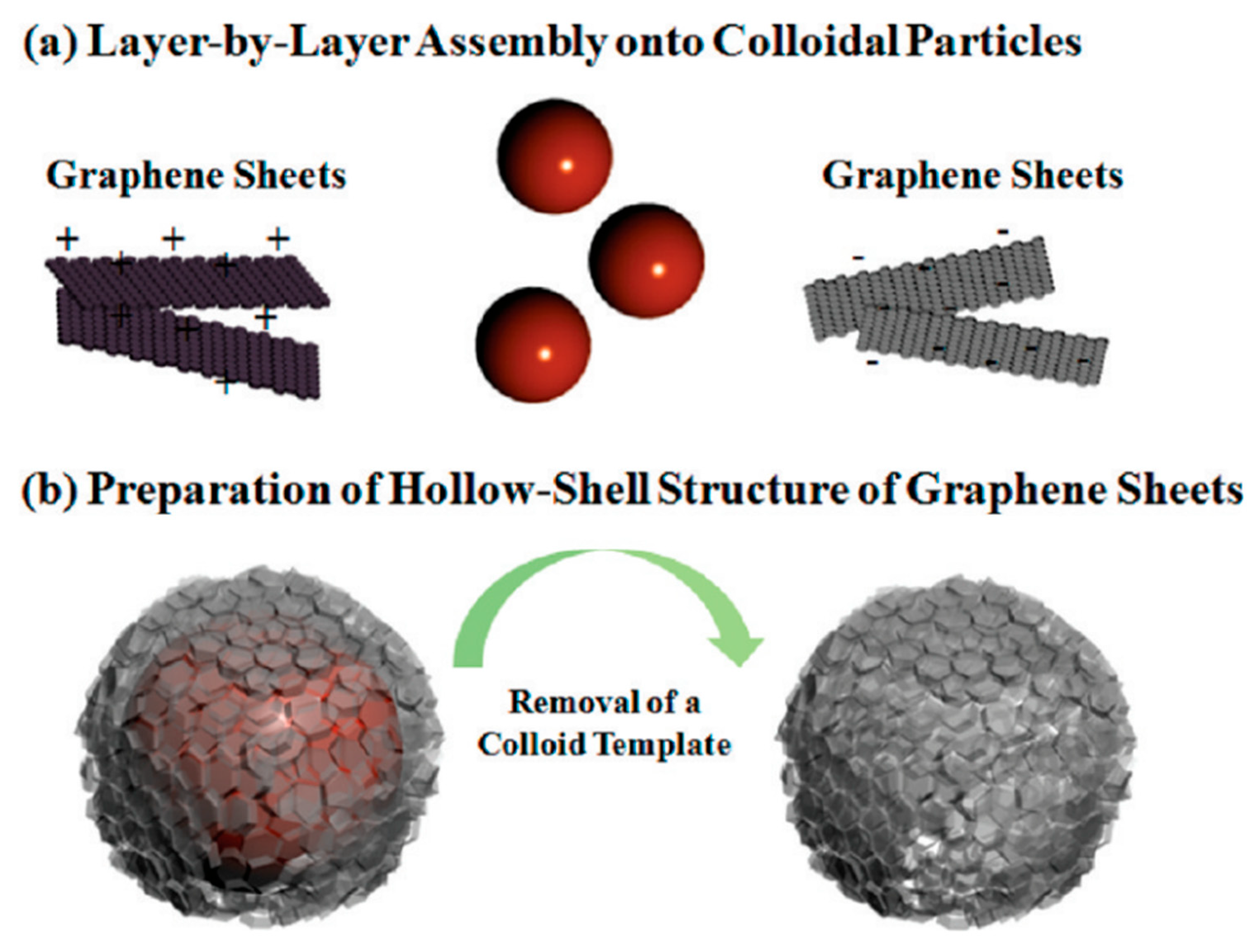
2. Graphene Oxide Multilayer Films
3. Graphene Oxide as Carrier in Drug Delivery
4. Graphene Oxide for Controlled Drug Release
5. Graphene Oxide: Biocompatible or Cytotoxic?
5.1. In Vitro Assays for Graphene Oxide
5.2. In Vivo Assays for Graphene Oxide
6. Graphene Oxide in Biomedical Applications
7. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s Disease |
| AF555 | Alexa Fluor |
| AFM | Atomic Force Microscopy |
| AGO | Amino-functionalized GO |
| APTES | ((3-aminopropyl) triethoxysilane) |
| AuNPs | Gold Nanoparticles |
| BBB | Blood Brain Barrier |
| Brim | Brimonidine |
| CD | Circular Dichroism |
| CEF | Cephalexin |
| CG | Claisen Graphene |
| Chi | Chitosan |
| CLSM | Confocal Laser Scanning Microscopy |
| CMC | Carboxymethylcellulose |
| CNT | Carbon Nanotubes |
| CSF | Cerebrospinal Fluid |
| Cur | Curcumin |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DDS | Drug Delivery System |
| DI | Deionized |
| DLS | Dynamic Light Scattering |
| DOX | Doxorubicin |
| FA | Folic Acid Antibody |
| FBS | Fetal Bovine Serum |
| Fe3O4@GO | Iron Oxide decorated Graphene Oxide |
| FGMs | Functional Graphenic Materials |
| FITIC | Fluorescein Isothiocyanate |
| FL | Fluorescein |
| FTIR | Fourier-Transform Infrared Spectroscopy |
| G(IIKK)4I-NH2 | Antibacterial peptide |
| GCE | Glassy Carbon Electrode |
| GO | Graphene Oxide |
| GOChi | Chitosan functionalized Graphene Oxide |
| GO-IONP | Graphene Oxide—Iron Oxide nanocomposites |
| GOn | Nano Graphene Oxide |
| HA | Hyaluronic Acid |
| HDF | Human Dermal Fibroblast |
| Hep | Heparin |
| hFOB | Human Fetal Osteoblast |
| h-MC | Hybrid Microcapsule |
| HOG | Human Oligodendroglia |
| HSCs | Hematopoietic Stem Cells |
| IO | Iron Oxide |
| LbL | Layer-by-layer |
| Lf | Lactoferrin |
| mGO | Magnetic Graphene Oxide |
| NIR | Near Infrared Radiation |
| NP’s | Nanoparticles |
| OVA | Ovalbumin |
| P(glu) | Poly glutamate |
| P(lys) | Poly lysine |
| PAA | Poly (acrylic acid) |
| PAA-Cys | Poly(acrylic acid)-cysteine |
| PAH | Poly(allylamine hydrochloride) |
| PB | 1-Pyrenebutyrate |
| PBAE | Poly(β-amino ester) |
| PBS | Phosphate Buffered Saline |
| PC12 | Pheochromocytoma Cells |
| PD | Parkinson’s Disease |
| PEDOT:PSS | Poly(3,4-ethylenedioxythiophene) polystyrene sulfonate |
| PEG | Polyethylene glycol |
| PEI | Poly(ethylene imine) |
| PEMf | Polyelectrolyte Multilayer films |
| Pep | Synthetic peptides |
| PLL | Poly L-Lysine |
| PMMA | Poly(methylmethacrylate) |
| POAA | Polyoxyalkyleneamine |
| PolyCD | Polycyclodextrins |
| PS | Polystyrene |
| Pue | Puerarin |
| PVP | Poly N-vinylpyrrolidone |
| PYM | Pingyangmycin |
| QCM | Quartz Crystal Microbalance |
| rGO | Reduced Graphene Oxide |
| RS | Raman Spectroscopy |
| SA | Sodium Alginate |
| SEM | Scanning Electron Microscopy |
| Si | Silicon |
| SPR | Surface Plasmon Resonance |
| STM | Scanning Tunneling Microscopy |
| TEM | Transmission Electron Microscopy |
| THF | Tetrahydrofuran |
| ThS | Thioflavin S |
| TR | Texas Red |
| XPS | X-ray Photoelectron Spectroscopy |
References
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-Based Composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Samulski, E.T. Synthesis of Water Soluble Graphene. Nano Lett. 2008, 8, 1679–1682. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Char, K.; Kim, B.-S. Hollow Capsules of Reduced Graphene Oxide Nanosheets Assembled on a Sacrificial Colloidal Particle. J. Phys. Chem. Lett. 2010, 1, 3442–3445. [Google Scholar] [CrossRef]
- Lian, Y.; Liu, Y.; Jiang, T.; Shu, J.; Lian, H.; Cao, M. Enhanced Electromechanical Performance of Graphite Oxide-Nafion Nanocomposite Actuator. J. Phys. Chem. C 2010, 114, 9659–9663. [Google Scholar] [CrossRef]
- Hong, W.; Xu, Y.; Lu, G.; Li, C.; Shi, G. Transparent Graphene/PEDOT–PSS Composite Films as Counter Electrodes of Dye-sensitized Solar Cells. Electrochem. Commun. 2008, 10, 1555–1558. [Google Scholar] [CrossRef]
- Cao, M.; Zhao, W.; Wang, L.; Li, R.; Gong, H.; Zhang, Y.; Xu, H.; Lu, J.R. Graphene Oxide-Assisted Accumulation and Layer-by-Layer Assembly of Antibacterial Peptide for Sustained Release Applications. ACS Appl Mater. Interfaces 2018, 10, 24937–24946. [Google Scholar] [CrossRef]
- Gao, F.; Hu, Y.; Li, G.; Liu, S.; Quan, L.; Yang, Z.; Wei, Y.; Pan, C. Layer-by-layer Deposition of Bioactive Layers on Magnesium Alloy Stent Materials to Improve Corrosion Resistance and Biocompatibility. Bioact. Mater. 2020, 5, 611–623. [Google Scholar] [CrossRef]
- Sahne, F.; Mohammadi, M.; Najafpour, G.D. Single-Layer Assembly of Multifunctional Carboxymethylcellulose on Graphene Oxide Nanoparticles for Improving In Vivo Curcumin Delivery into Tumor Cells. ACS Biomater. Sci. Eng. 2019, 5, 2595–2609. [Google Scholar] [CrossRef]
- Assunção, I.C.C.; Sério, S.; Ferreira, Q.; Jones, N.C.; Hoffmann, S.V.; Ribeiro, P.A.; Raposo, M. Graphene Oxide Layer-by-Layer Films for Sensors and Devices. Nanomaterials 2021, 11, 1556. [Google Scholar] [CrossRef]
- Kurapati, R.; Raichur, A.M. Near-Infrared Light-Responsive Graphene Oxide Composite Multilayer Capsules: A Novel Route for Remote Controlled Drug Delivery. Chem. Commun. 2013, 49, 734–736. [Google Scholar] [CrossRef]
- Shin, S.R.; Aghaei-Ghareh-Bolagh, B.; Gao, X.; Nikkhah, M.; Jung, S.M.; Dolatshahi-Pirouz, A.; Kim, S.B.; Kim, S.M.; Dokmeci, M.R.; Tang, X.S.; et al. Layer-by-Layer Assembly of 3D Tissue Constructs with Functionalized Graphene. Adv. Funct. Mater. 2014, 24, 6136–6144. [Google Scholar] [CrossRef]
- Hong, B.J.; Compton, O.C.; An, Z.; Eryazici, I.; Nguyen, S.T. Successful Stabilization of Graphene Oxide in Electrolyte Solutions: Enhancement of Biofunctionalization and Cellular Uptake. ACS Nano 2012, 6, 63–73. [Google Scholar] [CrossRef]
- Xu, Y.; Bai, H.; Lu, G.; Li, C.; Shi, G. Flexible Graphene Films via the Filtration of Water-Soluble Noncovalent Functionalized Graphene Sheets. J. Am. Chem. Soc. 2008, 130, 5856–5857. [Google Scholar] [CrossRef]
- Demirel, E.; Karaca, E.; Yuksel Durmaz, Y. Effective PEGylation Method to Improve Biocompatibility of Graphene Derivatives. Eur. Polym. J. 2020, 124, 109504. [Google Scholar] [CrossRef]
- Li, M.; Yang, X.; Ren, J.; Qu, K.; Qu, X. Using Graphene Oxide High Near-Infrared Absorbance for Photothermal Treatment of Alzheimer’s Disease. Adv. Mater. 2012, 24, 1722–1728. [Google Scholar] [CrossRef]
- Su, S.; Wang, J.; Qiu, J.; Martinez-Zaguilan, R.; Sennoune, S.R.; Wang, S. In Vitro Study of Transportation of Porphyrin Immobilized Graphene Oxide Through Blood Brain Barrier. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110313. [Google Scholar] [CrossRef]
- Mendonça, M.C.; Soares, E.S.; de Jesus, M.B.; Ceragioli, H.J.; Ferreira, M.S.; Catharino, R.R.; da Cruz-Höfling, M.A. Reduced Graphene Oxide Induces Transient Blood-Brain Barrier Opening: An In Vivo Study. J. Nanobiotechnol. 2015, 13, 78. [Google Scholar] [CrossRef]
- Tadyszak, K.; Wychowaniec, J.K.; Litowczenko, J. Biomedical Applications of Graphene-Based Structures. Nanomaterials 2018, 8, 944. [Google Scholar] [CrossRef]
- Costa, R.R.; Alatorre-Meda, M.; Mano, J.F. Drug Nano-Reservoirs Synthesized using Layer-by-Layer Technologies. Biotechnol. Adv. 2015, 33, 1310–1326. [Google Scholar] [CrossRef]
- Li, X.; Zhang, G.; Bai, X.; Sun, X.; Wang, X.; Wang, E.; Dai, H. Highly Conducting Graphene Sheets and Langmuir–Blodgett Films. Nat. Nanotechnol. 2008, 3, 538–542. [Google Scholar] [CrossRef]
- Jeong, H.; Ranallo, S.; Rossetti, M.; Heo, J.; Shin, J.; Park, K.; Ricci, F.; Hong, J. Electronic Activation of a DNA Nanodevice using a Multilayer Nanofilm. Small 2016, 12, 5572–5578. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-Yield Production of Graphene by Liquid-Phase Exfoliation of Graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liang, T.; Ma, Q. Layer-by-Layer Assembled Nano-Drug Delivery Systems for Cancer Treatment. Drug Deliv. 2021, 28, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.J.; Pranantyo, D.; Neoh, K.-G.; Kang, E.-T.; Teo, S.L.-M.; Rittschof, D. Layer-by-Layer Click Deposition of Functional Polymer Coatings for Combating Marine Biofouling. Biomacromolecules 2012, 13, 2769–2780. [Google Scholar] [CrossRef]
- Sieber, S.; Siegrist, S.; Schwarz, S.; Porta, F.; Schenk, S.H.; Huwyler, J. Immobilization of Enzymes on PLGA Sub-Micrometer Particles by Crosslinked Layer-by-Layer Deposition. Macromol. Biosci. 2017, 17, 1700015. [Google Scholar] [CrossRef]
- Smith, R.; Riollano, M.; Leung, A.; Hammond, P. Layer-by-Layer Platform Technology for Small-Molecule Delivery. Angew. Chem. Int. Ed. 2009, 48, 8974–8977. [Google Scholar] [CrossRef]
- Shutava, T.G.; Livanovich, K.S.; Sharamet, A.A. Layer-by-Layer Films of Polysaccharides Modified with Polyethylene Glycol and Dextran. Colloids Surf. B Biointerfaces 2019, 173, 412–420. [Google Scholar] [CrossRef]
- Silva, D.P.B.; Miyazaki, C.M.; Mascagni, D.B.T.; Ferreira, M. Layer-by-Layer Films of Gold Nanoparticles and Carbon Nanotubes for Improved Amperometric Detection of Cholesterol. J. Nanosci. Nanotechnol. 2019, 19, 5483–5488. [Google Scholar] [CrossRef]
- Blacklock, J.; Mao, G.; Oupický, D.; Möhwald, H. DNA Release Dynamics from Bioreducible Layer-by-Layer Films. Langmuir 2010, 26, 8597–8605. [Google Scholar] [CrossRef]
- Hong, C.A.; Son, H.Y.; Nam, Y.S. Layer-by-Layer SiRNA/Poly(L-Lysine) Multilayers on Polydopamine-Coated Surface for Efficient Cell Adhesion and Gene Silencing. Sci. Rep. 2018, 8, 7738. [Google Scholar] [CrossRef]
- Srivastava, S.; Kotov, N.A. Composite Layer-by-Layer (LBL) Assembly with Inorganic Nanoparticles and Nanowires. Acc. Chem. Res. 2008, 41, 1831–1841. [Google Scholar] [CrossRef]
- Smith, R.C.; Leung, A.; Kim, B.-S.; Hammond, P.T. Hydrophobic Effects in the Critical Destabilization and Release Dynamics of Degradable Multilayer Films. Chem. Mater. 2009, 21, 1108–1115. [Google Scholar] [CrossRef][Green Version]
- Xie, M.; Zhang, F.; Peng, H.; Zhang, Y.; Li, Y.; Xu, Y.; Xie, J. Layer-by-Layer Modification of Magnetic Graphene Oxide by Chitosan and Sodium Alginate with Enhanced Dispersibility for Targeted Drug Delivery and Photothermal Therapy. Colloids Surf. B Biointerfaces 2019, 176, 462–470. [Google Scholar] [CrossRef]
- Machado, M.; Silva, G.A.; Bitoque, D.B.; Ferreira, J.; Pinto, L.A.; Morgado, J.; Ferreira, Q. Self-Assembled Multilayer Films for Time-Controlled Ocular Drug Delivery. ACS Appl. Bio Mater. 2019, 2, 4173–4180. [Google Scholar] [CrossRef]
- Tanum, J.; Heo, J.; Hong, J. Spontaneous Biomacromolecule Absorption and Long-Term Release by Graphene Oxide. ACS Omega 2018, 3, 5903–5909. [Google Scholar] [CrossRef]
- Wang, F.; Li, J.; Tang, X.; Huang, K.; Chen, L. Polyelectrolyte Three Layer Nanoparticles of Chitosan/Dextran Sulfate/Chitosan for Dual Drug Delivery. Colloids Surf. B Biointerfaces 2020, 190, 110925. [Google Scholar] [CrossRef]
- Guo, H.; Guo, Q.; Chu, T.; Zhang, X.; Wu, Z.; Yu, D. Glucose-Sensitive Polyelectrolyte Nanocapsules Based on Layer-by-Layer Technique for Protein Drug Delivery. J. Mater. Sci. Mater. Med. 2014, 25, 121–129. [Google Scholar] [CrossRef]
- Yang, M.-C.; Tsou, H.-M.; Hsiao, Y.-S.; Cheng, Y.-W.; Liu, C.-C.; Huang, L.-Y.; Peng, X.-Y.; Liu, T.-Y.; Yung, M.-C.; Hsu, C.-C. Electrochemical Polymerization of PEDOT–Graphene Oxide–Heparin Composite Coating for Anti-Fouling and Anti-Clotting of Cardiovascular Stents. Polymers 2019, 11, 1520. [Google Scholar] [CrossRef]
- Qi, W.; Xue, Z.; Yuan, W.; Wang, H. Layer-by-Layer Assembled Graphene Oxide Composite Films for Enhanced Mechanical Properties and Fibroblast Cell Affinity. J. Mater. Chem. B 2014, 2, 325–331. [Google Scholar] [CrossRef]
- Cao, W.; He, L.; Cao, W.; Huang, X.; Jia, K.; Dai, J. Recent Progress of Graphene Oxide as a Potential Vaccine Carrier and Adjuvant. Acta Biomater. 2020, 112, 14–28. [Google Scholar] [CrossRef]
- Mangadlao, J.D.; Santos, C.M.; Felipe, M.J.L.; de Leon, A.C.C.; Rodrigues, D.F.; Advincula, R.C. On the Antibacterial Mechanism of Graphene Oxide (GO) Langmuir–Blodgett Films. Chem. Commun. 2015, 51, 2886–2889. [Google Scholar] [CrossRef]
- Sharker, S.M.; Lee, J.E.; Kim, S.H.; Jeong, J.H.; In, I.; Lee, H.; Park, S.Y. PH Triggered in Vivo Photothermal Therapy and Fluorescence Nanoplatform of Cancer Based on Responsive Polymer-Indocyanine Green Integrated Reduced Graphene Oxide. Biomaterials 2015, 61, 229–238. [Google Scholar] [CrossRef]
- Gollavelli, G.; Ling, Y.-C. Multi-Functional Graphene as an In Vitro and In Vivo Imaging Probe. Biomaterials 2012, 33, 2532–2545. [Google Scholar] [CrossRef]
- Sheng, Z.; Song, L.; Zheng, J.; Hu, D.; He, M.; Zheng, M.; Gao, G.; Gong, P.; Zhang, P.; Ma, Y.; et al. Protein-Assisted Fabrication of Nano-Reduced Graphene Oxide for Combined In Vivo Photoacoustic Imaging and Photothermal Therapy. Biomaterials 2013, 34, 5236–5243. [Google Scholar] [CrossRef]
- Shi, S.; Yang, K.; Hong, H.; Valdovinos, H.F.; Nayak, T.R.; Zhang, Y.; Theuer, C.P.; Barnhart, T.E.; Liu, Z.; Cai, W. Tumor Vasculature Targeting and Imaging in Living Mice with Reduced Graphene Oxide. Biomaterials 2013, 34, 3002–3009. [Google Scholar] [CrossRef]
- Ahn, E.; Lee, T.; Gu, M.; Park, M.; Min, S.H.; Kim, B.-S. Layer-by-Layer Assembly for Graphene-Based Multilayer Nanocomposites: The Field Manual. Chem. Mater. 2017, 29, 69–79. [Google Scholar] [CrossRef]
- Araújo, M. Nanostructured Films for Controlled Release of Drugs for Glaucoma Treatment. Master’s Thesis, Universidade de Lisboa, Lisbon, Portugal, 2016. [Google Scholar]
- Morais, H. Nanostructured Films of Graphene for Controlled Ocular Drug Delivery. Master’s Thesis, Instituto Politécnico de Lisboa, Lisbon, Portugal, 2017. [Google Scholar]
- Pereira, T. Development of a Liquid Cell to Study the Release of Brimonidine in Real Time. Master’s Thesis, Instituto Pólitécnico de Lisboa, Lisbon, Portugal, 2019. [Google Scholar]
- Raposo, M.; Oliveira, O. Adsorption Mechanisms in Layer-by-Layer Films. Braz. J. Phys. 1998, 28. [Google Scholar] [CrossRef][Green Version]
- Han, U.; Seo, Y.; Hong, J. Effect of pH on the Structure and Drug Release Profiles of Layer-by-Layer Assembled Films Containing Polyelectrolyte, Micelles, and Graphene Oxide. Sci. Rep. 2016, 6, 24158. [Google Scholar] [CrossRef]
- Saurer, E.M.; Yamanouchi, D.; Liu, B.; Lynn, D.M. Delivery of Plasmid DNA to Vascular Tissue In Vivo using Catheter Balloons Coated with Polyelectrolyte Multilayers. Biomaterials 2011, 32, 610–618. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, D.; Li, Y.; Sun, J. Layer-by-Layer Assembled Highly Adhesive Microgel Films. Polymer 2013, 54, 4220–4226. [Google Scholar] [CrossRef]
- Wang, L.; Chen, D.; Sun, J. Layer-by-Layer Deposition of Polymeric Microgel Films on Surgical Sutures for Loading and Release of Ibuprofen. Langmuir 2009, 25, 7990–7994. [Google Scholar] [CrossRef] [PubMed]
- Sakr, O.S.; Jordan, O.; Borchard, G. Sustained Protein Release from Hydrogel Microparticles using Layer-by-Layer (LbL) Technology. Drug Deliv. 2016, 23, 2747–2755. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Kim, K.-G.; Heo, J.; Jeong, H.; Kim, S.Y.; Hong, J. Multilayered Graphene Nano-Film for Controlled Protein Delivery by Desired Electro-Stimuli. Sci. Rep. 2015, 5, 17631. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Shah, N.J.; Drake, A.C.; DeMuth, P.C.; Lee, J.B.; Chen, J.; Hammond, P.T. Graphene Multilayers as Gates for Multi-Week Sequential Release of Proteins from Surfaces. ACS Nano 2012, 6, 81–88. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, X.; Zhou, L.; Shang, L.; Su, Z. Reduced Graphene Oxide (rGO) Hybridized Hydrogel as a Near-Infrared (NIR)/pH Dual-Responsive Platform foCr ombined Chemo-Photothermal Therapy. J. Colloid Interface Sci. 2019 536, 160–170. [CrossRef]
- McAloney, R.A.; Sinyor, M.; Dudnik, V.; Goh, M.C. Atomic Force Microscopy Studies of Salt Effects on Polyelectrolyte Multilayer Film Morphology. Langmuir 2001, 17, 6655–6663. [Google Scholar] [CrossRef]
- Raposo, M.; Ferreira, Q.; Ribeiro, P. A Guide for Atomic Force Microscopy Analysis of Soft Condensed Matter. In Modern Research and Educational Topics in Microscopy; Formatex: Badajoz, Spain, 2007; Volume 1, pp. 758–769. [Google Scholar]
- Deng, L.; Li, Q.; Al-Rehili, S.; Omar, H.; Almalik, A.; Alshamsan, A.; Zhang, J.; Khashab, N.M. Hybrid Iron Oxide-Graphene Oxide-Polysaccharides Microcapsule: A Micro-Matryoshka for On-Demand Drug Release and Antitumor Therapy In Vivo. ACS Appl. Mater. Interfaces 2016, 8, 6859–6868. [Google Scholar] [CrossRef]
- Wang, K.; Ruan, J.; Song, H.; Zhang, J.; Wo, Y.; Guo, S.; Cui, D. Biocompatibility of Graphene Oxide. Nanoscale Res. Lett. 2010, 6, 8. [Google Scholar] [CrossRef]
- Thierry, B.; Kujawa, P.; Tkaczyk, C.; Winnik, F.M.; Bilodeau, L.; Tabrizian, M. Delivery Platform for Hydrophobic Drugs: Prodrug Approach Combined with Self-Assembled Multilayers. J. Am. Chem. Soc. 2005, 127, 1626–1627. [Google Scholar] [CrossRef]
- Chen, D.; Wu, M.; Chen, J.; Zhang, C.; Pan, T.; Zhang, B.; Tian, H.; Chen, X.; Sun, J. Robust, Flexible, and Bioadhesive Free-Standing Films for the Co-Delivery of Antibiotics and Growth Factors. Langmuir 2014, 30, 13898–13906. [Google Scholar] [CrossRef]
- Mascagni, D.B.T.; Miyazaki, C.M.; da Cruz, N.C.; de Moraes, M.L.; Riul, A., Jr.; Ferreira, M. Layer-by-Layer Assembly of Functionalized Reduced Graphene Oxide for Direct Electrochemistry and Glucose Detection. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 739–745. [Google Scholar] [CrossRef]
- Ferreira, Q.; Delfino, C.L.; Morgado, J.; Alcácer, L. Bottom-Up Self-Assembled Supramolecular Structures Built by STM at the Solid/Liquid Interface. Materials 2019, 12, 382. [Google Scholar] [CrossRef]
- Ferreira, Q.; Bragança, A.M.; Alcácer, L.; Morgado, J. Conductance of Well-Defined Porphyrin Self-Assembled Molecular Wires up to 14 Nm in Length. J. Phys. Chem. C 2014, 118, 7229–7234. [Google Scholar] [CrossRef]
- Kurapati, R.; Raichur, A.M. Graphene Oxide Based Multilayer Capsules with Unique Permeability Properties: Facile Encapsulation of Multiple Drugs. Chem. Commun. 2012, 48, 6013–6015. [Google Scholar] [CrossRef]
- Kumarasamy, J.; Camarada, M.B.; Venkatraman, D.; Ju, H.; Dey, R.S.; Wen, Y. One-Step Coelectrodeposition-Assisted Layer-by-Layer Assembly of Gold Nanoparticles and Reduced Graphene Oxide and its Self-Healing Three-Dimensional Nanohybrid for an Ultrasensitive DNA Sensor. Nanoscale 2018, 10, 1196–1206. [Google Scholar] [CrossRef]
- Agarwal, S.; Zhou, X.; Ye, F.; He, Q.; Chen, G.C.K.; Soo, J.; Boey, F.; Zhang, H.; Chen, P. Interfacing Live Cells with Nanocarbon Substrates. Langmuir 2010, 26, 2244–2247. [Google Scholar] [CrossRef]
- Liu, Y.; Han, J.; Pan, H.; Jia, D.; Chen, L.; Yang, X. Oral Delivery of Pingyangmycin by Layer-by-Layer (LbL) Self-Assembly Polyelectrolyte-Grafted Nano Graphene Oxide. J. Nanosci. Nanotechnol. 2019, 19, 2260–2268. [Google Scholar] [CrossRef]
- Kavinkumar, T.; Varunkumar, K.; Ravikumar, V.; Manivannan, S. Anticancer Activity of Graphene Oxide-Reduced Graphene Oxide-Silver Nanoparticle Composites. J. Colloid Interface Sci. 2017, 505, 1125–1133. [Google Scholar] [CrossRef]
- Angelopoulou, A.; Voulgari, E.; Diamanti, E.K.; Gournis, D.; Avgoustakis, K. Graphene Oxide Stabilized by PLA-PEG Copolymers for the Controlled Delivery of Paclitaxel. Eur. J. Pharm. Biopharm. 2015, 93, 18–26. [Google Scholar] [CrossRef]
- Wang, L.H.; Liu, J.Y.; Sui, L.; Zhao, P.H.; Ma, H.D.; Wei, Z.; Wang, Y.L. Folate-Modified Graphene Oxide as the Drug Delivery System to Load Temozolomide. Curr. Pharm. Biotechnol. 2020, 21, 1088–1098. [Google Scholar] [CrossRef]
- Barahuie, F.; Saifullah, B.; Dorniani, D.; Fakurazi, S.; Karthivashan, G.; Hussein, M.Z.; Elfghi, F.M. Graphene Oxide as a Nanocarrier for Controlled Release and Targeted Delivery of an Anticancer Active Agent, Chlorogenic Acid. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 74, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Hussein-Al-Ali, S.H.; Abudoleh, S.M.; Hussein, M.Z.; Bullo, S.; Palanisamy, A. Graphene Oxide-Ellagic Acid Nanocomposite as Effective Anticancer and Antimicrobial Agent. IET Nanobiotechnol. 2021, 15, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahi, F.; Varshosaz, J.; Khodadadi, A.A.; Lim, S.; Jahanian-Najafabadi, A. Targeted Delivery of Docetaxel by Use of Transferrin/Poly(allylamine hydrochloride)-Functionalized Graphene Oxide Nanocarrier. ACS Appl. Mater. Interfaces 2016, 8, 13282–13293. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Zhao, Q.; Dai, D.; Zhang, S.; Tian, Z.; Sun, L.; Ren, J.; Liu, Z. Functionalized Ultrasmall Fluorinated Graphene with High NIR Absorbance for Controlled Delivery of Mixed Anticancer Drugs. Chem.–A Eur. J. 2017, 23, 17531–17541. [Google Scholar] [CrossRef]
- Wang, X.; Hao, L.; Zhang, C.; Chen, J.; Zhang, P. High Efficient Anti-Cancer Drug Delivery Systems using Tea Polyphenols Reduced and Functionalized Graphene Oxide. J. Biomater. Appl. 2017, 31, 1108–1122. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, X.; Wang, Y.; Zhai, G. Heparin Modified Graphene Oxide for pH-Sensitive Sustained Release of Doxorubicin Hydrochloride. Mater. Sci. Eng. C 2017, 75, 198–206. [Google Scholar] [CrossRef]
- Farnaz, R.; Maryam, S.; Masoumeh, J.; Parvaneh, S. Colloidal HSA-Graphene Oxide Nanosheets for Sustained Release of Oxaliplatin: Preparation, Release Mechanism, Cytotoxicity and Electrochemical Approaches. Colloids Surf. B Biointerfaces 2018, 171, 10–16. [Google Scholar] [CrossRef]
- Han, L.; Hao, Y.-N.; Wei, X.; Chen, X.-W.; Shu, Y.; Wang, J.-H. Hollow Copper Sulfide Nanosphere–Doxorubicin/Graphene Oxide Core–Shell Nanocomposite for Photothermo-Chemotherapy. ACS Biomater. Sci. Eng. 2017, 3, 3230–3235. [Google Scholar] [CrossRef]
- Patil, T.V.; Patel, D.K.; Dutta, S.D.; Ganguly, K.; Lim, K.-T. Graphene Oxide-Based Stimuli-Responsive Platforms for Biomedical Applications. Molecules 2021, 26, 2797. [Google Scholar] [CrossRef]
- Lima-Sousa, R.; de Melo-Diogo, D.; Alves, C.G.; Cabral, C.S.D.; Miguel, S.P.; Mendonça, A.G.; Correia, I.J. Injectable In Situ Forming Thermo-Responsive Graphene Based Hydrogels for Cancer Chemo-Photothermal Therapy and NIR Light-Enhanced Antibacterial Applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 117, 111294. [Google Scholar] [CrossRef]
- Dembereldorj, U.; Choi, S.Y.; Ganbold, E.O.; Song, N.W.; Kim, D.; Choo, J.; Lee, S.Y.; Kim, S.; Joo, S.W. Gold Nanorod-Assembled PEGylated Graphene-Oxide Nanocomposites for Photothermal Cancer Therapy. Photochem. Photobiol. 2014, 90, 659–666. [Google Scholar] [CrossRef]
- Markovic, Z.M.; Harhaji-Trajkovic, L.M.; Todorovic-Markovic, B.M.; Kepić, D.P.; Arsikin, K.M.; Jovanović, S.P.; Pantovic, A.C.; Dramićanin, M.D.; Trajkovic, V.S. In Vitro Comparison of the Photothermal Anticancer Activity of Graphene Nanoparticles and Carbon Nanotubes. Biomaterials 2011, 32, 1121–1129. [Google Scholar] [CrossRef]
- Ma, X.; Tao, H.; Yang, K.; Feng, L.; Cheng, L.; Shi, X.; Li, Y.; Guo, L.; Liu, Z. A Functionalized Graphene Oxide-Iron Oxide Nanocomposite for Magnetically Targeted Drug Delivery, Photothermal Therapy, and Magnetic Resonance Imaging. Nano Res. 2012, 5, 199–212. [Google Scholar] [CrossRef]
- Pan, N.; Wang, Y.; Ren, X.; Huang, T.-S.; Kim, I.S. Graphene Oxide as a Polymeric N-halamine Carrier and Release Platform: Highly-Efficient, Sustained-Release Antibacterial Property and Great Storage Stability. Mater. Sci. Eng. C 2019, 103, 109877. [Google Scholar] [CrossRef]
- Kumar, G.; Chaudhary, K.; Mogha, N.K.; Kant, A.; Masram, D.T. Extended Release of Metronidazole Drug using Chitosan/Graphene Oxide Bionanocomposite Beads as the Drug Carrier. ACS Omega 2021, 6, 20433–20444. [Google Scholar] [CrossRef]
- Tran, L.; Phuong, L.; Hoang Thi, T.T.; Park, K. Graphene Oxide Immobilized Surfaces Facilitate the Sustained Release of Doxycycline for the Prevention of Implant Related Infection. Colloids Surf. B Biointerfaces 2019, 181. [Google Scholar] [CrossRef]
- Xiong, S.; Luo, J.; Wang, Q.; Li, Z.; Li, J.; Liu, Q.; Gao, L.; Fang, S.; Li, Y.; Pan, H.; et al. Targeted Graphene Oxide for Drug Delivery as a Therapeutic Nanoplatform Against Parkinson’s Disease. Biomater. Sci. 2021, 9, 1705–1715. [Google Scholar] [CrossRef]
- Bramini, M.; Alberini, G.; Colombo, E.; Chiacchiaretta, M.; DiFrancesco, M.L.; Maya-Vetencourt, J.F.; Maragliano, L.; Benfenati, F.; Cesca, F. Interfacing Graphene-Based Materials with Neural Cells. Front. Syst. Neurosci. 2018, 12. [Google Scholar] [CrossRef]
- Emadi, F.; Amini, A.; Gholami, A.; Ghasemi, Y. Functionalized Graphene Oxide with Chitosan for Protein Nanocarriers to Protect against Enzymatic Cleavage and Retain Collagenase Activity. Sci Rep. 2017, 7, 42258. [Google Scholar] [CrossRef]
- Yang, L.; Wang, F.; Han, H.; Yang, L.; Zhang, G.; Fan, Z. Functionalized Graphene Oxide as a Drug Carrier for Loading Pirfenidone in Treatment of Subarachnoid Hemorrhage. Colloids Surf. B Biointerfaces 2015, 129, 21–29. [Google Scholar] [CrossRef]
- Figueroa, T.; Aguayo, C.; Fernández, K. Design and Characterization of Chitosan-Graphene Oxide Nanocomposites for the Delivery of Proanthocyanidins. Int. J. Nanomed. 2020, 15, 1229–1238. [Google Scholar] [CrossRef]
- Mahajan, C.R.; Joshi, L.B.; Varma, U.; Naik, J.B.; Chaudhari, V.R.; Mishra, S. Sustainable Drug Delivery of Famotidine using Chitosan-Functionalized Graphene Oxide as Nanocarrier. Glob. Chall. 2019, 3, 1900002. [Google Scholar] [CrossRef]
- Rahmanian, N.; Hamishehkar, H.; Dolatabadi, J.E.; Arsalani, N. Nano Graphene Oxide: A Novel Carrier for Oral Delivery of Flavonoids. Colloids Surf. B Biointerfaces 2014, 123, 331–338. [Google Scholar] [CrossRef]
- Li, H.; Jia, Y.; Liu, C. Pluronic® F127 Stabilized Reduced Graphene Oxide Hydrogel for Transdermal Delivery of Ondansetron: Ex Vivo and Animal Studies. Colloids Surf. B Biointerfaces 2020, 195, 111259. [Google Scholar] [CrossRef]
- Zhou, M.; Lozano, N.; Wychowaniec, J.K.; Hodgkinson, T.; Richardson, S.M.; Kostarelos, K.; Hoyland, J.A. Graphene Oxide: A growth Factor Delivery Carrier to Enhance Chondrogenic Differentiation of Human Mesenchymal Stem Cells in 3D Hydrogels. Acta Biomater 2019, 96, 271–280. [Google Scholar] [CrossRef]
- Yang, C.; Li, T. Transdermal Delivery of Flurbiprofen from Polyoxypropylene-Polyoxyethylene Block Copolymer Stabilized Reduced Graphene Oxide to Manage Pain in Spondylitis: In Vitro and In Vivo Studies. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2021, 165, 105929. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Li, P.; Bai, M.; Qi, W. Transdermal Delivery of Buprenorphine from Reduced Graphene Oxide Laden Hydrogel to Treat Osteoarthritis. J. Biomater. Sci. Polym. Ed. 2021, 32, 874–885. [Google Scholar] [CrossRef]
- Li, W.; Zhang, G.; Wei, X. Lidocaine-Loaded Reduced Graphene Oxide Hydrogel for Prolongation of Effects of Local Anesthesia: In Vitro and In Vivo Analyses. J. Biomater. Appl. 2021, 35, 1034–1042. [Google Scholar] [CrossRef]
- Luo, S.; Jin, S.; Yang, T.; Wu, B.; Xu, C.; Luo, L.; Chen, Y. Sustained Release of Tulobuterol from Graphene Oxide Laden Hydrogel to Manage Asthma. J. Biomater. Sci. Polym. Ed. 2021, 32, 524–535. [Google Scholar] [CrossRef]
- Choi, M.; Chung, J.-H.; Cho, Y.; Hong, B.Y.; Hong, J. Nano-Film Modification of Collagen Hydrogels for Controlled Growth Factor Release. Chem. Eng. Sci. 2015, 137, 626–630. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Soni, P.D.; Patel, P.J.; Desai, A.R.; Desai, D.T.; Shukla, M.R.; Shah, S.A.; Shah, D.O.; Willcox, M.D.P. Controlled Bimatoprost Release from Graphene Oxide Laden Contact Lenses: In Vitro and In Vivo Studies. Colloids Surf. B Biointerfaces 2021, 208, 112096. [Google Scholar] [CrossRef] [PubMed]
- Laurenti, M.; Lamberti, A.; Genchi, G.G.; Roppolo, I.; Canavese, G.; Vitale-Brovarone, C.; Ciofani, G.; Cauda, V. Graphene Oxide Finely Tunes the Bioactivity and Drug Delivery of Mesoporous ZnO Scaffolds. ACS Appl. Mater. Interfaces 2019, 11, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.; Kwon, E.J.; Kang, J.; Skalak, M.; Anglin, E.J.; Mann, A.P.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Porous Silicon–Graphene Oxide Core–Shell Nanoparticles for Targeted Delivery of siRNA to the Injured Brain. Nanoscale Horiz. 2016, 1, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Borandeh, S.; Alimardani, V.; Abolmaali, S.S.; Seppälä, J. Graphene Family Nanomaterials in Ocular Applications: Physicochemical Properties and Toxicity. Chem. Res. Toxicol. 2021, 34, 1386–1402. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Peng, C.; Lv, M.; Li, X.; Zhang, Y.; Chen, N.; Fan, C.; Huang, Q. Protein Corona-Mediated Mitigation of Cytotoxicity of Graphene Oxide. ACS Nano 2011, 5, 3693–3700. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A. Graphene: Safe or Toxic? The Two Faces of the Medal. Angew. Chem. Int. Ed. 2013, 52, 4986–4997. [Google Scholar] [CrossRef] [PubMed]
- Moura, D.; Caridade, S.G.; Sousa, M.P.; Cunha, E.; Rocha, H.C.; Mano, J.F.; Paiva, M.C.; Alves, N.M. High Performance Free-Standing Films by Layer-by-Layer Assembly of Graphene Flakes and Ribbons with Natural Polymers. J. Mater. Chem. B 2016, 4, 7718–7730. [Google Scholar] [CrossRef]
- Liao, K.-H.; Lin, Y.-S.; Macosko, C.W.; Haynes, C.L. Cytotoxicity of Graphene Oxide and Graphene in Human Erythrocytes and Skin Fibroblasts. ACS Appl. Mater. Interfaces 2011, 3, 2607–2615. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S. Graphene Nanomaterials: Synthesis, Biocompatibility, and Cytotoxicity. Int. J. Mol. Sci. 2018, 19, 3564. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Z.; Liu, Y.; Chu, M.; Yang, C.; Li, W.; Shao, Y.; Yue, Y.; Xu, R. The Short- and Long-Term Effects of Orally Administered High-Dose Reduced Graphene Oxide Nanosheets on Mouse Behaviors. Biomaterials 2015, 68, 100–113. [Google Scholar] [CrossRef]
- Fu, C.; Liu, T.; Li, L.; Liu, H.; Liang, Q.; Meng, X. Effects of Graphene Oxide on the Development of Offspring Mice in Lactation Period. Biomaterials 2015, 40, 23–31. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, X.; Sun, J.; Zhou, Q. Specific Nanotoxicity of Graphene Oxide During Zebrafish Embryogenesis. Nanotoxicology 2016, 10, 42–52. [Google Scholar] [CrossRef]
- Dasmahapatra, A.K.; Dasari, T.P.S.; Tchounwou, P.B. Graphene-Based Nanomaterials Toxicity in Fish. Rev. Environ. Contam. Toxicol. 2019, 247, 1–58. [Google Scholar] [CrossRef]
- Guo, Q.; Yang, Y.; Zhao, L.; Chen, J.; Duan, G.; Yang, Z.; Zhou, R. Graphene Oxide Toxicity in W1118 Flies. Sci. Total Environ. 2022, 805, 150302. [Google Scholar] [CrossRef]
- Rhazouani, A.; Gamrani, H.; El Achaby, M.; Aziz, K.; Gebrati, L.; Uddin, M.S.; Aziz, F. Synthesis and Toxicity of Graphene Oxide Nanoparticles: A Literature Review of In Vitro and In Vivo Studies. BioMed. Res. Int. 2021, 2021, 1–19. [Google Scholar] [CrossRef]
- Ou, L.; Song, B.; Liang, H.; Liu, J.; Feng, X.; Deng, B.; Sun, T.; Shao, L. Toxicity of Graphene-Family Nanoparticles: A General Review of the Origins and Mechanisms. Part. Fibre Toxicol. 2016, 13, 57. [Google Scholar] [CrossRef]
- Li, J.; Zhou, C.; Luo, C.; Qian, B.; Liu, S.; Zeng, Y.; Hou, J.; Deng, B.; Sun, Y.; Yang, J.; et al. N-acetyl Cysteine-Loaded Graphene Oxide-Collagen Hybrid Membrane for Scarless Wound Healing. Theranostics 2019, 9, 5839–5853. [Google Scholar] [CrossRef]
- Rehman, S.R.u.; Augustine, R.; Zahid, A.A.; Ahmed, R.; Hasan, A. Graphene Oxide Loaded Hydrogel for Enhanced Wound Healing in Diabetic Patients. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 3943–3946. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Hu, T.; Chen, B.; Yin, Z.; Ma, P.X.; Guo, B. Adhesive Hemostatic Conducting Injectable Composite Hydrogels with Sustained Drug Release and Photothermal Antibacterial Activity to Promote Full-Thickness Skin Regeneration During Wound Healing. Small 2019, 15, 1900046. [Google Scholar] [CrossRef]
- Vincent, M.; de Lázaro, I.; Kostarelos, K. Graphene Materials as 2D Non-Viral Gene Transfer Vector Platforms. Gene Ther. 2017, 24, 123–132. [Google Scholar] [CrossRef]
- Hsieh, T.Y.; Huang, W.C.; Kang, Y.D.; Chu, C.Y.; Liao, W.L.; Chen, Y.Y.; Chen, S.Y. Neurotensin-Conjugated Reduced Graphene Oxide with Multi-Stage Near-Infrared-Triggered Synergic Targeted Neuron Gene Transfection In Vitro and In Vivo for Neurodegenerative Disease Therapy. Adv. Healthc. Mater. 2016, 5, 3016–3026. [Google Scholar] [CrossRef]
- Jalani, G.; Jeyachandran, D.; Bertram Church, R.; Cerruti, M. Graphene Oxide-Stabilized Perfluorocarbon Emulsions for Controlled Oxygen Delivery. Nanoscale 2017, 9, 10161–10166. [Google Scholar] [CrossRef]
- Castilho, C.J.; Li, D.; Liu, M.; Liu, Y.; Gao, H.; Hurt, R.H. Mosquito Bite Prevention through Graphene Barrier Layers. Proc. Natl. Acad. Sci. USA 2019, 116, 18304–18309. [Google Scholar] [CrossRef]
- Sha, J.; Gao, Y.; Wu, T.; Chen, X.; Cordie, T.; Zhao, H.; Xie, L.; Ma, Y.; Turng, L.-s. Biocompatible Graphene Nanosheets Grafted with Poly(2-Hydroxyethyl Methacrylate) Brushes via Surface-Initiated ARGET ATRP. RSC Adv. 2016, 6, 35641–35647. [Google Scholar] [CrossRef]
- Catt, K.; Li, H.; Cui, X.T. Poly (3,4-Ethylenedioxythiophene) Graphene Oxide Composite Coatings for Controlling Magnesium Implant Corrosion. Acta Biomater. 2017, 48, 530–540. [Google Scholar] [CrossRef]
- Andreeva, T.D.; Stoichev, S.; Taneva, S.G.; Krastev, R. Hybrid Graphene Oxide/Polysaccharide Nanocomposites with Controllable Surface Properties and Biocompatibility. Carbohydr. Polym. 2018, 181, 78–85. [Google Scholar] [CrossRef]
- Asgar, H.; Deen, K.M.; Rahman, Z.U.; Shah, U.H.; Raza, M.A.; Haider, W. Functionalized Graphene Oxide Coating on Ti6Al4V Alloy for Improved Biocompatibility and Corrosion Resistance. Mater. Sci. Eng. C 2019, 94, 920–928. [Google Scholar] [CrossRef]
- Raucci, M.G.; Giugliano, D.; Longo, A.; Zeppetelli, S.; Carotenuto, G.; Ambrosio, L. Comparative Facile Methods for Preparing Graphene Oxide–Hydroxyapatite for Bone Tissue Engineering. J. Tissue Eng. Regen. Med. 2017, 11, 2204–2216. [Google Scholar] [CrossRef]
- Guo, B.; Feng, X.; Wang, Y.; Wang, X.; He, Y. Biomimetic and Immunomodulatory Baicalin-Loaded Graphene Oxide-Demineralized Bone Matrix Scaffold for In Vivo Bone Regeneration. J. Mater. Chem. B 2021, 9, 9720–9733. [Google Scholar] [CrossRef]
- Arnold, A.M.; Holt, B.D.; Daneshmandi, L.; Laurencin, C.T.; Sydlik, S.A. Phosphate Graphene as an Intrinsically Osteoinductive Scaffold for Stem Cell-Driven Bone Regeneration. Proc. Natl. Acad. Sci. USA 2019, 116, 4855–4860. [Google Scholar] [CrossRef]
- Li, J.; Zheng, L.; Zeng, L.; Zhang, Y.; Jiang, L.; Song, J. RGD Peptide-Grafted Graphene Oxide as a New Biomimetic Nanointerface for Impedance-Monitoring Cell Behaviors. J. Nanomater. 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Lai, P.X.; Chen, C.W.; Wei, S.C.; Lin, T.Y.; Jian, H.J.; Lai, I.P.; Mao, J.Y.; Hsu, P.H.; Lin, H.J.; Tzou, W.S.; et al. Ultrastrong Trapping of VEGF by Graphene Oxide: Anti-Angiogenesis Application. Biomaterials 2016, 109, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhang, X.; Li, Y.; Yang, X. Hyaluronic Acid and Graphene Oxide Loaded Silicon Contact Lens for Corneal Epithelial Healing. J. Biomater. Sci. Polym. Ed. 2021, 32, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Lu, H.; Ye, Z.; Zhang, S.; Wang, W.; Gao, L. Slow-Release Lubrication of Artificial Joints using Self-Healing Polyvinyl Alcohol/Polyethylene Glycol/ Graphene Oxide Hydrogel. J. Mech. Behav. Biomed. Mater. 2021, 124, 104807. [Google Scholar] [CrossRef] [PubMed]
- Taniselass, S.; Arshad, M.K.M.; Gopinath, S.C.B. Graphene-Based Electrochemical Biosensors for Monitoring Noncommunicable Disease Biomarkers. Biosens. Bioelectron. 2019, 130, 276–292. [Google Scholar] [CrossRef] [PubMed]
- Eckhart, K.E.; Holt, B.D.; Laurencin, M.G.; Sydlik, S.A. Covalent Conjugation of Bioactive Peptides to Graphene Oxide for Biomedical Applications. Biomater. Sci. 2019, 7, 3876–3885. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, C.M.; Mishra, R.; Kinahan, D.J.; Ferreira, M.; Ducrée, J. Polyethylene Imine/Graphene Oxide Layer-by-Layer Surface Functionalization for Significantly Improved Limit of Detection and Binding Kinetics of Immunoassays on Acrylate Surfaces. Colloids Surf. B Biointerfaces 2017, 158, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.; Costa-Almeida, R.; Timochenco, L.; Amaral, S.I.; Pinto, S.; Gonçalves, I.C.; Fernandes, J.R.; Magalhães, F.D.; Sarmento, B.; Pinto, A.M. Graphene Oxide Topical Administration: Skin Permeability Studies. Materials 2021, 14, 2810. [Google Scholar] [CrossRef]
- Wu, P.-C.; Chen, H.-H.; Chen, S.-Y.; Wang, W.-L.; Yang, K.-L.; Huang, C.-H.; Kao, H.-F.; Chang, J.-C.; Hsu, C.-L.L.; Wang, J.-Y.; et al. Graphene Oxide Conjugated with Polymers: A Study of Culture Condition to Determine Whether a Bacterial Growth Stimulant or an Antimicrobial Agent? J. Nanobiotechnol. 2018, 16, 1. [Google Scholar] [CrossRef]
- Katuwavila, N.P.; Amarasekara, Y.; Jayaweera, V.; Rajaphaksha, C.; Gunasekara, C.; Perera, I.C.; Amaratunga, G.A.J.; Weerasinghe, L. Graphene Oxide-Based Nanocomposite for Sustained Release of Cephalexin. J. Pharm. Sci. 2020, 109, 1130–1135. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Mohanty, S.; Mukherjee, S.; Basu, S.; Mishra, M. Graphene and Graphene Oxide as Nanomaterials for Medicine and Biology Application. J. Nanostruct. Chem. 2018, 8, 123–137. [Google Scholar] [CrossRef]
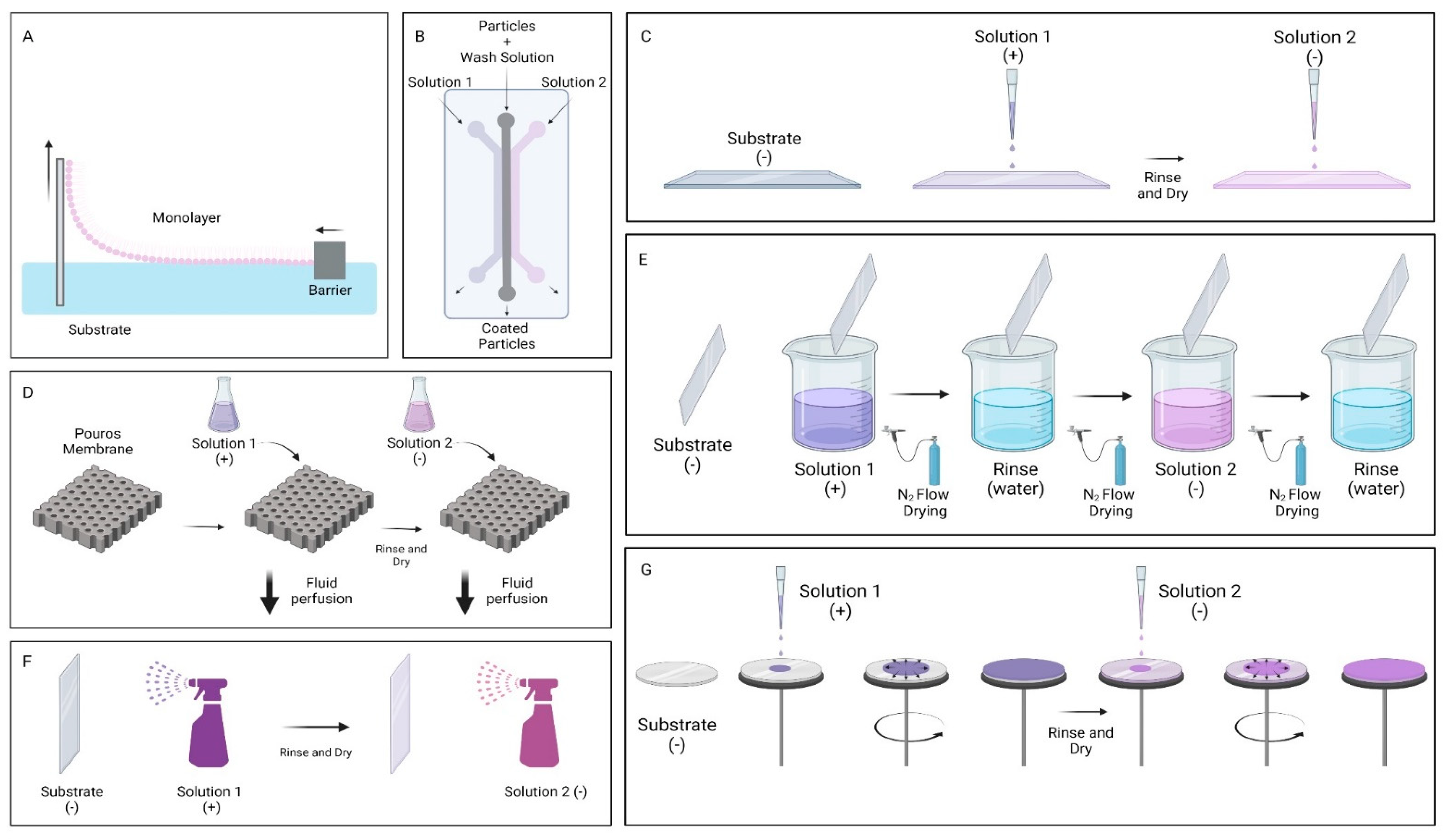
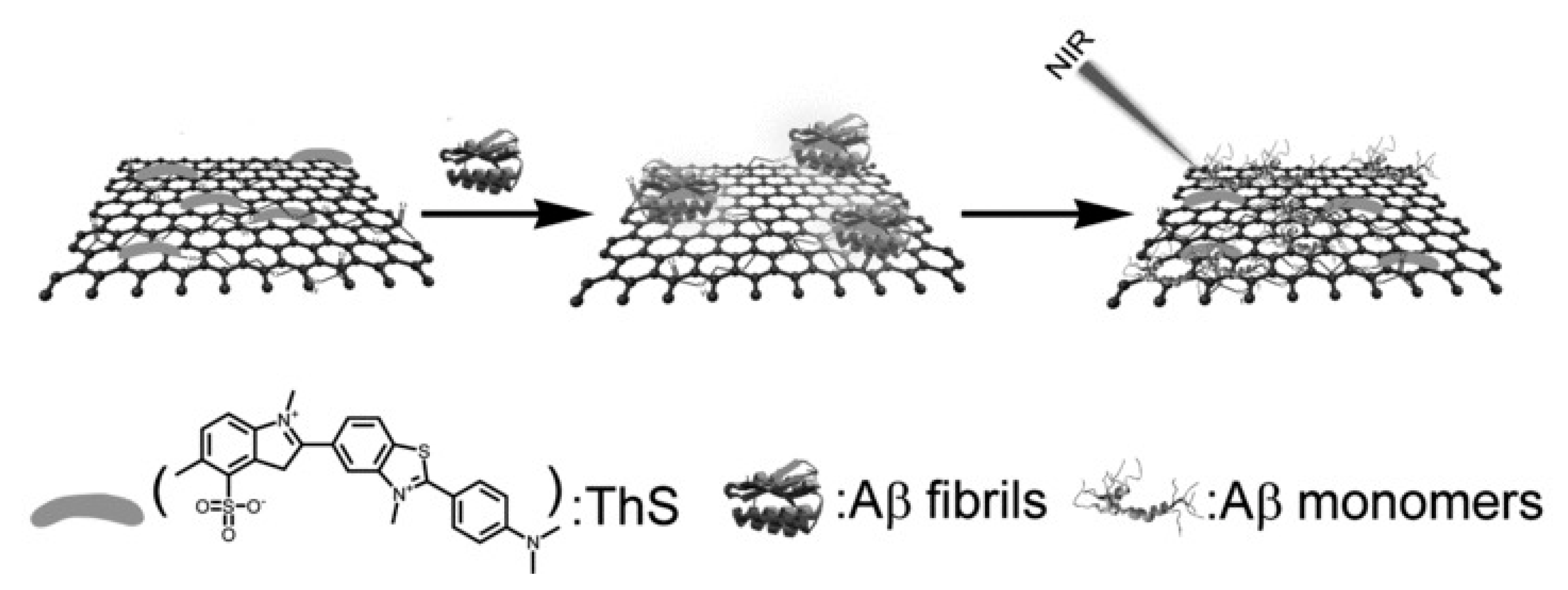
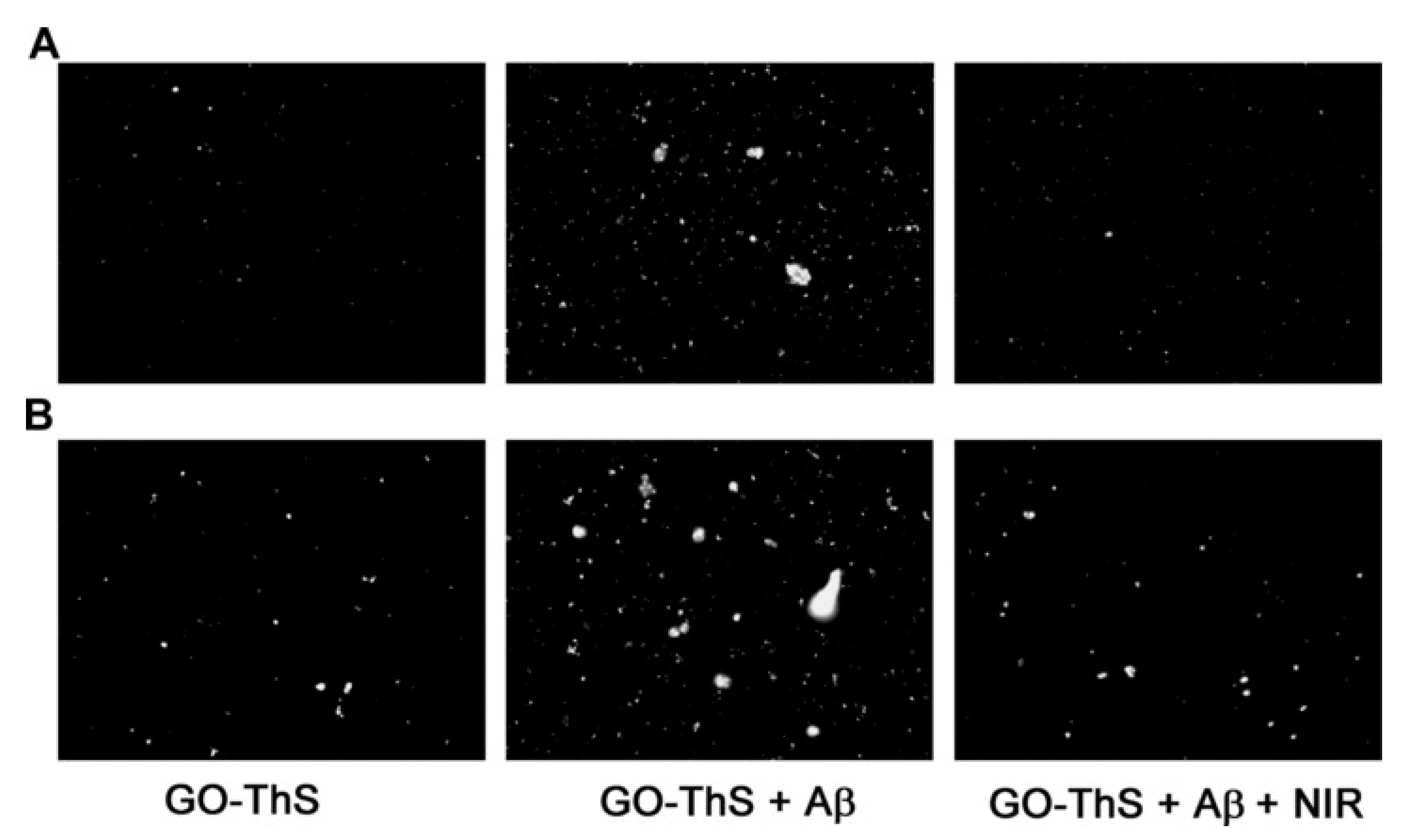
| Technique | Purpose |
|---|---|
| UV-Visible/Fluorescence Spectroscopy | GO structure [58] |
| Atomic Force Microscopy (AFM) | Surface morphology and roughness [21,32,34,35,57,58,59,60] |
| Scanning Electron Microscopy (SEM) | Surface morphology [21,35,36,54,55,56,57,58] |
| Transmission Electron Microscopy (TEM) | Surface morphology [36,37,61] GO structure [62] |
| Dynamic Light Scattering (DLS) method | Zeta potential and particle size and distribution [36,37,55,61] |
| Quartz Crystal Microbalance (QCM) | Layer adsorption [21,34,35,54,63] |
| Profilometry | Layer thickness [56,64] |
| Raman Spectroscopy (RS) | Layer deposition [61] GO structure [58] |
| SQUID—Field-dependent magnetization measurement | Magnetism measurement [61] |
| Fourier Transform Infrared Spectroscopy (FTIR) | GO structure [58] |
| Surface Plasmon Resonance (SPR) | Film growth [65] |
| Scanning Tunneling Microscopy (STM) | Characterization at molecular scale [66,67] |
| X-ray Diffraction | GO structure [33,58,68,69] Interlayer space [65] |
| X-ray Photoelectron Spectroscopy (XPS) | Film chemical characteristics [69,70] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, A.M.L.; Machado, M.; Silva, G.A.; Bitoque, D.B.; Tavares Ferreira, J.; Pinto, L.A.; Ferreira, Q. Graphene Oxide Thin Films with Drug Delivery Function. Nanomaterials 2022, 12, 1149. https://doi.org/10.3390/nano12071149
Oliveira AML, Machado M, Silva GA, Bitoque DB, Tavares Ferreira J, Pinto LA, Ferreira Q. Graphene Oxide Thin Films with Drug Delivery Function. Nanomaterials. 2022; 12(7):1149. https://doi.org/10.3390/nano12071149
Chicago/Turabian StyleOliveira, Alexandra M. L., Mónica Machado, Gabriela A. Silva, Diogo B. Bitoque, Joana Tavares Ferreira, Luís Abegão Pinto, and Quirina Ferreira. 2022. "Graphene Oxide Thin Films with Drug Delivery Function" Nanomaterials 12, no. 7: 1149. https://doi.org/10.3390/nano12071149
APA StyleOliveira, A. M. L., Machado, M., Silva, G. A., Bitoque, D. B., Tavares Ferreira, J., Pinto, L. A., & Ferreira, Q. (2022). Graphene Oxide Thin Films with Drug Delivery Function. Nanomaterials, 12(7), 1149. https://doi.org/10.3390/nano12071149