Single-Step Fabrication of Au-Fe-BaTiO3 Nanocomposite Thin Films Embedded with Non-Equilibrium Au-Fe Alloyed Nanostructures
Abstract
1. Introduction
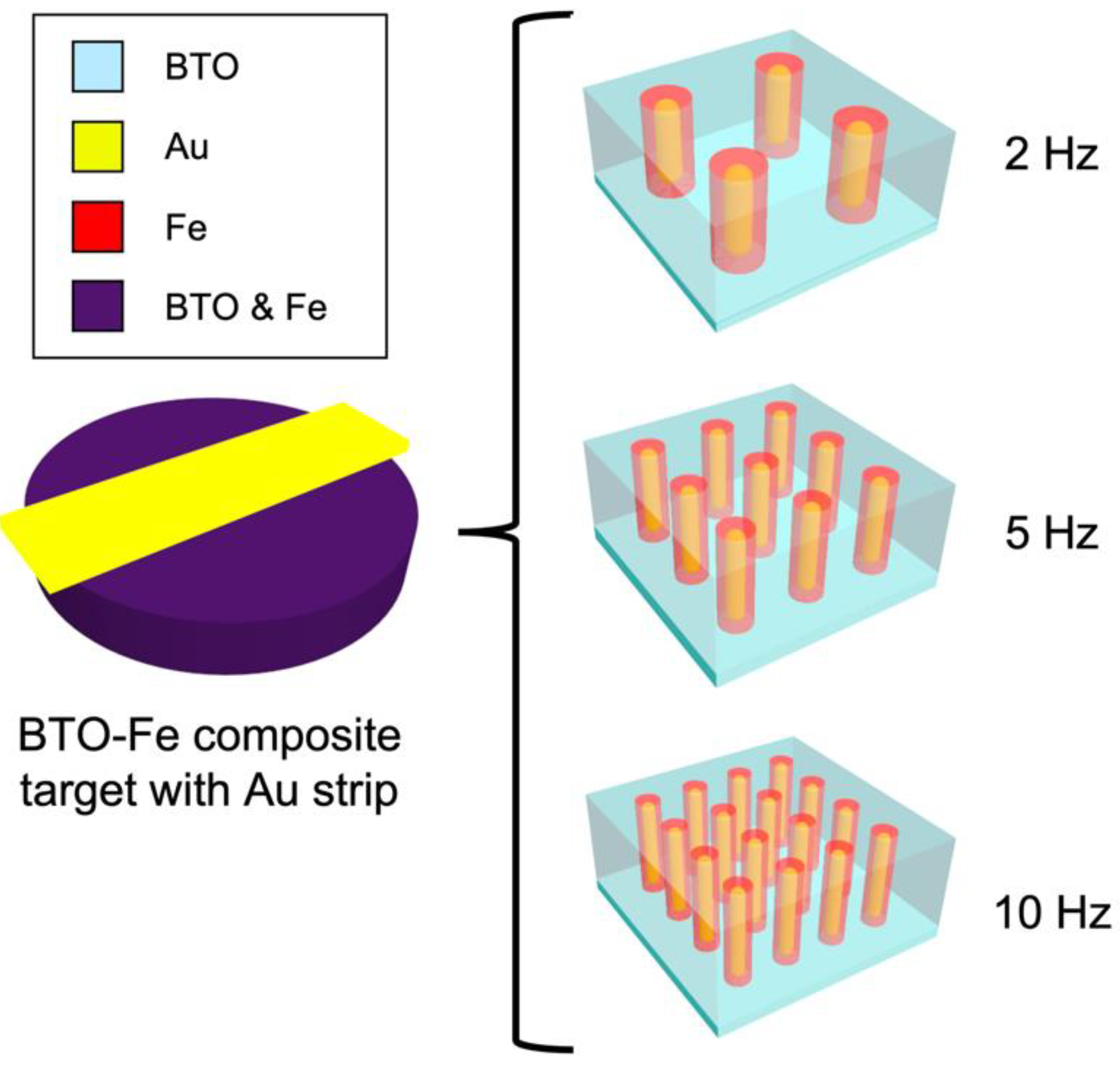
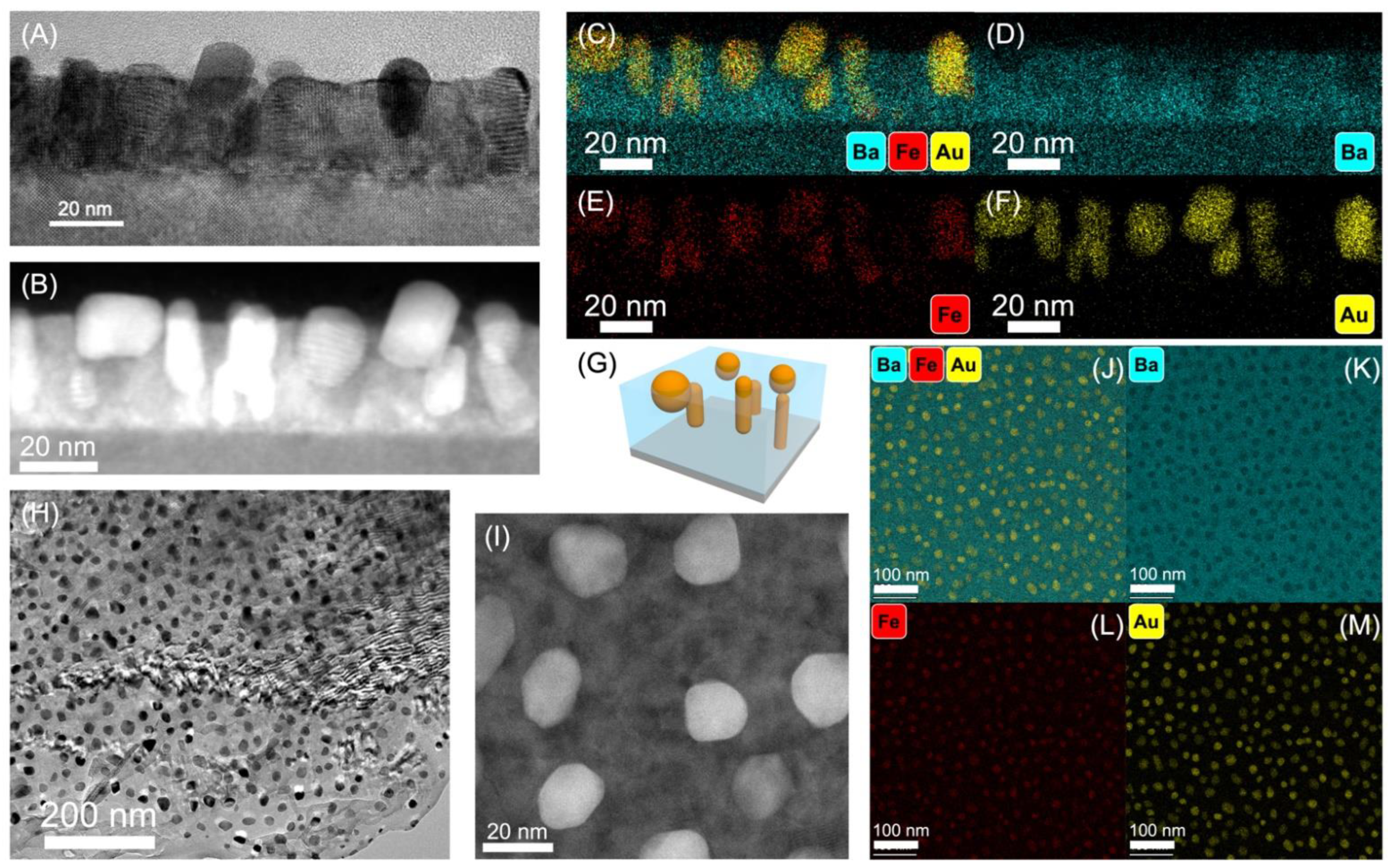
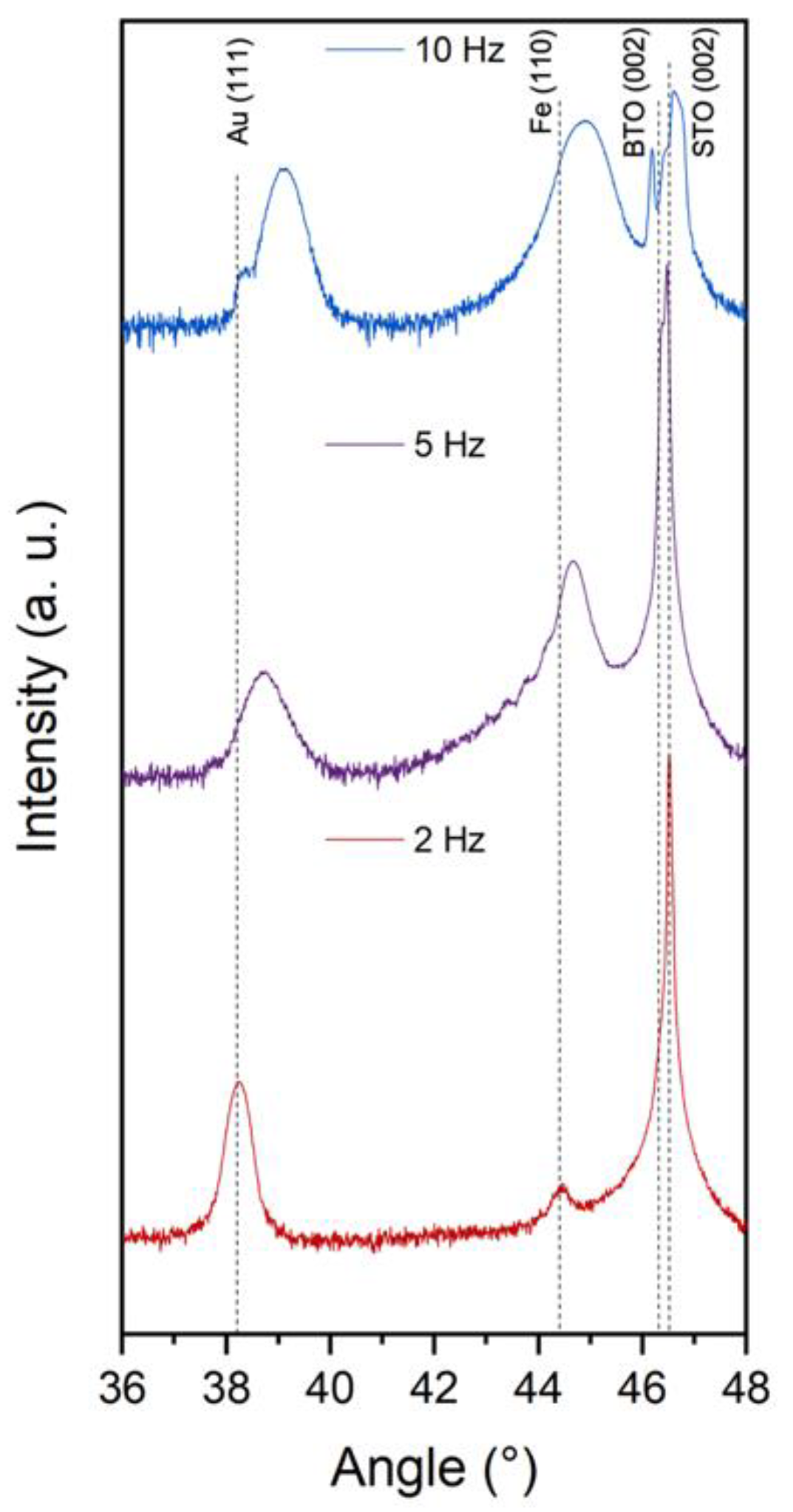
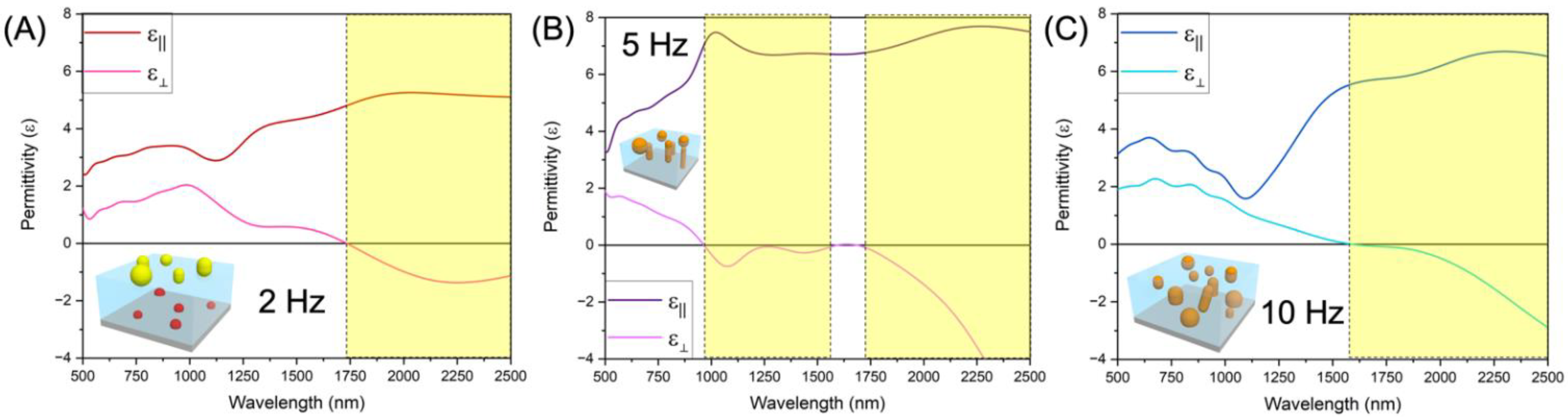
2. Results and Discussion
3. Conclusions
4. Experimental Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moore, G.E. Cramming More Components onto Integrated Circuits. IEEE Solid-State Circuits Soc. Newsl. 2006, 11, 33–35. [Google Scholar] [CrossRef]
- Phuah, X.L.; Wang, H.; Zhang, B.; Cho, J.; Zhang, X.; Wang, H. Ceramic Material Processing Towards Future Space Habitat: Electric Current-Assisted Sintering of Lunar Regolith Simulant. Materials 2020, 13, 4128. [Google Scholar] [CrossRef]
- Chen, A.; Bi, Z.; Jia, Q.; Macmanus-driscoll, J.L.; Wang, H. Microstructure, Vertical Strain Control and Tunable Functionalities in Self-Assembled, Vertically Aligned Nanocomposite Thin Films. Acta Mater. 2013, 61, 2783–2792. [Google Scholar] [CrossRef]
- Rutherford, B.X.; Zhang, B.; Wang, X.; Sun, X.; Qi, Z.; Wang, H.; Wang, H. Strain Effects on the Growth of La0.7Sr0.3MnO3 (LSMO)-NiO Nanocomposite Thin Films via Substrate Control. ACS Omega 2020, 5, 23793–23798. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, Q.; Huang, J.; Fan, M.; Rutherford, B.X.; Paldi, R.L.; Jian, J.; Zhang, X.; Wang, H. Strain-Driven Nanodumbbell Structure and Enhanced Physical Properties in Hybrid Vertically Aligned Nanocomposite Thin Films. Appl. Mater. Today 2019, 16, 204–212. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Jian, J.; Rutherford, B.X.; Gao, X.; Xu, X.; Zhang, X.; Wang, H. Metal-Free Oxide-Nitride Heterostructure as a Tunable Hyperbolic Metamaterial Platform. Nanoletters 2020, 20, 6614–6622. [Google Scholar] [CrossRef]
- Wang, X.; Jian, J.; Wang, H.; Liu, J.; Pachaury, Y.; Lu, P.; Rutherford, B.X.; Gao, X.; Xu, X.; El-azab, A.; et al. Nitride-Oxide-Metal Heterostructure with Self-Assembled Core—Shell Nanopillar Arrays: Effect of Ordering on Magneto-Optical Properties. Small 2021, 17, 2007222. [Google Scholar] [CrossRef]
- Misra, S.; Li, L.; Zhang, D.; Jian, J.; Qi, Z.; Fan, M.; Chen, H.; Zhang, X.; Wang, H. Self-Assembled Ordered Three-Phase Au-BaTiO3–ZnO Vertically Aligned Nanocomposites Achieved by a Templating Method. Adv. Mater. 2019, 31, 1806529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ramesh, R.; Macmanus-driscoll, J.L.; Wang, H. Multifunctional, Self-Assembled Oxide Nanocomposite Thin Films and Devices. MRS Bull. 2016, 40, 736–745. [Google Scholar] [CrossRef]
- Tran, D.H.; Putri, W.B.K.; Kang, B.; Lee, N.H.; Kang, W.N.; Seong, W.K. Reducing Thickness Dependence of Critical Current Density in GdBa2Cu3O7-δ Thin Films by Addition of Nanostructured Defects. J. Appl. Phys. 2013, 113, 1–3. [Google Scholar] [CrossRef]
- Chiang, B.I.-C.; Chen, D.-H. Synthesis of Monodisperse FeAu Nanoparticles with Tunable Magnetic and Optical Properties. Adv. Funct. Mater. 2007, 17, 1311–1316. [Google Scholar] [CrossRef]
- Mukherjee, P.; Manchanda, P.; Kumar, P.; Zhou, L.; Kramer, M.J.; Kashyap, A.; Skomski, R.; Sellmyer, D.; Shield, J.E. Size-Induced Chemical and Magnetic Ordering in Individual Fe-Au Nanoparticles. ACS Nano 2014, 8, 8113–8120. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Scaramuzza, S.; Agnoli, S.; Polizzi, S.; Meneghetti, M. Strong Dependence of Surface Plasmon Resonance and Surface Enhanced Raman Scattering on the Composition of Au–Fe Nanoalloys. Nanoscale 2014, 6, 1423–1433. [Google Scholar] [CrossRef]
- Ishmukhametov, I.; Batasheva, S.; Rozhina, E.; Akhatova, F.; Mingaleeva, R.; Rozhin, A.; Fakhrullin, R. DNA/Magnetic Nanoparticles Composite to Attenuate Glass Surface Nanotopography for Enhanced Mesenchymal Stem Cell Differentiation. Polymers 2022, 14, 344. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, P.; Atkinson, J.; Jacob, Z. Hyperbolic Metamaterials: Fundamentals and Applications. Nano Converg. 2014, 1, 1–17. [Google Scholar] [CrossRef]
- Yang, B.H.; Wang, H.; Yoon, J.; Wang, Y.; Jain, M.; Feldmann, D.M.; Dowden, P.C.; Macmanus-driscoll, J.L.; Jia, Q. Vertical Interface Effect on the Physical Properties of Self-Assembled Nanocomposite Epitaxial Films. Adv. Mater. 2009, 21, 3794–3798. [Google Scholar] [CrossRef]
- MacManus-Driscoll, J.L.; Zerrer, P.; Wang, H.; Yang, H.; Yoon, J.; Fouchet, A.; Yu, R.; Blamire, M.G.; Jia, Q. Strain Control and Spontaneous Phase Ordering in Vertical Nanocomposite Heteroepitaxial Thin Films. Nat. Mater. 2008, 7, 314–320. [Google Scholar] [CrossRef]
- Park, J.H.; Jang, H.M.; Kim, H.S.; Park, C.G.; Lee, S.G. Strain-Mediated Magnetoelectric Coupling in BaTiO3-Co Nanocomposite Thin Films. Appl. Phys. Lett. 2008, 92, 1–4. [Google Scholar] [CrossRef]
- Zhang, B.; Fan, M.; Li, L.; Jian, J.; Huang, J.; Wang, H.; Kalaswad, M.; Wang, H. Tunable Magnetic Anisotropy of Self-Assembled Fe Nanostructures within a La0.5Sr0.5FeO3 Matrix. Appl. Phys. Lett. 2018, 112, 013104. [Google Scholar] [CrossRef]
- Huang, J.; Li, L.; Lu, P.; Qi, Z.; Sun, X. Self-Assembled Co–BaZrO3 Nanocomposite Thin Films with Ultra-Fine Vertically Aligned Co Nanopillars. R. Soc. Chem. 2017, 7970–7976. [Google Scholar] [CrossRef] [PubMed]
- Myagkov, V.G.; Zhigalov, V.S.; Bykova, L.E.; Zharkov, S.M.; Matsynin, A.A.; Volochav, M.N.; Tambasov, I.A. Thermite Synthesis and Characterization of Co-ZrO2 Ferromagnetic Nanocomposite Thin Films. J. Alloys Compd. 2016, 665, 197–203. [Google Scholar] [CrossRef]
- Li, D.Y.; Zeng, Y.J.; Batuk, D.; Pereira, L.M.C.; Ye, Z.Z.; Fleischmann, C.; Menghini, M.; Nikitenko, S.; Hadermann, J.; Temst, K.; et al. Relaxor Ferroelectricity and Magnetoelectric Coupling in ZnO−Co Nanocomposite Thin Films: Beyond Multiferroic Composites. ACS Appl. Mater. Interfaces 2014, 6, 4737–4742. [Google Scholar] [CrossRef] [PubMed]
- Paldi, R.L.; Sun, X.; Wang, X.; Zhang, X.; Wang, H. Strain-Driven In-Plane Ordering in Vertically Aligned ZnO—Au Nanocomposites with Highly Correlated Metamaterial Properties. ACS Omega 2020, 5, 2234–2241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Kalaswad, M.; Rutherford, B.X.; Misra, S.; He, Z.; Wang, H.; Qi, Z.; Wissel, A.E.; Xu, X.; Wang, H. Au-Encapsulated Fe Nanorods in Oxide Matrix with Tunable Magneto-Optic Coupling Properties. Appl. Mater. Interfaces 2020, 12, 51827–51836. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, B.X.; Zhang, B.; Kalaswad, M.; He, Z.; Zhang, D.; Wang, X.; Liu, J.; Wang, H. Tunable Three-Phase Co−CeO2−BaTiO3 Hybrid Metamaterials with Nano-Mushroom-Like Structure for Tailorable Multifunctionalities. ACS Appl. Mater. Interfaces 2022, 5, 6297–6304. [Google Scholar] [CrossRef]
- Zhang, D.; Lu, P.; Misra, S.; Wissel, A.; He, Z.; Qi, Z.; Gao, X.; Sun, X.; Liu, J.; Lu, J.; et al. Design of 3D Oxide–Metal Hybrid Metamaterial for Tailorable Light–Matter Interactions in Visible and Near-Infrared Region. Adv. Opt. Mater. 2021, 9, 2001154. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, B.; Zhao, J. Enhanced Photocatalytic Performance of Au-Ag Alloy Modified ZnO Nanocomposite Films. J. Alloys Compd. 2014, 586, 663–668. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, J.; Mohaddes-Ardabili, L.; Wuttig, M.; Salamanca-Riba, L.; Schlom, D.G.; Ramesh, R. Three-Dimensional Heteroepitaxy in Self- Assembled BaTiO3-CoFe2O4 Nanostructures. Appl. Phys. Lett. 2004, 85, 2035–2037. [Google Scholar] [CrossRef]
- Khatkhatay, F.; Chen, A.; Lee, J.H.; Zhang, W.; Abdel-Raziq, H.; Wang, H. Ferroelectric Properties of Vertically Aligned Nanostructured BaTiO3−CeO2 Thin Films and Their Integration on Silicon. Appl. Mater. Interfaces 2013, 5, 12541–12547. [Google Scholar] [CrossRef]
- Chen, A.; Khatkhatay, F.; Zhang, W.; Jacob, C.; Jiao, L.; Wang, H. Strong Oxygen Pressure Dependence of Ferroelectricity in BaTiO3/SrRuO3/SrTiO3 Epitaxial Heterostructures. J. Appl. Phys. 2013, 114, 124101. [Google Scholar] [CrossRef]
- Kim, T.; Ram, B.; Lee, W.; Ha, J.; Lee, B.; Hyeok, J. Integration of Artificial SrTiO3/BaTiO3 Superlattices on Si Substrates Using a TiN Buffer Layer by Pulsed Laser Deposition Method. J. Cryst. Growth 2006, 289, 540–546. [Google Scholar] [CrossRef]
- Fuse, H.; Koshizaki, N.; Ishikawa, Y.; Swiatkowska-warkocka, Z. Determining the Composite Structure of Au-Fe-Based Submicrometre Spherical Particles Fabricated by Pulsed-Laser Melting in Liquid. Nanomaterials 2019, 9, 198. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H.; Massalski, T.B.; Swartzendruber, L.J.; Beck, P.A. The Au-Fe (Gold-Iron) System. Bull. Alloy. Phase Diagr. 1984, 5, 592–600. [Google Scholar] [CrossRef]
- Hume-Rothery, W. The Structure of Metals and Alloys; The Institute of Metals: London, UK, 1936. [Google Scholar]
- Bao, Q.; Chen, C.; Wang, D.; Ji, Q.; Lei, T. Pulsed Laser Deposition and Its Current Research Status in Preparing Hydroxyapatite Thin Films. Appl. Surf. Sci. 2005, 252, 1538–1544. [Google Scholar] [CrossRef]
- Pamler, W.; Marinero, E.E.; Chen, M. Laser-Induced Metal Cluster Growth and Segregation in Granular Metal-Insulator Systems. Phys. Rev. B 1986, 33, 5736–5743. [Google Scholar] [CrossRef]
- Lie, H.L.; Wu, J.H.; Min, J.H.; Kim, Y.K. Synthesis of Monosized Magnetic-Optical AuFe Alloy Nanoparticles. J. Appl. Phys. 2008, 103, 1–4. [Google Scholar] [CrossRef]
- Srinoi, P.; Chen, Y.; Vittur, V.; Marquez, M.D.; Lee, T.R. Bimetallic Nanoparticles: Enhanced Magnetic and Optical Properties for Emerging Biological Applications. Appl. Sci. 2018, 8, 1106. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rutherford, B.X.; Dou, H.; Zhang, B.; He, Z.; Barnard, J.P.; Paldi, R.L.; Wang, H. Single-Step Fabrication of Au-Fe-BaTiO3 Nanocomposite Thin Films Embedded with Non-Equilibrium Au-Fe Alloyed Nanostructures. Nanomaterials 2022, 12, 3460. https://doi.org/10.3390/nano12193460
Rutherford BX, Dou H, Zhang B, He Z, Barnard JP, Paldi RL, Wang H. Single-Step Fabrication of Au-Fe-BaTiO3 Nanocomposite Thin Films Embedded with Non-Equilibrium Au-Fe Alloyed Nanostructures. Nanomaterials. 2022; 12(19):3460. https://doi.org/10.3390/nano12193460
Chicago/Turabian StyleRutherford, Bethany X., Hongyi Dou, Bruce Zhang, Zihao He, James P. Barnard, Robynne L. Paldi, and Haiyan Wang. 2022. "Single-Step Fabrication of Au-Fe-BaTiO3 Nanocomposite Thin Films Embedded with Non-Equilibrium Au-Fe Alloyed Nanostructures" Nanomaterials 12, no. 19: 3460. https://doi.org/10.3390/nano12193460
APA StyleRutherford, B. X., Dou, H., Zhang, B., He, Z., Barnard, J. P., Paldi, R. L., & Wang, H. (2022). Single-Step Fabrication of Au-Fe-BaTiO3 Nanocomposite Thin Films Embedded with Non-Equilibrium Au-Fe Alloyed Nanostructures. Nanomaterials, 12(19), 3460. https://doi.org/10.3390/nano12193460





