Responsive Liquid Metal Droplets: From Bulk to Nano
Abstract
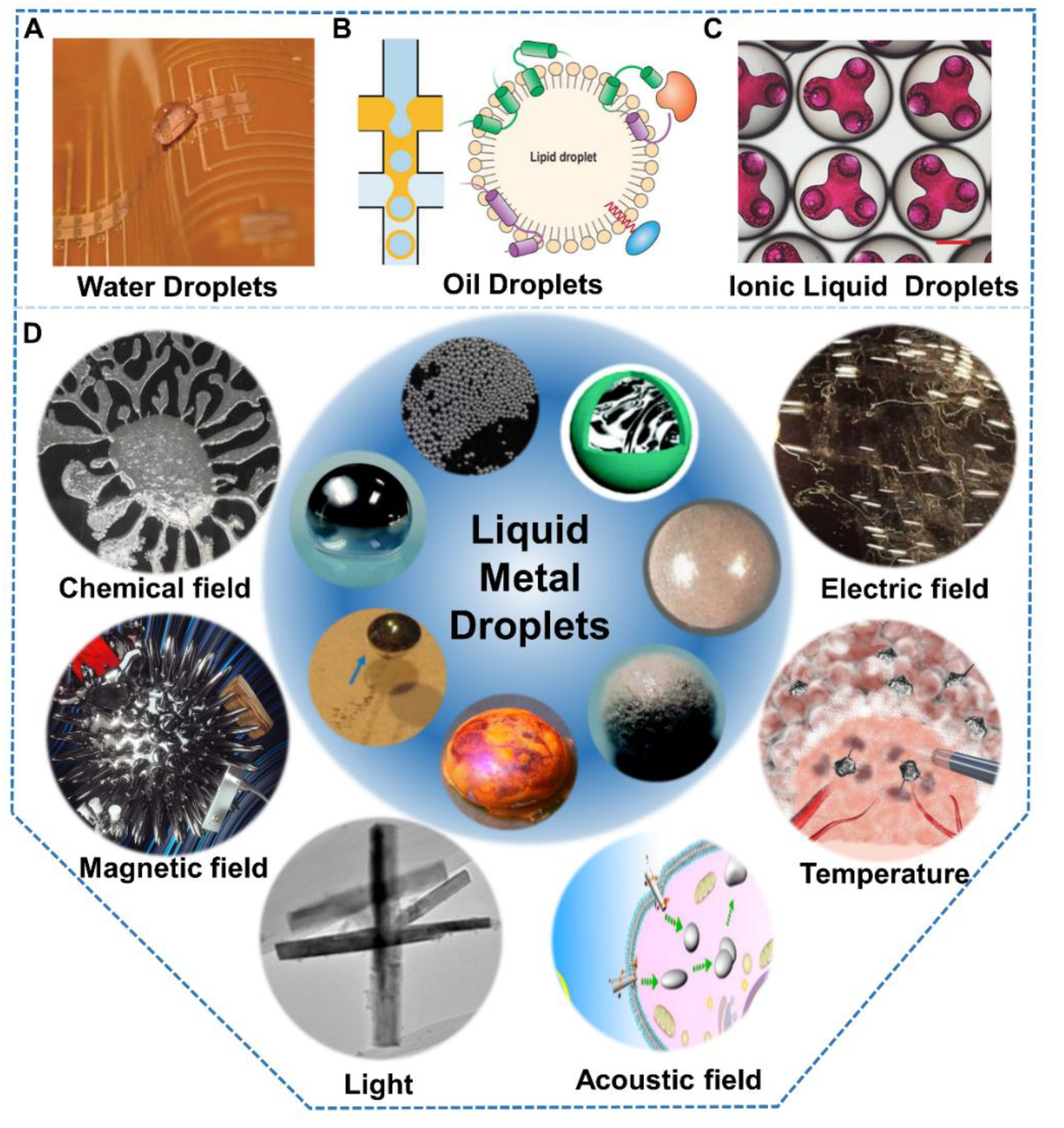
:1. Introduction
2. Liquid Metal Droplets
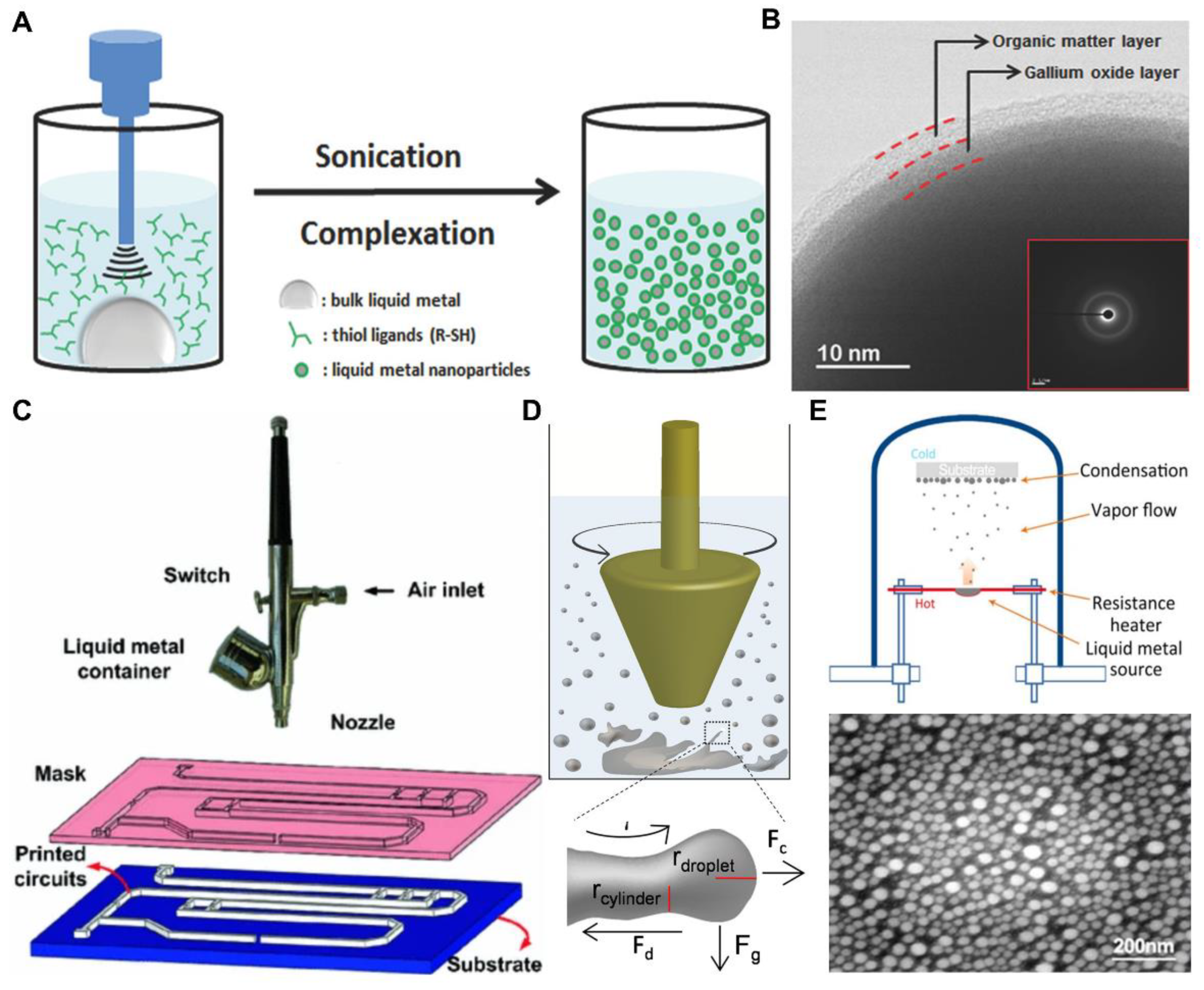
2.1. Fabrication of Pure Liquid Metal Droplets
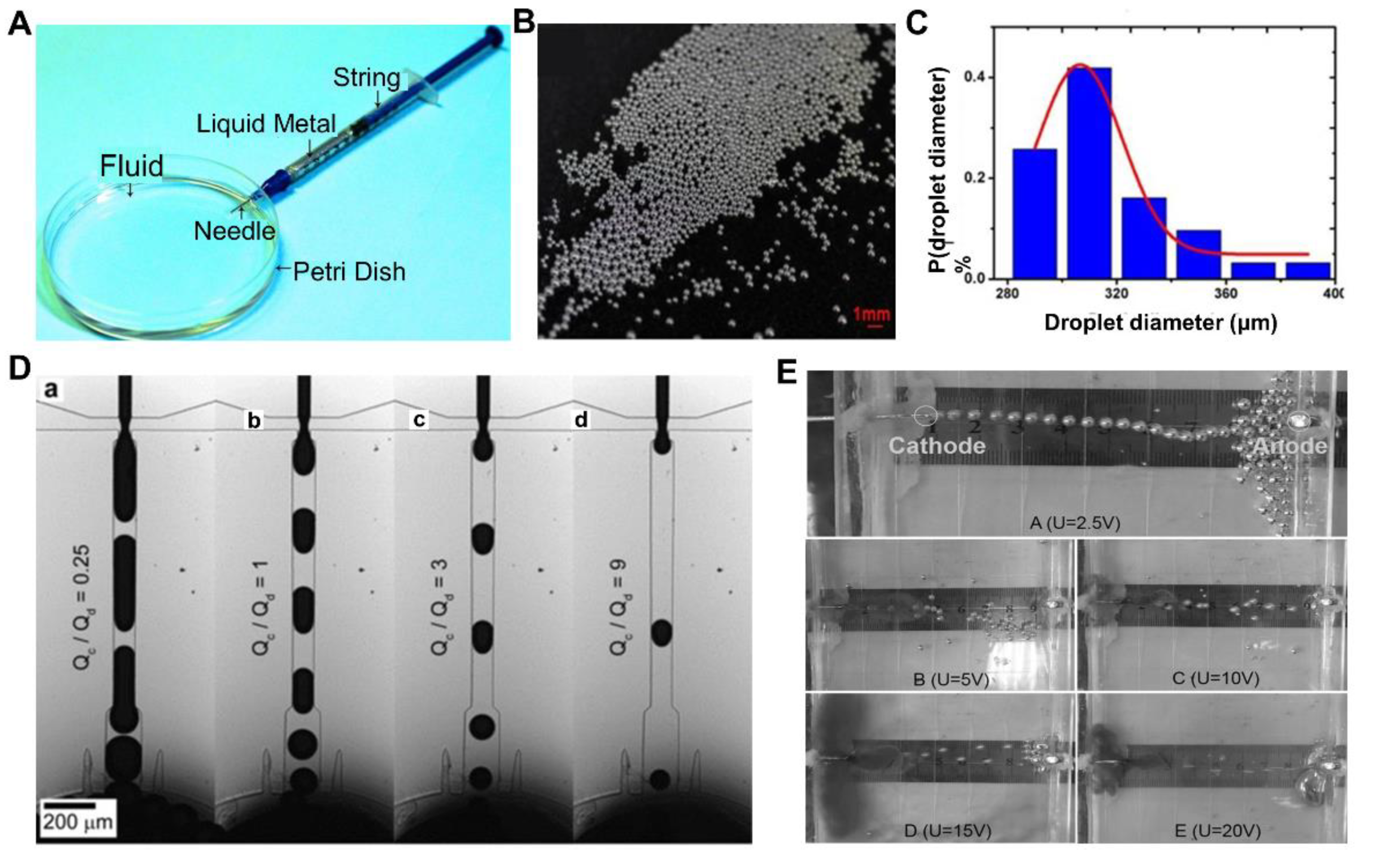
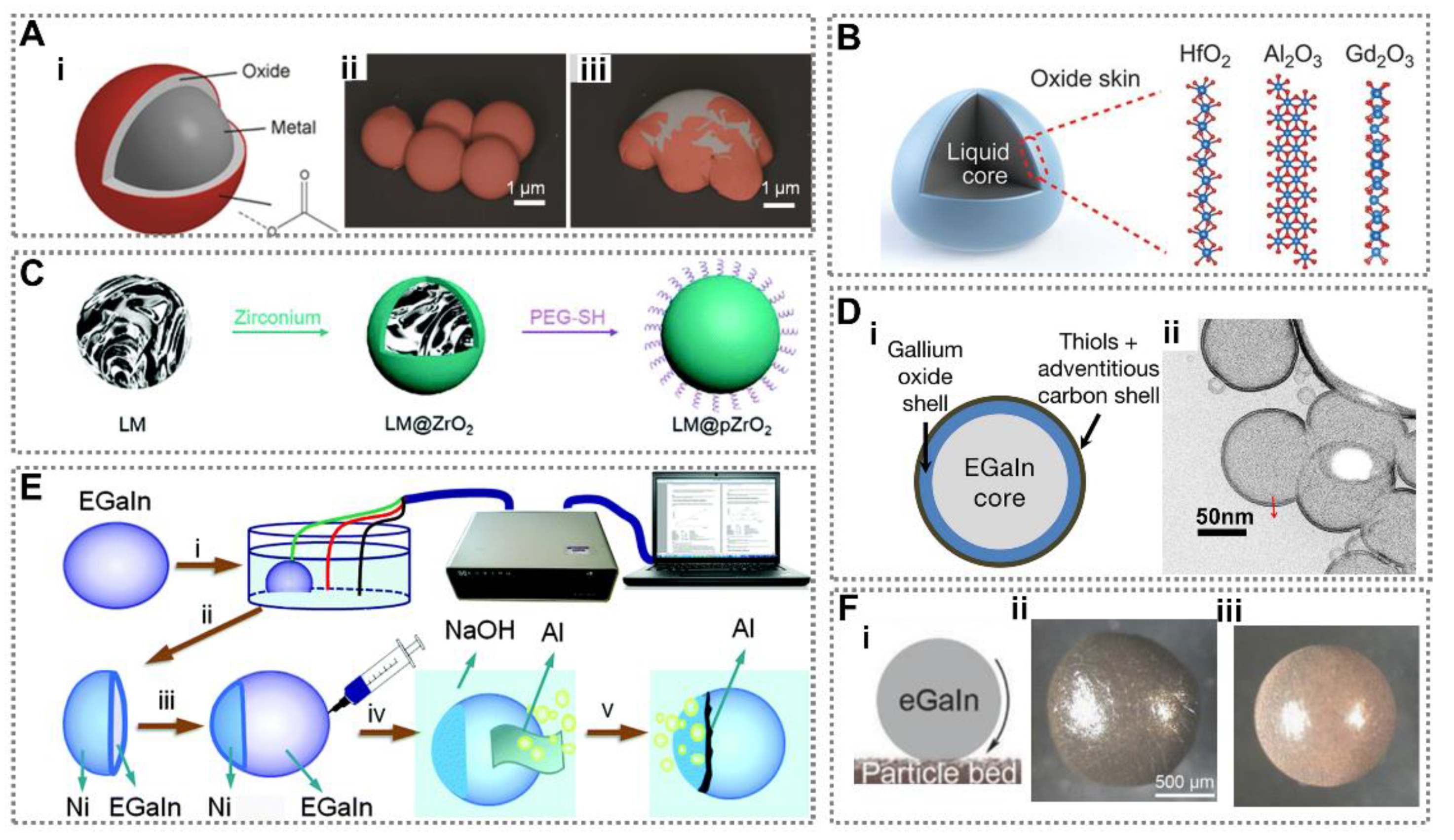
2.2. Liquid Metal Droplets with Core-Shell Structure
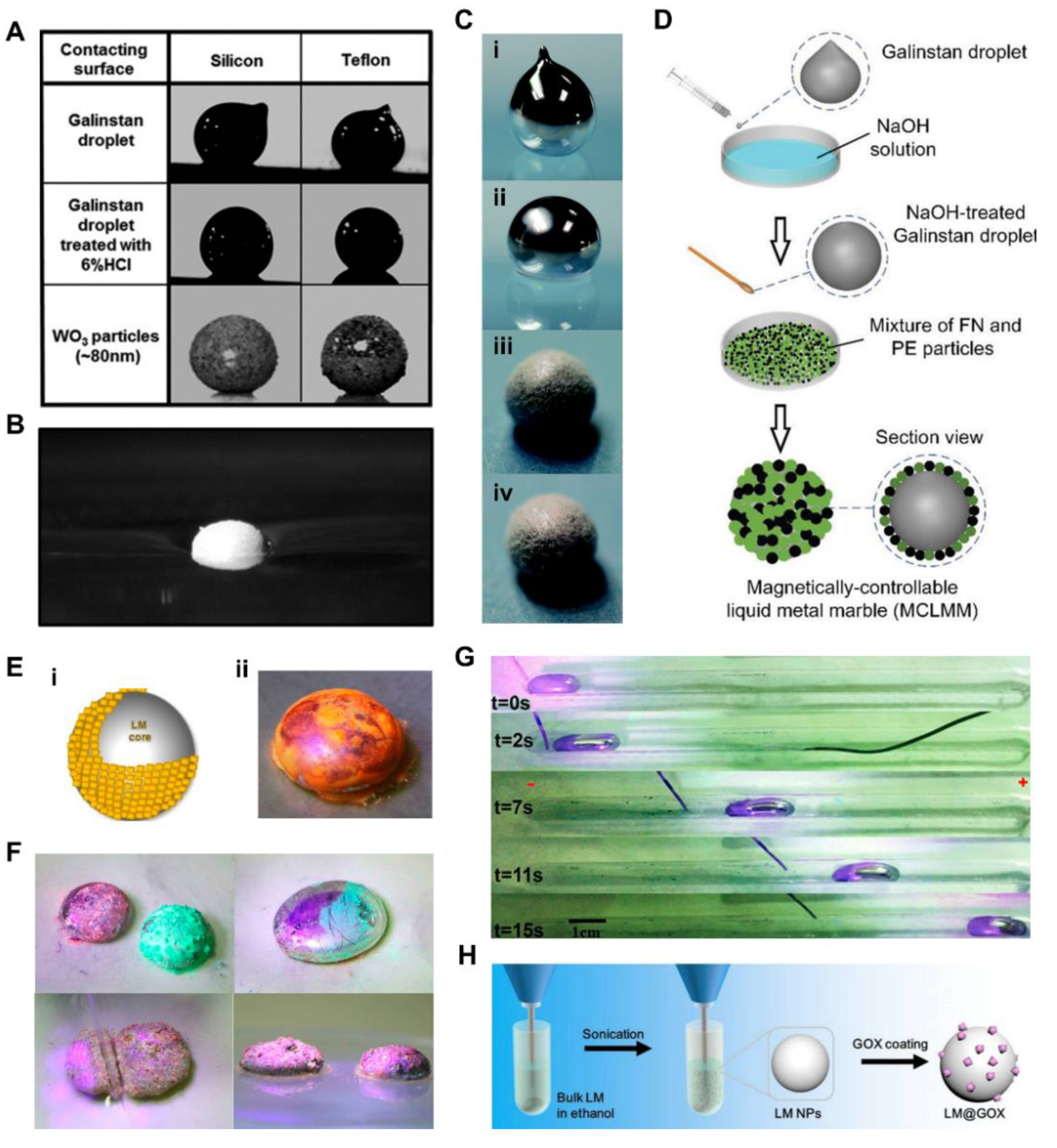
2.3. Liquid Metal Marbles
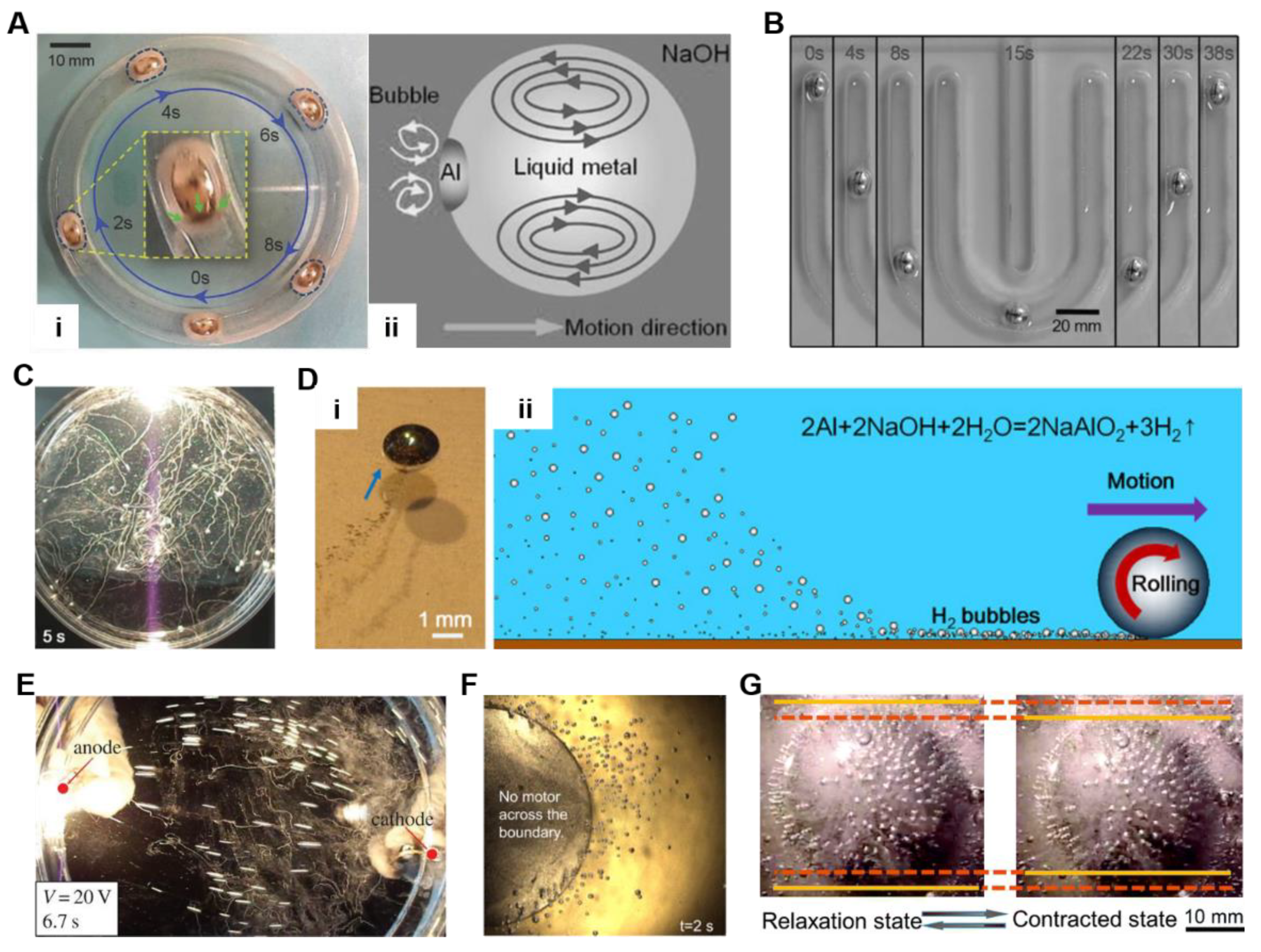
2.4. Self-Powered Liquid Metal Droplet Motors
3. Response Behaviors of Liquid Metal Droplets
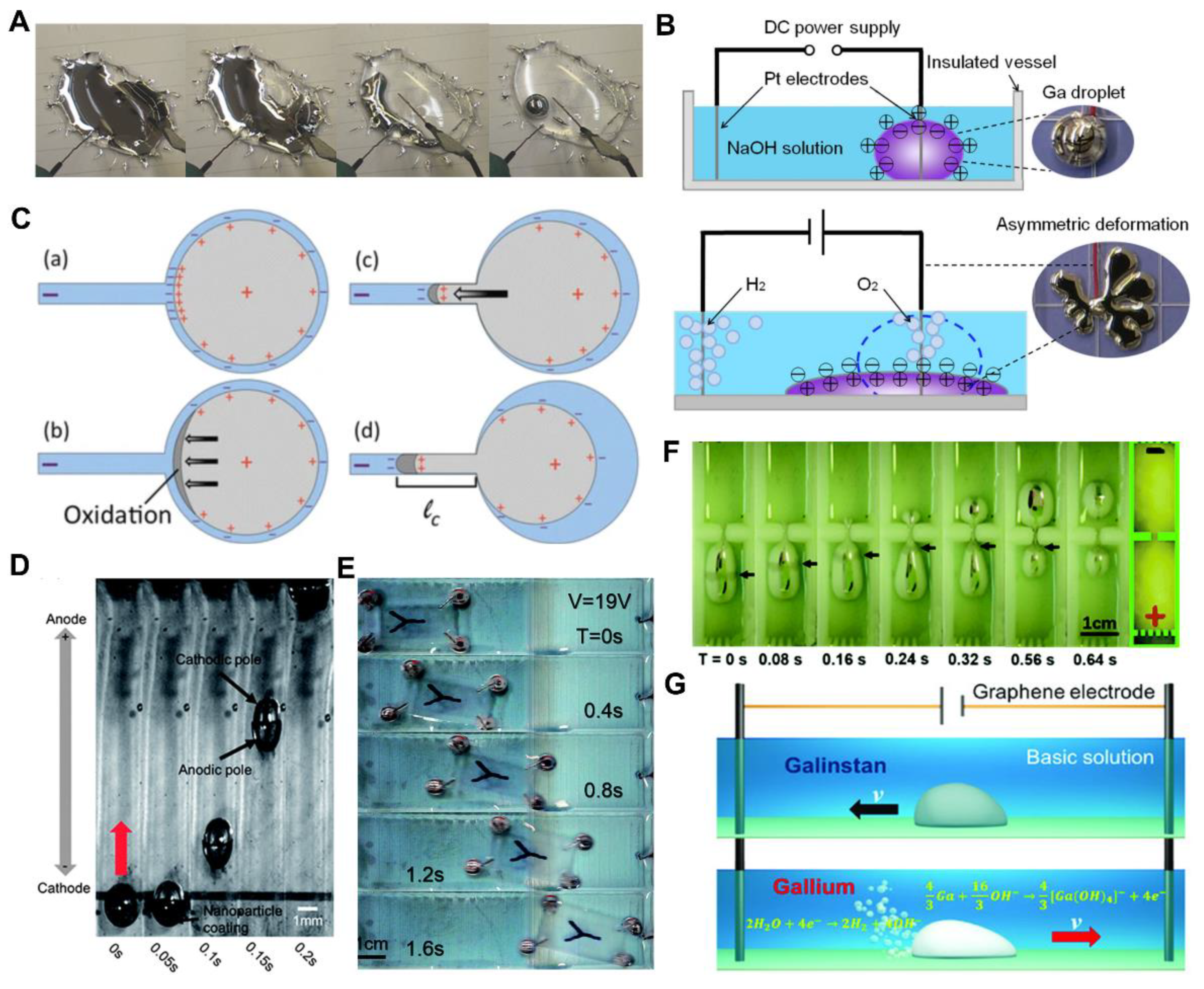
3.1. Chemically Induced Response
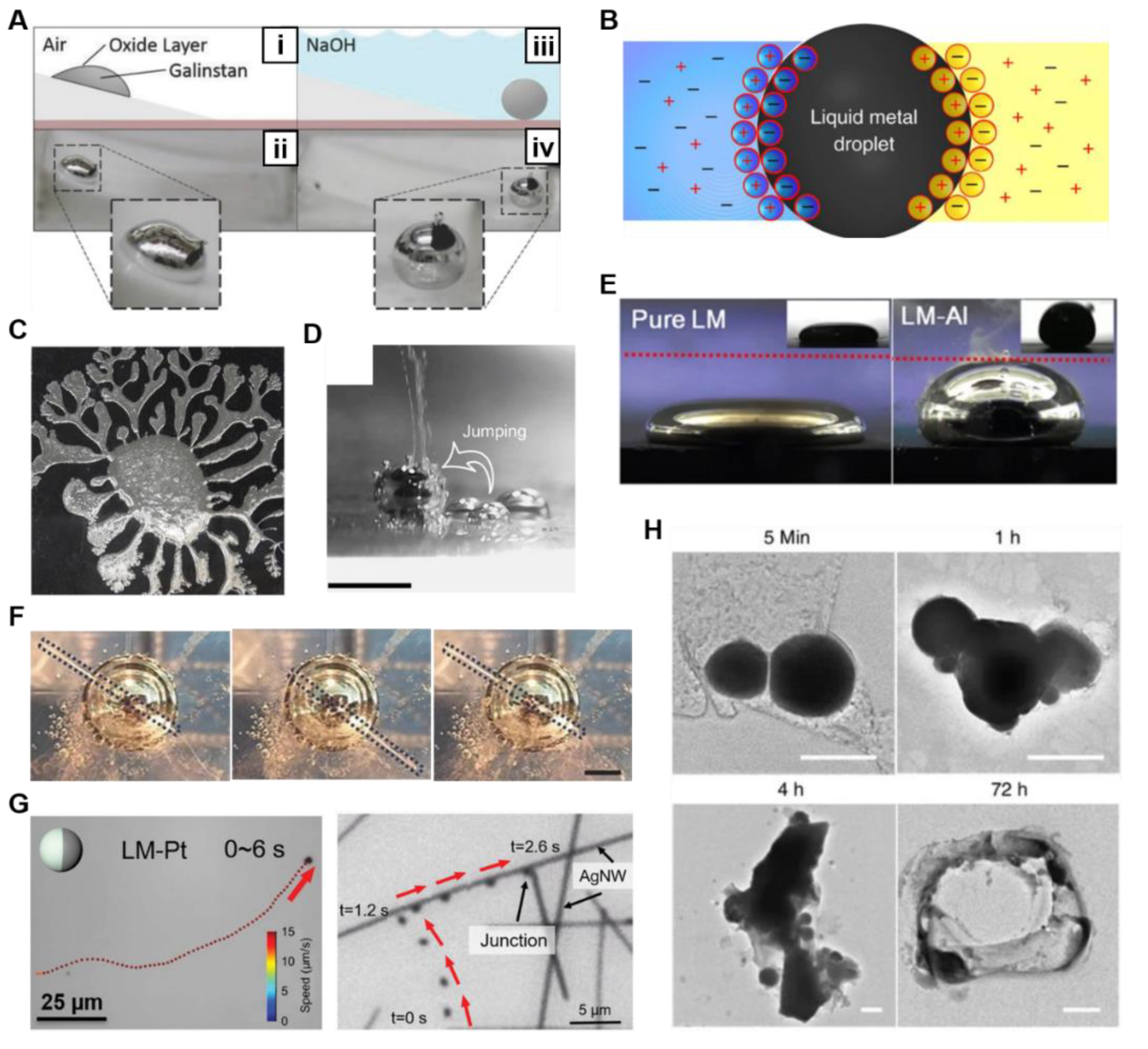
3.2. Electrically Induced Response
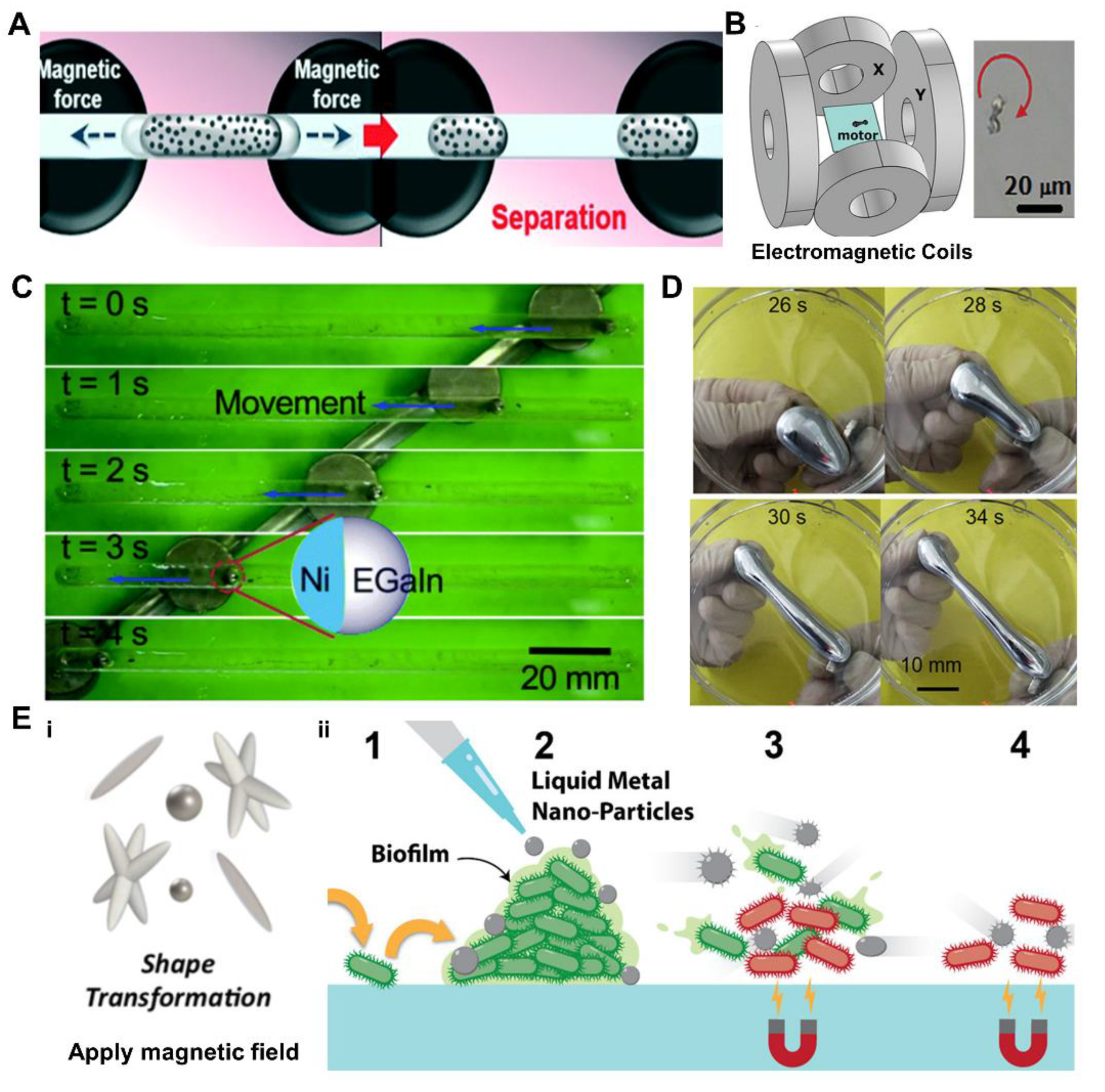
3.3. Magnetic Field Induced Response
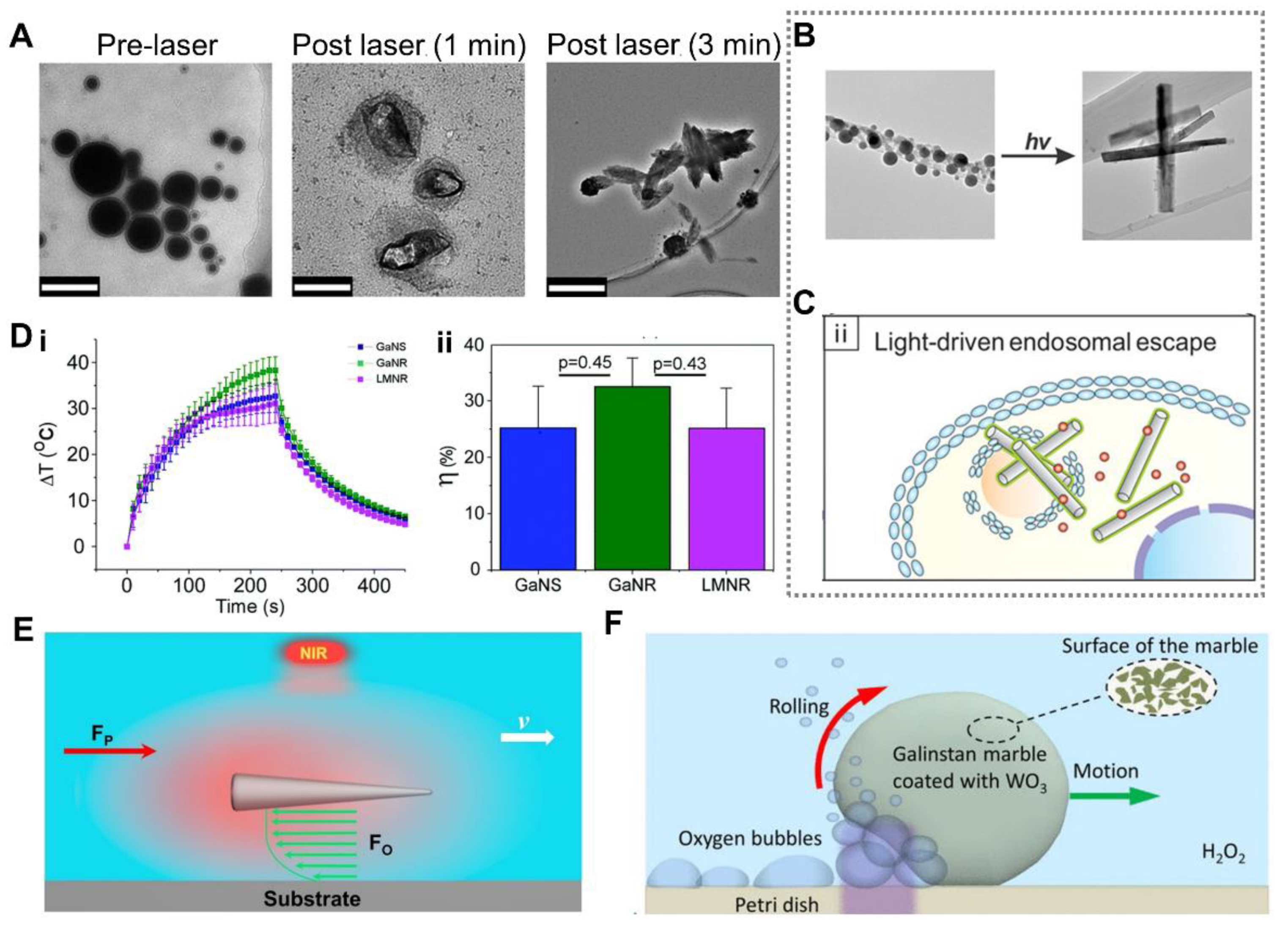
3.4. Optically Induced Response
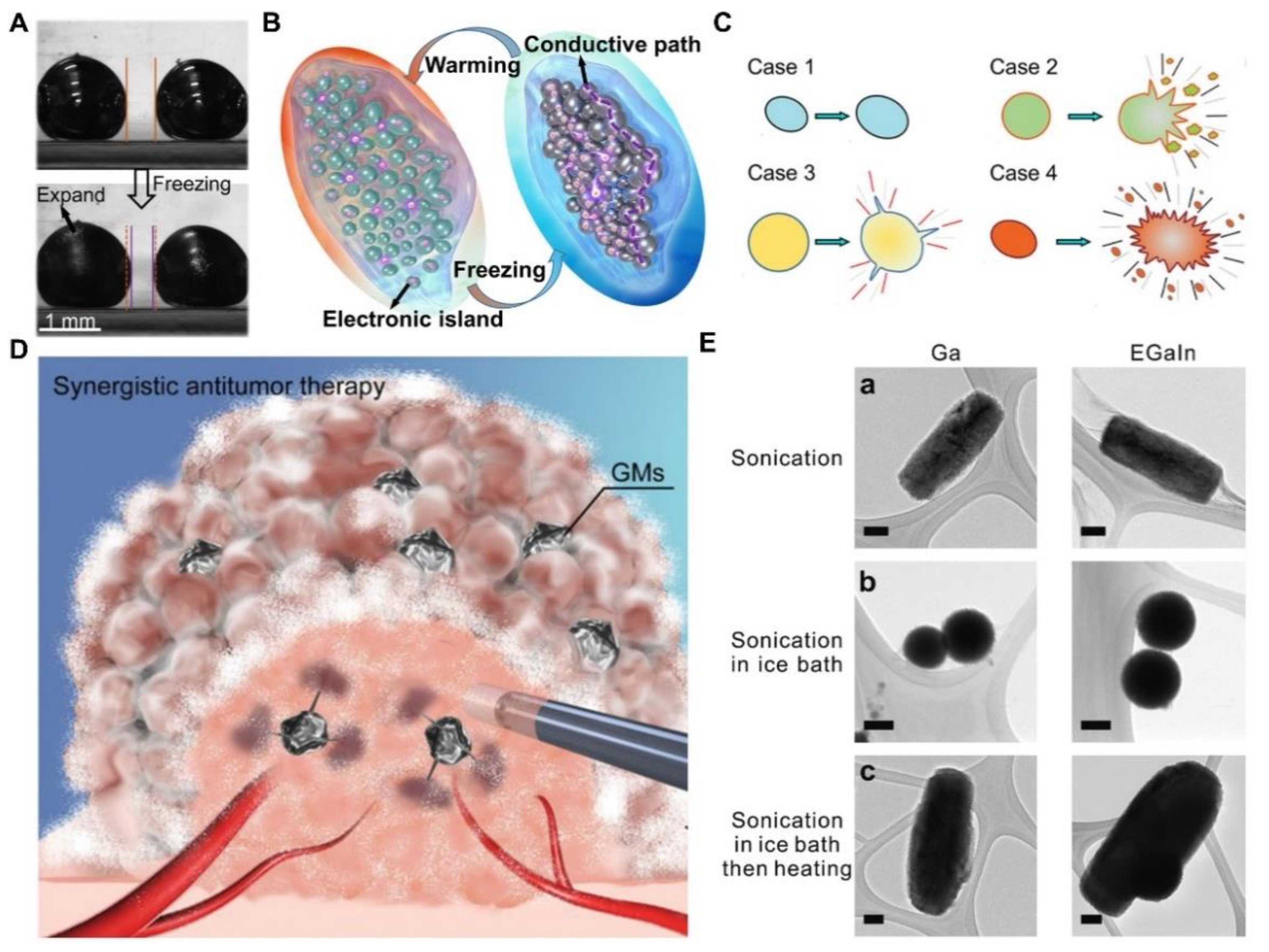
3.5. Temperature Induced Response
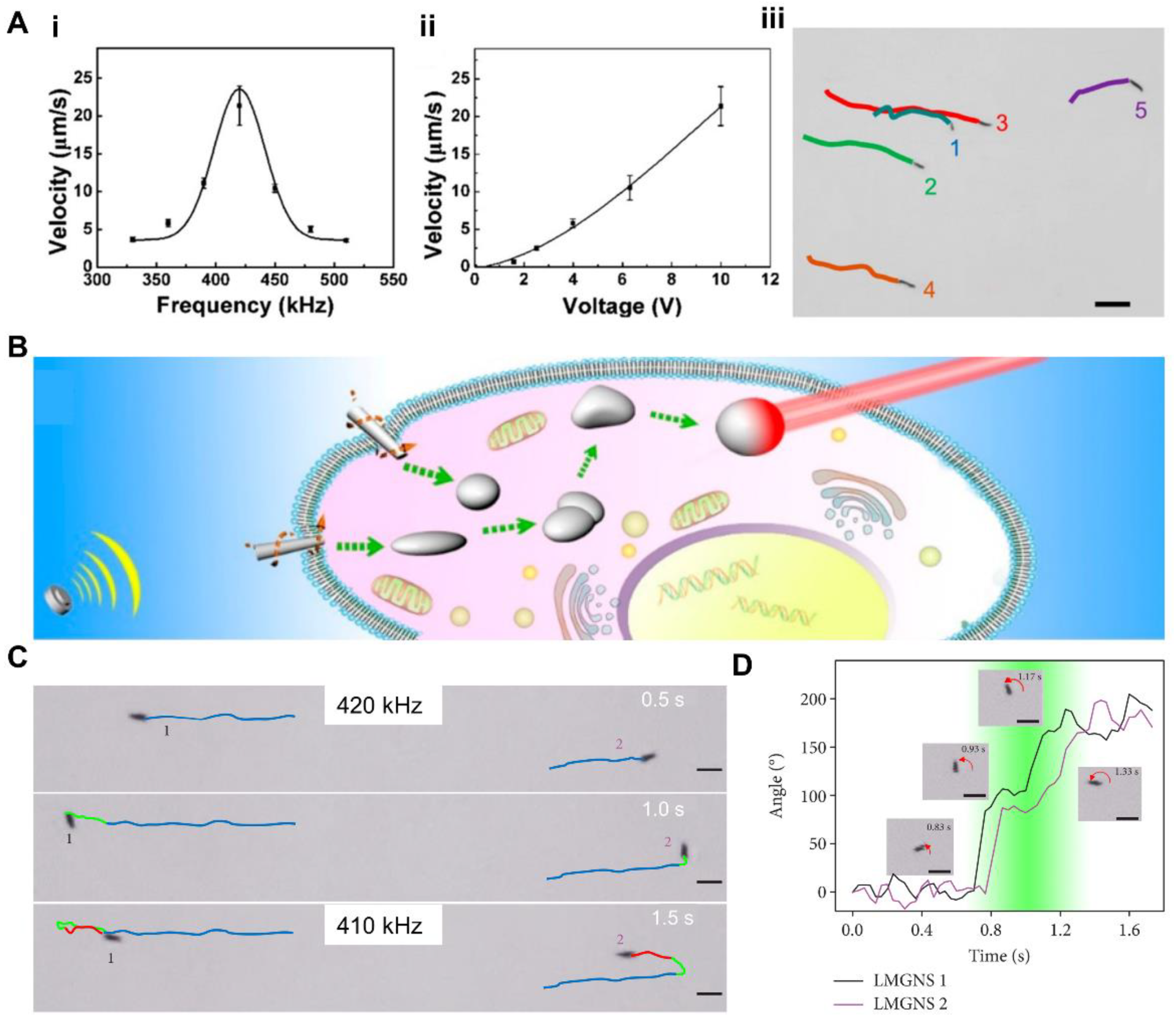
3.6. Ultrasound Induced Response
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rekvig, L.; Frenkel, D. Molecular Simulations of Droplet Coalescence in Oil/Water/Surfactant Systems. J. Chem. Phys. 2007, 127, 134701. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, Q.; Yi, L.; Liu, J. Channelless Fabrication for Large-Scale Preparation of Room Temperature Liquid Metal Droplets. Adv. Eng. Mater. 2014, 16, 255–262. [Google Scholar] [CrossRef]
- Boulet, P.; Tissot, J.; Trinquet, F.; Fournaison, L. Enhancement of Heat Exchanges on a Condenser Using an Air Flow Containing Water Droplets. Appl. Therm. Eng. 2013, 50, 1164–1173. [Google Scholar] [CrossRef]
- Shin, G.; Yong, H.; Chung, J.; Cho, E.; Ju, J.H.; Lin, Z.H.; Kim, D.; Lee, H.; Koo, B.; Lee, S. Condensed Droplet-Based Electricity Generation Via Water-Phase Change. Nano Energy 2021, 82, 105713. [Google Scholar] [CrossRef]
- Liu, Y.C.; Xu, Y.; Avedisian, C.T.; Hicks, M.C. The Effect of Support Fibers on Micro-Convection in Droplet Combustion Experiments. Proc. Combust. Inst. 2015, 35, 1709–1716. [Google Scholar] [CrossRef]
- Jokinen, V.; Kostiainen, R.; Sikanen, T. Multiphase Designer Droplets for Liquid-Liquid Extraction. Adv. Mater. 2012, 24, 6240–6243. [Google Scholar] [CrossRef]
- Asakawa, D.; Hiraoka, K. Study on the Redox Reactions for Organic Dyes and S-Nitrosylated Peptide in Electrospray Droplet Impact. J. Mass Spectrom. 2009, 44, 461–465. [Google Scholar] [CrossRef]
- Ghojel, J.; Honnery, D.; Al-Khaleefi, K. Performance, Emissions and Heat Release Characteristics of Direct Injection Diesel Engine Operating on Diesel Oil Emulsion. Appl. Therm. Eng. 2006, 26, 2132–2141. [Google Scholar] [CrossRef]
- Christopher, G.F.; Anna, S.L. Microfluidic Methods for Generating Continuous Droplet Streams. J. Phys. D Appl. Phys. 2007, 40, R319–R336. [Google Scholar] [CrossRef]
- Xu, S.; Yuan, B.; Hou, Y.; Liu, T.; Fu, J.; Liu, J. Self-Fueled Liquid Metal Motors. J. Phys. D Appl. Phys. 2019, 52, 353002. [Google Scholar] [CrossRef]
- Martin, S.; Parton, R.G. Lipid Droplets: A Unified View of a Dynamic Organelle. Nat. Rev. Mol. Cell Biol. 2006, 7, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zheng, H.; Liu, Y.; Zhou, X.; Zhang, C.; Song, Y.; Deng, X.; Leung, M.; Yang, Z.; Xu, R.X.; et al. A Droplet-Based Electricity Generator with High Instantaneous Power Density. Nature 2020, 578, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ning, X.; Li, L.; Wang, X.; Li, B.; Li, J.; Yin, J.; Guo, W. Performance and Power Management of Droplets-Based Electricity Generators. Nano Energy 2022, 92, 106705. [Google Scholar] [CrossRef]
- Wu, H.; Mendel, N.; van den Ende, D.; Zhou, G.; Mugele, F. Energy Harvesting from Drops Impacting onto Charged Surfaces. Phys. Rev. Lett. 2020, 125, 078301. [Google Scholar] [CrossRef]
- Feng, W.; Ueda, E.; Levkin, P.A. Droplet Microarrays: From Surface Patterning to High-Throughput Applications. Adv. Mater. 2018, 30, e1706111. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, P. The New Face of the Lipid Droplet: Lipid Droplet Proteins. Proteomics 2019, 19, e1700223. [Google Scholar] [CrossRef]
- Dubois, P.; Marchand, G.; Fouillet, Y.; Berthier, J.; Douki, T.; Hassine, F.; Gmouh, S.; Vaultier, M. Ionic Liquid Droplet as E-Microreactor. Anal. Chem. 2006, 78, 4909–4917. [Google Scholar] [CrossRef]
- Barikbin, Z.; Rahman, M.T.; Parthiban, P.; Rane, A.S.; Jain, V.; Duraiswamy, S.; Lee, S.S.; Khan, S.A. Ionic Liquid-Based Compound Droplet Microfluidics for ‘on-Drop’ Separations and Sensing. Lab Chip 2010, 10, 2458–2463. [Google Scholar] [CrossRef]
- Feng, K.; Gao, N.; Li, W.; Dong, H.; Sun, F.; He, G.; Zhou, K.; Zhao, H.; Li, G. Arrested Coalescence of Ionic Liquid Droplets: A Facile Strategy for Spatially Organized Multicompartment Assemblies. Small 2021, 17, e2104385. [Google Scholar] [CrossRef]
- Guo, M.T.; Rotem, A.; Heyman, J.A.; Weitz, D.A. Droplet Microfluidics for High-Throughput Biological Assays. Lab Chip 2012, 12, 2146–2155. [Google Scholar] [CrossRef]
- Zhu, Z.; Yang, C.J. Hydrogel Droplet Microfluidics for High-Throughput Single Molecule/Cell Analysis. Acc. Chem. Res. 2017, 50, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, M.; Wheeler, A.R. The Digital Revolution: A New Paradigm for Microfluidics. Adv. Mater. 2009, 21, 920–925. [Google Scholar] [CrossRef]
- Wang, J.; Hahn, S.; Amstad, E.; Vogel, N. Tailored Double Emulsions Made Simple. Adv. Mater. 2022, 34, e2107338. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Li, N.; Rao, W.; Yu, J.; Wu, Q.; Tan, L.; Li, H.; Gou, L.; Liang, P.; Li, L.; et al. Multifunctional and Flexible Zro2-Coated Egain Nanoparticles for Photothermal Therapy. Nanoscale 2019, 11, 10183–10189. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhao, X.; Li, J.; Liu, J. Thin, Porous, and Conductive Networks of Metal Nanoparticles through Electrochemical Welding on a Liquid Metal Template. Adv. Mater. Interfaces 2018, 5, 1800406. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Z.; Zhu, D.; Handschuh-Wang, S.; Liang, S.; Yang, J.; Kong, T.; Zhou, X.; Liu, Y.; Zhou, X. Liquid Metal Droplets with High Elasticity, Mobility and Mechanical Robustness. Mater. Horiz. 2017, 4, 591–597. [Google Scholar] [CrossRef]
- Liang, S.; Rao, W.; Song, K.; Liu, J. Fluorescent Liquid Metal as a Transformable Biomimetic Chameleon. ACS Appl. Mater. Interfaces 2018, 10, 1589–1596. [Google Scholar] [CrossRef]
- Yuan, B.; Tan, S.; Zhou, Y.; Liu, J. Self-Powered Macroscopic Brownian Motion of Spontaneously Running Liquid Metal Motors. Sci. Bull. 2015, 60, 1203–1210. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Wang, L.; Zhang, Q.; Liu, J. Liquid Metal Fractals Induced by Synergistic Oxidation. Sci. Bull. 2018, 63, 1513–1520. [Google Scholar] [CrossRef]
- Tan, S.C.; Yuan, B.; Liu, J. Electrical Method to Control the Running Direction and Speed of Self-Powered Tiny Liquid Metal Motors. Proc. R. Soc. A Math. Phys. Eng. Sci. 2015, 471, 20150297. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Lin, Y.; Chen, Z.; Hu, Q.; Liu, Y.; Yu, S.; Gao, W.; Dickey, M.D.; Gu, Z. Enhanced Endosomal Escape by Light-Fueled Liquid-Metal Transformer. Nano Lett. 2017, 17, 2138–2145. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Cui, B.; Yuan, B.; Wang, X.; Fan, L.; Yu, D.; He, Z.; Sheng, L.; Liu, J.; Lu, J. Liquid Metal Microparticles Phase Change Medicated Mechanical Destruction for Enhanced Tumor Cryoablation and Dual-Mode Imaging. Adv. Funct. Mater. 2020, 30, 2003359. [Google Scholar] [CrossRef]
- Wang, D.; Gao, C.; Wang, W.; Sun, M.; Guo, B.; Xie, H.; He, Q. Shape-Transformable, Fusible Rodlike Swimming Liquid Metal Nanomachine. ACS Nano 2018, 12, 10212–10220. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, S.; Li, H.; Chen, X.; Cheng, J.; Shao, Y.; Zhang, C.; Zhang, J.; Fan, L.; Chang, H.; et al. A Liquid Gripper Based on Phase Transitional Metallic Ferrofluid. Adv. Funct. Mater. 2021, 31, 2100274. [Google Scholar] [CrossRef]
- Ren, L.; Xu, X.; Du, Y.; Kalantar-Zadeh, K.; Dou, S.X. Liquid Metals and Their Hybrids as Stimulus–Responsive Smart Materials. Mater. Today 2020, 34, 92–114. [Google Scholar] [CrossRef]
- Cao, L.; Yu, D.; Xia, Z.; Wan, H.; Liu, C.; Yin, T.; He, Z. Ferromagnetic Liquid Metal Putty-Like Material with Transformed Shape and Reconfigurable Polarity. Adv. Mater. 2020, 32, 2000827. [Google Scholar] [CrossRef]
- Ding, Y.R.; Zeng, M.Q.; Fu, L. Surface Chemistry of Gallium-Based Liquid Metals. Matter 2020, 3, 1477–1506. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Tang, J.; Daeneke, T.; O’Mullane, A.P.; Stewart, L.A.; Liu, J.; Majidi, C.; Ruoff, R.S.; Weiss, P.S.; Dickey, M.D. Emergence of Liquid Metals in Nanotechnology. ACS Nano 2019, 13, 7388–7395. [Google Scholar] [CrossRef]
- Chen, S.; Wang, H.-Z.; Zhao, R.Q.; Rao, W.; Liu, J. Liquid Metal Composites. Matter 2020, 2, 1446–1480. [Google Scholar] [CrossRef]
- Xu, Q.; Oudalov, N.; Guo, Q.T.; Jaeger, H.M.; Brown, E. Effect of Oxidation on the Mechanical Properties of Liquid Gallium and Eutectic Gallium-Indium. Phys. Fluids 2012, 24, 063101. [Google Scholar] [CrossRef]
- Dong, J.; Zhu, Y.Y.; Liu, Z.F.; Wang, M. Liquid Metal-Based Devices: Material Properties, Fabrication and Functionalities. Nanomaterials 2021, 11, 3400. [Google Scholar] [CrossRef] [PubMed]
- Veerapandian, S.; Jang, W.; Seol, J.B.; Wang, H.B.; Kong, M.; Thiyagarajan, K.; Kwak, J.; Park, G.; Lee, G.; Suh, W.; et al. Hydrogen-Doped Viscoplastic Liquid Metal Microparticles for Stretchable Printed Metal Lines. Nat. Mater. 2021, 20, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Fassler, A.; Majidi, C. Liquid-Phase Metal Inclusions for a Conductive Polymer Composite. Adv. Mater. 2015, 27, 1928–1932. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Ou, M.; Huang, K.; Chu, S. Multilayer Graphite-Ga68.5in21.5sn10 Composites as Highly Thermal Conductive and Low-Cost Material. Energy Technol. 2020, 8, 2000240. [Google Scholar] [CrossRef]
- Ji, Y.; Yan, H.; Xiao, X.; Xu, J.; Li, Y.; Chang, C. Excellent Thermal Performance of Gallium-Based Liquid Metal Alloy as Thermal Interface Material between Aluminum Substrates. Appl. Therm. Eng. 2020, 166, 114649. [Google Scholar] [CrossRef]
- Wei, S.; Yu, Z.F.; Zhou, L.J.; Guo, J.D. Investigation on Enhancing the Thermal Conductance of Gallium-Based Thermal Interface Materials Using Chromium-Coated Diamond Particles. J. Mater. Sci. Mater. Electron. 2019, 30, 7194–7202. [Google Scholar] [CrossRef]
- Yan, J.; Lu, Y.; Chen, G.; Yang, M.; Gu, Z. Advances in Liquid Metals for Biomedical Applications. Chem. Soc. Rev. 2018, 47, 2518–2533. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, S.; So, J.H.; Kim, K.; Koo, H.J. Cytotoxicity of Gallium-Indium Liquid Metal in an Aqueous Environment. ACS Appl. Mater. Interfaces 2018, 10, 17448–17454. [Google Scholar] [CrossRef]
- Foremny, K.; Nagels, S.; Kreienmeyer, M.; Doll, T.; Deferme, W. Biocompatibility Testing of Liquid Metal as an Interconnection Material for Flexible Implant Technology. Nanomaterials 2021, 11, 3251. [Google Scholar] [CrossRef]
- Chen, S.; Liu, J. Pervasive Liquid Metal Printed Electronics: From Concept Incubation to Industry. iScience 2021, 24, 102026. [Google Scholar] [CrossRef]
- Zheng, R.M.; Peng, Z.F.; Fu, Y.; Deng, Z.F.; Liu, S.Q.; Xing, S.T.; Wu, Y.Y.; Li, J.Y.; Liu, L. A Novel Conductive Core-Shell Particle Based on Liquid Metal for Fabricating Real-Time Self-Repairing Flexible Circuits. Adv. Funct. Mater. 2020, 30, 1910524. [Google Scholar] [CrossRef]
- Li, Q.; Lin, J.; Liu, T.Y.; Zhu, X.Y.; Yao, W.-H.; Liu, J. Gas-Mediated Liquid Metal Printing toward Large-Scale 2d Semiconductors and Ultraviolet Photodetector. Npj 2D Mater. Appl. 2021, 5, 36. [Google Scholar] [CrossRef]
- Sun, X.; Yuan, B.; Wang, H.; Fan, L.; Duan, M.; Wang, X.; Guo, R.; Liu, J. Nano-Biomedicine Based on Liquid Metal Particles and Allied Materials. Adv. NanoBiomed Res. 2021, 1, 2000086. [Google Scholar] [CrossRef]
- Fan, L.; Duan, M.; Xie, Z.; Pan, K.; Wang, X.; Sun, X.; Wang, Q.; Rao, W.; Liu, J. Injectable and Radiopaque Liquid Metal/Calcium Alginate Hydrogels for Endovascular Embolization and Tumor Embolotherapy. Small 2020, 16, e1903421. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, X.; Liu, Y.; Ye, Y.; Yu, J.; Chen, Q.; Wang, J.; Zhang, Y.; Hu, Q.; Kang, Y.; et al. Shape-Controlled Synthesis of Liquid Metal Nanodroplets for Photothermal Therapy. Nano Res. 2019, 12, 1313–1320. [Google Scholar] [CrossRef]
- Chen, S.; Deng, Z.; Liu, J. High Performance Liquid Metal Thermal Interface Materials. Nanotechnology 2021, 32, 092001. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, H.; Chen, X.; Chu, S.; Liu, H.; Lin, Z.; Li, Q.; Chu, G.; Zhang, H. Liquid Metal Nano/Micro-Channels as Thermal Interface Materials for Efficient Energy Saving. J. Mater. Chem. C 2018, 6, 10611–10617. [Google Scholar] [CrossRef]
- Gao, J.Y.; Yan, Q.W.; Tan, X.; Lv, L.; Ying, J.F.; Zhang, X.X.; Yang, M.H.; Du, S.Y.; Wei, Q.P.; Xue, C.; et al. Surface Modification Using Polydopamine-Coated Liquid Metal Nanocapsules for Improving Performance of Graphene Paper-Based Thermal Interface Materials. Nanomaterials 2021, 11, 1236. [Google Scholar] [CrossRef]
- Liang, S.T.; Wang, H.Z.; Liu, J. Progress, Mechanisms and Applications of Liquid-Metal Catalyst Systems. Chemistry 2018, 24, 17616–17626. [Google Scholar] [CrossRef]
- Zuraiqi, K.; Zavabeti, A.; Allioux, F.M.; Tang, J.B.; Nguyen, C.K.; Tafazolymotie, P.; Mayyas, M.; Ramarao, A.V.; Spencer, M.; Shah, K.; et al. Liquid Metals in Catalysis for Energy Applications. Joule 2020, 4, 2290–2321. [Google Scholar] [CrossRef]
- Taccardi, N.; Grabau, M.; Debuschewitz, J.; Distaso, M.; Brandl, M.; Hock, R.; Maier, F.; Papp, C.; Erhard, J.; Neiss, C.; et al. Gallium-Rich Pd-Ga Phases as Supported Liquid Metal Catalysts. Nat. Chem. 2017, 9, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.F.; Xia, J.; Zhang, N.; Cheng, H.; Bi, W.; Zu, X.L.; Chu, W.S.; Wu, H.A.; Wu, C.Z.; Xie, Y. Solid-Liquid Phase Transition Induced Electrocatalytic Switching from Hydrogen Evolution to Highly Selective Co2 Reduction. Nat. Catal. 2021, 4, 202–211. [Google Scholar] [CrossRef]
- Li, F.; Shu, J.; Zhang, L.; Yang, N.; Xie, J.; Li, X.; Cheng, L.; Kuang, S.; Tang, S.Y.; Zhang, S.; et al. Liquid Metal Droplet Robot. Appl. Mater. Today 2020, 19, 100597. [Google Scholar] [CrossRef]
- Wang, H.; Chen, S.; Yuan, B.; Liu, J.; Sun, X. Liquid Metal Transformable Machines. Acc. Mater. Res. 2021, 2, 1227–1238. [Google Scholar] [CrossRef]
- Wu, J.; Tang, S.Y.; Fang, T.; Li, W.; Li, X.; Zhang, S. A Wheeled Robot Driven by a Liquid-Metal Droplet. Adv. Mater. 2018, 30, 1805039. [Google Scholar] [CrossRef] [PubMed]
- Handschuh-Wang, S.; Chen, Y.; Zhu, L.; Zhou, X. Analysis and Transformations of Room-Temperature Liquid Metal Interfaces—A Closer Look through Interfacial Tension. Chemphyschem 2018, 19, 1584–1592. [Google Scholar] [CrossRef]
- Liu, L.; Wang, D.; Rao, W. Mini/Micro/Nano Scale Liquid Metal Motors. Micromachines 2021, 12, 280. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, Y.; Sheng, L.; Liu, J. Self-Fueled Biomimetic Liquid Metal Mollusk. Adv. Mater. 2015, 27, 2648–2655. [Google Scholar] [CrossRef]
- Chen, S.; Liu, J. Spontaneous Dispersion and Large-Scale Deformation of Gallium-Based Liquid Metal Induced by Ferric Ions. J. Phys. Chem. B 2019, 123, 2439–2447. [Google Scholar] [CrossRef]
- Zhang, J.; Sheng, L.; Liu, J. Synthetically Chemical-Electrical Mechanism for Controlling Large Scale Reversible Deformation of Liquid Metal Objects. Sci. Rep. 2014, 4, 7116. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Guo, R.; Yuan, B.; Chen, S.; Wang, H.; Dou, M.; Liu, J.; He, Z.Z. Low-Temperature Triggered Shape Transformation of Liquid Metal Microdroplets. ACS Appl. Mater. Interfaces 2020, 12, 38386–38396. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Tang, S.-Y.; Sivan, V.; Zhang, W.; Mitchell, A.; Kalantar-zadeh, K.; Khoshmanesh, K. Photochemically Induced Motion of Liquid Metal Marbles. Appl. Phys. Lett. 2013, 103, 174104. [Google Scholar] [CrossRef]
- Finkenauer, L.R.; Lu, Q.; Hakem, I.F.; Majidi, C.; Bockstaller, M.R. Analysis of the Efficiency of Surfactant-Mediated Stabilization Reactions of Egain Nanodroplets. Langmuir 2017, 33, 9703–9710. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; He, Z.; Yao, Y.; Liu, J. Transient State Machine Enabled from the Colliding and Coalescence of a Swarm of Autonomously Running Liquid Metal Motors. Small 2015, 11, 5253–5261. [Google Scholar] [CrossRef] [PubMed]
- Valencia, P.M.; Farokhzad, O.C.; Karnik, R.; Langer, R. Microfluidic Technologies for Accelerating the Clinical Translation of Nanoparticles. Nat. Nanotechnol. 2012, 7, 623–629. [Google Scholar] [CrossRef] [Green Version]
- Whitesides, G.M. The Origins and the Future of Microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Seemann, R.; Brinkmann, M.; Pfohl, T.; Herminghaus, S. Droplet Based Microfluidics. Rep. Prog. Phys. 2012, 75, 016601. [Google Scholar] [CrossRef] [PubMed]
- Hutter, T.; Bauer, W.A.C.; Elliott, S.R.; Huck, W.T.S. Formation of Spherical and Non-Spherical Eutectic Gallium-Indium Liquid-Metal Microdroplets in Microfluidic Channels at Room Temperature. Adv. Funct. Mater. 2012, 22, 2624–2631. [Google Scholar] [CrossRef]
- Thelen, J.; Dickey, M.D.; Ward, T. A Study of the Production and Reversible Stability of Egain Liquid Metal Microspheres Using Flow Focusing. Lab Chip 2012, 12, 3961–3967. [Google Scholar] [CrossRef]
- Gol, B.; Tovar-Lopez, F.J.; Kurdzinski, M.E.; Tang, S.Y.; Petersen, P.; Mitchell, A.; Khoshmanesh, K. Continuous Transfer of Liquid Metal Droplets across a Fluid-Fluid Interface within an Integrated Microfluidic Chip. Lab Chip 2015, 15, 2476–2485. [Google Scholar] [CrossRef]
- Kocourek, V.; Karcher, C.; Conrath, M.; Schulze, D. Stability of Liquid Metal Drops Affected by a High-Frequency Magnetic Field. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2006, 74, 026303. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wu, X.; Lee, D.W. A Galinstan-Based Inkjet Printing System for Highly Stretchable Electronics with Self-Healing Capability. Lab Chip 2016, 16, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.Q.; He, Z.Z.; Liu, J. Electro-Hydrodynamic Shooting Phenomenon of Liquid Metal Stream. Appl. Phys. Lett. 2014, 105, 134104. [Google Scholar] [CrossRef] [Green Version]
- Friedman, H.; Porat, Z.E.; Halevy, I.; Reich, S. Formation of Metal Microspheres by Ultrasonic Cavitation. J. Mater. Res. 2011, 25, 633–636. [Google Scholar] [CrossRef]
- Friedman, H.; Reich, S.; Popovitz-Biro, R.; von Huth, P.; Halevy, I.; Koltypin, Y.; Gedanken, A.; Porat, Z. Micro- and Nano-Spheres of Low Melting Point Metals and Alloys, Formed by Ultrasonic Cavitation. Ultrason. Sonochem. 2013, 20, 432–444. [Google Scholar] [CrossRef]
- Kumar, V.B.; Gedanken, A.; Kimmel, G.; Porat, Z. Ultrasonic Cavitation of Molten Gallium: Formation of Micro- and Nano-Spheres. Ultrason. Sonochem. 2014, 21, 1166–1173. [Google Scholar] [CrossRef]
- Ren, L.; Zhuang, J.; Casillas, G.; Feng, H.; Liu, Y.; Xu, X.; Liu, Y.; Chen, J.; Du, Y.; Jiang, L.; et al. Nanodroplets for Stretchable Superconducting Circuits. Adv. Funct. Mater. 2016, 26, 8111–8118. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Mashima, Y.; Iyoda, T. Reversible Size Control of Liquid-Metal Nanoparticles under Ultrasonication. Angew. Chem. Int. Ed. 2015, 54, 12809–12813. [Google Scholar] [CrossRef]
- Yun, Q.; Kimura, A.; Taguchi, M.; Miyako, E. Sonication- and Γ-Ray-Mediated Biomolecule-Liquid Metal Nanoparticlization in Cancer Optotheranostics. Appl. Mater. Today 2022, 26, 101302. [Google Scholar] [CrossRef]
- Zhang, Q.; Gao, Y.; Liu, J. Atomized Spraying of Liquid Metal Droplets on Desired Substrate Surfaces as a Generalized Way for Ubiquitous Printed Electronics. Appl. Phys. A 2013, 116, 1091–1097. [Google Scholar] [CrossRef]
- Tevis, I.D.; Newcomb, L.B.; Thuo, M. Synthesis of Liquid Core-Shell Particles and Solid Patchy Multicomponent Particles by Shearing Liquids into Complex Particles (Slice). Langmuir 2014, 30, 14308–14313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, F.; Xu, J.; Li, H.; Wang, Z.; Sun, L.; Deng, T.; Tao, P.; Liang, Q. Ga-in Liquid Metal Nanoparticles Prepared by Physical Vapor Deposition. Prog. Nat. Sci. Mater. Int. 2018, 28, 28–33. [Google Scholar] [CrossRef]
- Cinar, S.; Tevis, I.D.; Chen, J.; Thuo, M. Mechanical Fracturing of Core-Shell Undercooled Metal Particles for Heat-Free Soldering. Sci. Rep. 2016, 6, 21864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Li, Y. Colloidal Carbon Spheres and Their Core/Shell Structures with Noble-Metal Nanoparticles. Angew. Chem. Int. Ed. 2004, 43, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yao, S.; Rao, W.; Liu, J. Transformable Soft Liquid Metal Micro/Nanomaterials. Mater. Sci. Eng. R Rep. 2019, 138, 1–35. [Google Scholar] [CrossRef]
- Zavabeti, A.; Ou, J.Z.; Carey, B.J.; Syed, N.; Orrell-Trigg, R.; Mayes, E.L.; Xu, C.; Kavehei, O.; O’Mullane, A.P.; Kaner, R.B.; et al. A Liquid Metal Reaction Environment for the Room-Temperature Synthesis of Atomically Thin Metal Oxides. Science 2017, 358, 332–335. [Google Scholar] [CrossRef] [Green Version]
- Farrell, Z.J.; Tabor, C. Control of Gallium Oxide Growth on Liquid Metal Eutectic Gallium/Indium Nanoparticles Via Thiolation. Langmuir 2018, 34, 234–240. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, R.; Liu, J. Self-Propelled Liquid Metal Motors Steered by a Magnetic or Electrical Field for Drug Delivery. J. Mater. Chem. B 2016, 4, 5349–5357. [Google Scholar] [CrossRef]
- Lear, T.R.; Hyun, S.H.; Boley, J.W.; White, E.L.; Thompson, D.H.; Kramer, R.K. Liquid Metal Particle Popping: Macroscale to Nanoscale. Extrem. Mech. Lett. 2017, 13, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Sen, P.; Kim, C.J. Characterization of Nontoxic Liquid-Metal Alloy Galinstan for Applications in Microdevices. J. Microelectromech. Syst. 2012, 21, 443–450. [Google Scholar] [CrossRef] [Green Version]
- Surmann, P.; Zeyat, H. Voltammetric Analysis Using a Self-Renewable Non-Mercury Electrode. Anal. Bioanal. Chem. 2005, 383, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Sivan, V.; Tang, S.Y.; O’Mullane, A.P.; Petersen, P.; Eshtiaghi, N.; Kalantar-zadeh, K.; Mitchell, A. Liquid Metal Marbles. Adv. Funct. Mater. 2013, 23, 144–152. [Google Scholar] [CrossRef]
- Chen, R.; Xiong, Q.; Song, R.Z.; Li, K.L.; Zhang, Y.X.; Fang, C.; Guo, J.L. Magnetically Controllable Liquid Metal Marbles. Adv. Mater. Interfaces 2019, 6, 1901057. [Google Scholar] [CrossRef]
- Hu, J.J.; Liu, M.D.; Gao, F.; Chen, Y.; Peng, S.Y.; Li, Z.H.; Cheng, H.; Zhang, X.Z. Photo-Controlled Liquid Metal Nanoparticle-Enzyme for Starvation/Photothermal Therapy of Tumor by Win-Win Cooperation. Biomaterials 2019, 217, 119303. [Google Scholar] [CrossRef]
- Tan, S.C.; Gui, H.; Yuan, B.; Liu, J. Magnetic Trap Effect to Restrict Motion of Self-Powered Tiny Liquid Metal Motors. Appl. Phys. Lett. 2015, 107, 071904. [Google Scholar] [CrossRef]
- Chen, S.; Yang, X.; Wang, H.; Wang, R.; Liu, J. Al-Assisted High Frequency Self-Powered Oscillations of Liquid Metal Droplets. Soft Matter 2019, 15, 8971–8975. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, Y.; Liu, J. Autonomous Convergence and Divergence of the Self-Powered Soft Liquid Metal Vehicles. Sci. Bull. 2015, 60, 943–951. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.C.; Yang, X.H.; Gui, H.; Ding, Y.J.; Wang, L.; Yuan, B.; Liu, J. Galvanic Corrosion Couple-Induced Marangoni Flow of Liquid Metal. Soft Matter 2017, 13, 2309–2314. [Google Scholar] [CrossRef]
- Ye, J.; Tan, S.C.; Wang, L.; Liu, J. A New Hydrodynamic Interpretation of Liquid Metal Droplet Motion Induced by an Electrocapillary Phenomenon. Soft Matter 2021, 17, 7835–7843. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J. Electromagnetic Rotation of a Liquid Metal Sphere or Pool within a Solution. Proc. R. Soc. A Math. Phys. Eng. Sci. 2015, 471, 20150177. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Wang, H.Z.; Wang, X.F.; Liu, X.; Guo, J.R.; Liu, J. Magnetic Liquid Metals Manipulated in the Three-Dimensional Free Space. ACS Appl. Mater. Inter. 2019, 11, 8685–8692. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Hwang, J.; Choi, Y.; Kwon, Y.; Jang, J.; Yoon, S.; Choi, J. Effective Delivery of Anti-Cancer Drug Molecules with Shape Transforming Liquid Metal Particles. Cancers 2019, 11, 1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Wang, H.Z.; Sun, X.Y.; Wang, Q.; Wang, X.J.; Chen, L.B.; Zhang, L.J.; Guo, R.; Liu, J. Generalized Way to Make Temperature Tunable Conductor-Insulator Transition Liquid Metal Composites in a Diverse Range. Mater. Horiz. 2019, 6, 1854–1861. [Google Scholar] [CrossRef]
- Bilodeau, R.A.; Zemlyanov, D.Y.; Kramer, R.K. Liquid Metal Switches for Environmentally Responsive Electronics. Adv. Mater. Interfaces 2017, 4, 1600913. [Google Scholar] [CrossRef]
- Zavabeti, A.; Daeneke, T.; Chrimes, A.F.; O’Mullane, A.P.; Zhen Ou, J.; Mitchell, A.; Khoshmanesh, K.; Kalantar-Zadeh, K. Ionic Imbalance Induced Self-Propulsion of Liquid Metals. Nat. Commun. 2016, 7, 12402. [Google Scholar] [CrossRef]
- Gough, R.C.; Dang, J.H.; Moorefield, M.R.; Zhang, G.B.; Hihara, L.H.; Shiroma, W.A.; Ohta, A.T. Self-Actuation of Liquid Metal Via Redox Reaction. ACS Appl. Mater. Interfaces 2016, 8, 6–10. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J.; Liu, J.; Zhou, Y. Jumping Liquid Metal Droplet in Electrolyte Triggered by Solid Metal Particles. Appl. Phys. Lett. 2016, 108, 223901. [Google Scholar] [CrossRef]
- Yi, L.; Ding, Y.; Yuan, B.; Wang, L.; Tian, L.; Chen, C.; Liu, F.; Lu, J.; Song, S.; Liu, J. Breathing to Harvest Energy as a Mechanism Towards Making a Liquid Metal Beating Heart. RSC Adv. 2016, 6, 94692–94698. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J. Graphite Induced Periodical Self-Actuation of Liquid Metal. RSC Adv. 2016, 6, 60729–60735. [Google Scholar] [CrossRef]
- Hu, L.; Li, J.; Tang, J.; Liu, J. Surface Effects of Liquid Metal Amoeba. Sci. Bull. 2017, 62, 700–706. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Yuan, B.; Liu, J. Liquid Metal Amoeba with Spontaneous Pseudopodia Formation and Motion Capability. Sci. Rep. 2017, 7, 7256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, B.; Wang, L.; Yang, X.; Ding, Y.; Tan, S.; Yi, L.; He, Z.; Liu, J. Liquid Metal Machine Triggered Violin-Like Wire Oscillator. Adv. Sci. 2016, 3, 1600212. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Duan, W.; Zhou, C.; Liu, Q.; Gu, J.; Ye, H.; Li, M.; Wang, W.; Ma, X. Phoretic Liquid Metal Micro/Nanomotors as Intelligent Filler for Targeted Microwelding. Adv. Mater. 2019, 31, e1905067. [Google Scholar] [CrossRef]
- Lu, Y.; Hu, Q.; Lin, Y.; Pacardo, D.B.; Wang, C.; Sun, W.; Ligler, F.S.; Dickey, M.D.; Gu, Z. Transformable Liquid-Metal Nanomedicine. Nat. Commun. 2015, 6, 10066. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yang, X.; Cui, Y.; Liu, J. Self-Growing and Serpentine Locomotion of Liquid Metal Induced by Copper Ions. ACS Appl. Mater. Interfaces 2018, 10, 22889–22895. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Chen, S.; Wu, Y.; Yuan, H. Liquid Metal Droplet Series Based Wire Oscillation. In International Conference on Artificial Intelligence and Security; Sun, X., Zhang, X., Xia, Z., Bertino, E., Eds.; Springer: Cham, Switzerland, 2021; Volume 1424, pp. 440–447. [Google Scholar] [CrossRef]
- Sheng, L.; Zhang, J.; Liu, J. Diverse Transformations of Liquid Metals between Different Morphologies. Adv. Mater. 2014, 26, 6036–6042. [Google Scholar] [CrossRef]
- Gough, R.C.; Morishita, A.M.; Dang, J.H.; Moorefield, M.R.; Shiroma, W.A.; Ohta, A.T. Rapid Electrocapillary Deformation of Liquid Metal with Reversible Shape Retention. Micro Nano Syst. Lett. 2015, 3, 4. [Google Scholar] [CrossRef] [Green Version]
- Yuan, B.; He, Z.Z.; Liu, J. Effect of Electric Field on the Wetting Behavior of Eutectic Gallium–Indium Alloys in Aqueous Environment. J. Electron. Mater. 2018, 47, 2782–2790. [Google Scholar] [CrossRef]
- Wang, M.F.; Jin, M.J.; Jin, X.J.; Zuo, S.G. Modeling of Movement of Liquid Metal Droplets Driven by an Electric Field. Phys. Chem. Chem. Phys. 2017, 19, 18505–18513. [Google Scholar] [CrossRef]
- Tang, S.Y.; Sivan, V.; Khoshmanesh, K.; O’Mullane, A.P.; Tang, X.; Gol, B.; Eshtiaghi, N.; Lieder, F.; Petersen, P.; Mitchell, A.; et al. Electrochemically Induced Actuation of Liquid Metal Marbles. Nanoscale 2013, 5, 5949–5957. [Google Scholar] [CrossRef]
- Sivan, V.; Tang, S.Y.; O’Mullane, A.P.; Petersen, P.; Kalantar-Zadeh, K.; Khoshmanesh, K.; Mitchell, A. Influence of Semiconducting Properties of Nanoparticle Coating on the Electrochemical Actuation of Liquid Metal Marble. Appl. Phys. Lett. 2014, 105, 121607. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Wang, L.; Ding, Y.; Zhan, S.; Liu, J. Manipulation of Liquid Metals on a Graphite Surface. Adv. Mater. 2016, 28, 9210–9217. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.L.; Dong, H.X.; He, Z.Z. Electrochemically Enabled Manipulation of Gallium-Based Liquid Metals within Porous Copper. Mater. Horiz. 2018, 5, 675–682. [Google Scholar] [CrossRef]
- Tan, S.; Zhou, Y.; Wang, L.; Liu, J. Electrically Driven Chip Cooling Device Using Hybrid Coolants of Liquid Metal and Aqueous Solution. Sci. China Technol. Sci. 2015, 59, 301–308. [Google Scholar] [CrossRef]
- Yao, Y.y.; Liu, J. Liquid Metal Wheeled Small Vehicle for Cargo Delivery. RSC Adv. 2016, 6, 56482–56488. [Google Scholar] [CrossRef]
- Yao, Y.Y.; Liu, J. A Polarized Liquid Metal Worm Squeezing across a Localized Irregular Gap. RSC Adv. 2017, 7, 11049–11056. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Lin, Z.; Zhou, C.; Gao, C.; He, Q. Liquid Metal Gallium Micromachines Speed up in Confining Channels. Adv. Intell. Syst. 2019, 1, 1900064. [Google Scholar] [CrossRef]
- Shu, J.; Tang, S.Y.; Feng, Z.; Li, W.; Li, X.; Zhang, S. Unconventional Locomotion of Liquid Metal Droplets Driven by Magnetic Fields. Soft Matter 2018, 14, 7113–7118. [Google Scholar] [CrossRef] [Green Version]
- Jeon, J.; Lee, J.B.; Chung, S.K.; Kim, D. On-Demand Magnetic Manipulation of Liquid Metal in Microfluidic Channels for Electrical Switching Applications. Lab Chip 2016, 17, 128–133. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.; Kuai, Y.; Cong, J.; Xu, Y.; Piao, H.G.; Pan, L.; Liu, Y. Magnetically Powered Shape-Transformable Liquid Metal Micromotors. Small 2019, 15, e1905446. [Google Scholar] [CrossRef]
- Elbourne, A.; Cheeseman, S.; Atkin, P.; Truong, N.P.; Syed, N.; Zavabeti, A.; Mohiuddin, M.; Esrafilzadeh, D.; Cozzolino, D.; McConville, C.F.; et al. Antibacterial Liquid Metals: Biofilm Treatment Via Magnetic Activation. ACS Nano 2020, 14, 802–817. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.J.; Liu, M.D.; Chen, Y.; Gao, F.; Peng, S.Y.; Xie, B.R.; Li, C.X.; Zeng, X.; Zhang, X.Z. Immobilized Liquid Metal Nanoparticles with Improved Stability and Photothermal Performance for Combinational Therapy of Tumor. Biomaterials 2019, 207, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Gao, S.; Lin, H.; Lu, X.; Yang, B.; Zhang, L.; Chen, Y.; Shi, J. Inorganic Nanoshell-Stabilized Liquid Metal for Targeted Photonanomedicine in Nir-Ii Biowindow. Nano Lett. 2019, 19, 2128–2137. [Google Scholar] [CrossRef] [PubMed]
- Chechetka, S.A.; Yu, Y.; Zhen, X.; Pramanik, M.; Pu, K.; Miyako, E. Light-Driven Liquid Metal Nanotransformers for Biomedical Theranostics. Nat. Commun. 2017, 8, 15432. [Google Scholar] [CrossRef] [Green Version]
- Gan, T.; Shang, W.; Handschuh-Wang, S.; Zhou, X. Light-Induced Shape Morphing of Liquid Metal Nanodroplets Enabled by Polydopamine Coating. Small 2019, 15, e1804838. [Google Scholar] [CrossRef]
- Sun, X.; Sun, M.; Liu, M.; Yuan, B.; Gao, W.; Rao, W.; Liu, J. Shape Tunable Gallium Nanorods Mediated Tumor Enhanced Ablation through near-Infrared Photothermal Therapy. Nanoscale 2019, 11, 2655–2667. [Google Scholar] [CrossRef]
- Wang, D.; Gao, C.; Si, T.; Li, Z.; Guo, B.; He, Q. Near-Infrared Light Propelled Motion of Needlelike Liquid Metal Nanoswimmers. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125865. [Google Scholar] [CrossRef]
- Kumar, V.B.; Porat, Z.e.; Gedanken, A. Dsc Measurements of the Thermal Properties of Gallium Particles in the Micron and Sub-Micron Sizes, Obtained by Sonication of Molten Gallium. J. Therm. Anal. Calorim. 2015, 119, 1587–1592. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, Y.; Genzer, J.; Dickey, M.D. Shape-Transformable Liquid Metal Nanoparticles in Aqueous Solution. Chem. Sci. 2017, 8, 3832–3837. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Gao, C.; Zhou, C.; Lin, Z.; He, Q. Leukocyte Membrane-Coated Liquid Metal Nanoswimmers for Actively Targeted Delivery and Synergistic Chemophotothermal Therapy. Research 2020, 2020, 3676954. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Zu, J.S.; Liu, J. Insights into Fluidic Endogenous Magnetism and Magnetic Monopoles from A Liquid Metal Droplet Machine. Soft Sci. 2021, 1, 15. [Google Scholar] [CrossRef]
| Ga | GaIn24.5 (EGaIn) | Ga67In20.5Sn12.5 (Galinstan) | Ga61In25Sn13Zn1 | |
|---|---|---|---|---|
| Melting point (°C) | 29.8 | 15.7 | 10.5 | 7.6 |
| Boiling point (°C) | 2204 | 2000 | 1300 | 900 |
| Density (103 kg/m3) | 6.08 | 6.28 | 6.36 | 6.5 |
| Viscosity (10−7 m2/s) | 3.24 | 2.7 | 2.98 | 0.71 |
| Surface tension (N/m1) | 0.72 | 0.624 | 0.533 | 0.5 |
| Conductivity (106 S/m) | 3.7 | 3.4 | 3.1 | 2.8 |
| Thermal conductivity (W/m °C) | 29.4 | 42.2 | 44.8 | 48.2 |
| Response Factors | Mechanism | Characteristics | Applications |
|---|---|---|---|
| Chemical field | Marangoni effects [108], | Self energy supply, | Soft robots, |
| Interfacial tension gradient | large scale deformation, | drug delivery, | |
| Electric field | Solution viscous force drive [109] | Good controllability | Cargo transportation |
| Magnetic field | Lorentz force [110], | Non-contact manipulation, | Reconfigurable electronics, |
| Magnetic force [111] | large driving force | antibacterial | |
| Light | Heat generation [112], | Photothermal synergy, | Photothermal therapy, |
| bubble propulsion [72] | non-contact | drug release | |
| Temperature field | Phase transition [113] | Rapid response | Functional circuit, tumor therapy |
| Acoustic field | Acoustic radiation force [34] | Biocompatible | Photothermal cancer cell therapy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, M.; Zhu, X.; Shan, X.; Wang, H.; Chen, S.; Liu, J. Responsive Liquid Metal Droplets: From Bulk to Nano. Nanomaterials 2022, 12, 1289. https://doi.org/10.3390/nano12081289
Duan M, Zhu X, Shan X, Wang H, Chen S, Liu J. Responsive Liquid Metal Droplets: From Bulk to Nano. Nanomaterials. 2022; 12(8):1289. https://doi.org/10.3390/nano12081289
Chicago/Turabian StyleDuan, Minghui, Xiyu Zhu, Xiaohui Shan, Hongzhang Wang, Sen Chen, and Jing Liu. 2022. "Responsive Liquid Metal Droplets: From Bulk to Nano" Nanomaterials 12, no. 8: 1289. https://doi.org/10.3390/nano12081289
APA StyleDuan, M., Zhu, X., Shan, X., Wang, H., Chen, S., & Liu, J. (2022). Responsive Liquid Metal Droplets: From Bulk to Nano. Nanomaterials, 12(8), 1289. https://doi.org/10.3390/nano12081289






