Aspartic Acid Stabilized Iron Oxide Nanoparticles for Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
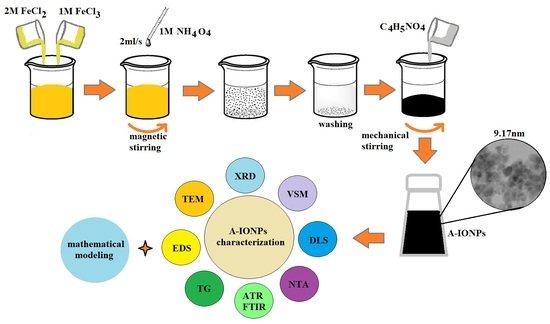
2.1. Yielding of A-IONP Aqueous Suspensions
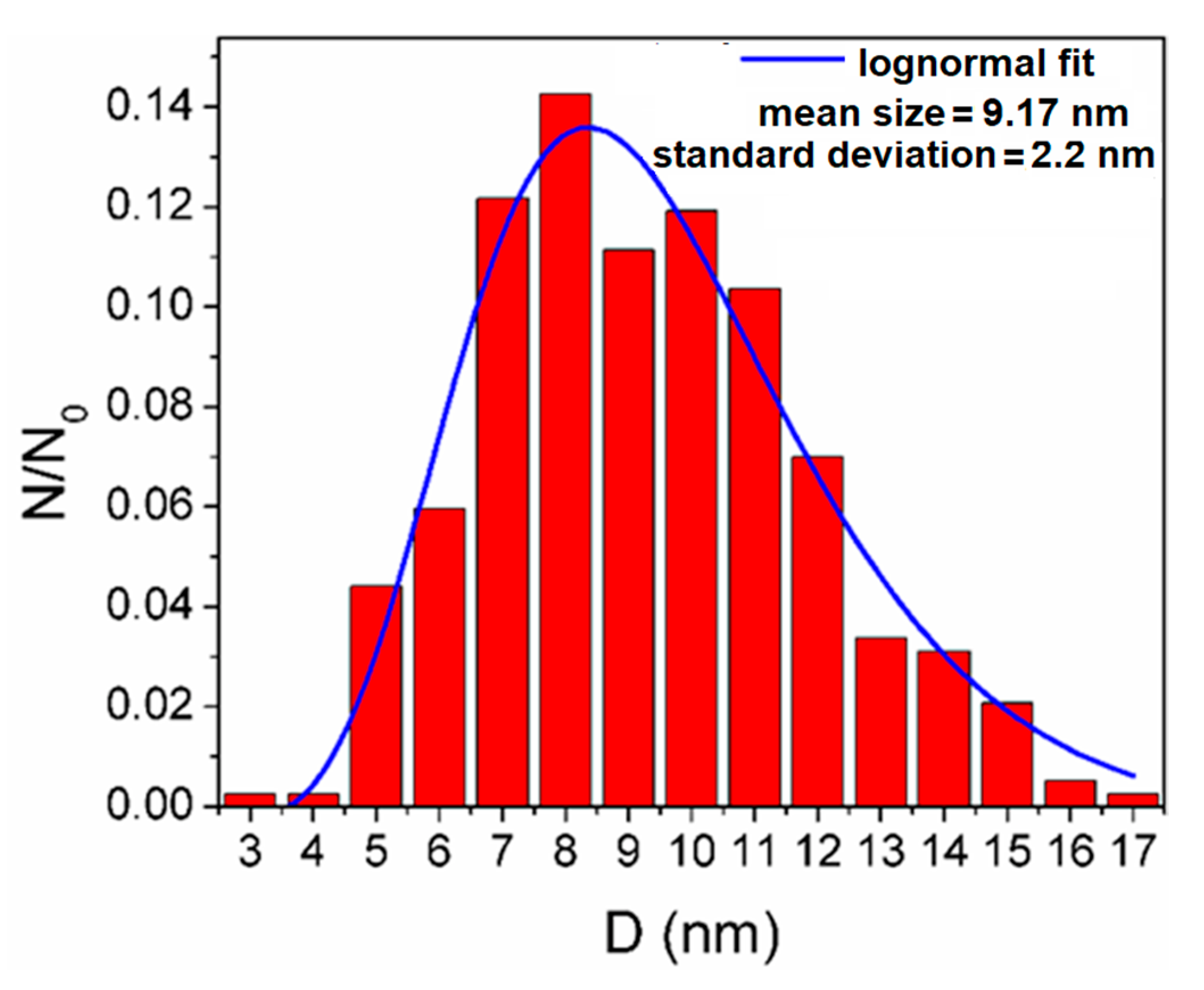
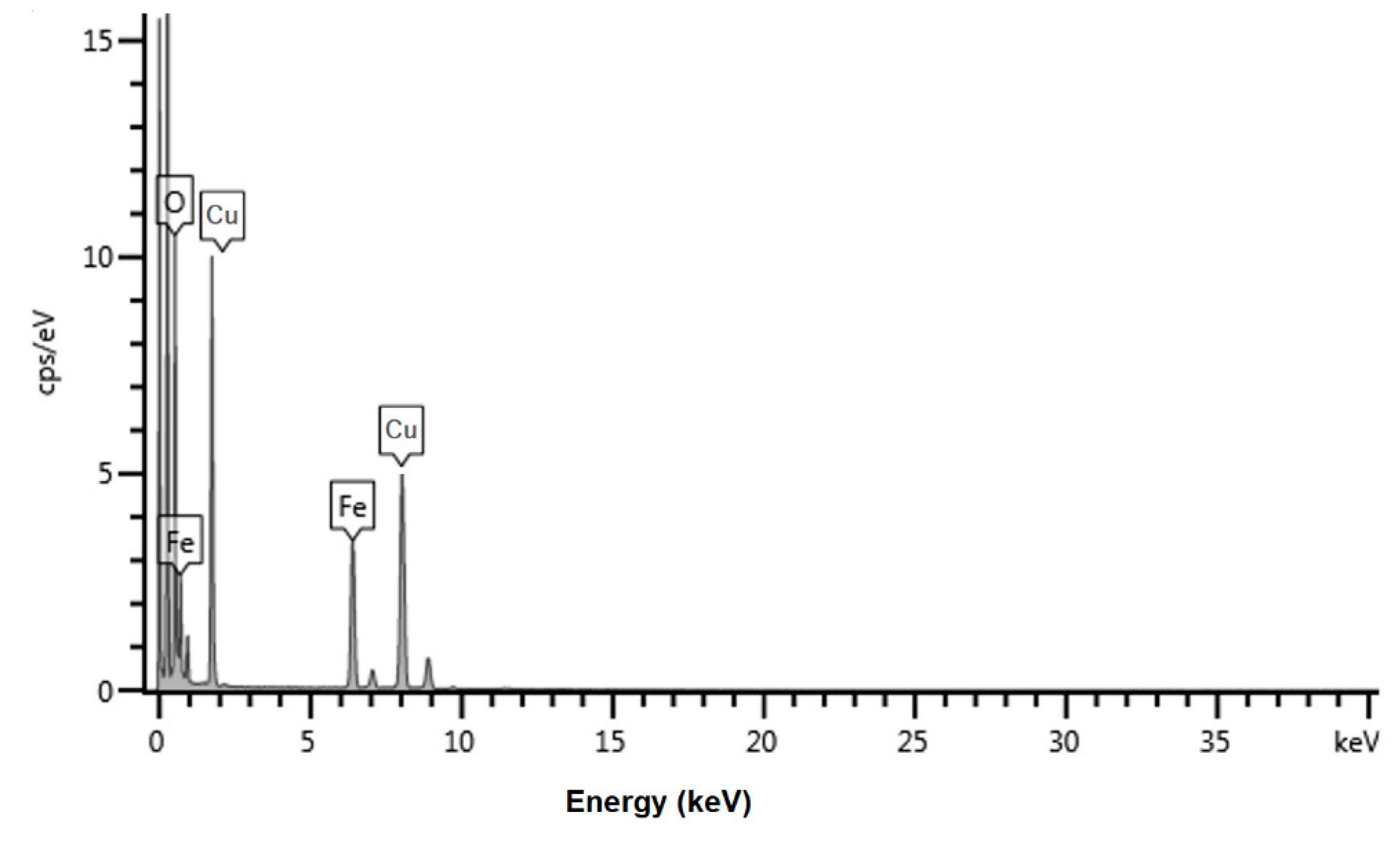
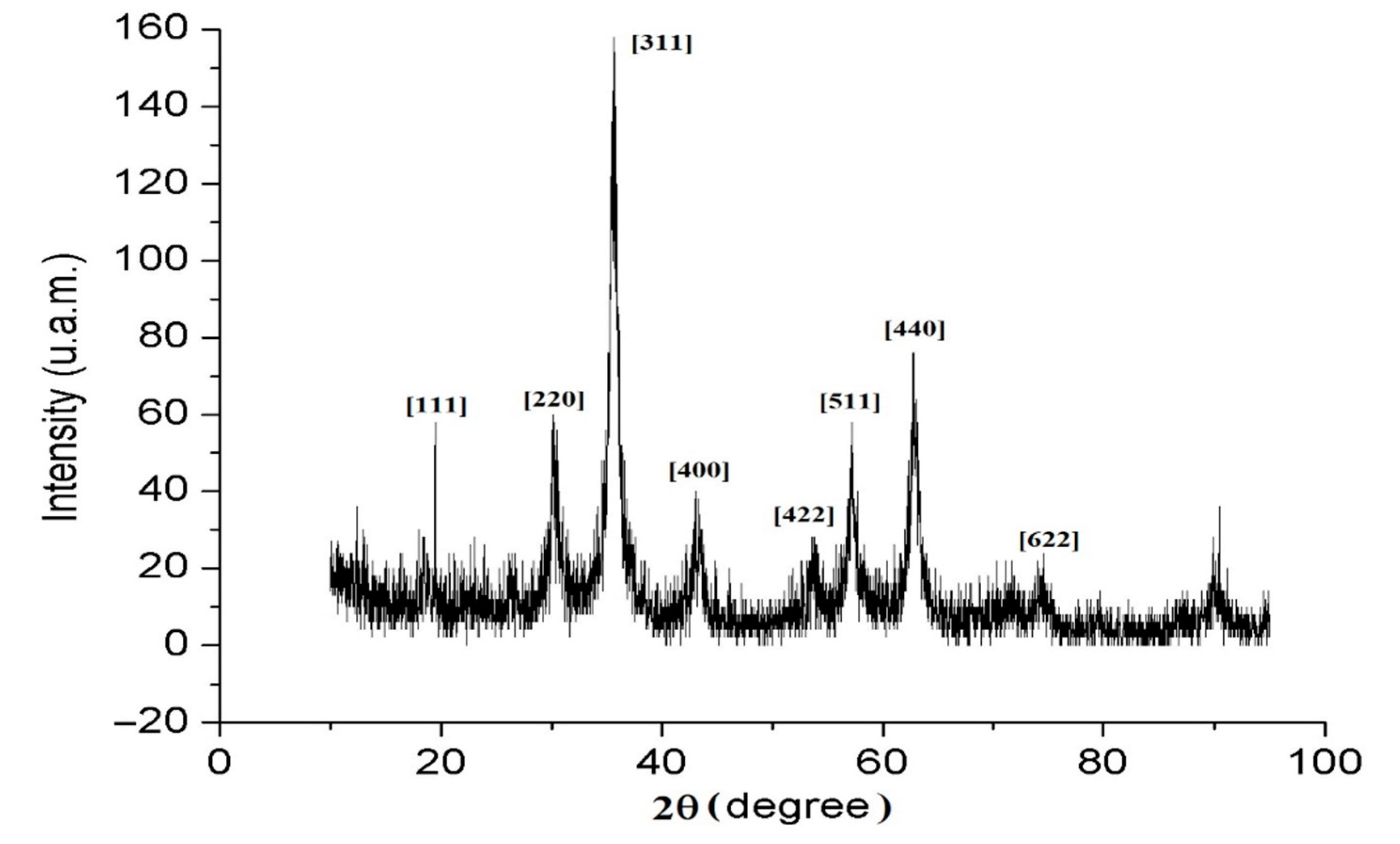
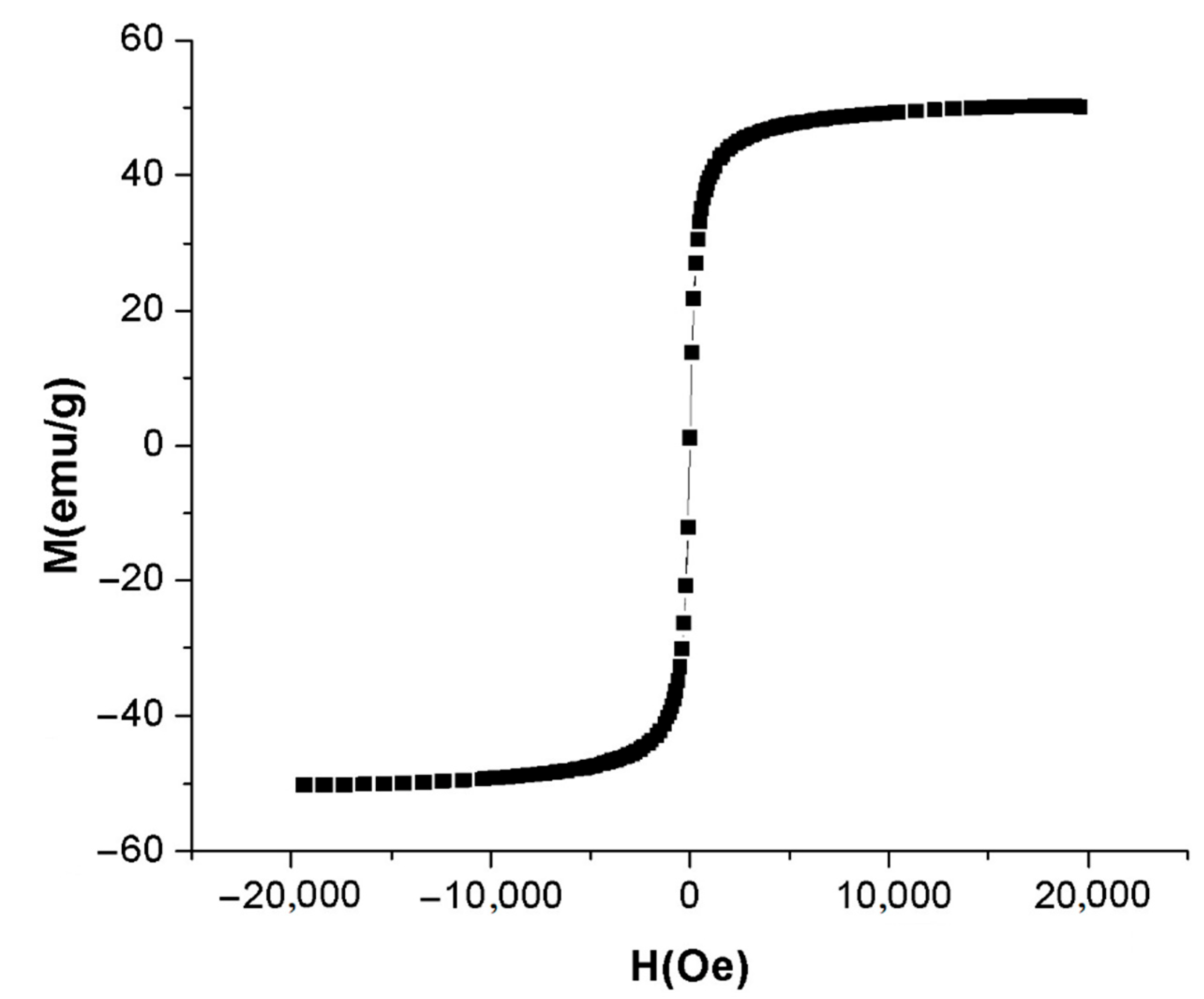
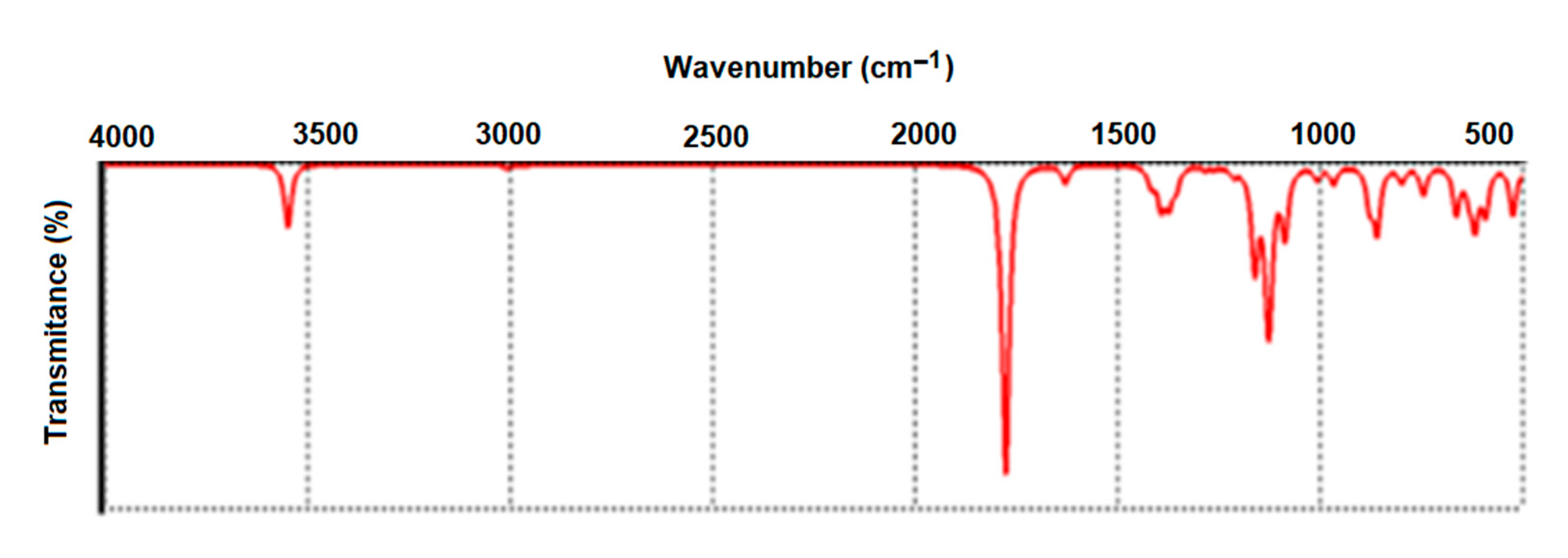
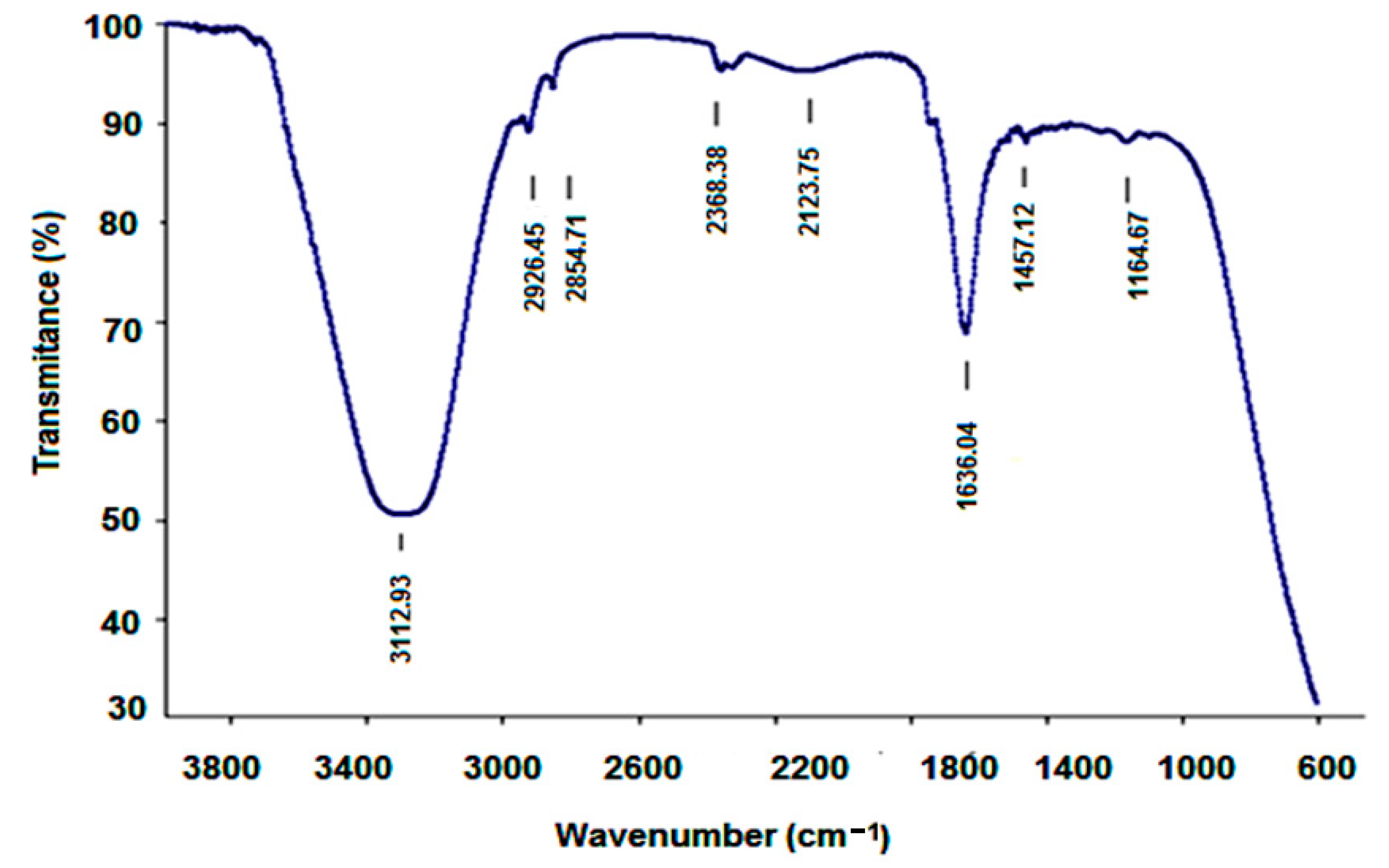
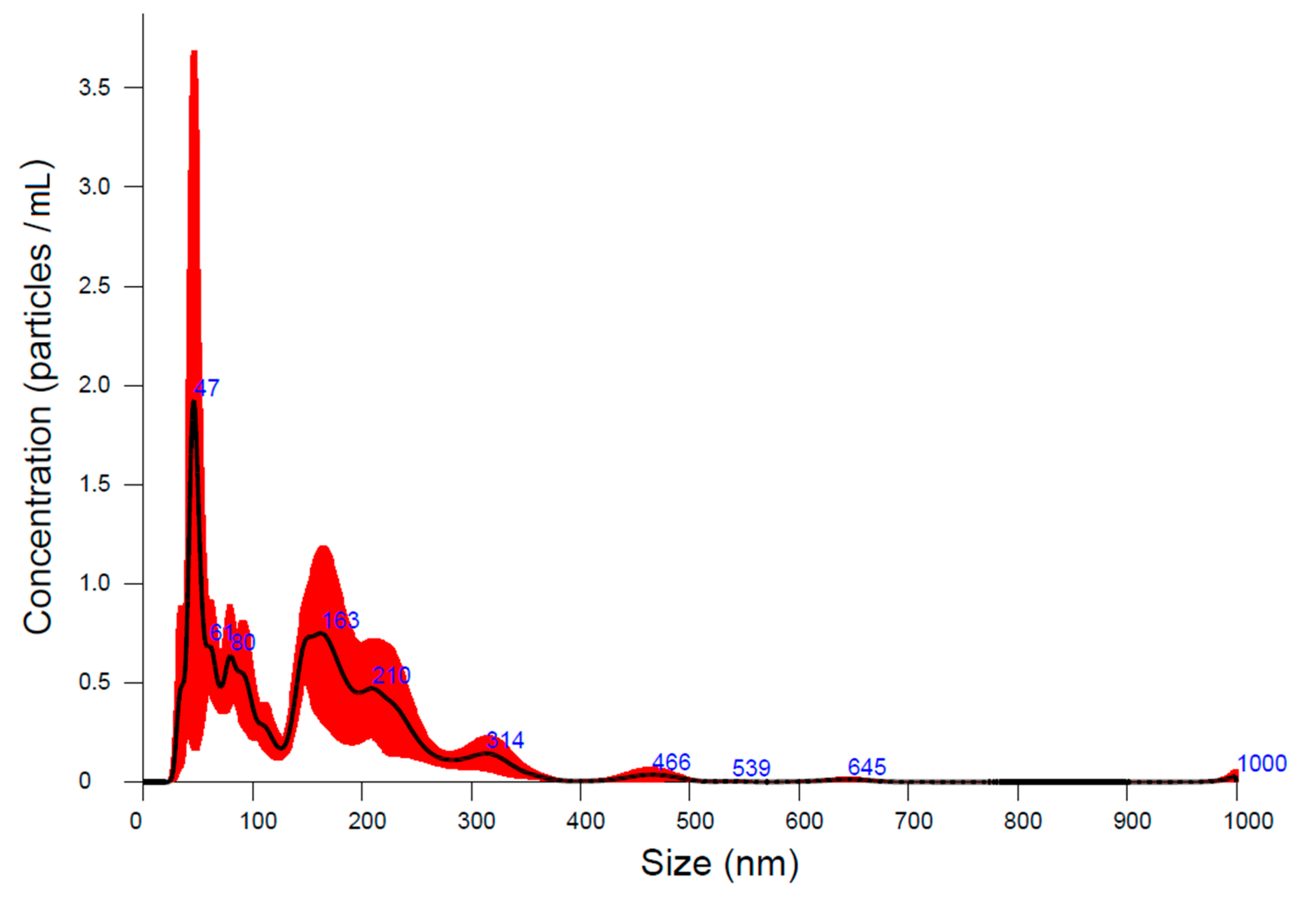
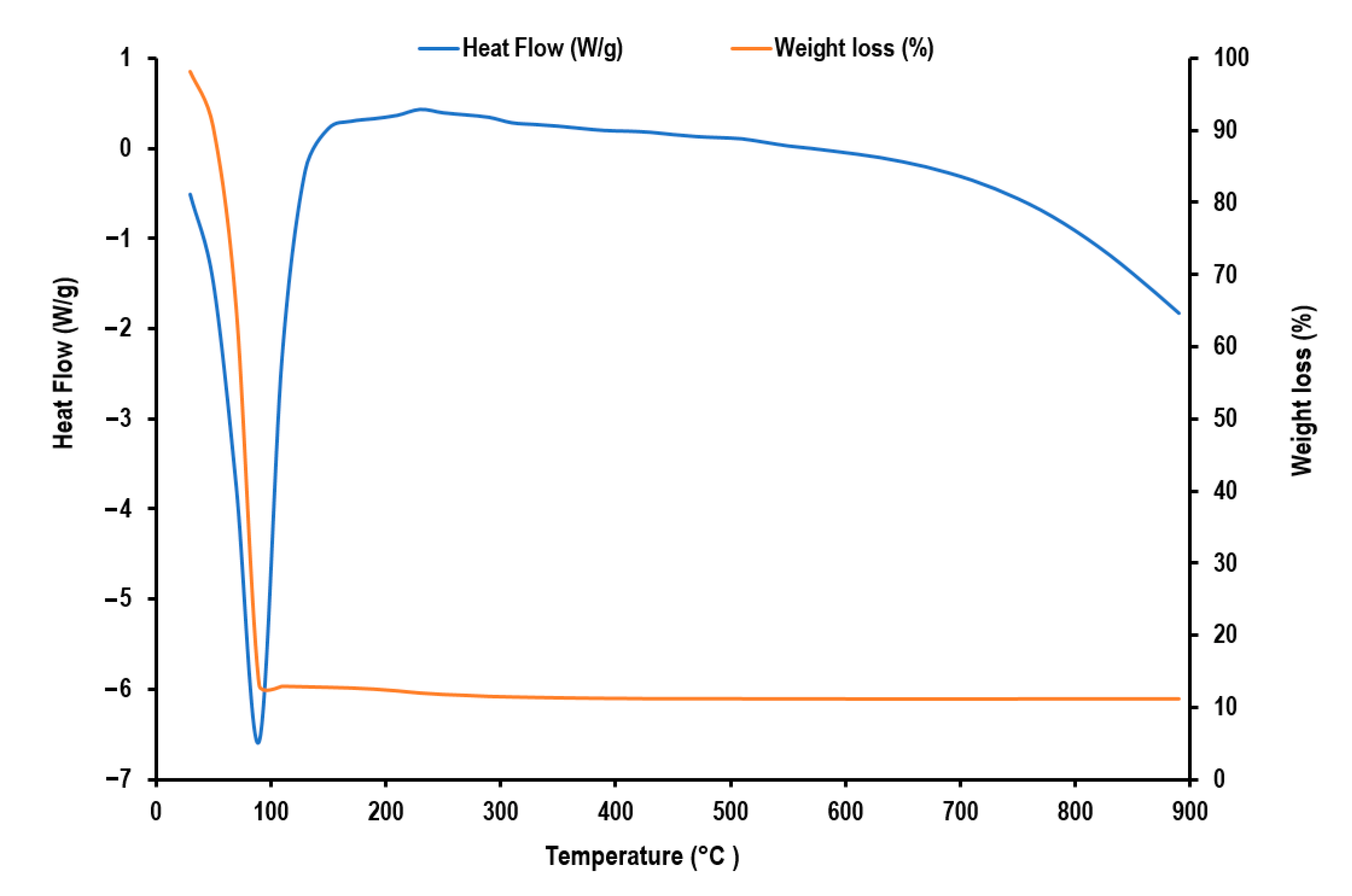
2.2. Characterization of A-IONPs
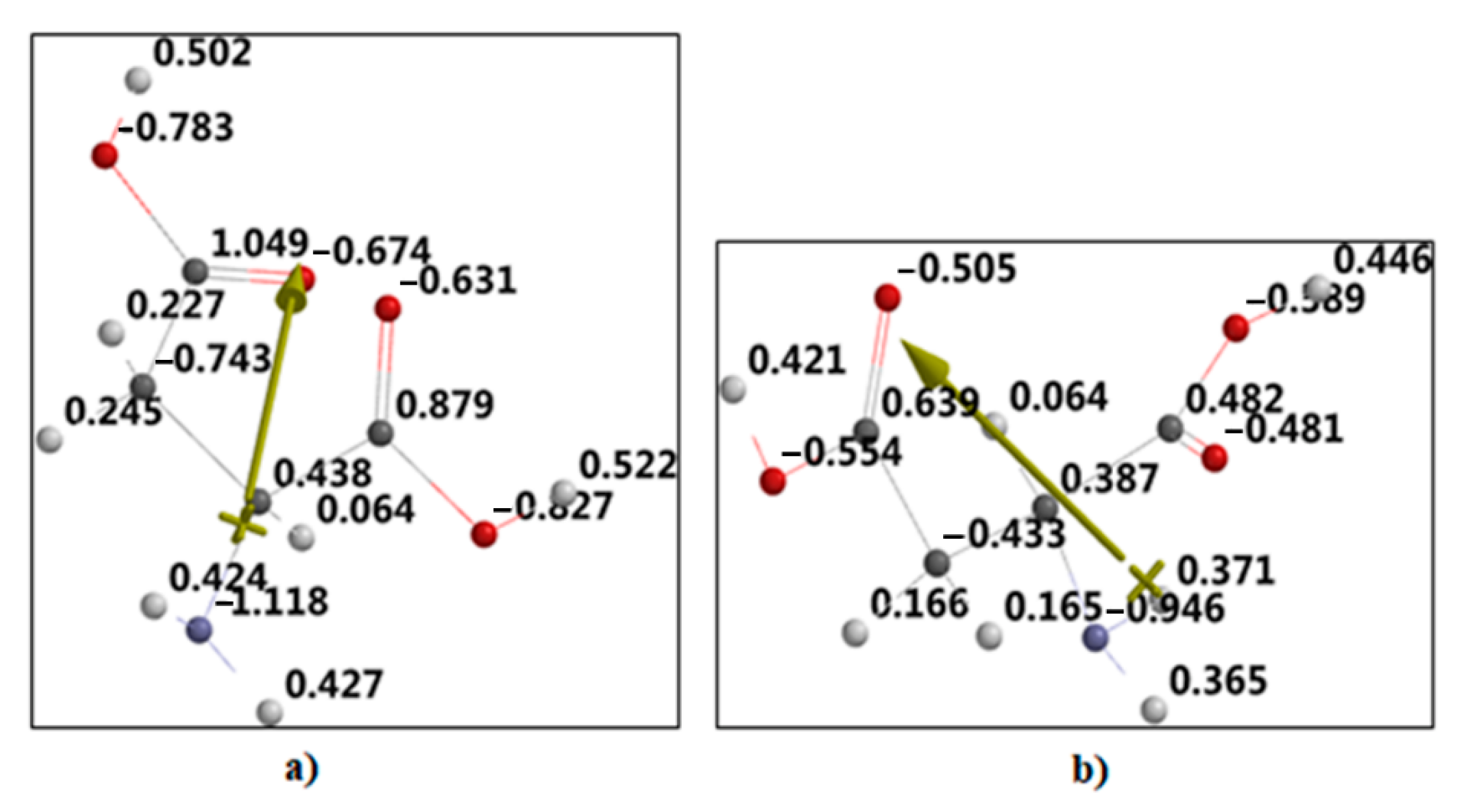
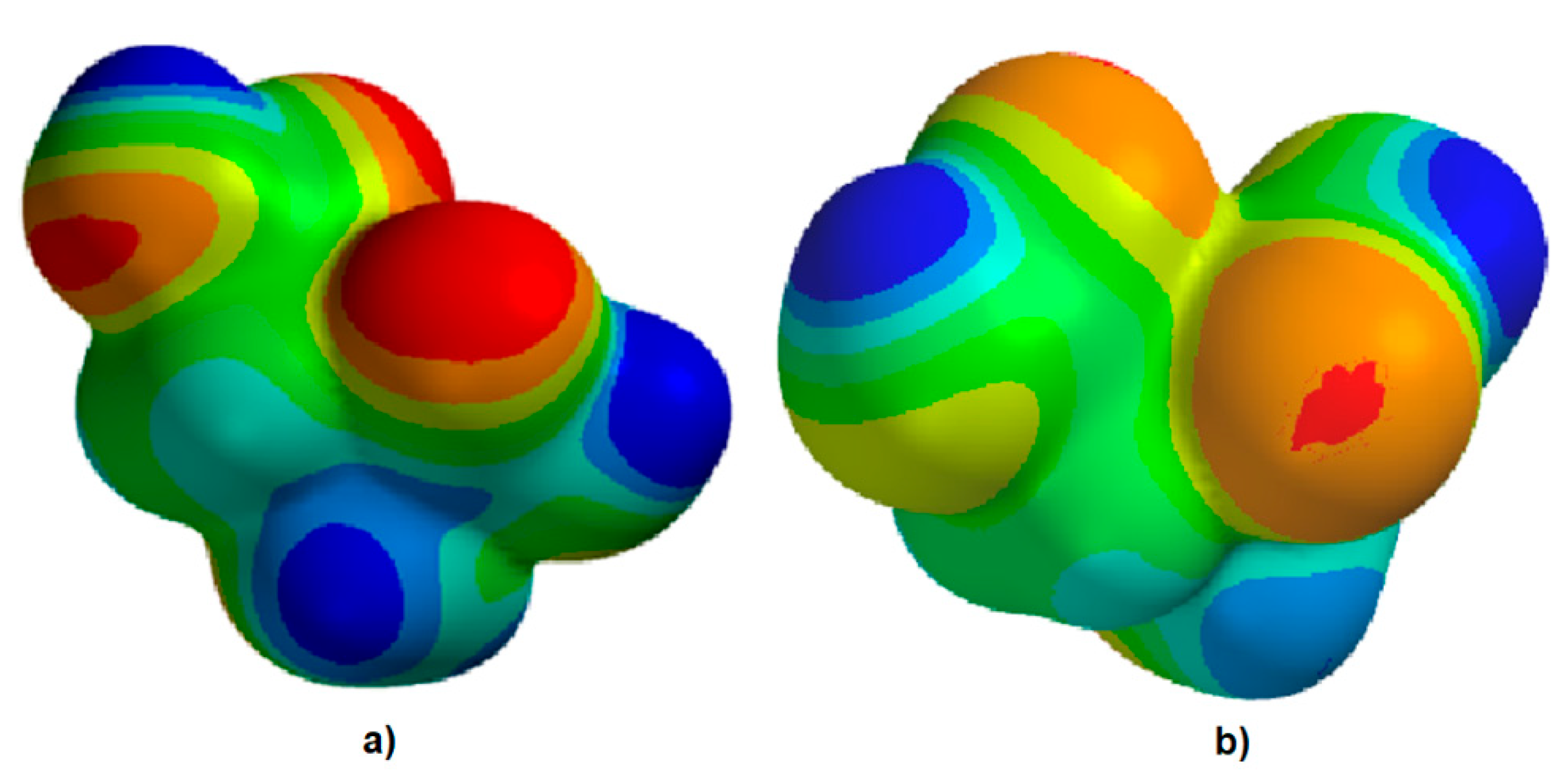
2.3. Theoretical Approach of Molecule Structure and Properties
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, T.; Sun, M.; Wu, S. State-of-the-Art review of electrospun gelatin-based nanofiber dressings for wound healing applications. Nanomaterials 2022, 12, 784. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.F.; Gui, D.; Wong, M.; Döring, A.; Rogach, A.L.; He, T.; Wong, W.T. A self-indicating cellulose-based gel with tunable performance for bioactive agent delivery. J. Drug Deliv. Sci. Technol. 2021, 63, 102428. [Google Scholar] [CrossRef]
- Yan, J.; Yao, Y.; Yan, S.; Gao, R.; Lu, W.; He, W. Chiral protein supraparticles for tumor suppression and synergistic immunotherapy: An enabling strategy for bioactive supramolecular chirality construction. Nano Lett. 2020, 20, 5844–5852. [Google Scholar] [CrossRef] [PubMed]
- Salarpour, S.; Barani, M.; Pardakhty, A.; Khatami, M.; Chauhan, N.P.S. The application of exosomes and exosome-nanoparticle in treating brain disorders. J. Mol. Liq. 2022, 350, 118549. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Xu, K.; Xu, C.; Xu, B. Biofunctional magnetic nanoparticles for protein separation and pathogen detection. Chem. Commun. 2006, 2006, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Mashhadi Malekzadeh, A.; Ramazani, A.; Tabatabaei Rezaei, S.J.; Niknejad, H. Design and construction of multifunctional hyperbranched polymers coated magnetite nanoparticles for both targeting magnetic resonance imaging and cancer therapy. J. Colloid Interface Sci. 2017, 490, 64–73. [Google Scholar] [CrossRef]
- Li, L.; Gao, F.; Jiang, W.; Wu, X.; Cai, Y.; Tang, J.; Gao, X.; Gao, F. Folic acid-conjugated superparamagnetic iron oxide nanoparticles for tumor-targeting MR imaging. Drug Deliv. 2016, 23, 1726–1733. [Google Scholar] [CrossRef]
- Hafeli, U.; Schutt, W.; Teller, J.; Zborowski, M. Scientific and Clinical Applications of Magnetic Carriers; Plenum Press: New York, NY, USA, 1997; pp. 323–340, 399–417. [Google Scholar]
- Demirer, G.S.; Okur, A.C.; Kizilel, S. Synthesis and design of biologically inspired biocompatible iron oxide nanoparticles for biomedical applications. J. Mater. Chem. B 2015, 3, 7831–7849. [Google Scholar] [CrossRef]
- Arias, L.S.; Pessan, J.P.; Vieira, A.; Lima, T.; Delbem, A.; Monteiro, D.R. Iron Oxide Nanoparticles for Biomedical Applications: A Perspective on Synthesis, Drugs, Antimicrobial Activity, and Toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef]
- Qu, H.; Ma, H.; Zhou, W.; O’Connor, C. In situ surface functionalization of magnetic nanoparticles with hydrophilic natural amino acids. Inorg. Chim. Acta 2012, 389, 60–65. [Google Scholar] [CrossRef]
- Berry, C. Possible exploitation of magnetic nanoparticle-cell interaction for biomedical applications. J. Mater. Chem. 2005, 15, 543–547. [Google Scholar] [CrossRef]
- Patel, D.; Chang, Y.; Lee, G.H. Amino Acid Functionalized Magnetite Nanoparticles in Saline Solution. Curr. Appl. Phys. 2009, 9, S32–S34. [Google Scholar] [CrossRef]
- Marinescu, G.; Patron, L.; Culita, D.C.; Neagoe, C.; Lepadatu, C.I.; Balint, I.; Bessais, L.; Cizmas, C.B. Synthesis of Magnetite Nanoparticles in the Presence of Aminoacids. J. Nanopart. Res. 2006, 8, 1045–1051. [Google Scholar] [CrossRef]
- Sousa, M.H.; Rubim, J.C.; Sobrinho, P.G.; Tourinho, F.A. Biocompatible magnetic fluid precursors based on aspartic and glutamic acid modified maghemite nanostructures. J. Magn. Magn. Mater. 2001, 225, 67–72. [Google Scholar] [CrossRef]
- Durmus, Z.; Kavas, H.; Toprak, M.S.; Baykal, A.; Altınçekiç, T.G.; Aslan, A.; Bozkurt, A.; Coşgun, S. L-lysine coated iron oxide nanoparticles: Synthesis, structural and conductivity characterization. J. Alloys Compd. 2009, 484, 371–376. [Google Scholar] [CrossRef]
- Park, J.Y.; Choi, E.S.; Baek, M.J.; Lee, G.H. Colloidal stability of amino acid coated magnetite nanoparticles in physiological fluid. Mater. Lett. 2009, 63, 379–381. [Google Scholar] [CrossRef]
- Berovic, M.; Berlot, M.; Kralj, S.; Makovec, D. A New Method for the Rapid Separation of Magnetized Yeast in Sparkling Wine. Biochem. Eng. J. 2014, 88, 77–84. [Google Scholar] [CrossRef]
- Ebrahiminezhad, A.; Varma, V.; Yang, S.; Ghasemi, Y.; Berenjian, A. Synthesis and Application of Amine Functionalized Iron Oxide Nanoparticles on Menaquinone-7 Fermentation: A Step towards Process Intensification. Nanomaterials 2016, 6, 1. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, F.; Shan, C.; Tong, M.; Hou, Y. Efficient bacterial capture with amino acid modified magnetic nanoparticles. Water Res. 2014, 50, 124–134. [Google Scholar] [CrossRef]
- Rhee, J.E.; Sheng, W.; Morgan, L.K.; Nolet, R.; Liao, X.; Kenney, L.J. Amino acids important for DNA recognition by the response regulator OmpR. J. Biol. Chem. 2008, 283, 8664–8677. [Google Scholar] [CrossRef]
- Dalangin, R.; Kim, A.; Campbell, R.E. The Role of Amino Acids in Neurotransmission and Fluorescent Tools for Their Detection. Int. J. Mol. Sci. 2020, 21, 6197. [Google Scholar] [CrossRef]
- Singh, D.; Singh, S.K.; Atar, N.; Krishna, V. Amino acid functionalized magnetic nanoparticles for removal of Ni(II) from aqueous solution. J. Taiwan Inst. Chem. Eng. 2016, 67, 148–160. [Google Scholar] [CrossRef]
- Ebrahiminezhad, A.; Ghasemi, Y.; Rasoul-Amini, S.; Barar, J.; Davaran, S. Impact of amino-acid coating on the synthesis and characteristics of iron-oxide nanoparticles (IONs). Bull. Korean Chem. Soc. 2012, 33, 3957–3962. [Google Scholar] [CrossRef]
- Ebrahiminezhad, A.; Davaran, S.; Rasoul-Amini, S.; Barar, J.; Moghadam, M.; Ghasemi, Y. Synthesis, characterization and anti-Listeria monocytogenes effect of amino acid coated magnetite nanoparticles. Curr. Nanosci. 2012, 8, 868–874. [Google Scholar] [CrossRef]
- Nosrati, H.; Salehiabar, M.; Davaran, S.; Ramazani, A.; Manjili, H.K.; Danafar, H. New advances strategies for surface functionalization of iron oxide magnetic nano particles (IONPs). Res. Chem. Intermed. 2017, 43, 7423–7442. [Google Scholar] [CrossRef]
- Afradi, N.; Foroughifar, N.; Pasdar, H.; Qomi, M. Aspartic-acid-loaded starch-functionalized Mn–Fe–Ca ferrite magnetic nanoparticles as novel green heterogeneous nanomagnetic catalyst for solvent-free synthesis of dihydropyrimidine derivatives as potent antibacterial agents. Res. Chem. Intermed. 2019, 45, 1–21. [Google Scholar] [CrossRef]
- Berger, P.; Adelman, N.B.; Beckman, K.J.; Campbell, D.J.; Ellis, A.B.; Lisensky, G.C. Preparation and properties of an aqueous ferrofluid. J. Chem. Educ. 1999, 76, 943–948. [Google Scholar] [CrossRef]
- Răcuciu, M. Biocompatible Magnetic Nanofluids: Synthesis, Characterisation and Applications; Lucian Blaga University Publishing: Sibiu, Romania, 2011; pp. 24–27. [Google Scholar]
- Hehre, W.J.; Radom, L.; Schleyer, P.V.R.; Pople, J.A. Ab Initio Molecular Orbital Theory; John Wiley & Sons Ltd.: New York, NY, USA, 1986; pp. 153–197. [Google Scholar]
- Young, D. Computational Chemistry; Wiley-Interscience: Hoboken, NJ, USA, 2001; p. 330. [Google Scholar]
- Movre Šapić, I.; Vidak, A.; Dananić, V. Experimental and theoretical study of aspartic acid. Bull. Chem. Technol. Bosnia Herzeg. 2019, 52, 17–22. [Google Scholar]
- Stefaniu, A.; Savoiu, V.G.; Lupescu, I.; Iulian, O. Computational study on 3D structure of L-aspartic acid and L-glutamic acid: Molecular descriptors and properties. Ovidius Univ. Ann. Chem. 2016, 27, 48–52. [Google Scholar] [CrossRef][Green Version]
- Patterson, A. The Scherrer formula for X-Ray particle size determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction, 3rd ed.; Prentice-Hall: New York, NY, USA, 2001; pp. 88–106. [Google Scholar]
- Blaney, L. Magnetite (Fe3O4): Properties, Synthesis, and Applications. Lehigh Rev. Lehigh Preserv. 2007, 15, 33–81. [Google Scholar]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Kákay, A.; Gutowski, M.W.; Takacs, L.; Franco, V.; Varga, L.K. Langevin granulometry of the particle size distribution. J. Phys. A Math. Gen. 2004, 37, 6027–6041. [Google Scholar] [CrossRef]
- Rosensweig, R.E. Ferrohydrodynamics; Cambridge University Press: New York, NY, USA, 1985; pp. 55–60. [Google Scholar]
- Kaiser, R.; Miskolczy, G. Magnetic properties of stable dispersions of subdomain magnetite particles. J. Appl. Phys. 1970, 41, 1064–1072. [Google Scholar] [CrossRef]
- Predoi, D. A study on iron oxide nanoparticles coated with dextrin obtained by coprecipitation. Dig. J. Nanomater. Biostruct. 2007, 2, 169–173. [Google Scholar]
- Cornwell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Mikhaylova, M.; Kim, D.K.; Berry, C.C.; Zagorodni, A.; Toprak, M.; Curtis, A.S.G.; Muhammed, M. BSA immobilization on amine-functionalized superparamagnetic iron oxide nanoparticles. Chem. Mater. 2004, 16, 2344–2354. [Google Scholar] [CrossRef]
- Lunardi, C.N.; Gomes, A.J.; Rocha, F.S.; de Tommaso, J.; Patience, G.S. Experimental methods in chemical engineering: Zeta potential. Can. J. Chem. Eng. 2021, 99, 627–639. [Google Scholar] [CrossRef]
- Ostolska, I.; Wiśniewska, M. Application of the zeta potential measurements to explanation of colloidal Cr2O3 stability mechanism in the presence of the ionic polyamino acids. Colloid Polym. Sci. 2014, 292, 2453–2464. [Google Scholar] [CrossRef]
- Nădejde, C.; Puscașu, E.; Brinza, F.; Ursu, L.; Creangă, D.; Stan, C. Preparation of soft magnetic materials and characterization with investigation methods for fluid samples. U. Politech. Buch. Ser. A 2015, 77, 277–284. [Google Scholar]
- Weiss, I.M.; Muth, C.; Drumm, R.; Kirchner, H.O.K. Thermal decomposition of the amino acids glycine, cysteine, aspartic acid, asparagine, glutamic acid, glutamine, arginine and histidine. BMC Biophys. 2018, 11, 2. [Google Scholar] [CrossRef]
- Salehiabar, M.; Nosrati, H.; Davaran, S.; Danafar, H.; Manjili, H.K. Facile synthesis and characterization of L-Aspartic acid coated iron oxide magnetic nanoparticles (IONPs) for biomedical ap-plications. Drug Res. 2018, 68, 280–285. [Google Scholar] [CrossRef]
- Pušnik, K.; Peterlin, M.; Cigić, I.K.; Marolt, G.; Kogej, K.; Mertelj, A.; Gyergyek, S.; Makovec, D. Adsorption of amino acids, aspartic acid, and lysine onto iron-oxide nanoparticles. J. Phys. Chem. C 2016, 120, 14372–14381. [Google Scholar] [CrossRef]
- Karimzadeh, I.; Aghazadeh, M.; Doroudi, T.; Ganjali, M.R.; Kolivand, P.H.; Gharailou, D. Amino Acid Coated Superparamagnetic Iron Oxide Nanoparticles for Biomedical Applications Through a Novel Efficient Preparation Method. J. Clust. Sci. 2016, 28, 1259–1271. [Google Scholar] [CrossRef]
- Ebrahiminezhad, A.; Ghasemi, Y.; Rasoul-Amini, S.; Barar, J.; Davaran, S. Preparation of novel magnetic fluorescent nanoparticles using amino acids. Colloids Surf. B Biointerfaces 2013, 102, 534–539. [Google Scholar] [CrossRef]
- Răcuciu, M. Iron oxide nanoparticles coated with aspartic acid and their genotoxic impact on root cells of Zea mays embryos. Rom. Rep. Phys. 2020, 72, 701. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Răcuciu, M.; Barbu-Tudoran, L.; Oancea, S.; Drăghici, O.; Morosanu, C.; Grigoras, M.; Brînză, F.; Creangă, D.E. Aspartic Acid Stabilized Iron Oxide Nanoparticles for Biomedical Applications. Nanomaterials 2022, 12, 1151. https://doi.org/10.3390/nano12071151
Răcuciu M, Barbu-Tudoran L, Oancea S, Drăghici O, Morosanu C, Grigoras M, Brînză F, Creangă DE. Aspartic Acid Stabilized Iron Oxide Nanoparticles for Biomedical Applications. Nanomaterials. 2022; 12(7):1151. https://doi.org/10.3390/nano12071151
Chicago/Turabian StyleRăcuciu, Mihaela, Lucian Barbu-Tudoran, Simona Oancea, Olga Drăghici, Cezarina Morosanu, Marian Grigoras, Florin Brînză, and Dorina E. Creangă. 2022. "Aspartic Acid Stabilized Iron Oxide Nanoparticles for Biomedical Applications" Nanomaterials 12, no. 7: 1151. https://doi.org/10.3390/nano12071151
APA StyleRăcuciu, M., Barbu-Tudoran, L., Oancea, S., Drăghici, O., Morosanu, C., Grigoras, M., Brînză, F., & Creangă, D. E. (2022). Aspartic Acid Stabilized Iron Oxide Nanoparticles for Biomedical Applications. Nanomaterials, 12(7), 1151. https://doi.org/10.3390/nano12071151