Bifunctional Single-Atom Cobalt Electrocatalysts with Dense Active Sites Prepared via a Silica Xerogel Strategy for Rechargeable Zinc–Air Batteries
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Co-N-C SAC Catalyst
3. Characterizations
3.1. Electrochemical Measurements
3.2. Zn–Air Battery
4. Results and Discussion
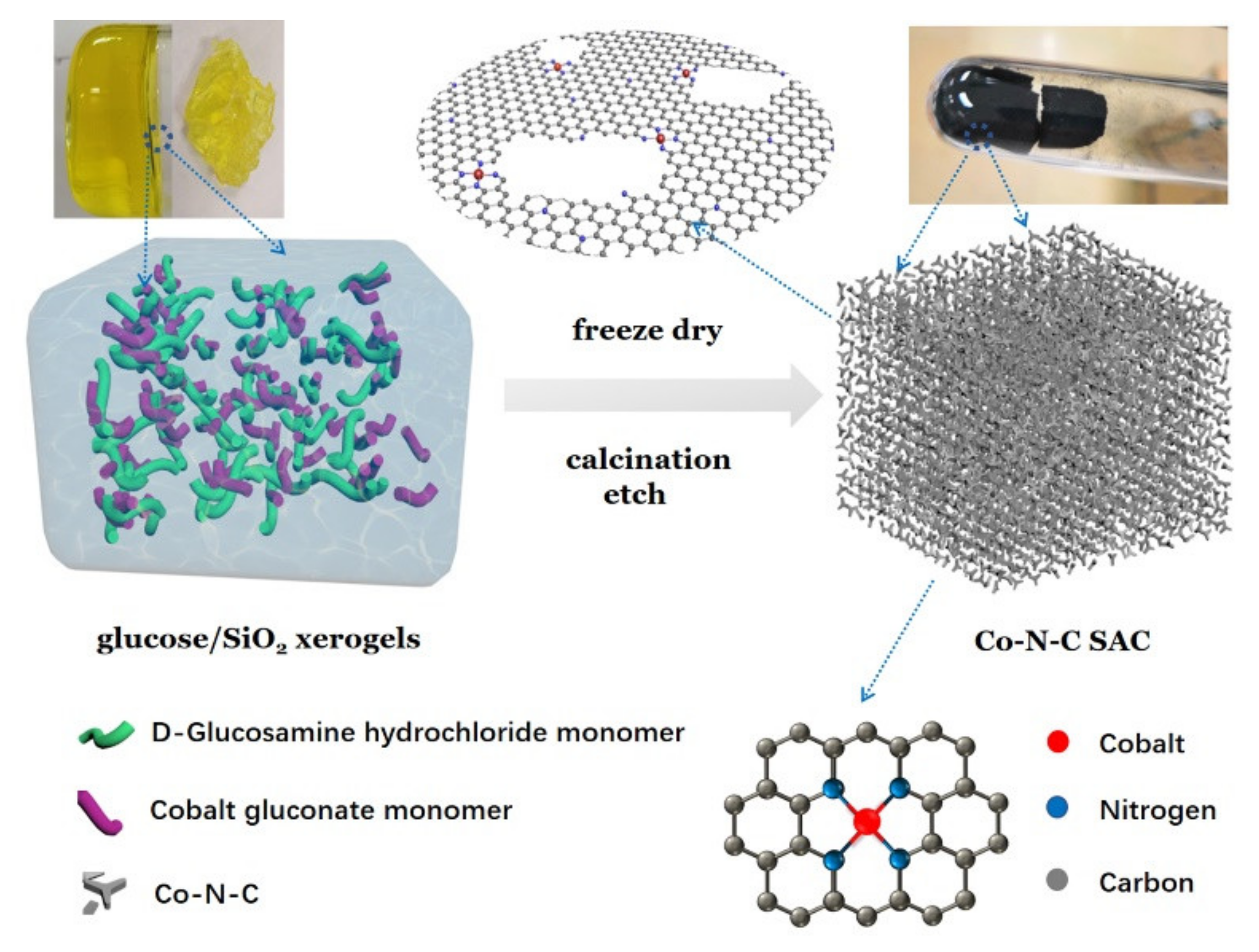
4.1. Synthesis Protocol
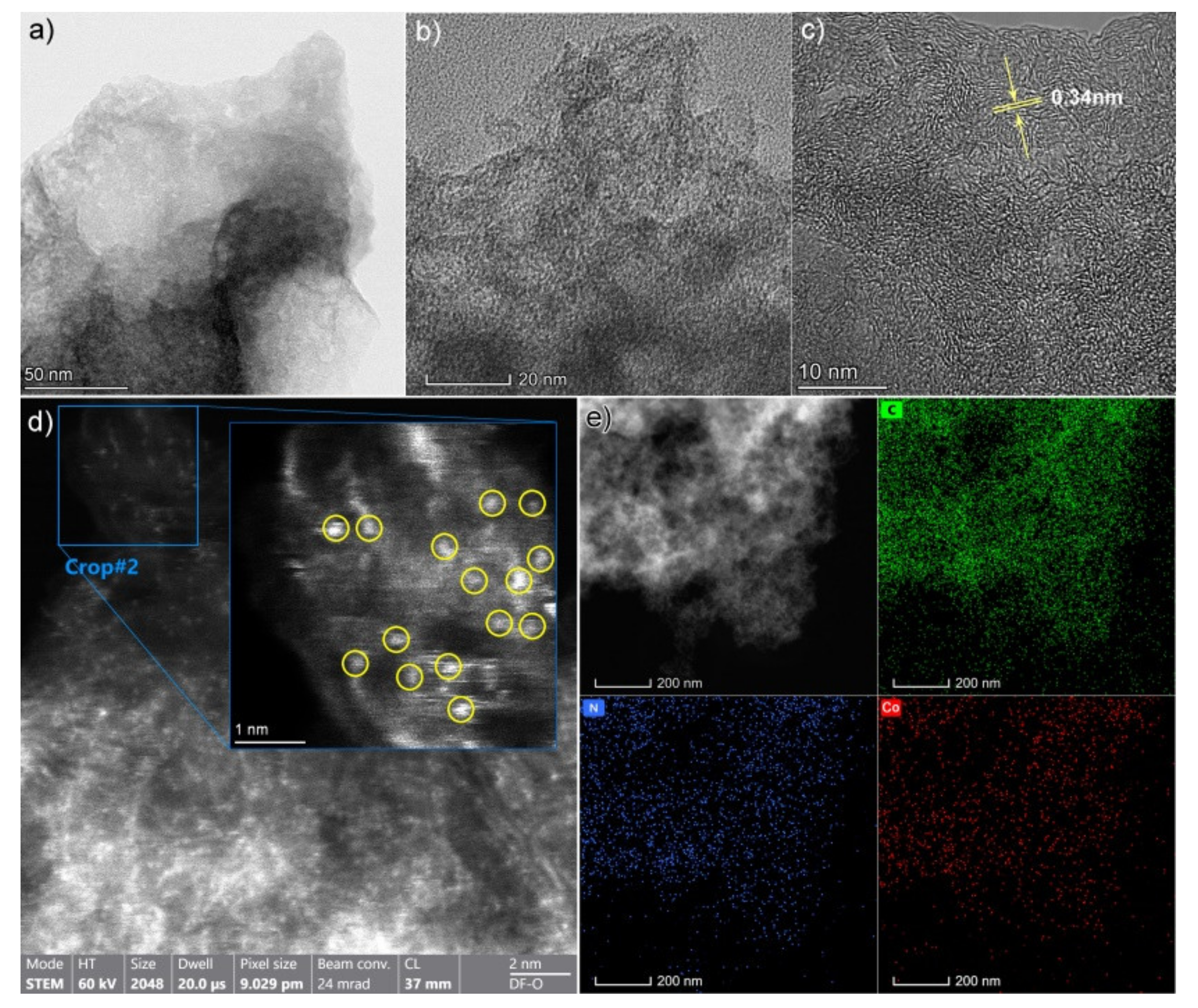
4.2. Morphological Features
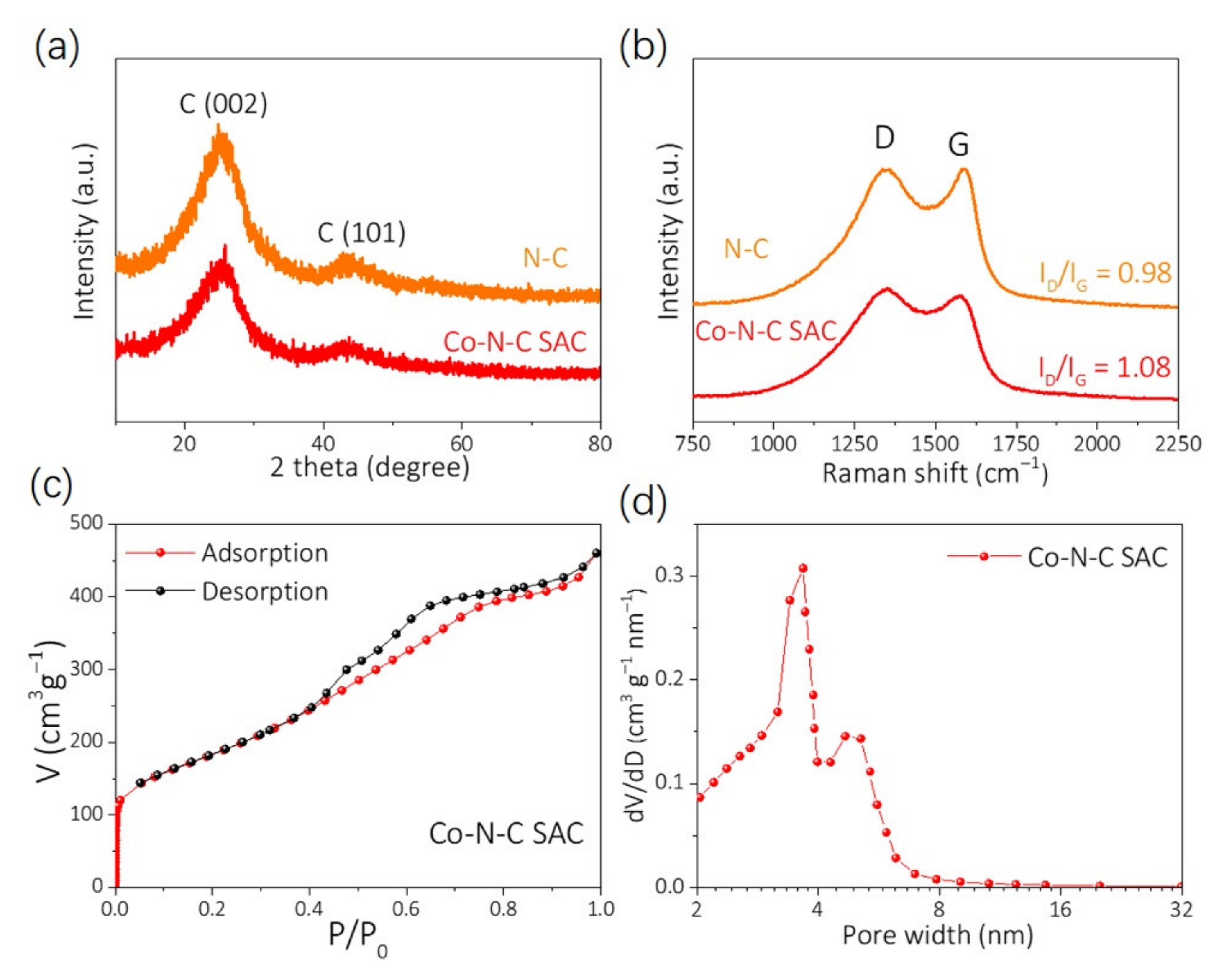
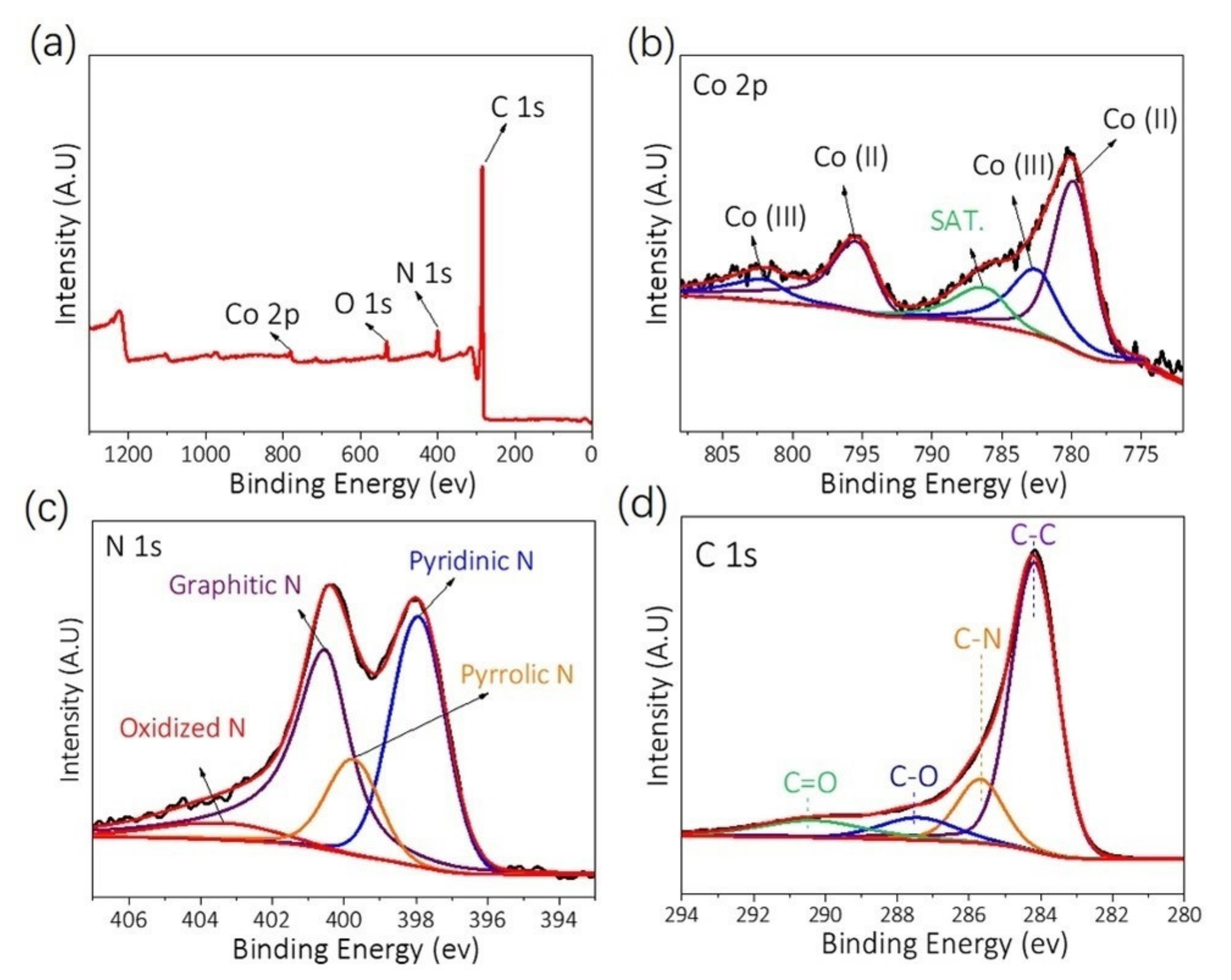
4.3. Structural Features
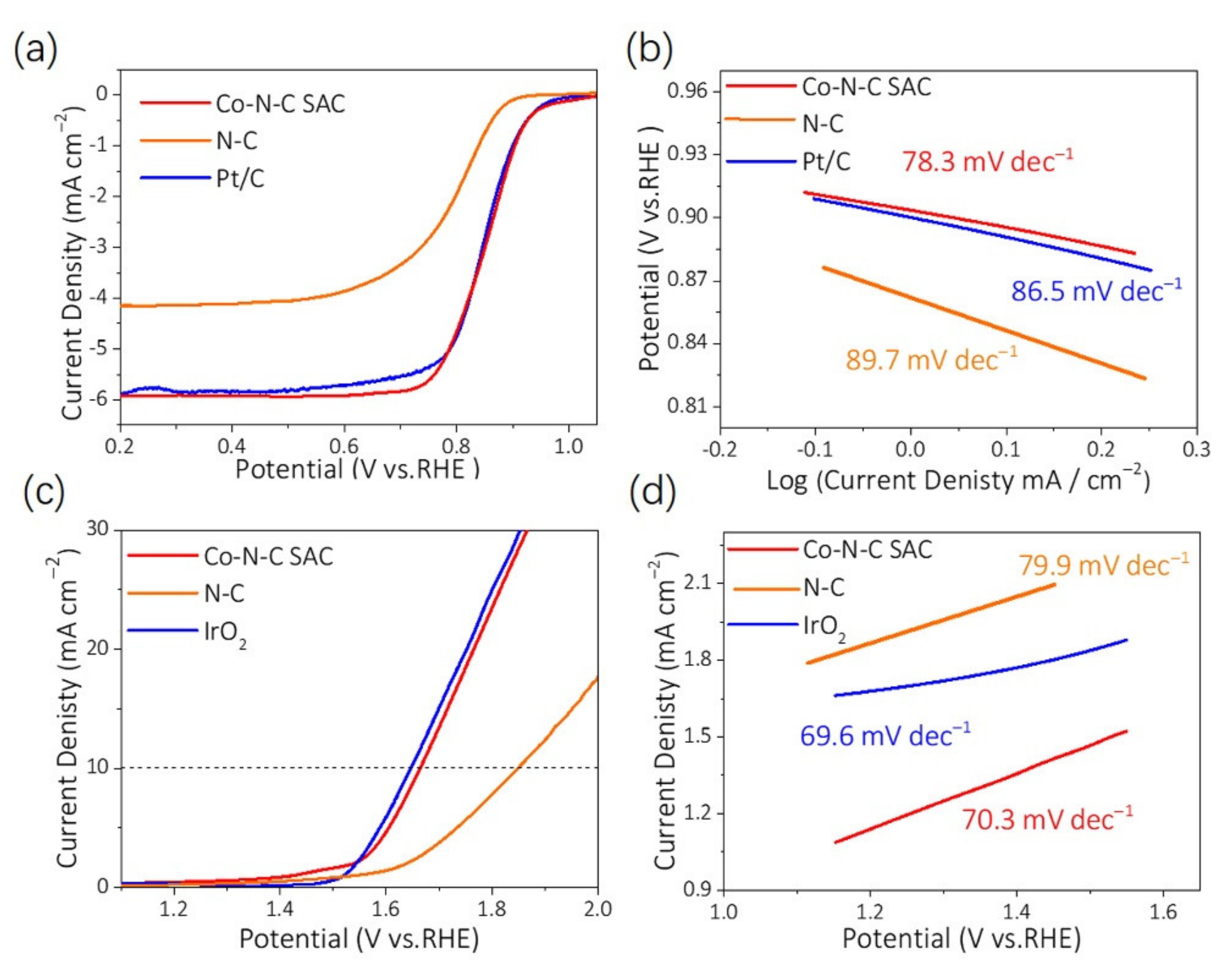
4.4. Electrochemical Measurements
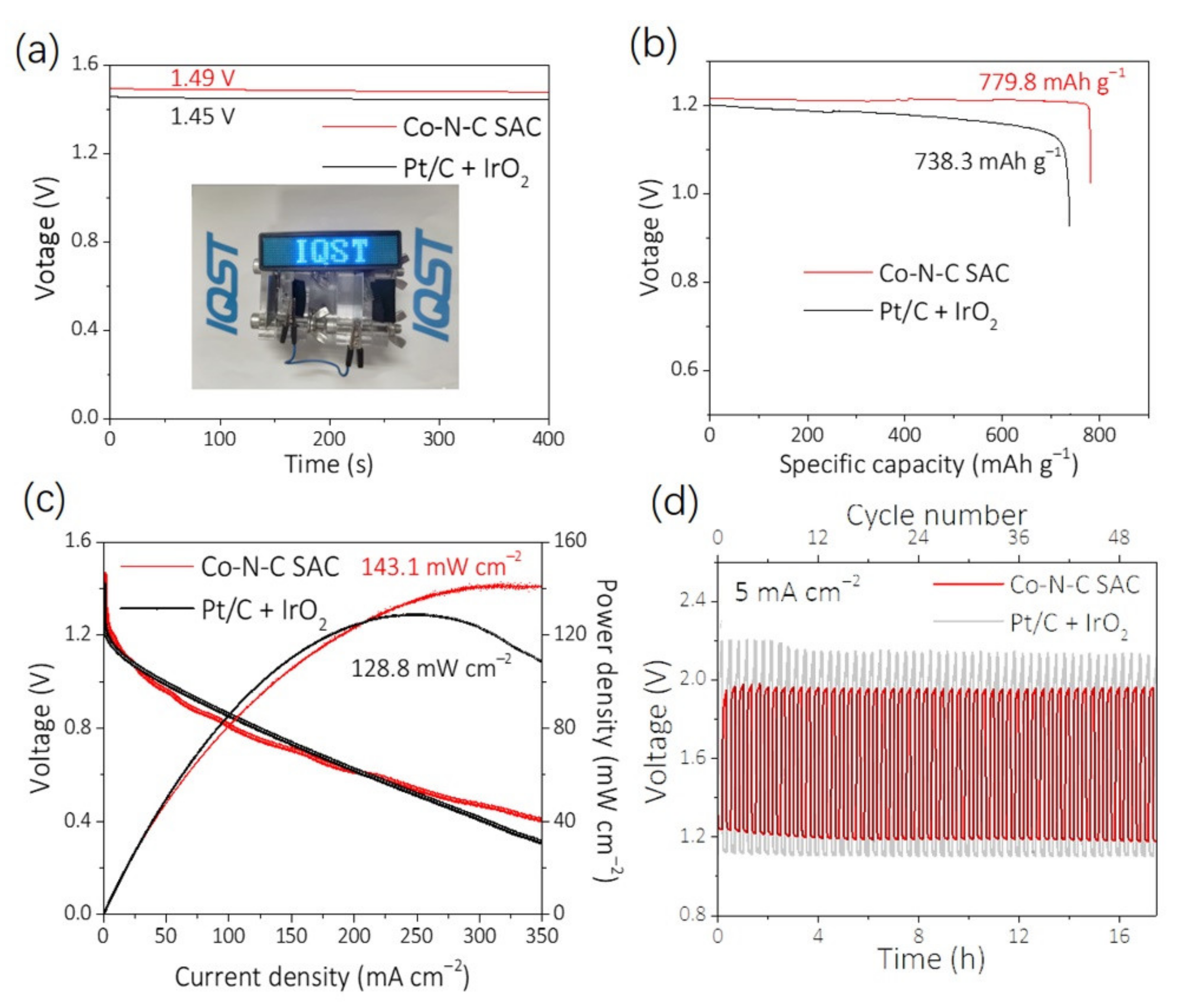
4.5. Zn–Air Battery Performance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Gong, M.; Liang, Y.; Feng, J.; Kim, J.-E.; Wang, H.; Hong, G.; Zhang, B.; Dai, H. Advanced Zinc–Air Batteries Based on High-Performance Hybrid Electrocatalysts. Nat. Commun. 2013, 4, 1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, F.; Zhong, H.; Bao, D.; Yan, J.; Zhang, X. In Situ Coupling of Strung Co4N and Intertwined N–C Fibers toward Free-Standing Bifunctional Cathode for Robust, Efficient, and Flexible Zn–Air Batteries. J. Am. Chem. Soc. 2016, 138, 10226–10231. [Google Scholar] [CrossRef] [PubMed]
- Mistry, H.; Varela, A.S.; Kühl, S.; Strasser, P.; Cuenya, B.R. Nanostructured Electrocatalysts with Tunable Activity and Selectivity. Nat. Rev. Mater. 2016, 1, 16009. [Google Scholar] [CrossRef]
- Ratso, S.; Walke, P.R.; Mikli, V.; Ločs, J.; Šmits, K.; Vītola, V.; Šutka, A.; Kruusenberg, I. CO2 Turned into a Nitrogen Doped Carbon Catalyst for Fuel Cells and Metal–Air Battery Applications. Green Chem. 2021, 23, 4435–4445. [Google Scholar] [CrossRef]
- Tang, C.; Wang, B.; Wang, H.-F.; Zhang, Q. Defect Engineering toward Atomic Co–Nx–C in Hierarchical Graphene for Rechargeable Flexible Solid Zn-Air Batteries. Adv. Mater. 2017, 29, 1703185. [Google Scholar] [CrossRef]
- Yang, L.; Shi, L.; Wang, D.; Lv, Y.; Cao, D. Single-Atom Cobalt Electrocatalysts for Foldable Solid-State Zn-Air Battery. Nano Energy 2018, 50, 691–698. [Google Scholar] [CrossRef]
- Xu, R.; Wang, X.; Zhang, C.; Zhang, Y.; Jiang, H.; Wang, H.; Su, G.; Huang, M.; Toghan, A. Engineering Solid–Liquid-Gas Interfaces of Single-Atom Cobalt Catalyst for Enhancing the Robust Stability of Neutral Zn-Air Batteries under High Current Density. Chem. Eng. J. 2021, 431, 133685. [Google Scholar] [CrossRef]
- Jiang, W.-J.; Gu, L.; Li, L.; Zhang, Y.; Zhang, X.; Zhang, L.-J.; Wang, J.-Q.; Hu, J.-S.; Wei, Z.; Wan, L.-J. Understanding the High Activity of Fe–N–C Electrocatalysts in Oxygen Reduction: Fe/Fe3C Nanoparticles Boost the Activity of Fe–Nx. J. Am. Chem. Soc. 2016, 138, 3570–3578. [Google Scholar] [CrossRef]
- Lai, Q.; Zheng, L.; Liang, Y.; He, J.; Zhao, J.; Chen, J. Metal–Organic-Framework-Derived Fe-N/C Electrocatalyst with Five-Coordinated Fe-Nx Sites for Advanced Oxygen Reduction in Acid Media. ACS Catal. 2017, 7, 1655–1663. [Google Scholar] [CrossRef]
- Li, J.-C.; Hou, P.-X.; Zhao, S.-Y.; Liu, C.; Tang, D.-M.; Cheng, M.; Zhang, F.; Cheng, H.-M. A 3D Bi-functional Porous N-doped Carbon Microtube Sponge Electrocatalyst for Oxygen Reduction and Oxygen Evolution Reactions. Energy Environ. Sci. 2016, 9, 3079–3084. [Google Scholar] [CrossRef]
- Sumboja, A.; Lübke, M.; Wang, Y.; An, T.; Zong, Y.; Liu, Z. All-Solid-State, Foldable, and Rechargeable Zn-Air Batteries Based on Manganese Oxide Grown on Graphene-Coated Carbon Cloth Air Cathode. Adv. Energy Mater. 2017, 7, 1700927. [Google Scholar] [CrossRef]
- Wang, H.-F.; Tang, C.; Wang, B.; Li, B.-Q.; Zhang, Q. Bifunctional Transition Metal Hydroxysulfides: Room-Temperature Sulfurization and Their Applications in Zn–Air Batteries. Adv. Mater. 2017, 29, 1702327. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Li, L.; Wei, Z. Recent Advancements in Pt and Pt-free Catalysts for Oxygen Reduction Reaction. Chem. Soc. Rev. 2015, 44, 2168–2201. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, W.; Wang, H.; Xie, L.; Liang, Y.; Wei, F.; Idrobo, J.-C.; Pennycook, S.J.; Dai, H. An Oxygen Reduction Electrocatalyst Based on Carbon Nanotube–Graphene Complexes. Nat. Nanotech. 2012, 7, 394–400. [Google Scholar] [CrossRef]
- Wang, J.; Liu, W.; Luo, G.; Li, Z.; Zhao, C.; Zhang, H.; Zhu, M.; Xu, Q.; Wang, X.; Zhao, C.; et al. Synergistic Effect of Well-Defined Dual Sites Boosting the Oxygen Reduction Reaction. Energy Environ. Sci. 2018, 11, 3375–3379. [Google Scholar] [CrossRef]
- Li, B.-Q.; Zhang, S.-Y.; Tang, C.; Cui, X.; Zhang, Q. Anionic Regulated NiFe (Oxy)Sulfide Electrocatalysts for Water Oxidation. Small 2017, 13, 1700610. [Google Scholar] [CrossRef]
- Lee, D.U.; Xu, P.; Cano, Z.P.; Kashkooli, A.G.; Park, M.G.; Chen, Z. Recent Progress and Perspectives on Bi-functional Oxygen Electrocatalysts for Advanced Rechargeable Metal–Air Batteries. J. Mater. Chem. A 2016, 4, 7107–7134. [Google Scholar] [CrossRef]
- Cheng, F.; Chen, J. Metal–Air Batteries: From Oxygen Reduction Electrochemistry to Cathode Catalysts. Chem. Soc. Rev. 2012, 41, 2172–2192. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Xu, D.; Xu, J.-J.; Zhang, X.-B. Oxygen Electrocatalysts in Metal–Air Batteries: From Aqueous to Nonaqueous Electrolytes. Chem. Soc. Rev. 2014, 43, 7746–7786. [Google Scholar] [CrossRef]
- Zitolo, A.; Ranjbar-Sahraie, N.; Mineva, T.; Li, J.; Jia, Q.; Stamatin, S.; Harrington, G.F.; Lyth, S.M.; Krtil, P.; Mukerjee, S.; et al. Identification of Catalytic Sites in Cobalt-Nitrogen-Carbon Materials for the Oxygen Reduction Reaction. Nat. Commun. 2017, 8, 957. [Google Scholar] [CrossRef]
- Ratso, S.; Kruusenberg, I.; Käärik, M.; Kook, M.; Saar, R.; Kanninen, P.; Kallio, T.; Leis, J.; Tammeveski, K. Transition Metal-Nitrogen Co-doped Carbide-Derived Carbon Catalysts for Oxygen Reduction Reaction in Alkaline Direct Methanol Fuel Cell. Appl. Catal. B Environ. 2017, 219, 276–286. [Google Scholar] [CrossRef]
- Kaare, K.; Yu, E.; Käämbre, T.; Volperts, A.; Dobele, G.; Zhurinsh, A.; Niaura, G.; TamasauskaiteTamasiunaite, L.; Norkus, E.; Kruusenberg, I. Biomass-Derived Graphene-like Catalyst Material for Oxygen Reduction Reaction. ChemNanoMat 2021, 7, 307–313. [Google Scholar] [CrossRef]
- Sathiskumar, C.; Ramakrishnan, S.; Vinothkannan, M.; Kim, A.R.; Karthikeyan, S.; Yoo, D.J. Nitrogen-Doped Porous Carbon Derived from Biomass Used as Trifunctional Electrocatalyst toward Oxygen Reduction, Oxygen Evolution and Hydrogen Evolution Reactions. Nanomaterials 2020, 10, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zang, W.; Kou, Z.; Pennycook, S.J.; Wang, J. Heterogeneous Single Atom Electrocatalysis, Where “Singles” Are “Married”. Adv. Energy Mater. 2020, 10, 1903181. [Google Scholar] [CrossRef]
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-Atom Catalysis of CO Oxidation Using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef]
- Choi, C.H.; Kim, M.; Kwon, H.C.; Cho, S.J.; Yun, S.; Kim, H.-T.; Mayrhofer, K.J.J.; Kim, H.; Choi, M. Tuning Selectivity of Electrochemical Reactions by Atomically Dispersed Platinum Catalyst. Nat. Commun. 2016, 7, 10922. [Google Scholar] [CrossRef] [Green Version]
- Yin, P.; Yao, T.; Wu, Y.; Zheng, L.; Lin, Y.; Liu, W.; Ju, H.; Zhu, J.; Hong, X.; Deng, Z.; et al. Single Cobalt Atoms with Precise N-Coordination as Superior Oxygen Reduction Reaction Catalysts. Angew. Chem. Int. Ed. 2016, 55, 10800–10805. [Google Scholar] [CrossRef]
- Borghei, M.; Lehtonen, J.; Liu, L.; Rojas, O.J. Advanced Biomass-Derived Electrocatalysts for the Oxygen Reduction Reaction. Adv. Mater. 2018, 30, 1703691. [Google Scholar] [CrossRef]
- Mahbub, M.A.A.; Adios, C.G.; Xu, M.; Prakoso, B.; LeBeau, J.M.; Sumboja, A. Red Bean Pod Derived Heterostructure Carbon Decorated with Hollow Mixed Transition Metals as a Bifunctional Catalyst in Zn-Air Batteries. Chem-Asian J. 2021, 16, 2559–2567. [Google Scholar] [CrossRef]
- Sumboja, A.; Prakoso, B.; Ma, Y.; Irwan, F.R.; Hutani, J.J.; Mulyadewi, A.; Mahbub, M.A.A.; Zong, Y.; Liu, Z. FeCo Nanoparticle-Loaded Nutshell-Derived Porous Carbon as Sustainable Catalyst in Al-Air Batteries. Energy Mater. Adv. 2021, 2021, 7386210. [Google Scholar] [CrossRef]
- Wu, G.; Zelenay, P. Single-Atom Catalysts: A New Frontier in Heterogeneous Catalysis. Acc. Chem. Res. 2013, 46, 1878–1889. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Shui, J.-L.; Chen, C.; Chen, X.; Reprogle, B.M.; Wang, D.; Liu, D.-J. Iron imidazolate Framework as Precursor for Electrocatalysts in Polymer Electrolyte Membrane Fuel Cells. Chem. Sci. 2012, 3, 3200–3205. [Google Scholar] [CrossRef]
- Jiao, L.; Zhang, R.; Wan, G.; Yang, W.; Wan, X.; Zhou, H.; Shui, J.; Yu, S.-H.; Jiang, H.-L. Nanocasting SiO2 into Metal-Organic Frameworks Imparts Dual Protection to High-Loading Fe Single-Atom Electrocatalysts. Nat. Commun. 2020, 11, 2831. [Google Scholar] [CrossRef]
- Martinez, U.; Babu, S.K.; Holby, E.F.; Chung, H.T.; Yin, X.; Zelenay, P. Progress in the Development of Fe-Based PGM-Free Electrocatalysts for the Oxygen Reduction Reaction. Adv. Mater. 2019, 31, 1806545. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Swihart, M.T.; Wu, G. Performance Enhancement and Degradation Mechanism Identification of a Single-Atom Co-N-C Catalyst for Proton Exchange Membrane Fuel Cells. Nat. Catal. 2019, 2, 578–589. [Google Scholar] [CrossRef]
- Chen, G.; Liu, P.; Liao, Z.; Sun, F.; He, Y.; Zhong, H.; Zhang, T.; Zschech, E.; Chen, M.; Wu, G.; et al. Zinc-Mediated Template Synthesis of Fe-N-C Electrocatalysts with Densely Accessible Fe-Nx Active Sites for Efficient Oxygen Reduction. Adv. Mater. 2020, 32, 1907399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, B.-C.; Wu, Z.-Y.; Chu, S.-Q.; Zhu, H.-W.; Liang, H.-W.; Zhang, J.; Yu, S.-H. SiO2-Protected Shell Mediated Templating Synthesis of Fe–N-doped Carbon Nanofibers and Their Enhanced Oxygen Reduction Reaction Performance. Energy Environ. Sci. 2018, 11, 2208–2215. [Google Scholar] [CrossRef]
- Fu, X.; Zamani, P.; Choi, J.-Y.; Hassan, F.M.; Jiang, G.; Higgins, D.C.; Zhang, Y.; Hoque, M.A.; Chen, Z. In Situ Polymer Graphenization Ingrained with Nanoporosity in a Nitrogenous Electrocatalyst Boosting the Performance of Polymer-Electrolyte-Membrane Fuel Cells. Adv. Mater. 2017, 29, 1604456. [Google Scholar] [CrossRef]
- Zhu, C.; Shi, Q.; Xu, B.Z.; Fu, S.; Wan, G.; Yang, C.; Yao, S.; Song, J.; Zhou, H.; Du, D.; et al. Hierarchically Porous M–N–C (M = Co and Fe) Single-Atom Electrocatalysts with Robust MNx Active Moieties Enable Enhanced ORR Performance. Adv. Energy Mater. 2018, 8, 1801956. [Google Scholar] [CrossRef]
- Ye, Y.; Cai, F.; Li, H.; Wu, H.; Wang, G.; Li, Y.; Miao, S.; Xie, S.; Si, R.; Wang, J.; et al. Surface Functionalization of ZIF-8 with Ammonium Ferric Citrate toward High Exposure of Fe-N Active Sites for Efficient Oxygen and Carbon Dioxide Electroreduction. Nano Energy 2017, 38, 281–289. [Google Scholar] [CrossRef]
- Jiang, R.; Li, L.; Sheng, T.; Hu, G.; Chen, Y.; Wang, L. Edge-Site Engineering of Atomically Dispersed Fe-N4 by Selective C-N Bond Cleavage for Enhanced Oxygen Reduction Reaction Activities. J. Am. Chem. Soc. 2018, 140, 11594–11598. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, B.; Zhang, Q.; Liu, Q.; Wang, Z.; Zhao, Y.; Shui, J.; Xiang, Z. Highly Accessible Atomically Dispersed Fe-Nx Sites Electrocatalyst for Proton-Exchange Membrane Fuel Cell. Adv. Sci. 2021, 8, 2002249. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, J.; Chung, D.Y.; Yoo, J.M.; Lee, H.S.; Kim, M.J.; Mun, B.S.; Kwon, S.G.; Sung, Y.-E.; Hyeon, T. Design Principle of Fe–N–C Electrocatalysts: How to Optimize Multimodal Porous Structures? J. Am. Chem. Soc. 2019, 141, 2035–2045. [Google Scholar] [CrossRef]
- Wan, X.; Liu, X.; Li, Y.; Yu, R.; Zheng, L.; Yan, W.; Wang, H.; Xu, M.; Shui, J. Fe–N–C Electrocatalyst with Dense Active Sites and Efficient Mass Transport for High-Performance Proton Exchange Membrane Fuel Cells. Nat. Catal. 2019, 2, 259–268. [Google Scholar] [CrossRef]
- Ghosh, S.; Basu, R.N. Multifunctional Nanostructured Electrocatalysts for Energy Conversion and Storage: Current Status and Perspectives. Nanoscale 2018, 10, 11241–11280. [Google Scholar] [CrossRef]
- Wang, H.-F.; Chen, L.; Pang, H.; Kaskel, S.; Xu, Q. MOF-Derived Electrocatalysts for Oxygen Reduction, Oxygen Evolution and Hydrogen Evolution Reactions. Chem. Soc. Rev. 2020, 49, 1414–1448. [Google Scholar] [CrossRef]
- Cai, X.; Lai, L.; Lin, J.; Shen, Z. Correction: Recent Advances in Air Electrodes for Zn–air Batteries: Electrocatalysis and Structural Design. Mater. Horiz. 2017, 4, 945–976. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, K.; Tang, D.; Liu, W.; Meng, F.; Huang, Q.; Liu, J. Recent Progress in Electrolytes for Zn–Air Batteries. Front. Chem. 2020, 8, 372. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Liang, R.; Liu, G.; Yu, A.; Bai, Z.; Yang, L.; Chen, Z. Recent Progress in Electrically Rechargeable Zinc–Air Batteries. Adv. Mater. 2019, 31, 1805230. [Google Scholar] [CrossRef]
- Sa, Y.J.; Seo, D.-J.; Woo, J.; Lim, J.T.; Cheon, J.Y.; Yang, S.Y.; Lee, J.M.; Kang, D.; Shin, T.J.; Shin, H.S.; et al. A General Approach to Preferential Formation of Active Fe–Nx Sites in Fe–N/C Electrocatalysts for Efficient Oxygen Reduction Reaction. J. Am. Chem. Soc. 2016, 138, 15046–15056. [Google Scholar] [CrossRef]
- Malonzo, C.D.; Shaker, S.M.; Ren, L.; Prinslow, S.D.; Platero-Prats, A.E.; Gallington, L.C.; Borycz, J.; Thompson, A.B.; Wang, T.C.; Farha, O.K.; et al. Thermal Stabilization of Metal–Organic Framework-Derived Single-Site Catalytic Clusters through Nanocasting. J. Am. Chem. Soc. 2016, 138, 2739–2748. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.-W.; Zhuang, X.; Brüller, S.; Feng, X.; Müllen, K. Hierarchically Porous Carbons with Optimized Nitrogen Doping as Highly Active Electrocatalysts for Oxygen Reduction. Nat. Commun. 2014, 5, 4973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaouen, F.; Lefèvre, M.; Dodelet, J.-P.; Cai, M. Heat-Treated Fe/N/C Catalysts for O2 Electroreduction: Are Active Sites Hosted in Micropores? J. Phys. Chem. B 2006, 110, 5553–5558. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Z.; Qu, Y.; Yuan, T.; Wang, W.; Wu, Y.; Li, Y. Review of Metal Catalysts for Oxygen Reduction Reaction: From Nanoscale Engineering to Atomic Design. Chem 2019, 5, 1486–1511. [Google Scholar] [CrossRef]
- Zhang, H.; Hwang, S.; Wang, M.; Feng, Z.; Karakalos, S.; Luo, L.; Qiao, Z.; Xie, X.; Wang, C.; Su, D.; et al. Single Atomic Iron Catalysts for Oxygen Reduction in Acidic Media: Particle Size Control and Thermal Activation. J. Am. Chem. Soc. 2017, 139, 14143–14149. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhu, Q.; Xu, A.-W. Noble-Metal-Free Fe–N/C Catalyst for Highly Efficient Oxygen Reduction Reaction under Both Alkaline and Acidic Conditions. J. Am. Chem. Soc. 2014, 136, 11027–11033. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Xu, Z.; Peng, T.; Liu, M.; Zhang, L.; Zhang, J. Bifunctional Single-Atom Cobalt Electrocatalysts with Dense Active Sites Prepared via a Silica Xerogel Strategy for Rechargeable Zinc–Air Batteries. Nanomaterials 2022, 12, 381. https://doi.org/10.3390/nano12030381
Wang L, Xu Z, Peng T, Liu M, Zhang L, Zhang J. Bifunctional Single-Atom Cobalt Electrocatalysts with Dense Active Sites Prepared via a Silica Xerogel Strategy for Rechargeable Zinc–Air Batteries. Nanomaterials. 2022; 12(3):381. https://doi.org/10.3390/nano12030381
Chicago/Turabian StyleWang, Lijuan, Zixiang Xu, Tingyu Peng, Maosong Liu, Long Zhang, and Jianming Zhang. 2022. "Bifunctional Single-Atom Cobalt Electrocatalysts with Dense Active Sites Prepared via a Silica Xerogel Strategy for Rechargeable Zinc–Air Batteries" Nanomaterials 12, no. 3: 381. https://doi.org/10.3390/nano12030381
APA StyleWang, L., Xu, Z., Peng, T., Liu, M., Zhang, L., & Zhang, J. (2022). Bifunctional Single-Atom Cobalt Electrocatalysts with Dense Active Sites Prepared via a Silica Xerogel Strategy for Rechargeable Zinc–Air Batteries. Nanomaterials, 12(3), 381. https://doi.org/10.3390/nano12030381






