Defect Chemistry and Chemical Looping Performance of La1−xMxMnO3 (M = Sr, Ca, (x = 0–0.5)) Perovskites
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. La1−xMxMnO3 (M = Sr or Ca): Oxygen Excess Region
3.2. La1−xMxMnO3 (M = Sr or Ca): Oxygen-Deficient Region
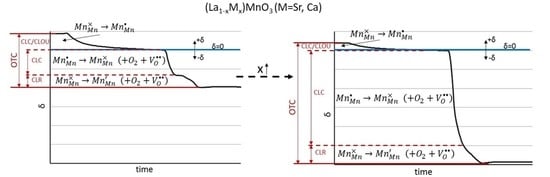
4. Discussion
4.1. LaMnO3
4.2. LaMnO3 + ΜO (Μ = Sr, Ca)
4.3. Oxygen Excess in LaMnO3 + ΜO (Μ = Sr, Ca)
4.4. Oxygen Deficiency in LaMnO3 + ΜO (Μ = Sr, Ca)
4.5. Complementary Remarks
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, X.; Imtiaz, Q.; Donat, F.; Müller, C.R.; Li, F. Chemical looping beyond combustion—A perspective. Energy Environ. Sci. 2020, 13, 772–804. [Google Scholar] [CrossRef]
- Czakiert, T.; Krzywanski, J.; Zylka, A.; Nowak, W. Chemical looping combustion: A brief overview. Energies 2022, 15, 1563. [Google Scholar] [CrossRef]
- Abuelgasim, S.; Wang, W.; Abdalazeez, A. A brief review for chemical looping combustion as a promising CO2 capture technology: Fundamentals and progress. Sci. Total Environ. 2021, 764, 142892. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Yang, Y.; Yang, S.; Zhang, Q.; Zhao, J.; Fang, Y.; Hao, X.; Guan, G. Iron-based oxygen carriers in chemical looping conversions: A review. Carbon Resour. Convers. 2019, 2, 23–34. [Google Scholar] [CrossRef]
- Argyris, P.A.; Leeuwe, C.; Abbas, S.Z.; Amieiro, A.; Poultson, S.; Wails, D.; Spallina, V. Chemical looping reforming for syngas generation at real process conditions in packed bed reactors: An experimental demonstration. Chem. Eng. J. 2022, 435, 134883. [Google Scholar] [CrossRef]
- Li, L.; Jiang, B.; Tang, D.; Zheng, Z.; Zhao, C. Hydrogen production from chemical Looping reforming of ethanol using Ni/CeO2 nanorod oxygen carrier. Catalysts 2018, 8, 257. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, L.; Xie, L.; Sun, C.; Zhao, W.; Liu, X.; Zhuang, Z.; Liu, S.; Zhao, Q. Defect-driven selective oxidation of MoS2 nanosheets with photothermal effect for photo-catalytic hydrogen evolution reaction. Chem. Eng. J. 2022, 439, 135757. [Google Scholar] [CrossRef]
- Chen, J.; Tang, Y.; Wang, S.; Xie, L.; Chang, C.; Cheng, X.; Liu, M.; Wang, L.; Wang, L. Ingeniously designed Ni-Mo-S/ZnIn2S4 composite for multi-photocatalytic reaction systems. Chin. Chem. Lett. 2022, 33, 1468–1474. [Google Scholar] [CrossRef]
- Mattison, T.; Lyngfelt, A.; Leion, H. Chemical looping with oxygen uncoupling for combustion of solid fuels. Int. J. Greenh. Gas Control 2009, 3, 11–19. [Google Scholar] [CrossRef]
- Vos, Y.; Jacobs, M.; Voort, P.; Driessche, I.; Snijkers, F.; Verberckmoes, A. Development of stable oxygen carrier materials for chemical looping processes—A review. Catalysts 2020, 10, 926. [Google Scholar] [CrossRef]
- Zhao, K.; He, F.; Huang, Z.; Zheng, A.; Zhao, Z. Three-dimensionally ordered macroporous LaFeO3 perovskites for chemical-looping steam reforming of methane. Int. J. Hydrog. Energy 2014, 39, 3243–3252. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, R.; Gao, Y.; Lin, Y.; Liu, A.; Wang, X.; Zheng, A.; Huang, Z.; Zhao, Z. High syngas selectivity and near pure hydrogen production in perovskite oxygen carriers for chemical looping steam methane reforming. Fuel Processing Technol. 2022, 236, 107398. [Google Scholar] [CrossRef]
- Huang, Z.; Wei, G.; Zheng, A.; Li, H.; Zhao, Z. Perovskite-type oxides LaFe1−xCoxO3 for chemical looping steam methane reforming to syngas and hydrogen co-production. Appl. Energy 2016, 168, 193–203. [Google Scholar]
- Ryden, M.; Lyngfelt, A.; Mattison, T.; Chen, D.; Holmen, A.; Bjørgum, E. Novel oxygen-carrier materials for chemical-looping combustion and chemical-looping reforming: LaxSr1−xFeyCo1−yO3−δ perovskites and mixed-metal oxides of NiO, Fe2O3 and Mn3O4. Int. J. Greenh. Gas Control 2008, 2, 21–36. [Google Scholar] [CrossRef]
- Liu, L.; Taylor, D.D.; Rodriquez, E.E.; Zachariah, M.R. Influence of transition metal electronegativity on the oxygen storage capacity of perovskite oxides. Chem. Commun. 2016, 52, 10369. [Google Scholar] [CrossRef] [PubMed]
- Hona, R.K.; Ramezanipour, F. Effect of the oxygen vacancies and structural order on the oxygen evolution activity: A case study of SrMnO3−δ featuring four different structure types. Inorg. Chem. 2020, 59, 4685–4692. [Google Scholar] [CrossRef]
- CaO, W.; Hu, Z.; Lin, Z.; Guo, X.; Su, J.; Chang, J.; Hao, Y. Defects and doping engineering towards high performance lead-free or lead-less perovskite solar cells. J. Energy Chem. 2022, 68, 420–438. [Google Scholar] [CrossRef]
- Hona, R.K.; Huq, A.; Ramezanipour, F. Charge transport properties of Ca2FeGaO6−δ and CaSrFeGaO6−δ: The effect of defect-order. Mater. Chem. Phys. 2019, 238, 121924. [Google Scholar] [CrossRef]
- Murray, E.P.; Barnett, S.A. (La,Sr)MnO3-(Ce,Gd)O2−x composite cathodes for solid oxide fuel cells. Solid State Ion. 2002, 143, 265–273. [Google Scholar] [CrossRef]
- Haile, S.M. Fuel cell materials and components. Acta Mater. 2003, 52, 5981–6000. [Google Scholar] [CrossRef]
- Shen, M.; Zhao, Z.; Chen, J.; Su, Y.; Wang, J.; Wang, X. Effects of calcium substitute in LaMnO3 perovskites for NO catalytic oxidation. J. Rare Earths 2013, 31, 119–123. [Google Scholar] [CrossRef]
- Tulloch, J.; Donne, S.W. Activity of perovskite La1−xSrxMnO3 catalysts towards oxygen reduction in alkaline electrolytes. J. Power Sources 2009, 188, 359–366. [Google Scholar] [CrossRef]
- Hallberg, P.; Källén, M.; Jing, D.; Snijkers, F.; van Noyen, J.; Rydén, M.; Lyngfelt, A. Experimental investigation of CaMnO3−δ based oxygen carriers used in continuous chemical-looping combustion. Int. J. Chem. Eng. 2014, 2014, 412517. [Google Scholar] [CrossRef]
- Haron, W.; Wisitsoraat, A.; Wongnawa, S. Comparison of monocrystalline LaMO3 (M = Co, Al) Perovskite Oxide Prepared by Co-Precipitation Method. Int. J. Chem. Eng. Appl. 2014, 5, 123–126. [Google Scholar]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chaleogenides. Acta Crystallogr. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Kröger, F.A.; Vink, H.J. Relations between the Concentrations of Imperfections in Crystalline Solids. Solid State Phys. 1956, 3, 307–435. [Google Scholar]
- Roosmalen, J.A.M.; Cordfunke, E.H.P. The defect chemistry of LaMnO3+δ. J. Solid State Phys. 1994, 110, 109–112. [Google Scholar]
- Nakamura, K.; Ogawa, K. Excess Oxygen in LaMnO3+δ. J. Solid State Chem. 2002, 163, 65–76. [Google Scholar] [CrossRef]
- Nowotny, J.; Rekas, M. Defect Chemistry of (La,Sr)MnO3. J. Am. Ceram. Soc. 1998, 81, 67–80. [Google Scholar] [CrossRef]
- Heuer, S.A.; Schierholz, R.; Alekseev, E.V.; Peters, L.; Mueller, D.N.; Duchoň, T.; Vibhu, V.; Tempel, H.; Haart, L.G.J.; Kungl, H.; et al. Oxygen nonstoichiometry and valence state of Manganese in La1−xCaxMnO3+δ. ACS Omega 2021, 6, 9638–9652. [Google Scholar] [CrossRef]
- Zuev, A.Y.; Tsvetkov, D.S. Oxygen nonstoichiometry, defect structure and defect-induced expansion of undoped perovskite LaMnO3±δ. Solid State Ion. 2010, 181, 557–563. [Google Scholar] [CrossRef]
- Nalbandian, L.; Evdou, A.; Matsouka, C.; Zaspalis, V. Assessment of (La1−xSrx)MnO3±δ perovskites as oxygen- carrier materials in chemical-looping processes. Fuel Processing Technol. 2022, 226, 107086. [Google Scholar] [CrossRef]
- Iliopoulou, E.F.; Matsouka, C.; Pachatouridou, E.; Papadopoulou, F.; Psarras, A.; Evdou, A.; Zaspalis, V.; Nalbandian, L. Novel La1−xCaxMnO3 perovskite materials for chemical looping combustion applications. Int. J. Energy Res. 2022, 1–15. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, L.; Wang, Y.; Wang, Y.; Wang, H.; Wang, Y.; Jiang, L.; Zhu, X.; Wei, Y.; Li, K. Enhanced activity of La1−xMnCuxO3 perovskite oxides for chemical looping steam methane reforming. Fuel Processing Technol. 2021, 215, 106744. [Google Scholar] [CrossRef]
- Yin, X.; Wang, S.; Wang, B.; Shen, L. Chemical looping steam methane reforming using Al doped LaMnO3+δ perovskites as oxygen carriers. Int. J. Hydrog. Energy 2021, 46, 33375–33387. [Google Scholar] [CrossRef]
- Yin, X.; Wang, S.; Wang, B.; Shen, L. Perovskite-type LaMn1−xBxO3+δ (Β=Fe, Co, Ni) as oxygen carriers for chemical looping steam methane reforming. Chem. Eng. J. 2021, 422, 128751. [Google Scholar] [CrossRef]
- Drosou, C.; Goergakopoulou, T.; Fanourgiakis, S.; Nikolaraki, E.; Artemakis, G.; Stratakis, A.; Matsouka, C.; Nalbandian, L.; Zaspalis, V.; Charisiou, N.; et al. Catalytic Oxidation of CH4 under oxygen excess conditions on Ir/LaxSr1−xMnO3 catalysts. In Proceedings of the 13th Panhellenic Conference on Chemical Engineering, Patras, Greece, 2–4 June 2022; p. 174. [Google Scholar]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evdou, A.; Georgitsis, T.; Matsouka, C.; Pachatouridou, E.; Iliopoulou, E.; Zaspalis, V. Defect Chemistry and Chemical Looping Performance of La1−xMxMnO3 (M = Sr, Ca, (x = 0–0.5)) Perovskites. Nanomaterials 2022, 12, 3461. https://doi.org/10.3390/nano12193461
Evdou A, Georgitsis T, Matsouka C, Pachatouridou E, Iliopoulou E, Zaspalis V. Defect Chemistry and Chemical Looping Performance of La1−xMxMnO3 (M = Sr, Ca, (x = 0–0.5)) Perovskites. Nanomaterials. 2022; 12(19):3461. https://doi.org/10.3390/nano12193461
Chicago/Turabian StyleEvdou, Antigoni, Theofilos Georgitsis, Charitini Matsouka, Eleni Pachatouridou, Eleni Iliopoulou, and Vassilios Zaspalis. 2022. "Defect Chemistry and Chemical Looping Performance of La1−xMxMnO3 (M = Sr, Ca, (x = 0–0.5)) Perovskites" Nanomaterials 12, no. 19: 3461. https://doi.org/10.3390/nano12193461
APA StyleEvdou, A., Georgitsis, T., Matsouka, C., Pachatouridou, E., Iliopoulou, E., & Zaspalis, V. (2022). Defect Chemistry and Chemical Looping Performance of La1−xMxMnO3 (M = Sr, Ca, (x = 0–0.5)) Perovskites. Nanomaterials, 12(19), 3461. https://doi.org/10.3390/nano12193461